(C) 2012 Andreas Hertz. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
We describe the frog species Diasporus citrinobapheus sp. n. from the Cordillera Central of western Panama. The new species differs from all other species in its genus in coloration, disk cover and disk pad shape, skin texture, advertisement call, and size. It is most similar to Diasporus tigrillo, from which it differs in dorsal skin texture, relative tibia length, number of vomerine teeth, ventral coloration, dorsal markings, and relative tympanum size, and to Diasporus gularis, from which it can be distinguished by the lack of membranes between the toes, adult size, posterior thigh coloration, and position of the choanae. We provide data on morpho- logy, vocalization, and distribution of the new species, as well as brief information on its natural history.
ResumenDescribimos la especie de rana Diasporus citrinobapheus sp. n. de la Cordillera Central, occidente de Panamá. La nueva especie se distingue de otras especies del género por su coloración, su forma de la cubierta y la almohadilla de los discos, textura de la piel, canto de anúncio, y tamaño corporal. Se asemeja mas a Diasporus tigrillo, del cual se distingue por la textura de la piel dorsal, longitud relativa de la tibia, número de dientes vomerianos, coloración ventral, patrón dorsal, y tamaño relativo del tímpano, y a Diasporus gularis, del cual se diferencia por la ausencia de membranas entre los dedos de pie, tamaño corporal, coloración de la parte trasera del muslo, y posición de las coanas. Presentamos datos de la morfología, vocalización, y distribución de la nueva especie, así como notas concisas de su historia natural.
Central America, Anura, diversity of species, taxonomy, vocalization
América Central, Anura, diversidad de especies, taxonomía, vocalización
Panama’s herpetofauna is known to be the most diverse in consideration of its size in Central America, with only Mexico being more diverse in absolute species count (
The genus Diasporus (
Field work was carried out between May and August 2010 at several sites along both slopes of the Serranía de Tabasará between the Fortuna depression and Santa Fé, Veraguas, Panama. All specimens were encountered during opportunistic searches at night. Preparation and preservation of voucher specimens follows
We took additional morphological data from all Central American species currently assigned to the genus Diasporus in the SMF collection. We list all specimens examined for comparison in the Appendix I. Abbreviations for museum collections follow those of
The sex of the male holotype and the paratypes was determined by the presence of vocal slits and vocal sac. Measurements were made with a dial caliper with the aid of a dissecting microscope and rounded to the nearest 0.1 mm. Measurements of the holotype (LACM 146212) and paratype (LACM 146241) of Diasporus tigrillo were taken from
Advertisement calls were recorded using a Sennheiser ME 66 shotgun microphone capsule with a Sennheiser K6 powering module in combination with the Marantz PMD 620 solid-state recorder. A minimum distance from microphone to frog of one meter was kept while recording to prevent disturbance. As needed, the microphone was attached to branches with the aid of a Joby Gorillapod in order to minimize disturbance of the calling frog. Calls were recorded in PCM format at a sampling rate of 48 kHz with 24 bit resolution and stored as wav files on a SD Card. Call editing and analysis were performed using SOUND RULER 0.9.6.0 (
For the complementary molecular analysis, we extracted DNA following the protocol of
urn:lsid:zoobank.org:act:4A526693-CA45-44FC-9D9D-4F3064A47341
http://species-id.net/wiki/Diasporus_citrinobapheus
Figures 1 A, B; 2; 3; 5Adult male SMF 89814: collected on June 26, 2010 at 19:13 by Andreas Hertz and Sebastian Lotzkat at Quebrada Rasca (8.4851°N, 81.1727°W, 790 m elevation), near Paredón, Comarca Ngöbe-Buglé, western Panama, approximately 50 airline km NNW of the city of Santiago and 20 airline km N of Cañazas, Veraguas.
All collected by Andreas Hertz and Sebastian Lotzkat at the type locality on June 26, 2010: MHCH 2370-71; SMF 89816; all adult males.
Adult males SMF 89817 and MHCH 2372: collected on July 01, 2010 by Andreas Hertz and Sebastian Lotzkat at the private reserve Willie Mazú, Comarca Ngöbe-Buglé (8.7903°N, 82.1989°W, 681 m elevation); female SMF 89820: collected on March 31, 2009 by Andreas Hertz, Sebastian Lotzkat and Arcadio Carrizo at Cerro Negro, Parque Nacional Santa Fé, Veraguas (8.5691°N, 81.0988°W, 730 m elevation).
A member of the genus Diasporus based on the following combination of characters: vocal slits and a single subgular vocal sac present, adult males without nuptial thumb pads; Finger I shorter than Finger II; Toe III much shorter than Toe V; subarticular tubercles on hands and feet flattened; no supernumerary tubercles on hands and feet; no tarsal fold or tubercle. Diasporus citrinobapheus differs from alldescribed members of its genus by the following combination of characters (for accounts, see Table 1): coloration bright yellow to orange in life (Fig. 1 A); head almost as broad as long, but comparatively broad in relation to SVL; skin of dorsum smooth; venter coarsely areolate; tympanum covered by skin but annulus clearly visible; TD about 41% of ED; EL on average narrower than IOD; snout subacuminate in profile and rounded to subovoid in dorsal outline; disks of fingers and toes slightly expanded, disk covers of most fingers and toes spadate, but lacking papillae; disk pads of most fingers and toes triangular; subarticular tubercles of hands and feet rounded, very flat, almost not visible; vomerine odonthophores longish oval and widely separated; vomerine teeth weakly developed; upper eyelid usually smooth, very low pustules in some individuals; heel smooth. Its bright yellow to orange coloration distinguishes Diasporus citrinobapheus from almost all described Central American Diasporus, which, in spite of considerable variation, are all tan to gray or brownish to almost black. In Diasporus hylaeformis and Diasporus ventrimaculatus, the dorsal ground color can be suffused with pink or red. Only Diasporus tigrillo from Costa Rica, a species known only from two specimens, shows a yellowish coloration in life according to the original description (
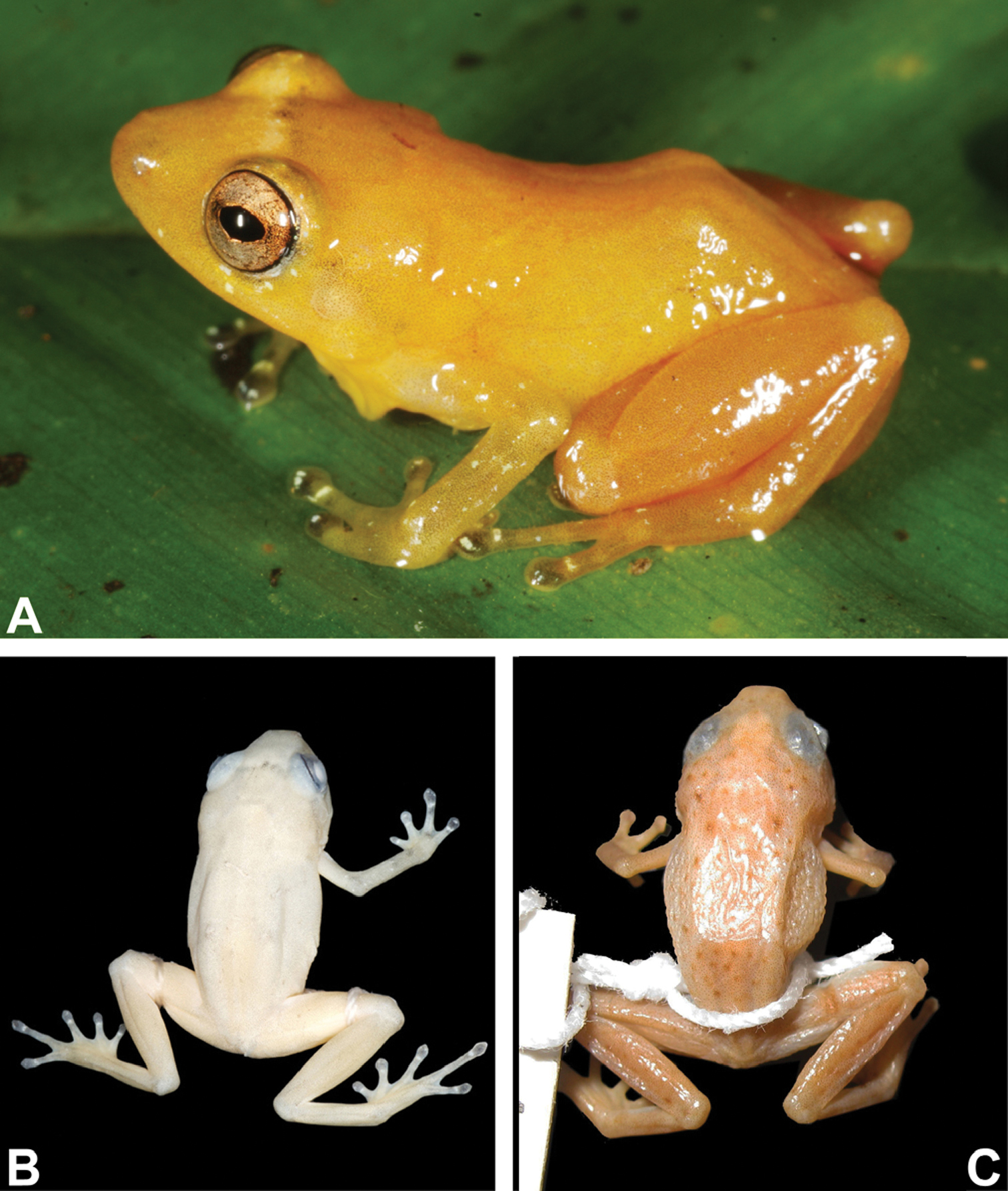
A–B Holotype ofDiasporus citrinobapheus (SMF 89814, adult male): Ain life B in preservative. C Diasporus tigrillo in preservative (LACM 146212, holotype, adult male), note dark brown spots. Pictures are not at the same scale.
Morphological measurements of Diasporus citrinobapheus in comparison with other described species of the genus from western Panama and southern Costa Rica (mean±SD, min–max). See Materials and Methods for abbreviations.
| Character | Diasporus citrinobapheus | Diasporus diastema | Diasporus hylaeformis | Diasporus ventrimaculatus | Diasporus vocator | Diasporus tigrillo | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| male (n=6) | female (n=1) | male (n=20) | female (n=22) | male (n=9) | female (n=5) | male (n=6) | female (n=2) | male (n=4) | female (n=6) | male (n=2) | |
| SVL (mm) | 18.7±0.63 (17.3–19.7) | 21.8 | 18.7±1.62 (15.9–22.1) | 18.7±2.58 (15.0–23.5) | 19.1±1.30 (16.9–20.9) | 21.2±0.97 (19.2–21.7) | 21.8±1.2 (20.2–23.5) | 23.9±0.8 (23.2–24.7) | 15.3±2.18 (12.2–17.2) | 14.7±2.18 (13.6–15.7) | 16.8 (16.0–17.5) |
| DW/LF III | 0.23±0.03 (0.18–0.26) | 0.23 | 0.36±0.06 (0.21–0.44) | 0.32±0.07 (0.21–0.44) | 0.31±0.03 (0.27–0.36) | 0.32±0.02 (0.29–0.34) | - | - | 0.26±0.06 (0.19–0.32) | 0.32±0.06 (0.24–0.42) | - |
| DW/LT IV | 0.14±0.03 (0.11–0.18) | 0.17 | 0.23±0.05 (0.15–0.32) | 0.22±0.05 (0.11–0.29) | 0.22±0.03 (0.18–0.26) | 0.22±0.02 (0.20–0.24) | - | - | 0.17±0.03 (0.13–0.19) | 0.17±0.03 (0.14–0.19) | - |
| HL/SVL | 0.41±0.01 (0.39–0.44) | 0.40 | 0.39±0.02 (0.35–0.44) | 0.41±0.02 (0.36–0.44) | 0.39±0.02 (0.35–0.43) | 0.39±0.02 (0.37–0.42) | 0.33 | 0.35 | 0.38±0.02 (0.35–0.41) | 0.38±0.02 (0.35–0.42) | 0.39 (0.38–0.40) |
| HW/SVL | 0.37±0.01 (0.35–0.38) | 0.36 | 0.36±0.02 (0.33–0.39) | 0.36±0.02 (0.32–0.39) | 0.37±0.01 (0.35–0.39) | 0.36±0.02 (0.35–0.40) | 0.39 | 0.40 | 0.34±0.02 (0.31–0.36) | 0.34±0.02 (0.31–0.36) | 0.36 (0.34–0.37) |
| HW/HL | 0.91±0.03 (0.88–0.97) | 0.90 | 0.91±0.06 (0.79–1.01) | 0.90±0.06 (0.78–1.04) | 0.94±0.05 (0.85–1.00) | 0.92±0.04 (0.85–0.96) | 1.15 | 1.14 | 0.89±0.08 (0.79–0.96) | 0.89±0.08 (0.86–0.95) | 0.92 (0.85–0.99) |
| TL/SVL | 0.41±0.01 (0.40–0.42) | 0.42 | 0.40±0.04 (0.35–0.51) | 0.42±0.05 (0.36–0.56) | 0.39±0.01 (0.37–0.42) | 0.39±0.05 (0.35–0.47) | 0.50 | 0.51 | 0.40±0.02 (0.38–0.43) | 0.38±0.02 (0.36–0.42) | 0.48 (0.46–0.50) |
| EL/IOD | 0.98±0.12 (0.83–1.12) | 0.94 | 1.04±0.10 (0.89–1.24) | 1.12±0.18 (0.89–1.62) | 1.01±0.12 (0.88–1.24) | 1.07±0.10 (0.88–1.19) | 0.86 | 1.00 | 1.07±0.12 (0.95–1.24) | 1.43±0.12 (1.25–1.59) | - |
| ED/HL | 0.32±0.03 (0.28–0.36) | 0.32 | 0.29±0.04 (0.22–0.35) | 0.29±0.04 (0.21–0.37) | 0.30±0.03 (0.27–0.35) | 0.28±0.03 (0.22–0.30) | 0.37 | 0.39 | 0.33±0.01 (0.32–035) | 0.34±0.01 (0.33–0.37) | 0.32 (0.28–0.35) |
| TD/ED | 0.39±0.07 (0.32–0.45) | 0.32 | 0.38±0.09 (0.27–0.65) | 0.37±0.08 (0.19–0.52) | 0.42±0.07 (0.30–0.52) | 0.45±0.03 (0.42–0.50) | 0.48 | 0.47 | 0.36±0.07 (0.30–0.44) | 0.43±0.07 (0.33–0.50) | 0.55 (0.54–0.57) |
| ED/SVL | 0.13±0.01 (0.11–0.15) | 0.13 | 0.11±0.20 (0.08–0.13) | 0.12±0.13 (0.09–0.15) | 0.12±0.01 (0.10–0.14) | 0.11±0.01 (0.09–0.12) | 0.12 | 0.13 | 0.13±0.01 (0.12–0.13) | 0.13±0.01 (0.12–0.14) | 0.12 (0.11–0.13) |
An adult male; measurements (in mm): SVL 18.4, LF III 2.4, LT IV 4.2, DWF III 0.6, DWT IV 0.5, HL 7.2, HW 7.0, TL 7.8, EL 2.6, IOD 2.9, TD 0.8, ED 2.4; dorsal skin smooth; venter coarsely areolate; no discoidal fold; upper eyelid smooth; snout subovoid in dorsal outline and subacuminate in profile; nostrils weakly protuberant, directed dorsolaterally; head slightly longer than wide, width 97% of length; HW 38% of SVL; canthus rostralis indistinct; ED 36% of HL and 13% of SVL; EL 90% of IOD; TD 33% of ED (Fig. 2 A); choanae round, orientated extremely laterally on palate, partially concealed by palatal shelf of maxillary arch; elliptical vomerine odonthophores, posteromedian to choanae, which are widely separated from each other, with four rows of weakly developed, short teeth; legs short in relation to body; TL 42% of SVL; relative finger length: I<II=IV<III; all fingers with disks, slightly wider than digits, on Fingers II–IV wider than on Finger I; relative toe length: I<II<III<V<IV, Toe V much longer than toe III; tip of Toe V extending to distal subarticular tubercle on Toe IV; tip of Toe III extending to penultimate subarticular tubercle on Toe IV; disks on Toes III–V larger than on I–II; disk covers spadate, lacking papillae; no supernumerary tubercles (Figs 2 B, C).
Holotype ofDiasporus citrinobapheus (SMF 89814, adult male): A Lateral view of head B Ventral view of right hand. C Ventral view of right foot. Scale bars = 1 mm.
The specific name citrinobapheus is a noun in apposition and is derived from the Greek words citrinos (citrin-yellow) and bapheus (dyer) referring to the yellow body color that dyes one’s fingers yellowish when the frog is handled. Although we could observe this phenomenon in a few other species of Diasporus too, it is notably evident in the new species.
All examined specimens show shades of bright yellow and orange dorsally; some have dark grayish and/or whitish-grayish spots (Fig. 3). Ventral surfaces are almost achlorophyllaceous and transparent apart from the yellow male vocal sac.
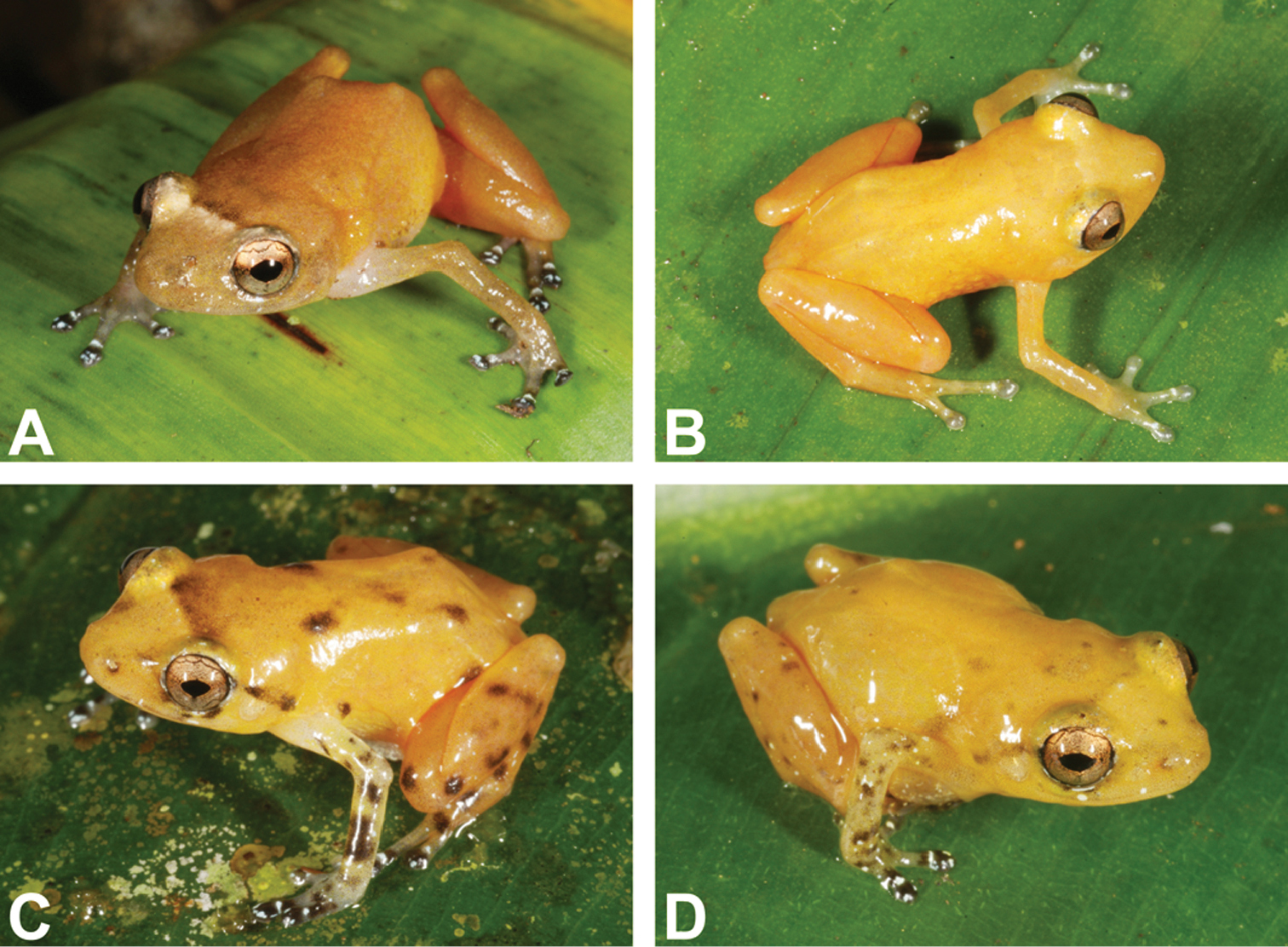
Variation in coloration pattern in life of Diasporus citrinobapheus from different localities: A Female SMF 89820 from Cerro Negro, Parque Nacional de Santa Fé (Veraguas, Panama) with dirty orange coloration B Male SMF 89816 from type locality Paredón (Comarca Ngöbe-Buglé, Panama) with immaculate yellow coloration C Male MHCH 2372 from Willie Mazú (Comarca Ngöbe-Buglé, Panama) with intense mottling D Male SMF 89817 from Willie Mazú (Comarca Ngöbe-Buglé, Panama) with intermediate mottling.
MHCH 2372 (Fig. 3 C): Dorsal ground color Orange Yellow (18); posterior and anterior surfaces of thighs Chrome Orange (16); Raw Umber (23) interorbital and postocular stripes formed by very fine mottling; dorsum with five Dark Grayish Brown (20) blotches, forming a pattern like the five dots on a dice; scattered Dark Grayish Brown (20) blotches on dorsal surfaces of limbs; disk covers Blackish Neutral Gray (82), with white rings at the base; ventral surface of hind limbs Chrome Orange (16); ventral surface of body transparent with dirty white mottling; vocal sac white with a suggestion of Spectrum Yellow (55).
SMF 89820 (Fig. 3 A): In the only female, coloration in life has been recorded as follows: Dorsal surface Yellow Ocher (123 C); a Chamois (123 D) interorbital bar; anterior and posterior surfaces of thighs Chrome Orange (16); venter almost transparent; upper surfaces of disks Sepia (119) with dirty white spots and a dirty white ring around base; gular region Smoke Gray (44).
In preservation the bright yellow and orange colors fade rapidly to a pale grayish yellow (Fig. 1 B) with scattered dark grayish blotches in some individuals. Legs pale orange; vocal sac pale yellow in males; gular area in females pale gray; tips of digits dark grayish black. Dark grayish black eyeballs shining through skin when head is viewed dorsally.
Compared to other species of this genus, the individuals of Diasporus citrinobapheus available to us exhibit only little variation in their coloration (Fig. 3). All show a yellow to orange dorsal ground color in life. This can either appear bright and clear or somewhat dirty, depending on the pigment translocation within the melanophores in the frog’s skin. In some individuals, higher concentrations of melanophores in certain areas of the dorsum form dark blotches or stripes. This is especially the case in the two specimens from Willie Mazú (Figs 3 C, D). The most frequent pattern of this type is an interorbital bar, which in most cases is darker than ground color along the anterior edge of the bar and lighter than ground color along the posterior edge. In addition, some individuals show dark brown blotches on the limbs and less frequently also on the dorsum. Most individuals show additional small whitish spots, in particular under and around the eyes, as well as scattered across the forelimbs. In the male SMF 89816 from the type locality (Fig. 3 B) the dark and white markings on and around the disk covers are not as pronouncedly contrasting as in the other individuals examined.
The distinctiveness of Diasporus citrinobapheus is supported by the analysis of the 16S mitochondrial rRNA gene (Fig. 4). The four individuals we examined form a distinct cluster that appears separated from the other members for which 16S sequences are available. The mean genetic distance among the four specimens of Diasporus citrinobapheus is 0.3%. In our consensus tree (Fig. 4 A) it appears to be most closely related to the candidate species Diasporus aff. diastema from El Copé, from which it is separated by a mean genetic distance of 1.8%. In the haplotype network analysis (Fig. 4 B) both clades form unconnected subnetworks, indicating a differentiation at species level (Fig. 4 C). The mean genetic distance to the next closest relative Diasporus quidditus is 6.6% for Diasporus citrinobapheus and 7% for Diasporus aff. diastema.
Results of 16S mtDNA analysis. A Consensus tree from Maximum Likelihood analysis. Scale bar refers to substitutions per site. Bootstrap support values before the slash correspond to Maximum Likelihood analysis, those after the slash to the Maximum Parsimony consensus tree of exactly the same topology. Numbers behind branches refer to respective museum numbers B Parsimony network derived from the same alignment, with each node representing a unique haplotype separated by one substitutional step from its nearest neighbor. Rectangles are haplotypes of analyzed sequences, circles are haplotypes missing in our sample C Tentative taxonomic implication. Bar breaks indicate assumed species boundaries. Names refer to morphological determination or GenBank taxonomic identity.
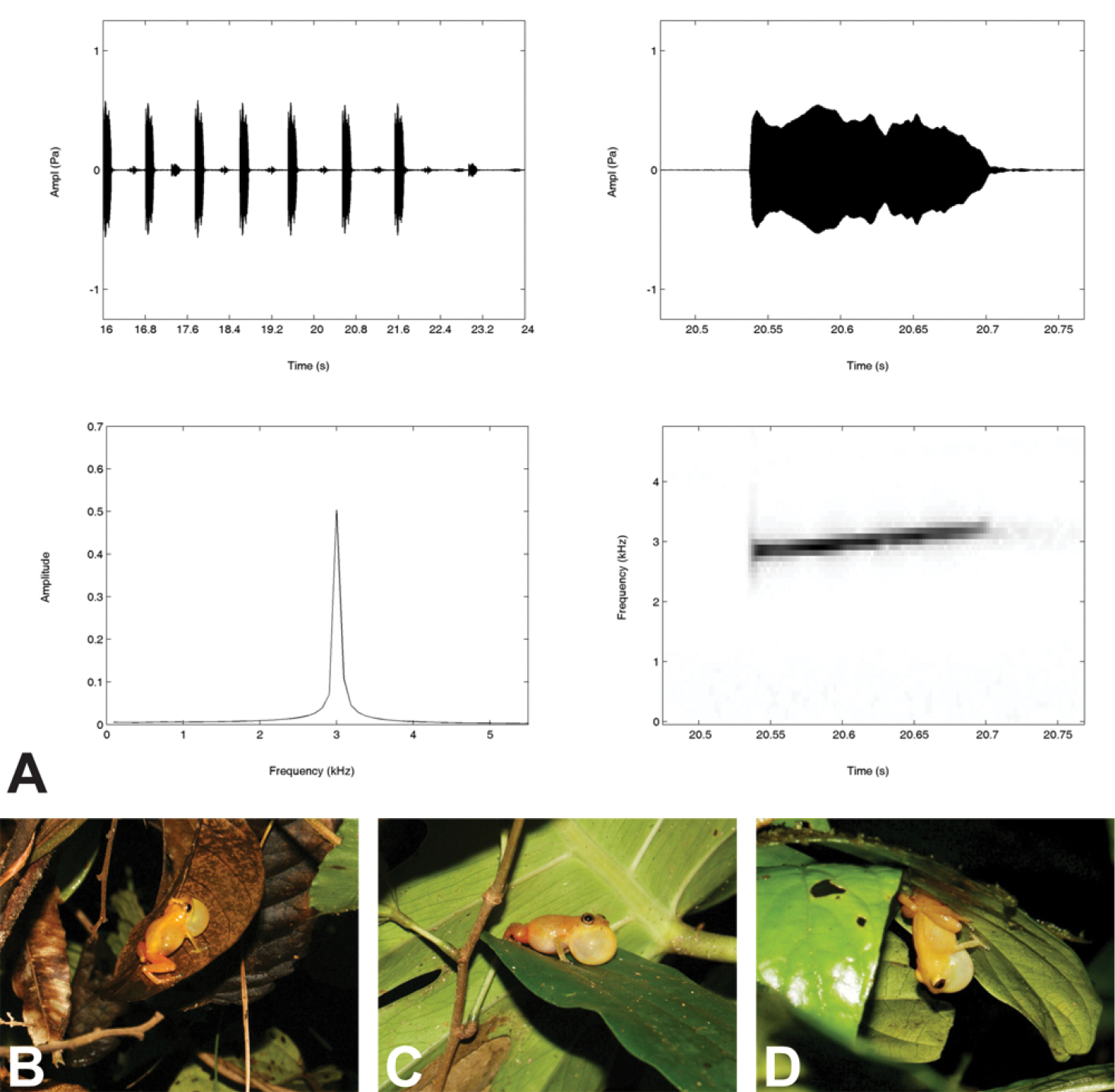
We recorded a 3 min, 43.5 seconds portion of the advertisement call of the holotype that yielded a total of 63 calls. An exemplary visualization of the call structure is given in Fig. 5 A. Relative humidity during recording was 95.3% at an air temperature of 24.5 °C. As in other members of the genus, the call consists of a single note, even though calls sound like a “whistle, ” rather than the typical “tink” usually emitted by members of the genus Diasporus (
A Visualizations of an advertisement call (Hanning window function, FFT 512, 0.8 overlap) of Diasporus citrinobapheus (holotype, SMF 89814) recorded in Paredón, Comarca Ngöbe-Buglé, Panama, at 24.5°C air temperature and 95.3% relative humidity. Clockwise from top left: Oscillogram of a call group; Oscillogram of the penultimate call in the shown call group; Power spectrum showing the dominant frequency of the penultimate call in the shown call group; Spectrogram of the penultimate call in the shown call group B–D Different call positions of male Diasporus citrinobapheus: B Male holotype (SMF 89814) from Paredón calling on dead leaves in dense vegetation about 2 meters above ground level; C Male paratype (MHCH 2371) from Paredón on green leaf about 3 m above ground level D Male specimen (SMF 89817) from Willie Mazú referred to as Diasporus citrinobapheus calling from an elevated position on the underside of a leaf.
In addition to the holotype, we recorded and analyzed the advertisement calls of two paratypes (SMF 89816, MHCH 2371) and one referred specimen (SMF 89817). Summing up, the advertisement call of Diasporus citrinobapheus sounds like a whistle, is organized in call groups, has a call duration of 0.14–0.16 s in average and a dominant frequency of 2860–3040 Hz (see all parameters in Table 2). While the paratypes vary only little in call parameters, SMF 89817 shows obvious differences regarding call duration, call interval, and call rate (see Discussion section for details).
Variation in advertisement call parameters in four male specimens referred to as Diasporus citrinobapheus (mean±SD, min–max).
| SMF 89814 | SMF 89816 | MHCH 2371 | SMF 89817 | |
|---|---|---|---|---|
| Temperature / RH during recording | 24.5° C/95.3 % | 24.3° C/93.5 % | 24.6° C/93.6 % | 21.8° C/100 % |
| Total recording time (min) | 3.73 | 1.35 | 1.66 | 3.03 |
| Number of call groups recorded | 5 | 2 | 1 | 4 |
| Number of calls recorded | 63 | 26 | 11 | 68 |
| Call group rate (call groups / min) | 1.34 | 1.48 | 0.6 | 1.32 |
| Call group duration (s) | 25.0±5.7 19.8–34.1 | 23.0±9.5 16.3–29.7 | 19.0 | 20.6±8.5 12.5–28.5 |
| Calls per group | 12.8±3.2 8–17 | 11–15 | 11 | 16.6±5.7 10–22 |
| Call group interval (s) | 21.6±8.0 15.7–33.2 | 26.84 | >78 | 25±16.7 10.9–43.5 |
| Call rate over entire recording (calls/min) | 16.9 | 19.3 | 6.63 | 22.4 |
| Call rate within a call group (calls/min) | 32±6.3 23.4–40.8 | 35.4±7.1 30.3–40.4 | 35 | 50±6.8 44.2–60 |
| Call duration (s) | 0.157±0.01 0.126–0.178 | 0.162±0.01 0.143–0.174 | 0.156±0.003 0.151 – 0.162 | 0.141±0.01 0.114–0.167 |
| Call interval (s) | 1.93±1.2 0.57–5.77 | 1.74±1.4 0.63–3.77 | 1.71±0.75 0.85 – 2.85 | 1.15±0.49 0.55–2.58 |
| Dominant frequency (Hz) | 2953±0 | 3010±75 2859–3140 | 2859±0 | 2965±32 2953–3046 |
| Minimum frequency (Hz) | 2889±44 2859–2953 | 2776±31 2765–2859 | 2671±0 | 2939±33 2895–2953 |
| Maximum frequency (Hz) | 3257±44 3140–3328 | 3184±61 3064–3234 | 3029±38 2953–3046 | 3290±74 3140–3421 |
| Frequency modulation (Hz) | 367 188–469 | 407 281–468 | 358 281–375 | 351 281–375 |
So far, Diasporus citrinobapheus has been found on the Caribbean slopes of the western Serranía de Tabasará and on both Pacific and Caribbean slopes of the eastern Serranía de Tabasará (Fig. 6) at intermediate elevations from 680 to 790 m.a.s.l. Males call from very dense vegetation and are difficult to spot. They are almost only detectable by following their characteristic vocalization. Vocal activity is highest just after dusk and finally stops when it becomes dark. Calling height ranges from near ground level up to three meters above ground. Calling position can be either on the upper side of a leaf or on its underside (Figs 4 B–D). The only female (SMF 89820) was found at daytime (15:00 h) inside an involute, young plantain leaf that apparently served as a daytime hiding place. The species does not seem to be limited to mature forest, but is also found in secondary growth and plantations. However, it appears to avoid open habitats like pasture land.
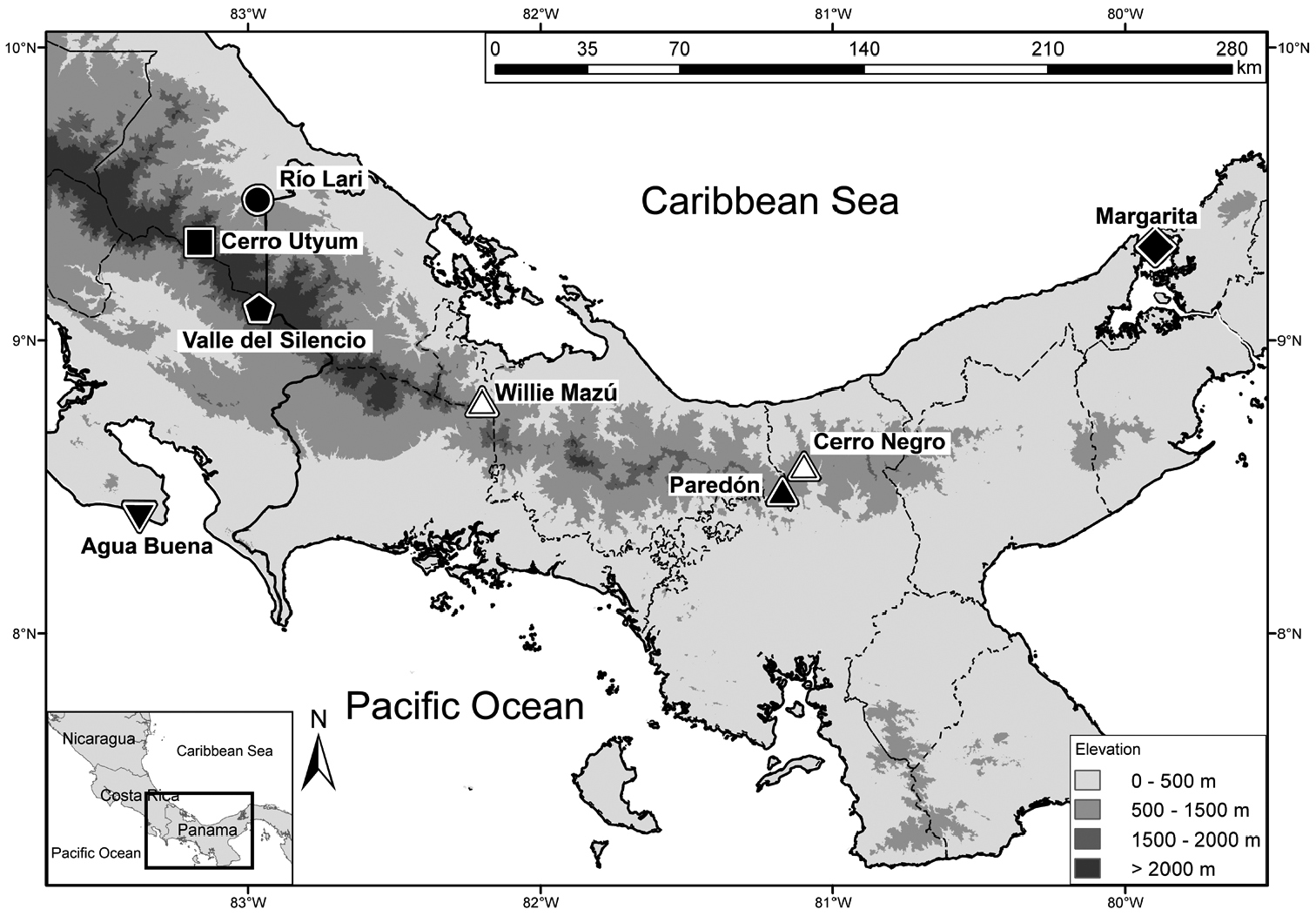
Distribution map of Diasporus citrinobapheus and type localities of other species in the genus in Panama and Costa Rica. Solid triangle: Paredón, Comarca Ngöbe-Buglé, type locality of Diasporus citrinobapheus. Hollow triangles: Additional collection sites of Diasporus citrinobapheus: Private Reserve Willie Mazú in the west, and Cerro Negro (Parque Nacional Santa Fé, Veraguas) in the east. Inverted triangle: Agua Buena, Puntarenas, Costa Rica, type locality of Diasporus vocator. Pentagon: Valle del Silencio, at the provincial boarder between Puntarenas and Limón, Costa Rica, type locality of Diasporus ventrimaculatus. Square: Cerro Utyum, Limón, Costa Rica, type locality of Diasporus hylaeformis. Circle: Río Lari, Limón, Costa Rica, type locality of Diasporus tigrillo. Diamond: Margarita, Colón, Panama, type locality of Diasporus diastema. Dashed lines represent provincial borders. Solid lines represent coast line and national border.
Diasporus citrinobapheus is easily distinguishable from all other known frogs of the genus in Lower Central America by its bright yellow to orange coloration. The only described species of the genus that somewhat resemble the new species in coloration are Diasporus gularis from Colombia and Ecuador and Diasporus tigrillo from the Caribbean slopes of the Costa Rican part of the Serranía de Talamanca. The latter species is known only from two specimens, both collected in 1964 at a single locality and there are no photographs of the species in life, tissue samples, or call recordings available to clarify the systematic relationships of this species. The different ground coloration in preservative between Diasporus tigrillo and Diasporus citrinobapheus is certainly due to different preservation techniques, because the fixation process in 10% formalin darkens the complete specimen. However, this does not influence the general color pattern, so we treat the dark brown spots on the dorsum of Diasporus tigrillo as a diagnostic feature to differentiate between Diasporus tigrillo and Diasporus citrinobapheus. Additional material is required, preferably from near the type locality of Diasporus tigrillo to conduct further studies. In contrast, Diasporus gularis is known from a number of specimens from Colombia and Ecuador. However, the presence and development of papillae at the apex of the pad on the underside of the disk cover, one of the main characters that has been used to distinguish this species from its congeners, is a controversial issue.
Besides various records of other amphibians and reptiles, we found no additional species of the genus Diasporus at the type locality. At Willie Mazú, a locality approximately 120 km NW of the type locality of Diasporus citrinobapheus, we collected a single specimen of Diasporus that we refer to Diasporus vocator based on size, coloration, disk shape, and male advertisement call. At Cerro Negro, Diasporus citrinobapheus occurs sympatrically with Diasporus diastema. Based on our current concept of its distribution, the possibility remains that also Diasporus vocator occurs at this locality, although the species has not been recorded from this site.
The eponymous, readily soluble yellow coloration of Diasporus citrinobapheus lead us to the assumption that this might serve a defensive function against predators. On this account, an alcohol extraction was analyzed for alkaloids, but no active substances were found. Probably, the yellow pigment is just highly soluble and therefore easily washed out. Nevertheless, one could speculate that it has a bitter or otherwise unpalatable taste that might deter certain predators.
Various studies have shown that the advertisement call represents a premating isolating mechanism in anurans (e.g.,
Nevertheless, there is also an intraspecific variation among calls of specimens referred to Diasporus citrinobapheus. The call of the single male recorded at Willie Mazú (SMF 89817) differs from the calls of the members of the type series in temporal parameters, such as shorter call duration and call interval that result in a higher call rate. These differences are minor, but lead us to not include specimens from localities other than the type locality in the type series. However, various studies have shown that call parameter variation is linked to ambient temperature (e.g.,
Apart from morphology which apparently is not the best tool to identify species of Diasporus, neither DNA nor bioacoustics, both of paramount importance for contemporary anuran taxonomy, have been consistently analyzed among geographically and taxonomically wide-ranging samples. While the Panamanian and Costa Rican 16S barcodes compared in this study reveal the existence of more infrageneric lineages than names are available, the doubtless assignation of a given Diasporus “aff. hylaeformis” or “aff. diastema” is likely to be highly challenging if one is to rely on the existing treatments, which mostly provide only partial or even contradicting information. In conclusion, the complex and cryptic diversity within the genus Diasporus requires a thorough revision of as many “quality vouchers” (collected specimens associated with both well-preserved tissue samples and call recordings) from as many localities throughout the generic range as possible.
Key to the species of Diasporus in Central America
| 1a | Disk covers lanceolate or papillate | 2 |
| 1b | Disk covers palmate or spadate | 3 |
| 2a | Dorsum shagreened; fingers without thick lateral fringes; Toe V not partially fused with Toe IV; SVL of adult males 14.0–16.0 mm, of adult females 16.5–18.0 mm | Diasporus vocator |
| 1b | Dorsum with scattered low warts; fingers with thick lateral fringes; Toe V partially fused with Toe IV; SVL of adult males 10.9–14.8 mm, of adult females 13.2–16.9 mm | Diasporus quidditus |
| 3a | Fingers II and III with palmate disk covers and broadened, non-triangular disk pads; adults with vomerine teeth | Diasporus diastema |
| 3b | Fingers II and III with spadate disk covers and triangular disk pads; adults with or without vomerine teeth | 4 |
| 4a | Venter in most individuals with distinct black and white blotches; dorsum and dorsal surfaces of arms and legs in some individuals bright red in life | Diasporus ventrimaculatus |
| 4b | Venter patternless or with a few small black dots; dorsum and dorsal surfaces of arms and legs brown, cream, or yellow in life | 5 |
| 5a | Posterior surface of thigh pigmented (brownish, often suffused with red in life); overall dorsal coloration bright cream, grayish or reddish brown in life; adults without vomerine teeth | Diasporus hylaeformis |
| 5b | Posterior surface of thigh unpigmented (yellow in life); overall dorsal coloration bright yellow to orange in life; adults with vomerine teeth | 6 |
| 6a | Dorsum with scattered low pustules; ratio tympanum length / eye length 0.54–0.57; distal subarticular tubercle on Fingers and Toes I weakly bifid; dorsum yellow to orange with dark brown spots confined to pustules; SVL of adult males 16.0–17.5 mm | Diasporus tigrillo |
| 6b | Dorsum smooth; ratio tympanum length / eye length 0.32–0.45; distal subarticular tubercle on Fingers and Toes I flat and rounded; dorsum uniformly bright yellow to orange, sometimes with irregularly distributed dark blotches; SVL of adult males 17.3–19.7 mm | Diasporus citrinobapheus |
Collecting permits SC/A-8-09 and SC/A-21-10, as well as the corresponding exportation permits were issued by the Dirección de Áreas Protegidas y Vida Silvestre of the Autoridad Nacional del Ambiente (ANAM), Panama City, Panama. An additional permit for the Comarca Ngöbe-Buglé was issued by Rogelio Moreno (Cazique General of the Ngöbe), San Félix. We are especially grateful to the inhabitants of Paredón for their exceptional hospitality, to Wilberto Martínez, owner of the private reserve Willie Mazú, for his permission to survey on his ground, as well as to the family Peña Solís, Alto de Piedra, for logistical support. For assistance in the field we thank Arcadio Carrizo, Joe Bienentreu and Smelin Abrego. Particular thanks go to Dietrich Mebs and Werner Pogoda, Center for Forensic Medicine, University of Frankfurt am Main, who kindly performed an analysis of the new species’ skin secretions, to Neftali Camacho, Natural History Museum of Los Angeles County, Los Angeles, who provided photographs of the type material of Diasporus tigrillo, and to Linda Acker, Senckenberg Forschungsinstitut and Naturmuseum, who prepared the illustrations of the holotype of Diasporus citrinobapheus. We thank Franco Andreone, Larry Wilson and two anonymous reviewers for insightful comments on an early draft of the manuscript. This paper is based upon work funded to AH by the FAZIT-Stiftung, and to SL by the Studienstiftung des deutschen Volkes, as well as the Vereinigung von Freunden und Förderern der Goethe-Universität.
Diasporus diastema — Costa Rica: Heredia: Puerto Viejo de Sarapiquí, entrance to La Selva, 30 m: SMF 81812; Rara Avis, 700 m: SMF 81811; Honduras: Gracias a Dios: Region La Mosquitia, Rio PlátanoBiosphere Reserve, Raudal Kiplatara, 162 m: SMF 85938; El Ocotilla, 410 m: SMF 85939; Nicaragua: Atlántico Norte: PN Saslaya, 920 m: SMF 82031-82035; Jinotega: Bosawas Biosphere Reserve: SMF 78561; National Park Saslaya, 188 m: SMF 78965; Matagalpa: Selva Negra, 1300 m: SMF 77231, 77235, 78184–78191; Río San Juan: Bartola, 30–70 m: SMF 80977–80979, SMF 79799–79800; Río Sarnoso, 25 m: SMF 79796–79798; Boca de San Carlos, 20 m: SMF 79794–5; ridge near Río Las Cruces, near Caño Negro, 415 m: SMF 83389; Cerro el Bolívar, near Río Machado, 280 m: SMF 83390; Lomas de Tambor, 210 m: SMF 83391; Panama: Bocas del Toro: Bosque Protector Palo Seco, 1148 m: SMF 84997; Archipelago Bocas del Toro, Isla Colón, 30 m: SMF 85068; Chiriquí: El Chorogo, 295 m: SMF 92008; Coclé: Cerro Gaitál, El Valle de Antón, 800 m: SMF 80781; Comarca Kuna Yala: Reserva Natural Nusagandi, 280 m: SMF 81961; Panamá: Canal Zone: SMF 29859, 29874.
Diasporus aff. hylaeformis — Panama: Bocas del Toro: Parque Internacional La Amistad, northern slope of Cerro Pando, 2400 m: SMF 89868, 89869, 89873, 89874, AH 263, AH 266. Chiriquí: Parque Internacional La Amistad, Jurutungo, southern slope of Cerro Pando, 2070–2330 m: SMF 89867, 89872, 89875, 89876, AH 126, 127; Cerro Punta, Las Nubes Ranger Station, 2070 m: SMF 89870, 89871.
Diasporus vocator — Panama: Bocas del Toro: Humedal de San San Pond Sak, 5 m: SMF 89865, AH 364; Comarca Kuna Yala: Reserva Natural Nusagandi, 350 m: SMF 81970–81976.
Corresponding information of sequenced Diasporus specimens.
| Species | Collection number | Field number | GenBank accession no. | Country | Province | Latitude, Longitude |
|---|---|---|---|---|---|---|
| Diasporus citrinobapheus | SMF 89814 | AH 449 | JQ927333 | Panama | Comarca Ngöbe-Buglé | 8.485, -81.173 |
| Diasporus citrinobapheus | SMF 89820 | AH 211 | JQ927334 | Panama | Veraguas | 8.569, -81.099 |
| Diasporus citrinobapheus | MHCH 2370 | AH 450 | JQ927335 | Panama | Comarca Ngöbe-Buglé | 8.485, -81.173 |
| Diasporus citrinobapheus | MHCH 2371 | AH 452 | JQ927336 | Panama | Comarca Ngöbe-Buglé | 8.485, -81.173 |
| Diasporus aff. hylaeformis | SMF 89868 | AH 267 | JQ927337 | Panama | Bocas del Toro | 8.931, -82.714 |
| Diasporus aff. hylaeformis | SMF 89869 | AH 268 | JQ927338 | Panama | Bocas del Toro | 8.931, -82.714 |
| Diasporus aff. hylaeformis | SMF 89872 | AH 124 | JQ927339 | Panama | Chiriquí | 8.911, -82.713 |
| Diasporus aff. hylaeformis | SMF 89875 | AH 282 | JQ927340 | Panama | Chiriquí | 8.912, -82.713 |
| Diasporus aff. diastema ‘orange’ | USNM 572442 | KRL 0902 | FJ784425 | Panama | Coclé | 8.667, -80.592 |
| Diasporus aff. diastema ‘orange’ | USNM 572443 | KRL 1181 | FJ784484 | Panama | Coclé | 8.667, -80.592 |
| Diasporus aff. diastema ‘orange’ | USNM 572454 | KRL 0900 | FJ784423 | Panama | Coclé | 8.667, -80.592 |
| Diasporus aff. diastema ‘orange’ | USNM 572455 | KRL 0901 | FJ784424 | Panama | Coclé | 8.667, -80.592 |
| Diasporus aff. diastema ‘orange’ | MVUP 1783 | KRL 0694 | FJ784338 | Panama | Coclé | 8.667, -80.592 |
| Diasporus aff. diastema ‘orange’ | MVUP 1830 | KRL 0840 | FJ784395 | Panama | Coclé | 8.667, -80.592 |
| Diasporus quidditus | USNM 572444 | KRL 0647 | FJ784326 | Panama | Coclé | 8.667, -80.592 |
| Diasporus quidditus | MVUP 1832 | KRL 0856 | FJ784405 | Panama | Coclé | 8.667, -80.592 |
| Diasporus vocator | FMNH 257769 | AJC 0127 | JN991419 | Costa Rica | Puntarenas | 8.79, -82.96 |
| Diasporus aff. diastema | USNM 572546 | KRL 0782 | FJ784369 | Panama | Coclé | 8.667, -80.592 |
| Diasporus aff. diastema | MVUP 1826 | KRL 0831 | FJ784390 | Panama | Coclé | 8.667, -80.592 |
| Diasporus diastema | MVZ 203844 | 1999 | EU186682 | Costa Rica | Cartago | 9.75, -83.804 |
| Diasporus hylaeformis | UCR 16264 | AJC 0468 | JN991418 | Costa Rica | Alajuela | 10.22, -84.54 |
| Pristimantis ridens | UTA-A 57014 | ENS 10722 | JN991464 | Honduras | Olancho | 14.93, -86.14 |
Audio samples of the advertisement calls of specimens of Diasporus citrinobapheus. (doi: 10.3897/zookeys.196.2774.app1) File format: MP3 Audio file (MP3).
Explanation note: MP3 audio samples of specimens of Diasporus citrinobapheus recorded at the type locality (Paredón) and Willie Mazú, Serranía de Tabasará, Panama.