(C) 2011 Christer Hansson. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Three new species of Eulophidae associated, or presumed to be associated with ants are described: two species of Horismenus Walker and one species of Microdonophagus Schauff. Information on the biology is also included. The two Horismenus species are from Chiapas, Mexico. Horismenus myrmecophagus sp. n. is known only from females and is a gregarious endoparasitoid in larvae of the weaver ant Camponotus sp. ca. textor. The parasitoids pupate inside the host larva, and an average of 6.7 individuals develops per host. This is the second time a species of genus Horismenus is found parasitizing the brood of a formicine ant of genus Camponotus. Horismenus microdonophagus sp. n. is described from both males and females, and is a gregarious endoparasitoid attacking the larvae of Microdon sp. (Diptera: Syrphidae), a predator on ant brood found in nests of Camponotus sp. ca. textor. The new species of Microdonophagus, Microdonophagus tertius, is from Costa Rica, and known only from the female. Nothing is known about its biology but since another species in same genus, Microdonophagus woodleyi Schauff, is associated with ants through its host, Microdon larva (with same biology as Horismenus microdonophagus), it is possible that also Microdonophagus tertius has this association. A new distributional record for Microdonophagus woodleyi is also reported, extending its distribution from Panama and Colombia to Brazil.
Horismenus, Microdonophagus, Camponotus, Microdon, ant parasitism, myrmecophile, taxonomy
Natural enemies of ants include dipteran, strepsipteran and hymenopteran parasitoids (for a review see
Four associations involving a eulophid wasp and an ant host have been reported to date, and all are genera belonging to the subfamily Entedoninae: an unidentified gregarious parasitoid, apparently closely related to the genus Paracrias Ashmead (identified by Gahan), was recorded parasitizing larvae of the myrmicine Crematogaster acuta (Fabr.) in Guyana (
Here we describe two species of Horismenus, one parasitizing the brood of the weaver ant Camponotus sp. ca. textor, and the other parasitizing a syrphid myrmecophile associated with this ant species. A new species of Microdonophagus Schauff presumed to be associated with ants is also described. A new distributional record for Microdonophagus woodleyi is provided.
MethodsSpecimens for this study were either reared (Horismenus species) or collected manually (Microdonophagus), killed in alcohol, and subsequently critical point dried and mounted on cards for further studies. Observations of the specimens were made through a stereomicroscope, Nikon© SMZ 1500 with a halogen ring light as light source. The colour photos were taken with a DS-Fi1 camera mounted on the stereomicroscope and the light source for the photos was a dome light manufactured from a description on http://www.cdfa.ca.gov/. Each picture was made from several photos taken at different levels of focus, and merged using Helicon Focus©. Micrographs are from uncoated specimens analyzed in low vacuum, with a JEOL© JSM 5600 LV scanning microscope.
Morphological abbreviations and acronymsAbbreviations for morphological terms: DE = shortest distance between eyes in frontal view; DO = diameter of median ocellus; HE = height of eye; HW = height of fore wing; LC = length of median carina on propodeum; LG = length of gaster; LM = length of marginal vein; LS = length of hind tibial spur; LT = length of hind tarsus; LW = length of fore wing, measured from base of marginal vein to apex of wing; MM = length of mesosoma; MS = malar space; OOL = distance between one posterior ocellus and eye; PM = length of postmarginal vein; POL = distance between posterior ocelli; POO = distance between posterior ocelli and occipital margin; ST = length of stigmal vein; WC = greatest width of median carina on propodeum; WH = width of head; WM = width of mouth; WT = width of thorax. For illustrations of the morphological terms see http://www.neotropicaleulophidae.com/.
Collection acronyms used are: BMNH = The Natural History Museum, London, England; CH = collection of Christer Hansson; ECO-CH-AR = Arthropod Collection El Colegio de la Frontera Sur-Chetumal, Mexico; USNM = the United States National Museum of Natural History, Washington, D.C., USA.
Taxonomy Genus Horismenus WalkerThere are 400 species described of the almost exclusively New World genus Horismenus Walker, 1843, mostly from the Neotropical region (
urn:lsid:zoobank.org:act:CE88218A-4A94-4FD3-B87C-09C5442AA1AA
http://species-id.net/wiki/Horismenus_myrmecophagus
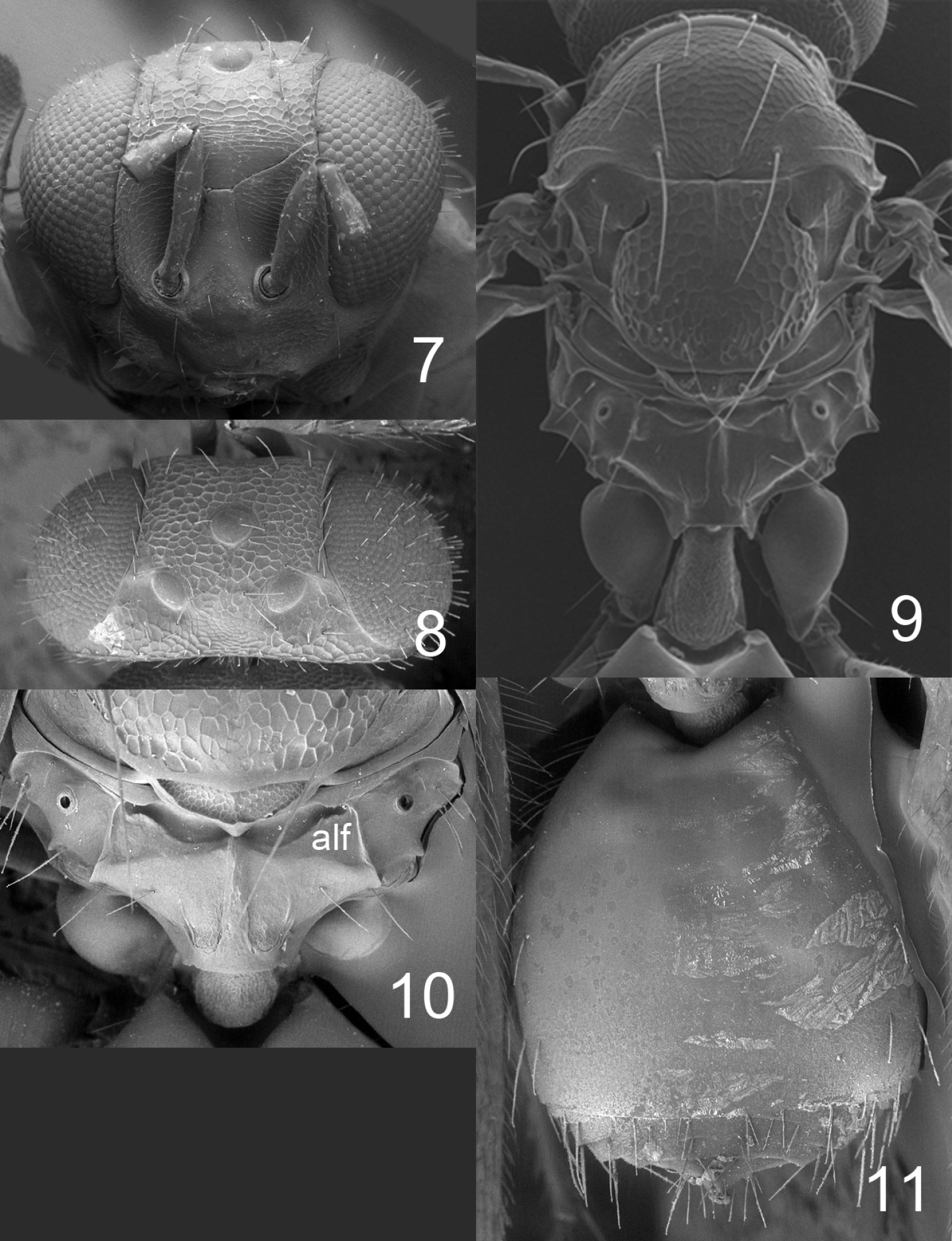
Figures 1, 2–6, 17, 21, 23–24HOLOTYPE female (BMNH), glued to a card, labelled “MEXICO: Chiapas, Tuxtla Chico, Rosario Izapa, 14°58'25"N, 92°09'19"W, 430 m, 25.ii.2010, G. Pérez-Lachaud & J.-P. Lachaud, reared from Camponotus sp. ca. textor pupa, nest no. 2, on mandarine (Citrus reticulata)”.PARATYPES. 1♀ with same label data as holotype (BMNH); 29♀ with same label and host data as holotype but collected from nest #3 28.ii.2010 (22♀ in BMNH, 2 ♀ in CH, 5♀ in ECO-CH-AR). Several paratypes have opaque and somewhat distorted wings due to premature killing in alcohol, i.e. before the wing membranes had hardened.
Frons with interscrobal area protruding and carinate (Fig. 2); scutellum entirely reticulate, without median groove and lateral mesh–rows (Fig. 4); fore wing speculum small and closed below (Fig. 21); all coxae white; propodeum with submedian grooves strongly reticulate and with anterolateral foveae weakly indicated anteriorly (Fig. 5); propodeal callus with five setae.
The species is very similar to Horismenus alienus Hansson, but differs mainly in the shape of the petiole which in Horismenus alienus has a strongly raised transverse carina dorsally, but Horismenus myrmecophagushas two strong and rounded projections dorsolaterally (Fig. 5); it differs also in sculpture of median propodeum: smooth in Horismenus alienus, but strongly reticulate in Horismenus myrmecophagus(Fig. 5).
Female. Length of body 1.1–1.4 mm. Scape white; pedicel and flagellum pale brown. Frons golden–green with purple tinges (Fig. 23). Vertex metallic bluish–green. Mesoscutum metallic bluish–green (Fig. 24). Scutellum dark golden–purple with green tinges (Fig. 24). Propodeum dark golden–purple (Fig. 24). Legs white. Wings hyaline. Petiole dark golden–purple. Gaster dark brown with metallic purple tinges.
Antenna as in Fig. 17. Frons (Fig. 2) with part just above frontal suture with raised and weak reticulation, remaining parts with raised and strong reticulation; frontal suture V–shaped, incomplete and not reaching eyes; antennal scrobes joining frontal suture separately. Vertex (Fig. 3) with raised and strong reticulation; without a median groove. Occipital margin rounded.
Mesoscutum with raised and strong reticulation (Fig. 4); notauli indistinct. Scutellum with raised and strong reticulation (Fig. 4), without median groove and lateral mesh–rows. Dorsellum slightly concave and with raised and strong reticulation. Propodeum with raised and strong reticulation (Fig. 5); propodeal callus with five setae. Coxae with raised and weak reticulation. Fore wing speculum small and closed below (Fig. 21); with 12 admarginal setae.
Gaster (Fig. 6) with first tergite with very weak reticulation posteriorly and laterally, otherwise smooth.
Camponotus sp. ca. textor larva parasitized by Horismenus myrmecophagus. H. myrmecophagus develops as a gregarious endoparasitoid. The ant larva has been cut open (its head is at the bottom of the picture). Several pupae of the eulophid parasitoid may be observed, some of them still inside the ant larva.
Horismenus myrmecophagus female: 2 head in frontal view 3 vertex 4 thoracic dorsum 5 propodeum 6 gaster in dorsal view.
DE/DO 6.9; WH/DE 1.9; HE/MS/WM 2.4/1.0/2.0; POL/OOL/POO 2.5/1.0/1.1; WH/WT 1.2; LW/LM/HW 1.8/1.0/1.0; PM/ST 1.4; LC/WC 1.4; WG/WC 2.0; LS/LT 0.22; MM/LG 1.0.
Male. Unknown.
Named after the feeding habits of the larva (from the Greek myrmecophagus = ant eater).
Mexico (Chiapas).
Horismenus myrmecophagusis a gregarious endoparasitoid of the larvae of Camponotus sp. ca. textor, a neotropical weaver ant. Parasitized host larvae spin a cocoon before their development is arrested, but no pupation occurs. Parasitized ant larvae are not modified in external form or color by the developing parasitoids, but changes in appearance were observed in the host at the end of the wasp larval development. In material preserved in alcohol, late instar larvae, pupae and teneral adults of the wasps can be readily observed inside ant larvae, within the host cocoon, but earlier developmental stages of the parasitoids could not be detected. The wasp larvae pupate inside the host larva. Horismenus individuals occupy almost the entire body of the host. Wasp pupae were found aligned on either part of the middle of the body of the host, their heads converging to the center, while the cephalic and caudal portions of the host larva were occupied by the host remains and the parasitoids meconia (Fig. 1). An average of 6.7 individuals developed per host (range: 4–12, mode: 7, n=27 parasitized cocoons examined). Adults emerge from the host cocoon through a unique, common hole pierced in the host larval cuticle and through the cocoon wall, but it is unknown whether adult wasps leave the nests to mate. Only females have been observed to date (all broods examined, where the sex of the parasitoid could be ascertained, were constituted by females (n=10 parasitized hosts)). The facts that only single sex broods parasitize any one host, and that only females are known, suggest that Horismenus myrmecophagus is a thelythokous species. Large ant larvae (presumably queens) have never been observed to be parasitized.
Camponotus sp. ca. textor (until now referred to in the literature as Camponotus senex textor Forel) is a common, dominant ant in shade coffee plantations in the Soconusco Region of Chiapas, Mexico (
The host range of Horismenus myrmecophagus is unknown. It is possible that this species may attack other ant species occupying similar niches, given that certain species of Horismenus are known to be polyphagous (e.g. Horismenus aeneicollis, Horismenus apantelivorus, Horismenus opsiphanis or Horismenus sardus, see
The similar species Horismenus alienus is known only from the female and its host/biology is unknown, but due to its morphological similarity to Horismenus myrmecophagusit is possible that Horismenus alienus is also a parasitoid of ants.
urn:lsid:zoobank.org:act:1848E913-005D-48C1-8431-870D4FACB80F
http://species-id.net/wiki/Horismenus_microdonophagus
Figures 7–11, 18–19, 22, 25–27HOLOTYPE female (BMNH) glued to a card, labelled “MEXICO: Chiapas, Tuxtla, Chico, Rosario Izapa, 14°58'25"N, 92°09'19"W, 430 m, 28.ii.2010, G. Pérez-Lachaud & J.-P. Lachaud, reared from larva of Microdon sp., predator inside Camponotus sp. ca. textor, nest no. 3”. PARATYPES. 10 ♀ 2♂ with same label data as holotype (4 ♀ 1♂ in BMNH, 1♀ in CH, 5♀ 1♂ in ECO-CH-AR).
Fore wing speculum covered with setae (Fig. 22); scutellum transverse, 0.75X as long as wide, entirely reticulate with raised and strong reticulation and with a narrow median groove in anterior half (Fig. 9); propodeum with a median carina but without submedian grooves (Fig. 10). This species is easy to recognize through these diagnostic features.
Female. Length 2.0 mm. Scape yellowish–brown, pedicel pale brown, flagellum dark brown. Frons dark golden–green (Fig. 25). Vertex golden–red. Mesoscutum golden–red with posterior 2/3 of midlobe metallic bluish–green (Fig. 26), to predominantly metallic bluish–green or golden–green. Scutellum golden with a median spot metallic bluish–green (Fig. 26), to predominantly metallic bluish–green. Propodeum metallic purple (Fig. 26). Coxae black to dark brown with golden–green tinges; femora, tibiae and tarsi yellowish–brown. Wings hyaline. Petiole black, shiny. Gaster metallic dark purple.
Antenna as in Fig. 18. Frons (Fig. 7) with interscrobal and clypeal areas and part just above frontal suture smooth, remaining parts with raised and strong reticulation with small meshes; frontal suture V–shaped, incomplete not reaching eyes; antennal scrobes join with frontal suture separately. Vertex (Fig. 8) with raised and strong reticulation, areas just behind posterior ocelli smooth; posterior part without median groove. Occipital margin rounded (Fig. 8).
Mesoscutum and scutellum with raised and strong reticulation (Fig. 9); notauli as indistinct impressions, forming posterior part of midlobe to an indistinct triangle. Dorsellum concave with raised and strong reticulation. Propodeum smooth (Fig. 10) or with raised and weak reticulation; median carina narrow and weak; propodeal callus with 5–7 setae and with 2–3 additional setae on median part of propodeum. Coxae smooth. Fore wing speculum absent or very small, obliterated by setae (Fig. 22); with 15 admarginal setae.
Gaster (Fig. 11) with first tergite smooth and shiny with a very weak reticulate band close to posterior margin.
Horismenus microdonophagus female: 7 head in frontal view 8 vertex 9 thoracic dorsum and petiole 10 propodeum 11 gaster in dorsal view. Abbreviation alf = anterolateral fovea.
DE/DO 4.2; WH/DE 2.4; HE/MS/WM 2.7/1.0/1.4; POL/OOL/POO 3.1/1.0/1.6; WH/WT 0.9; LW/LM/HW 1.9/1.2/1.0; PM/ST 1.7; LC/WC 4.0; WG/WC 1.5; LS/LT 0.32; LP/WP 1.5; MM/LG 1.3–1.4.
Male. Length 1.6 mm. The male is similar to the female except: scape inflated (Fig. 19) and dark brown, slightly longer petiole and shorter gaster.
HE/MS/WM 2.4/1.0/1.2; LP/WP 1.6; MM/LG 1.6.
Named after the feeding habits of larvae (from the Greek microdonophagus = eater of Microdon).
Mexico (Chiapas).
Horismenus microdonophagus is a gregarious endoparasitoid of Microdon larvae (Diptera: Syrphidae), a predator on the brood of Camponotus sp. ca. textor. One Microdon sp. larva that was about to pupate was found inside a Camponotus nest. From this single host 79 females and 6 males of Horismenus microdonophagus emerged.
One of the two males has the flagellum of both antennae missing, as have also some of the female paratypes, and the other male has the entire right flagellum and apical two flagellomeres of the left antenna missing. Only specimens in fair condition were included in the description, i.e. are type material. The remaining specimens were too fragmented to be included.
This is an exclusively Neotropical genus recorded from Brazil, Colombia, Costa Rica and Panama (
urn:lsid:zoobank.org:act:5DB16050-0C5D-41AE-A2EA-6D16981D2E2C
http://species-id.net/wiki/Microdonophagus_tertius
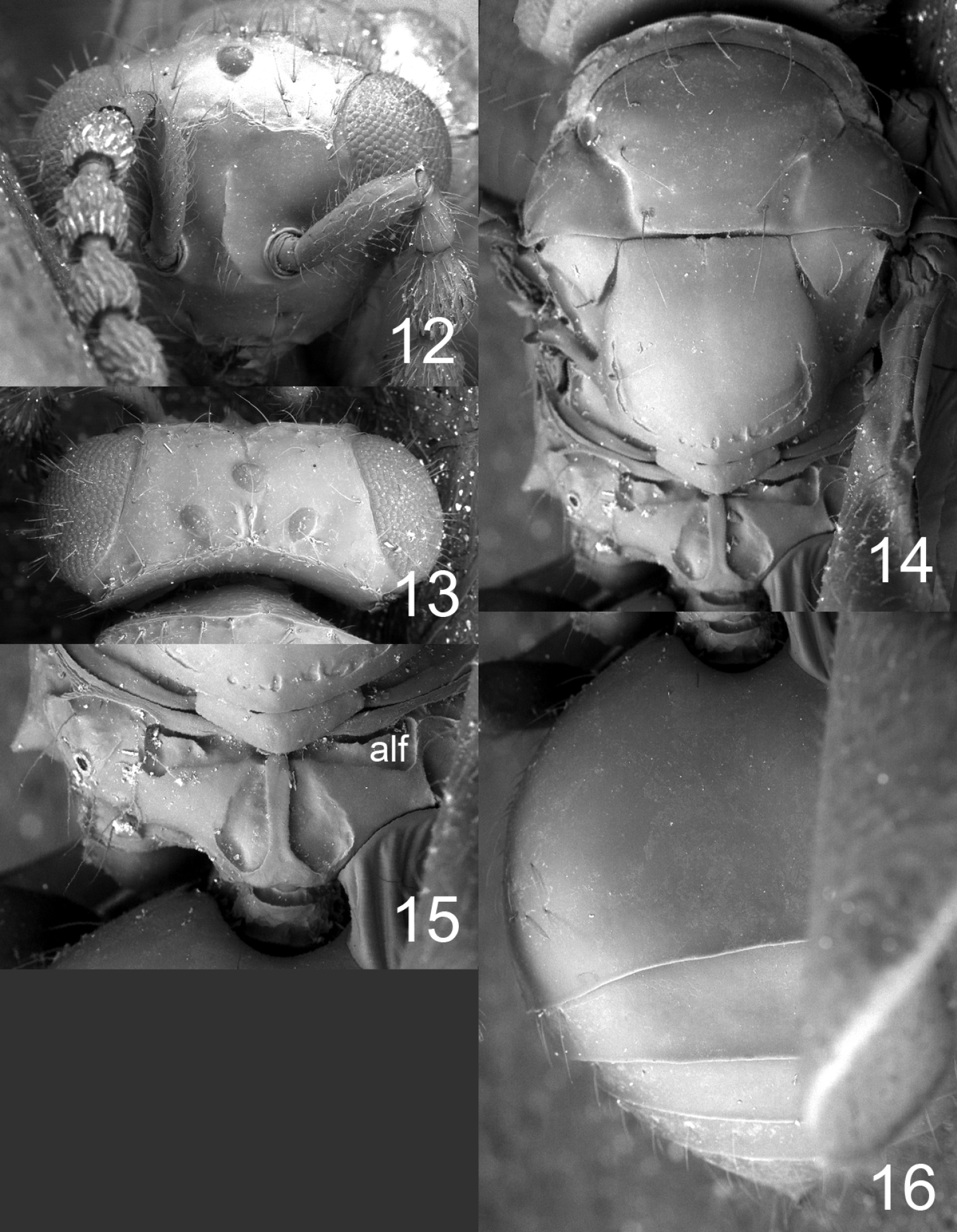
Figures 12–16, 20, 28HOLOTYPE female (BMNH) glued to a card, labelled “COSTA RICA, Puntarenas, Parque Nacional Corcovado, Mosokha, Quebrada Hedionda, 15.iii–15.iv.2003, Khanaki.”
This species is similar to Microdonophagus levis Hansson (
Female. Length 2.0 mm. Scape yellowish–brown, pedicel and flagellum pale brown. Head and body including gaster dark brown and shiny. Coxae pale brown; femora, tibiae and tarsi yellowish–brown. Wings hyaline.
Flagellum without anelli, with three funicular segments and a two-segmented clava (Fig. 20). Frons smooth and shiny (Fig. 12), without antennal scrobes and frontal suture, with a narrow and sharp process (an interantennal crest) between toruli. Vertex smooth and shiny (Fig. 13). Occipital margin sharp (Fig. 13). Eyes with scattered long hairs (longer than in Microdonophagus levis).
Mesoscutum smooth and shiny (Fig. 14); midlobe with two pairs of setae; notauli as distinct grooves throughout. Scutellum smooth and shiny (Fig. 14); with one pair of setae; with sublateral grooves in posterior half. Propodeum with a narrow median carina (Fig. 15); with wide sublateral grooves; with distinct anterolateral foveae; propodeal callus with about eight setae; propodeal surface smooth. Fore wing with four setae (right fore wing) and six setae (left fore wing) respectively on dorsal surface of submarginal vein; costal cell bare; speculum small and closed below; postmarginal vein not visible.
Petiole hidden behind inflated gaster but appears to be about as long as wide, dorsal surface with strong sculpture. Gaster circular; gastral tergites smooth (Fig. 16).
Microdonophagus tertius female: 12 head in frontal view 13 vertex 14 thoracic dorsum 15 propodeum 16 gaster in dorsal view. Abbreviation alf = anterolateral fovea.
17–20 antenna in lateral view: 17 Horismenus myrmecophagus female 18 H. microdonophagus female 19 H. microdonophagus male (apical 2 flagellomeres missing) 20 Microdonophagus tertius female. 21–22 right fore wing female: 21 H. myrmecophagus 22 H. microdonophagus.
23–24 Horismenus myrmecophagus female: 23 head in frontal view 24 thoracic dorsum. 25–26 Horismenus microdonophagus female: 25 head in frontal view 26 thoracic dorsum.
HE/MS/WM 1.7/1.0/1.3; POL/OOL/POO 6.0/4.4/1.0; WH/WT 1.0; LW/LM/HW 1.7/1.0/1.0; LP/WP ca 1; MM/LG 1.0.
Male. Unknown.
From the Latin tertius = third. Named for this being the third species described in the genus.
Costa Rica.
Unknown, but possibly associated with ants, as is the type species of Microdonophagus, Microdonophagus woodleyi.
http://species-id.net/wiki/Microdonophagus_woodleyi
Figure 30 Brazil (new record, 1♀ from Santa Catarina, Nova Teutonia, 7.iv.1938, F. Plaumann, in BMNH), Colombia (
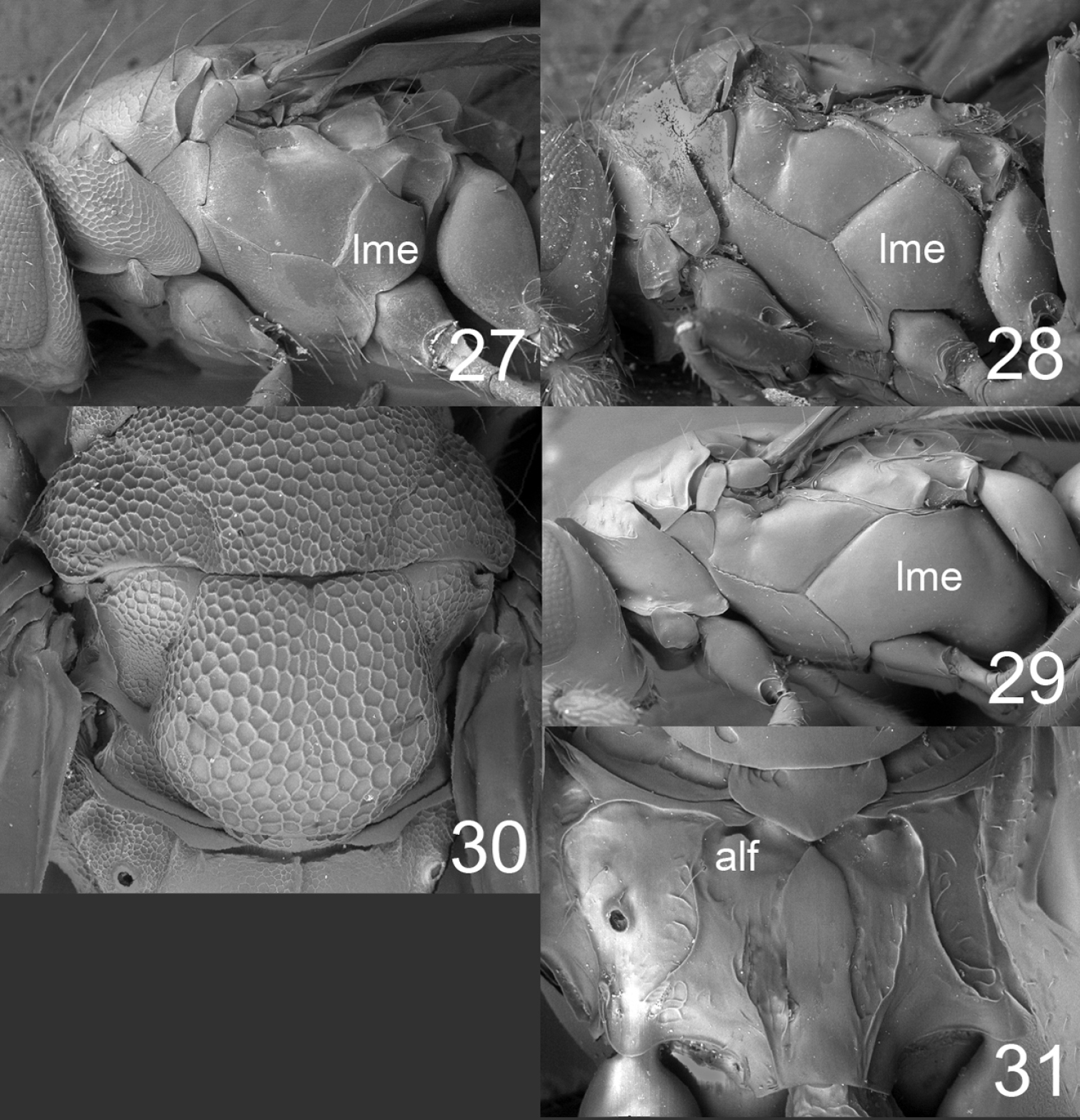
27–29 mesosoma in lateral view females: 27 Horismenus microdonophagus 28 Microdonophagus tertius 29 M. levis Hansson 30 M. woodleyi Schauff, part of thoracic dorsum with scutellum. 31 M. levis, propodeum. Abbreviations alf = anterolateral fovea; lme = lower mesepimeron.
Horismenus and Microdonophagus can be identified using the matrix key to Entedoninae genera on the website http://www.neotropicaleulophidae.com/. These two genera share certain characters that indicate that they are sister-groups: multiporous plate sensilla on flagellomeres with upper surface concave; propodeum with median carina, submedian grooves and anterolateral foveae. However, they also possess characters that show that each genus is a distinct monophyletic group:
| Horismenus | Microdonophagus |
| Antennal spicule with apical seta | Antennal spicule with apical peg (apomorphy) |
| Antennal scrobes as grooves | Antennal scrobes not visible (apomorphy) |
| Frontal suture as groove | Frontal suture not visible (apomorphy) |
| Axillar fovea present (apomorphy) | Axillar fovea missing |
| Lower mesepimeron normal | Lower mesepimeron enlarged (apomorphy) |
(using modifications of the key in
Horismenus myrmecophagusfemales run to subkey B and from there to couplet 5, first alternative, where Horismenus myrmecophagusis differentiated from Horismenus alienus as indicated above under diagnosis for Horismenus myrmecophagus.
Horismenus microdonophagus females run to subkey D and from there to couplet 8 where the second alternative is chosen and this leads to 9a instead of 9, and then:
| 9a | Fore wing speculum small (Fig. 22) and propodeum with a complete median carina (Fig. 10) | Horismenus microdonophagus sp. n. |
| – | Fore wing speculum large, or median carina on propodeum mainly obliterated by reticulation | 9 |
Horismenus microdonophagus males are difficult to include in the key because the antennal clava is missing. However, the combination of strongly inflated scape, transverse scutellum that is completely reticulate, small fore wing speculum, and propodeum without submedian grooves make males of this species easy to recognize (true also for females).
Key to all species of Microdonophagus
| 1 | Scutellum with raised and strong reticulation (Fig. 30) | Horismenus woodleyi Schauff (female) |
| – | Scutellum predominantly smooth and shiny (Fig. 14) | 2 |
| 2 | Lower mesepimeron (lme) strongly enlarged (Fig. 29); propodeum with indistinct anterolateral foveae (alf) (Fig. 31) | Microdonophagus levis Hansson |
| – | Lower mesepimeron (lme) smaller (Fig. 28); propodeum with distinct anterolateral foveae (alf) (Fig. 15) | Microdonophagus tertius sp. n. |
Parasitization of ants is uncommon among Eulophidae and a survey of published literature showed that only four (possibly just three) eulophid genera, all Entedoninae, are recorded as parasitoids of ants. Apart from Horismenus (Horismenus floridensis (
Systematic surveys of macro- and microfauna biodiversity directly within ant colonies are lacking (
One hypothesized evolutionary path to parasitism of nest-building Hymenoptera, e.g. ants, by Hymenoptera parasitoids is via their non-hymenopterous symbionts, and then at some point in time a host shift has occurred (
We thank J.H.C. Delabie (Laboratório de Mirmecologia, CEPEC/CEPLAC, Itabuna-BA, Brazil) for confirming the identification of the ant host. We also acknowledge the Department of Biology, Lund University for the use of their SEM facility.