(C) 2012 Terry L. Erwin. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Adults and larvae of Leptotrachelus dorsalis (Fabricius), the Sugarcane Savior Beetle, live in association with grasses, the larvae in the appressed leaf axils. Both adult and larval Leptotrachelus dorsalis eat larvae of the Sugarcane Borer, Diatraea saccharalis (Fabricius), and perhaps other insects living in the confines of the leaf sheaths of that and other grass-like species. The geographic range of Leptotrachelus dorsalis extends from Kansas in the west to the Atlantic seaboard, north as far as Ontario, Canada and south to Cuba; it is an eastern species of North America and the Caribbean. Larval character attributes that are shared with a related ctenodactyline, Askalaphium depressum (Bates), provide a preliminary basis for characterization of the immatures of tribe Ctenodactylini.
Sugarcane Savior Beetle, Louisiana, commensalism, Sugarcane, Saccharum officinarum L. , Sugarcane Borer, Diatraea saccharalis (Fabricius)
Leptotrachelus dorsalis (Fabricius) is known to occur in Canada – ON; Cuba; and the USA – AL, AR, CT, DC, DE, FL, GA, IA, IL, IN, KS, KY, LA, MD, MI, MN, MO, MS, NC, NJ, NY, OH, PA, SC, SD, TN, VA, and WV. According to
A stand of Cattails, Typha latifolia L. (foreground) near the edge of a sugarcane field (background) in the environs of Houma, LA. Insert: Photo credit: Randy Richard of the USDA, ARS Sugarcane Research Unit.
Adult, dorsal aspect, of Leptotrachelus dorsalis (Fabricius); adult from Wittman, Talbot County, MD. Apparent body length (ABL) = 8.1mm.
Now that we have reared specimens (all stages except egg), we realize that Van Emden’s description is not that of purely Leptotrachelus dorsalis individuals.
Bionomics of Leptotrachelus dorsalis are discussed in a separate paper (
Specimens were initially obtained from experiments to determine economic thresholds for Sugarcane Borer in new sugarcane cultivars (
Descriptive and larval preparation methods follow those suggested by the classic carabid larval method paper of
(The following is based on larvae of Askalaphium depressum (Bates) and Leptotrachelus dorsalis (Fabricius), the only confirmed described larvae in the Tribe).
Recognition. (See
(Modified from
| 27 (25) | Blade of mandible and/orretinaculum denticulate or crenulate | 28 |
| – | Blade of mandible andretinaculum not denticulate or crenulate | 36 |
| 28 (27) | Cervicalgroove present, short | 29 |
| – | Cervical groove absent | 32 |
| 29 (28) | Antennae distinctly longer than mandibles | Panagaeini (in part) |
| – | Antennae subequal to mandibular length 30 | |
| 30 (29) | Nasale medially produced, equally quatro-dentate; maxilla with inner lobe (L3); pygopod with dense patch of setae ventrally (L3); cerci not articulated | Ctenodactylini |
| – | Nasale not produced, margin medially microdentate, two lateral teeth larger than medial dents; maxilla without inner lobe (L3); pygopod without dense patch of setae ventrally (L3); cerci articulated | Odacanthini |
| 1 | Head and body markedly depressed; head with definitive neck; tarsus multispinose, spines robust; pygopod multisetiferous medio-ventrally, setae curved, decumbent posteriorly | Askalaphium Liebke, 1938 |
| – | Head and body slightly depressed; head without definitive neck; tarsus bisetose, setae fine; pygopod without medio-ventral patch of curved setae, general setae normal, straight, not decumbent | Leptotrachelus Latreille, 1829 |
Coloration (as in Fig. 3). Mostly pale cream color with infuscated head capsule, mandibles, and urogomphi, the latter with pale spots; other mouthparts, antennae, and pronotum slightly darker than rest of body.
Head capsule without visible sculpticels.
Head (Figs 3, 4, 8). Nasale moderately produced, quarto-dentate, teeth coequal in length; mandibles robust and with obvious serrations medially on blade and posterior to retinaculum; genae not prominent, very slightly wider than distance across stemmata, slightly narrowed to broad neck. Eyes of 6 barely prominent stemmata. Antennomere slightly shorter than porrect mandible; antennomere 2 slightly shorter than 1, 3, and 4. Mandible with prominent retinaculum, curved dentiform; terebral blade obviously serrate, pensillus absent. Ligula of labium slightly produced, unisetose, labrum ventrally sextasetose. Ratios of palpomere lengths can be deduced from the illustrations.
Larva, 3rd instar (top), 2nd instar (bottom), dorsal aspect, of Leptotrachelus dorsalis (Fabricius). Apparent body length (ABL) (L3 = 8.9mm; L2 = 7.0)
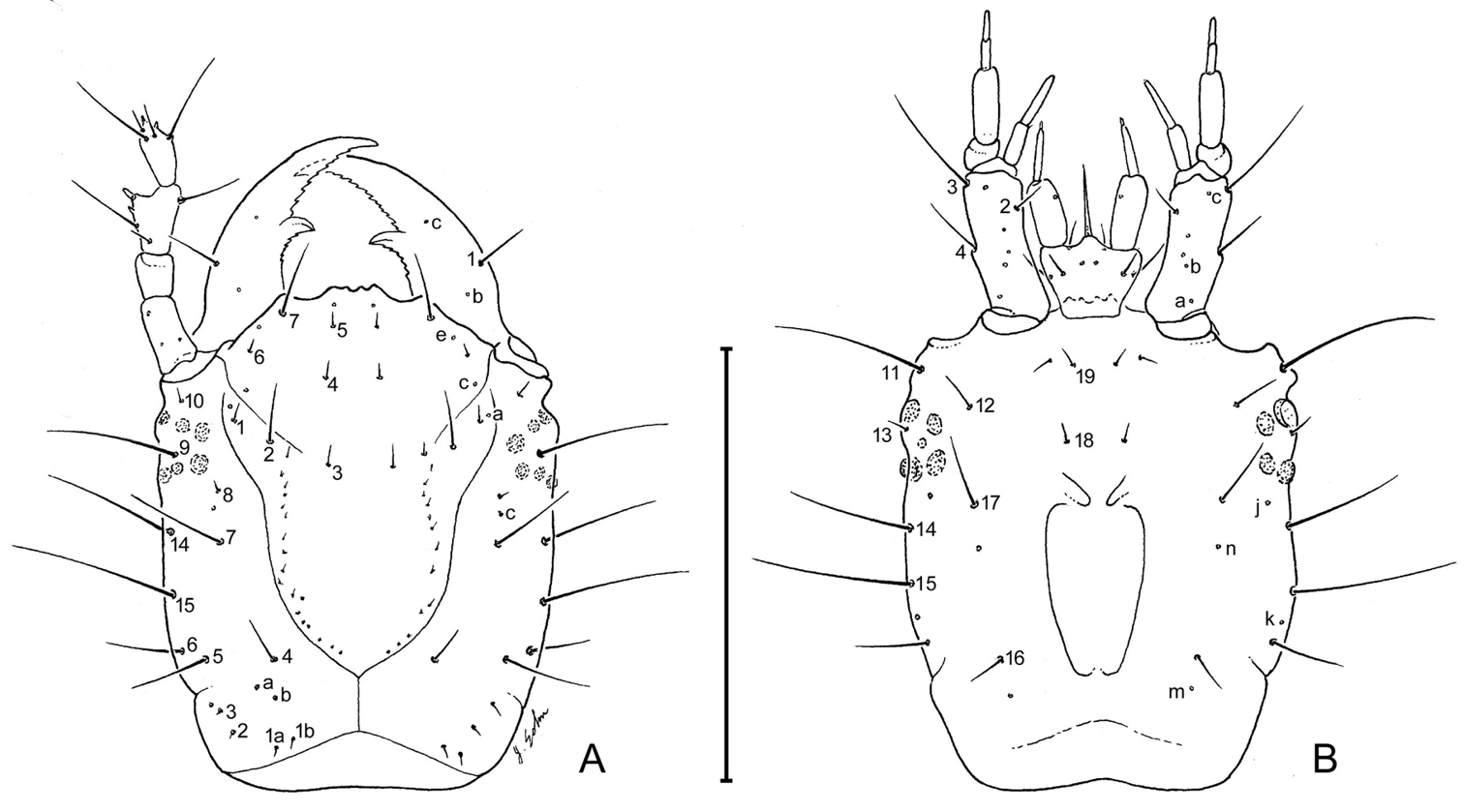
Larval head capsule (L1) of Leptotrachelus dorsalis (Fabricius). A dorsal aspect B ventral aspect. Scale line equals 0.5 mm.
(Figs 3, 5, 9). Prothorax narrowly quadrate (L1), more broadly quadrate (L3); meso- and metathorax transverse trapezoid, narrow anteriad, broader posteriorly.
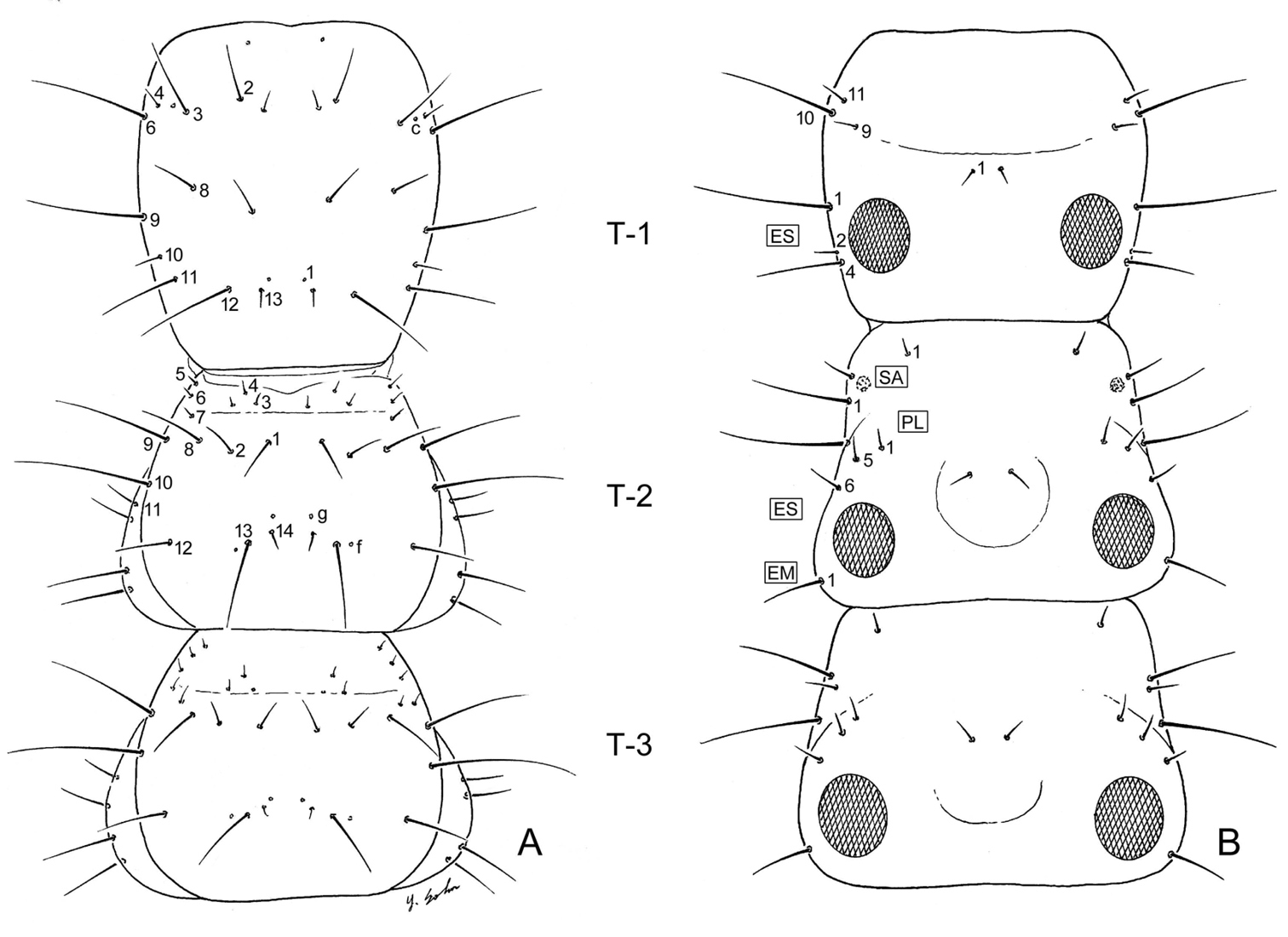
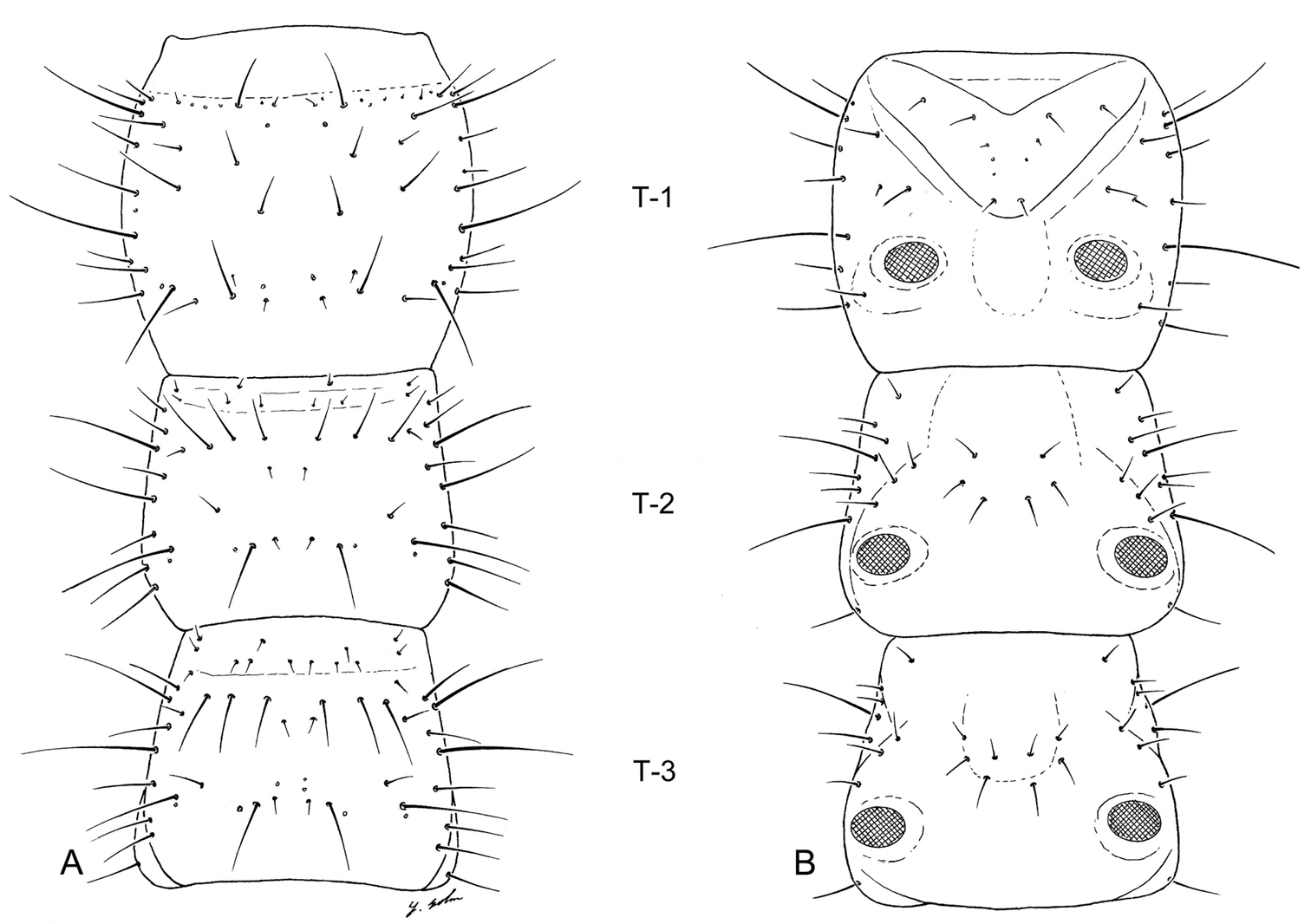
Larval thorax (L1), of Leptotrachelus dorsalis (Fabricius). A dorsal aspect B ventral aspect. Prothorax T-1 Mesothorax T-2 Metathorax T-3 Episternum ES Epimeron EM. Scale line equals 0.5 mm.
(Figs 3, 6, 7, 10, 11). Segments hexagonal, broad. Urogomphi about one and a half times as long as prothorax is long.
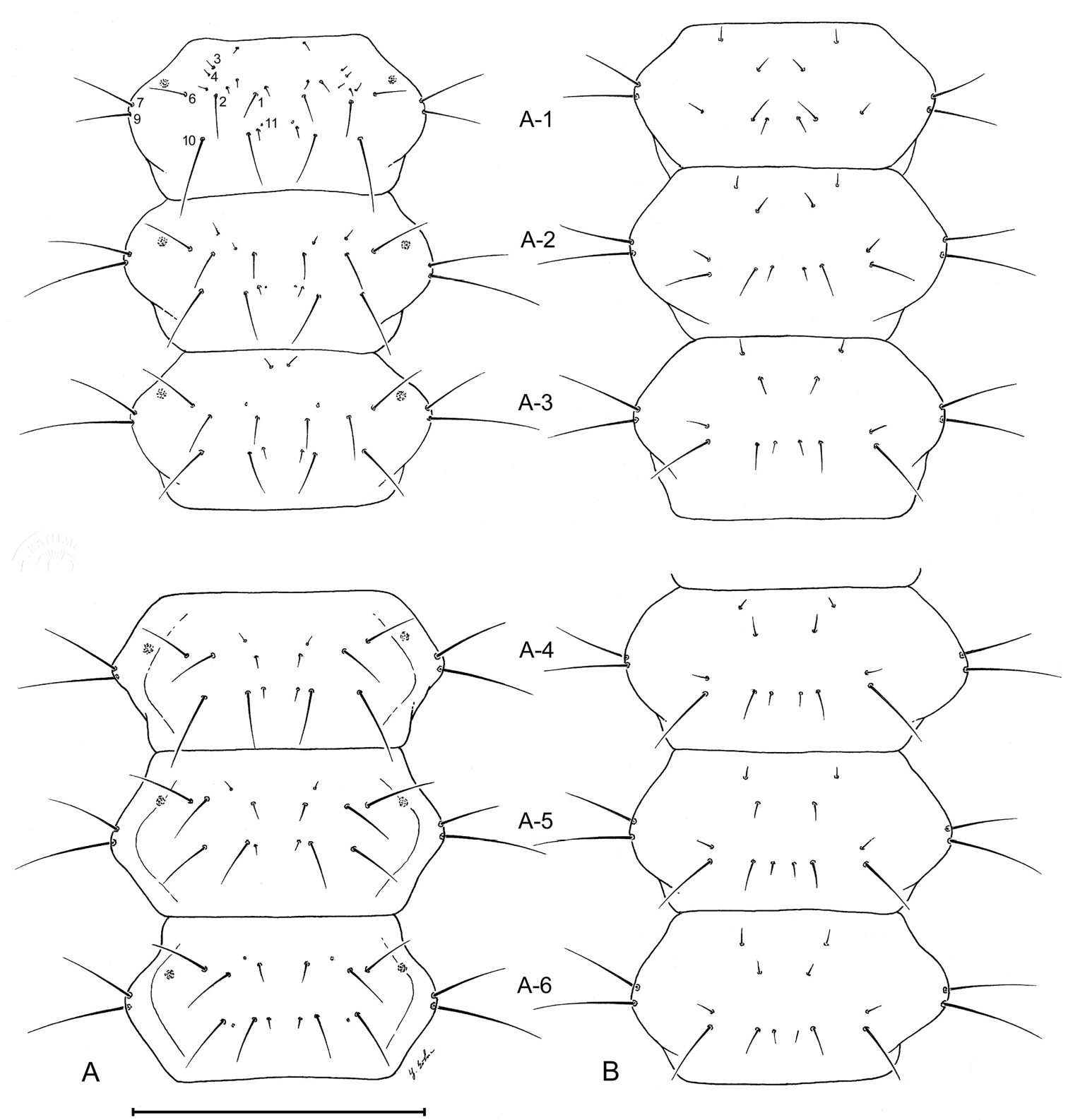
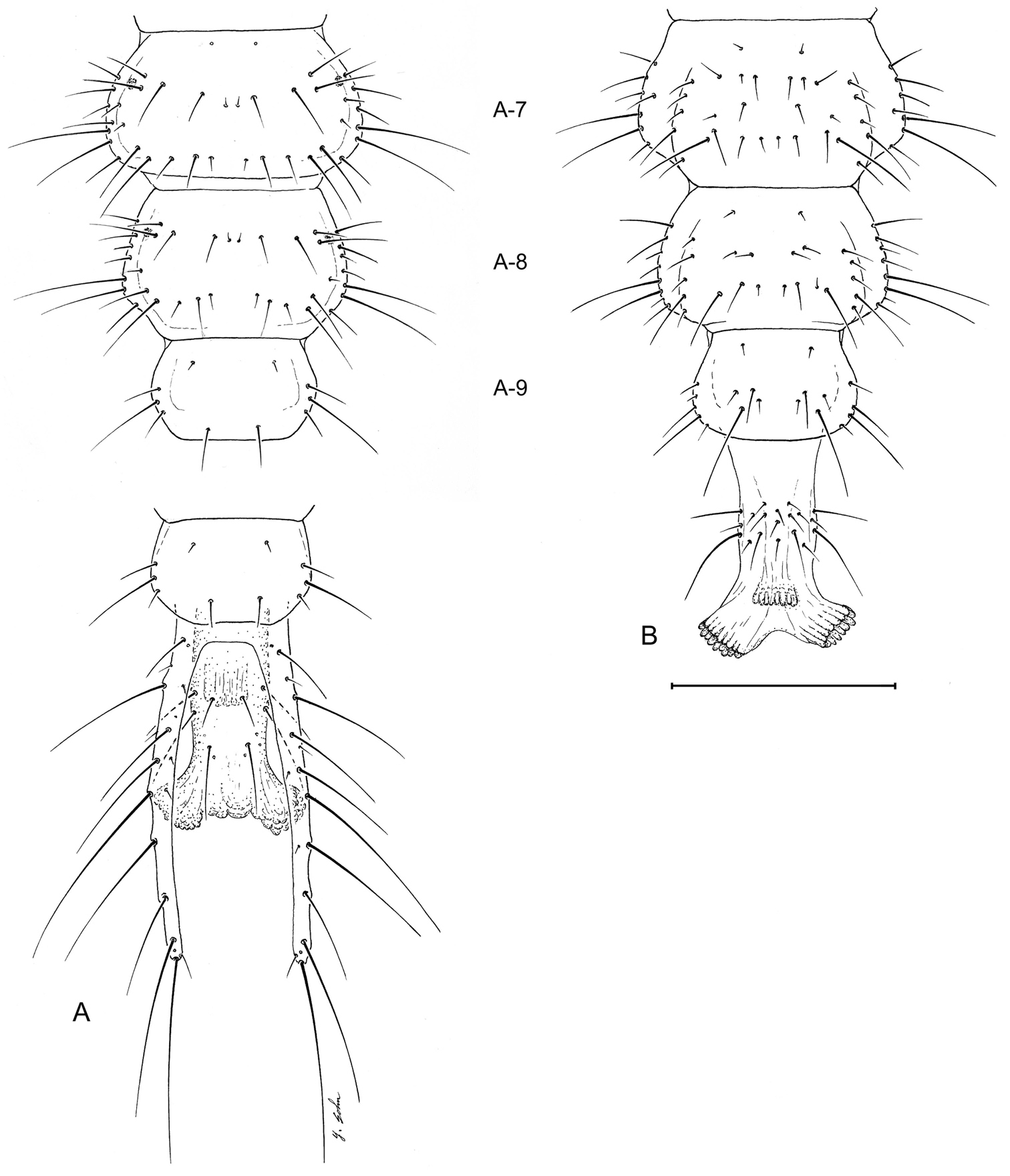
Larval abdomen (L1), of Leptotrachelus dorsalis (Fabricius). A dorsal aspect B ventral aspect. Abdominal segments A-1 through A-6. Scale line equals 0.5 mm.
(Figs 3, 12). Tarsus unispinose at apex and with a single seta at midpoint dorsally.
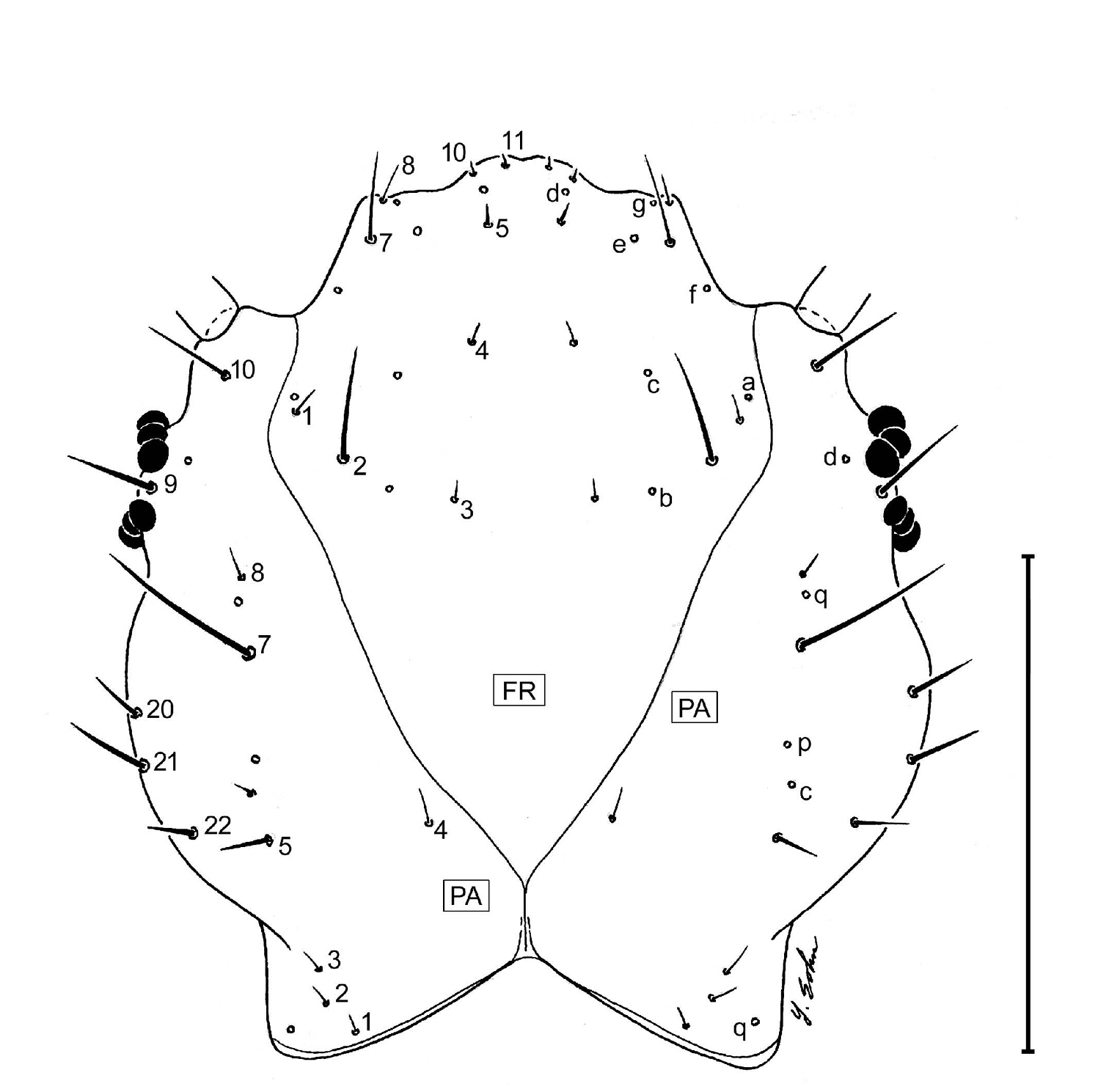
Head. Frontale (Fig. 4) with 7 setae (FR1 – FR7) on each side; and 2 pores (FRc &FRe) on each side; egg burster a lyre-shaped row of short setae. Parietale (Figs 4A, 4B) with 19 setae (PA1 – PA19) and 5 pores (PAc, PAj, PAk, PAm, PAn) on each side. Antenna (Fig. 4A): antennomere 1 with 3 pores (unlabeled); antennomeres 2 – 4 with no pores; antennomere 3 with 3 setae (AN1 – AN3) and 1 small sensilla near base of sensorial appendage (Fig. 4A); antennomere 4 with 4 setae (AN1 – AN4) and 2 small apical sensillae (Fig. 4A). Mandible (Fig. 4A) with 1 seta (MN1) and 2 pores (MNb – MNc). Labium (Fig. 4B): prementum with 3 setae (LA2, LA3, LA7) and 1 pore (LAa) on each side; palpomere 1 with 1 pore (LAb); palpomere 2 and 3 without visible features. Maxilla (Fig. 4B): cardo without setae; stipes with 3 constant setae (MX2, MX3, MX4); 5 pores (MXa – MXc), others not labeled; lacinia and galeomeres without setae and pores; maxillary palpomeres without visible sensatory features.
Prothorax: Notum (Fig. 5A) with 10 major setae (PR2 – 4, 6, 8 – 13), PR1, 5, 7 absent, and 3 pores (PRc only named one) on each side; pleurite (Fig. 5B) with 3 setae (PL9, 10, 11), and no pores on each side; episternum (Fig. 5B) with 3 setae (unnumbered).
Mesothorax: Notum (Fig. 5A) with 14 setae (ME1 – ME14), 3 small auxiliary setae, and 1 pores (MEg) on each side; episternum (Fig. 5B) with no setae and no pores; epimeron (Fig. 5B) with 1 seta (EM1); pleurite (Fig. 5B) with 3 posterior seta (PL1, 5, 6); sternum (Fig. 5B) with 1 seta (not numbered) and no pores on each side.
Tergite I (Fig. 7A) with 10 setae (TE1 – TE10, TE8 missing and several accessory setae present) and 1 pore on each side. Tergites II – VIII as in Tergite 1 but with less accessory setae. Tergite IX and urogomphi (Fig. 7A) with 8 setae (UR1 – UR8, UR1 missing) and no pores. Epipleurite (Fig. 7B) with 2 setae (unnumbered) and no pores. Hypopleurite (Fig. 7B) with 7 setae (unnumbered) and no pores. Sterna 1 – 9 (Fig. 7B) with 5 or 6 setae each side (unnumbered) all in the same pattern. Sternum IX with 4 setae (ST2 – ST5) on each side.
Larval abdomen (L1), of Leptotrachelus dorsalis (Fabricius). A dorsal aspect B ventral aspect. Abdominal segments A-7 through A-10; and cerci and pygidium. Scale line equals 0.5 mm.
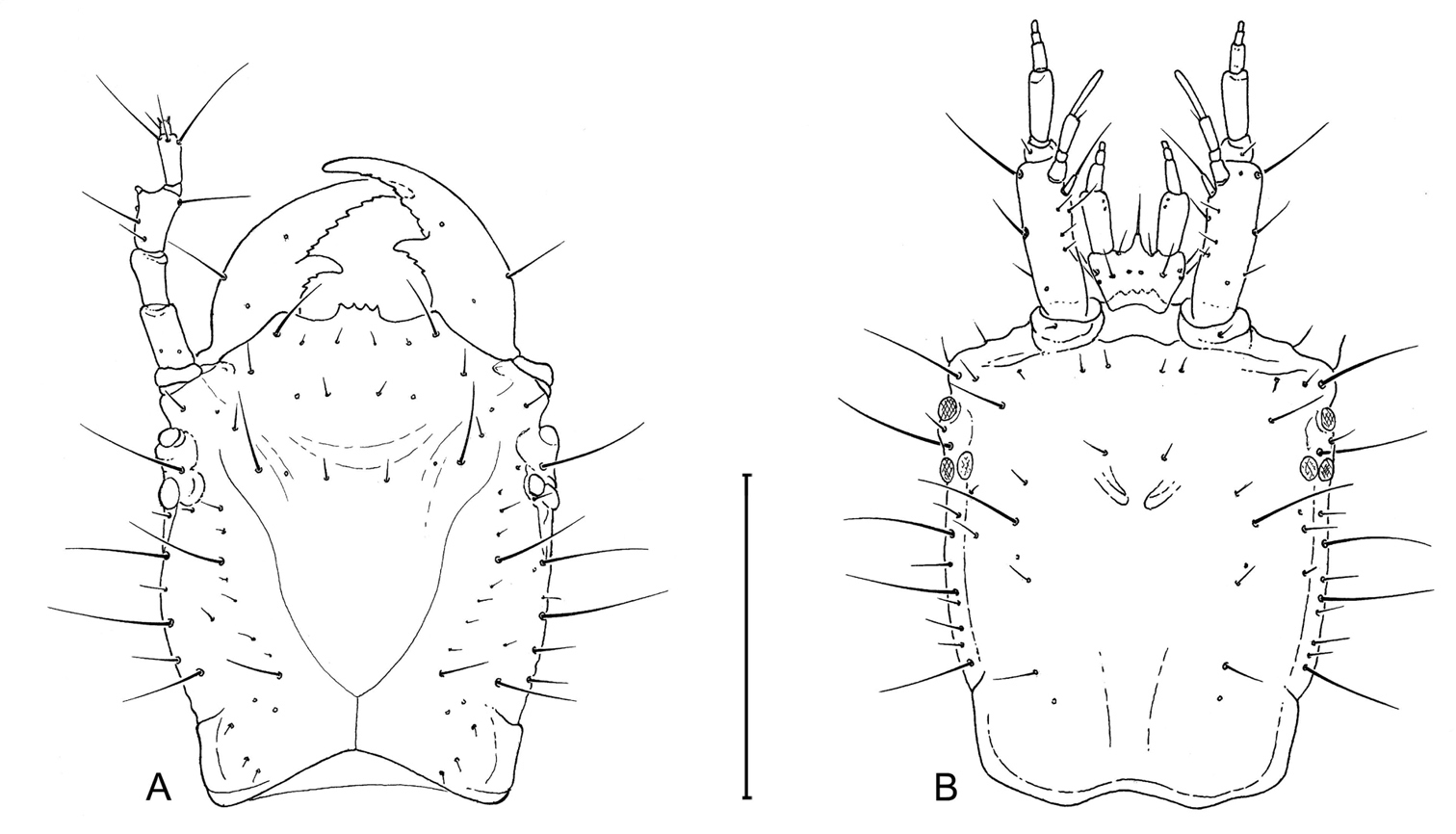
Larval head capsule (L3) of Leptotrachelus dorsalis (Fabricius). A dorsal aspect B ventral aspect. Scale line equals 0.5 mm.
Larval thorax (L3), of Leptotrachelus dorsalis (Fabricius). A dorsal aspect B ventral aspect. Prothorax T-1 Mesothorax T-2 Metathorax T-3. Scale line equals 0.5 mm.
Larval abdomen (L3), of Leptotrachelus dorsalis (Fabricius). A dorsal aspect B ventral aspect. Abdominal segments A-1 through A-6. Scale line equals 0.5 mm.
Larval abdomen (L3), of Leptotrachelus dorsalis (Fabricius). A dorsal aspect B ventral aspect. Abdominal segments A-7 through A-10 and cerci and pygidium. Scale line equals 0.5 mm.
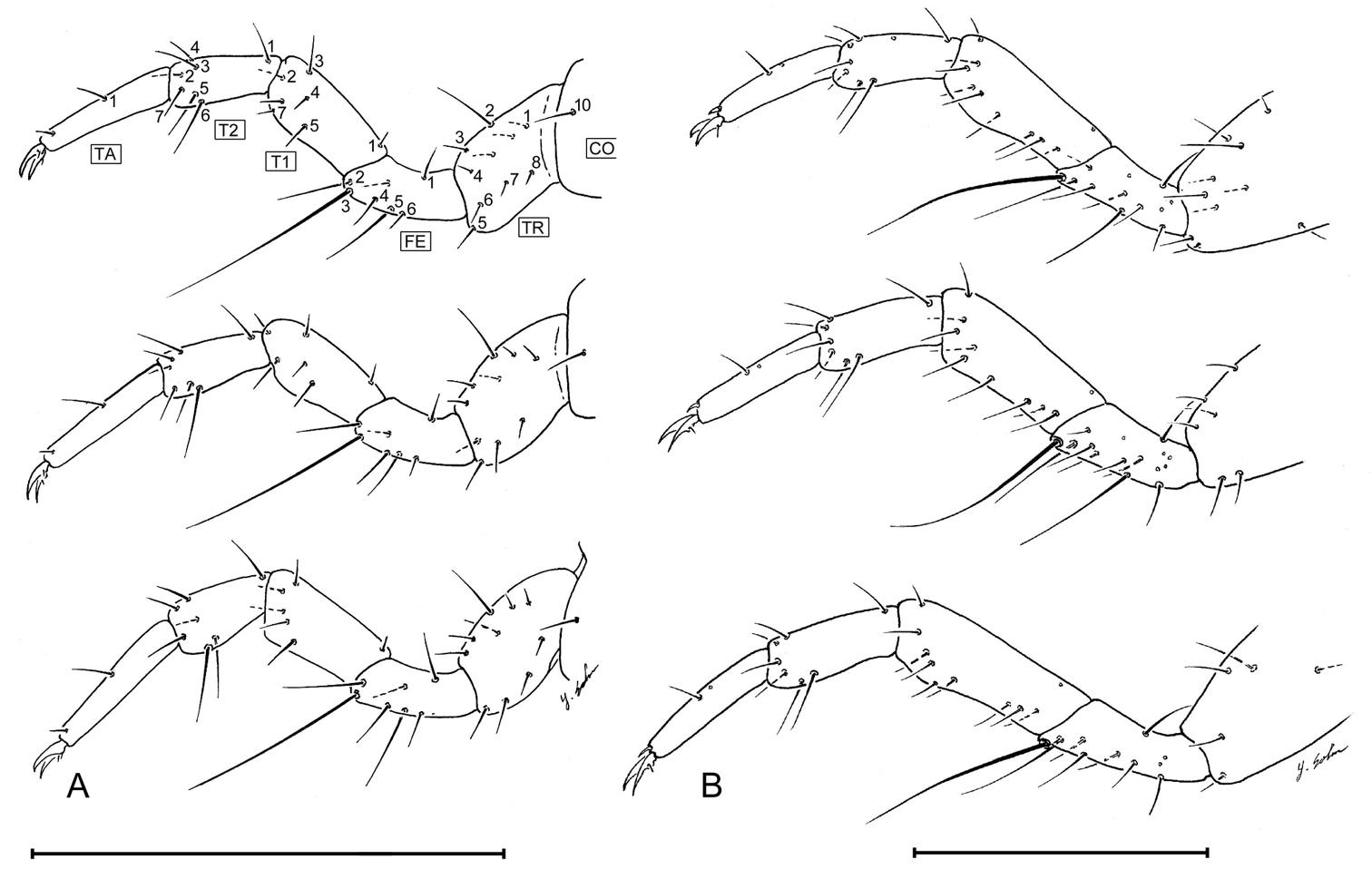
Coxa (Fig. 12) with 1 setae (CO10). Trochanter (Fig. 12) with 8 setae (TR1 – TR8), 2 unnumbered accessory setae and no pores. Femur (Fig. 12) with 6 setae (FE1 – FE6), 1unnumbered accessory seta and no pores. Tibia (Fig. 12) with 6 setae (TI1 – TI7, TI6 missing) and no pores. Tarsus (Fig. 12) 2 segmented, with 6 seta (T21 – T26) and 1 unnumbered accessory seta and no pores on T2, and 1 constant seta on T1 and 1 unnumbered accessory seta and no pores. Claws (Fig. 12) with 1 seta near base.
Coxa CO trochanter TR femur FE and tarsus T1, T2, TA of Leptotrachelus dorsalis (Fabricius), posterior lateral aspect. A L1, top – anterior leg; middle – middle leg; bottom – posterior leg B L3, top – anterior leg; middle – middle leg; bottom – posterior leg. Scale line equals 0.5 mm.
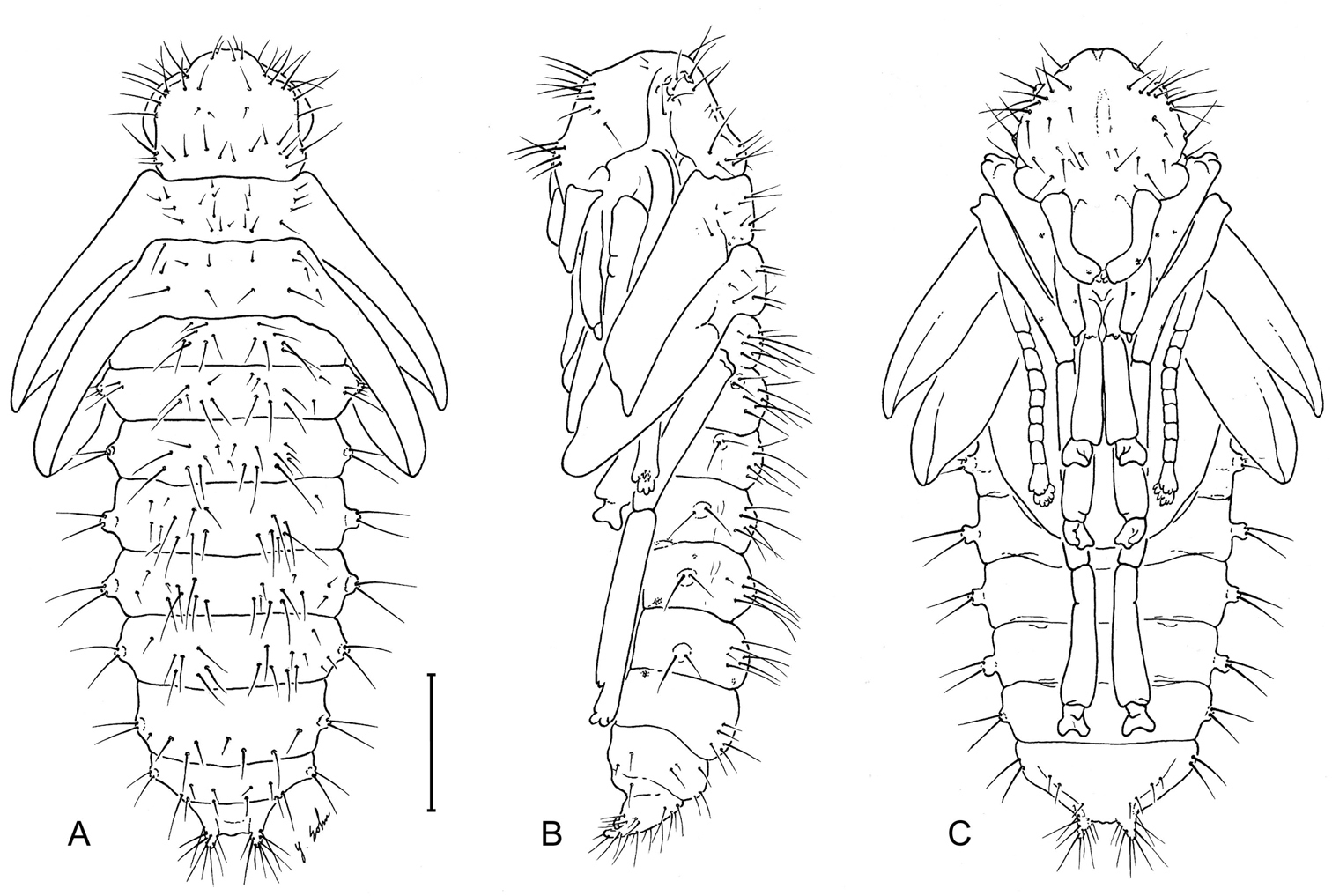
See Fig. 13. Typical of known carabid pupae, not many of which have been illustrated and described. Note the exceedingly setiferous ocular area of head and cerci.
Pupa of Leptotrachelus dorsalis (Fabricius). A dorsal aspect B left lateral aspect C ventral aspect. Scale line equals 1.0 mm.
Larval head capsule (L3), parietale (PA), frontale (FR), dorsal aspect of Askalaphium depressum (Bates). This illustration was inadvertently left out of
The hypothesized “home reed, ” Typha latifolia L., as a microhabitat for this commensal species of carabids is classified in the Poales, Typhaceae. This reed, commonly called bulrushes or cattails, is an obligate wetland species and has been found in a variety of climates, including tropical, subtropical, southern and northern temperate, humid coastal, and dry continental up to 2300 m altitude in North, Middle, and South America. However, we point out that species of Leptotrachelus are known to occur commonly as adults on the culms of marsh grasses such as Panicum dichotomiflorum (Leptotrachelus dorsalis:
We thank Randy Richard and Elta Duet of the USDA, ARS Sugarcane Research Unit for valuable assistance in the field and laboratory. We also thank Warren Steiner for specimen preparation and general collection assistance, Young Sohn who provided the excellent larval illustrations, Charyn Micheli for literature research and critical review of the manuscript, as well as Karolyn Darrow for her assistance with rendering and arranging the many illustration plates and photos; all four individuals are part of the important technical staff of the Department of Entomology, Smithsonian Institution. Lourdes Chamorro (USDA Staff in NMNH) also provided a critical review of the manuscript. Funding for this study was received from the American Sugar Cane League of the U.S.A., Inc. and publication costs borne by the National Museum of Natural History, Smithsonian Institution. We also thank two excellent anonymous reviewers who contributed much to the final product, although we are responsible for any errors that might still be maintained in the final product.