(C) 2012 Sarah Atherton. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new species of Acanthodasys (Gastrotricha, Macrodasyida, Thaumastodermatidae) is described from sublittoral sediments off the Atlantic coast of Florida. Acanthodasys paurocactus sp. n. is a relatively small species (to 450 µm long) with a strap-shaped outline, a series of anterior, lateral, and ventrolateral adhesive tubes, paired caudal pedicles with posterior adhesive tubes, and a morphologically diverse cuticle. The cuticle contains both spined and unspined scales. Unspined scales are present in two general shapes: lanceolate and eye shaped, with some transitional shapes. All scales have a thickened rim and depressed central region; some scales of both shapes bear either one or more central bumps, a parallel ridge, or a perpendicular ridge that gives the appearance of a cross-shaped pattern under transmitted light. Spined scales are somewhat quadrangular in shape and bear uniancres to 15 µm long with a cross-shaped sectional profile. The new species is now one of five described species to possess both spined and spineless scales, and only one of two species to possess two types of spineless scales (the second species is an incompletely described specimen from Norway).
Meiofauna, Caribbean, gastrotrich, taxonomy, Macrodasyida, cuticle
Gastrotrichs are microscopic invertebrates found in all oceans, seas and inland water bodies. The phylum Gastrotricha is composed of two orders, Chaetonotida, which includes 322 freshwater species (
To date, little is known of gastrotrich biodiversity in the tropics and subtropics, particularly the Tropical Northwestern Atlantic (TNWA, aka wider Caribbean), which extends from South Florida to the French Guiana-Brazil border.
In this study, we document a new species of Acanthodasys (Macrodasyida, Thaumastodermatidae) from sublittoral sediments off the Atlantic coast of Florida. This description forms part of a larger study that aims to classify the meiofauna from Capron Shoal, an offshore sandy shoal known to harbor diverse meiofauna (
Gastrotrichs were collected from Capron Shoal (27°26'52"N, 80°13'81"W), a 3 m deep station approximately 7 km off the coast of Fort Pierce, Florida. Samples were collected via anchor dredge in March, 2005 and August 2011 and analyzed back at the Smithsonian Marine Station in Fort Pierce, Florida. Extraction of gastrotrichs followed a standard protocol: 1) approximately 100 cm3 of sediment was combined with 900 cm3 of 7% aqueous MgCl2 solution in a 1 L Erlenmeyer flask and allowed to rest for 10 min; 2) the flask was gently shaken and the supernatant was decanted over a 48 µm mesh; and 3) the mesh was gently washed with seawater into a Petri dish. Specimens were sorted under a Leica EZ4 stereomicroscope, transferred to a glass slide, and viewed with a compound microscope (Zeiss A1) equipped with DIC (differential interference contrast). Light micrographs and digital videos were captured with a Sony Handycam digital camera. Measurements of individual specimens were performed with an ocular micrometer. Lengths and positions of organ systems are described in terms of percentage body units, where total body length from anterior (U00) to posterior (U100) is 100 units.
Specimens were prepared for scanning electron microscopy with the following protocol: fixation in 3% glutaraldehyde in 0.1M cacodylate buffer (pH 7.2) for 24h; rinsing four times (15 min each); postfixation in 1% OsO4 in 0.1 M cacodylate buffer for 1 h; rinsing in 0.1M cacodylate buffer (4 × 15 m); dehydration in an ethanol series; transfering to BEEM capsules and dehydration in a critical point dryer. Specimens were then sputter coated with gold and examined on a JEOL 6400 SEM at 10 kV.
One specimen was prepared for museum archival using the following protocol, which is deemed more permanent than standard glycerin mounts: fixation in 2.5% glutaraldehyde in 0.1M phosphate buffer saline (PBS; pH 7.4) for 24 hr; rinsing with PBS for 1 hr; postfixation in 1% OsO4 in 0.1M PBS for 30 sec (to increase contrast); rinsing in PBS for 15 min; dehydration through an ethanol series; transfering to propylene oxide for 30 min; and embedding in epon resin on a glass microscope slide (coverslipped and placed in an oven at 60o C for 24 hr). Type specimen is deposited in the National Museum of Natural History, Smithsonian Institution, Washington, DC.
Abbreviations: PIJ, pharyngeointestinal junction; TbA, anterior adhesive tubes below ventral mouth rim; TbL, lateral adhesive tubes; TbP, posterior adhesive tubes on caudal pedicles; TbVl, ventrolateral adhesive tubes.
Results Order Macrodasyida Remane, 1925 [Rao and Clausen, 1970] Family Thaumastodermatidae Remane, 1927 Subfamily Diplodasyinae Ruppert, 1978 Genus Acanthodasys Remane, 1927urn:lsid:zoobank.org:act:16C6323A-A944-4C69-9FBE-3F060876360F
Capron Shoal, Florida (27°26'52"N, 80°13'81"W), 3m depth, coarse sand. Sediments collected via anchor dredge by Hugh Reichardt and Woody Lee in March 2005; also in August 2011.
Florida: Five adult specimens observed with DIC optics on 4 August 2011; two specimens prepared during an earlier expedition (March 2005) for scanning electron miocroscopy.
Adult specimen, ~ 375 µm long, curled, lateral orientation. Epidermal glands are artifically swollen. Cat no. USNM 1179053. Also, digital video of same specimen, live, deposited at the Smithsonian.
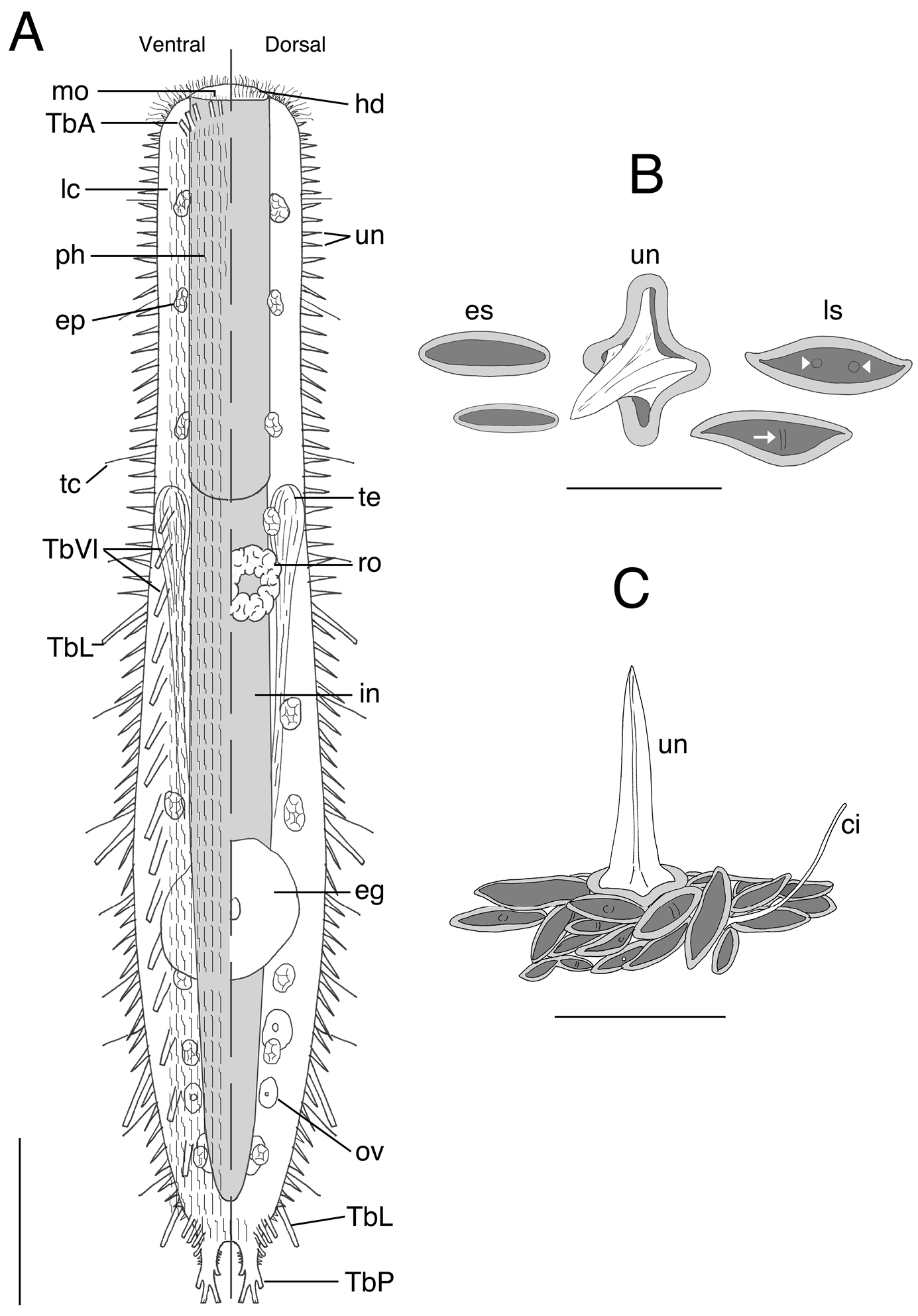
Acanthodasys with body length 300–450 µm (mature specimens at ~ 325 µm length). Body mostly strap-shaped with a distinct pair of caudal pedicles curled under body. Maximum body width at mouth/PIJ/midpoint of body is 35/42/67 µm. Pharynx to 136 µm long with pharyngeal pores near base. Area around mouth naked (no scales or spines) and up to 12 µm long, bearing numerous sensory cilia to 10 µm long. Scales cover entire body with oblique and transverse orientations; scales of two shapes, elongate lanceolate and short eye shaped, each with a centrally depressed region. Some scales have a small bump(s) or ridge at the center. Spined scales of dorsal and lateral cuticle bear uniancres 4–15 µm long; ventral uniancres 2–4 µm long scattered in ciliary fields and in median columns between locomotory cilia. Scales extend on to the caudal pedicles. Lateral sensory cilia to 15 µm long. Epidermal glands to 13 µm in diameter, 15–20 per side. Five TbA per side inserting directly on body surface at mouth rim. Up to 4 robust and elongate TbL per side, present only in trunk region. Up to 20 TbVl per side beginning posterior of PIJ, with the most posterior group of five TbVl becoming distinctly lateral in position close to the caudal pedicles. Caudal pedicles distinct with one lateral, two terminal, and one medial tube per lobe. Hermaphroditic, with paired testes and single glandular caudal organ. Rosette gland on dorsolateral left side of body; large egg present (~50 µm diameter); ovaries paired at caudal end.
This species is named for its spiky appearance, reminiscent of cactus (pauro, Greek: little, small; cactus, Greek: a prickly plant).
The description is based on specimens measured in vivo; most specimens were dorsoventrally curled (see Fig. 1B). Body strap-shaped and 300–450 µm long (subadults ~ 300 µm long, most specimens 350–400 µm long) (Fig. 1). Terminal mouth 30–35 µm wide; body width increasing slightly to 43 µm at PIJ and to 67 µm in adults with developing ova. The trunk gradually tapers and leads to a pair of distinct caudal pedicles (Fig 1. inset). The entire body is covered with scales and spined scales except for the hood-like region around the mouth (Fig 1, 4). Epidermal glands to 13 µm diameter, up to 15–20 per side (Figs. 1A, 2).
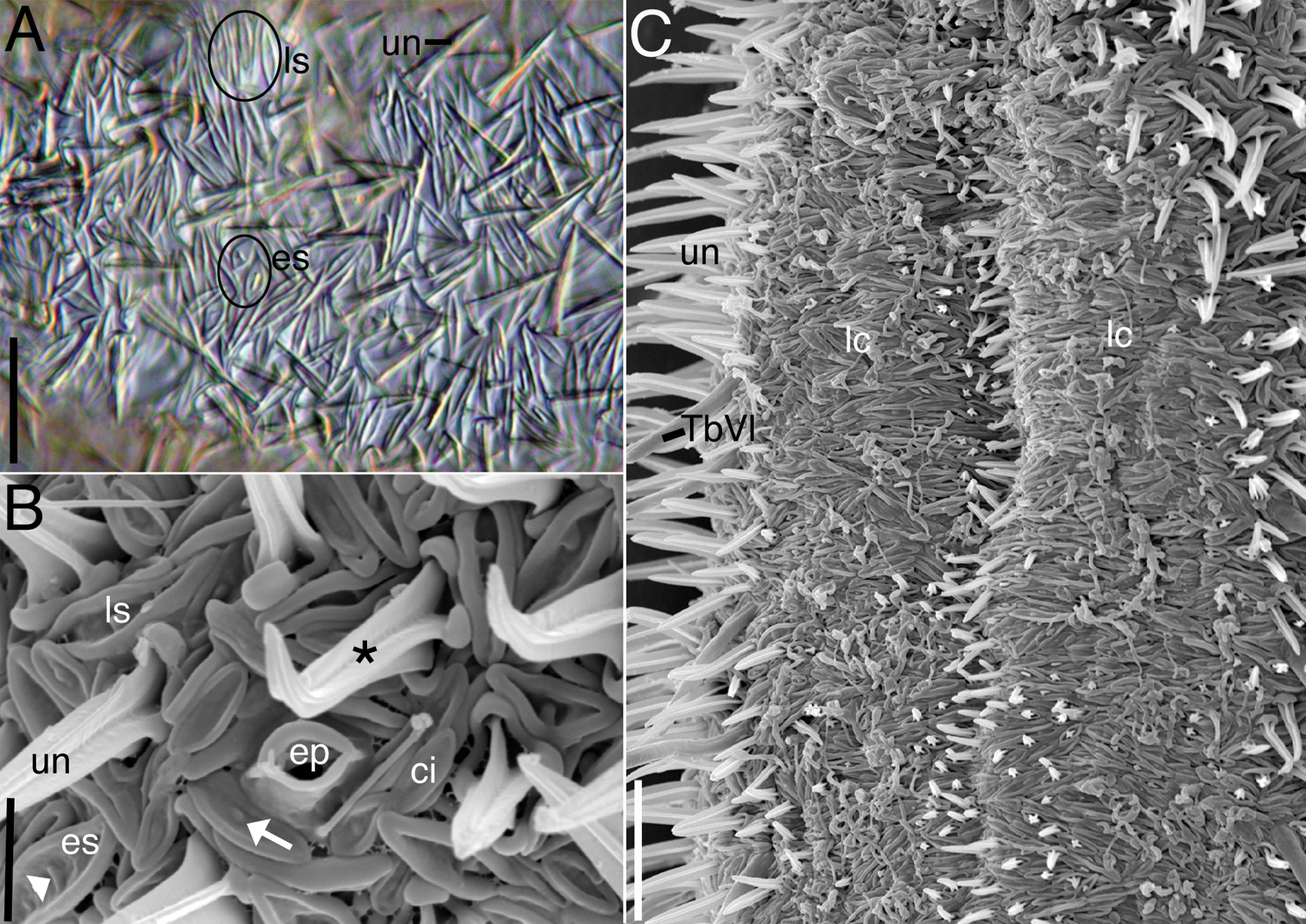
Cuticlular armature.Scales and spined scales present (Figs. 1A, 2–4). Scales often appear as interwoven fibers in brightfield optics, but DIC reveals numerous scales in between the spined scales (uniancres); several scales with various raised structures at their center (es, Fig. 3A). At high magnification with DIC (1000X) and SEM (> 1000X), at least two types of scales are observed: elongate, lanceolate-shaped scales (ls) and shorter, eye-shaped scales (es, Figs. 3, 4B); scales of intermediate size and shape are also present (Fig. 4B). All scales have a slightly thickened rim and central depression that extends along the longitudinal axis of the scale (Figs. 3B, 4B). Scales are arranged in several different orientations (longitudinal, transverse, oblique) across the dorsal and lateral body walls (Fig. 4C). SEM reveals that several scales, both lanceolate and eye shaped, have either a raised, oval bump at the center of the depression (white arrow, Fig. 3B) or a raised, bar-shaped ridge that is parallel (es, Fig. 3A) or perpendicular (white arrow, Fig. 3B) to the long axis of the scale. Lanceolate scales measure to 7 µm long and eye-shaped scales to 4 µm long with a maximum width to 1.5 µm. Spined scales bearing uniancres are interspersed among spineless scales (Fig. 3A–C). Uniancres with a cross-shaped (cruciform) sectional profile (asterisk, Fig. 3B) arise from the center of thick-rimmed scales that also have a somewhat quadrangular shape (Figs. 3B, 4B). Dorsal and lateral uniancres close to the oral hood are 3–5 µm long and increase in length along the trunk and reach a maximum of 15 µm long. Several small uniancres (2–3 µm) extend onto the caudal pedicles. Uniancres are mostly straight and oriented perpendicular to the body surface or in a slightly posterior direction; some uniancres had a bent tip that might have been the result of dehydration during preparation for SEM. Openings to the epidermal glands were surrounded by a raised cuticular ridge. Ventrolaterally, the uniancres decrease in size to 4 µm long where they border the locomotory cilia (Fig. 3C). Several very small uniancres, 1–3 µm long, are scattered among the cilia on the ventral body wall (Fig. 3C). Several tiny (1–2 µm) and slightly larger (2–4 µm) uniancres are present in between the ciliary columns in the trunk region.
Cilia.Sensory cilia to 10 µm long extend across the oral hood and form a thin corona around the head (Figs. 1A, 2A). A thicker patch of sensory cilia on either side of the head extends to 15 µm length. Smaller cilia 5–8 µm long line the mouth rim on the ventral body wall. At least ten stiff, hair-like cilia to 12 µm long extend down the length of the body on either side. Sensory cilia were observed to project out between the scales under SEM (Fig. 3B). Ventral locomotory cilia cover most of the pharyngeal region, extending from approximately U05 to the PIJ (Figs. 3C, 4A). At the PIJ, the cilia continues as a series of continuous rows to the posterior end but with a narrow column of naked cuticle (and uniancres) in between (Fig. 3C).
Adhesive tubes.Five pairs of anterior adhesive tubes (TbA) up to 5 µm long are present at the mouth margin: one either side of the midline is a close-set pair of tubes that is present medially and three tubes that form a group that is oriented diagnonally and closer to the lateral margin of the body (Fig. 4). Four pairs of TbL are present in the trunk region. Each tube is 21–25 µm long and robust in appearance. One specimen showed tubes at U45, U54, U70 and U80; three specimens were curled and difficult to measure. One specimen only had two TbL at positions U68 and U79. Up to twenty ventrolateral adhesive tubes (TbVl) to 12 µm long are inserted posterior to the PIJ. Most TbVl appear evenly spaced down the trunk; five TbVl become slightly more lateral in position and are clustered anterior to the caudal pedicles. The pedicles reach a maximum of 16 µm long including the posterior adhesive tubes (TbP) and bear a total of four TbP each: one lateral (6 µm), two terminal (4–5 µm), and one medial (4 µm) (Fig. 1 inset).
Digestive tract.Mouth terminal and circular to 35 um wide (Figs. 1B, 2B), surrounded by naked cuticle that forms a dorsal oral hood with a 12 µm rim (Fig. 2A, B); the naked cuticle around the ventral rim of the mouth is only 6 µm wide (hd, Figs. 2C). Pharynx to 136 µm long and 22 µm wide. Pharyngeal pores near base of pharynx (~ U34), not observable in all specimens. Intestine narrow and tapering at posterior; anus not observed.
Reproductive system.Hermaphroditic, with paired, bilateral testes beginning at the PIJ around U36 (Fig. 4A). Vasa deferentia extend posteriorly but could not be followed beyond mid-trunk region. Caudal organ observed in one specimen (body length: 400 µm), and pear-shaped, but the animal was too damaged for measurements. Rosette organ to 28 µm in diameter at U43–U46 in largest specimen (Fig. 4A). Paired ova were observed on either side of the posterior intestine in one specimem, with one large egg dorsal to intestine at approximately U65.
Acanthodasys paurocactus sp. n. A Adult specimen, dorsolaterally curled, DIC optics. Scale bar = 30 µm. B Adult specimen, ventral view, SEM. Note that the caudal pedicles are curled thereby obscuring the TbP. Scale bar = 50 µm. InsetPosterior end showing arrangement of TbVl and TbP. Scale bar = 14 µm. Abbreviatons: ep epidermal gland oh oral hood lc locomotory cilia mo mouth TbL lateral adhesive tube un uniancres.
Acanthodasys paurocactus sp. n. A Closeup of anterior end of adult specimen, dorsal view, DIC optics. Scale bar = 15 µm. B Closeup of anterior end of adult specimen, lateral view, SEM. Scale bar = 15 µm. C Closeup of anterior end of adult specimen, ventral view, DIC optics. Scale bar = 12 µm. Abbreviations: ep opening of epidermal gland hd oral hood mo mouth ph pharynx ss spineless scales un uniancres.
Acanthodasys paurocactus sp. n. A Closeup of dorsal cuticle of adult specimen, with focus on two types of spineless scales (circled) and uniancres (un), DIC optics. Scale bar = 12 µm. B Closeup of lateral cuticle of specimen showing lanceolate-shaped scales and eye-shaped scales, SEM. Some scales have perpendicular ridges (white arrowhead) or bumps (white arrow). Uniancres (*) arise from quadrangular-shaped scales. Scale bar = 4 µm. C Closeup of ventral trunk region of adult specimen showing location of ventral locomotory cilia (lc) and small ventral uniancres. Scale bar = 12 µm. Abbreviations: ci sensory cilium next to scales ep epidermal gland opening with raised cuticular ridge es eye-shaped scales lc locomotory cilia ls lanceolate scales TbVl ventrolateral adhesive tube un uniancre.
Acanthodasys paurocactus sp. n. A Composite sketch showing ventral (left) and dorsal (right) features. Scale bar = 40 µm. B Sketches of some scales based on SEM photographs of the dorsal cuticle. C Sketch of the general orientation of various spineless scales around a single spined scale based on SEM micrographs. Scale bar = 12 µm. Abbreviations: ci sensory cilium eg mature egg ep epidermal gland es eye-shaped scale hd oral hood in intestine lc locomotory cilia ls lanceolate-shaped scale mo mouth ov developing ova ph pharynx ro rosette organ TbA anterior adhesive tubes TbL lateral adhesive tubes TbVl ventrolateral adhesive tubes TbP posterior adhesive tubes tc lateral tactile cilia te testis un uniancres.
At present, there are sixteen species of Acanthodasys known from several oceans and inland seas including the Atlantic ocean (e.g.,
In general, Acanthodasys paurocactus sp. n. can be easily distinguished from its congeners by the structure of the cuticle, while most other characteristics overlap with those of previously described species. For example, the strap-shaped body outline is characteristic of most species in the genus, while the presence of a pair of distinct caudal pedicles (lobes) is known from Acanthodasys aculeatus, Acanthodasys carolinensis, Acanthodasys caribbeanensis, Acanthodasys fibrosus, Acanthodasys lineatus and Acanthodasys sp. 1. Among these species, Acanthodasys paurocactus sp. n. shows the most overall similarity with Acanthodasys aculeatus sp. n. regarding body shape and general distribution of TbVl and TbP. Unfortunately, details about the number and distribution of adhesive tubes in Acanthodasys aculeatus are questionable as the original description by Remane is incomplete: “Die Verteilung der Haftröhrchen konnte ich nur teilweise feststellen” (
Acanthodasys paurocactus sp. n. is now one of five species that is known to possess both spined scales (uniancres) and spineless scales. The other species are Acanthodasys aculeatus, Acanthodasys arcassonensis, Acanthodasys caribbeanensis and Acanthodasys sp. 2. The uniancres of the new species are larger than those reported for Acanthodasys aculeatus (variable: up to 9 µm,
There were two general types of spineless scales revealed with SEM. One type of scale was lanceolate in shape, very thin and up to 7 µm long. Interspersed among these scales were eye-shaped scales that were somewhat wider and to 4 µm long. Scales of intermediate size and shape were also present. All scales had a central depressed region that extended the length of the longitudinal axis of the scale; some of these scales also had raised regions (e.g., bumps, a single parallel ridge, a single perpendicular ridge) in the depressed region. The rim of all scales, which appeared thicker than than the rest of the scale body, was always elevated above the central depression. We hypothesize that the raised ridge and depressed center of each scales alters their refractive index under transmitted light, thereby imparting the fiber-like appearance of the scales at low magnification. A similar case may also be found in Acanthodasys fibrosus once that species is viewed with SEM. Interestingly, Acanthodasys paurocactus sp. n. is now only the second known species to possess two types of spineless scales, the other species being an undescribed specimen (Acanthodasys sp. 2) from Norway (
As noted for Acanthodasys arcassonensis (
The authors acknowledge the helpful comments of Dr. M. Antonio Todaro, Dr. Alexander Kieneke, and one anonymous reviewer for improving this manuscript. The authors would also like to thank Hugh Reichardt, Woody Lee, Julie Piraino and Dr. Michael Boyle at the Smithsonian Marine Station in Fort Pierce, FL for their assistance in field collections and SEM work. This is Smithsonian Marine Station at Fort Pierce contribution number 845. This material is based upon work supported by the National Science Foundation under Grant No. DEB 0918499.