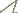(C) 2011 Weiting Zhang. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new genus with a new species of Saldidae, Brevrimatus pulchalifer gen. et sp. n., is described and illustrated. The fossil specimen was found from the Early Cretaceous Yixian Formation of Duolun County, Inner Mongolia, China. Phylogenetic analyses within Saldidae were performed, and the results indicate Brevrimatus pulchalifer gen. et sp. n. should be assigned to the subfamily Chiloxanthinae.
Saldidae, fossil, phylogeny, Early Cretaceous, China
The Saldidae is a small family of insects belonging to Heteroptera. About 335 extant species have been described in this cosmopolitan family (
To date, 6 incontrovertible fossil species in 3 generahave been reported: Oligosaldina Statz & Wagner, 1950 with three species, Oligosaldina rottensis, Oligosaldina rhenana and Oligosaldina aquatilis, found from Upper Oligocene deposits in Germany; Propentacora froeschneri (= Oreokora froeschneri) found in Miocene Latah Formation in USA (
However, 2 genera assigned to this group previously are not saldids. Leptosalda chiapensis (Cobben, 1971) from Mexico amber was assigned to the subfamily Leptosaldinae within Saldidae first, but was later transferred to Leptopodidae by
In this paper, we described a new fossil shore bug, Brevrimatus pulchalifer
gen. et sp. n., from the Yixian Formation, Baitugou, Nanyingpan
Village, Sanbeigou Town, Duolun County, Inner Mongolia, China.
Our fossil specimen is deposited in the Key Laboratory of
Insect Evolution and Environmental Changes, Capital Normal University,
Beijing, China. It was examined with the LEICA MZ 12.5 dissecting
microscope. The specimens were examined without alcohol and under
alcohol. Photos were taken by a Nikon Digital Camera DXM1200C. Line
drawings were made with Photoshop graphic software. Morphological
terminology used here follows that of
The body length was measured from the apex of head to the apex of abdomen; body width, at the maximal width of body; pronotum length, along the midline; pronotum width, across the broadest part at its posterior angles; wing length, from the basal to the apex of anterior margin; wing width, at the maximal width of the wing. All measurements are in millimeters (mm).
Systematic paleontologyOrder Hemiptera Linnaeus, 1758
Suborder Heteroptera Latreille, 1810
Infraorder Leptopodomorpha Popov, 1971
Family Saldidae Amyot & Serville, 1843
Subfamily Chiloxanthinae Cobben, 1959
urn:lsid:zoobank.org:act:B57D1B53-16FE-421A-BCA6-8F3141142A84
Brevrimatus pulchalifer sp. n.
Body ovate, moderate in size, macropterous. Head relatively short. Rostrum reaching to the base of hind coxae. Corium with large pale spots, medial fracture short, costal fracture of hemelytra very long, hypocostal ridge and associated secondary hypocostal ridge present on hemelytra, membrane with five closed cells. Posterior margin of female sternum VII concave along the midline. Base of ovipositor exposed.
The generic name is a combination of the Latin prefix “brev-” (short) and Latin word “rimatus” (fracture), which indicated the genus with short medial fracture. Gender masculine.
China.
urn:lsid:zoobank.org:act:80999FB3-E52E-459A-8A25-46CEAF6D947E
http://species-id.net/wiki/Brevrimatus_pulchalifer
Figs 1, 2Holotype, ♀, CNU-HET-ND2010334 p/c (part and counterpart).
Baitugou, Nanyingpan Village, Sanbeigou Town, Duolun County, Inner Mongolia, China, Yixian Formation. Early Cretaceous.
Head relatively short. The last segment of antennae slightly swollen. Corium with three large pale spots, medial fracture short, costal fracture of hemelytra very long; membrane with five cells, apex of innermost cell of membrane extending past apex of outermost cell. Posterior margin of female sternum VII extremely concave along the midline.
Body ovate, about 2.4 times as long as wide.
Head 1.4 times as wide as long. Antennae slender, 4-segmented, first segment shortest, second segment longest, 1.47 times as long as the third segment, fourth segment slightly shorter than third segment. Eyes reniform, moderately protrusive, located at the posterolateral angles of the head. Ocelli round, raised slightly, ocelli separated by 1.3 times the width of an ocellus, ocelli closer to each other than to margins of eyes. Rostrum reaching to the hind coxae. Length of head subequal to the length of pronotum on midline.
Pronotum transverse, 3.2 times as wide as long, Anterior and posterior margins of pronotum concave, lateral margins straight, anterior and posterior angles feebly rounded. Scutellum distinctly longer than pronotum on midline, triangular, 1.3 times as wide as long. Tarsal formula: 3–3–3. Fore tibiae about 2.0 times as long as corresponding tarsi, fore tarsomere I shortest, tarsomeres II and III almost subequal in length; mid femora 1.3 times as long as tibiae, tibiae 2.3 times as long as tarsi, tarsomere I shortest, tarsomere II slightly longer than tarsomere III; hind tibiae long, almost 1.5 times as long as hind femora, and 2.3 times as long as tarsi. Fore wing macropterous, 0.6 times as long as body; corium and membrane clearly delimited; corium with embolium; medial fracture short, 0.3 times as long as fore wing; costal fracture of hemelytra very long, reaching to the middle of the corium; venation of corium weakly indicated; membrane large, with five closed cells, cells reduced gradually from the inner to the outer. Claval commissure shorter than scutellum length at median line. Hemelytra with only slight modification for mating, the embolar region slightly thickened.
Anterior margin of female sternum VII curve; posterior margin of female sternum VII extremely concave along the midline. Base of ovipositor exposed ventrally.
Measurements (in mm). Body length 8.00, width 3.18. Head length 0.84, width 1.24. Antennal measurements I–IV: 0.56, 1.30, 0.92, 0.85. Interocular space of ocelli 0.12. Interocular space of eyes 0.84. Pronotum length 0.78, width 2.52. Scutellum length 1.43, width 1.78. Length fore leg: tibia 1.22, tarsomeres I–III: 0.13, 0.23, 0.23; length mid leg: femur 1.91, tibia 1.57, tarsomeres I–III: 0.18, 0.27, 0.23; length hind leg: femur 2.14, tibia 3.15, tarsomeres I–III: 0.22, 0.69, 0.52. Hemelytron length 5.14, width 1.73.
Brevrimatus pulchalifer gen. et sp. n., line drawings. Holotype, CNU-HET-ND2010334 p/c. A dorsal view B ventral view. Scale bar=2 mm.
Brevrimatus pulchalifer gen. et sp. n., photographs. Holotype, CNU-HET-ND2010334 p/c. Apart and B counterpart. Scale bar=2 mm.
The species name is a combination of the Latin prefix “pulch-” (beautiful) and Latin word “alifer” (wing), meaning beautiful wing. Gender masculine.
The Leptopodomorpha consists of four extant families (Saldidae, Aepophilidae, Leptopodidae, Omaniidae) and three extinct families (Archegocimicidae, Mesolygaeidae, Palaeoleptidae).
The new genus possesses some typical Chiloxanthinae characters, such as costal fracture very long, female sternum VII truncate with mesal concavity and base of ovipositor exposed. On the other hand, it possesses short medial fracture as Saldinae. Therefore, we carried out phylogenetic analyses to determine the placement of our new genus.
For the phylogenetic analyses, we selected three extant genera from Chiloxanthinae, five extant genera from Saldinae, our new fossil genus, and an unambiguous fossil species Oligosaldina aquatilis as in-group. Following previous studies (
Taxa included in the phylogenetic analysis (*: only included when we carried out phylogenetic analysis with Oligosaldina aquatilis)
| Family | Subfamily | Tribe | Species | |
|---|---|---|---|---|
| out-group | Leptopodidae | Patapius thaiensis Cobben, 1968 | ||
| Aepophilidae | Aepophilus bonnairei Signoret, 1879 | |||
| in-group | Saldidae | Saldinae | Saldini | Salda lugubris (Say, 1832) |
| Teloleuca altaica Vinokurov, 2009 | ||||
| Saldoidini | Saldula montana Cobben, 1966 | |||
| Calacanthia sichuanicus Chen & Zheng, 1987 | ||||
| Saldunculini | Salduncula swezeyi (Usinger, 1946) | |||
| Chiloxanthinae | Chiloxanthus pilosus (Fallén, 1807) | |||
| Pentacora ligata (Say, 1832) | ||||
| Paralosalda innova Polhemus & Evans, 1969 | ||||
| *Oligosaldina aquatilis Statz & Wagner, 1950 | ||||
| Brevrimatus pulchalifer gen. et sp. n. |
Most character information of the extent taxa was extracted from literatures (
Matrix of 17 characters and the 12 taxa used for phylogenetic analysis (*: only included when we carried out phylogenetic analysis with Oligosaldina aquatilis)
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Taxon/Character | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Patapius thaiensis | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | – | 1 | 0 | ? | 0 | 0 | 0 | – |
| Aepophilus bonnairei | – | 2 | 0 | 0 | 2 | – | 0 | 0 | 0 | – | 0 | 0 | 0 | 0 | 0 | 0 | – |
| Salda lugubris | 1 | ? | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | – |
| Teloleuca altaica | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | – |
| Saldula montana | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 |
| Calacanthia sichuanicus | 1 | 2 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 |
| Salduncula swezeyi | 2 | ? | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 |
| Chiloxanthus pilosus | 2 | ? | 0 | 1 | 0 | 1 | 2 | 2 | 1 | 1 | 2 | 1 | 0 | 0 | 1 | 1 | 1 |
| Pentacora ligata | 1 | 2 | 0 | 1 | 0 | 1 | 2 | 2 | 1 | 1 | 2 | 1 | 0 | 0 | 1 | 1 | 1 |
| Paralosalda innova | 2 | 2 | 0 | 1 | 0 | 0 | 2 | 2 | 1 | 1 | 2 | 1 | 0 | 0 | 1 | 1 | 1 |
| Brevrimatus pulchalifer gen. et sp. n. | 2 | 2 | ? | 1 | 0 | 1 | 2 | 1 | ? | ? | 2 | 1 | ? | ? | ? | ? | ? |
| *Oligosaldina aquatilis | ? | 2 | 0 | 1 | 0 | 1 | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
For the phylogenetic analyses excluding fossil species Oligosaldina aquatilis, we got two equally most parsimonious trees (Fig. 3A, B), with the following main characteristics: tree length = 28, consistency index (CI) = 82, retention index (RI) = 87. The strict consensus tree is shown in Figure 3C. Phylogenetic resultsindicate Saldidae is a monophyletic group, which is supported by four synapomorphies: posterior pronotal margin indented distinctly (Character 4:1); eversible glands present posterolaterally between sterna VI and VII (Character 9:1); eggs with aeropyles (Character 15:1); larval organ present (Character 16:1). Some synapomorphic characters, such as apicolateral sclerotized structures of penis present (Character 13:1) and filum gonopori coiled one to four times, like a watch-spring (Character 14:1) supported the monophyly of the subfamily Saldinae. Chiloxanthinae with our fossil species included is a monophyletic group, which is supported by four synapomorphies: five well defined cells in membrane (Character 6:1); medial fracture long (Character 8:2); female subgenital plate truncate with concavity along the midline (Character 11:2); base of ovipositor exposed (Character 12:1). In summary, phylogenetic results suggest our new fossil genus is in Chiloxanthinae and short medial fracture was treated as a reversal character.
Phylogeny of Saldidae. A, Bthe most parsimonious trees based on 11 taxa and 17 characters. C the strict consensus tree based on 11 taxa and 17 characters D the most parsimonious trees based on 12 taxa and 17 characters. (●) non-homoplasious; (○) homoplasious.
For the phylogenetic analysis including fossil species Oligosaldina aquatilis, we got one most parsimonious tree (Fig. 3D), tree length = 28, CI = 82, RI = 88. The monophyly of Saldidae is supported by four synapomorphies (Character 4:1, 9:1, 15:1 and 16:1) as the results above. In this phylogenetic result, besides Character 13:1 and Character 14:1, short costal fracture of hemelytra (Character 7:1) supports the monophyly of the subfamily Saldinae. Five well defined cells in membrane (Character 6:1) indicate that our new genus should be in the branch of Chiloxanthinae. Therefore, both of the phylogenetic analyses suggest our fossil species should be classified into Chiloxanthinae.
Comparison with Chiloxanthinae indicates the new fossil species differs from other extant chiloxanthines in its short medial fracture. Besides this character, the boundary between corium and membrane is not clear in Enalosalda, which is different from Brevrimatus gen. n. with clear boundary. Paralosalda has four membrane cells, which is different from Brevrimatus gen. n. with five cells. Embolar modification of female is well developed in Pentacora, but in Brevrimatus gen. n. the embolar region is slightly thickened. Sublateral cell of membrane is shortest in Chiloxanthus, which differs from Brevrimatus gen. n. with the lateralmost cell is shortest. We further compared it with other fossil Saldidae. The arrangement of the cells of Brevrimatus gen. n. is similar to that of Oligosaldina, but lateralmost cell of membrane is distinctly smaller than that of Oligosaldina. Long costal fracture is present on Brevrimatus gen. n., but absent on Oligosaldina. A deep furrow is present in the pronotum of Oligosaldina, while it is absent in our new genus. Propentacora contains five closed cells in the wing membrane, but the corial veinof Propentacora appears to continue between the third and fourth membrane cells, which is different from the new genus. Brevrimatus gen. n. is distinctly different from Salda, which can be seen in phylogenetic result. Comparing our fossils with the fossil species Salda exigua, we can separate them in the following characters: Brevrimatus gen. n. possesses five closed cells in the forewing membrane, while Saldonia exigua has three closed cells and rostrum of Brevrimatus gen. n. reaches to the base of hind coxae, while in Saldonia exigua, rostrum just reaches to the fore coxae. Therefore Brevrimatus gen. n. is different from all other fossil genera. In geological age, all of the previously recorded fossil saldids are from Cenozoic. So far, Brevrimatus gen. n. found in the Lower Cretaceous sedimentary stratum is the oldest saldid.
We make a grateful acknowledgement for Alexandr Rasnitsyn’s contribution to paleoentomology. We sincerely thank Dr. Nikolai N. Vinokurov (Institute for Biological Problems of Cryolithozone, Siberian Branch, Russian Academy of Sciences), Ganyang Zhang (Entomology Department, University of California) and Hui Liu (Entomological Laboratory, Faculty of Agriculture, Kyushu University) for sending papers to us. Thanks to Dr. Shih ChungKun (College of Life Science, Capital Normal University) for his improvement of our manuscript, and to two anonymous reviewers and the editor for constructive comments. This research was supported by grants from the National Natural Science Foundation of China (No. 40872022, 31071964, 30800095), Nature Science Foundation of Beijing (No. 5082002), Beijing Talented Scholar Program Foundation (No. 20081D050160092) and the PHR20090509 Project of Beijing Municipal Commission of Education.
List of characters and character states used in phylogenetic analysis
Distance between ocelli: equal to width of ocellus (0); less than width of ocellus (1); more than width of ocellus (2). [We treat this character inapplicable in Aepophilidae that doesn’t have ocellus.]
fore coxae (0); middle coxae (1); hind coxae (2).
[Rostrum of Leptopodidae is very short, reaching to fore coxae at most.
Rostrum of Saldidae is relatively long, reaching to middle coxae or
hind coxae. Rostrum of Brevrimatus pulchalifer sp. n. and Oligosaldina aquatilis reaches to hind coxae. Long rostrum is the primitive character (
Postclypeus: absent (0); present (1). [Postclypeus present in S. lugubris, T. altaica, S. montana, C. sichuanicus. This character cannot be identified in our fossil specimen. In other groups postclypeus is absent.]
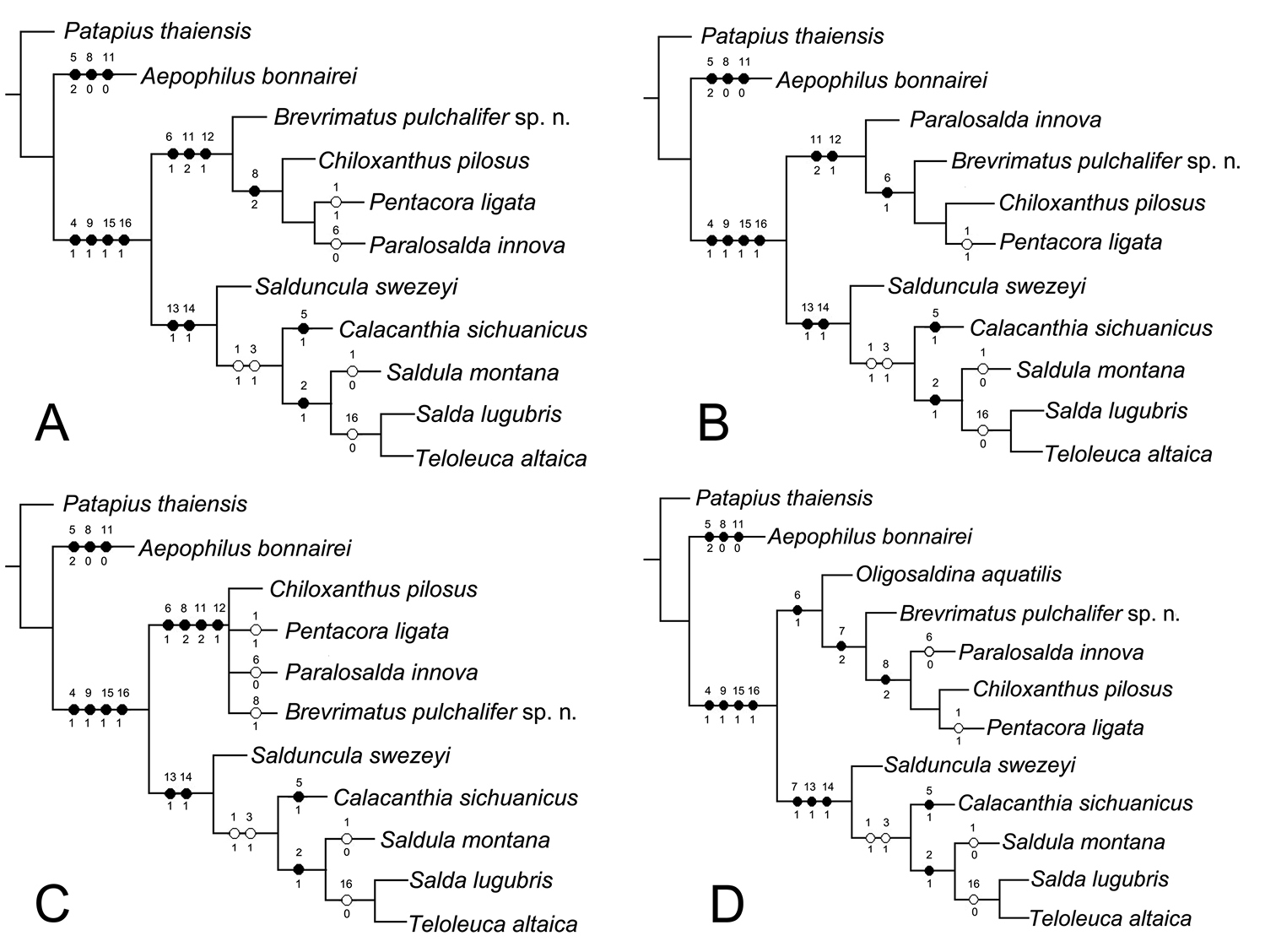
Posterior pronotal margin: non-indented (0); indented distinctly (1). [Posterior pronotal margin nearly straight in Aepophilidae, so we threat this character in Aepophilidae as non-indented. Posterior pronotal margin of Saldidae is demonstrated briefly in Figure 4A showing the indented posterior pronotal margin and Leptopodidae in Figure 4B showing the non-indented posterior pronotal margin. This character is considered as the synapomorphy for Saldidae.]
Forewing: macroptery or submacroptery (0); semibrachypterous moderately or strongly (1); brachyptery (2). [Wing polymorphism occurs in Saldidae. Wing pattern can be separated into five categories based on the reduction of the membrane of fore wing (Cobben 1980). The membrane of Aepophilidae completely reduced, so it is brachyptery. C. sichuanicus with the semibrachypterous forewing.]
Cells: Four well defined cells in membrane (0); five well defined cells in membrane (1). [In Aepophilidae, forewing greatly reduced, in form of pads without membrane, so we treated this character inapplicable in this family. Usually, Saldinae have four cells and Chiloxanthinae have five cells, but the distinction in hemelytral cells between the Saldinae and Chiloxanthinae is not constant (Polhemus and Chapman 1979). The fossil species, B. pulchalifer sp. n. and O. aquatilis have five cells.]
Costal fracture of hemelytra: absent (0); present, short (1); present, very long (2). [Costal fracture length is a stable character within subfamily. Saldinae usually possesses short costal fracture and Chiloxanthinae usually possesses long costal fracture. Polhemus (1977) consider the long costal fracture in Chiloxanthinae to be a derived character providing a synapomorphy for the group. Costal fracture is absent in P. thaiensis, A. bonnairei and O. aquatilis. Costal fracture in our fossil species, B. pulchalifer sp. n., is long.]
Medial fracture: absent (0); present, short
(1); present, very long (2). [Long medial fracture reaches at least to
level of posterior end of claval suture, and short medial fracture not
reaches anteriorly more than half the distance from costal fracture to
posterior end of claval suture (
Eversible glands: absent (0); present posterolaterally between sterna VI and VII (1). [Saldidae has paired abdominal eversible glands with their openings locating between sterna VI and VII. Eversible glands is absent in Leptopodidae and Aepophilidae. This structure is unknown in B. pulchalifer sp. n. and O. aquatilis. Eversible glands is considered to be a apomorphic character in Saldidae (Polhemus 1977).]
Sclerite adjacent to eversible gland: present (0); absent (1). [This sclerite is present in Saldinae, but absent in Chiloxanthinae.]
Female subgenital plate: truncate without concavity along the midline (0); triangular, posterior margin of produced caudad along the midline (1); truncate with concave along the midline (2). [In A. bonnairei female subgenital plate just like a normal abdominal sternite. Subgenital plate is truncate with concave along the midline is found in the members of Chiloxanthinae and B. pulchalifer sp. n. as Figure 4C. Subgenital plate is triangular in Saldinae as Figure 4D]
Base of ovipositor: hidden by posterior medial prolongation of subgenital plate (0); exposed (1). [The base of ovipositor is hidden in Aepophilidae and Saldinae, but exposed in Chiloxanthinae and B. pulchalifer sp. n. The exposed ovipositor is considered a derived character (Polhemus 1977)]
Apicolateral sclerotized structures of penis: absent (0), present (1). [This structure absent in Aepophilidae and Chiloxanthinae, but present in Saldinae.]
Filum gonopori: base of penis-filum not curled or at most forming one closed ring (0); filum gonopori coiled one to four times, like a watch-spring (1). [Base of penis-filum not curled in Leptopodidae and Aepophilidae, and coiled less than one ring in Chiloxanthinae. In Saldinae, base of penis-filum like a watch-spring.]
Eggs: without aeropyles (0); with aeropyles (1). [Eggs with aeropyles is regarded as a synapomorphy for Saldidae.]
Larval organ: absent (0); present (1). [Larval organ is absent in Leptopodidae and Aepophilidae, S. lugubris and T. altaica. Larval organ present in S. montana, C. sichuanicus, C. pilosus, P. ligata, P. innova. Larval organ present in most members of Saldidae. The absence condition in Saldini is presumed to be the secondary lost (Polhemus 1977).]
Larval organ: larval organ lateral, adjacent to spiracle (0); larval organ located medially, some distance from the spiracle (1). [In Saldinae, larval organ when present adjacent to spiracle, while the later condition occurred in Chiloxanthinae.]
A, B pronotum A posterior pronotal margin indented B posterior pronotal margin non-indented C, D female subgenital plate C truncate with concave along the midline D posterior margin of produced caudad along the midline (modified from Polhemus and Chapman 1979).