(C) 2011 Vladimir N. Makarkin. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Two new species of the genus Sinosmylites Hong are described from the Middle Jurassic locality at Daohugou (Inner Mongolia, China): Sinosmylites fumosus sp. n. and Sinosmylites rasnitsyni sp. n. This is the oldest known occurrence of the family Berothidae. The berothid affinity of this genus is confirmed by examination of the hind wing venation characteristic of the family. The Late Triassic family Mesoberothidae may represent an early group of Berothidae.
Neuroptera, Berothidae, Mesoberothidae, Daohugou, China, Middle Jurassic
Today, the Berothidae (including Rhachiberothinae) is a small neuropteran family comprised of about 100 species discontinuously distributed mainly across tropical and warm-temperate regions of the world (
Currently, 34 fossil berothid species have been described from various localities (listed in Table 1; others are described but unnamed, only illustrated or represented by larvae). The family was previously only known tentatively from the Jurassic:
A list of known fossil Berothidae.
| Species | Age | Locality | References | |
|---|---|---|---|---|
| 1 | Berothidae gen. et sp. n. | Early Cretaceous (Early Berriasian) | Durlston Bay, England (Lulworth Formation) |
|
| 2 | Banoberotha enigmatica Whalley, 1980 | Early Cretaceous (Valanginian/Hauterivian) | Lebanese amber (Jezzine) |
|
| 3 | Paraberotha acra Whalley, 1980 | Early Cretaceous (Valanginian/Hauterivian) | Lebanese amber (Jezzine) |
|
| 4 | Berothidae indet. (larva) | Early Cretaceous (Valanginian/Hauterivian) | Lebanese amber (Jezzine) |
|
| 5 | Chimerhachiberotha acrasarii Nel et al., 2005 | Early Cretaceous (Valanginian/Hauterivian) | Lebanese amber (Jezzine) |
|
| 6 | Raptorapax terribilissima Petrulevicius et al., 2010 | Early Cretaceous (Neocomian) | Lebanese amber (Houarij) |
|
| 7 | Spinoberotha mickaelacrai Nel et al., 2005 | Early Cretaceous (Barremian/Aptian) | Lebanese amber (Hammana) |
|
| 8 | Oloberotha sinica Ren et Guo, 1996 | Early Cretaceous (Barremian) | Yixian Formation, China |
|
| 9 | Araripeberotha fairchildi Martins-Neto et Vulcano, 1990 | Early Cretaceous (Late Aptian) | Crato Formation, Brazil |
|
| 10 | Caririberotha martinsiMartins-Neto & Vulcano, 1990 | Early Cretaceous (Late Aptian) | Crato Formation, Brazil |
|
| 11 | Berothidae indet. | Early Cretaceous (Early Aptian) | Spanish amber (El Sophao) |
|
| 12 | Alboberotha petrulevicii Nel et al., 2005 | Early Cretaceous (Late Albian) | Archingeay, France |
|
| 13 | Eorhachiberotha burmitica Engel, 2004 | Early Cretaceous (Late Albian) | Burmese amber |
|
| 14 | Dasyberotha eucharis Engel et Grimaldi, 2008 | Early Cretaceous (Late Albian) | Burmese amber |
|
| 15 | Ethiroberotha elongata Engel et Grimaldi, 2008 | Early Cretaceous (Late Albian) | Burmese amber |
|
| 16 | Haploberotha persephone Engel et Grimaldi, 2008 | Early Cretaceous (Late Albian) | Burmese amber |
|
| 17 | Iceloberotha kachinensis Engel et Grimaldi, 2008 | Early Cretaceous (Late Albian) | Burmese amber |
|
| 18 | Iceloberotha simulatrix Engel et Grimaldi, 2008 | Early Cretaceous (Late Albian) | Burmese amber |
|
| 19 | Jersiberotha myanmarensis Engel et Grimaldi, 2008 | Early Cretaceous (Late Albian) | Burmese amber |
|
| 20 | Jersiberotha tauberorum Engel et Grimaldi, 2008 | Early Cretaceous (Late Albian) | Burmese amber |
|
| 21 | Scoloberotha necatrix Engel et Grimaldi, 2008 | Early Cretaceous (Late Albian) | Burmese amber |
|
| 22 | Systenoberotha magillae Engel et Grimaldi, 2008 | Early Cretaceous (Late Albian) | Burmese amber |
|
| 23 | Telistoberotha libitina Engel et Grimaldi, 2008 | Early Cretaceous (Late Albian) | Burmese amber |
|
| 24 | Berothidae indet. (larva) | Early Cretaceous (Late Albian) | Burmese amber |
|
| 25 | Retinoberotha stuermeri Schlüter, 1978 | Late Cretaceous (Cenomanian) | Bezonnais, France |
|
| 26 | Plesiorobius sibiricus Makarkin, 1994 | Late Cretaceous (Cenomanian) | Obeshchayushchiy, NE Siberia (Ola Formation) |
|
| 27 | Jersiberotha luzzii Grimaldi, 2000 | Late Cretaceous (Turonian) | Raritan (New Jersey) amber |
|
| 28 | Jersiberotha similis Grimaldi, 2000 | Late Cretaceous (Turonian) | Raritan (New Jersey) amber |
|
| 29 | Nascimberotha picta Grimaldi, 2000 | Late Cretaceous (Turonian) | Raritan (New Jersey) amber |
|
| 30 | Rhachibermissa phenax Engel et Grimaldi, 2008 | Late Cretaceous (Turonian) | Raritan (New Jersey) amber |
|
| 31 | Rhachibermissa splendida Grimaldi, 2000 | Late Cretaceous (Turonian) | Raritan (New Jersey) amber |
|
| 32 | Plesiorobius cf. canadensis | Late Cretaceous (Santonian) | Yantardakh, N Siberia |
|
| 33 | Plesiorobius canadensis Klimaszewski et Kevan, 1986 | Late Cretaceous (Campanian) | Canadian amber |
|
| 34 | Albertoberotha leuckorum McKellar et Engel, 2009 | Late Cretaceous (Campanian) | Canadian amber |
|
| 35 | Berothidae indet. | Late Cretaceous (Campanian) | Canadian amber |
|
| 36 | Oisea celinea (Nel et al., 2005) | Early Eocene | Oise amber, France |
|
| 37 | Microberotha macculloughi Archibald et Makarkin, 2004 | Early Eocene | Hat Creek amber, British Columbia |
|
| 38 | Whalfera venatrix (Whalley, 1983) | Late Eocene | English amber |
|
| 39 | Proberotha prisca Krüger, 1923 | Late Eocene | Baltic amber |
|
| 40 | Whalfera wiszniewskii Makarkin et Kupryjanowicz, 2010 | Late Eocene | Baltic amber |
|
| 41 | Berothidae indet. | Late Eocene | Baltic amber |
|
| 42 | Berothidae indet. | Late Eocene | Baltic amber |
|
| 43 | Berothinae indet. | Late Eocene | Baltic amber |
|
| 44 | Berothinae indet. (larva) | Late Eocene | Baltic amber |
|
| 45 | Berothinae indet. (larva) | Late Eocene | Baltic amber | V.Makarkin, S.Wedmann, T.Weiterschan (ongoing research) |
| 46 | Berothinae indet. (larva) | Late Eocene | Rovno amber, Ukraine | E.Perkovsky, V.Makarkin (ongoing research) |
This study is based on three specimens collected from Daohugou Village (Shantou Township, Ningcheng County, Inner Mongolia, China) and housed in the Key Laboratory of Insect Evolution and Environmental Changes, College of Life Sciences, Capital Normal University, Beijing, China (CNUB; Dong Ren, curator). These insect-bearing beds are here considered as belonging to the Jiulongshan Formation and are dated Bathonian, Middle Jurassic (
Specimens were examined using a Leica MZ12.5 dissecting microscope; line drawings were prepared with CorelDraw 12 graphics software with the aid of Adobe Photoshop; photographed by a Nikon SMZ1000 stereomicroscope.
Venational terminology principally follows
Abbreviations used in the text and figures are as the follows: 1A–3A, first to third anal veins; CuA, CuP, anterior and posterior branches of the cubital vein (Cu); MA, MP, anterior and posterior branches of the medial vein (M); R1, anterior branch of the radial vein (R); Rs1, most proximal branch of the radial sector (Rs); Rs2, branch of the radial sector located distal to Rs1; Rs3, branch of the radial sector located distal to Rs2; Sc, subcostal vein.
Taxonomy Family Berothidae Handlirsch, 1906http://species-id.net/wiki/Sinosmylites
Sinosmylites pectinatus Hong, 1983, by original designation.
Forewing: costal space strongly narrowed basally; humeral veinlet not recurrent and branched; Sc, R1 fused distally; Sc+R1 with 9-11 veinlets, mostly simple; all subcostal veinlets simple; M forked far distal to origin of Rs; CuA pectinate, with seven branches; few crossveins in radial space arranged mainly in 1-2 ‘inner’ gradate series.
Three species from the Middle Jurassic of China (Jiulongshan Formation): Sinosmylites pectinatus (Liaoning Province), Sinosmylites fumosus sp. n. and Sinosmylites rasnitsyni sp. n. (Inner Mongolia).
The venation of these two new species is very similar to that of Sinosmylites pectinatus. The latter species is represented by a nearly complete forewing (
urn:lsid:zoobank.org:act:8851F470-C9D3-4E72-87AA-B8D8639DB16F
http://species-id.net/wiki/Sinosmylites_rasnitsyni
Figs 1 –3Differs from both other species of Sinosmylites by more closely spaced subcostal veinlets, and more deeply forked CuP.
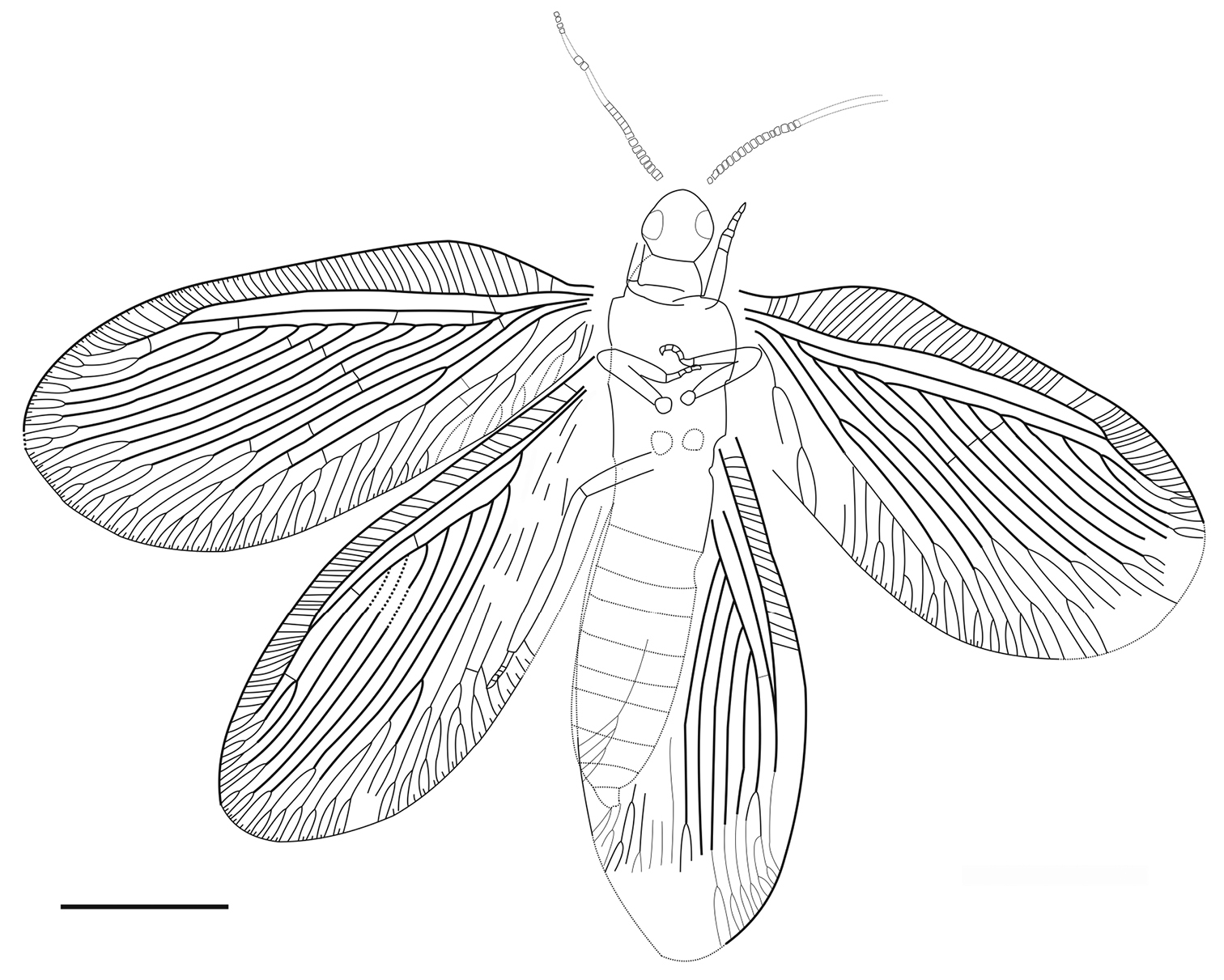
Body indistinctly preserved. Antennae moniliform, incomplete; preserved segments transverse (wider than long). Prothorax short. Mesonotum of usual neuropteran morphology. Legs covered with short hairs; fore-, mid-legs relatively short; hind-leg tibia long; fore-, hind-leg basitarsus longest segment of tarsus. Abdomen very poorly preserved.
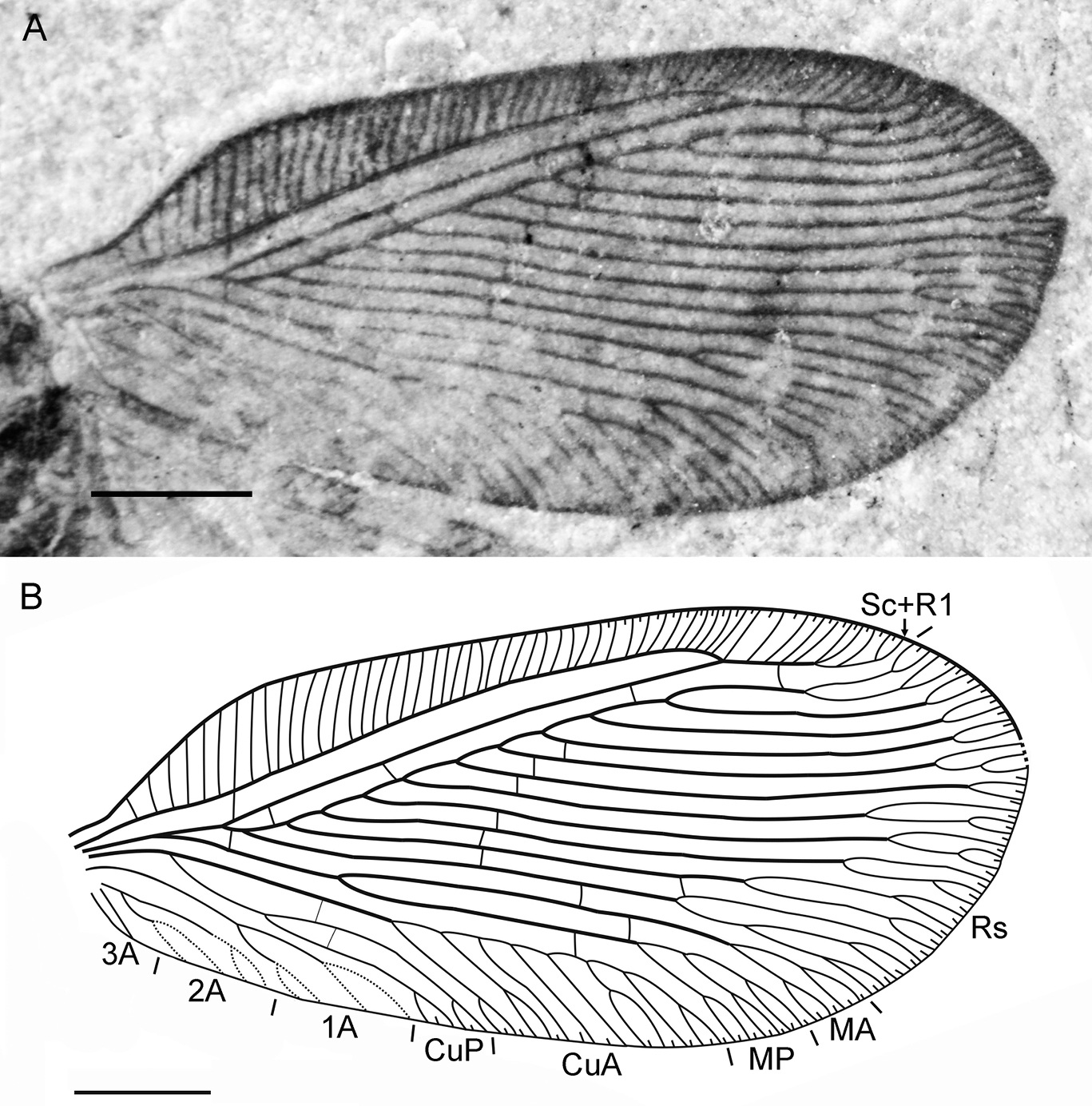
Forewing with broad-rounded apex, 6.7 mm long, 3.0 mm wide. Costal space moderately broad, strongly dilated at proximal 1/5 of wing length, narrowed basally. Subcostal veinlets simple, regularly arranged, closely spaced. Sc distally fused with R1 far from wing apex; Sc+R1 with 9-11 simple veinlets. Subcostal space broad, with one basal crossveins located immediately after origin of Rs. R1 space narrower than subcostal space; three widely-spaced crossveins before fusion of Sc, R1, one after. Rs with 11 (right wing), 10 (left wing) parallel pectinate regularly-spaced branches; six proximal branches with 2-4 terminal forks, other branches once forked. Rs1 originating near origin of Rs. M appears fused basally for short distance; forked much distal to origin of Rs1. MA, MP almost parallel, distally with one, two quite long forked branches respectively. Cu divided into CuA, CuP proximal to origin of Rs. CuA pectinate, with 7 branches, some once forked. CuP once deeply forked. Anal veins very poorly preserved; 1A, 2A apparently once deeply forked each; 3A simple. Four gradate series of crossveins posterior to stem of Rs partly preserved (series 1-4 of
Hind wing poorly preserved, approximately 6.5 mm long, 2.6 mm wide. Costal space narrow, distally only slightly dilated. Subcostal veinlets simple, rather closely spaced. Sc distally fused with R1 far from wing apex; Sc+R1 with 13 simple veinlets. Subcostal space relatively narrow; no crossveins detected. R1 space broad, dilated basally; two crossveins before fusion of Sc, R1, one after. Rs originating far from wing base, with eight branches, each forked distally 1-3 times except Rs1 which deeply forked four times. Fork of M not detected. MA once forked distally; MP dichotomously branched distally. CuA long, almost parallel to hind margin, its branches poorly preserved. CuA space relatively broad. CuP fragmentary preserved, quite short. Anal veins not preserved. Crossveins posterior to stem of Rs not detected except one distal between MP, CuA (4m-cu).
Sinosmylites rasnitsyni sp. n. Photograph of the holotype A part (CNU-NEU-NN2011002P; in alcohol) B counterpart (CNU-NEU-NN2011002C; dry).
Sinosmylites rasnitsyni sp. n. Drawing of the holotype CNU-NEU-NN2011002P. Scale bar is 2 mm.
Sinosmylites rasnitsyni sp. n. Forewing of the holotype CNU-NEU-NN2011002P (converted to the right). A photograph B drawing. Scale bar is 1 mm.
Holotype CNU-NEU-NN2011002P (part), CNU-NEU-NN2011002C (counterpart), deposited in CNUB. A nearly complete specimen.
Daohugou Village, Shantou township, Ningcheng county, Inner Mongolia, China. Jiulongshan Formation, Middle Jurassic.
The species is named in honor of the distinguished Russian paleoentomologist Prof. Alexandr Pavlovich Rasnitsyn.
urn:lsid:zoobank.org:act:544F55FC-01D7-4655-BD4F-CBEE79EA247F
http://species-id.net/wiki/Sinosmylites_fumosus
Figs 4A, BDiffers from Sinosmylites pectinatus by CuP once forked (twice forked in Sinosmylites pectinatus), by presence of one ‘inner’ gradate series of crossveins (two in Sinosmylites pectinatus) (see differences from Sinosmylites rasnitsyni sp. n. under that species.).
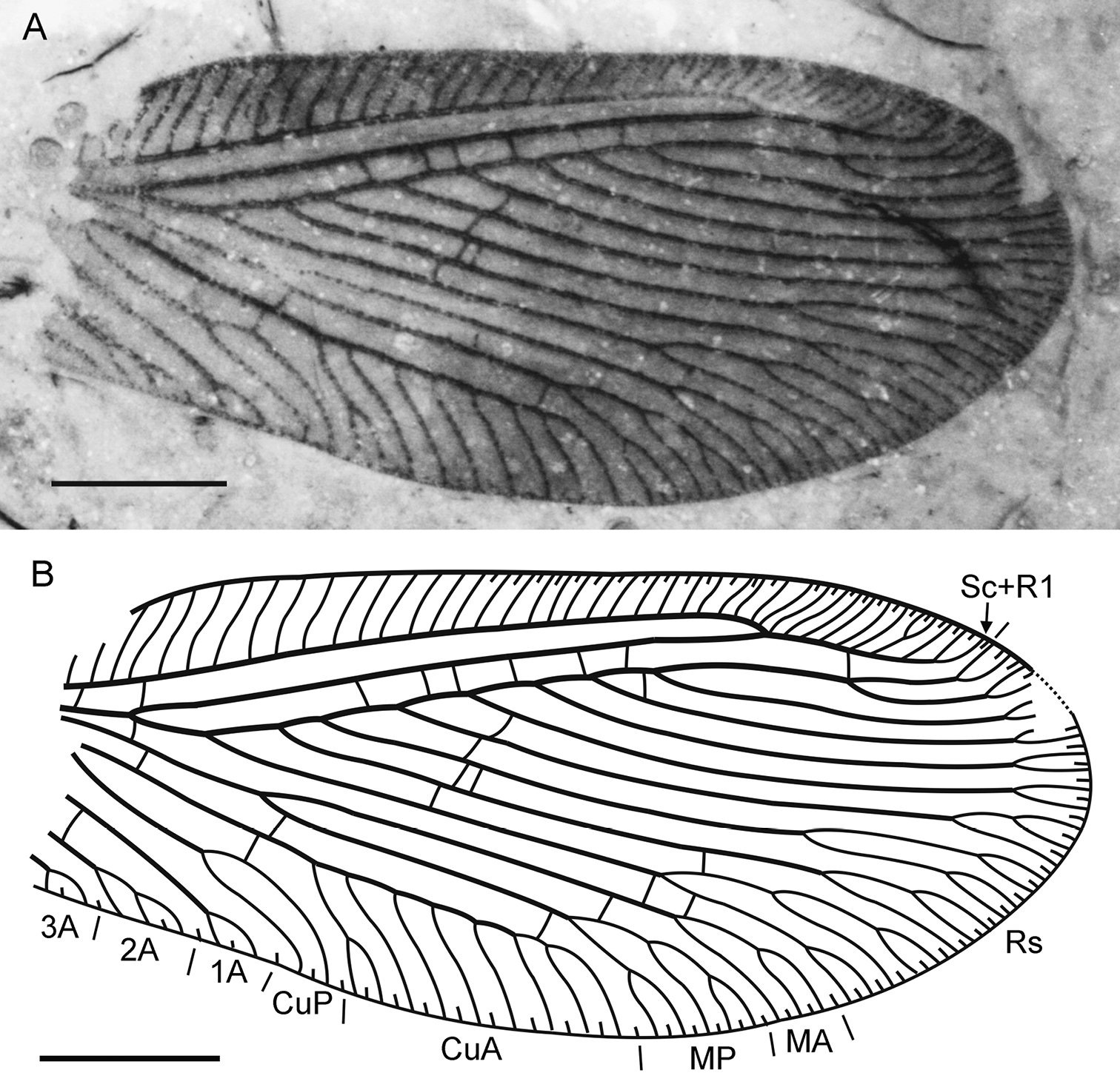
Forewing with broad-rounded apex, about 6.0 mm long (as preserved, estimated complete length about 6.5 mm), 2.6 mm wide. Costal space moderately broad, most dilated at proximal 1/5 of wing length. Subcostal veinlets simple, regularly arranged, less closely spaced than in previous species. Sc distally fused with R1 far from wing apex; Sc+R1 with nine veinlets (eight simple, one forked). Subcostal space broad, with two basal crossveins. R1 space nearly as wide as subcostal space; six crossveins before fusion of Sc and R1, one after. Rs with nine pectinate, regularly spaced branches; four proximal-most branches with 2-4 terminal forks, other branches once forked. Rs1 originating at some distance from origin of Rs. M not fused basally; forked much distal to origin of Rs1. MA, MP almost parallel, distally with one (simple) , two (one simple) branches respectively. Cu divided into CuA, CuP proximal to origin of Rs. CuA pectinate, with 7 branches; proximal-most branch once forked. CuP once deeply forked. Anal veins incompletely preserved; 1A with single marginal fork; 2A with two marginal short branches; 3A very incomplete, with single fork preserved. Four gradate series of crossveins posterior to stem of Rs, all incomplete. First series consists of three crossveins: 1r-m (located at origin of Rs), 1m-cu, 1a1-a2 (longer than previous); Second series includes two crossveins: 2m-cu (connecting MP, CuA), 2icu (connecting CuA, anterior branch of CuP). Third (‘inner’) series with six crossveins (3rs-rs7, 3rs5-rs4 to 3rs2-rs1; two between Rs3, Rs2). Fourth (‘outer’) series with five crossveins (from 4rs2-rs1 to 4m-cu; two between Rs1, MA). Wing one color, fuscous. Veins mainly dark brown as preserved.
Sinosmylites fumosus sp. n. Holotype CNU-NEU-NN2011003, the forewing. A photograph B drawing. Scale bar is 1 mm.
Holotype CNU-NEU-NN2011003, deposited in CNUB. A nearly complete forewing.
Daohugou Village, Shantou township, Ningcheng county, Inner Mongolia, China. Jiulongshan Formation, Middle Jurassic.
From the Latin fumosus, smoked, in reference to the coloration of wings.
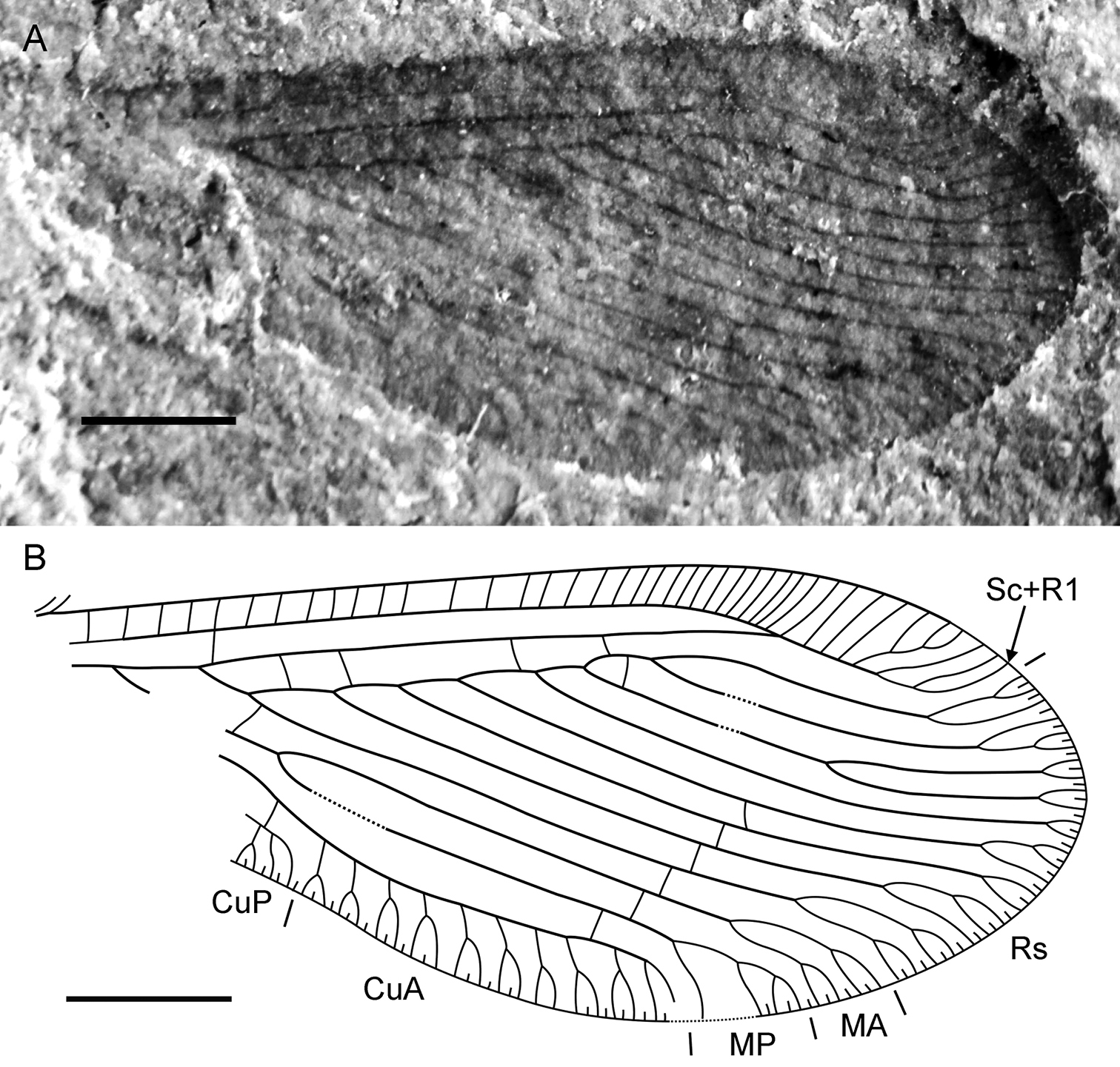
Hind wing approximately 6.5 mm long, 2.7 mm wide. Humeral lobe not extended; frenulum poorly-developed consisting of few bristles. Costal space narrow, dilated towards apex. Subcostal veinlets simple, more closely-spaced apically. Sc distally fused with R1 far from wing apex; Sc+R1 with seven long veinlets (one forked). Subcostal space relatively broad, with one basal crossveins. R1 space nearly as wide as subcostal space; four crossveins before fusion of Sc, R1. Rs with seven pectinate, regularly spaced branches; one branch deeply forked. Rs1 originating at some distance from origin of Rs. Proximal crossvein m-r long, connecting Rs1 near its origin with M. M forked distal to origin of Rs1. MA, MP almost parallel, distally with few branches. CuA long, slightly incurved, in general parallel to hind margin, with nine forkes branches originated at angle >45 degrees, one simple branch. CuP short, with two branched preserved. 1A–3A not preserved. Crossvein between CuA, 1A (or CuP). One crossvein between Rs, Rs6 in ‘inner’ gradate series (possibly anomalous). Six crossveins (from Rs4 to CuA) in ‘outer’ gradate series preserved. Wing one color, fuscous. Veins appear mainly dark brown.
Sinosmylites sp. Specimen CNU-NEU-NN2011004, the hind wing. A photograph B drawing. Scale bar is 1 mm.
Specimen CNU-NEU-NN2011004, deposited in CNUB. A nearly complete hind wing.
Daohugou Village, Shantou township, Ningcheng county, Inner Mongolia, China. Jiulongshan Formation, Middle Jurassic.
The venation of this hind wing is typical for Berothidae. In particular, the configuration of CuA is characteristic of this family; although this also occurs in the Nevrorthidae, nevrorthid venation is otherwise dissimilar. Also, the basal crossvein between R and M systems is straight, perpendicular to connecting veins; this is characteristic of all Berothidae except Rhachiberothinae. In the vast majority of extant Berothidae, the complete CuP is lost, but the basal or/and distal parts there are often present. CuP is entirely lost in some genera, both fossil (e.g., Microberotha macculloughi Archibald and Makarkin, 2004) and extant (e.g., Cyrenoberotha MacLeod and Adams, 1968, Berlekrumyia Aspöck and Aspöck, 1988). Therefore, it is hard to determine which vein is preserved in this hind wing, the distal part of CuP or 1A (see Fig. 5B, labeled CuP), as the proximal portion of the wing is not preserved. We tentatively consider this vein to be CuP.
This specimen is tentatively assigned to Sinosmylites. The hind wings of Sinosmylites rasnitsyni sp. n., the only species of the genus in which these are known, are quite poorly preserved and their venation does not enable its generic character states to be determined with confidence. However, this hind wing shares similar size, coloration, and venation (e.g., Sc and R1 are fused; Sc+R1 with many branches; several crossveins between R1 and Rs; the same configuration of the Rs branches) with the forewings of Sinosmylites species. Therefore, this generic affinity is most likely.
As the type species of the genus Sinosmylites is represented by a single forewing, its family affinity cannot be confidently determined. Its venation is more or less similar to that of such families as Berothidae, Sisyridae, Mesoberothidae, Archeosmylidae, and Prohemerobiidae, all except the first two are extinct and poorly understood. Their forewing venations show superficial similarities in the structure of the costal space (narrowed basally), Sc fused with R1 (only convergent distally but not fused in Prohemerobiidae), similar (in general) branching of Rs, M, and Cu, and their sparse cross-venation. One feature occurs rarely (if at all) in these taxa, i.e., the strongly pectinate CuA, complicating family determination. Therefore, based solely on the forewing, it may be theoretically associated with most of these families, at least provisionally. Fortunately, one of two new species described herein bears its hind wing, although poorly preserved. Its structure indicates that the berothid affinity of Sinosmylites is most probable, as its general venation does not conflict with that of Berothidae, and the presence of the long CuA running nearly parallel to the hind margin characteristic of the family. Moreover, the berothid affinity of a better preserved hind wing (“Sinosmylites sp.”) is doubtless, as all of its character states are characteristic only of the Berothidae.
The forewing venation of Sinosmylites differs rather greatly from that of the vast majority of extant (advanced) genera of Berothidae. Particularly, M is forked distinctly more distally than in most berothid genera (including Cretaceous genera: see
The hind wing venation of “Sinosmylites sp.” appears amazingly modern. Even if our generic attribution turns out to be incorrect, its berothid family affinity is doubtless.
Triassic berothid-like taxa have been treated as belonging to the family Mesoberothidae. This taxon was created by
Mesoberotha is represented by a single forewing specimen of Mesoberotha superba Riek, 1955, whose venation is similar to that of Sinosmylites (especially Sinosmylites fumatus sp. n., known from a better preserved forewing), i.e., the costal space is similarly constructed, narrowed basally; Sc and R1 are fused; the subcostal space is relatively broad; few crossveins are present, most of which are arranged in a gradate series of crossveins; M, Cu, CuA and CuP are branched in a similar manner; 1A with only marginal (shallow) branches. As mentioned above, this genus cannot be assigned to a particular family with confidence based only on characters of the forewing. However, the similarity of the venation between Sinosmylites and Mesoberotha is so distinct as to strongly suggest that Mesoberotha may belong to Berothidae, and that Mesoberothidae is, therefore, a synonym of Berothidae. However, it is necessary to find and examine the Mesoberotha hind wing in order to test this hypothesis.
‘Archeosmylus’ ?costalis Riek, 1955 from the same Australian locality as Mesoberotha probably also belongs to the Mesoberothidae. Its venation differs not sufficiently from that of Mesoberotha superba, and it may possibly belong to this genus. The family affinity of ‘Archeosmylus’ stigmatus Riek, 1955 is not yet clear.
We thank S. Bruce Archibald (Simon Fraser University, Burnaby, Canada) for correction of English. This research is supported by the National Natural Science Foundation of China (Nos. 40872022, 31071964), the Nature Science Foundation of Beijing (No. 5082002) and Scientific Research Key Program KZ200910028005, and PHR Project of Beijing Municipal Commission of Education.