(C) 2011 Elena D. Lukashevich. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Four new species of Chironomidae with well-developed elongate proboscises are described from a Late Jurassic site Shar Teg in SW Mongolia. These are named Cretaenne rasnicyni sp. n., Podonomius blepharis sp. n., Podonomius macromastix sp. n., ?Podonomius robustus sp. n.
Diptera, Chironomidae, fossil, proboscis, feeding, new species, Mongolia, Late Jurassic
The present paper continues a series of articles with descriptions of Diptera from the Late Jurassic Shar Teg site (e.g.
About 600 identifiable dipteran fossils are known among
3000 fossil insects collected at Shar Teg. Up to now, members of two
culicomorph families are described from this locality, Dixidae (
The Mesozoic records of Chironomidae are numerous, and usually it is the aquatic immatures that are dominant (
The adults of nearly all extant chironomid midges have
reduced mouthparts and so their common name is “non-biting midges”.
However the presence of toothed mandibles in a chironomid midge was
recognized first by
New chironomids with biting mouthparts from Shar Teg are
described herein. These fossils are housed in the Borissiak
Paleontological Institute, Russian Academy of Sciences, Moscow (PIN).
Photographs were made using a Leica MZ 9.5 stereomicroscope with a Leica
DFC420 digital camera, with further correction using Adobe Photoshop®
CS 9.0 software. Measurements were made with an ocular micrometer in a
Leica stereomicroscope. Morphological terminology and measurements
mainly follow
Subfamily ?Aenneinae Ansorge, 1999
http://species-id.net/wiki/Cretaenne
The genus was established based on two species fromEarly Cretaceous Lebanese amber. The specimens under description are assigned to this genus due to functional blade-like laciniae and mandibles in females, postnotum with a longitudinal groove, reduced hind tibial comb, and peculiarities of wing venation (vein C long and reaching wing tip; Sc not terminating in wing margin; R2 present; cell between divergent R2+3 and R4+5 very broad; R4+5 almost straight; bM3+4 present; r-m, bM3+4 and m-cu aligned; m-cu connecting CuA proximal to r-m). In the new species, tibial spurs are probably present; however, their structure remains unclear due to the state of preservation. The structure of claws is important for the determination of Lebanese species, but claws are not visible in the Mongolian specimens as well as the details of chaetotaxy (e.g., on pedicel) and therefore not mentioned in the description.
urn:lsid:zoobank.org:act:1F783221-19EE-43C3-8764-45B6B34E0CB4
Named in honour of an outstanding Russian palaeoentomologist Dr A.P. Rasnitsyn.
Holotype: part and counterpart of well-preserved female PIN 4270/2379±, SW Mongolia, Shar Teg (443/1); Late Jurassic. Paratypes: impressions of two females PIN 4270/2367±, 2459, from the same outcrop.
The new species is distinguished from both known species of Cretaenne by the longer proboscis (more than half of the head height and about twice the clypeus height) and the longer Rs stem.
Female. Measurements (mm): Total length 2.1–2.7 (holotype 2.3); thorax length ca. 0.8, width ca. 0.4; wing length 2.1–2.3 (holotype 2.1); abdomen length 1.35–1.8 (holotype 1.5). Total length / wing length 1.1.
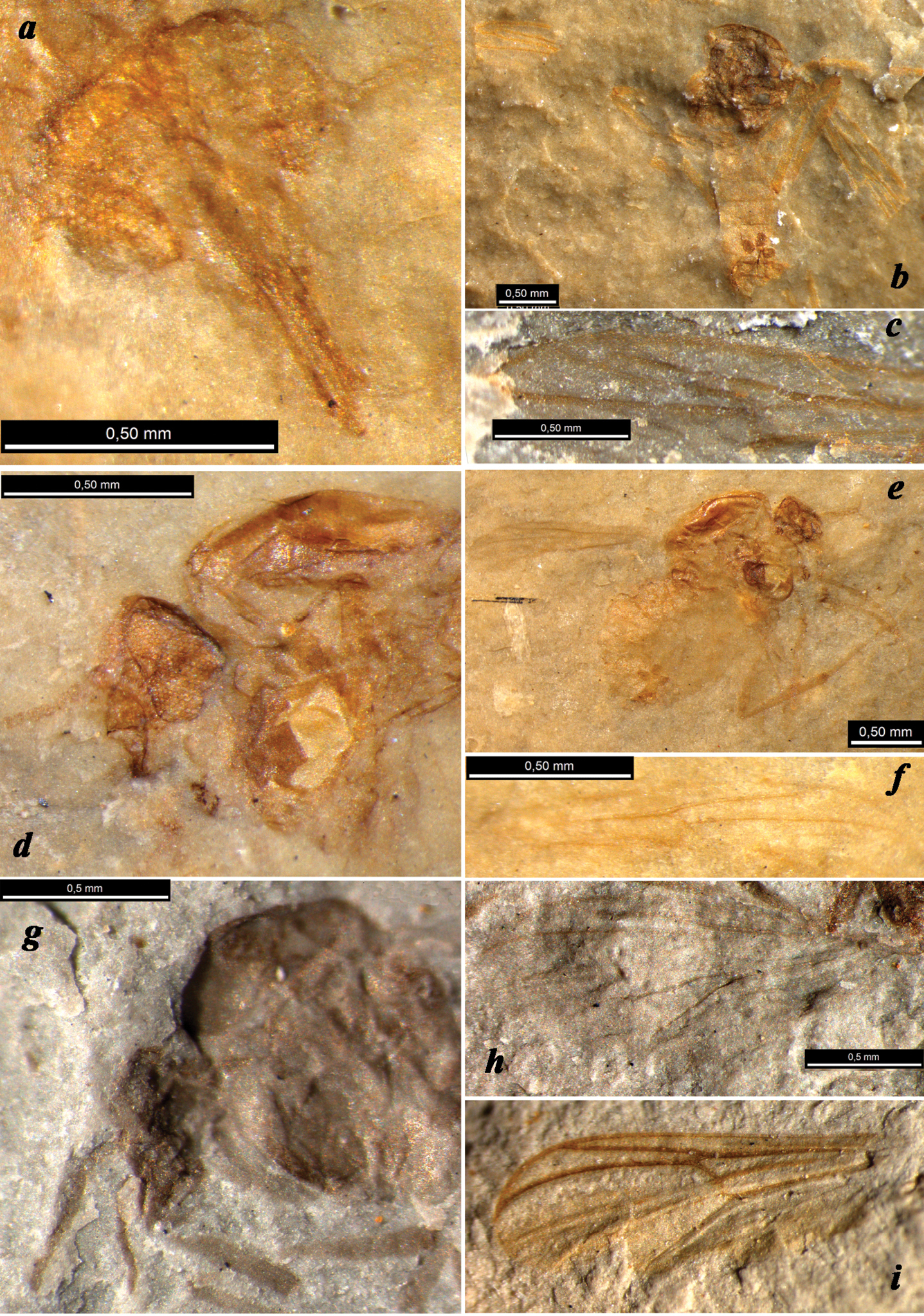
(Figs 1a–d, 2b–c). Coloration. Thorax dark, abdomen and legs lighter, at least some legs with slightly darker apices of femora and tibiae. Head ca. 550 μm wide, ca. 400 μm high to lower eye margin. Scape ca. 100 μm, pedicel ca. 30 μm in diameter. Clypeus ca. 200 μm wide, ca. 250 μm high. Proboscis with visible part ca. 350 μm long (possibly, without apex), tapering, sclerotized. Thorax. Postnotum wider than long, ca. 200 μm long, possibly with longitudinal median groove. Wing longer than abdomen, Sc clearly up to Rs level, thinning distally, possibly not reaching C (apical part of Sc not discernible). Vein C and radial veins strong, coloured (R2+3 thinner then others), as well as proximal sections of M and CuA, r-m and bM3+4. Long stem Rs subequal to r-m; R4+5 8–9 times as long as Rs; R2 distinctly longer than dR1. VR 1.1. Legs (lengths not measurable). Femora (mid- and hind) widened to apex, maximum 120 μm wide, with thin sclerotized ridge ventrally near apex. Tibiae (mid- and hind) apically 80–100 μm wide. Tarsi not preserved. Abdomen. Abdominal segments II–V: ca. 200 μm long, 500–600 μm wide. Three large subequal oval sclerotized spermathecae 140–150 μm long, ca. 100 μm wide, with necks (probably, short). Gonapophysis IX distinctly visible, sclerotized, with notum ca. 200 μm long, 15 μm wide at anterior end, with rami ca. 60 μm long. Probable gonocoxites VIII (gonacoxapodemes?) visible as moderately sclerotized small oval lobes near posterior end of notum. Cerci short, hardly visible.
a–h Cretaenne rasnicyni sp. n. a–d holotype (a total habitus b abdominal apex c, d wings) e–f paratype PIN 4270/2367 (head and wing) g–h paratype PIN 4270/2459 (apex of midtibia and wing) i–l Podonomius blepharis sp. n., holotype (i abdominal apex j hind tibial apex k wing l head and thorax) m–n Podonomius macromastix sp. n., holotype (wing and head) o Tanypodinae inc. sed., PIN 4270/2324; all from Shar Teg, J3. Scale bar 0.5 mm except for g, j without scale.
Jurassic Chironomidae: a–c Cretaenne rasnicyni sp. n. (a paratype PIN 4270/2367, female head under alcohol b–c holotype b female habitus, positive impression under alcohol c wing, negative impression) d–f Podonomius blepharis sp. n., holotype (d female head and thorax, negative impression under alcohol e female habitus, positive impression under alcohol f wing, negative impression under alcohol) g–h Podonomius macromastix sp. n., holotype (female head and wing); all from Shar Teg, J3 i holotype of ?Podonomius rotundatus Kalugina, 1985, Kubekovo, J2.
(Figs 1e–h, 2a). Visible characters as in holotype, with following additions. Head preserved only in PIN 4270/2367, 650 μm wide, 850 μm high with proboscis, ca. 400 μm high to lower eye margin. Eyes with medially narrowing dorsomedial extension, well separated by 60 μm. Coronal triangle ca. 130 μm high, ca. 100 μm wide; coronal suture clear, probably pair of poorly visible ocelli (ca. 30 μm in diameter) adjoining lower part ventrally to eye dorsomedial extension. Scape ca. 100 μm, pedicel ca. 40 μm in diameter. Flagellomeres (five distinctly visible lateral to eye margin) short-oval, 50–70 μm long, 30–40 μm wide. Clypeus ca. 200 μm wide, ca. 300 μm high. Proboscis ca. 550 μm long, sclerotized, stylet-like, pointed, with blade-like tapered laciniae and mandibles, labrum apically more sclerotized. Palpi poorly visible, looking widened (ca. 70 μm in distal parts); visible parts of palpi reaching about 4/5 of proboscis. Thorax. Scutum weakly, evenly convex; anterior part ca. 300 μm wide. Antepronotals narrowed medially. Scutellum ca. 120 μm long; postnotum ca. 200 μm long, 300 μm wide, with distinct longitudinal median groove. Legs. Measurements (μm). p1(?): ti 880, ta1-5 1200; p2: ti 1375, ta1 750–850, ta2 ca. 400, ta3-5 ca. 700, LR2 0.55; p3: fe 1000, ti 1450–1500, ta1-5 > 1250. Tibiae 60–100 μm wide near apex. Apices of mid-, hind tibiae with combs of separate spiniform setae (visible only in PIN 4270/2459): midtibia with no less than 3–4 slenderer setae ca. 50 μm long, hind tibia with no less than 3 thicker setae, longest ca. 50 μm. Hind tibia probably with two spurs, 70 and 50 μm long.
The pattern of the female mouthparts is poorly visible in the holotype and unknown in paratype PIN 4270/2459; paratype PIN 4270/2367 has a well-preserved proboscis and its wing venation is very pale and incomplete; the setae at the tibial apices are distinctly visible only in paratype PIN 4270/2459; in both paratypes the spermathecae are not visible. Hence it is possible that these specimens are not conspecific. However, we suggest that all these specimens belong to the same species due to visible peculiarities of venation (particularly the long Rs stem and R2 position).
The genus Cretaenne was described in the subfamily Aenneinae
with reservations, due to the vein Sc not terminating in the wing
margin and short Rs stem, as distinct from the type genus of the
subfamily, Aenne Ansorge, 1999 from the Late Triassic and Early Jurassic of Europe (
http://species-id.net/wiki/Podonomius
The genus was described for six species
fromthe Early and Middle Jurassic of Siberia, with only two species
being attributed with certainty. Later, one more species from the Early
Jurassic of Germany was tentatively included (
We re-examined the type material of the six species described by Kalugina (1985) from Siberia (Fig. 2i) but did not examine ?Podonomius tumidus Ansorge, 1996 from Grimmen. A postnotum without longitudinal median groove was recorded in the original diagnosis of Podonomius (for German species, such information was absent;
urn:lsid:zoobank.org:act:EE993F1F-6B84-4BA7-80B6-9905C90DA6B2
From Greek “blepharis” for eyelash, after the pattern of the tibial comb.
Holotype: part and counterpart of well-preserved female PIN 4270/2357±, SW Mongolia, Shar Teg (443/1); Late Jurassic.
The new species is distinguished by its small size (wing length l.4 mm), well-developed elongate proboscis, weakly convex scutum without a hump, wing with broad cell c, and pale legs with darker junction of femur with trochanter and tibia, and with combs of dark closely-spaced spiniform setae at tibial apices.
Female (Figs 1i–l, 2d–f). Measurements (mm): Total length 2.0; thorax length 0.8, height 0.9; abdomen length 1.0; wing length 1.4. Total length / wing length 1.4. Coloration. Head and thorax dark, abdomen lighter, legs pale with darker junction of femur with trochanter and tibia, with darker apices of tibiae. Head no less than 600 μm wide, no less than 550 μm high with proboscis, 380 μm high to lower eye margin. Eyes large, with wide dorsomedial extension, looking narrowly separated by ca. 30 μm. Facets equal. Coronal triangle ca. 100 μm high, coronal suture clear near upper eye margin. Scape 90 μm, pedicel 45 μm in diameter; proximal flagellomeres short-oval to rounded, ca. 35 μm wide and 40–50 μm long. Clypeus ca. 100 μm wide, ca. 150 μm high, possibly with longitudinal groove. Proboscis well-developed, elongate, sclerotized at least in distal part, ca. 200 μm long (projecting distally of clypeus for no less than 120 μm), ca. 30 μm wide at visible apex. Thorax. Scutum weakly, evenly convex, without hump or tubercle. Scutellum ca. 150 μm long, not projecting. Postnotum ca. 200 μm long. Wing. Vein C probably not produced beyond R4+5; cell c at r-m level subequal to cell r1, which only slightly narrower than r5 cell at level of R1 tip; R1 approximately 2/3 as long as almost straight R4+5; r-m inclined to M. All veins mentioned strong, coloured. Legs. Measurements (μm). p1(?): ta1 380, ta2-5 ca. 405; p2: fe 700, ti 680; p3: fe 560, ti 680, ta1 ca. 500, ta2-5 ca. 650, LR3 ca. 0.75. Femora maximum ca. 110–120 μm wide, with thin sclerotized ridge ventrally near apex. Tibiae ca. 80 μm wide. Apices of mid-, hind tibiae with combs of dark closely-spaced spiniform setae ca. 50 μm long; in midtibial comb, no less than 10 setae, in hind tibial comb, 8 setae. Spurs not observed. Abdomen. Segments III–VII: tergites 150–180 μm long. Three large subequal oval moderately sclerotized spermathecae 100 μm long, ca. 80 μm wide, with necks (probably, long), in compact group. Sternite VIII with posteromedian sclerotized plate, its posterior margin bilobate; probable gonocoxites VIII (gonacoxapodemes?) visible as moderately sclerotized small oval lobes approximating each other. Cerci not visible.
The new species is similar to Podonomius splendidus Kalugina, 1985 (J1/2, Novospasskoye, Transbaikalia) in its venation (C length, ratio R1/ R4+5), colour pattern of legs and elongated mouthparts, which are visible on paratype PIN 3000/1857 (in the other type specimens of Podonomius from Siberia, the mouthparts are not visible due to the state of preservation). Podonomius blepharis sp. n. differs from Podonomius splendidus in broader cell c and smaller size. As for tibial combs, Kalugina noted (1985) that in Podonomius tugnuicus and Podonomius splendidus the tibial apices are darkened but without mentioning combs. According to our re-examination of the type material of Podonomius splendidus, the hind tibia has a reduced comb consisting of a row of separate dark points, which may be minute setae or possibly bases of missing long bristles (these seem to be visible near the tibial apex in the holotype). In the latter case, a well-developed tibial comb is not unique for Podonomius blepharis.
urn:lsid:zoobank.org:act:AD0202E9-83D8-4462-B354-5D9697CA7485
From Greek “makros” for long and “mastix” for whip, after the long antenna.
Holotype: part and counterpart of well-preserved female PIN 4270/2314±, SW Mongolia, Shar Teg (423/6); Late Jurassic.
The new species is distinguished by its small size (wing length 1.9), well-developed elongate proboscis, strongly convex scutum with a hump, comparatively long wings with R1 with arched tip and broad cell r5, and pale legs with darker junction of femur with trochanter and tibia.
Female (Figs 1m–n, 2g–h). Measurements (mm): Total length 2.0; thorax length 0.7, height 0.9; wing length 1.9, width 0.8; abdomen length 1.1. Total length / wing length ca. 1.05. Coloration pattern as in Podonomius blepharis sp. n. Head 480 μm wide, no less than 600 μm high with proboscis, 380 μm high to lower eye margin. Eyes large, with wide dorsomedial extension, narrowly separated by ca. 30 μm. Facets slightly increasing to lower eye parts. Coronal triangle ca. 50 μm high, coronal suture ca. 80 μm high. Antenna no less than 600 μm. Scape ca. 80 μm, pedicel ca. 60 μm in diameter; at least 12 flagellomeres, ca. 35 μm wide (proximal 6 flagellomeres moniliform, 40–45 μm long; others cylindrical, 50–70 μm long). Clypeus ca. 130 μm wide, ca. 150 μm high. Proboscis well-developed, elongated, tapering, ca. 180 μm long (about 1/3 of head height), ca. 70 μm wide at visible apex, with pair of separate sclerotized blades. Thorax. Scutum strongly convex, with hump before midlength, 700 μm long. Postnotum ca. 200 μm long. Wing much longer than abdomen. Vein C only slightly produced beyond R4+5, not reaching wing tip; cell c at r-m level broader than cell r1, which is almost half as wide as cell r5 at R1 tip level; R1 with arched tip, 2/3 as long as slightly curved down distally R4+5; r-m slightly inclined, bM3+4 almost perpendicular to M. Legs poorly visible. Measurements (μm). p2(?): fe ca. 550, ti ca. 700, ta1-5 ca. 700; p3(?): ti ca. 870, ta1-5 > 800. Hind femora ca. 110 μm wide; hind tibiae 70–80 μm wide at apex, with sclerotized apical traces, possibly of setae bases. Abdomen with three large unequal short-oval sclerotized spermathecae 60–90 μm long, 50–60 μm wide, with necks, in compact group. Cerci ca. 40 μm long.
The new species is similar to ?Podonomius rotundatus Kalugina, 1985 (J2, Kubekovo, South Siberia, Fig. 2i) in its venation (length of C, R1/R4+5 ratio, cells r1/r5 ratio) and size, but is distinguished by the arched tip of R1. The new species differs from Podonomius blepharis sp. n. in the longer wings with broader cell r5 and thoracic shape.
From Latin “robustus” for stout, after the total habitus.
Holotype: Part and counterpart of partly preserved female PIN 4270/2254±, SW Mongolia, Shar Teg (443/1); Late Jurassic.
The new species is distinguished by its medium size (wing length 3.7 mm), well-developed, strongly elongate proboscis, wing with C produced beyond R4+5 and reaching wing tip, strongly sclerotized abdomen and legs, and one spermatheca situated proximally of other two.
Female (Fig. 3). Measurements (mm): Total length 4.1; thorax length 1.5, width ca. 1.0; abdomen length 2.9; wing length 3.7. Total length / wing length 1.1. Coloration. Head, thorax, abdomen, legs uniformly dark. Head 700 μm wide, no less than 1100 μm high with proboscis, 550 μm high to lower eye margin. Eyes large, with wide dorsomedial extension, well-separated by ca. 100 μm. Facets equal. Frontal strip between eye extensions dark-coloured, long, sclerotized. Coronal triangle ca. 100 μm high, coronal suture ca. 70 μm high. Scape ca. 125 μm, pedicel ca. 40 μm in diameter. Clypeus ca. 200 μm high, ca. 150 μm wide. Proboscis very long, strong (longer than remainder of head), tapering, ca. 550 μm long, ca. 80 μm wide at (visible) apex; apical part with pair of sclerotized blades. Probable palpi no less than 400 μm long, two visible segments elongate, cylindrical, ca. 150 μm long, ca. 30 μm wide. Thorax. Scutum 900 μm long; postnotum ca. 250 μm long. Wing clearly longer than abdomen. Vein C produced beyond R4+5 (costal extension ca. 200 μm), reaching wing tip; cell c at r-m level broader than cell r1; cell r5 at R1 tip level almost twice as wide as cell r1; R1 straight, 2/3 as long as R4+5. Vein C, radial veins, M, r-m strong and coloured; other veins thin, pale. Legs. Mid- and hind femora ca. 180 μm wide; tibiae 100–120 μm wide near apex. Apex of mid- or hind tibia with traces of at least 5 spiniform setae. Abdomen. Segments II–VIII 250–300 μm long, 800–900 μm wide. Three rounded sclerotized spermathecae 70–90 μm in diameter, with long necks, largest spermatheca situated proximally of other two. Gonapophysis IX distinctly visible, sclerotized, with notum ca. 200 μm long and rami ca. 50 μm long. Posterior margin of sternite VIII bilobate: probable gonocoxites VIII (gonacoxapodemes?) visible distal to spermathecae. Cerci distinct, elongate-oval, 150 μm long, ca. 70 μm wide.
?Podonomius robustus sp. n., holotype (a, d head, positive impression b, e abdominal apex, negative impression c, h wing, negative impression f female habitus, negative impression g apex of hind tibia, positive impression; all photos made under alcohol except for f); Shar Teg, J3. Scale bar 0.5 mm
Adults of this new species are the largest among Podonomius (wing length is similar only in ?Podonomius simplex Kalugina, 1985 (J2, Kubekovo, South Siberia), but in the Siberian species R1 is curved up distally). ?Podonomius robustus
sp. n. differs from other species from Shar Teg also in the longer
costal extension. Such a long costa extending to the wing tip is an
important plesiomorphic character (
Among fifty adult chironomids from Shar Teg, only two incomplete females (PIN 4270/2324±, 4270/2431) and one male (PIN 4270/2384) can be determined as members of this subfamily (all from the same 443/1 outcrop) due to the typical venation on partly preserved wings (Fig. 1о). The poor state of their preservation does not allow us to place the specimens within a genus.
DiscussionThe four new species of Chironomidae
described in this paper are characterized by the elongate proboscis
with well-developed (probably sclerotized) mandibles and/or maxillae. To
date, a well-developed piercing proboscis has been described only in
two recent and no less than four extinct genera of the Chironomidae (
The proboscises of Cretaenne rasnicyni sp. n. and ?Podonomius robustus sp. n. are much longer than in any other fossil Chironomidae described to date (possibly except for an undeterminable culicomorphan from the Triassic Cow Branch Formation;
In the general appearance (very long and strong
proboscis, body size, shape and proportions; pattern and degree of
sclerotization, e.g. strongly sclerotized abdomen and legs) ?Podonomius robustus differs from “typical” Chironomidae as well as from other species assigned to the genus Podonomius andresembles some “robust” Ceratopogonidae, especially many Palpomyiini, but this advanced tribe is unknown from the Mesozoic (
However, in the holotype of ?Podonomius robustus the partly visible transverse vein under r-m
is undoubtedly coloured, that is not recorded for the basal part of
vein M2 in ceratopogonid wing, but typical for bM3+4 in podonomine wing
(Figs 2h–i). In addition, the venation pattern of the anterior part of the wing is more similar to Podonominae than to Ceratopogonidae (vein R1 long, cell r1 long and not narrow) and the vertex of Podonomius blepharis as well as ?Podonomius robustus possesses a coronal suture, which is a feature of the Chironomidae, absent in Ceratopogonidae (
The subfamily Podonominae is shown to be a dominant one in specimen abundance and diversity in the Jurassic deposits of Siberia (
Recently,
We are deeply indebted to Dmitry Shcherbakov (Moscow) for valuable discussions. We are grateful to Art Borkent (Canada) and Peter Cranston (Australia) for insightful comments on the manuscript and for improving the English. The research was partly supported by the programme of the Presidium of Russian Academy of Sciences “Biosphere origin and evolution of geobiological systems”.