(C) 2012 David C. Houghton. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The caddisfly fauna of Minnesota contains at least 277 species within 21 families and 75 genera. These species are based on examination of 312, 884 specimens from 2, 166 collections of 937 Minnesota aquatic habitats from 1890 to 2007. Included in these totals is my own quantitative sampling of 4 representative habitat types: small streams, medium rivers, large rivers, and lakes, from each of the 58 major Minnesota watersheds from June through September during 1999–2001. All species are illustrated herein, and their known Minnesota abundances, distributions, adult flight periodicities, and habitat affinities presented. Four species: Lepidostoma griseum (Lepidostomatidae), Psilotreta indecisa (Odontoceridae), and Phryganea sayi and Ptilostomis angustipennis (Phryganeidae) are added to the known fauna. An additional 31 dubious species records are removed for various reasons. Of the 5 determined caddisfly regions of the state, species richness per watershed was highest in the Lake Superior and Northern Regions, intermediate in the Southeastern, and lowest in the Northwestern and Southern. Of the 48 individual collections that yielded >40 species, all but 1 were from the Northern Region. Many species, especially within the families Limnephilidae and Phryganeidae, have appeared to decrease in distribution and abundance during the past 75 years, particularly those once common within the Northwestern and Southern Regions. Many species now appear regionally extirpated, and a few have disappeared from the entire state. The loss of species in the Northwestern and Southern Regions, and probably elsewhere, is almost certainly related to the conversion of many habitats to large-scale agriculture during the mid-20th century.
Trichoptera, Minnesota, caddisfly, caddisflies, fauna, biodiversity, identification
Biological diversity research is necessary for an understanding of ecosystem ecology, organism conservation, and cladistic biogeography (
Documenting the biodiversity of aquatic insects takes on yet an additional measure of importance due to the utility of the group in water quality biomonitoring. Freshwater resources continue to decline in the U.S. and elsewhere (e.g.,
The caddisflies (Trichoptera) are an order of holometabolous insects found on every continent except Antarctica. Larvae are aquatic and occupy virtually all types of freshwater ecosystems. There are currently approximately 15, 000 species of caddisflies known from the world (
Most adult caddisflies are nocturnally active during warm evenings throughout the summer and early fall, and the majority can be captured by attracting to ultraviolet lights (
Due to the taxonomic richness and ecological diversity of the caddisflies, along with their varying susceptibilities to pollution and abundance in virtually all freshwater ecosystems, the order has high potential value as a water quality biomonitoring taxon (
In most of the United States and adjacent Canadian provinces, caddisflies are either barely known, or known from only a basic species checklist. More comprehensive treatments of the Alabama (
The state of Minnesota is an ideal location to study caddisfly biological diversity. First, the state has an amazing wealth of freshwater resources, including nearly 12, 000 natural lakes >4 ha in size, >100, 000 km of streams and rivers, and nearly 4 million ha of wetlands (
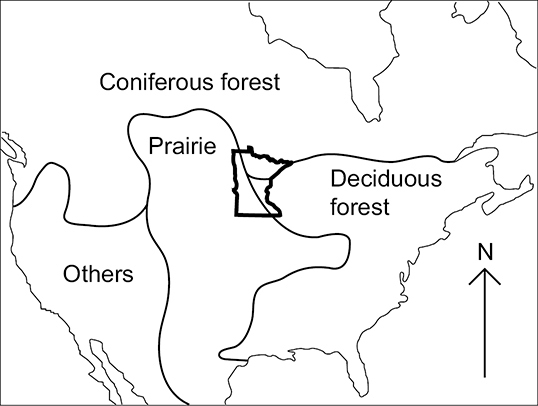
The USA and southern Canada showing the convergence of the Coniferous Forest, Deciduous Forest, and Prairie biotic provinces within the state of Minnesota (
Prior to the 2000s, caddisfly taxonomic research in Minnesota was generally that of basic checklists and taxonomic revisions citing Minnesota records.
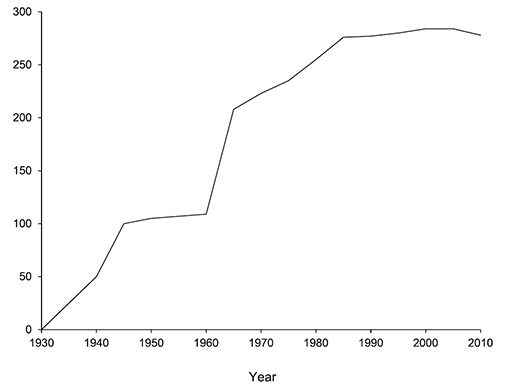
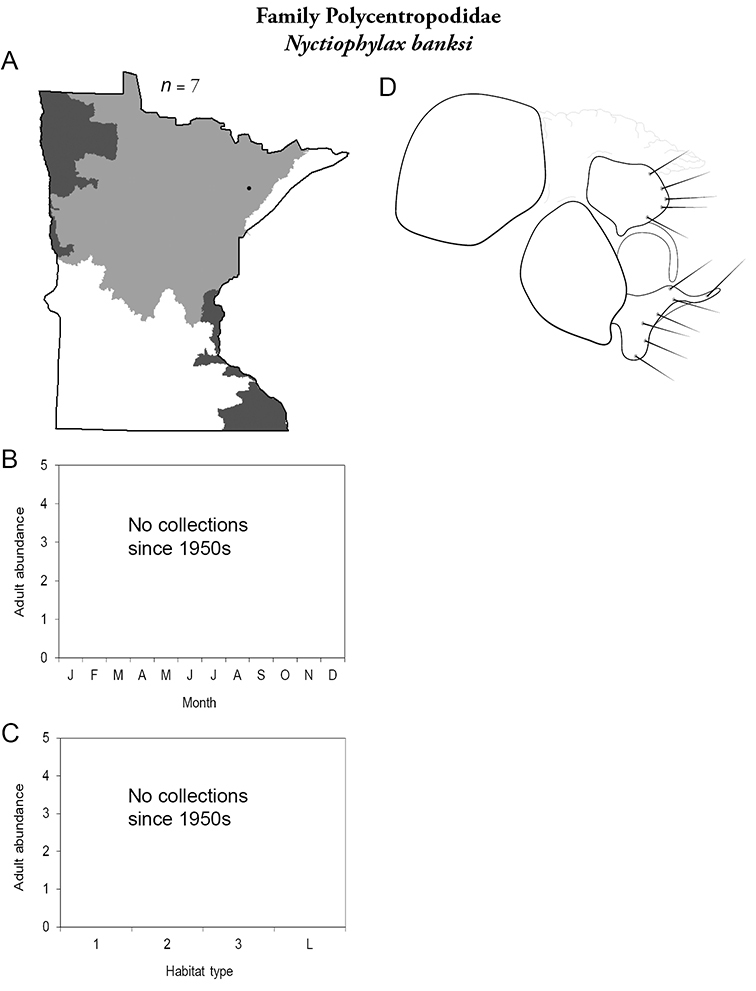
The progression of caddisfly species discovery in Minnesota. At least 31 other species have been reported from Minnesota, but are not included due to doubt about their identity or validity.
In the early 2000s, a more comprehensive and quantitative approach to caddisfly faunistic research in Minnesota was undertaken.
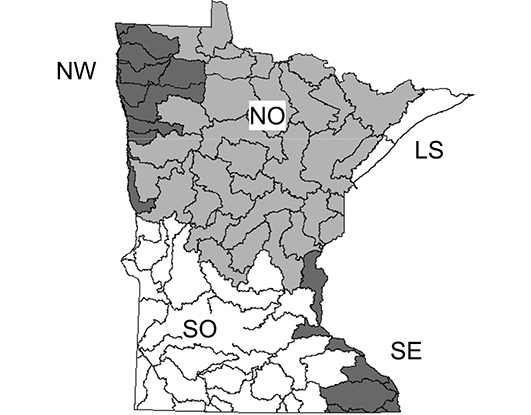
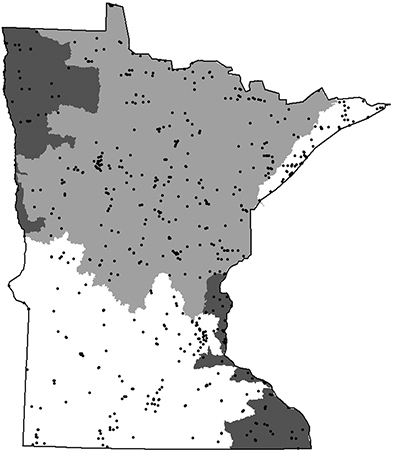
The 5 determined caddisfly regions based on species relative abundance (
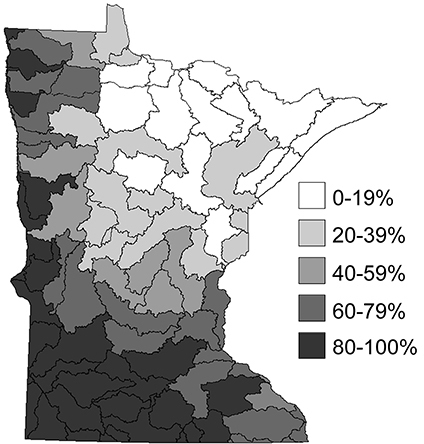
The relative level of disturbed habitat within the 58 major watersheds of Minnesota (
The general trends in caddisfly biological diversity within Minnesota are now as well known as anywhere in the U.S. The individual species, however, are not. The state still lacks a resource to identify individual species or predict their occurrence geographically and in different habitat types. The purpose of the current study, therefore, was to synthesize all known information about the individual Minnesota caddisflies into a single manual that allows for the identification of species, and the characterization of geographic range, adult flight periodicity, and habitat preference.
Materials and methods Collecting and databasingThis study reflects all specimens stored in the University of Minnesota Insect Collection (UMSP), dating back to the 1890s. Adult collecting techniques have included malaise trapping, sweep netting, aspirating from riparian rocks and vegetation, and suspending several 8-W ultraviolet lights in front of a white sheet for 2 h after dusk, with subsequent capture in a cyanide kill jar. Many of D.G. Denning’s adult collections from the 1930s and 1940s occurred at the nighttime lights at gas stations in the middle of small towns such as Crookston, Hallock, and Finland. Larvae were collected by various means, transported alive back to the laboratory and reared to adult in either standard aquaria, or in a Living Stream (Frigid Units, Sylvania, OH), with approximated photoperiod, temperature, and flow regime of the particular habitat. Such a technique was especially important in obtaining adults of certain species of Brachycentrus (Brachycentridae) and Glossosoma (Glossosomatidae), which are often diurnal with a highly synchronous emergence.
From 1999 to 2001, I sampled the entire state representatively with light traps. For this technique, an 8-W ultraviolet light was set on top of a white enamel pan filled with 80% ethanol. Lights placed near aquatic habitats for 2 h after dusk attract most caddisfly species. For an in-depth discussion of this technique see
Four aquatic habitat classes and the total number of samples taken from each during 1999-2001 using ultraviolet light traps. Stream width was estimated at each sampling site.
| Class | Description | Width | n |
|---|---|---|---|
| 1 | Small stream | <4 m | 61 |
| 2 | Medium river | 4–15 m | 81 |
| 3 | Large river | >15 m | 64 |
| 4 | Lentic | N/A | 69 |
A grand total of 312, 884 specimens from 2166 collections of 937 total Minnesota localities (Figure 5) were entered into the UMSP BIOTA database (
All known collecting localities associated with caddisfly specimens stored in the University of Minnesota Insect Museum.
Following the procedure originally described by
Pencil sketches of specimens were made using a microscope with an ocular grid corresponding to a similar grid scale on graphing paper. Sketches were scanned into the computer program Adobe Illustrator™ for final illustration preparation using the procedure described by
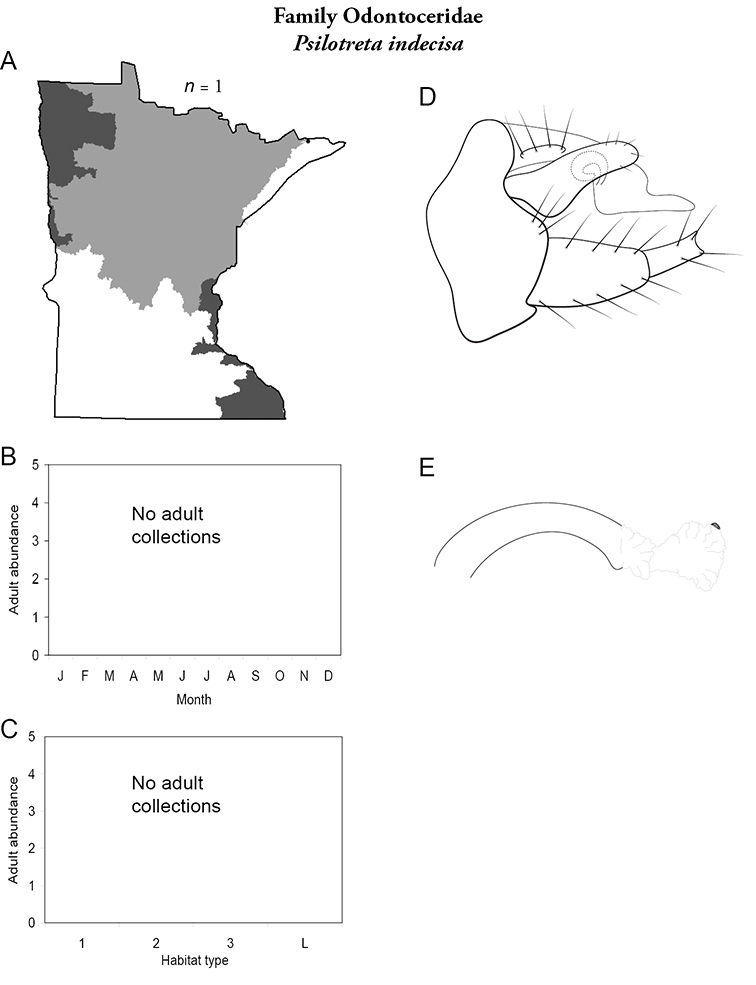
A total of 277 species are confirmed as occurring in Minnesota. These species are organized into 21 families and 75 genera. Four species: Lepidostoma griseum (Lepidostomatidae), Psilotreta indecisa (Odontoceridae), and Phryganea sayi and Ptilostomis angustipennis (Phryganeidae) are new additions to the state fauna since Houghton et al.’s (2001) checklist. Psilotreta indecisa, tentatively identified from larval sclerites, also represents a new genus and family record for the state. A total of 31 species is removed from the Minnesota fauna, mostly due to misidentifications, synonymies, nomina dubia, or an inability to locate the cited specimens. Due to these removals, fewer species are treated in this work than in the 2001 checklist (Figure 2). All dubious Minnesota species are listed in their respective genus chapters, including those already removed by
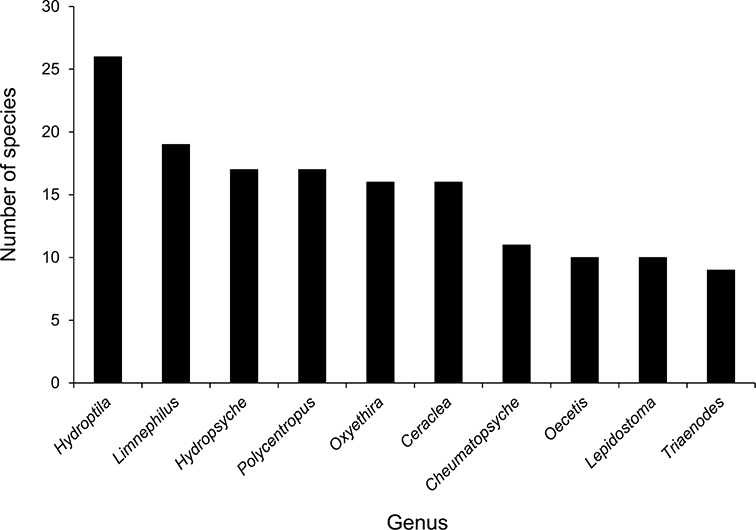
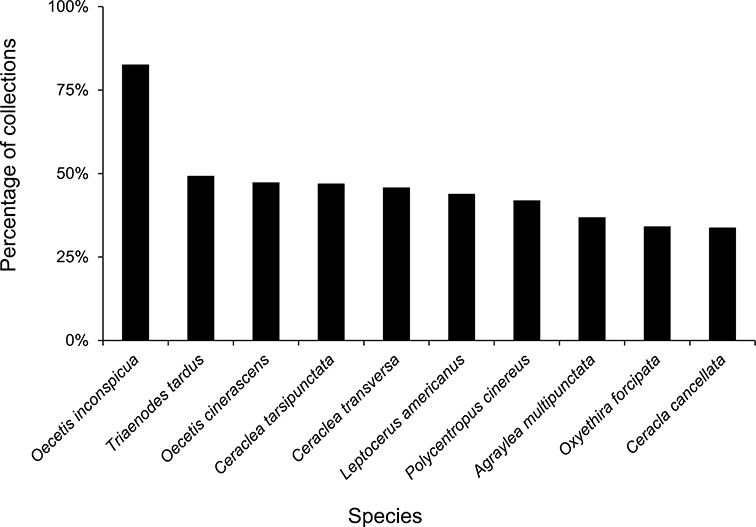
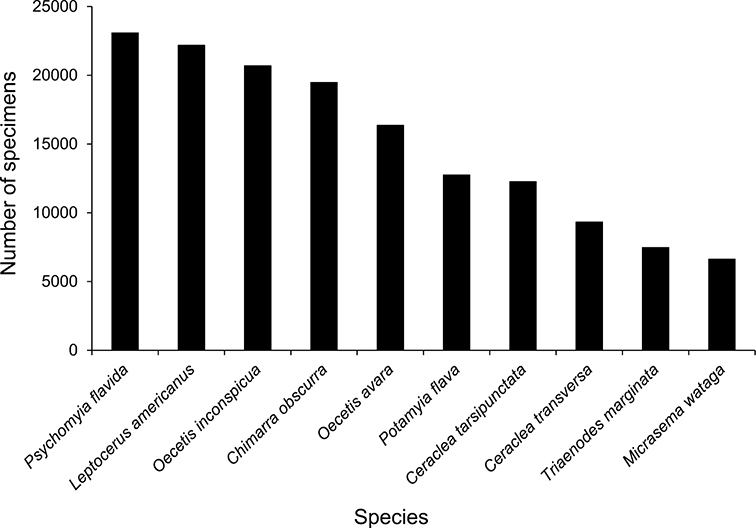
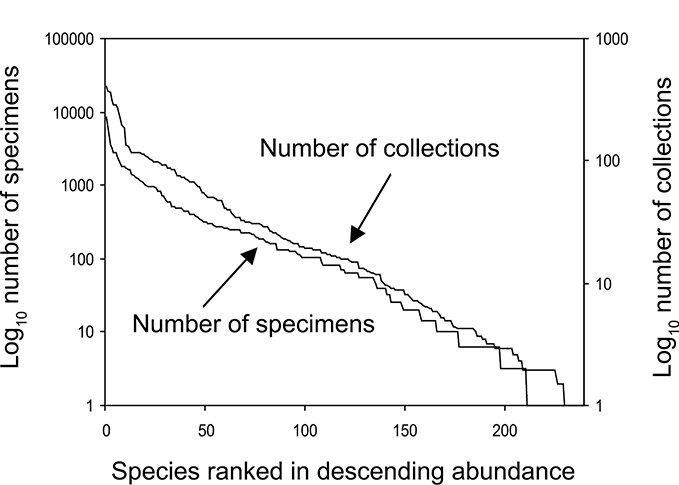
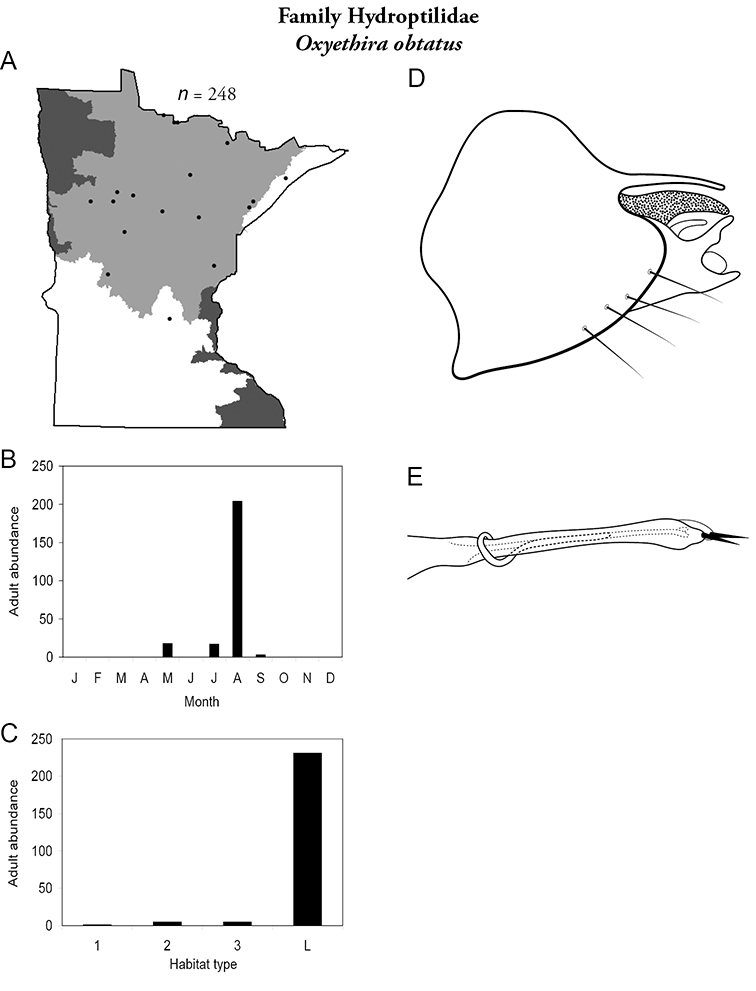
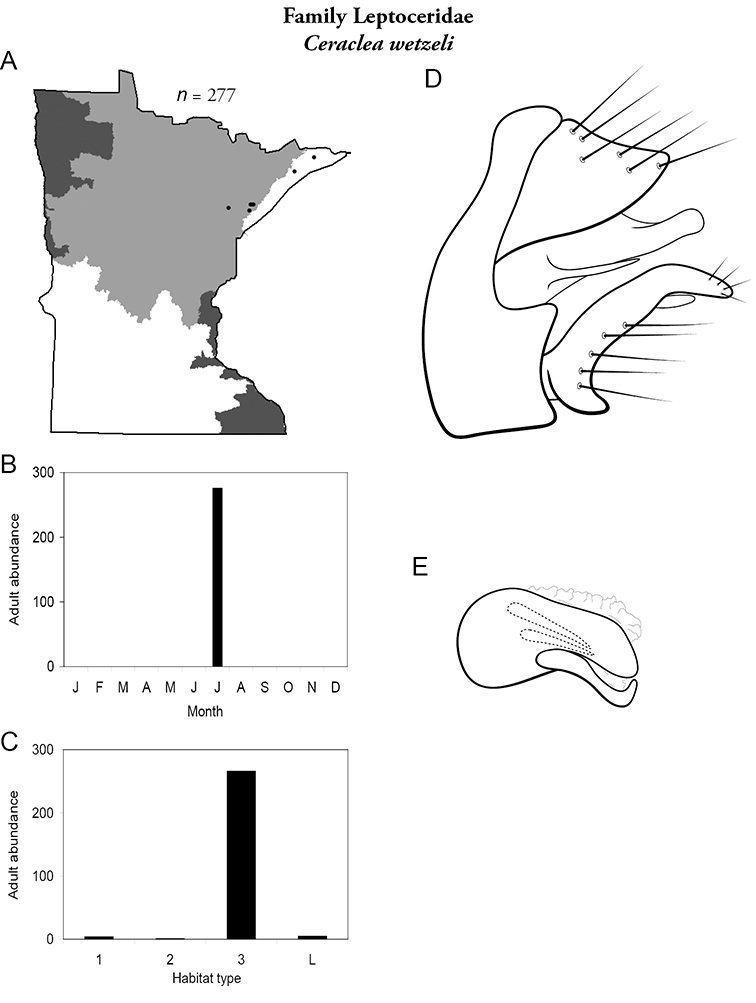
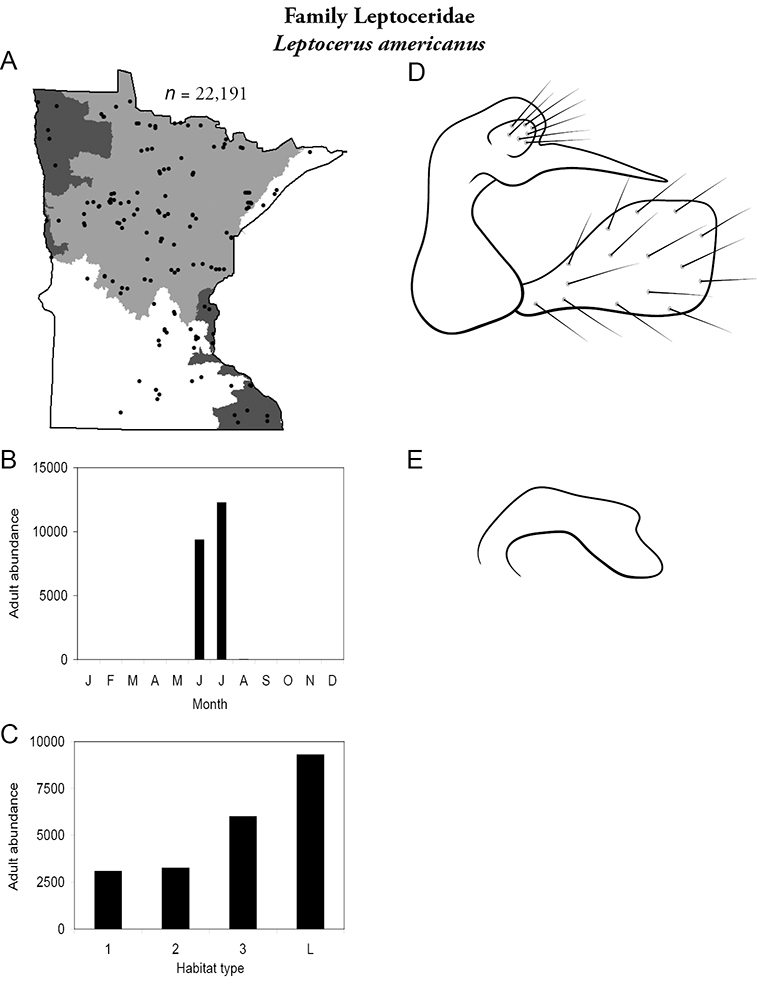
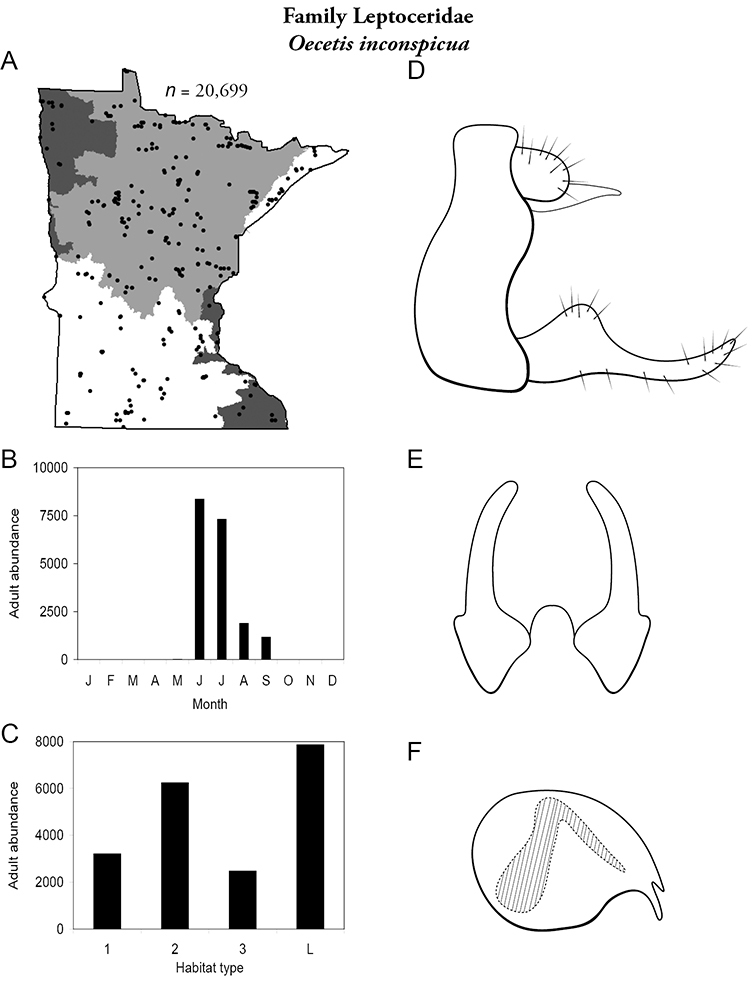
The families Hydroptilidae, Limnephilidae, and Leptoceridae collectively represented over half of the state fauna (Figure 6). Over 40% of the fauna was in 6 genera: Hydroptila and Oxyethira (Hydroptilidae), Limnephilus (Limnephilidae), Hydropsyche (Hydropsychidae), Polycentropus (Polycentropodidae), and Ceraclea (Leptoceridae) (Figure 7). Oecetis inconspicua (Leptoceridae) was, by far, the most widespread species in Minnesota, occurring at nearly 85% of all collecting localities in the state. In comparison, the 2nd most widespread species, Triaenodes tarda (Leptoceridae), occurred at <50% of all localities. Other widespread species are in Figure 8. Psychomyia flavida (Psychomyiidae) was the most abundant species based on all specimens collected, followed by Leptocerus americanus (Leptoceridae), and Oecetis inconspicua (Figure 9). The top 10 most abundant species represented >50% of all specimens examined. In contrast, almost 30% of the entire fauna has been found from <5 localities, and >25% of species are known from <10 specimens (Figure 10).
The total number of species known to occur in Minnesota for all of the Minnesota caddisfly families.
The 10 most species-rich genera in Minnesota.
The 10 most widespread caddisfly species in Minnesota based on all specimens in the University of Minnesota Insect Collection.
The 10 most abundant caddisfly species in Minnesota based on all specimens in the University of Minnesota Insect Collection.
The number of caddisfly specimens known for each Minnesota species, and the number of collections in which each species has been found based on sampling during 1999-2001.
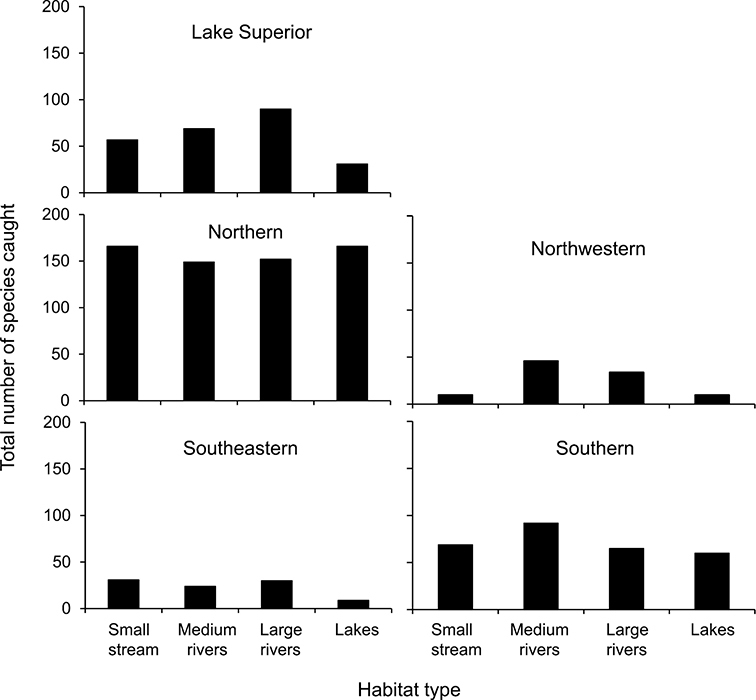
Total species richness from the 4 different habitat types for the 5 caddisfly regions based on all historical collecting is in Figure 11. Based on the representative sampling during 1999–2001, the Northern Caddisfly Region had the highest total caddisfly species richness, followed by the Southern, Lake Superior, Southeastern, and Northwestern Regions (Table 2). The Lake Superior and Northern Regions had the highest mean species richness per watershed sampling unit, the Southern and Northwestern regions the lowest, and Southeastern had an intermediate mean (One-way Analysis of Variance with post-hoc Students-Neuman-Keuls test, p < 0.001) (Table 2). In the Lake Superior, Northern, and Southeastern Regions, more species were collected after 1980 than had been collected historically. The Northwestern and Southern Regions, however, have yielded fewer species since the 1980s.
Summary statistics for 5 regions of Minnesota caddisfly biodiversity (Figure 4). Species per watershed was based on those from sampling during 1999-2001. Superscript letters denote statistically significant groupings based on a One-way Analysis of Variance with Student-Newman-Keuls test (a = 0.05). Unique species refer to those within Minnesota based on all historical and recent collecting, not true endemism.
| Region | Species prior to 1950 | Species after 1980 | Total species | Species/ watershed | Unique species |
|---|---|---|---|---|---|
| Lake Superior | 105 | 169 | 175 | 74A | 15 |
| Northern | 205 | 219 | 231 | 73A | 49 |
| Southeastern | 46 | 78 | 84 | 47B | 6 |
| Southern | 148 | 110 | 152 | 31C | 8 |
| Northwestern | 69 | 52 | 69 | 27C | 3 |
Total species richness for 4 defined habitat types within the 5 caddisfly regions (Table 1, Figure 3), based on all specimens stored in the UMSP.
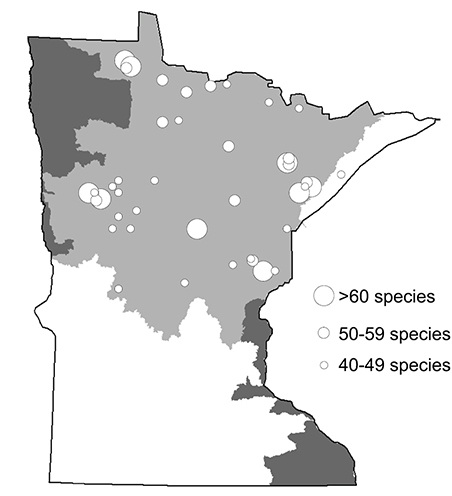
There were 48 individual collections that each yielded >40 species (Figure 12). All but 1 of these collections were from the Northern Region. A total of 9 collections yielded 60–69 species each, and 12 yielded 50–59 species. Based on these data, it appears that the most species-rich area of Minnesota is the Cloquet River watershed. Three sites within 30 km each yielded 60–69 species, 3 yielded 50–59, and 2 others yielded 40–49. These 8 sites collectively included 115 species collected over 2 nights during 2000—over 40% of total Minnesota caddisfly species richness. Other areas of high species richness include the upper Roseau River area (97 species) and the area around the White Earth Indian Reservation (78 species).
Individual collections from Minnesota during 1999-2001 that yielded >40 caddisfly species.
The Lake Superior Caddisfly Region encompasses almost 6, 000 km2 and is composed of two areas draining directly into Lake Superior. It was originally composed of entirely Coniferous Forest, much of which has been replaced by deciduous forest stands (
The Northern Caddisfly Region contains a total of 32 watersheds and over 100, 000 km2. It is composed of mostly Coniferous Forest with a band of Deciduous Forest in its southern portion. The Northern Region contains approximately 85% of Minnesota’s natural lakes, most of which are small, deep, and oligotrophic (
The Northwestern Caddisfly Region contains 10 watersheds and encompasses approximately 16, 000 km2; all of its streams drain into the Red River of the North. It is composed approximately equally of Prairie and Coniferous Forest. This region is now dominated by agriculture, with around 82% of the land area under cultivation, and has had almost all of its prairie vegetation removed and lakes, wetlands, and small streams modified to accommodate this landuse practice (
The Southeastern Caddisfly Region is made up of eight watersheds and almost 10, 000 kmt, primarily composed of Deciduous Forest. It is semi-discontinuous, containing most of the watersheds draining into the lower Saint Croix and Mississippi Rivers. The region is dominated by streams and has virtually no natural lakes except in its extreme northern portion (
The Southern region contains 29 watersheds and nearly 70, 000 km2. It is composed of approximately equal amounts of Deciduous Forest and Prairie. As with the Northwestern Region, much of the natural vegetation and many of the lakes, wetlands, and small streams have been replaced with agriculture, which accounts for 85% of landuse (
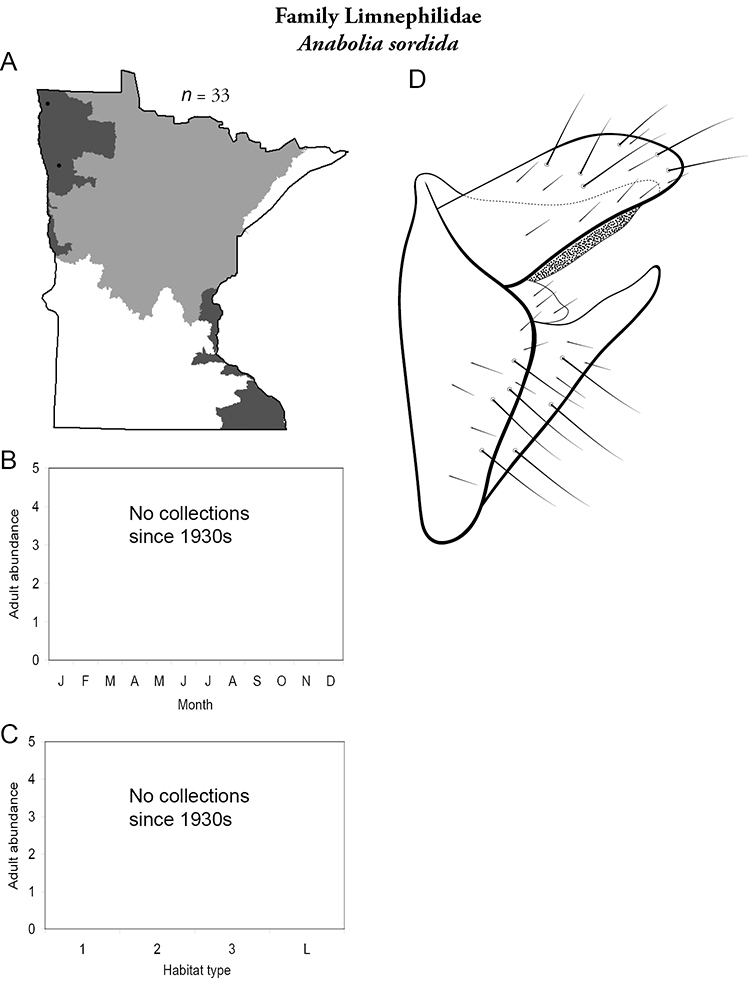
Differences in regional caddisfly biodiversity appear to reflect both the natural and anthropogenic differences among habitats of the 5 determined regions. The Northwestern Region, for example, had the lowest total species richness of any region, reflecting specimens collected before much of the region was converted to large-scale agriculture. Indeed, several single collections from the Northern Region approximate the entire known fauna of the Northwestern Region from all historical collecting. The prairie habitats that dominate much of this region are low-gradient and with little heterogeneity relative to the forested habitats of the other regions. Further, only 3 species from all historical collecting: Hydropsyche confusa (Hydropsychidae), and Anabolia sordida and Philarctus quaeris (Limnephilidae) are unique to this region (Table 2), again suggesting a natural lack of habitat heterogeneity and species richness.
The most likely cause of the observed decrease in caddisfly species richness in the Northwestern and Southern Regions since the 1940s is large-scale agriculture. Intensive agriculture probably has the most extensive impact of any human land use on aquatic ecosystems (
It is unlikely that the caddisfly faunas of the Lake Superior, Northern, and Southeastern regions are completely “natural”. Many of the watersheds throughout Minnesota that are now forested were previously logged or cultivated, with resulting loss of woody debris and sediment, and floodplain and channel modification, effects that can last for tens or hundreds of years (
In some regions of Minnesota, both species richness and the degree to which the contemporary fauna appeared similar to that of historical fauna was influenced by the occurrence of “refuge” habitats: relatively undisturbed ecosystems within a large disturbed area. Even small forested areas can be important for maintaining aquatic biological diversity in a disturbed landscape (
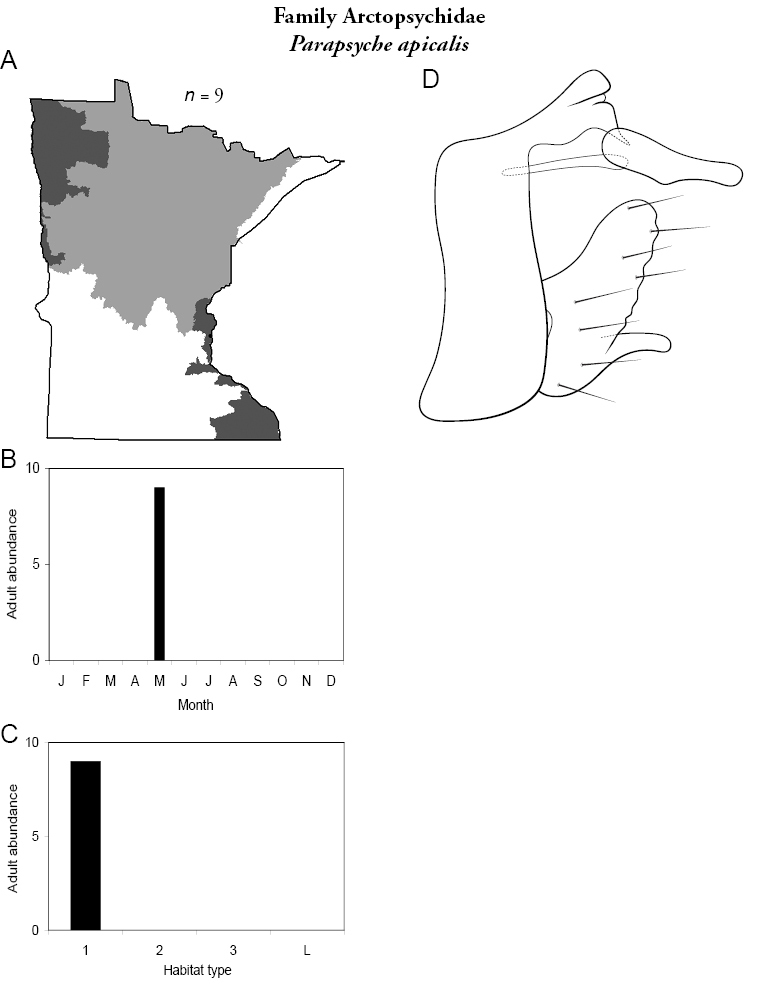
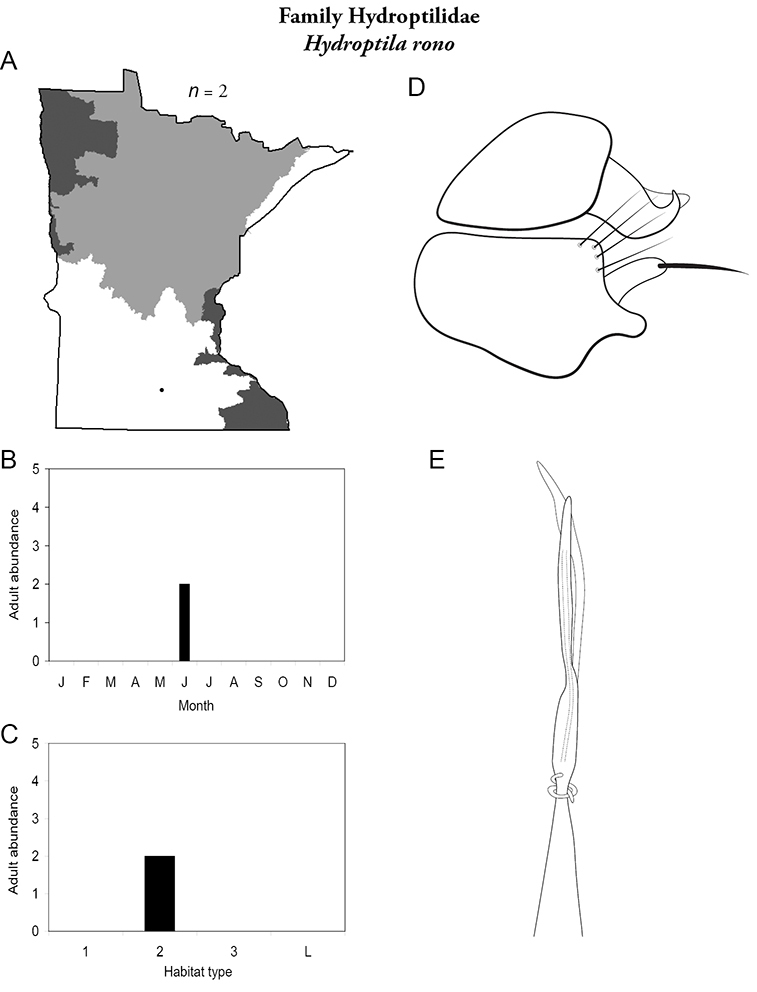
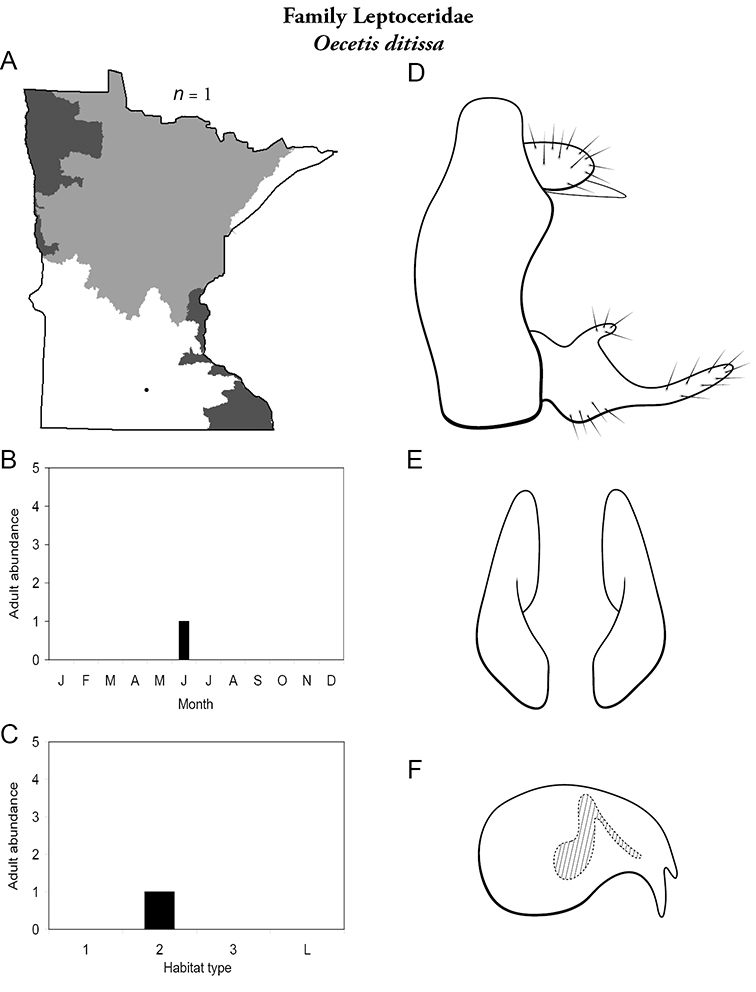
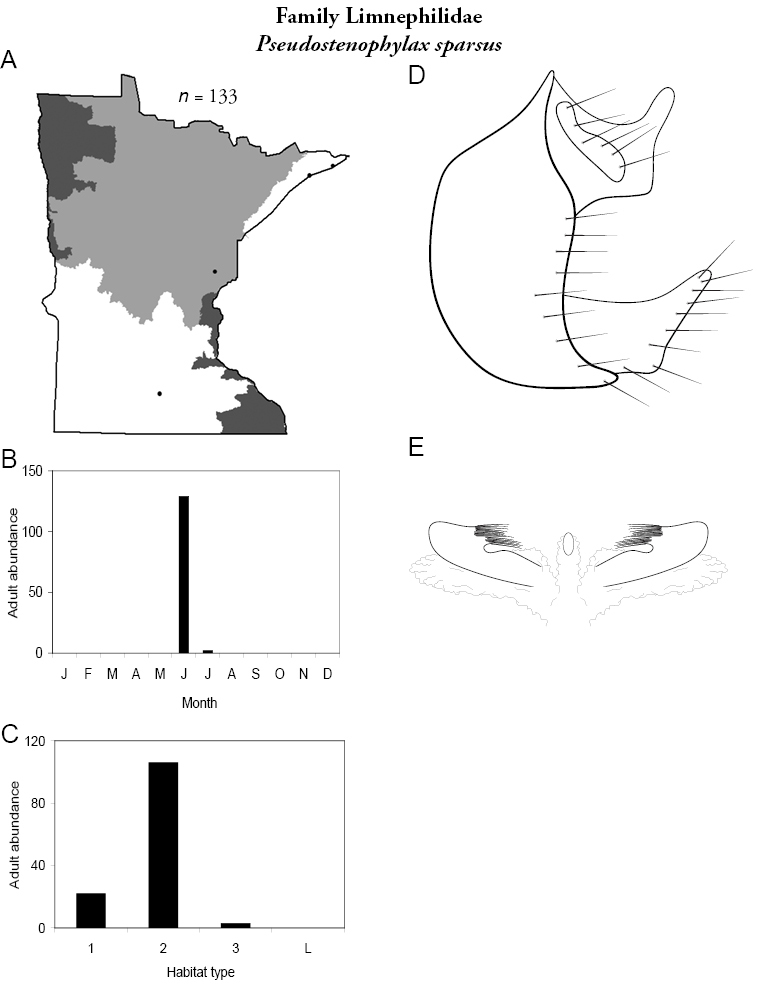
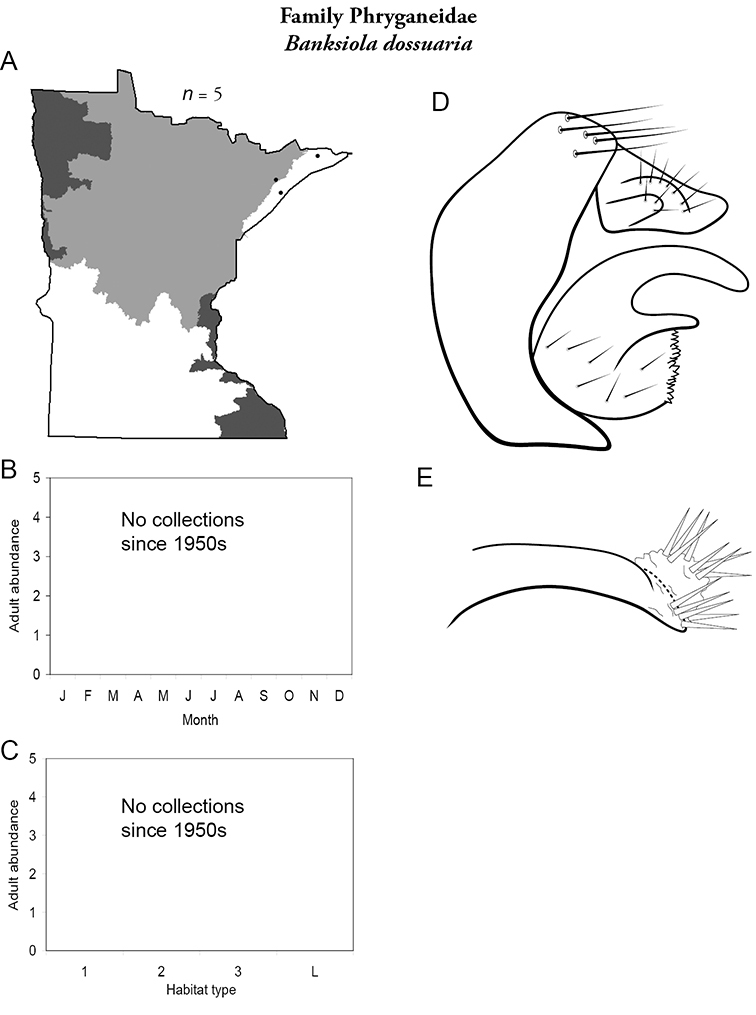
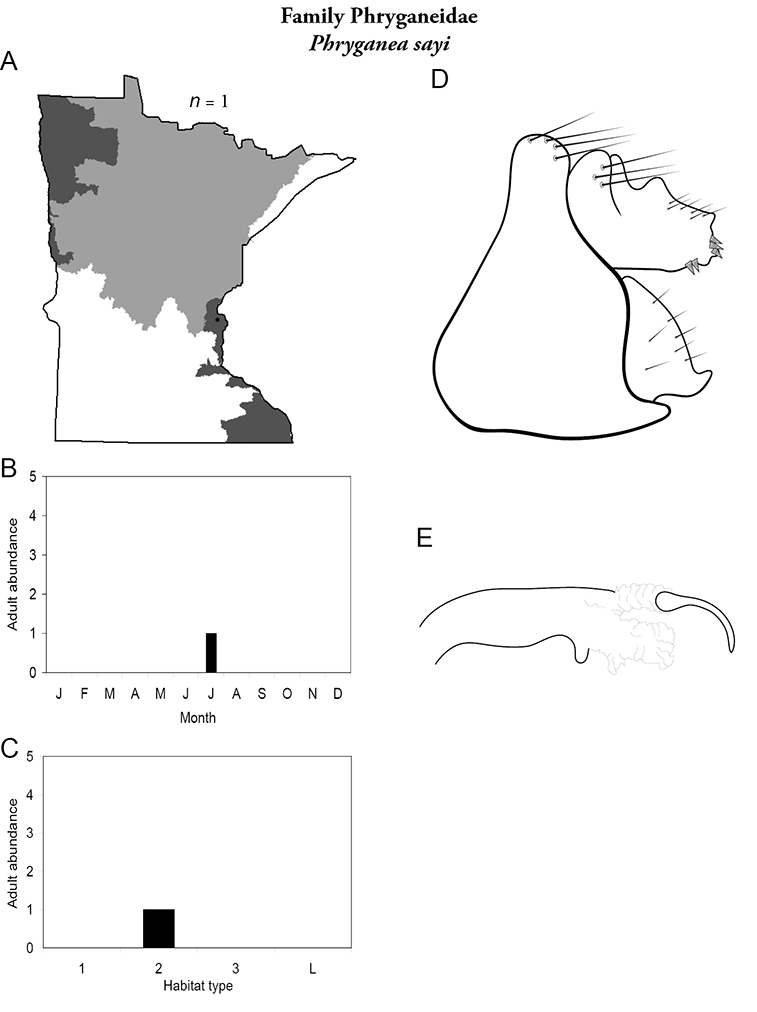
Refuge habitats also appear important for protecting species with limited distributions. For example, one of the only refuge habitats in the Southern Region, Minneopa Creek in Minneopa State Park, yielded 3 species: Hydroptila rono (Hydroptilidae), Lepidostoma libum (Lepidostomatidae), and Oecetis ditissa (Leptoceridae), found nowhere else in Minnesota, as well as populations of rare species such as Diplectrona modesta (Hydropsychidae) and Pseudostenophylax sparsus (Limnephilidae). Two of the new state records reported in this monograph: Lepidostoma griseum (Lepidostomatidae) and Ptilostomis angustipennis (Phryganeidae), were found during the same collection of a first-order intermittent stream, Mill Creek in William O’Brien State Park in the Southeastern Region, a site < 1 hour’s drive from the Twin Cities metropolitan area. The same stream also yielded the only known specimens of Parapsyche apicalis (Arctopsychidae) ever collected in Minnesota. Only 8 caddisfly species total have been found in this stream. Thus, nearly 40% of the caddisfly fauna of Mill Creek has been found nowhere else in the state.
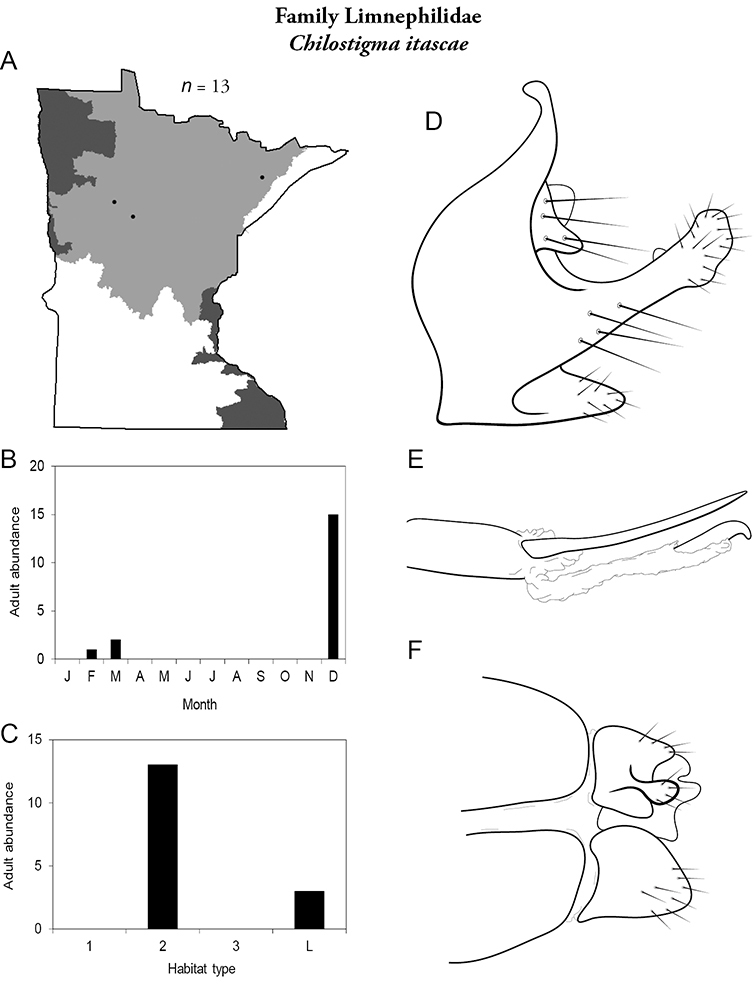
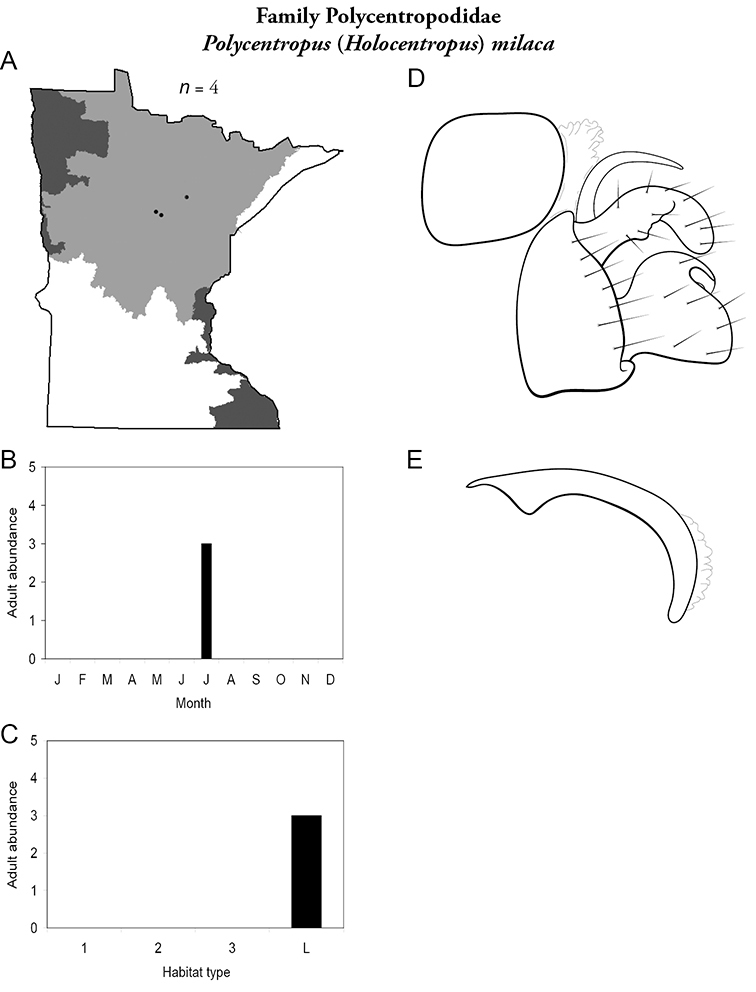
Despite a collecting history of >100 years, and an asymptotic species-sample surve, it is possible that additional species remain undiscovered in Minnesota. Most of these species will probably be found in novel habitats, such as intermittent streams, vernal pools, or wetlands. Refuge habitats within disturbed landscapes may also yield new records. Efforts are ongoing to locate adults of Oligostomis (Phryganeidae) and Psilotreta (Odontoceridae), which are currently only known from larvae, and to find additional populations of rare endemic species, such as Chilostigma itascae and Polycentropus milaca. The caddisfly fauna of Minnesota is now as well-known as the fauna of any other area of North American. With good baseline data now in place, any future changes to the fauna can be evaluated with greater confidence and precision.
Identification manual explanationThis manual intentionally avoids the use of dichotomous keys and detailed taxonomic descriptions for species identification. Instead, it relies on the premise that a collector is more likely to encounter a common species than a rare species. Descriptions of the 21 known families are listed in the order of collection likelihood on page 27. Common and abundant species for the 4 different habitat types of each of the 5 caddisfly regions are summarized in Tables 3–7. For example, 95% of all specimens collected from large rivers of the Northwestern Region are represented by the 7 species listed in Table 5. It is, obviously, recommended that users examine the illustrations of the listed species first. Taxonomic keys to Minnesota caddisfly families and genera can be found in
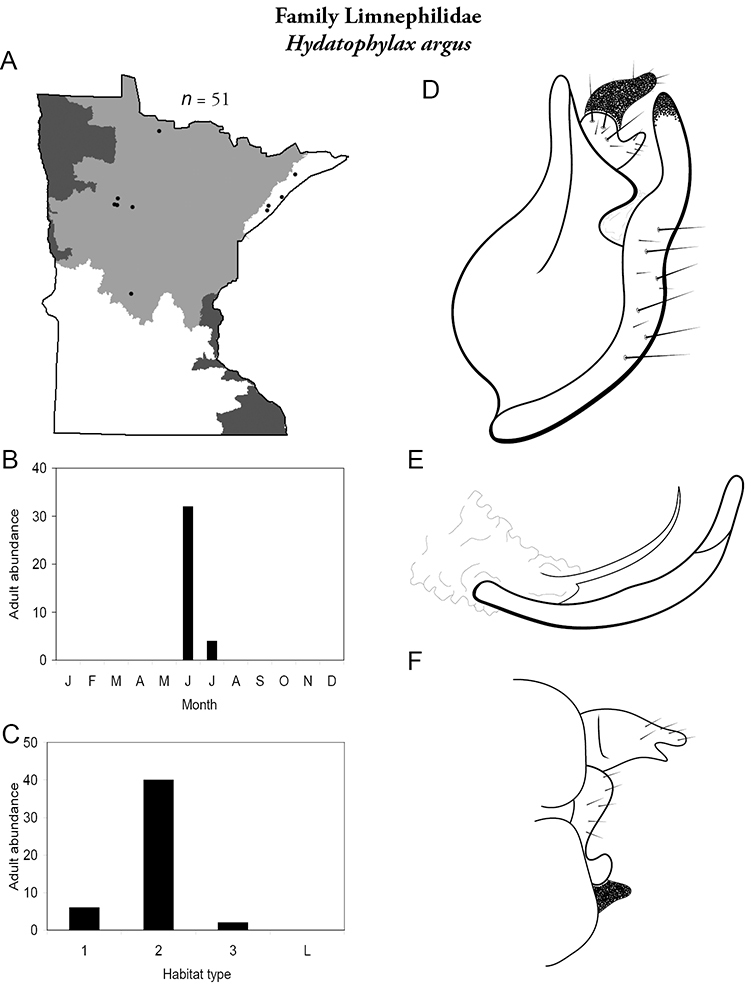
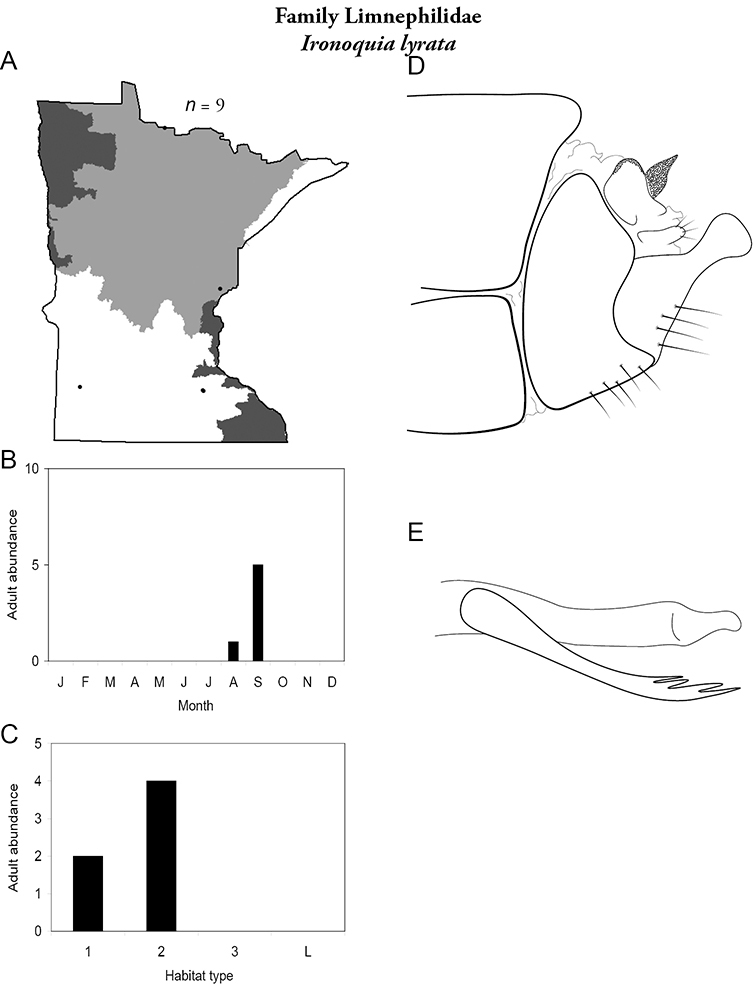
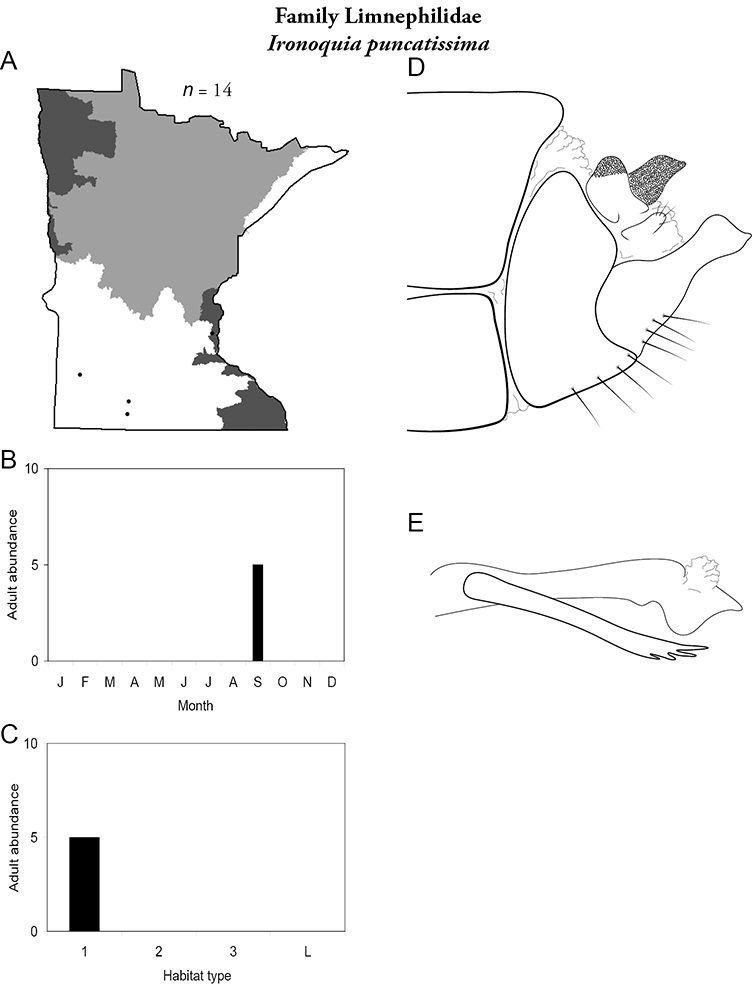
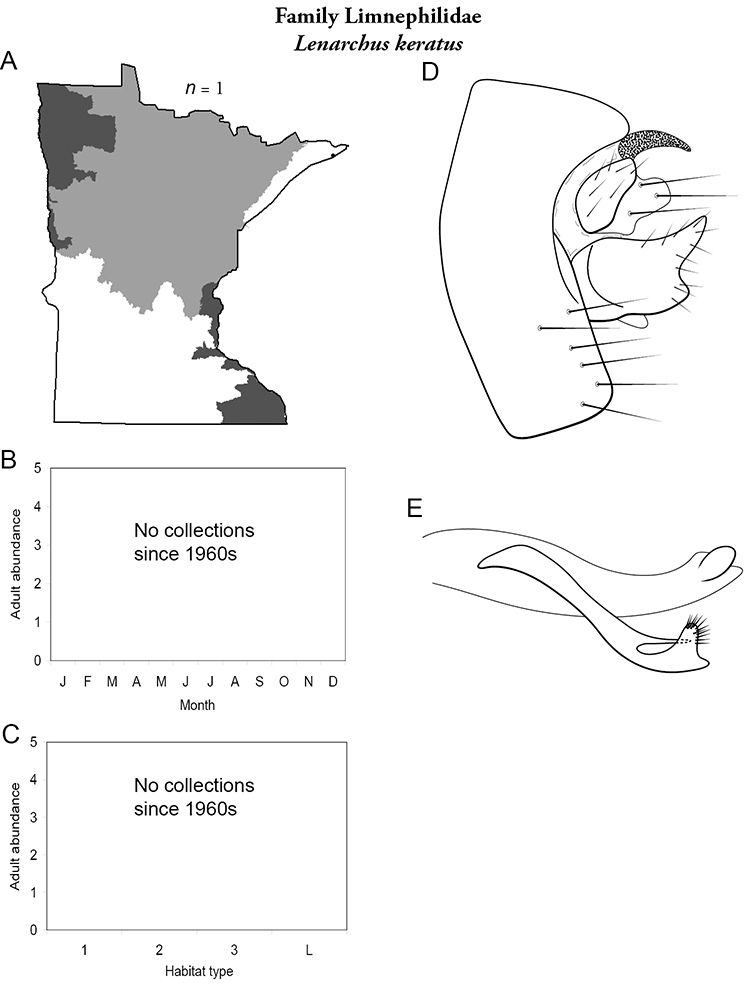
All species are organized alphabetically by family and genus. Each species plate includes illustrations of the important identification characteristics. For most species, this means a lateral view of the genital capsule and phallus of the male. Other male illustrations may include additional views of the genital capsule, or specific views of the inferior appendages, tergum X, or other characteristics necessary for identifying the species. Most female caddisflies are not readily identifiable. For species that can be identified (e.g., most limnephilids and psychomyiids), a lateral or ventral view of the female genital capsule is included.
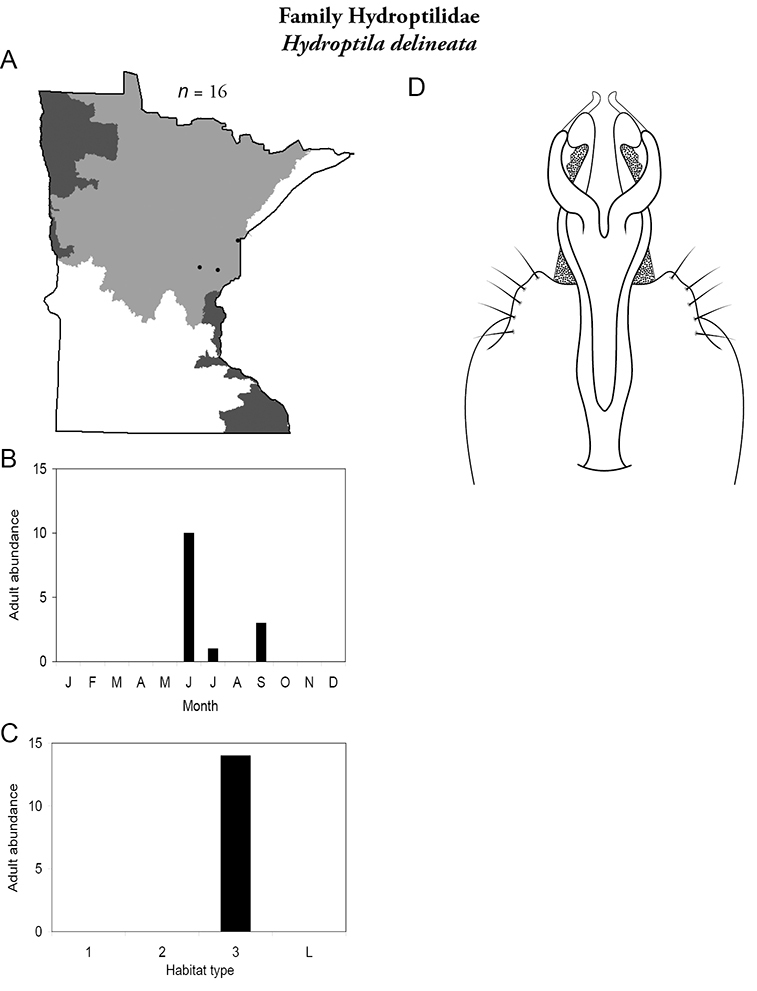
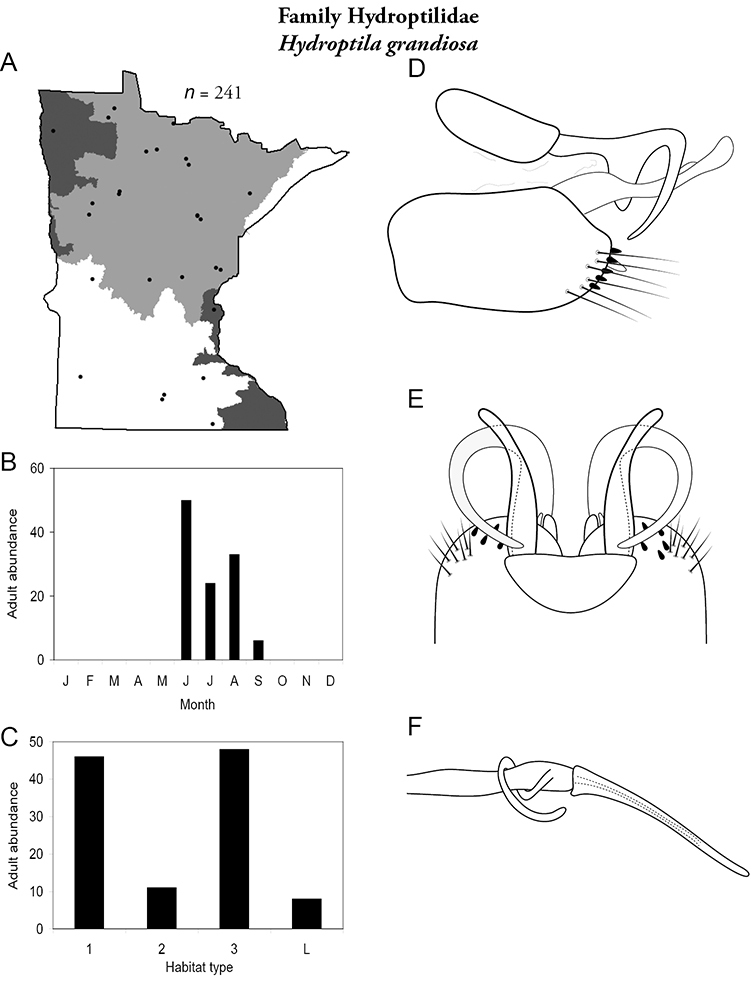
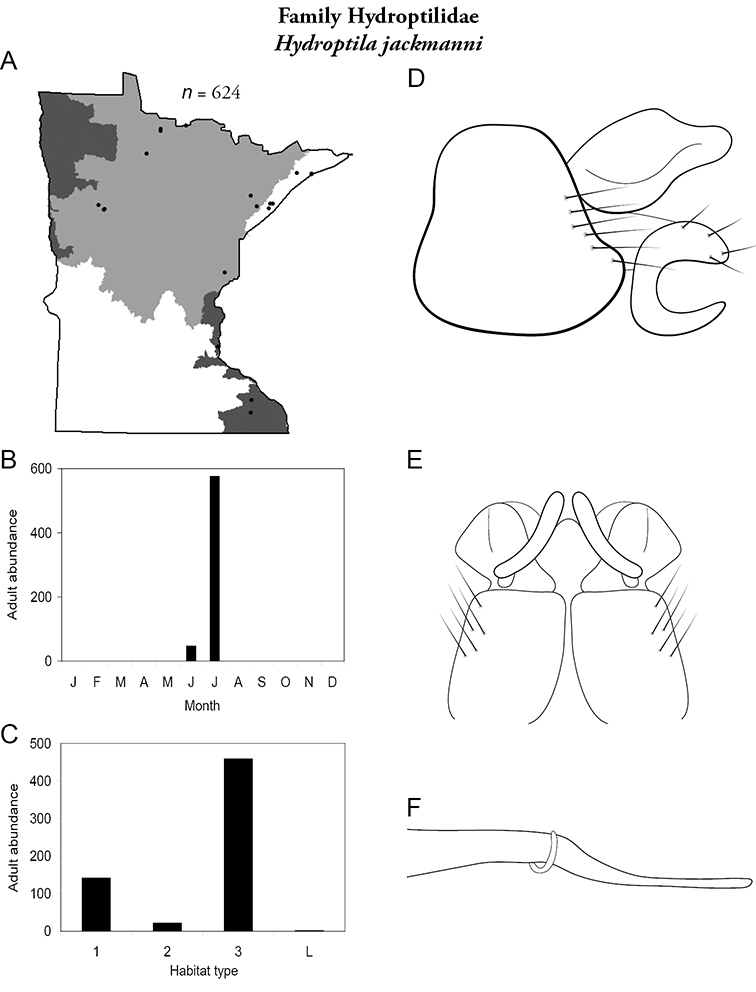
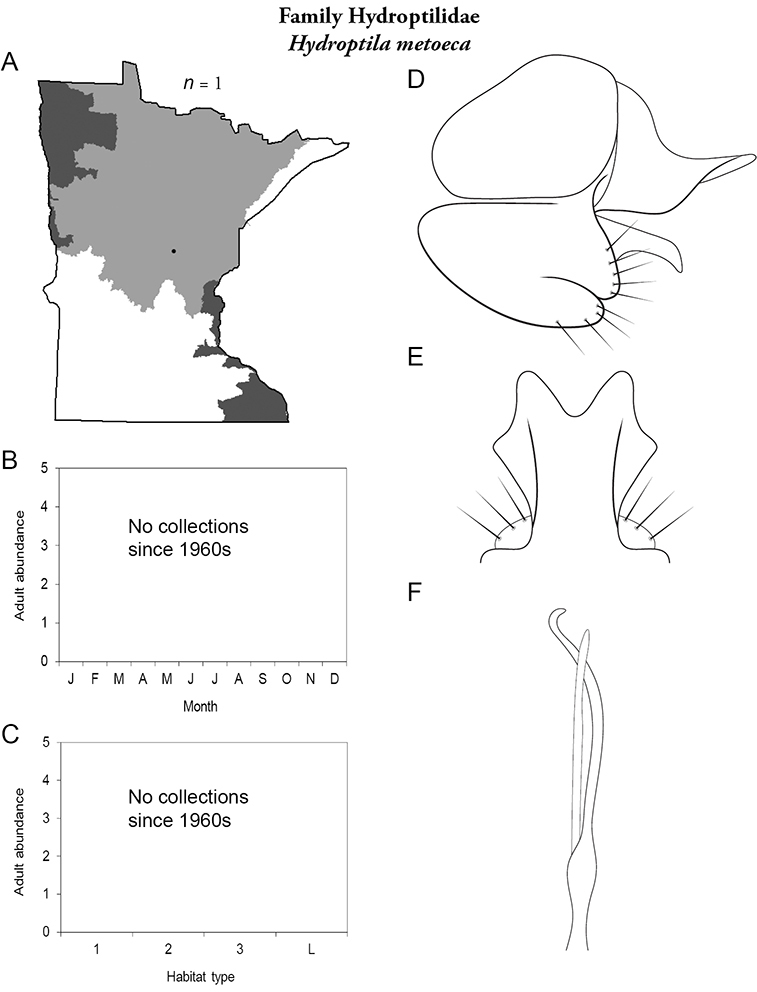
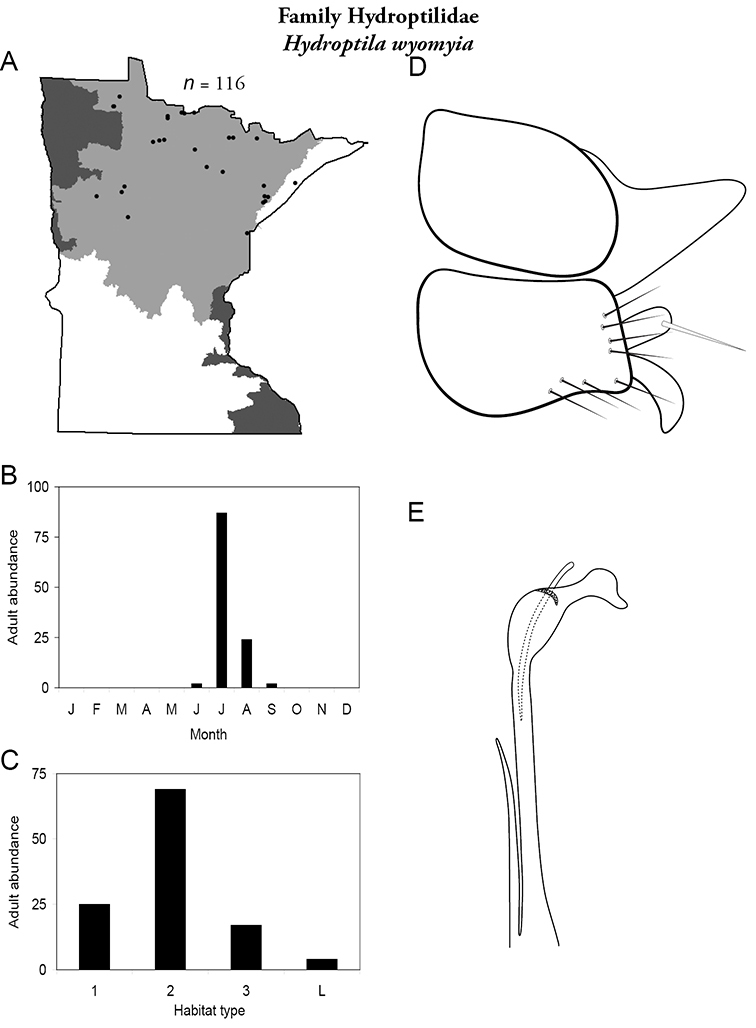
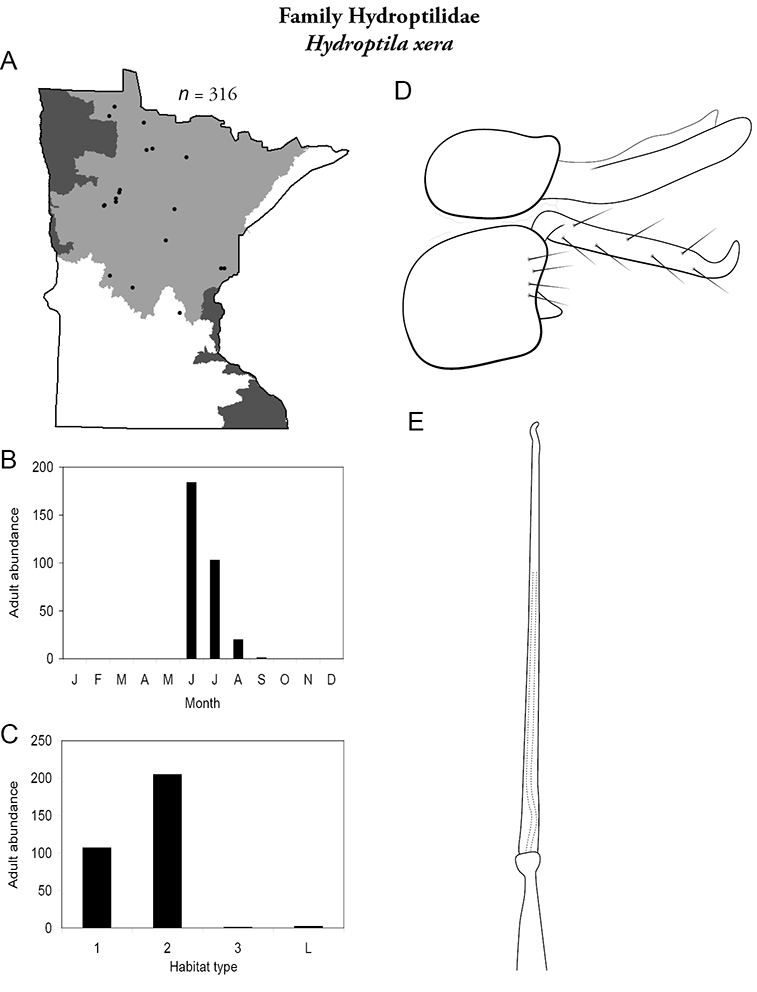
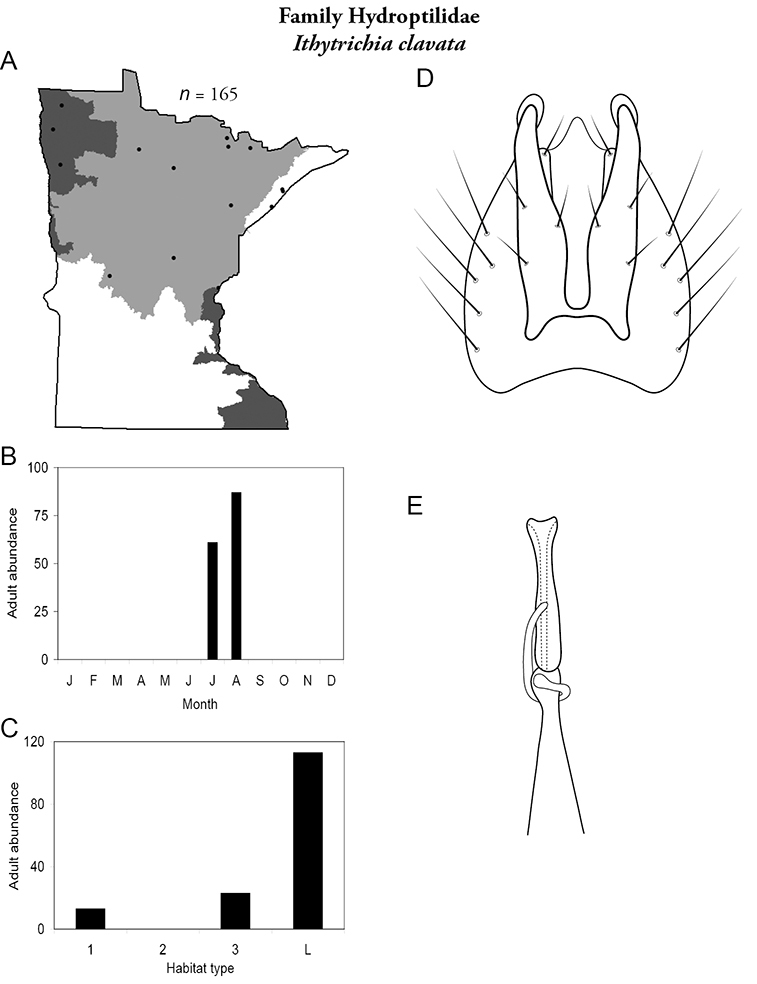
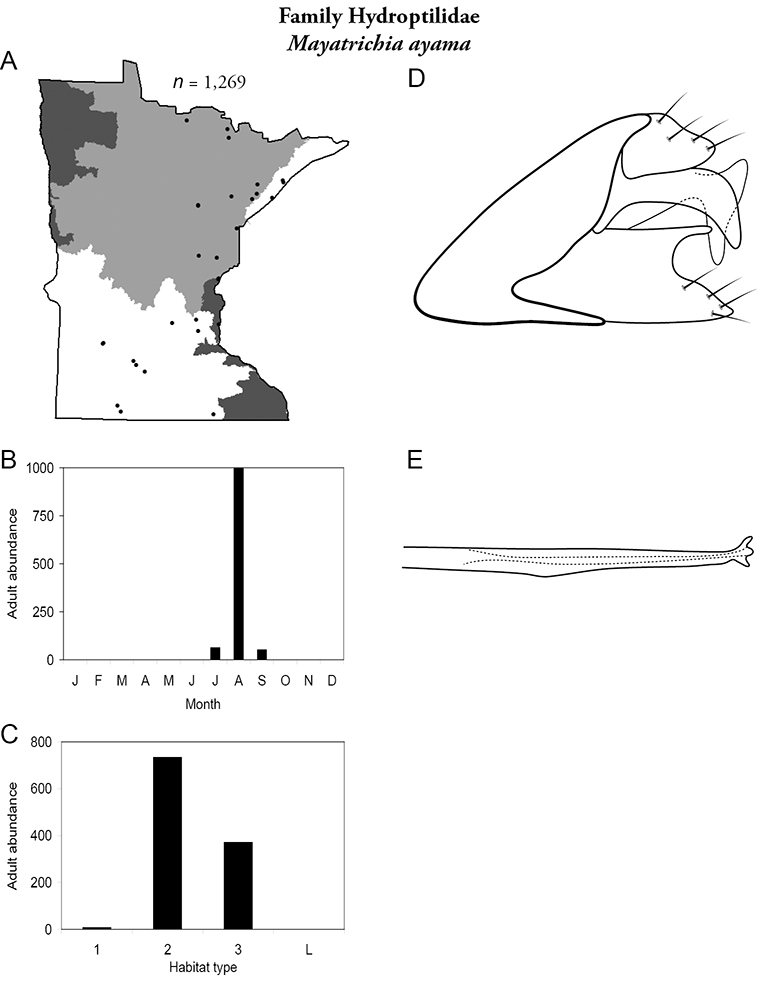
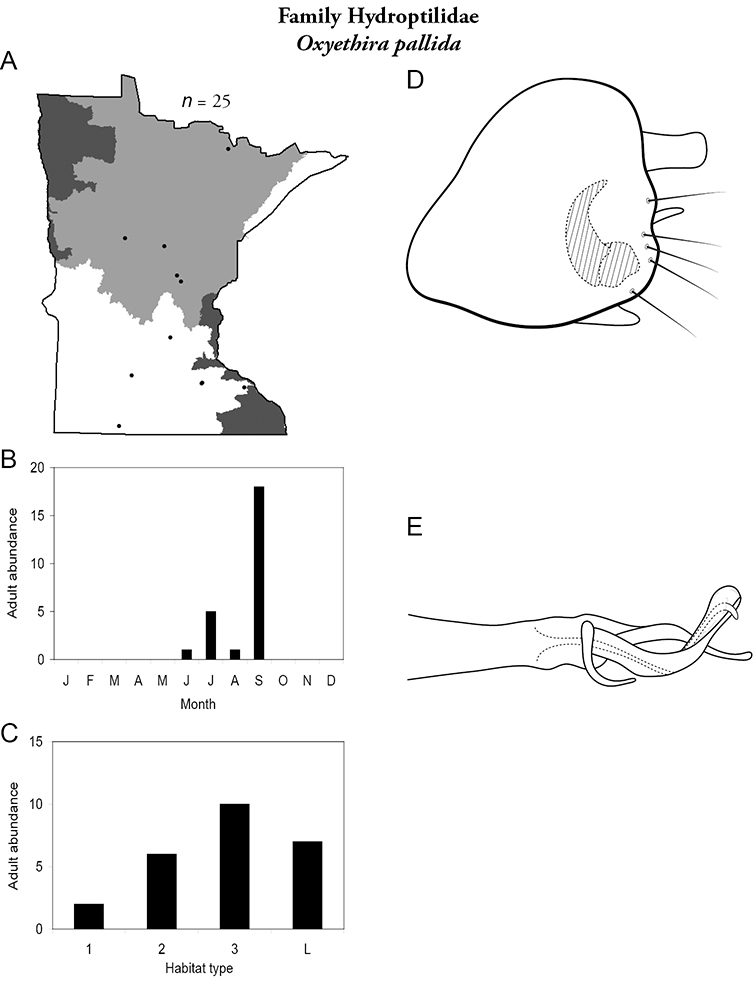
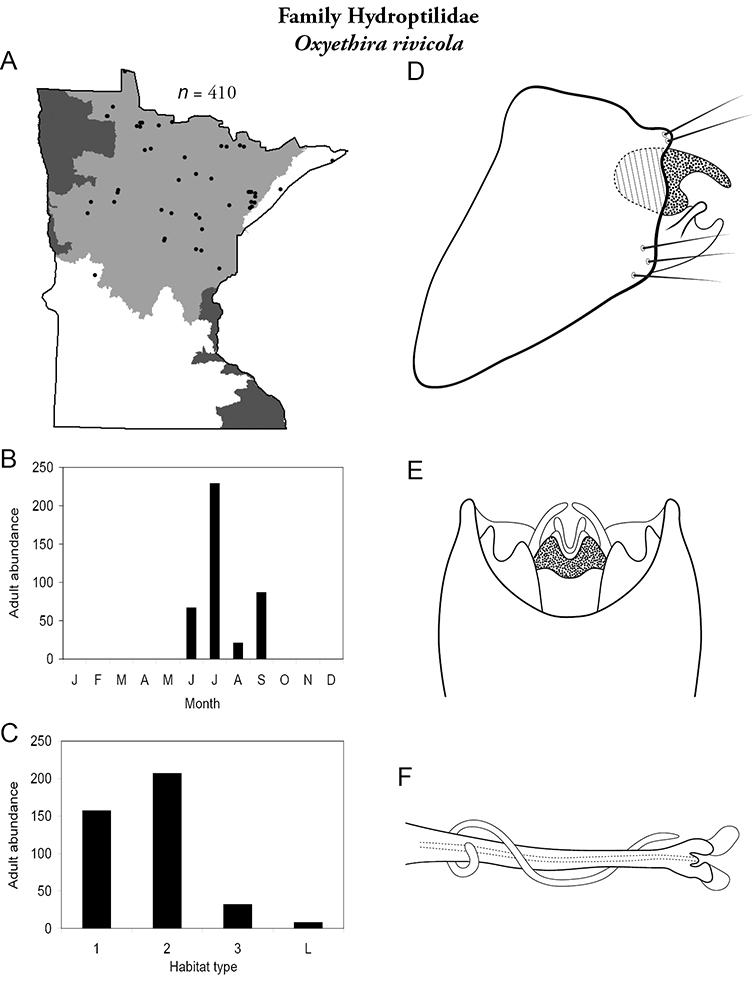
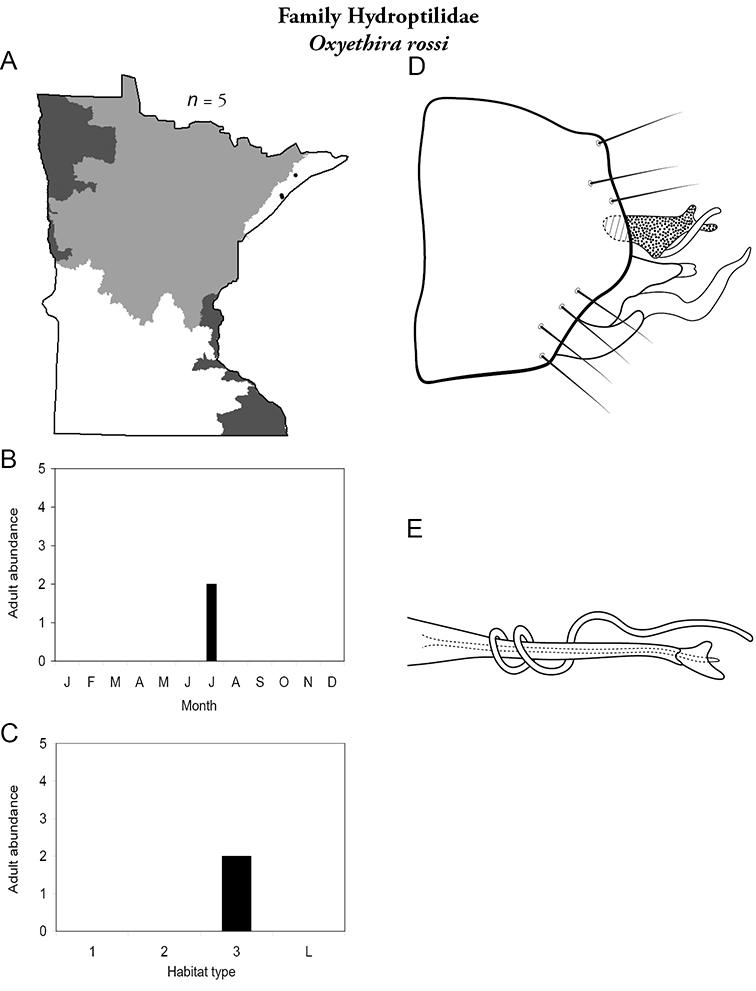
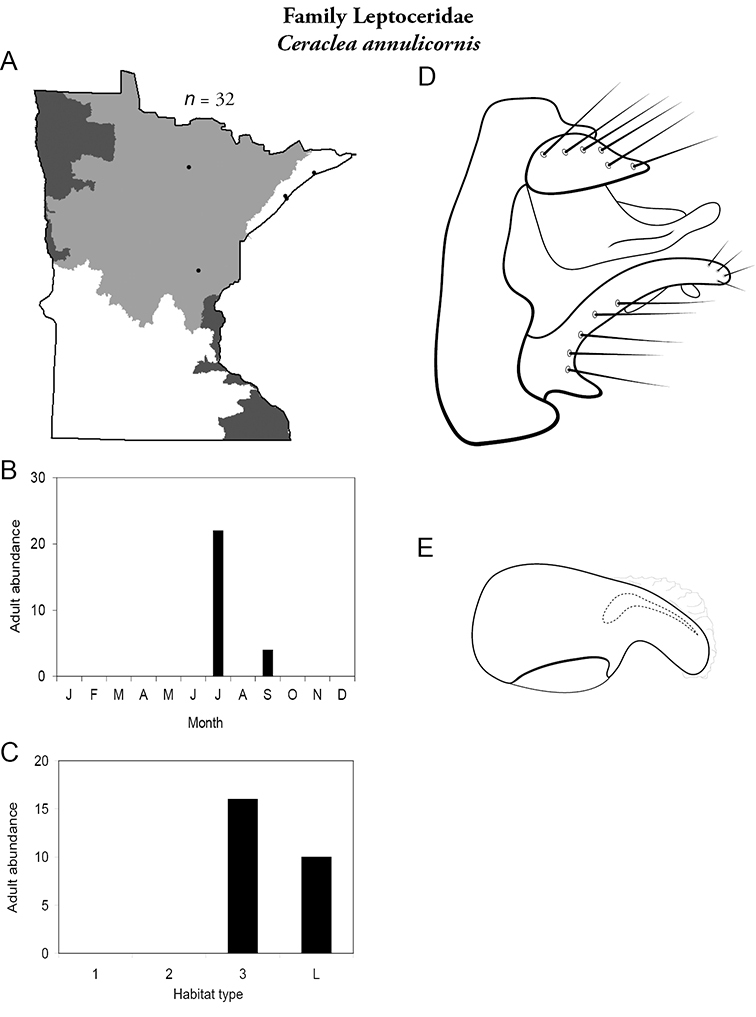
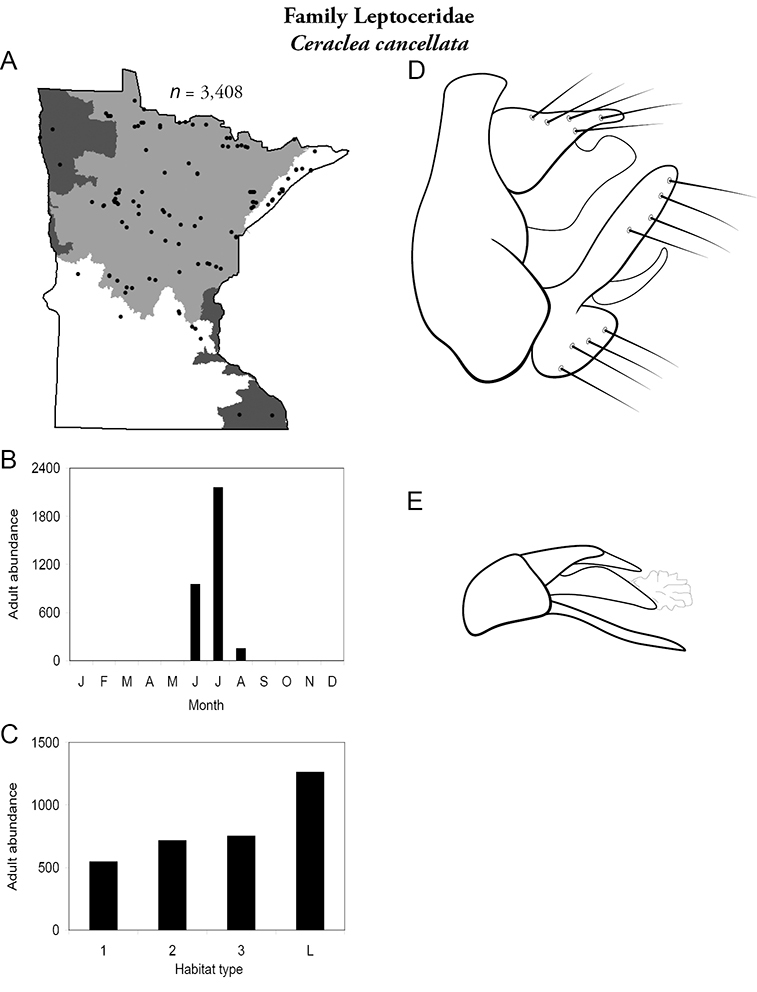
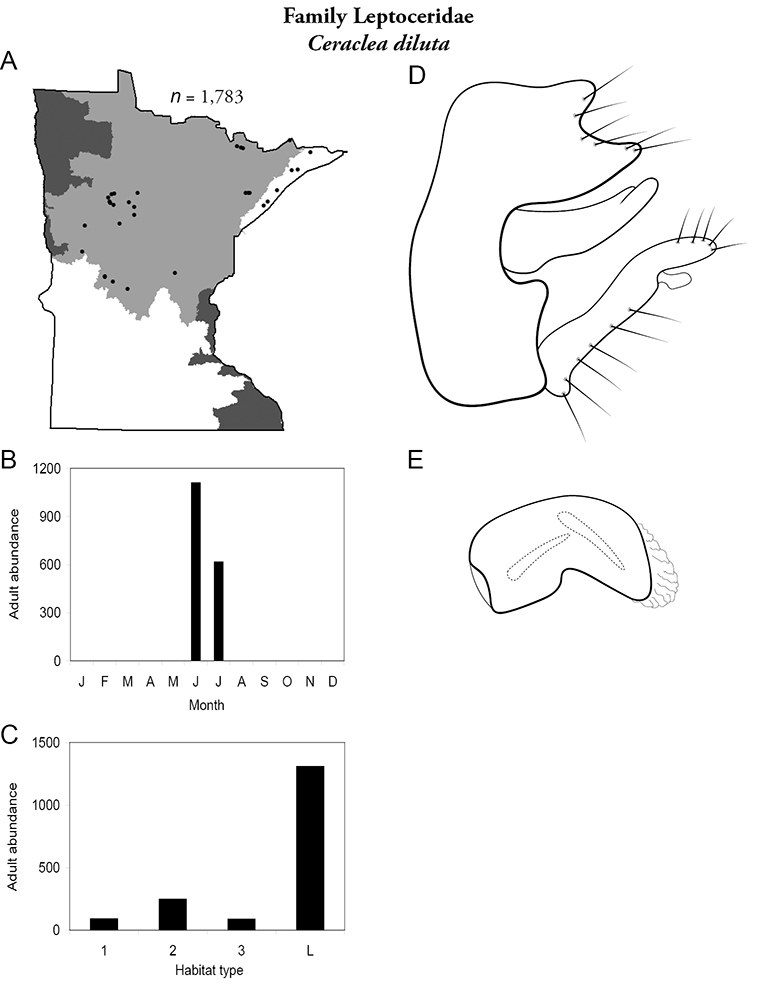
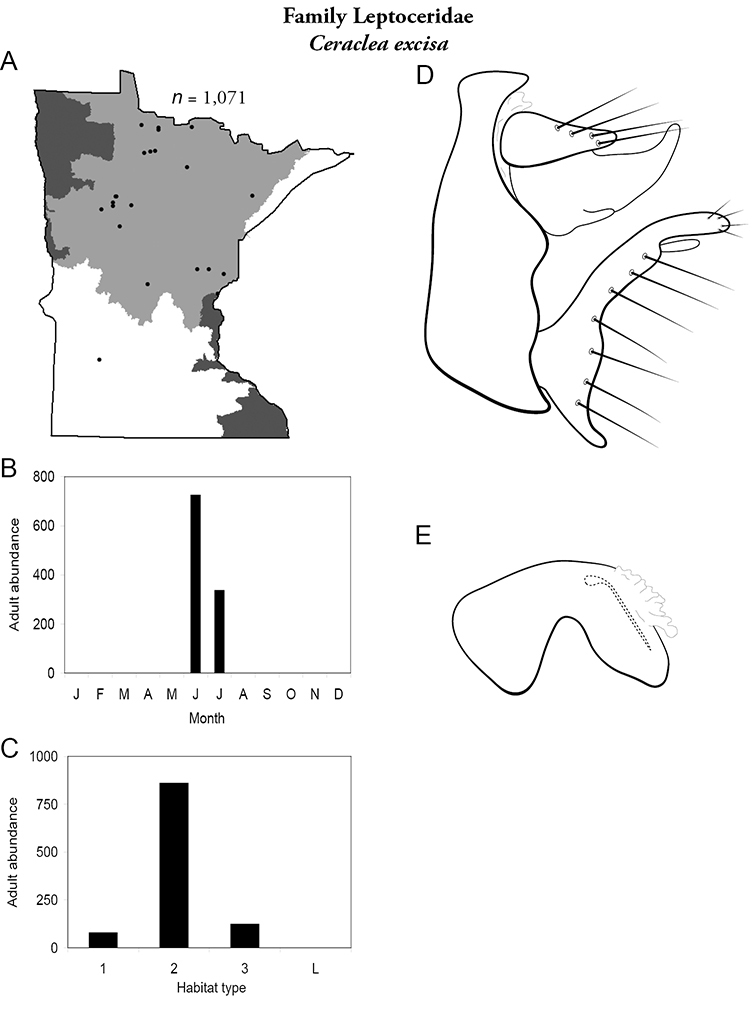
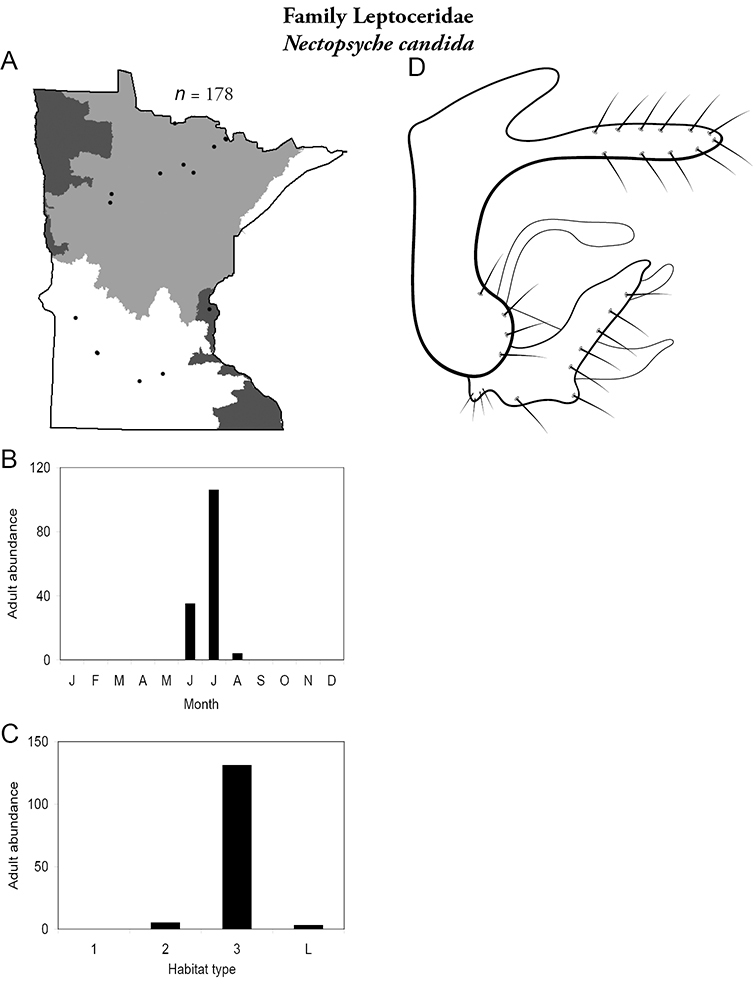
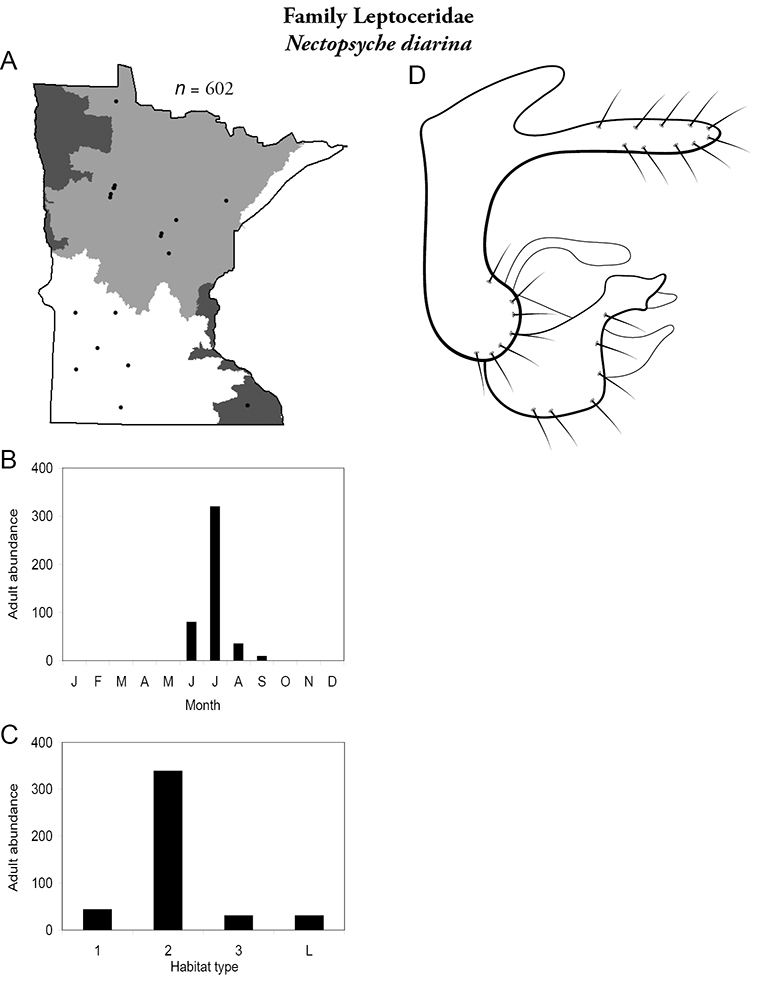
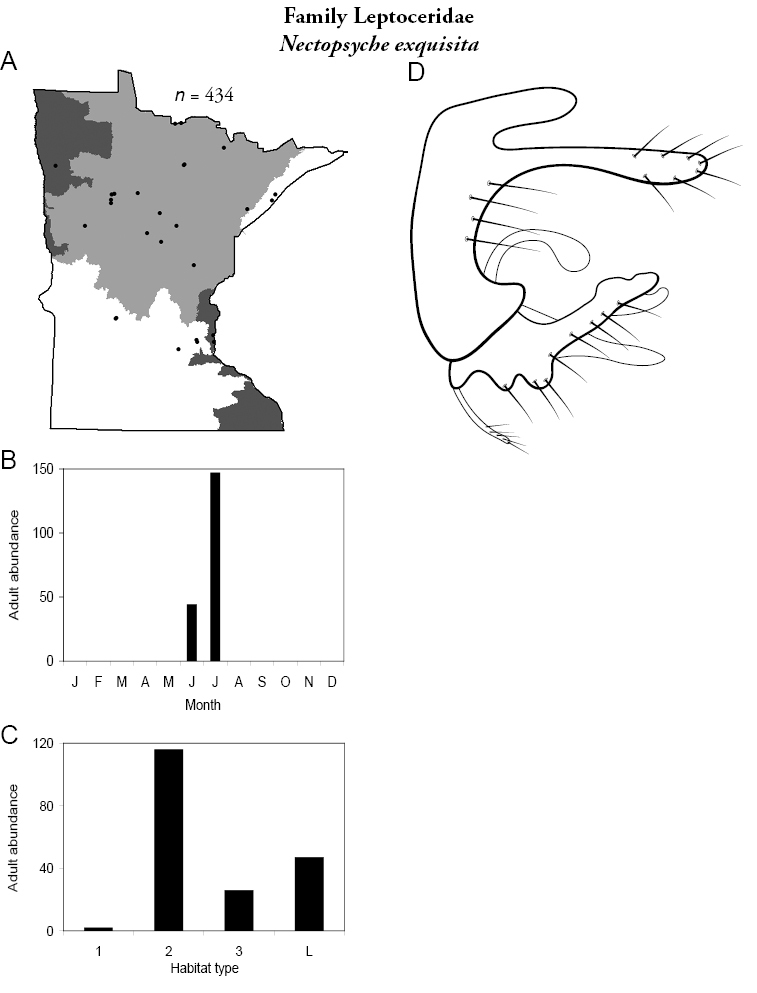
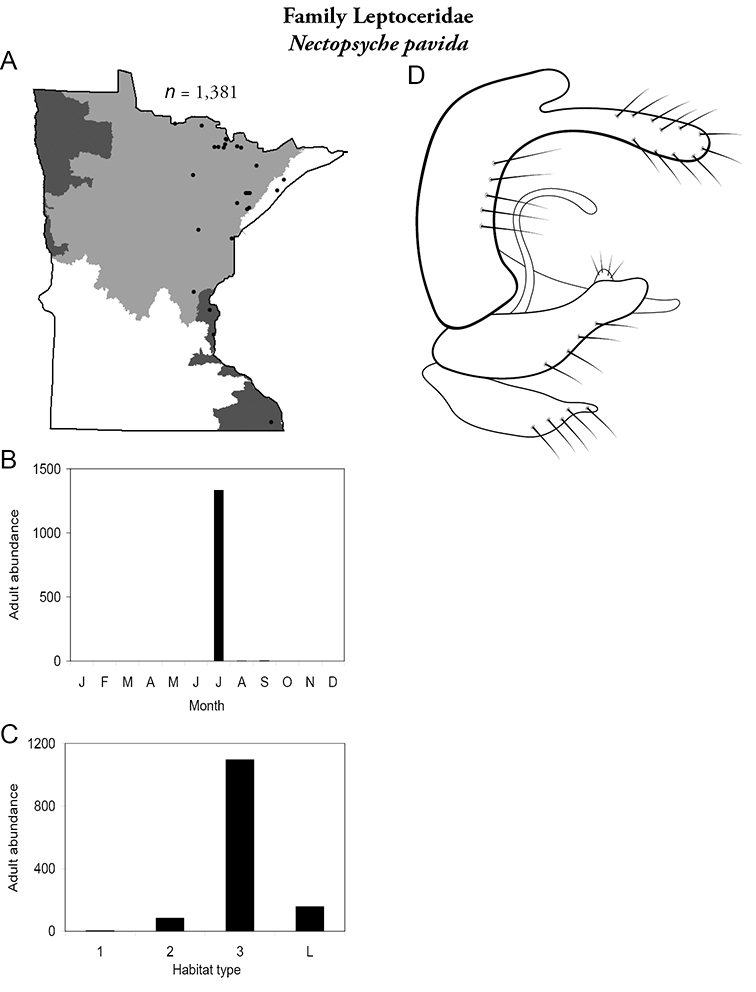
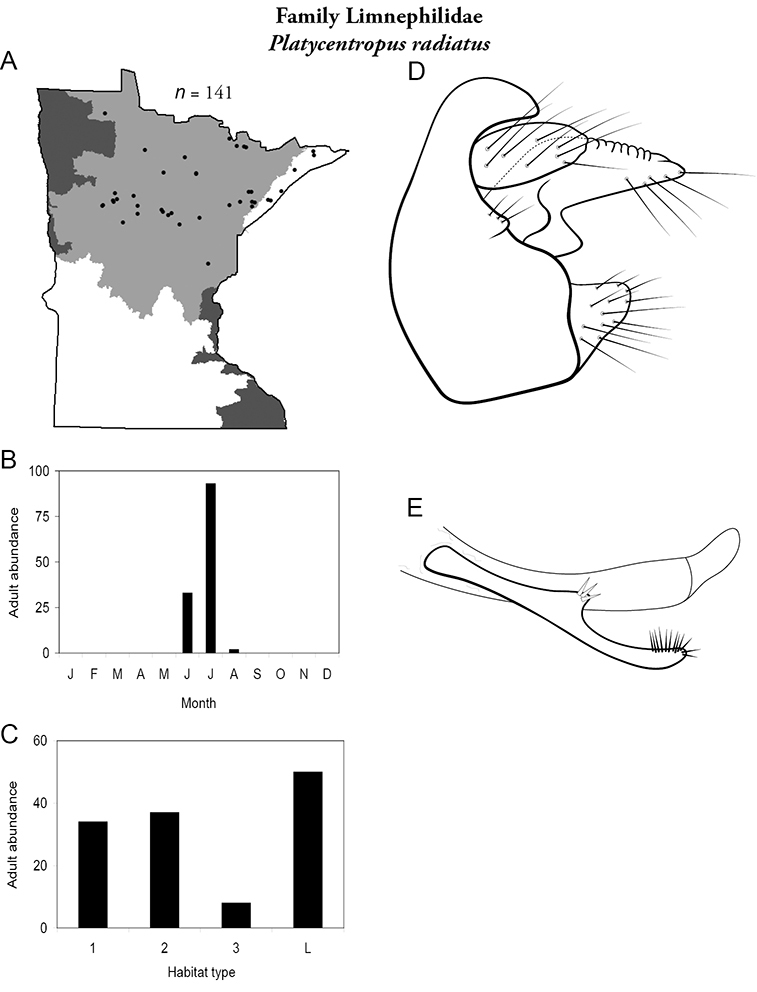
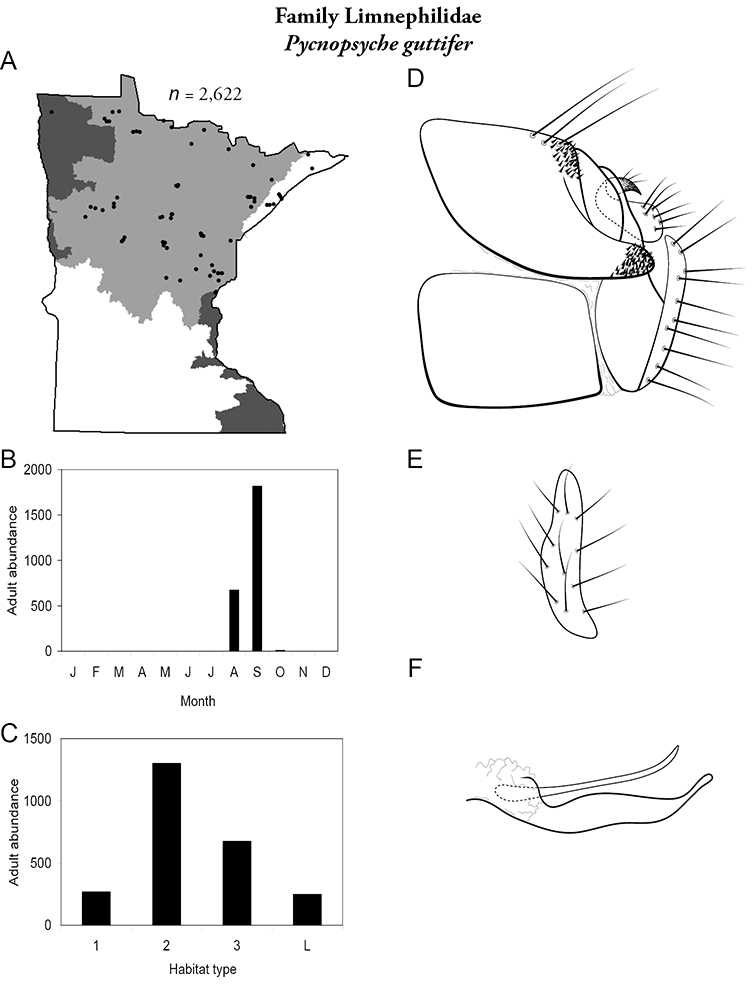
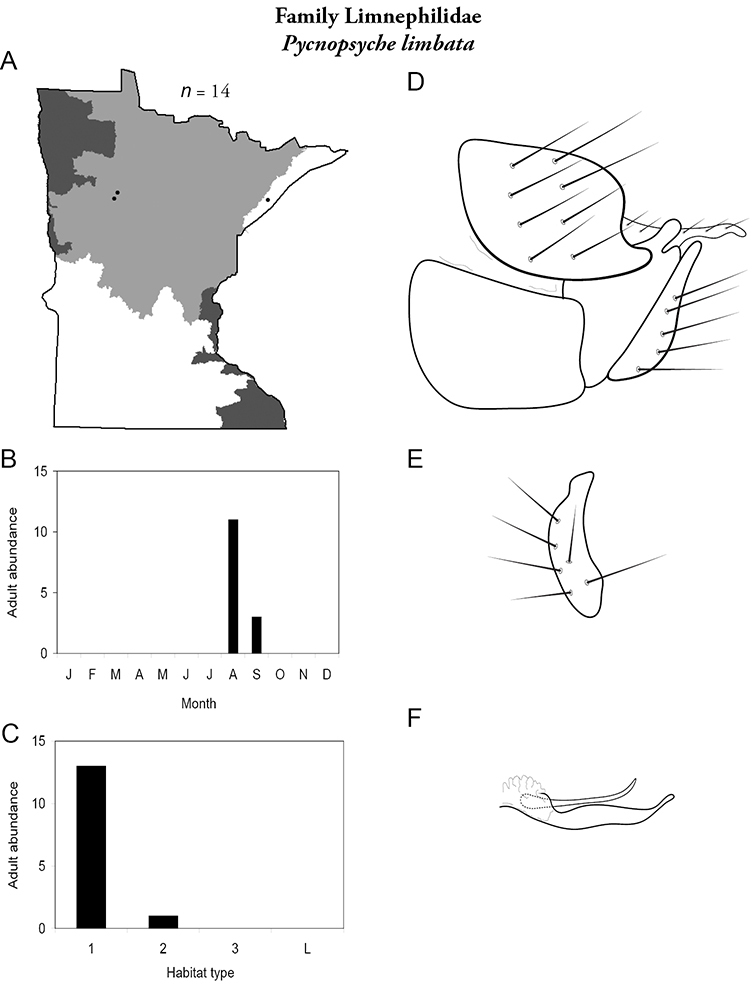
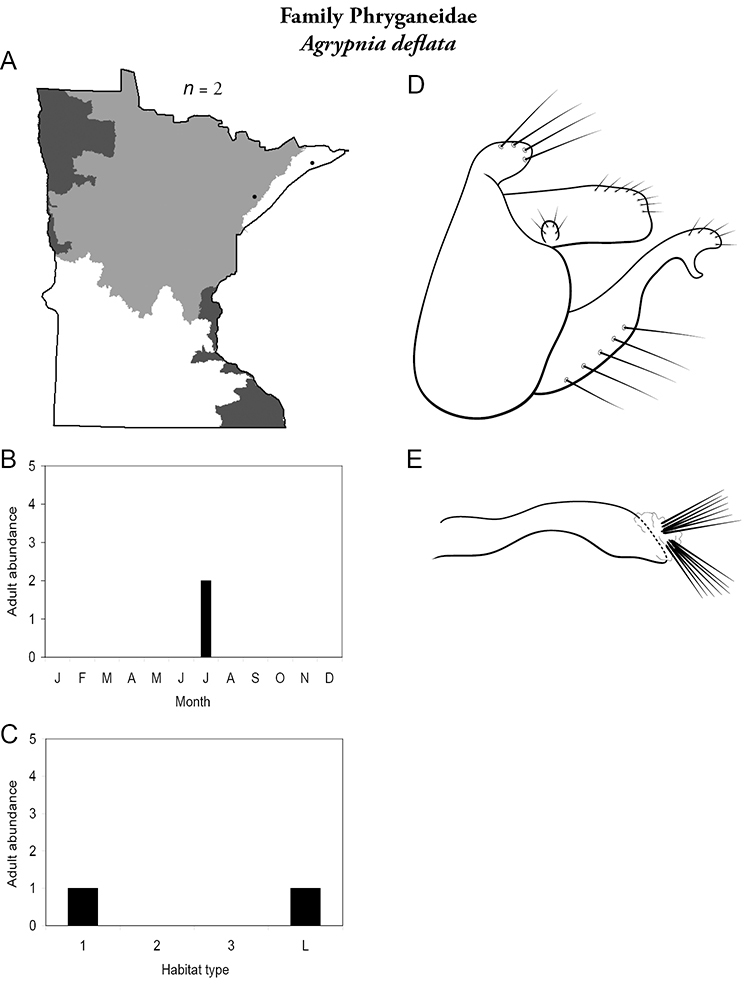
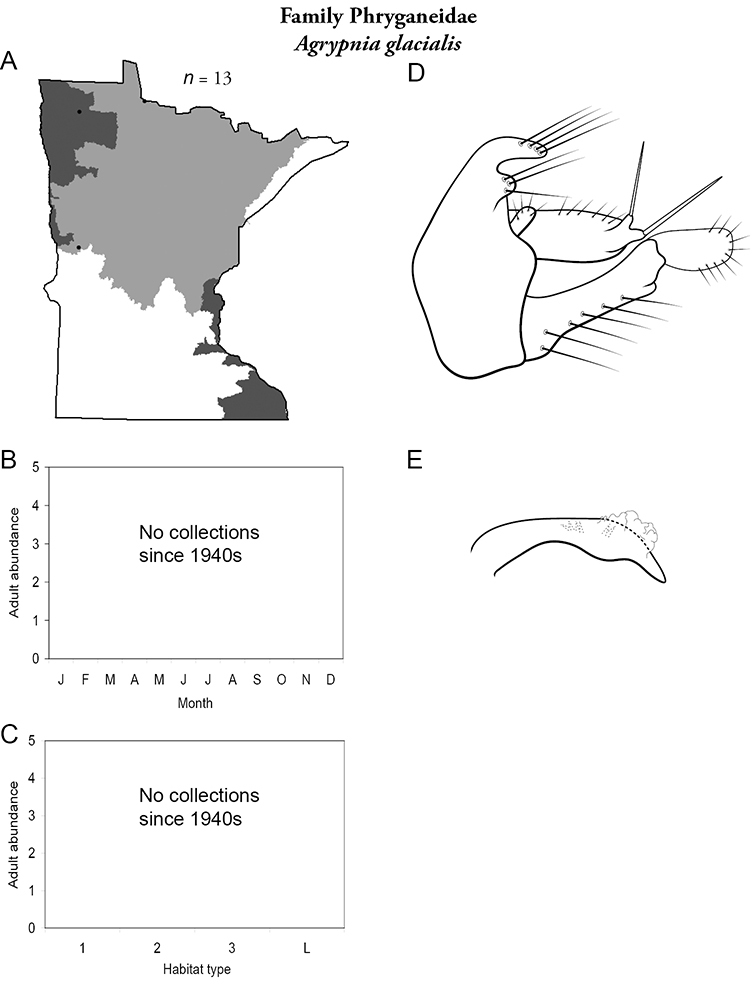
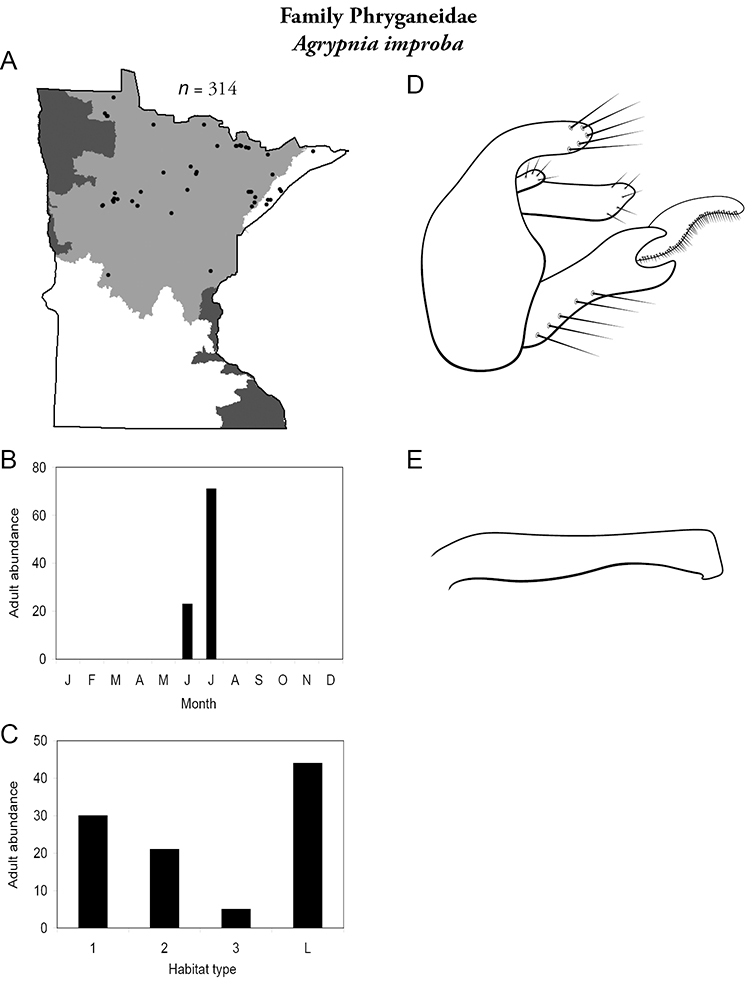
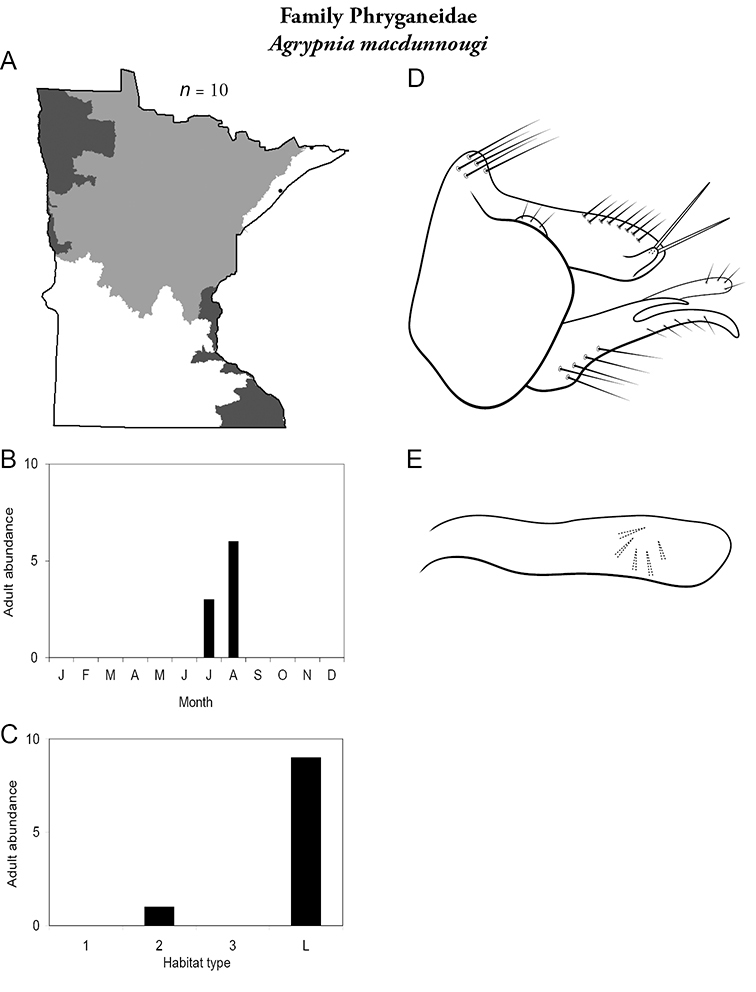
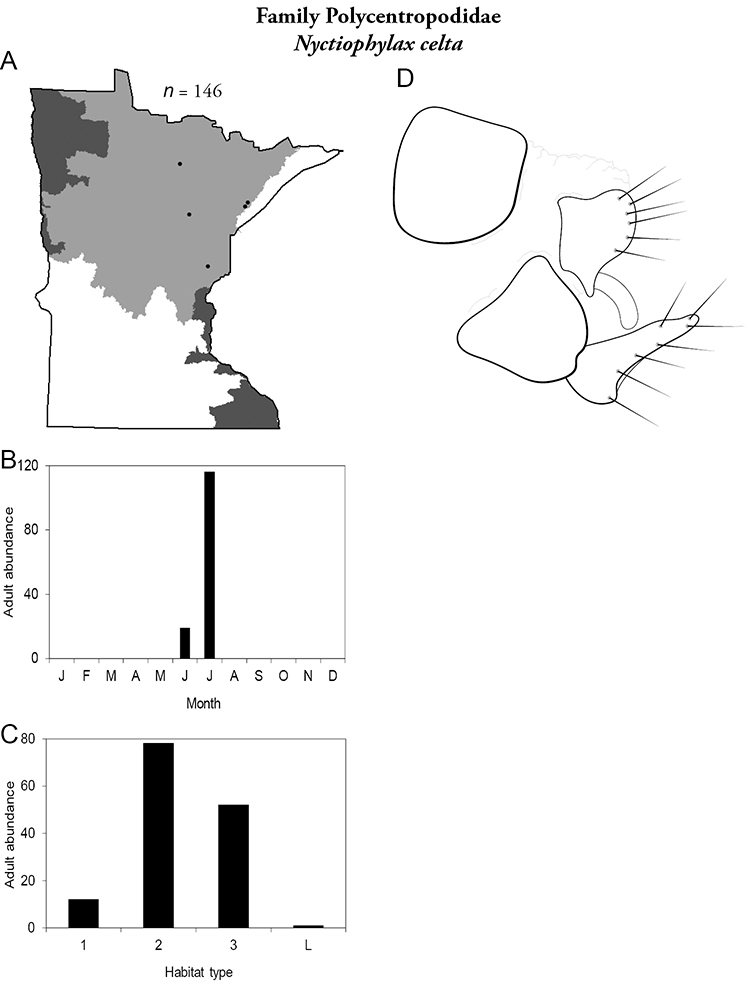
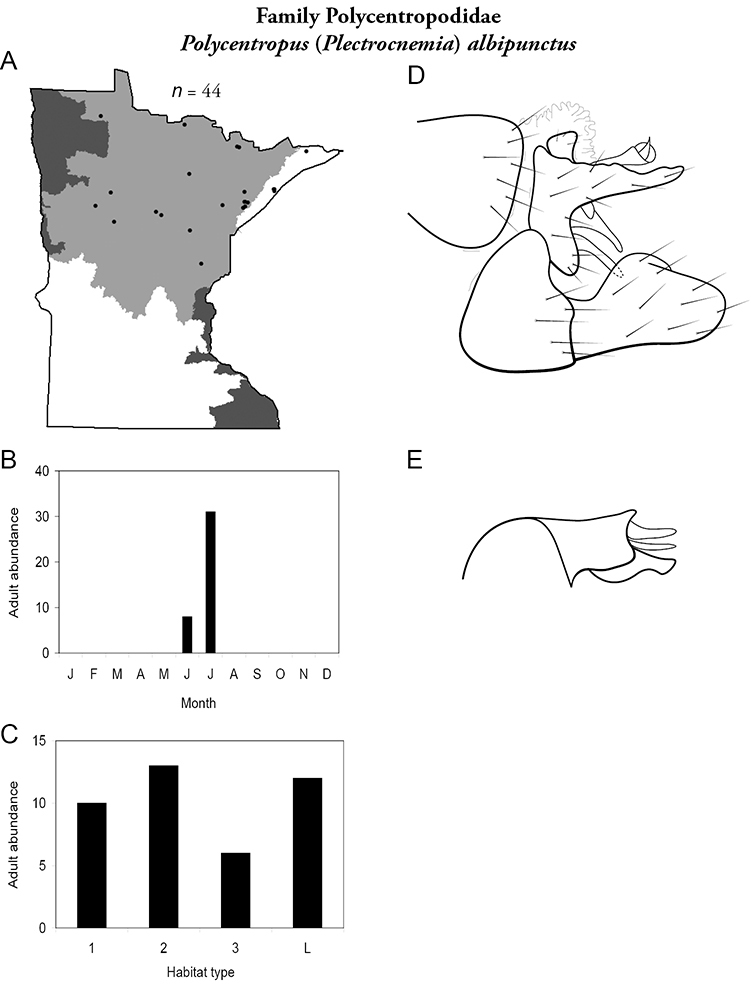
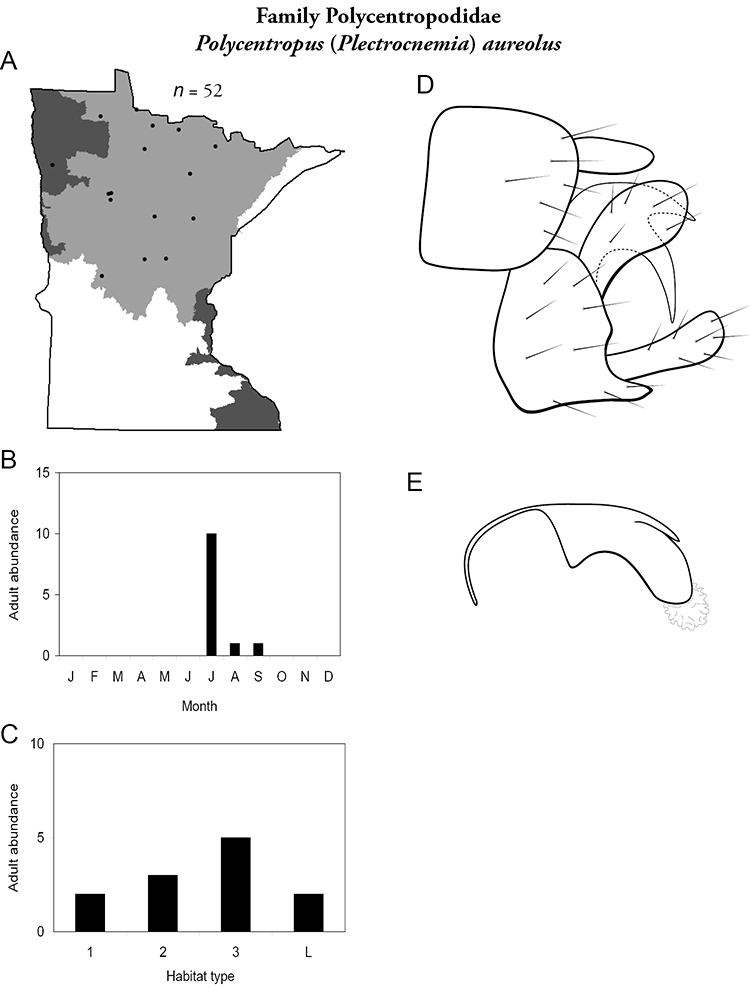
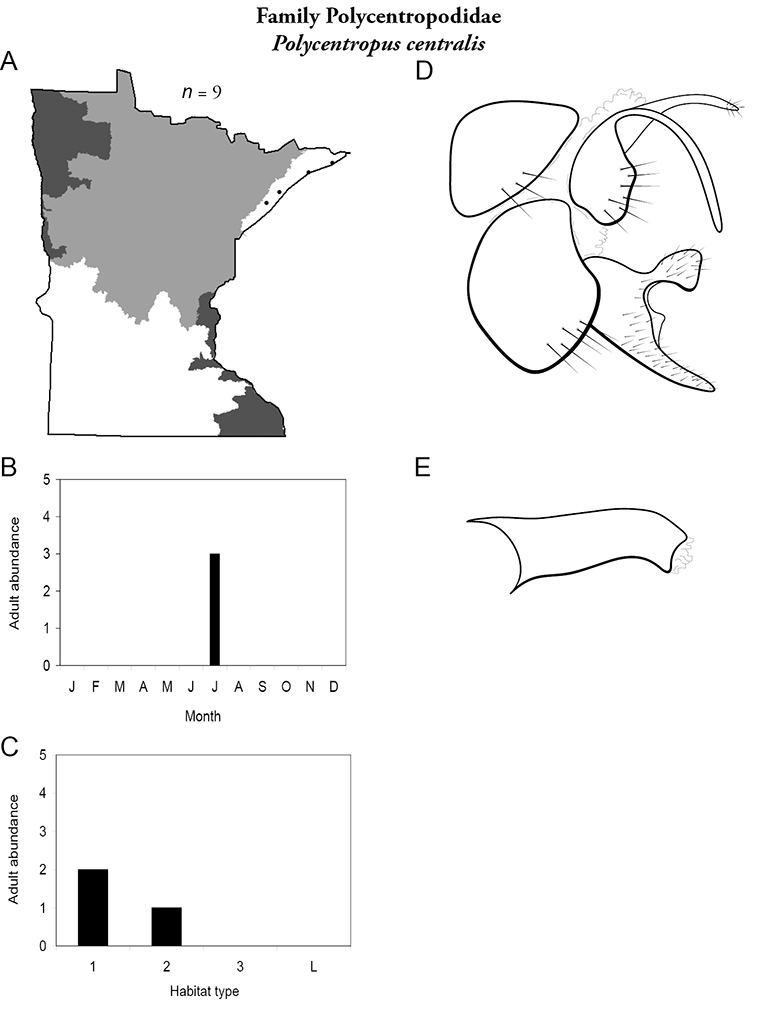
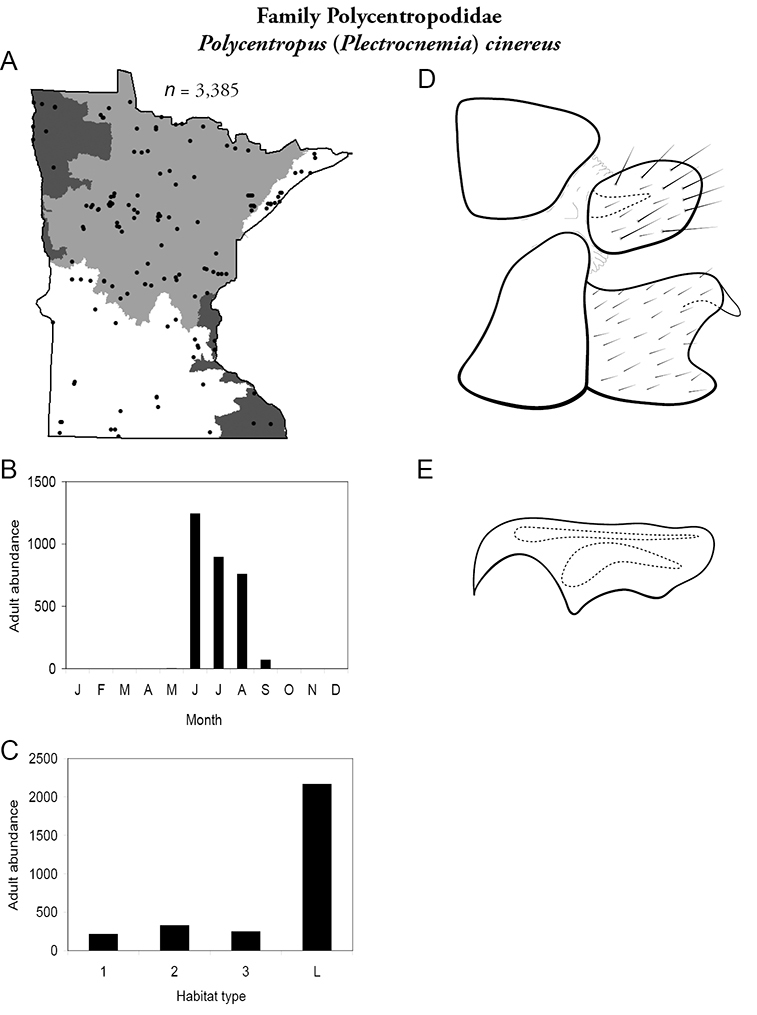
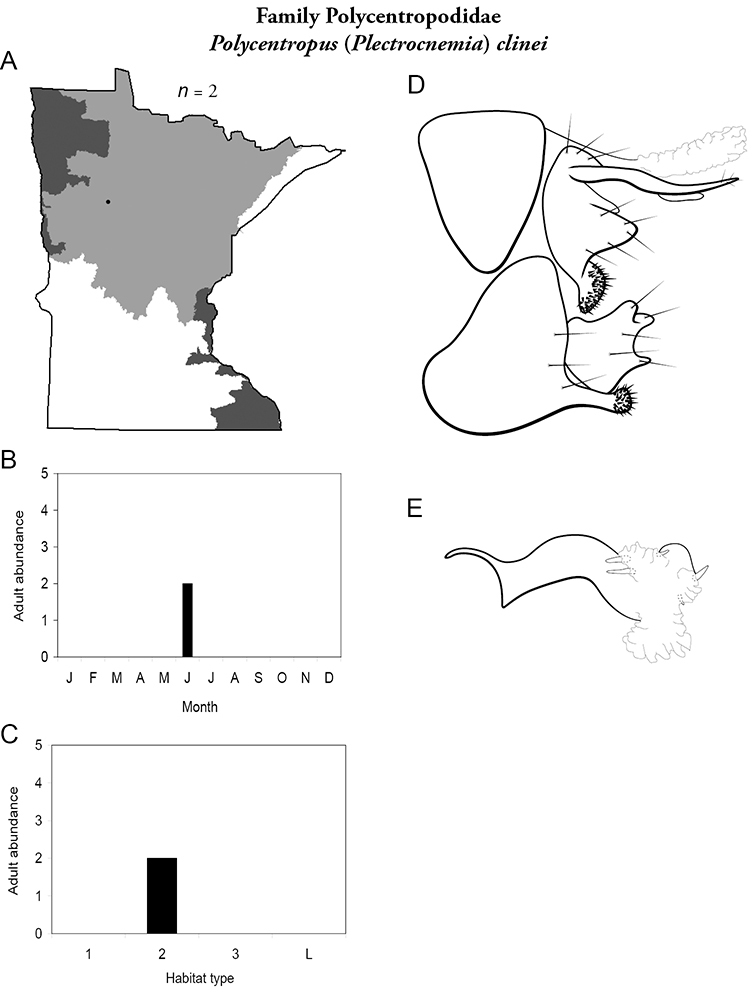
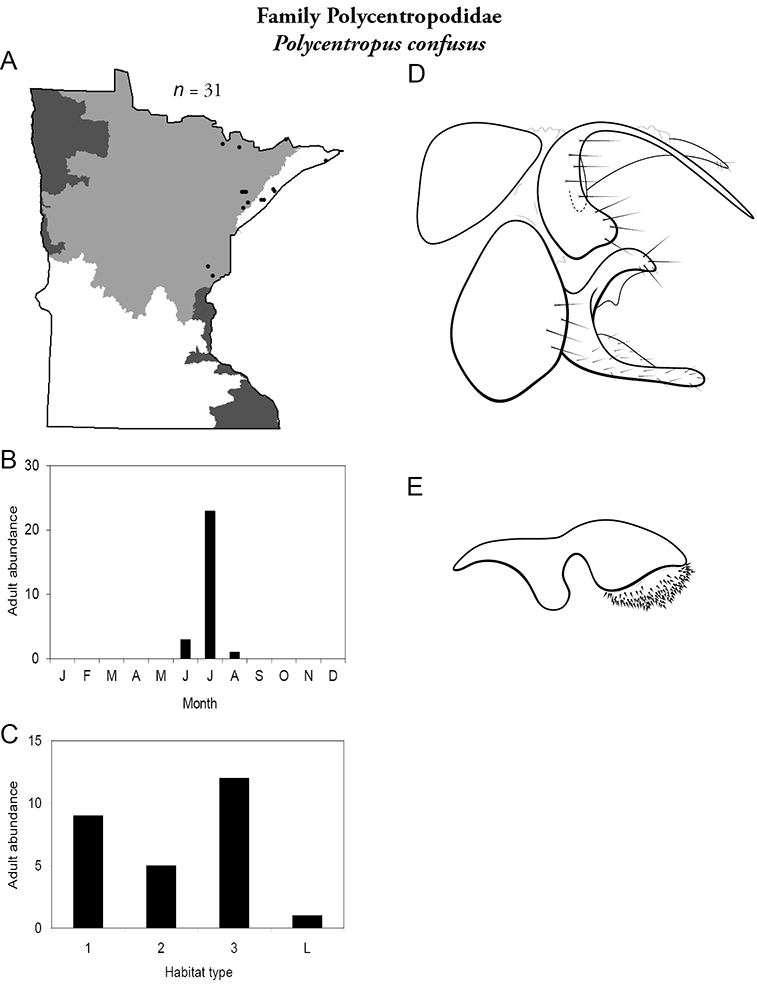
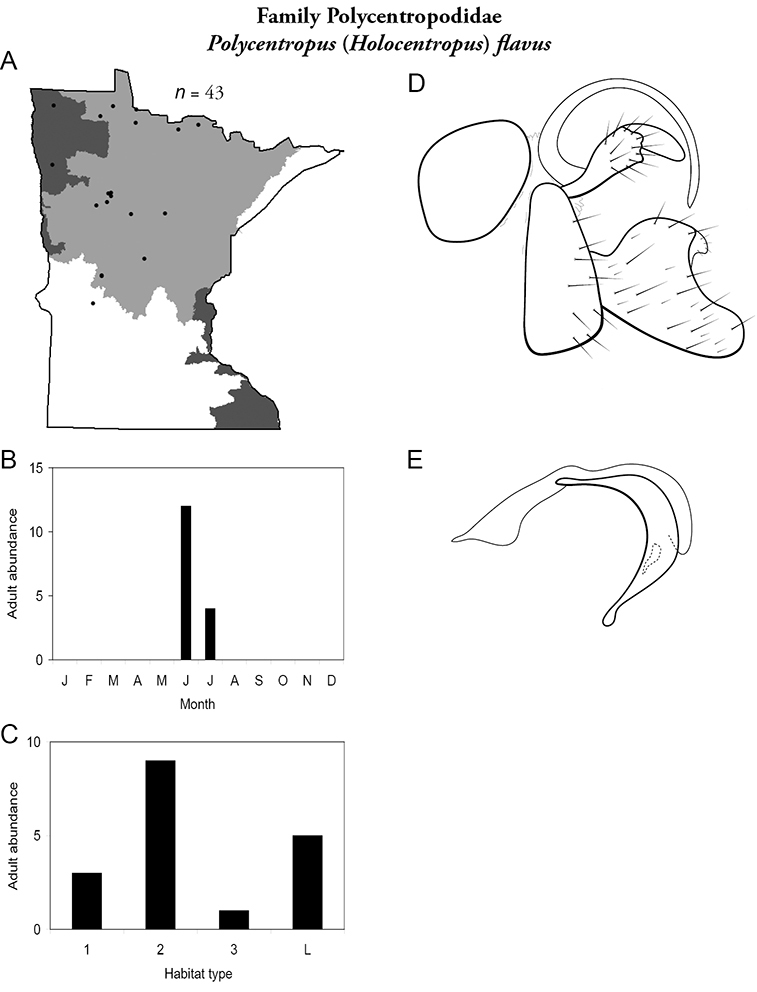
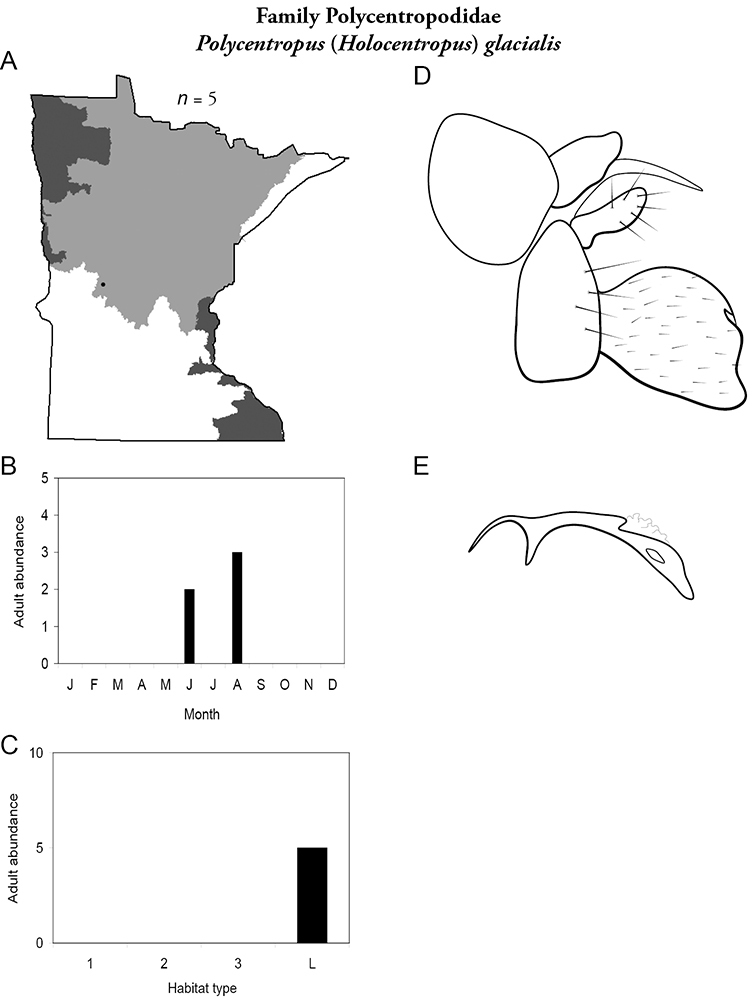
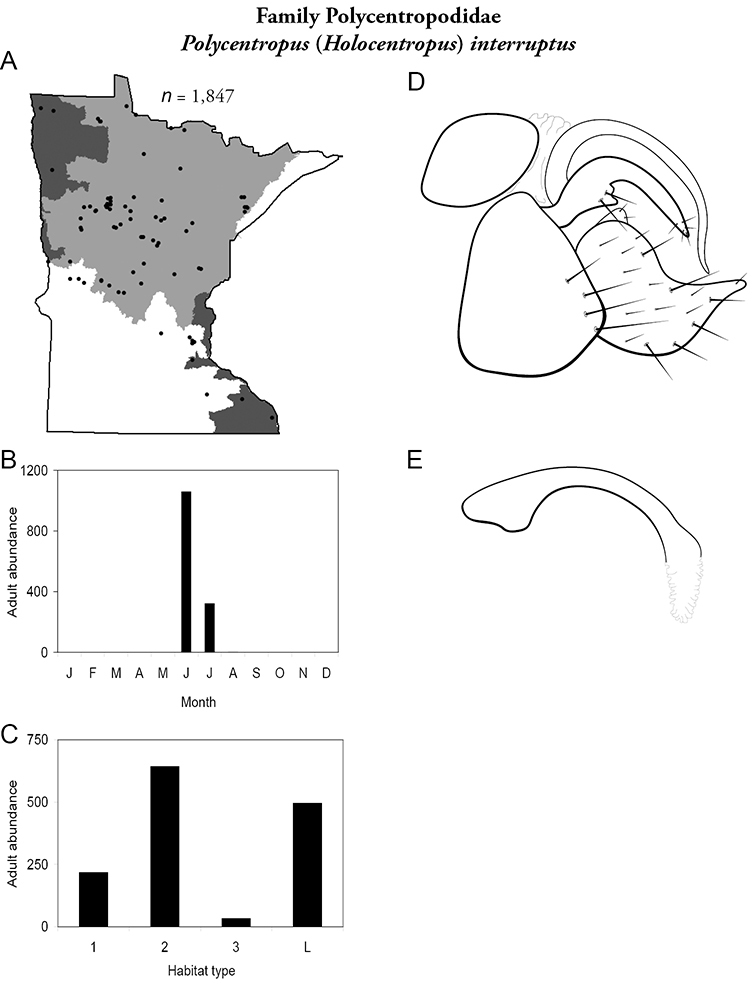
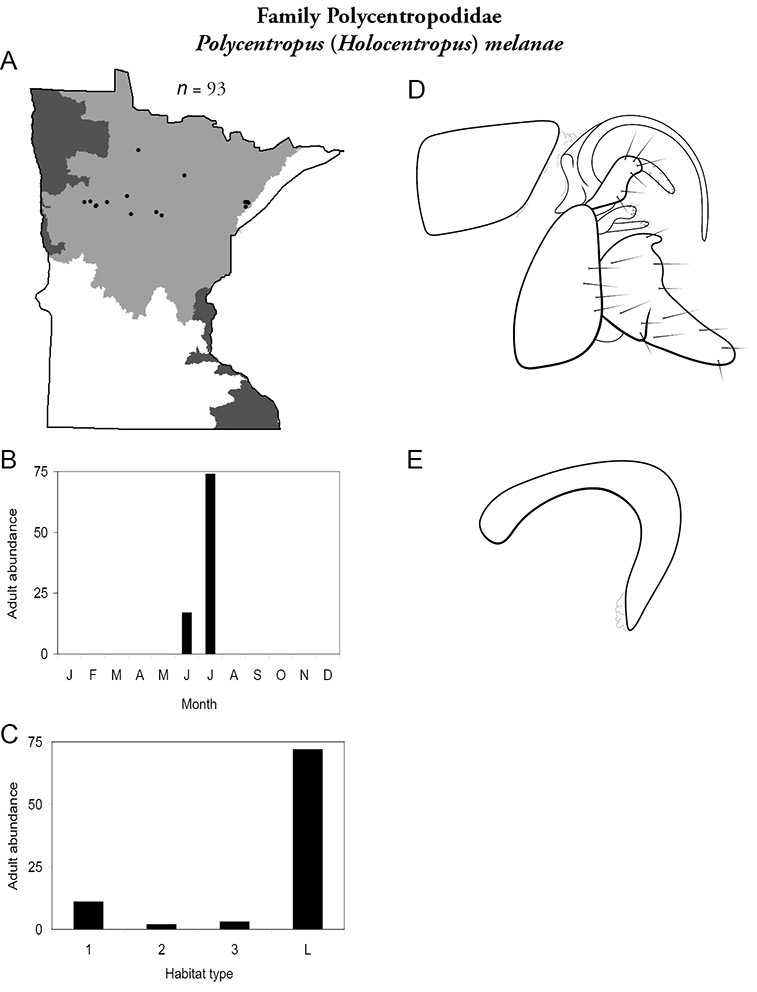
Each species plate also includes a distribution map which reflects the total number of specimens and all collecting localities known to yield those specimens from all historical and recent collecting. An explanation is given in the text for species that appear to have been reduced from a portion of their known historical range. Lastly, each species plate includes graphs reflecting the adult flight period and habitat preferences of the species. These graphs were based only on collections since 1980. Many historical collections were from cities not associated with any particular habitat, rendering inclusion into habitat preference graphs impossible. Most specimens collected since 1980 were obtained using quantitative sampling techniques, thus allowing users to accurately compare contemporary abundances of the different species in different habitats. Users can also compare the number of specimens collected since 1980 with the total number collected. For some species, the decrease in abundance and distribution since the 1940s is quite striking. Ideally, viewing all historical collecting localities in conjunction with recent quantitative habitat preference data, and reading the text for each species, should give the best predictive information on where each species should be expected to occur in Minnesota.
Species found in ≥ 50% of respective habitats of the Lake Superior Caddisfly Region (Figure 3) since 1980 and representing ≥ 1% of total specimen abundance in each habitat (Table 1). Species richness totals in Figure 11.
| Habitat | Species | Family | % of fauna | Figure |
|---|---|---|---|---|
| Small streams | Hydropsyche sparna | Hydropsychidae | 17% | 58 |
| Hydropsyche slossonae | Hydropsychidae | 10% | 57 | |
| Dolophilodes distinctus | Philopotamidae | 9% | 236 | |
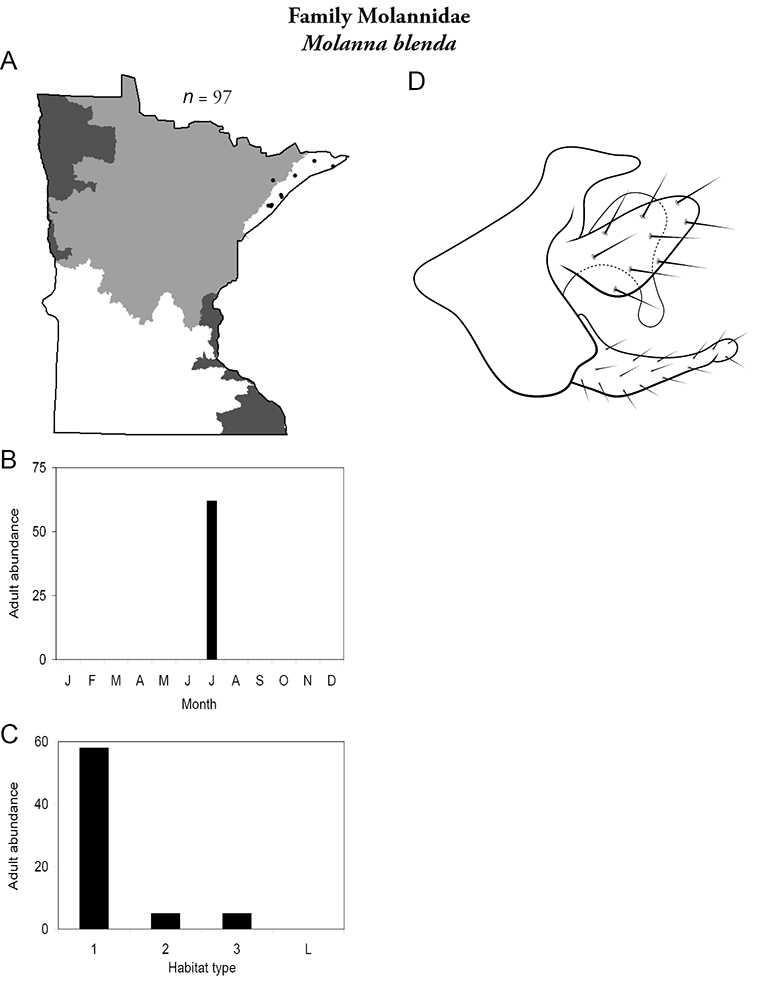
| Molanna blenda | Molannidae | 9% | 227 | |
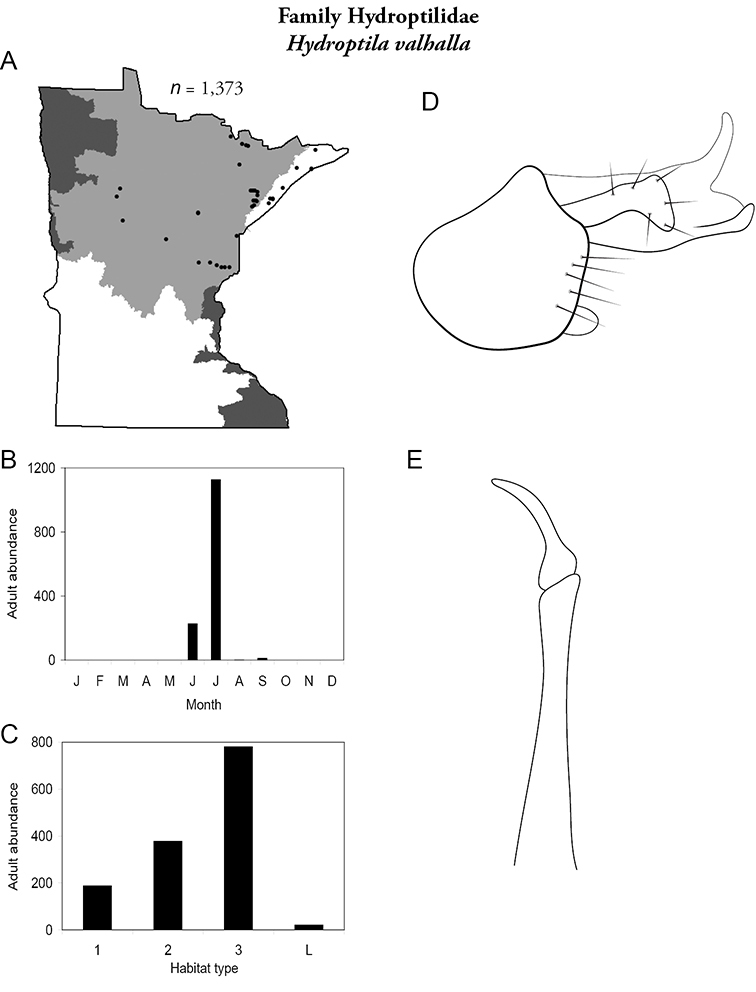
| Hydroptila valhalla | Hydroptilidae | 8% | 85 | |
| Nyctiophylax moestus | Polycentropodidae | 7% | 263 | |
| Lepidostoma togatum | Lepidostomatidae | 4% | 130 | |
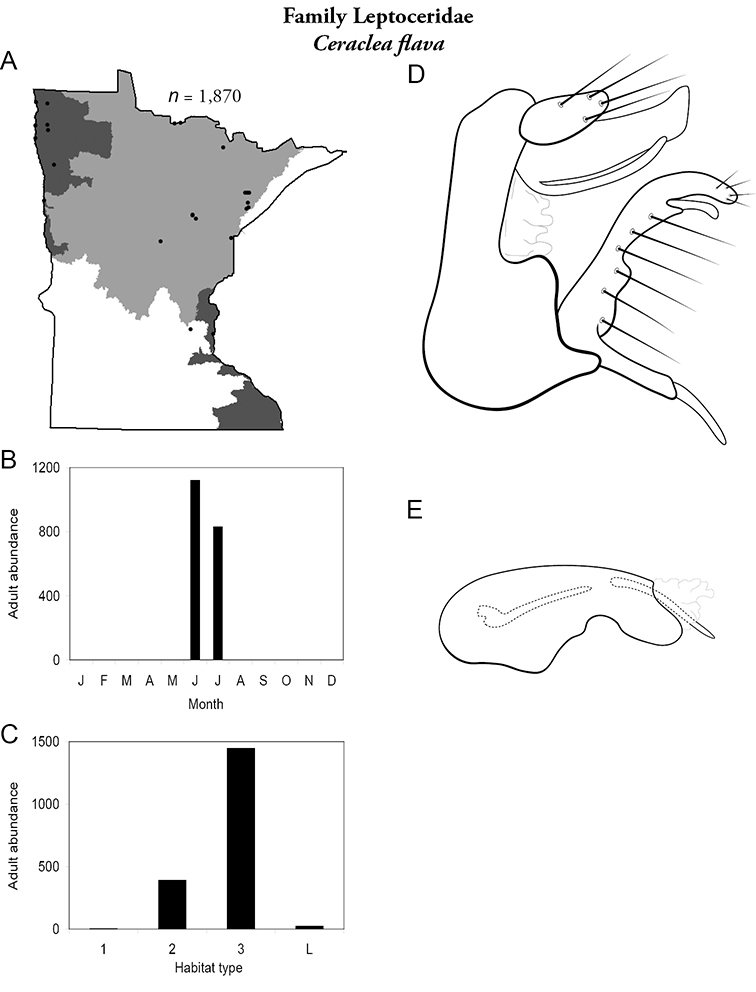
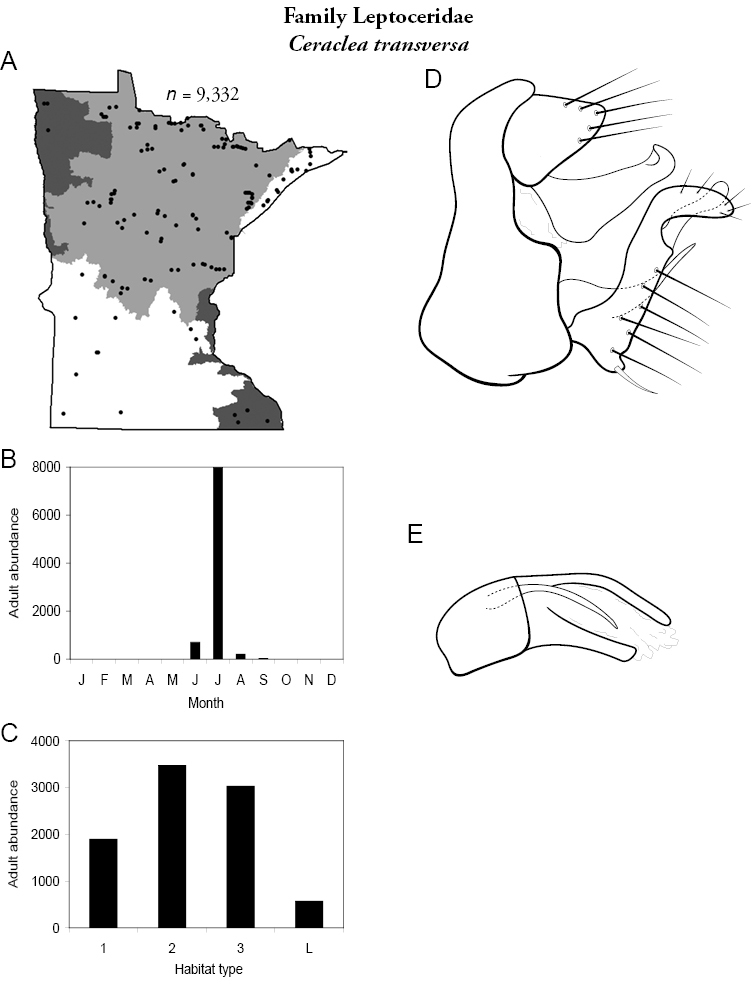
| Ceraclea transversa | Leptoceridae | 3% | 146 | |
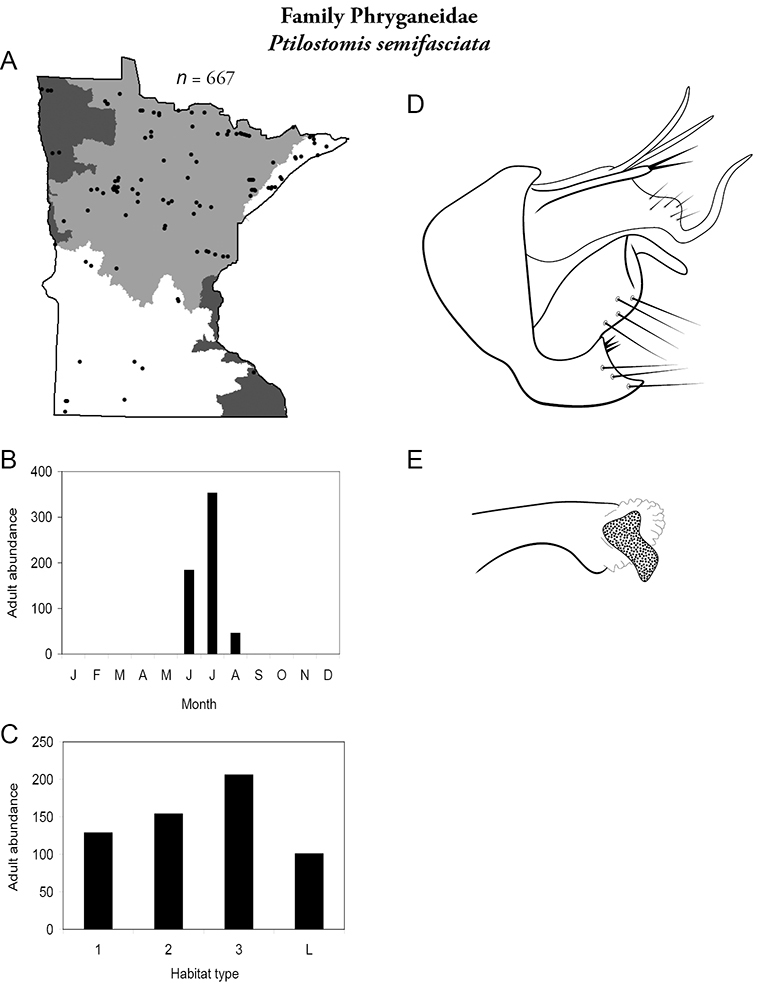
| Ptilostomis semifasciata | Phryganeidae | 2% | 255 | |
| Oecetis inconspicua | Leptoceridae | 2% | 160 | |
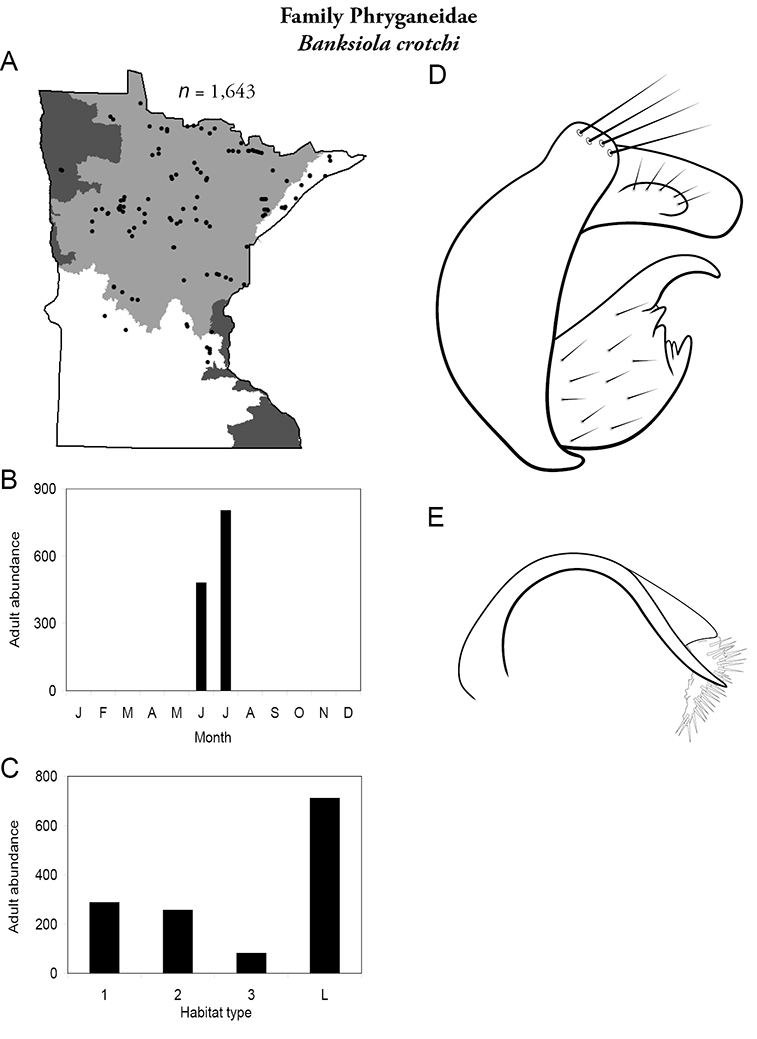
| Banksiola crotchi | Phryganeidae | 1% | 244 | |
| Glossosoma nigrior | Glossosomatidae | 1% | 26 | |
| Medium rivers | Lepidostoma togatum | Lepidostomatidae | 32% | 130 |
| Ceraclea transversa | Leptoceridae | 11% | 146 | |
| Ceraclea cancellata | Leptoceridae | 9% | 138 | |
| Cheumatopsyche gracilis | Hydropsychidae | 7% | 34 | |
| Hydroptila valhalla | Hydroptilidae | 7% | 85 | |
| Hydropsyche slossonae | Hydropsychidae | 5% | 57 | |
| Hydropsyche walkeri | Hydropsychidae | 4% | 59 | |
| Hydropsyche sparna | Hydropsychidae | 2% | 58 | |
| Banksiola crotchi | Phryganeidae | 2% | 244 | |
| Glossosoma nigrior | Glossosomatidae | 2% | 26 | |
| Nyctiophylax moestus | Polycentropodidae | 2% | 263 | |
| Hydropsyche dicantha | Hydropsychidae | 2% | 50 | |
| Helicopsyche borealis | Helicopsychidae | 1% | 31 | |
| Polycentropus cinereus | Polycentropodidae | 1% | 267 | |
| Oecetis inconspicua | Leptoceridae | 1% | 160 | |
| Dolophilodes distinctus | Philopotamidae | 1% | 236 | |
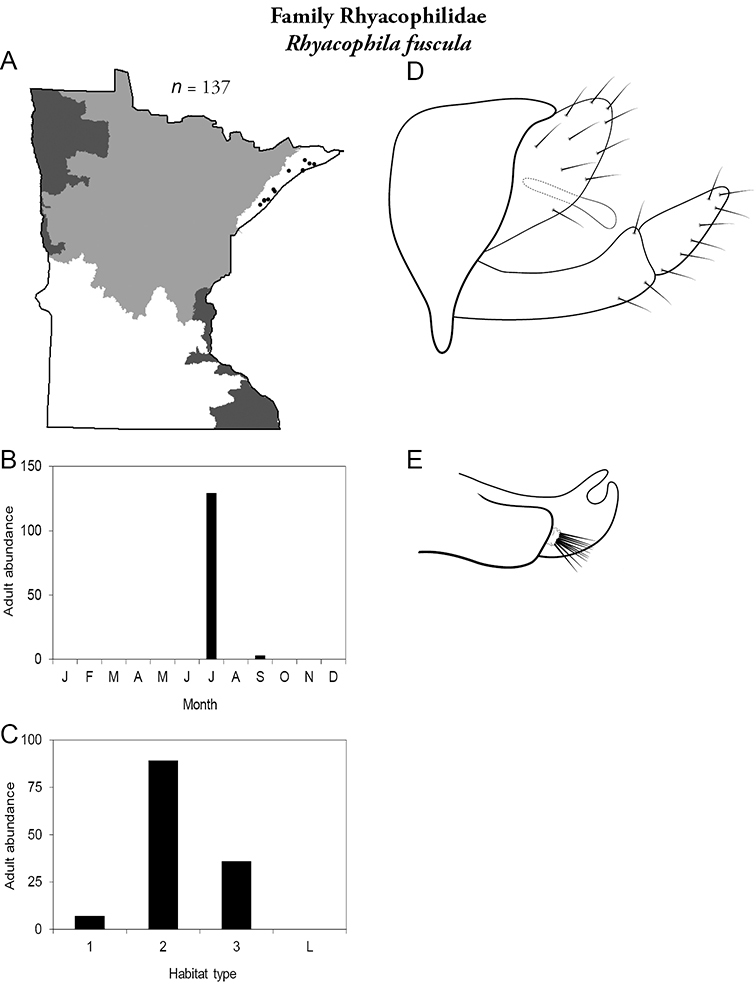
| Rhyacophila fuscula | Rhyacophilidae | 1% | 284 | |
| Large rivers | Ceraclea transversa | Leptoceridae | 18% | 146 |
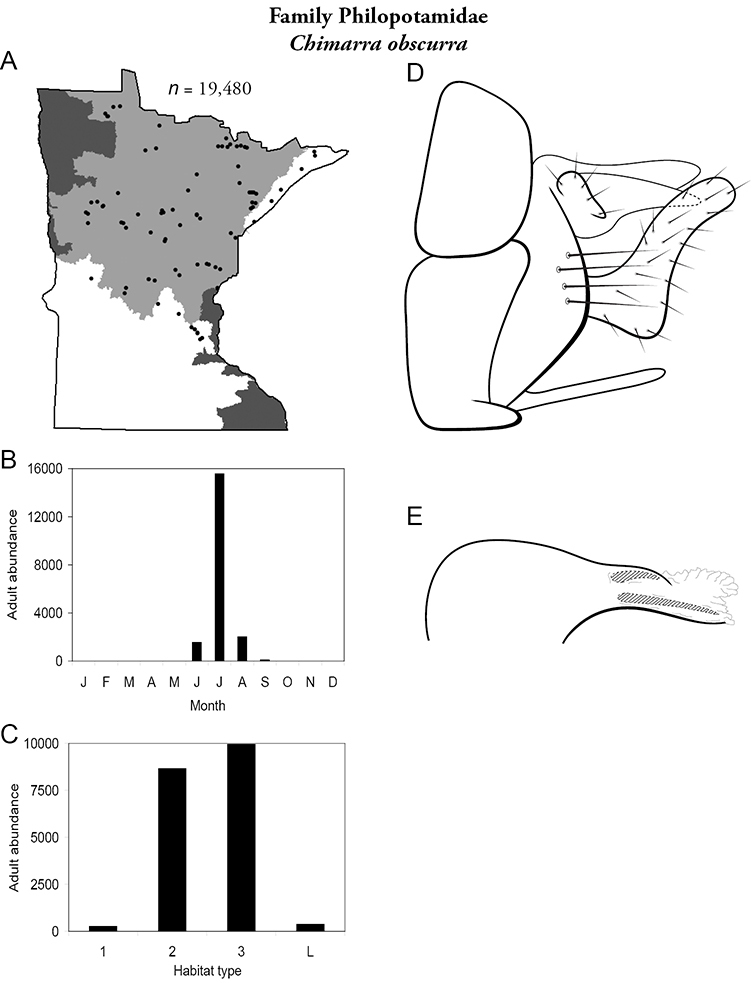
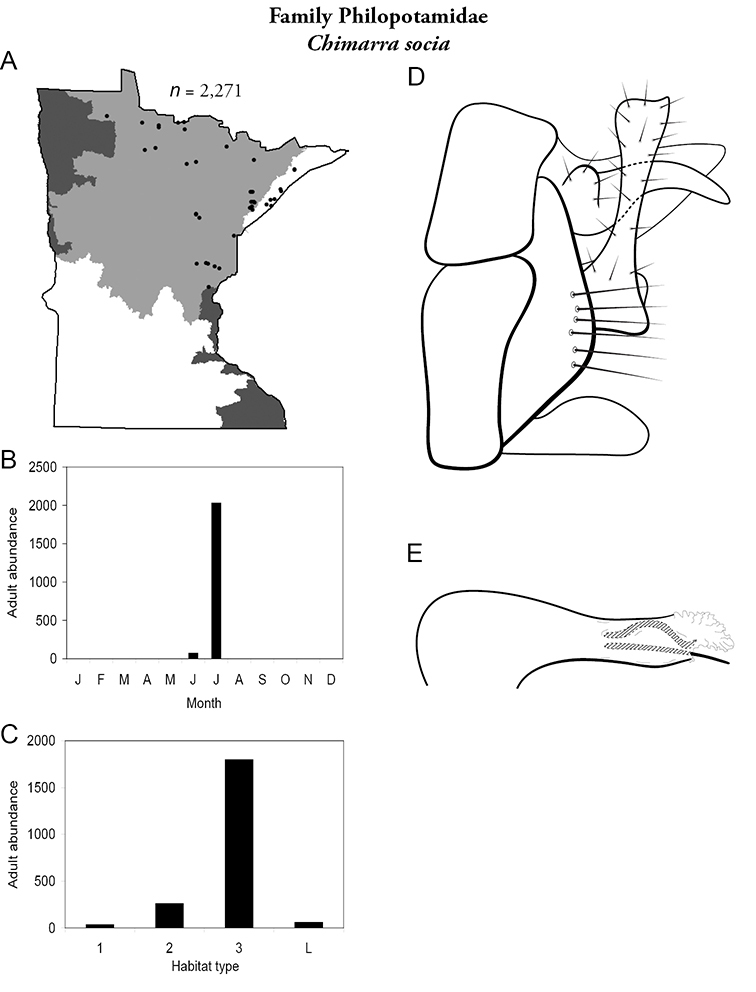
| Chimarra socia | Philopotamidae | 14% | 235 | |
| Lepidostoma togatum | Lepidostomatidae | 10% | 130 | |
| Leptocerus americanus | Leptoceridae | 9% | 148 | |
| Chimarra obscurra | Philopotamidae | 8% | 234 | |
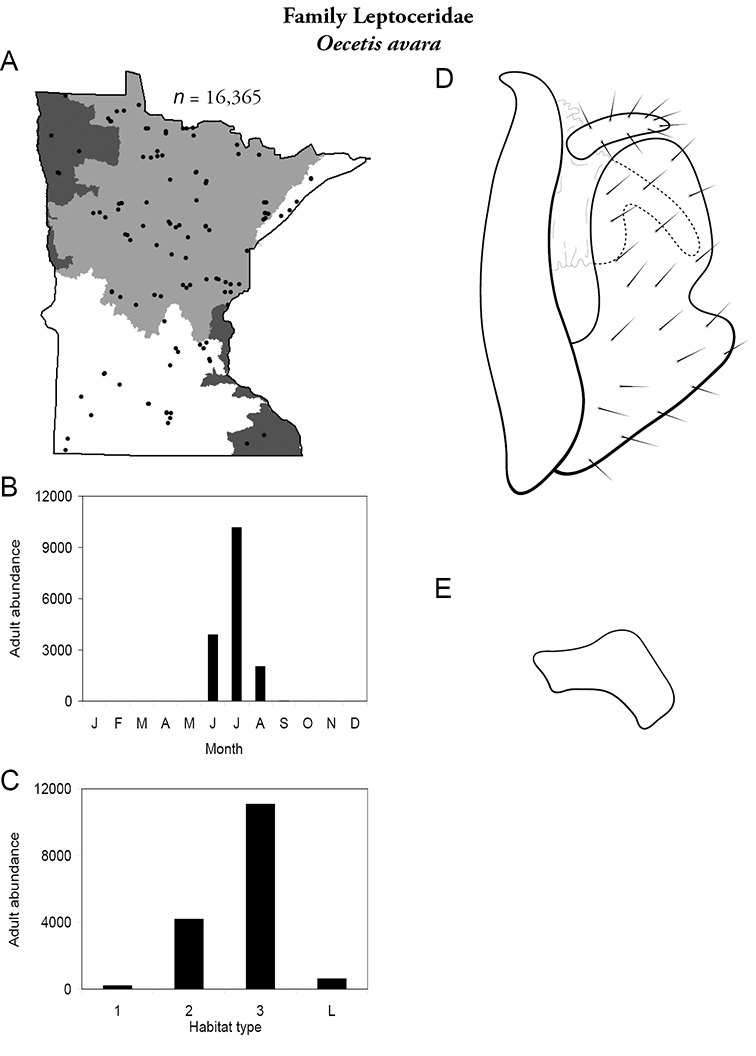
| Oecetis avara | Leptoceridae | 5% | 155 | |
| Psychomia flavida | Psychomyiidae | 5% | 282 | |
| Hydroptila valhalla | Hydroptilidae | 4% | 85 | |
| Ceraclea diluta | Leptoceridae | 4% | 139 | |
| Ceraclea resurgens | Leptoceridae | 2% | 144 | |
| Rhyacophila fuscula | Rhyacophilidae | 2% | 284 | |
| Ceraclea cancellata | Leptoceridae | 2% | 138 | |
| Glossosoma nigrior | Glossosomatidae | 2% | 26 | |
| Oecetis inconspicua | Leptoceridae | 1% | 160 | |
| Lakes | Triaenodes injustus | Leptoceridae | 38% | 172 |
| Polycentropus cinereus | Polycentropodidae | 27% | 267 | |
| Molanna flavicornis | Molannidae | 8% | 228 | |
| Nyctiophylax affinis | Polycentropodidae | 5% | 260 | |
| Oecetis inconspicua | Leptoceridae | 2% | 160 | |
| Phryganea cinerea | Phryganeidae | 2% | 251 | |
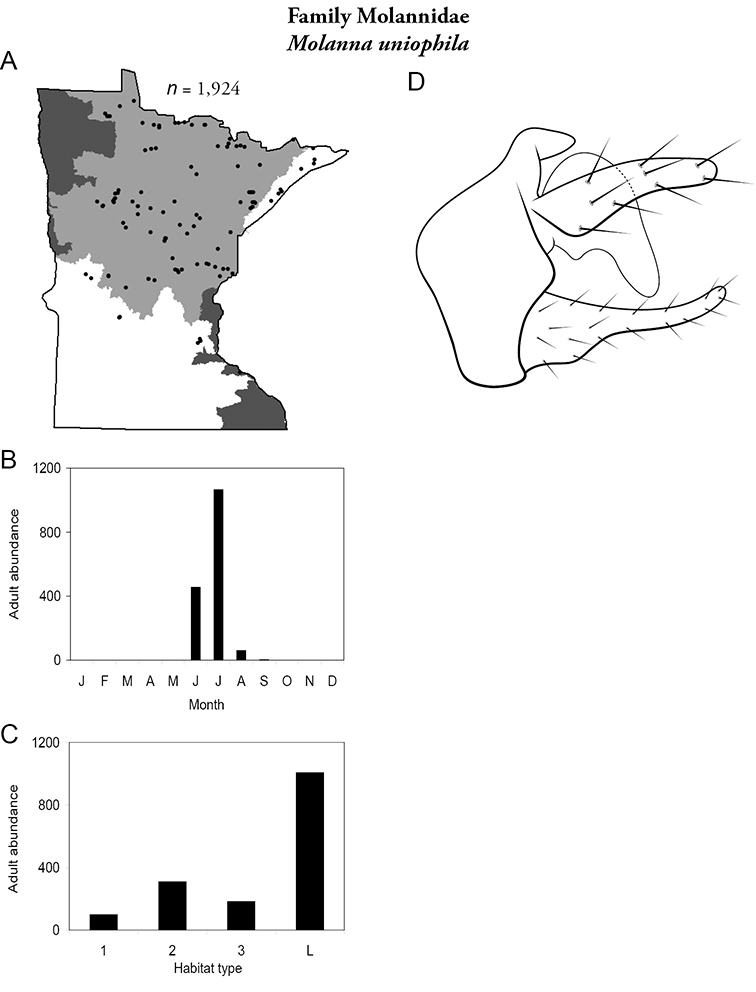
| Molanna uniophila | Molannidae | 2% | 230 | |
| Ceraclea cancellata | Leptoceridae | 2% | 138 | |
| Phylocentropus placidus | Dipseudopsidae | 1% | 22 |
Species found in ≥ 50% of respective habitats of the Northern Caddisfly Region (Figure 3) since 1980 and representing ≥ 1% of total specimen abundance in each habitat (Table 1). Species richness totals in Figure 11.
| Habitat | Species | Family | % of fauna | Figure |
|---|---|---|---|---|
| Small streams | Leptocerus americanus | Leptoceridae | 16% | 148 |
| Oecetis inconspicua | Leptoceridae | 14% | 160 | |
| Ceraclea transversa | Leptoceridae | 9% | 146 | |
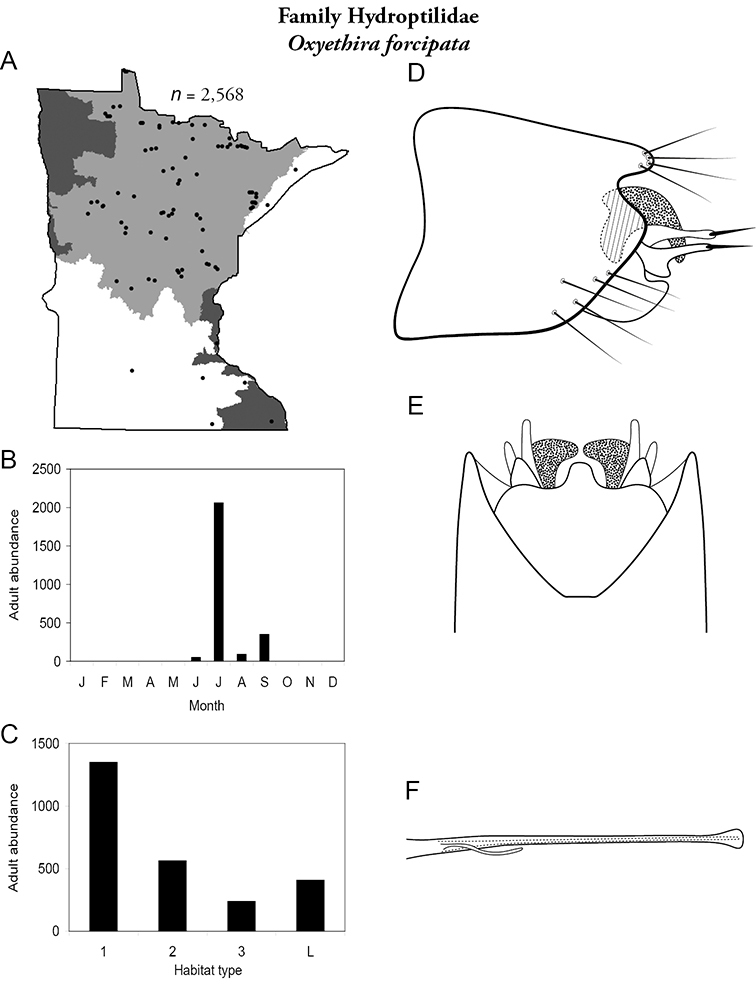
| Oxyethira forcipata | Hydroptilidae | 6% | 109 | |
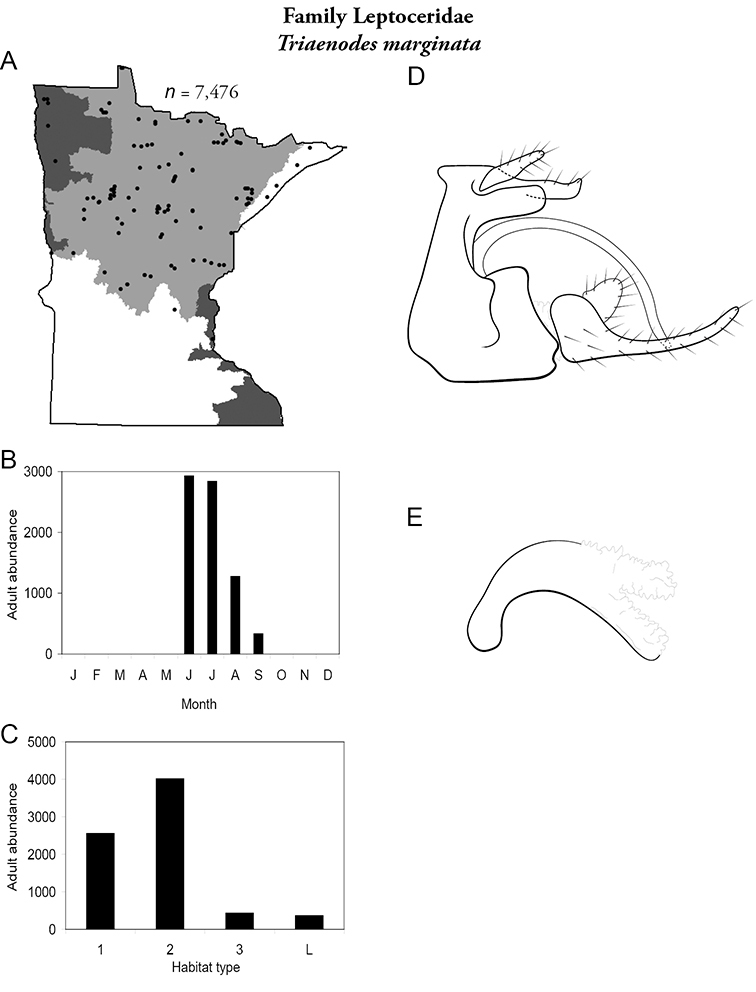
| Triaenodes marginata | Leptoceridae | 4% | 173 | |
| Banksiola crotchi | Phryganeidae | 1% | 244 | |
| Triaenodes tarda | Leptoceridae | 1% | 175 | |
| Cheumatopsyche pettiti | Hydropsychidae | 1% | 39 | |
| Medium rivers | Chimarra obscurra | Philopotamidae | 22% | 234 |
| Oecetis avara | Leptoceridae | 10% | 155 | |
| Ceraclea transversa | Leptoceridae | 6% | 146 | |
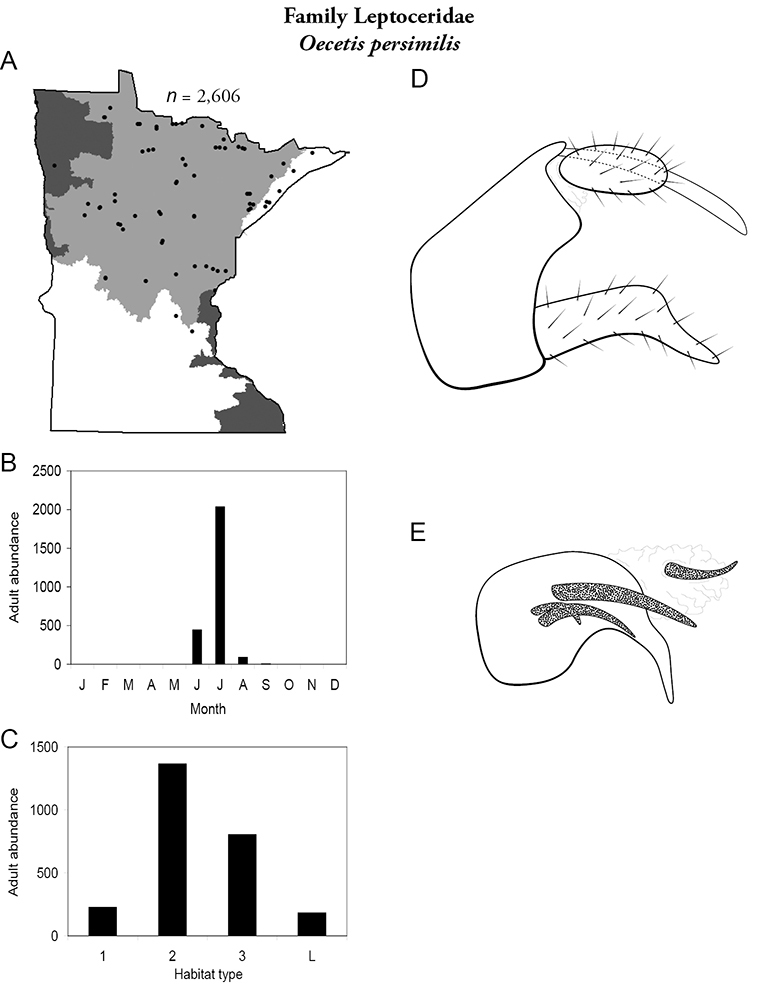
| Oecetis persimilis | Leptoceridae | 4% | 164 | |
| Leptocerus americanus | Leptoceridae | 3% | 148 | |
| Oecetis inconspicua | Leptoceridae | 3% | 160 | |
| Triaenodes marginata | Leptoceridae | 1% | 174 | |
| Oxyethira forcipata | Hydroptilidae | 1% | 109 | |
| Large rivers | Psychomyia flavida | Psychomyiidae | 20% | 282 |
| Oecetis avara | Leptoceridae | 14% | 155 | |
| Chimarra obscurra | Philopotamidae | 13% | 234 | |
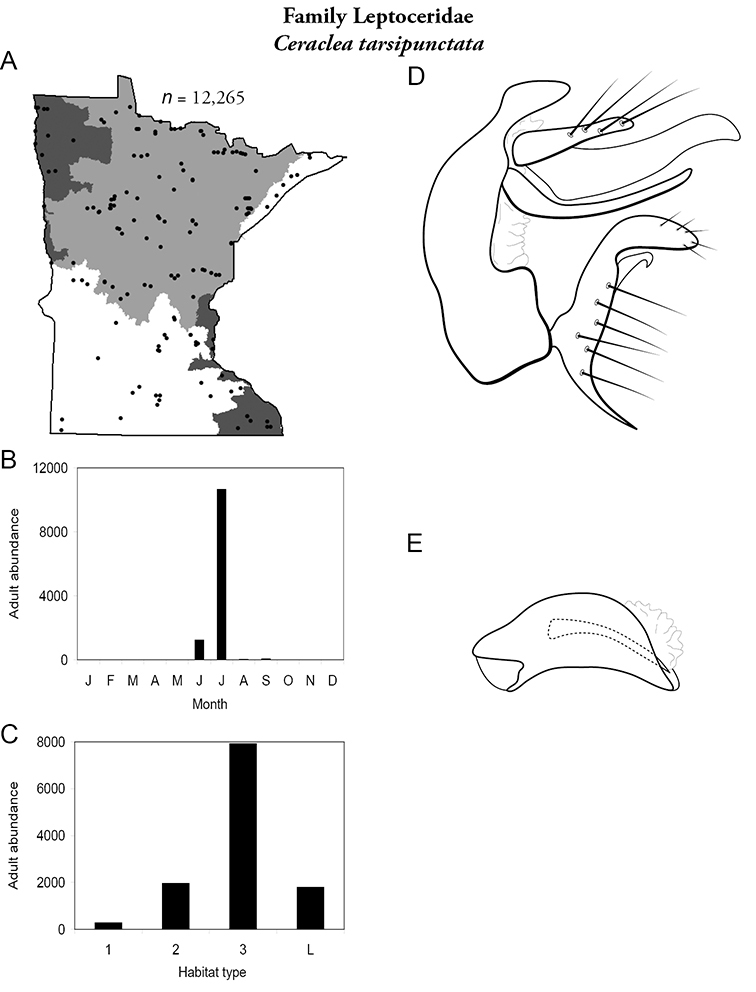
| Ceraclea tarsipunctata | Leptoceridae | 9% | 145 | |
| Cheumatopsyche speciosa | Hydropsychidae | 6% | 41 | |
| Leptocerus americanus | Leptoceridae | 4% | 148 | |
| Ceraclea transversa | Leptoceridae | 3% | 146 | |
| Oecetis inconspicua | Leptoceridae | 3% | 160 | |
| Helicopsyche borealis | Helicopsychidae | 3% | 31 | |
| Lepidostoma togatum | Lepidostomatidae | 2% | 130 | |
| Triaenodes injustus | Leptoceridae | 1% | 173 | |
| Ceraclea cancellata | Leptoceridae | 1% | 138 | |
| Oecetis persimilis | Leptoceridae | 1% | 164 | |
| Triaenodes marginata | Leptoceridae | 1% | 174 | |
| Agraylea multipunctata | Hydroptilidae | 1% | 63 | |
| Cheumatopsyche campyla | Hydropsychidae | 1% | 33 | |
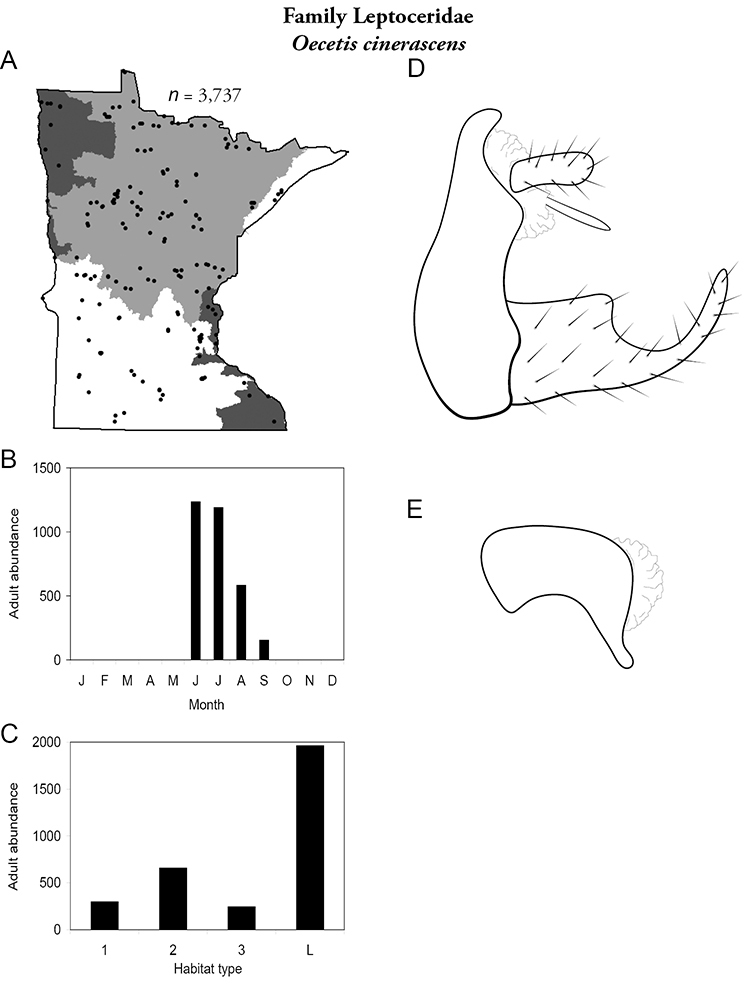
| Oecetis cinerascens | Leptoceridae | 1% | 156 | |
| Triaenodes tarda | Leptoceridae | 1% | 175 | |
| Lakes | Leptocerus americanus | Leptoceridae | 17% | 148 |
| Oecetis inconcpicua | Leptoceridae | 11% | 160 | |
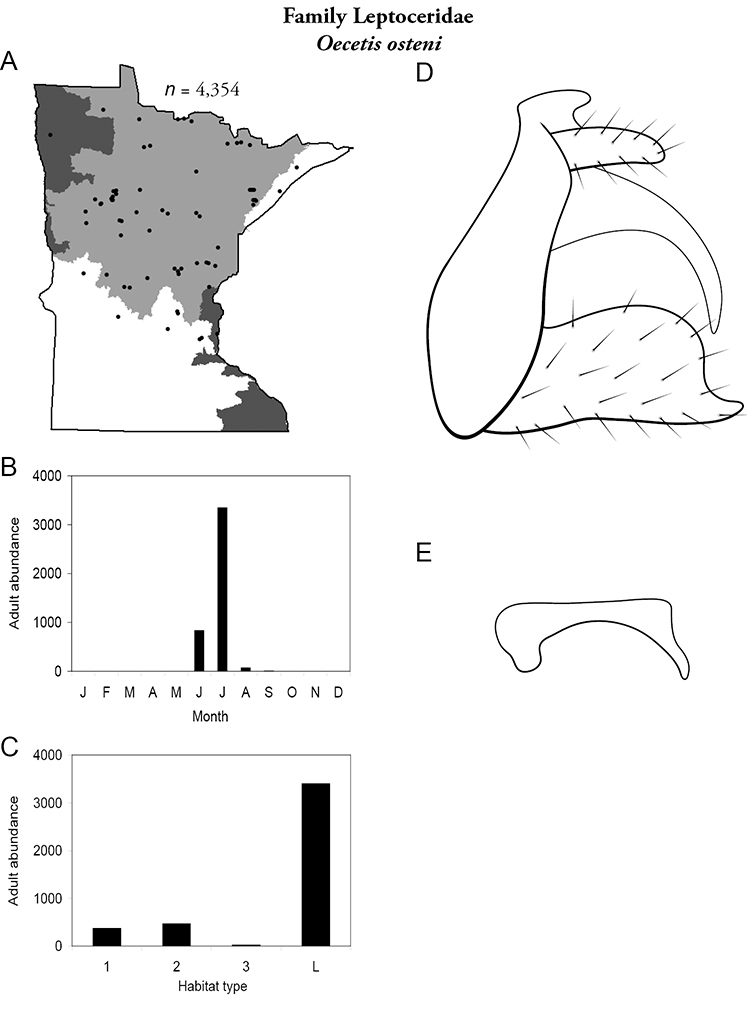
| Oecetis osteni | Leptoceridae | 7% | 163 | |
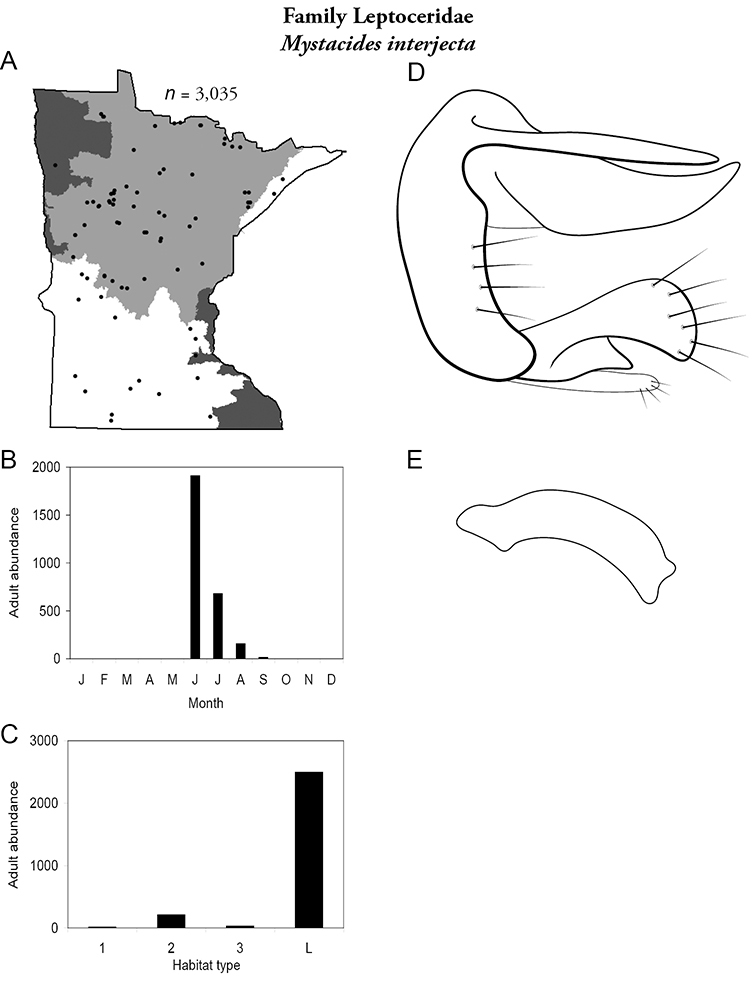
| Mystacides interjecta | Leptoceridae | 5% | 149 | |
| Ceraclea tarsipunctata | Leptoceridae | 4% | 145 | |
| Oecetis cinerascens | Leptoceridae | 3% | 156 | |
| Triaenodes injustus | Leptoceridae | 3% | 172 | |
| Molanna flavicornis | Molannidae | 3% | 228 | |
| Molanna uniophila | Molannidae | 2% | 230 | |
| Agraylea multipunctata | Hydroptilidae | 2% | 63 | |
| Ceraclea cancellata | Leptoceridae | 2% | 138 | |
| Ceraclea transversa | Leptoceridae | 1% | 146 | |
| Banksiola crotchi | Phryganeidae | 1% | 244 | |
| Oxyethira forcipata | Hydroptilidae | 1% | 109 |
Species found in ≥ 50% of respective habitats of the Northwestern Caddisfly Region (Figure 3) since 1980 and representing ≥ 1% of total specimen abundance in each habitat (Table 1). Species richness totals in Figure 11.
| Habitat | Species | Family | % of fauna | Figure |
|---|---|---|---|---|
| Small streams | Hydropsyche bidens | Hydropsychidae | 33% | 47 |
| Cheumatopsyche speciosa | Hydropsychidae | 17% | 41 | |
| Potamyia flava | Hydropsychidae | 17% | 62 | |
| Cheumatopsyche campyla | Hydropsychidae | 10% | 33 | |
| Hydropsyche simulans | Hydropsychidae | 6% | 56 | |
| Medium rivers | Potamyia flava | Hydropsychidae | 37% | 62 |
| Agraylea multipunctata | Hydroptilidae | 24% | 63 | |
| Cheumatopsyche speciosa | Hydropsychidae | 8% | 41 | |
| Oecetis inconspicua | Leptoceridae | 6% | 160 | |
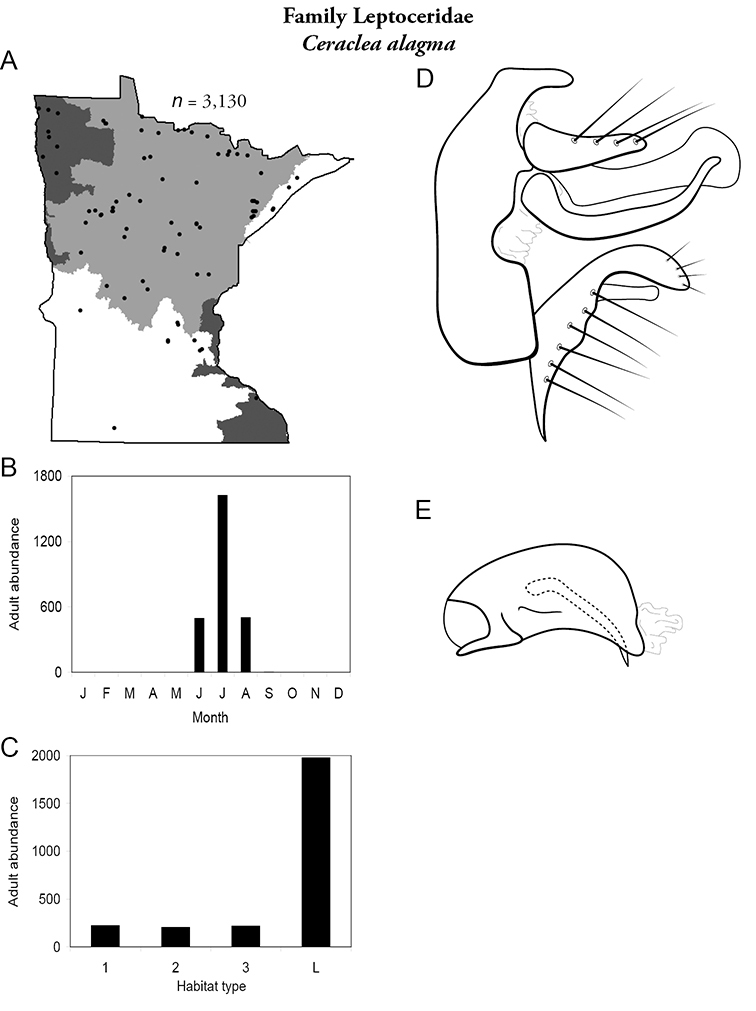
| Ceraclea alagma | Leptoceridae | 5% | 132 | |
| Cheumatopsyche campyla | Hydropsychidae | 3% | 33 | |
| Cheumatopsyche pettiti | Hydropsychidae | 2% | 39 | |
| Hydropsyche bidens | Hydropsychidae | 2% | 47 | |
| Hydropsyche simulans | Hydropsychidae | 1% | 56 | |
| Large rivers | Potamyia flava | Hydropsychidae | 32% | 62 |
| Hydropsyche confusa | Hydropsychidae | 31% | 49 | |
| Cheumatopsyche speciosa | Hydropsychidae | 14% | 41 | |
| Hydropsyche bidens | Hydropsychidae | 9% | 47 | |
| Hydropsyche simulans | Hydropsychidae | 6% | 56 | |
| Cheumatopsyche campyla | Hydropsychidae | 2% | 33 | |
| Oecetis inconspicua | Leptoceridae | 1% | 160 | |
| Lakes | Oecetis inconspicua | Leptoceridae | 85% | 160 |
| Ceraclea alagma | Leptoceridae | 3% | 132 | |
| Polycentropus cinereus | Polycentropodidae | 2% | 267 | |
| Cheumatopsyche campyla | Hydropsychidae | 1% | 33 |
Species found in ≥ 50% of respective habitats of the Southeastern Caddisfly Region (Figure 3) since 1980 and representing ≥ 1% of total specimen abundance in each habitat (Table 1). Species richness totals in Figure 11.
| Habitat | Species | Family | % of fauna | Figure |
|---|---|---|---|---|
| Small streams | Brachycentrus americanus | Brachycentridae | 38% | 16 |
| Hydropsyche alhedra | Hydropsychidae | 18% | 44 | |
| Hydropsyche slossonae | Hydropsychidae | 12% | 57 | |
| Glossosoma intermedium | Glossosomatidae | 4% | 25 | |
| Hydroptila consimilis | Hydroptilidae | 4% | 72 | |
| Micrasema gelidum | Brachycentridae | 3% | 19 | |
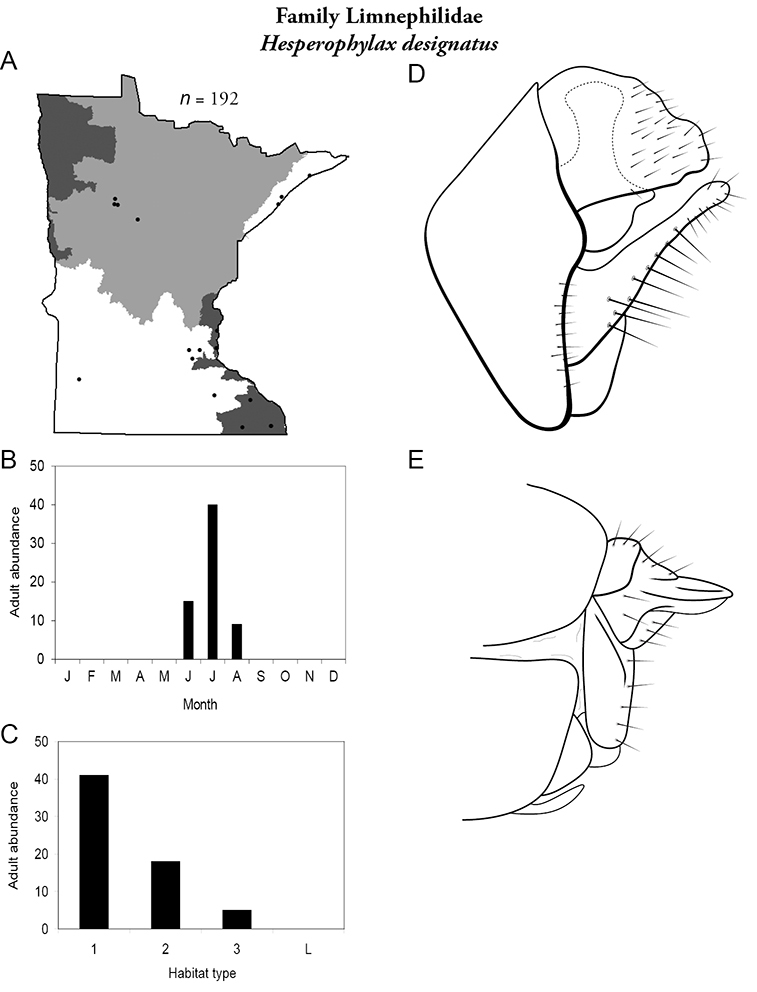
| Hesperophylax designatus | Limnephilidae | 2% | 190 | |
| Medium rivers | Ceraclea tarsipunctata | Leptoceridae | 49% | 145 |
| Brachycentrus americanus | Brachycentridae | 17% | 16 | |
| Triaenodes tarda | Leptoceridae | 9% | 176 | |
| Hydropsyche slossonae | Hydropsychidae | 5% | 57 | |
| Hydropsyche betteni | Hydropsychidae | 3% | 46 | |
| Hydropsyche alhedra | Hydropsychidae | 2% | 44 | |
| Glossosoma intermedium | Glossosomatidae | 1% | 25 | |
| Large rivers | Ceraclea tarsipunctata | Leptoceridae | 45% | 145 |
| Cheumatopsyche pasella | Hydropsychidae | 12% | 38 | |
| Hydropsyche alhedra | Hydropsychidae | 10% | 44 | |
| Potamyia flava | Hydropsychidae | 5% | 62 | |
| Hydropsyche bidens | Hydropsychidae | 5% | 47 | |
| Psychomyia flavida | Psychomyiidae | 4% | 282 | |
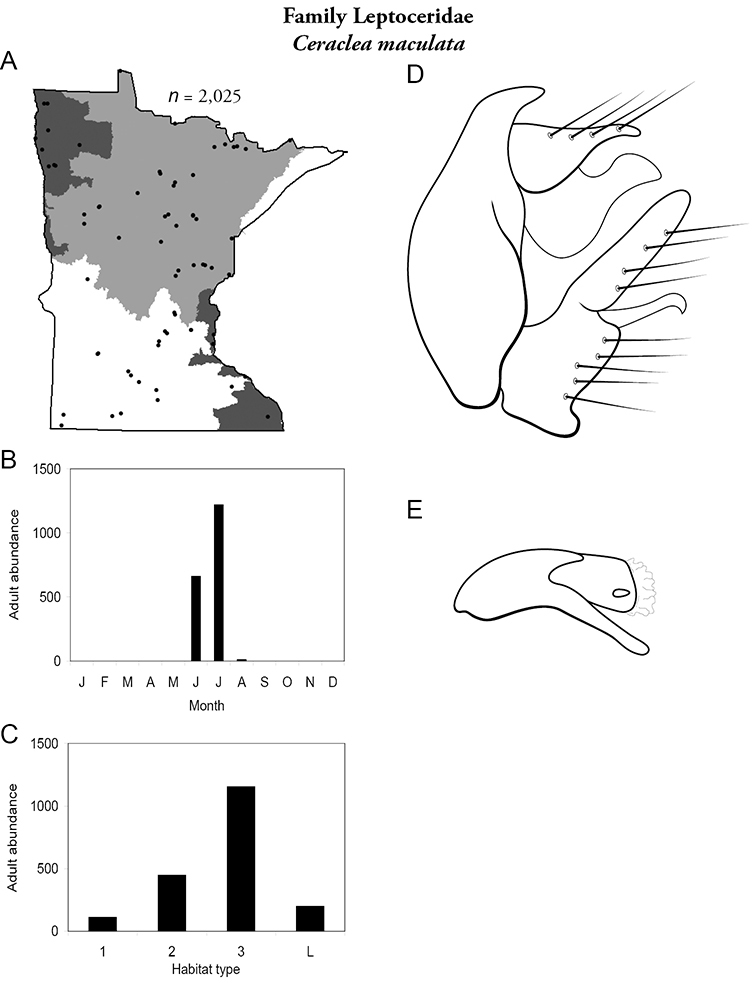
| Ceraclea maculata | Leptoceridae | 4% | 142 | |
| Leptocerus americanus | Leptoceridae | 3% | 148 | |
| Cheumatopsyche campyla | Hydropsychidae | 3% | 33 | |
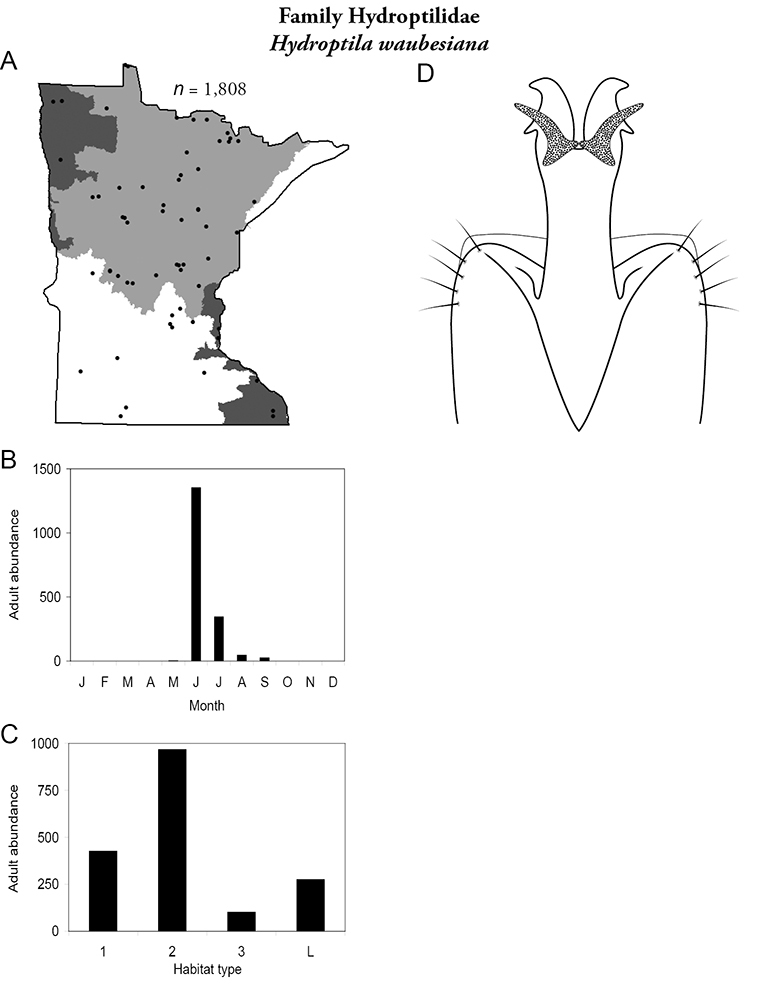
| Lakes | Hydroptila waubesiana | Hydroptilidae | 21% | 87 |
| Leptocerus americanus | Leptoceridae | 18% | 148 | |
| Ceraclea alagma | Leptoceridae | 14% | 132 | |
| Oecetis inconspicua | Leptoceridae | 14% | 160 | |
| Triaenodes tarda | Leptoceridae | 14% | 176 | |
| Polycentropus cinereus | Polycentropodidae | 7% | 267 |
Species found in ≥ 50% of respective habitats of the Southern Caddisfly Region (Figure 3) since 1980 and representing ≥ 1% of total specimen abundance in each habitat (Table 1). Species richness totals in Figure 11.
| Habitat | Species | Family | % of fauna | Figure |
|---|---|---|---|---|
| Small streams | Potamyia flava | Hydropsychidae | 28% | 62 |
| Hydropsyche simulans | Hydrpopsychidae | 14% | 56 | |
| Oecetis inconcpicua | Leptoceridae | 6% | 160 | |
| Hydropsyche morosa | Hydropsychidae | 6% | 51 | |
| Cheumatopsyche pettiti | Hydropsychidae | 4% | 39 | |
| Hydropsyche betteni | Hydropsychidae | 3% | 46 | |
| Cheumatopsyche campyla | Hydropsychidae | 2% | 33 | |
| Oecetis cinerascens | Leptoceridae | 1% | 156 | |
| Triaenodes tarda | Leptoceridae | 1% | 176 | |
| Ceraclea maculata | Leptoceridae | 1% | 142 | |
| Medium rivers | Potamyia flava | Hydropsychidae | 41% | 62 |
| Psychomyia flavida | Psychomyiidae | 18% | 282 | |
| Hydropsyche simulans | Hydropsychidae | 4% | 56 | |
| Cheumatopsyche campyla | Hydropsychidae | 3% | 33 | |
| Hydropsyche morosa | Hydropsychidae | 3% | 51 | |
| Oecetis inconcpicua | Leptoceridae | 2% | 160 | |
| Cheumatopsyche aphanta | Hydropsychidae | 2% | 32 | |
| Hydroptila consimilis | Hydropsychidae | 1% | 72 | |
| Oecetis cinerascens | Leptoceridae | 1% | 156 | |
| Large rivers | Potamyia flava | Hydropsychidae | 31% | 62 |
| Ceraclea tarsipunctata | Leptoceridae | 2% | 145 | |
| Hydropsyche simulans | Hydropsychidae | 1% | 56 | |
| Psychomyia flavida | Psychomyiidae | 1% | 282 | |
| Oecetis inconspicua | Leptoceridae | 1% | 160 | |
| Hydropsyche morosa | Hydropsychidae | 1% | 51 | |
| Oecetis avara | Leptoceridae | 1% | 155 | |
| Cheumatopsyche campyla | Hydropsychidae | 1% | 33 | |
| Oecetis cinerascens | Leptoceridae | 1% | 156 | |
| Agraylea multipunctata | Hydroptilidae | 1% | 63 | |
| Lakes | Leptocerus americanus | Leptoceridae | 30% | 148 |
| Potamyia flava | Hydropsychidae | 19% | 62 | |
| Mystacides interjecta | Leptoceridae | 7% | 150 | |
| Oecetis inconspicua | Leptoceridae | 6% | 160 | |
| Polycentropus cinereus | Polycentropodidae | 5% | 267 | |
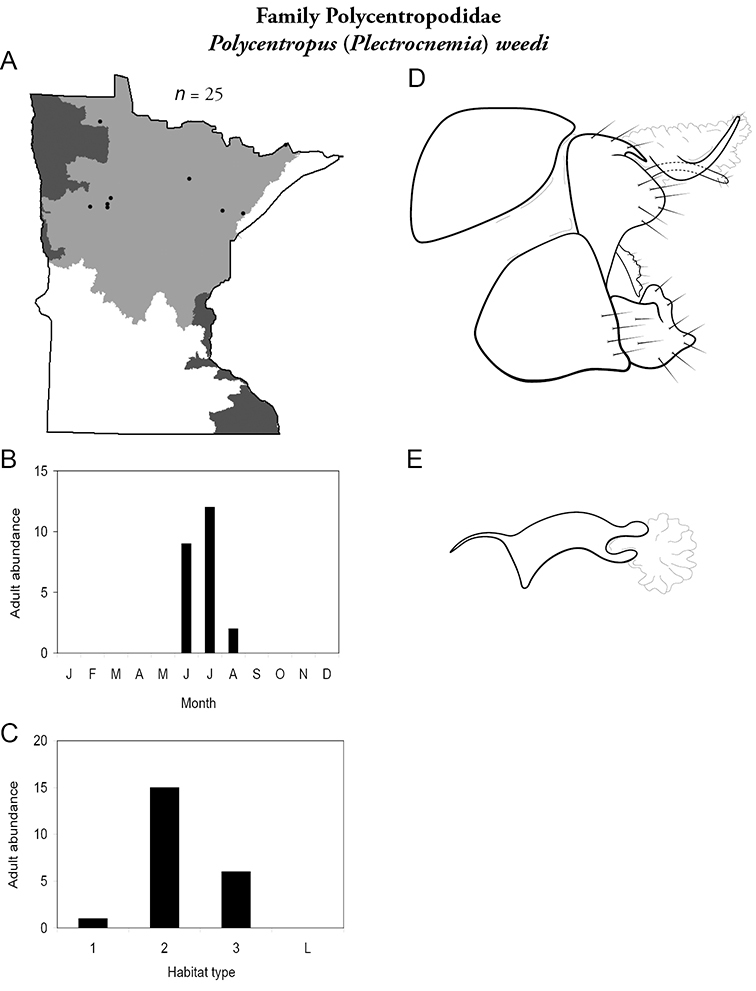
| Ceraclea tarsipunctata | Leptoceridae | 3% | 145 | |
| Oecetis cinerascens | Leptoceridae | 3% | 156 | |
| Agraylea multipunctata | Hydrpoptilidae | 2% | 63 |
The caddisfly families of Minnesota are ranked below based on their likelihood of being encountered by a general collector. The most useful diagnostic characters for each family are also listed, as well as general distribution and abundance information, and how to differentiate each family from similar families.
1. Leptoceridae. Forewing length 8–20 mm; usually light brown in color with darker blotches or reticulations; some species bright white or jet black. Antennae >2× length of body. Ocelli absent (Figure 13a). Common, widespread, and abundant throughout state; abundances can be extreme near any habitat type.
Similar families: two species of Hydropsychidae have antennae >2× body length, but have maxillary palpi 2× as long as preceding segment, flexible, and usually curved (Figure 13h). Antennal length is otherwise diagnostic, assuming they are intact.
2. Hydropsychidae. Forewing length 5–18 mm; usually light brown in color with darker reticulations; sometimes uniformly brown or straw-colored. Terminal segment of maxillary palpi 2× as long as preceding segment, flexible, and usually curved (Figure 13h). Ocelli absent (Figure 13a). Widespread, common, and abundant throughout state; abundances can be extreme near lotic habitats.
Similar families: Arctopsychidae is only found in small tributaries of Saint Croix River. Polycentropodidae has similar mouthparts, but with terminal segment of maxillary palpi not as long or curved (Figure 13i). Philopotamidae has similar mouthparts, but is darker in color and has ocelli (Figure 13b).
3. Hydroptilidae. Forewing length 2–5 mm; usually light brown in color; sometimes with darker reticulations. Forewings tapering to point and usually covered with dense setae (Figure 13e). Hindwings also tapering to point and with dense fringe of setae on posterior margin (Figure 13f). Ocelli present (Figure 13b). Widespread and abundant throughout state.
Similar families: some species of Glossosomatidae, Psychomyiidae, or Polycentropodidae may overlap in size. All of these families have forewings widening towards apex, not tapering (Figure 13c).
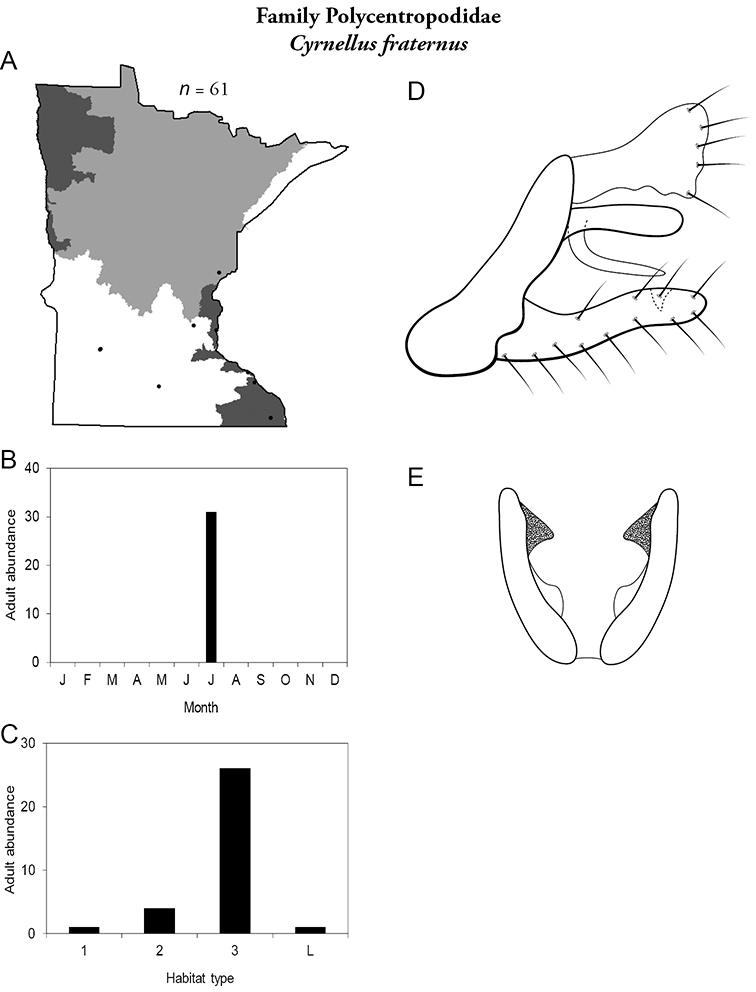
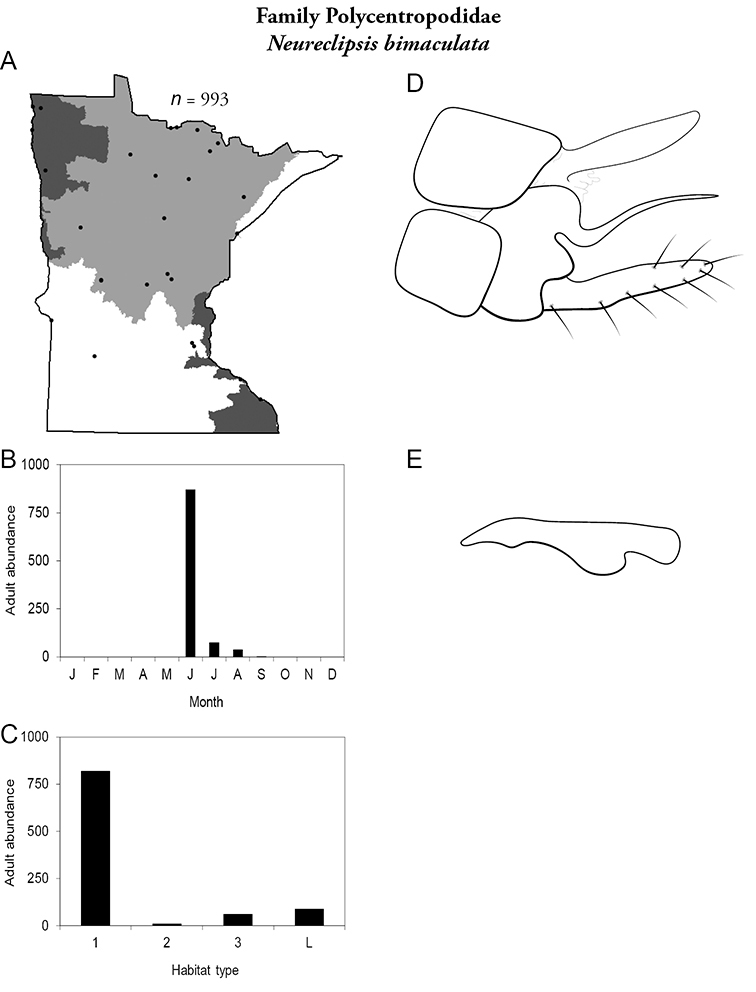
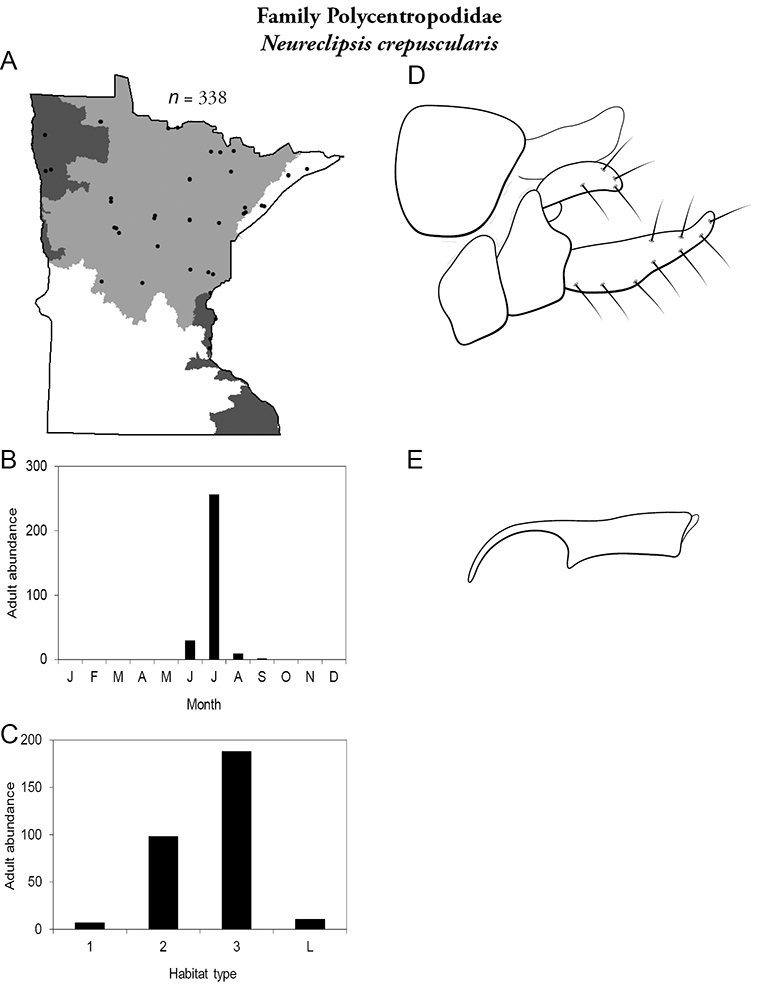
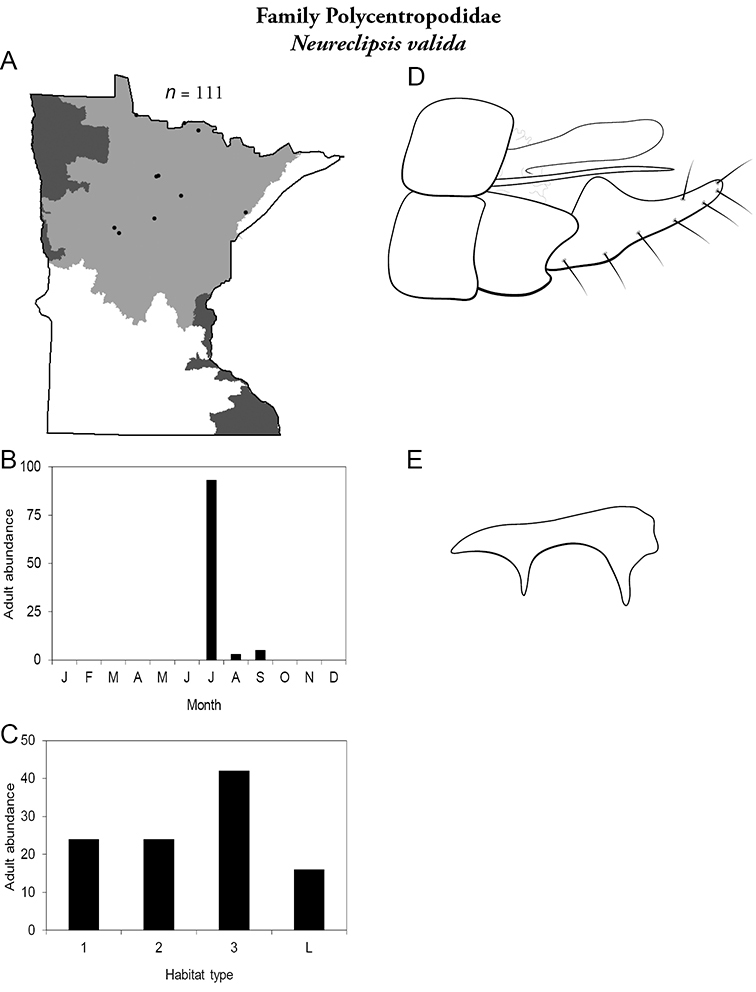
4. Polycentropodidae. Forewing length 5–12 mm; usually drab brown or grey in color; sometimes with darker reticulations. Ocelli absent (Figure 13a). Terminal segment of maxillary palpi 2× as long as preceding segment (Figure 13i). Widespread and abundant throughout state.
Similar families: Hydropsychidae has similar mouthparts, but with terminal segment of maxillary palpi longer and more flexible (Figure 13h). Philopotamidae has similar mouthparts, but is darker in color and has ocelli (Figure 13b). Hydroptilidae is almost always smaller and with forewings tapering to point (Figure 13e). Dipseudopsidae has R2 of wings branching from R3 near radial crossvein (Figure 13g), and is found only in northeastern MN. Glossosomatidae and Psychomyiidae may overlap in size, but don’t have long terminal segment of maxillary palpi.
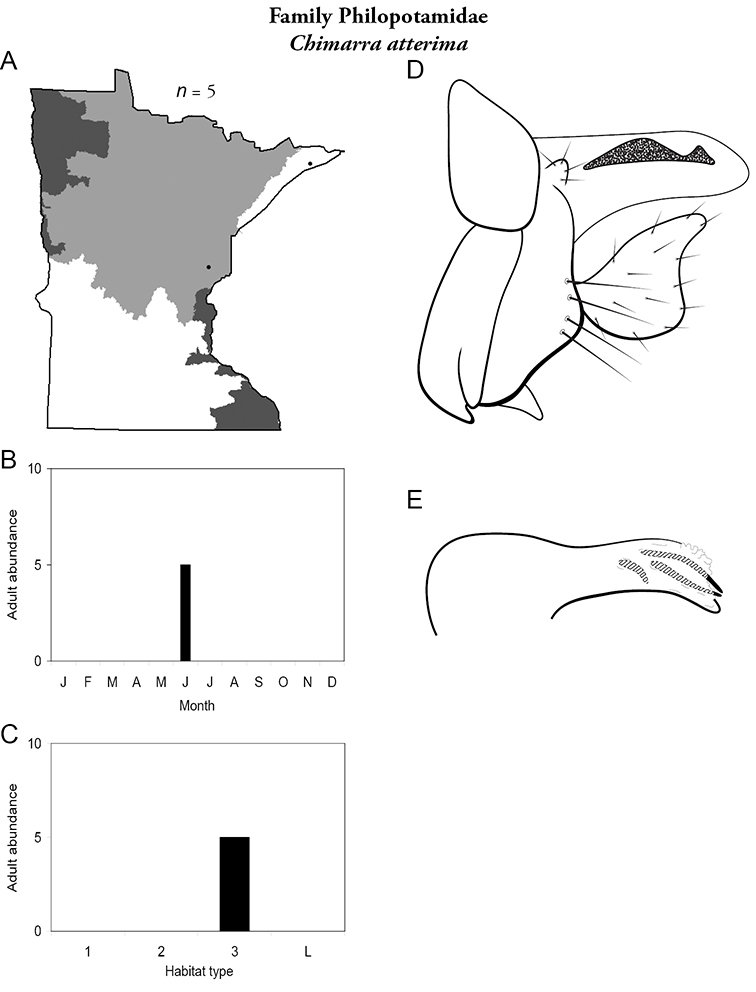
5. Philopotamidae. Forewing length 8–10 mm; black or dark brown in color. Terminal maxillary segment of maxillary palpi 2× as long as preceding segment (Figure 13i). Ocelli present (Figure 13b). Widespread throughout state; can be extremely abundant near lotic habitats.
Similar families: Hydropsychidae, Dipseudopsidae, and Polycentropodidae all have similar mouthparts but are lighter in color and lack ocelli (Figure 13a).
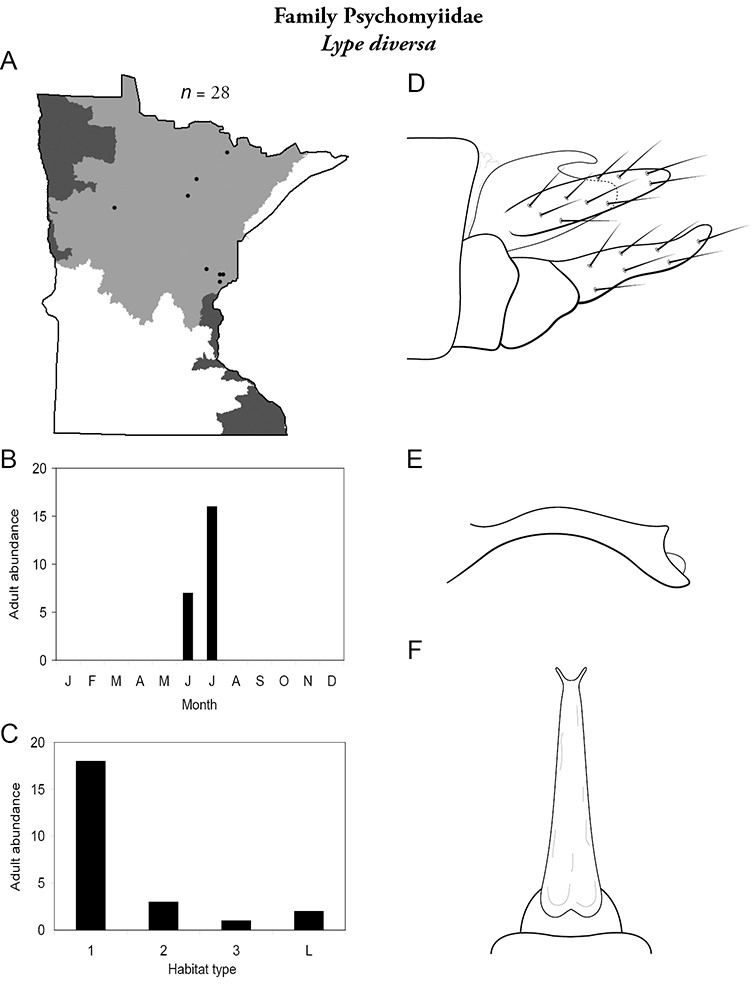
6. Psychomyiidae. Forewing length 4–6 mm; black or dark brown in color. Ocelli absent (Figure 13b). Widespread and abundant throughout state; abundances can be extreme near lotic habitats. Females are far more abundant than males.
Similar families: Glossosomatidae and Hydroptilidae frequently overlap in size, but have ocelli (Figure 13a). Polycentropodidae occasionally overlaps in size, but has elongate terminal segment of maxillary palpi (Figure 13i).
7. Helicopsychidae. Forewing length 6–8 mm; light brown in color. Scape >2× as long as pedicel. Rear of head with large quadrate setal warts (Figure 13a). Ocelli absent. Widespread through state; can be abundant near lotic habitats.
Similar families: superficially resembles the Lepidostomatidae and Sericostomatidae, which lack the enlarged quadrate posterior head warts.
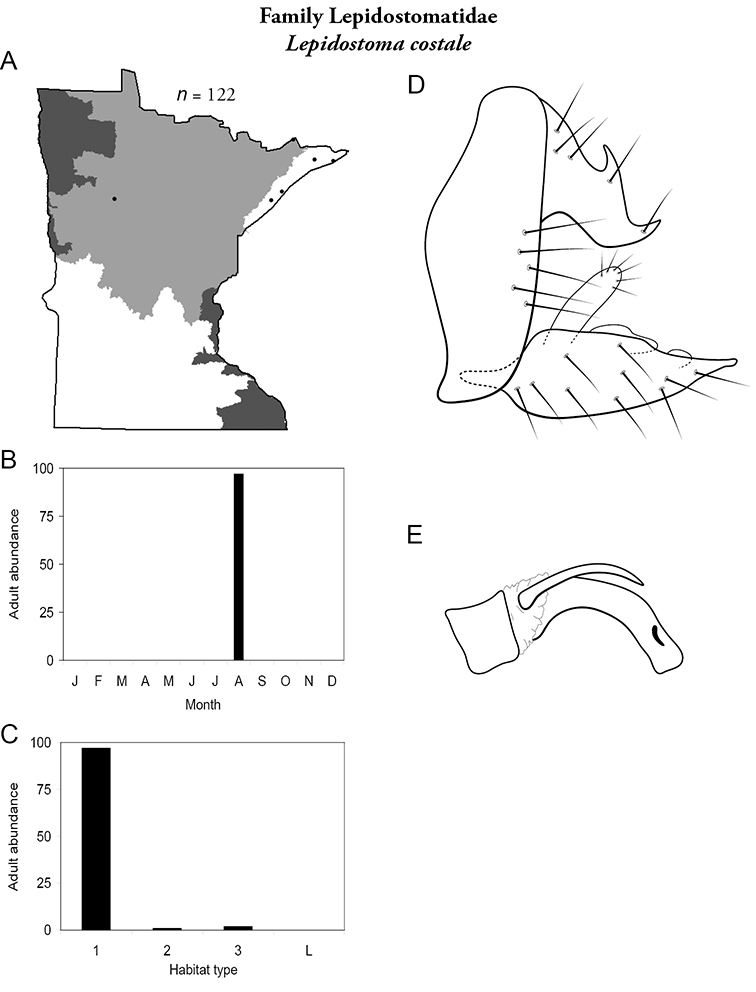
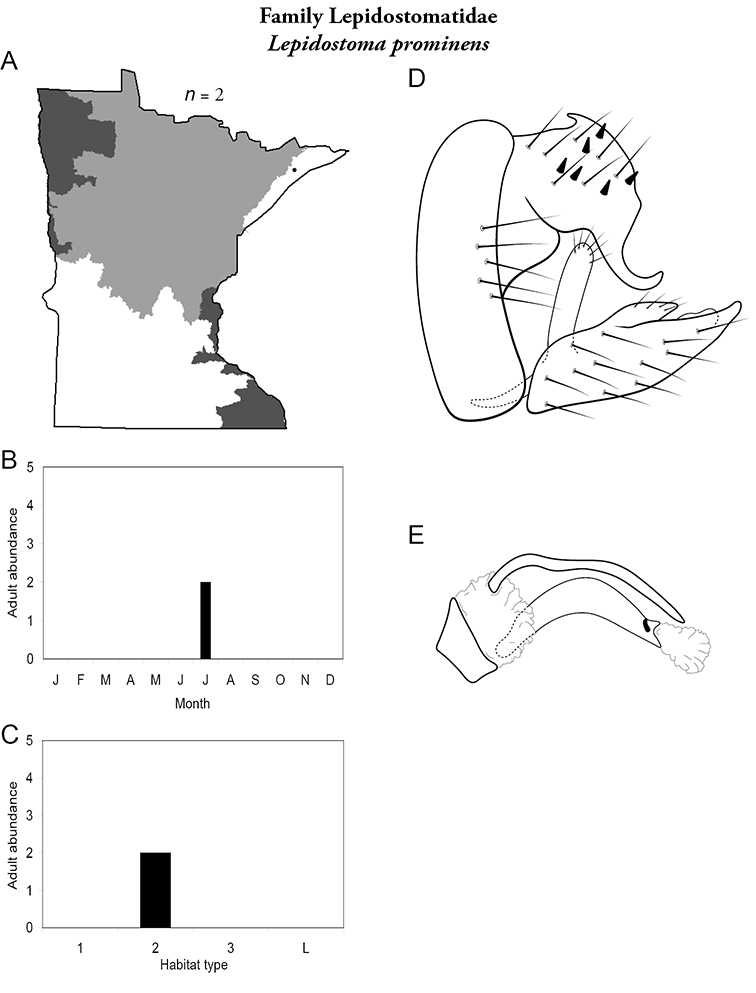
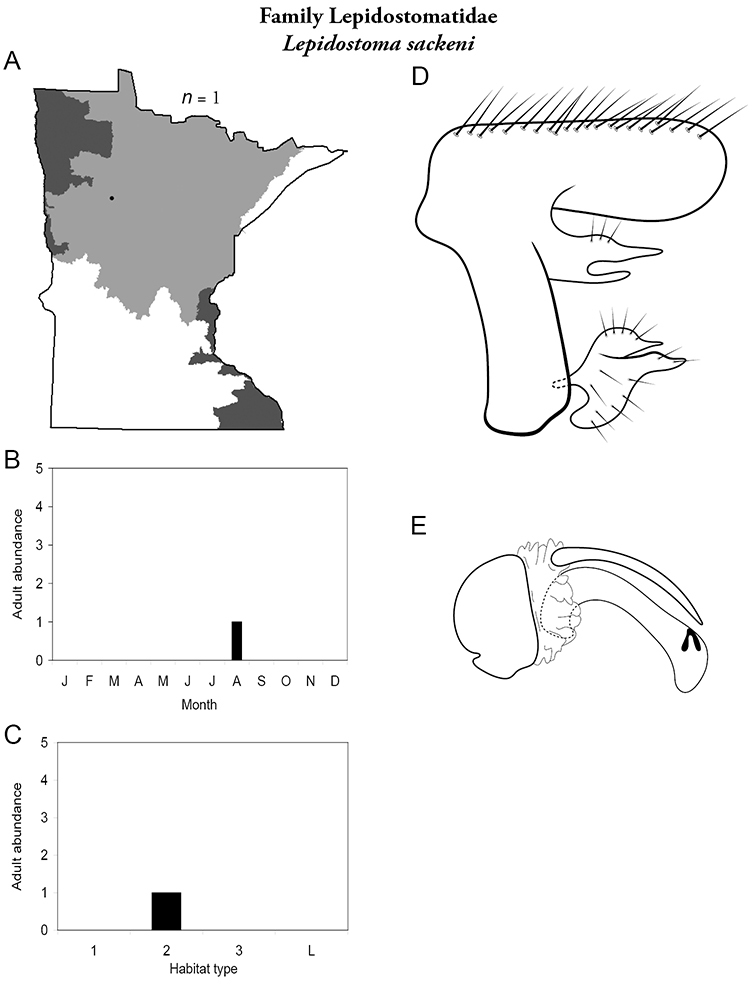
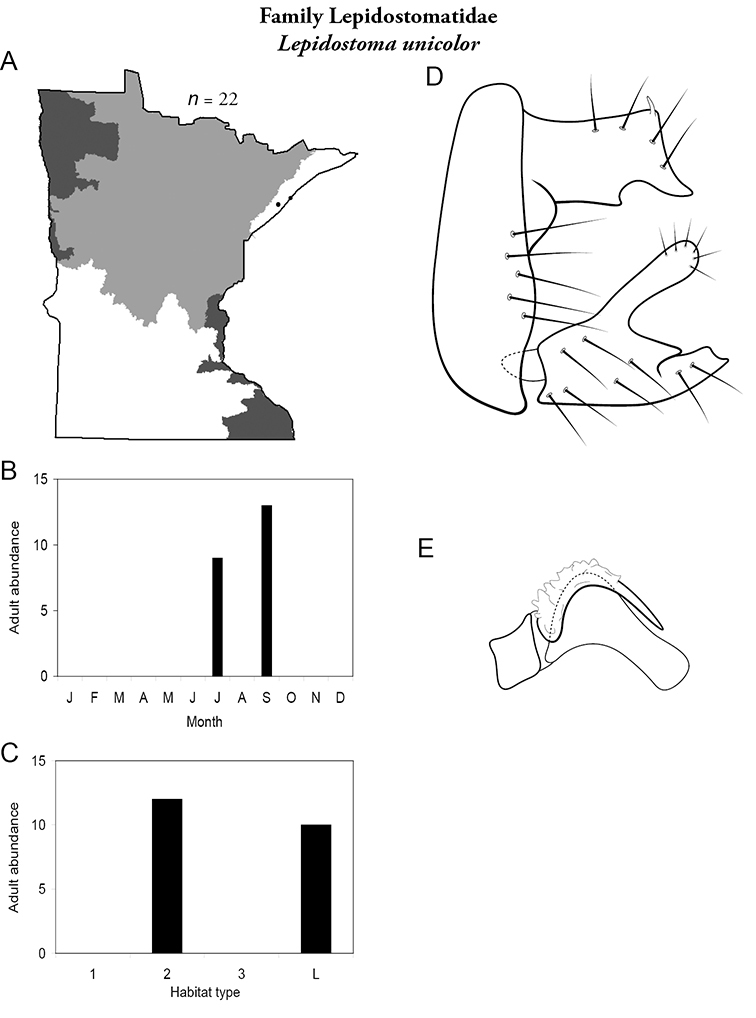
8. Lepidostomatidae. Forewing length 8–10 mm; light brown in color. Scape >2× as long as pedicel (Figure 13a). Ocelli absent. Widespread and abundant throughout Lake Superior, Northern, and Southeastern Regions.
Similar families: superficially resembles the Brachycentridae, but has scape >2× as long as pedicel. Similar to Helicopsychidae, but without quadrate posterior setal warts on head.
9. Limnephilidae. Forewing length 12–40 mm; usually light brown with darker reticulations, but can be nearly any color. Ocelli present (Figure 13b). Each middle tibia with 0 or 1 pre-apical spurs. Widespread throughout state but usually not abundant.
Similar families; frequently confused with the Phryganeidae due to large size; can be differentiated by the number of preapical spurs on middle tibiae.
10. Phryganeidae. Forewing length 10–35 mm; usually brown, grey or dark orange with dark reticulations. Ocelli present (Figure 13a). Each middle tibia with 2 pre-apical spurs (Figure 13k). Found throughout state, but abundant only in northern MN.
Similar families; frequently confused with the Limnephilidae due to large size; can be differentiated by the number of preapical spurs on middle tibiae.
11. Molannidae. Forewing length 8–12 mm; black or dark grey in color. Elongate body. Widespread throughout state. Scape >2× as long as pedicel (Figure 13a). Ocelli absent. Widespread throughout state but usually not abundant.
Similar families: Color and body shape are distinctive. Superficially resemble the Leptoceridae, but have antennae <2× length of body.
12. Glossosomatidae. Forewing length 4–8 mm; black or dark brown in color. Second segment of maxillary palpi globose in shape (Figure 13j). Ocelli present (Figure 13b). Widespread throughout state; abundant only in Southeastern Region.
Similar families: Rhyacophilidae has similar mouthparts, but is larger and confined to the Lake Superior Region. Hydroptilidae may overlap in size, but has forewings tapering to a point (Figure 13e)
13. Brachycentridae. Forewing length 5–12 mm; dark grey to black in color; often with light spots near apical margins. Ocelli absent (Figure 13b). Widespread throughout state and locally abundant.
Similar families: superficially resemble Lepidostomatidae and Sericostomatidae, but have scape <3× length of pedicel.
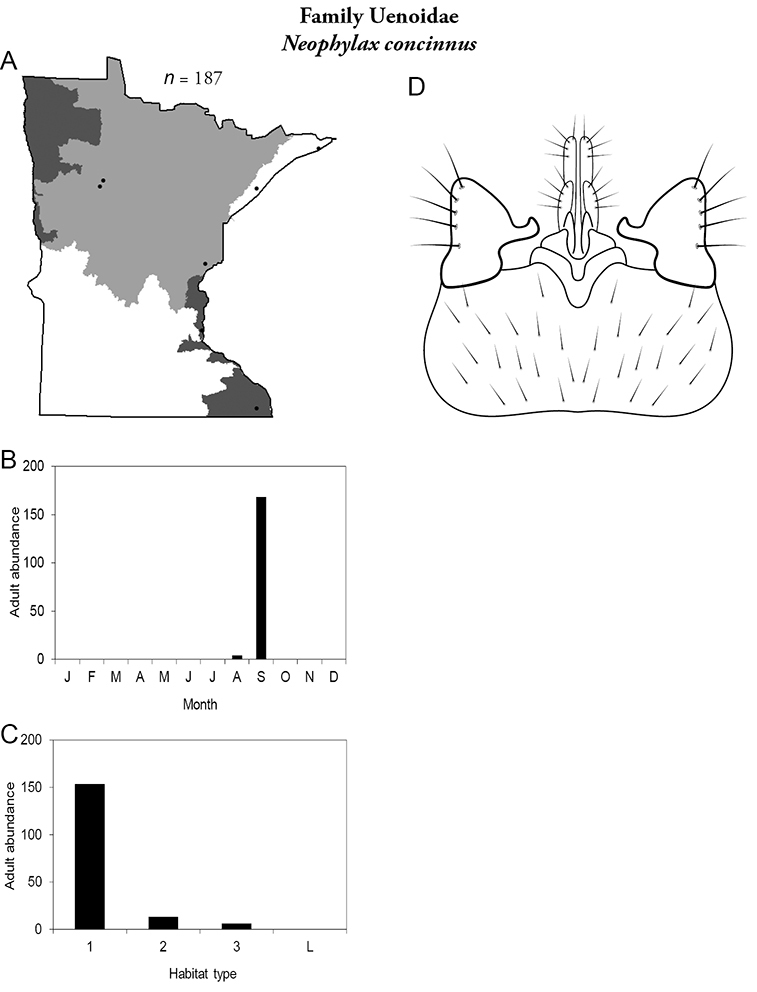
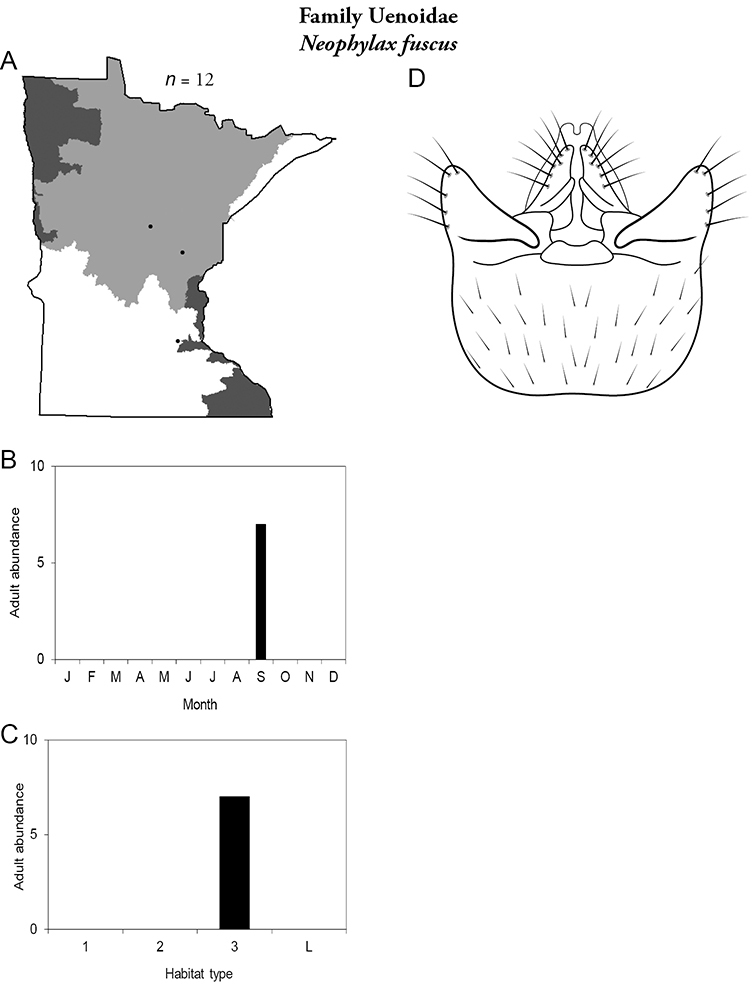
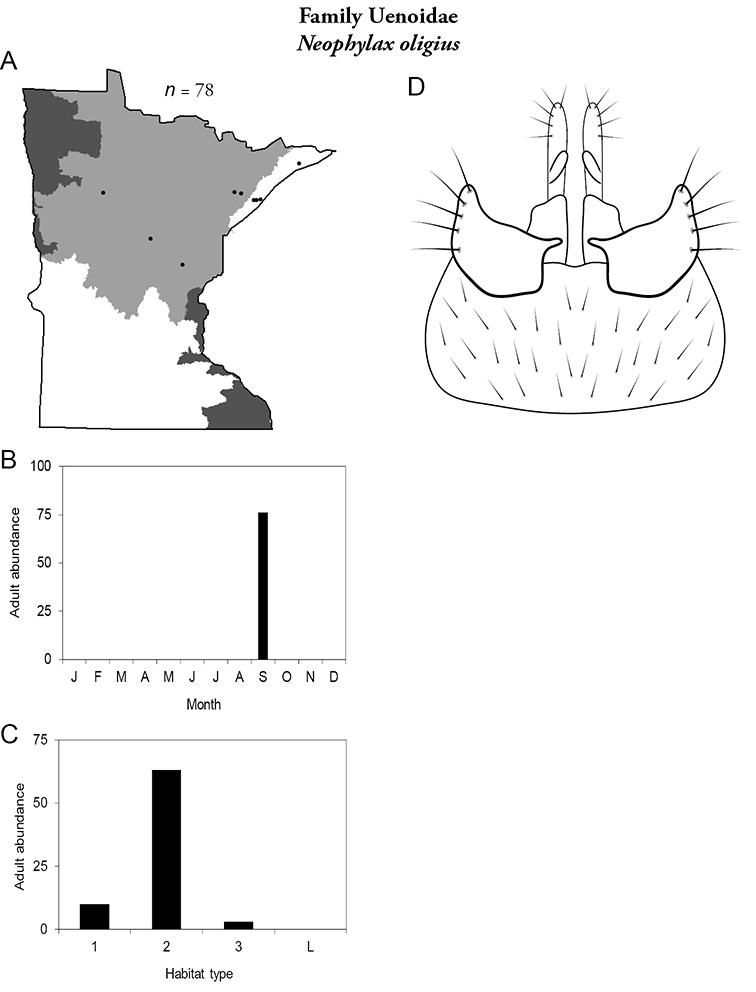
14. Uenoidae. Forewing length 8–10 mm; dark brown in color; sometimes with bright orange patches and reticulations. Ocelli present (Figure 13a). Widespread throughout Lake Superior, Northern, and Southeastern Regions. Only collected from mid-August to mid-October.
Similar families: superficially resembles Limnephilidae and Phryganeidae, but is smaller, usually with bright orange patches on forewings, and only emerges in the fall. Also superficially resembles Apataniidae, which is uniformly light brown and found only along the North Shore of Lake Superior.
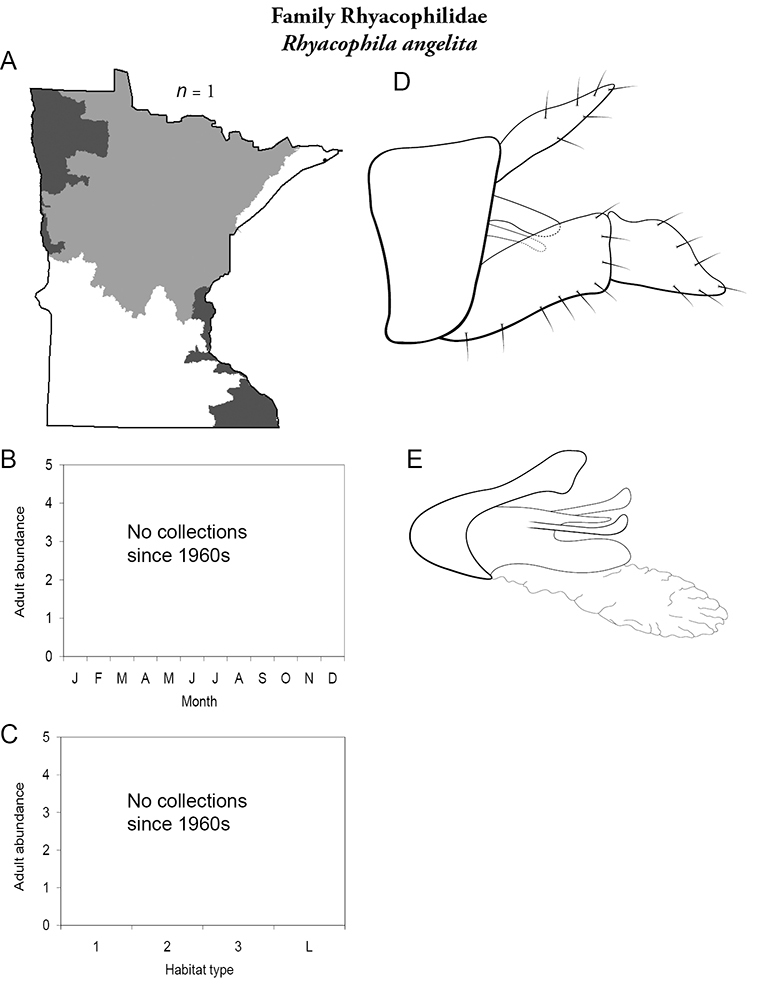
15. Rhyacophilidae. Forewing length 14–18 mm; black or dark brown in color without notable patterning. Second segment of maxillary palpi globose in shape (Figure 13j). Ocelli present (Figure 13a). Found only throughout Lake Superior Region.
Similar families: Glossosomatidae has similar mouthparts, but is smaller.
16. Dipseudopsidae. Forewing length 10–12 mm; drab brown in color with faint darker reticulations. Ocelli absent (Figure 13a). Found only in northeastern MN. Rarely abundant.
Similar families: Polycentropodidae is much more common and has R2 and R3 of wings either unbranched or else branching near wing margin (Figure 13c).
The following families are unlikely to be collected by a general collector.
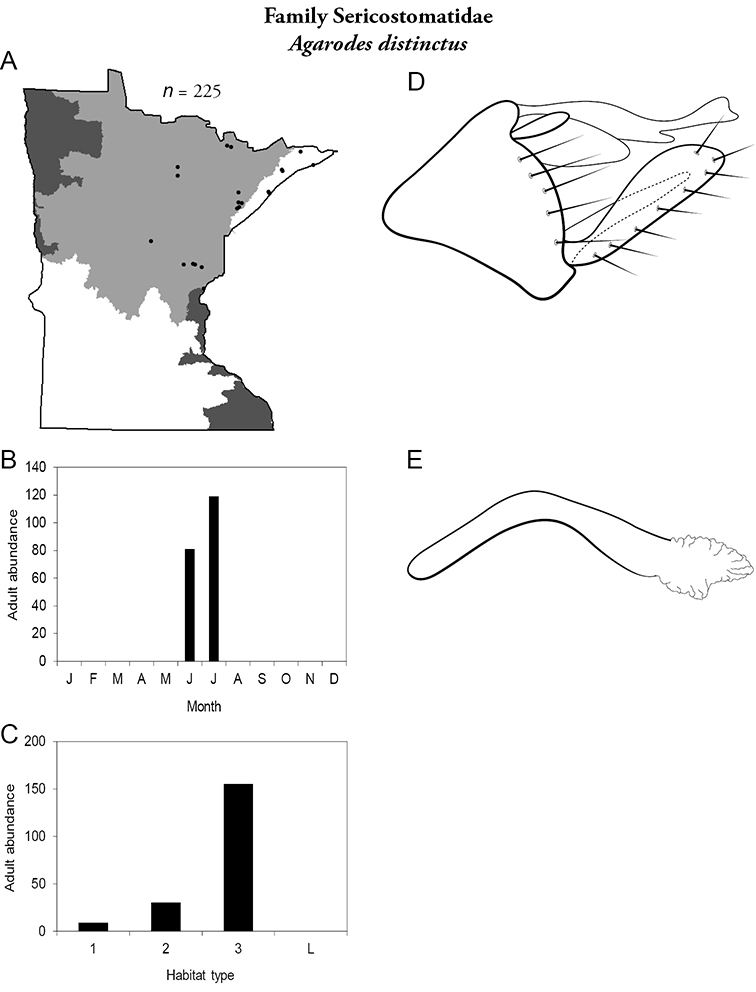
17. Sericostomatidae. Forewing length 8–12 mm; grey in color. Scape >2× as long as pedicel (Figure 13a). Ocelli absent. Found only in the northeastern portion of the state and rarely abundant.
Similar families: superficially resembles Lepidostomatidae.
18. Apataniidae. Forewing length 6–8 mm; light brown in color. Ocelli present (Figure 13b). Found only along north shore of Lake Superior.
Similar families: superficially resembles Uenoidae and Limnephilidae.
19. Arctopsychidae. Forewing length 10–12 mm; grey in color with darker reticulations. Terminal segment of maxillary palpi long and flexible, usually curved (Figure 13h). Ocelli absent (Figure 13a). Found only in small tributaries of the Saint Croix River.
Similar families: very similar to Hydropsychidae.
20. Goeridae. Forewing length 6–8 mm; light brown in color. Found only near Lake Itasca State Park. Ocelli absent (Figure 13a).
Similar families: superficially resembles the Lepidostomatidae.
21. Odontoceridae. Forewing length 6–8 mm; dark brown in color. Elongate body. Ocelli absent (Figure 13a). Found only in extreme northeastern MN.
Similar families: may superficially resemble the Molannidae.
Adult caddisflies A head of Helicopsyche borealis B head of Limnephilus canadensis C forewing of Polycentropus interruptus D hindwing of Polycentropus interruptus E forewing of of Hydroptila consimilis F hindwing of Hydroptila consimilis G forewing of Phylocentropus placidus H maxillary palp of Hydropsyche simulans I maxillary palp of Polycentropus interruptus J maxillary palpi of Rhyacophila fuscula K foreleg of Banksiola crotchi. Abbreviations: PaS = preapical spur, PHW: posterior head warts, Pd = pedicel, Oc: ocellus, R = radial vein, r = radial crossvein, Sc = scape.
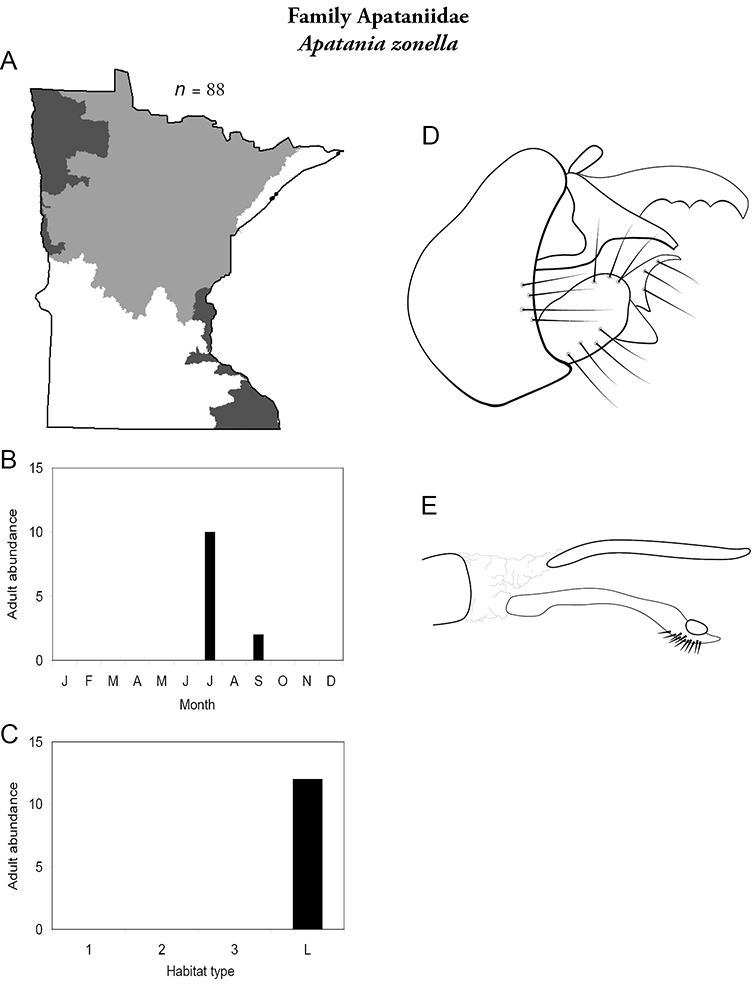
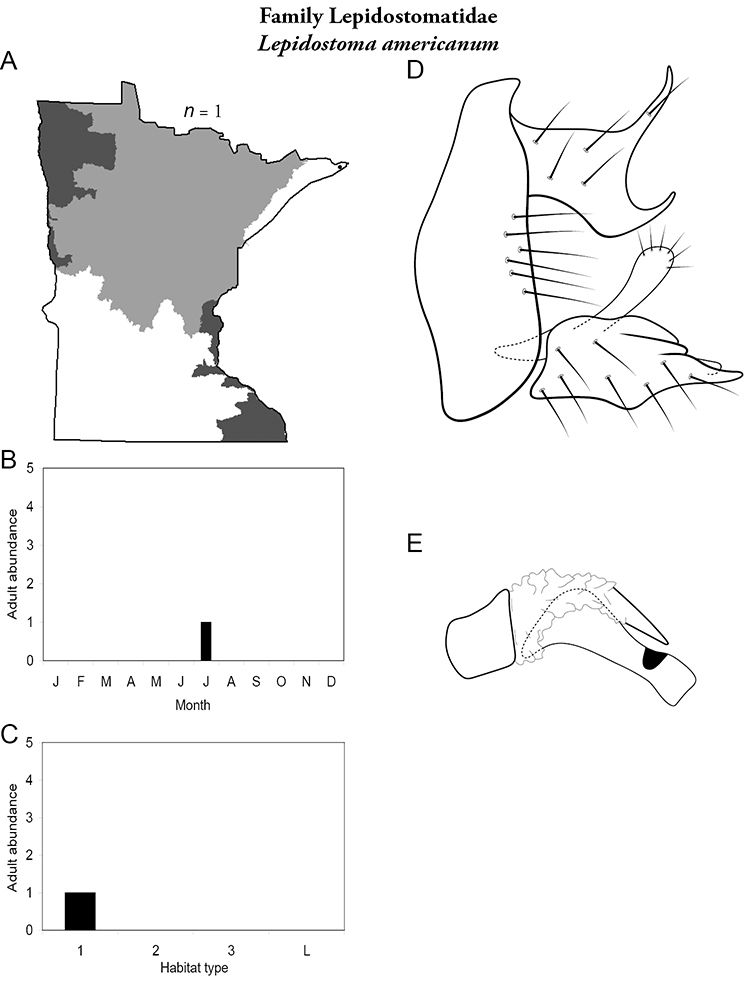
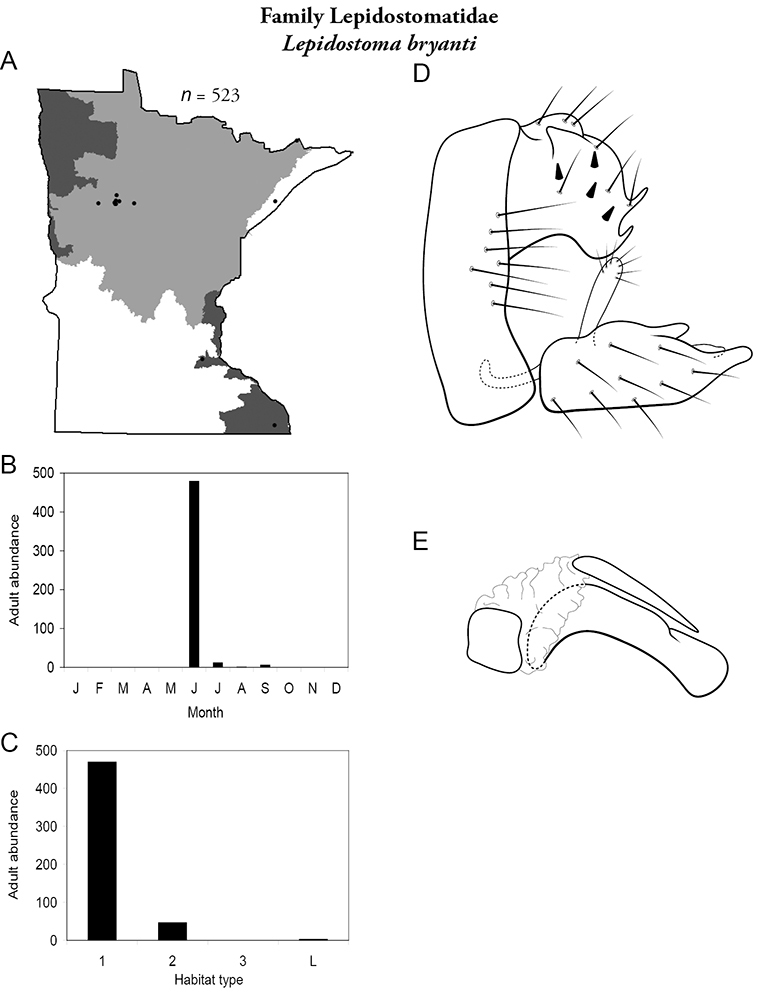
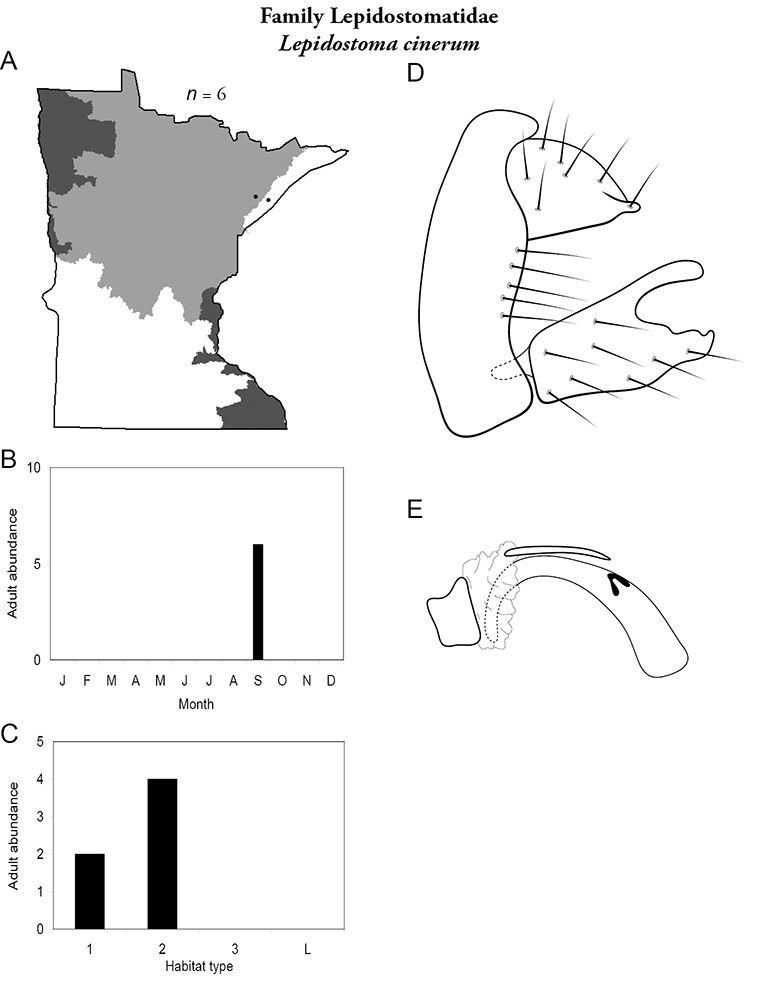
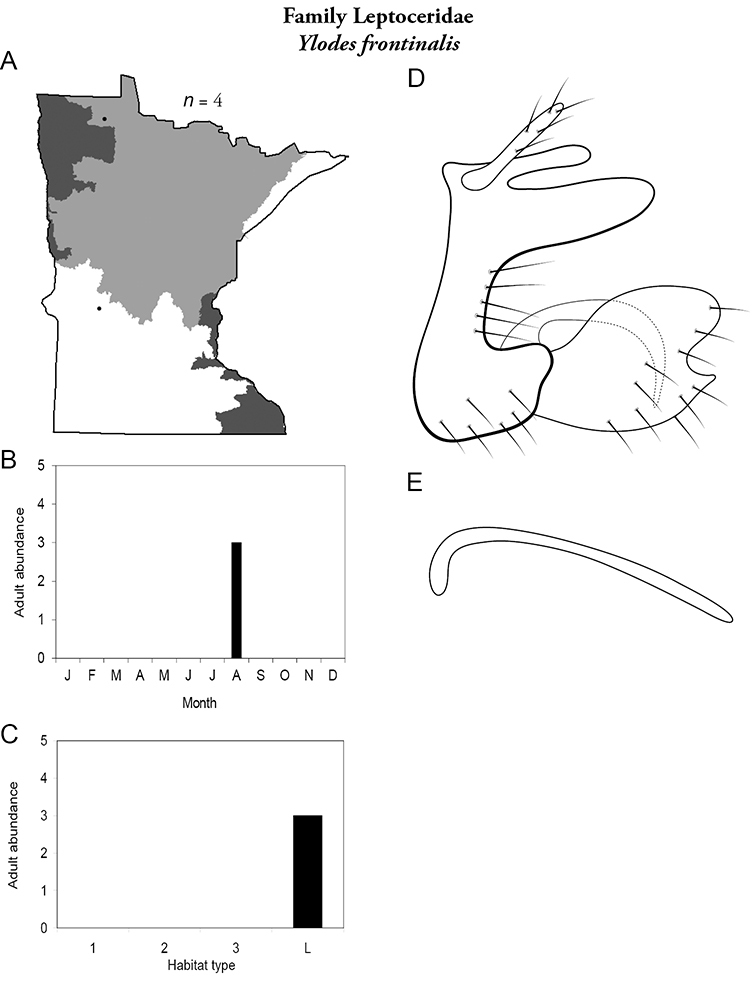
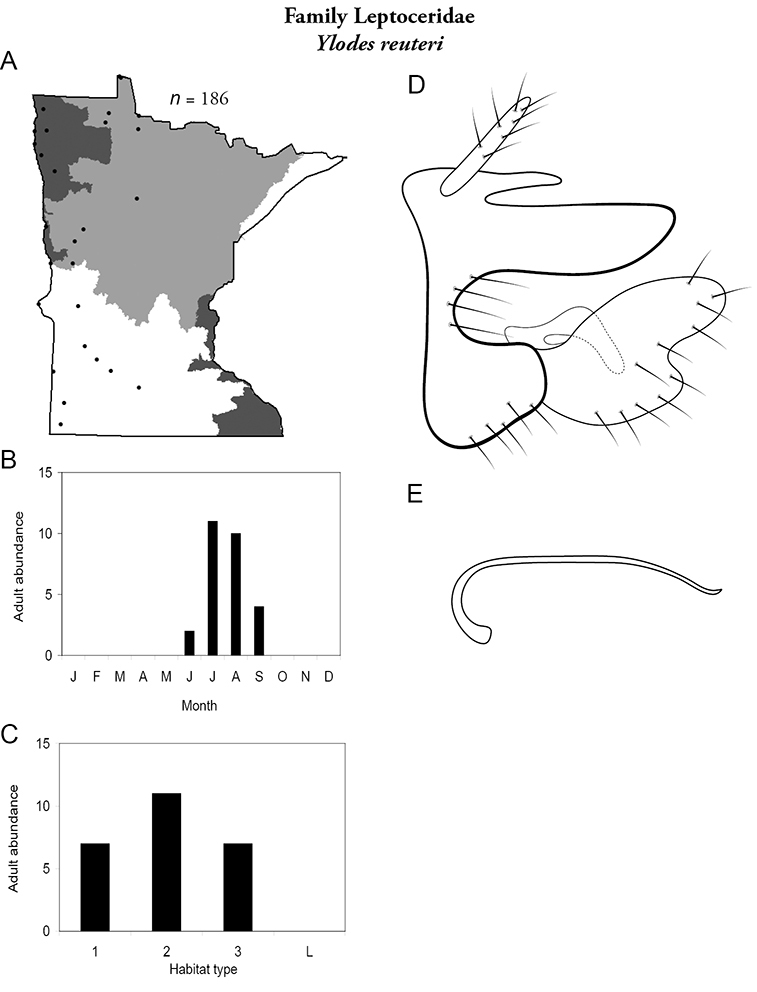
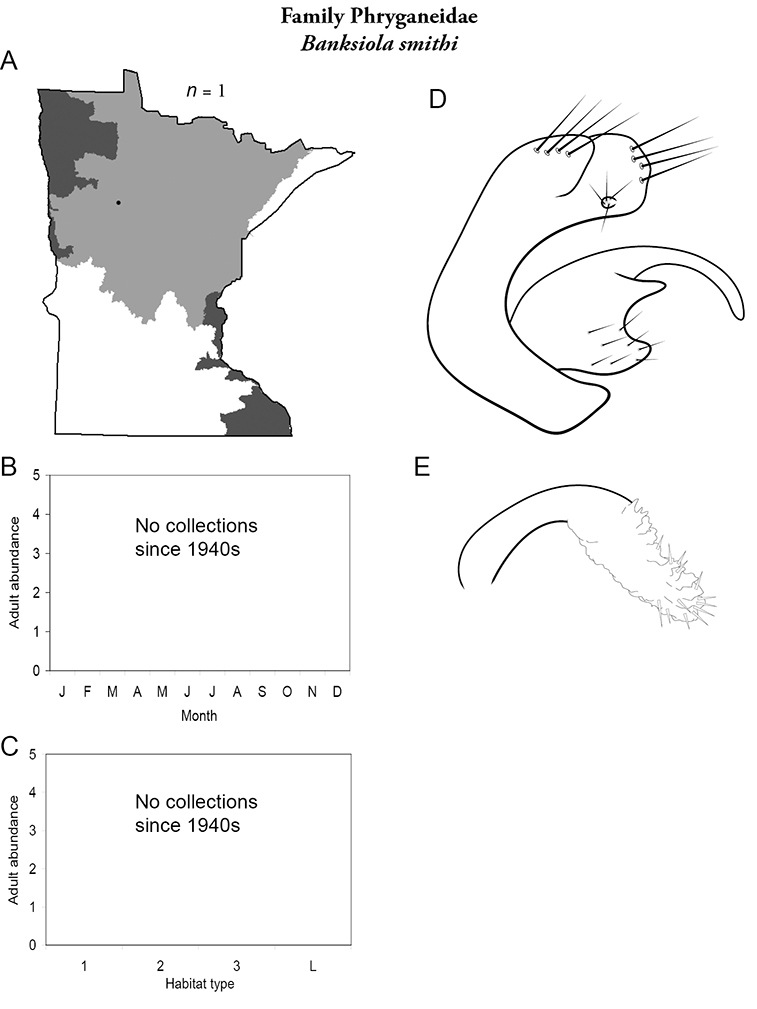
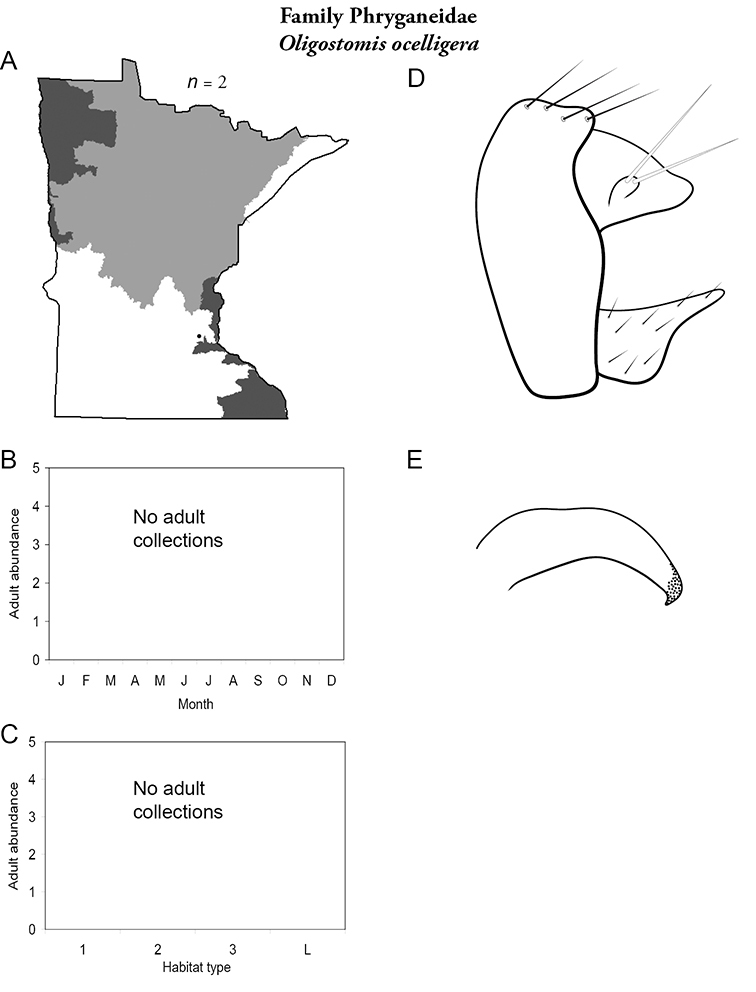
This family contains a single genus in Minnesota, Apatania, and a single species. For additional species, see
Apatania zonella (Figure 14) has been found only along the north shore of Lake Superior during July and September. Adults were abundant along riparian rocks and vegetation during the day; they were not attracted to lights at night. The apparent bivoltine adult periodicity is probably spurious, and instead likely reflects a lack of collecting during August.
Another Apatania species, Apatania incerta was reported from northeastern Minnesota based on a female specimen (
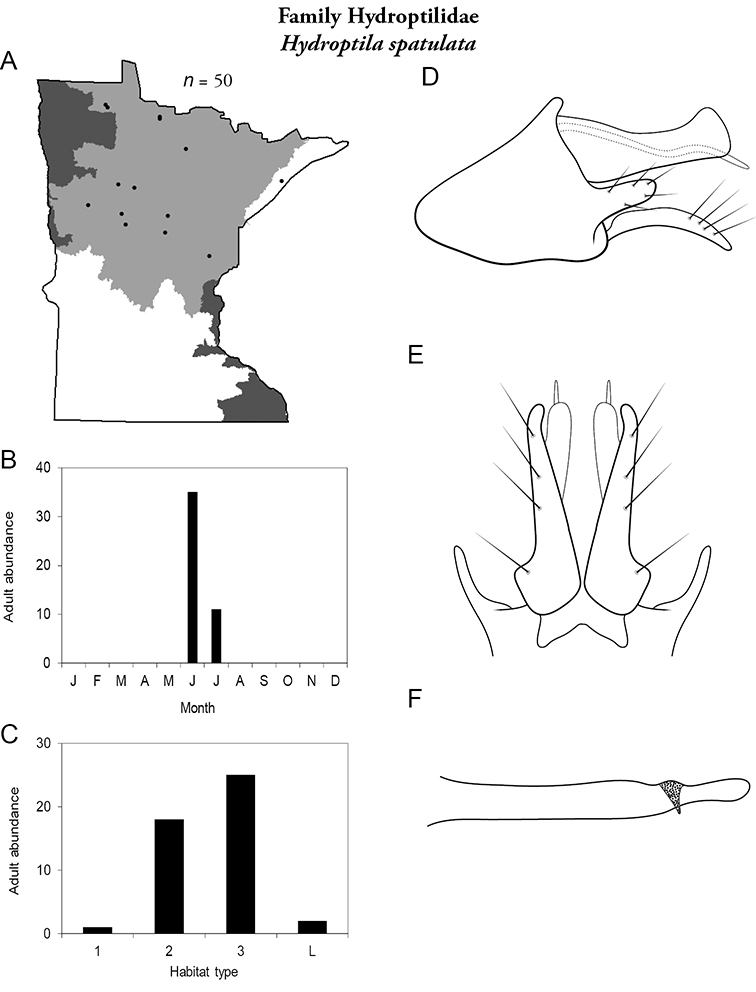
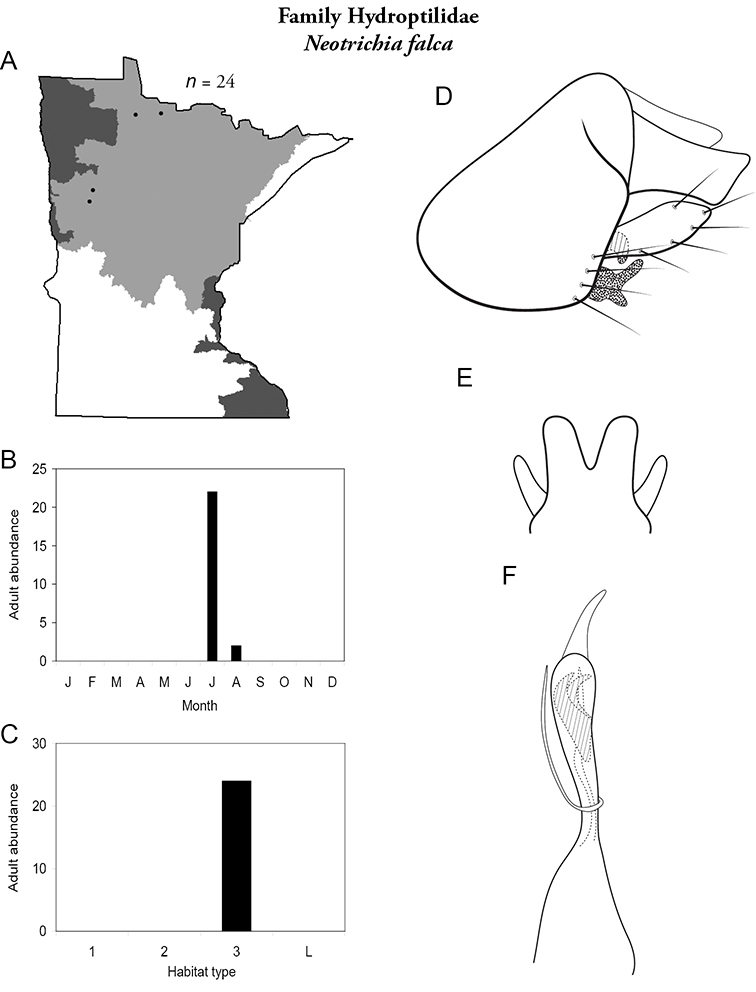
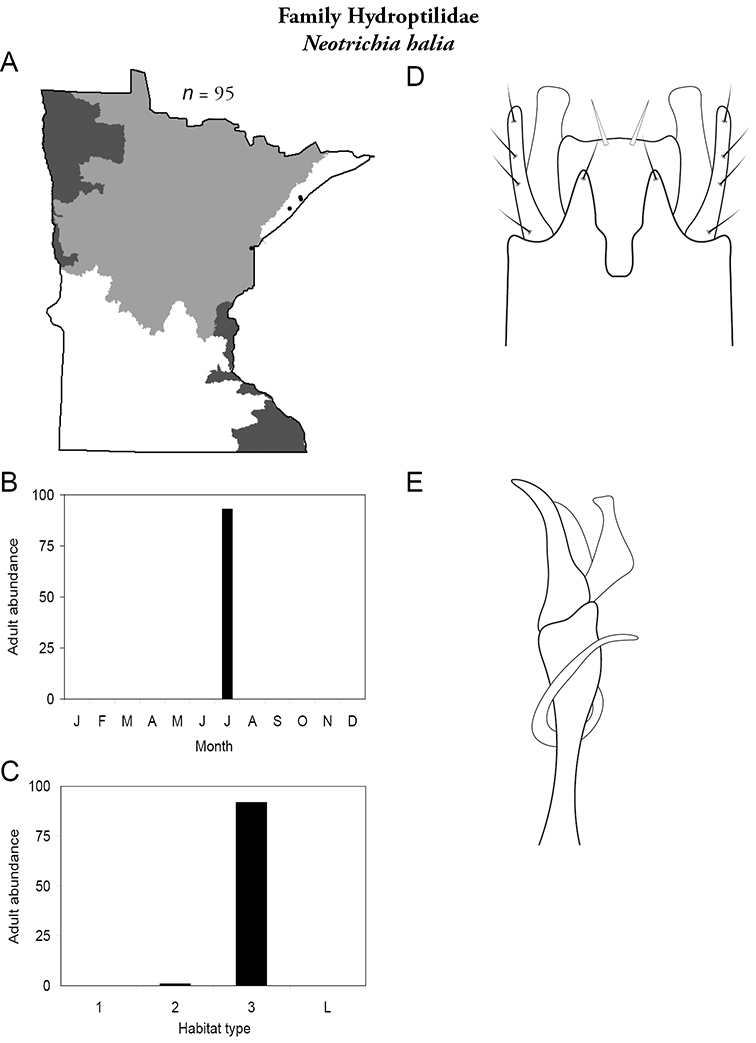
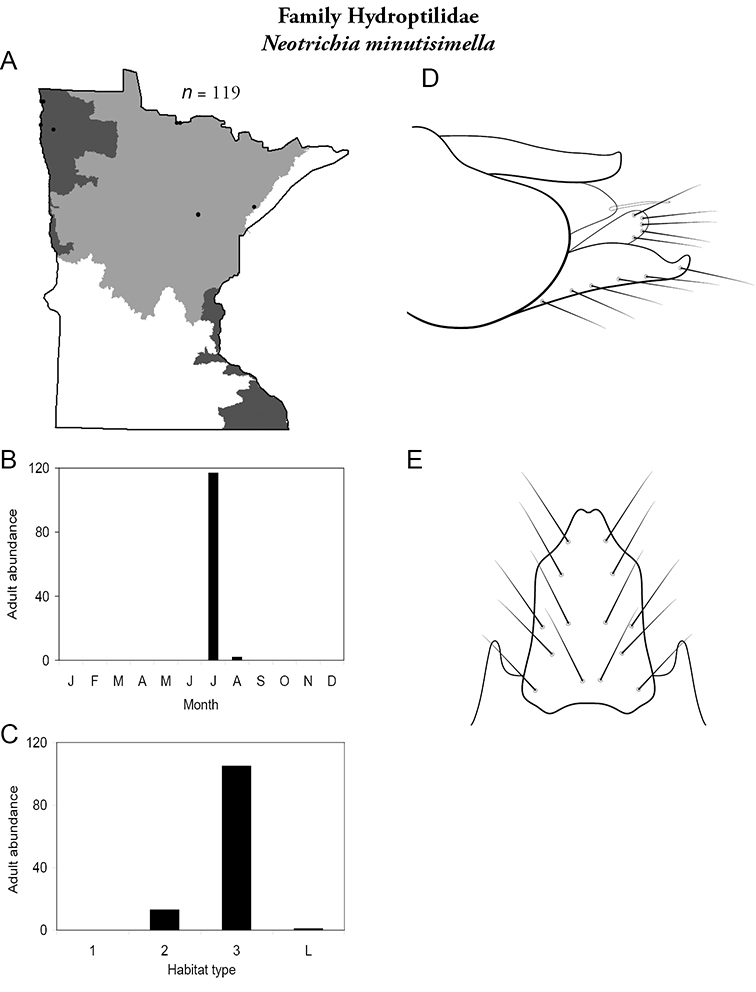
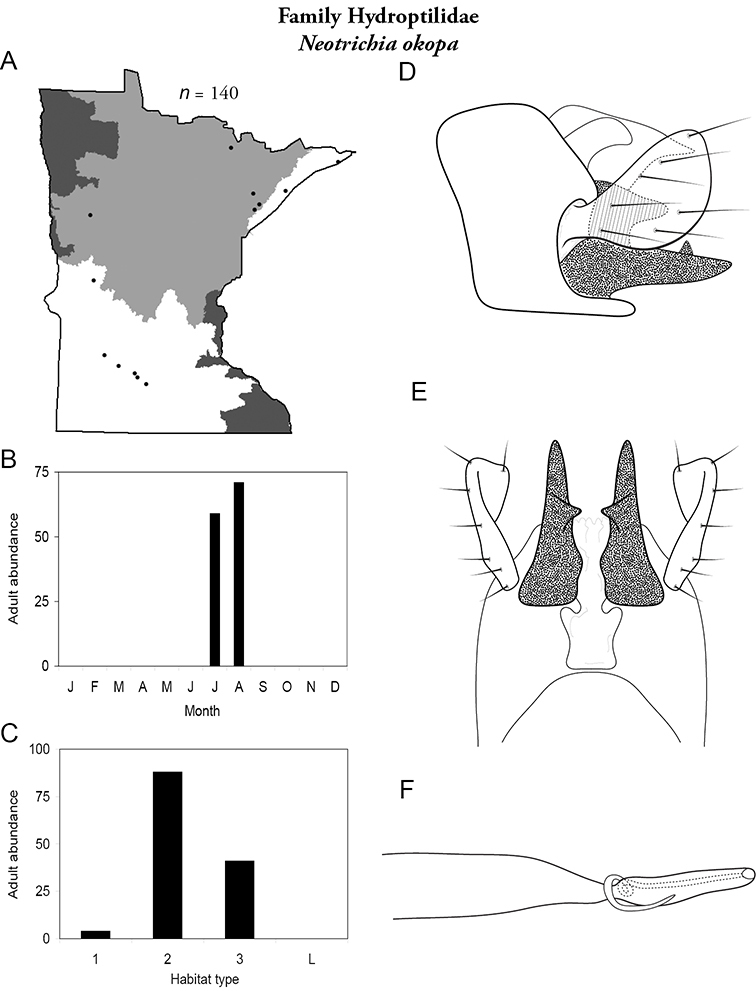
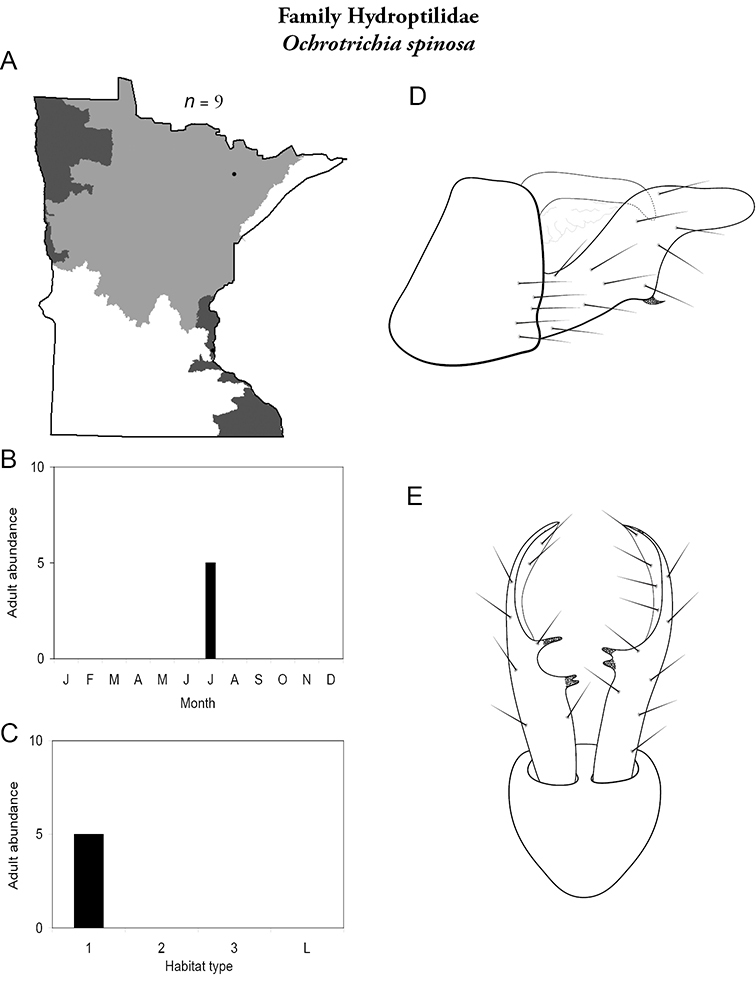
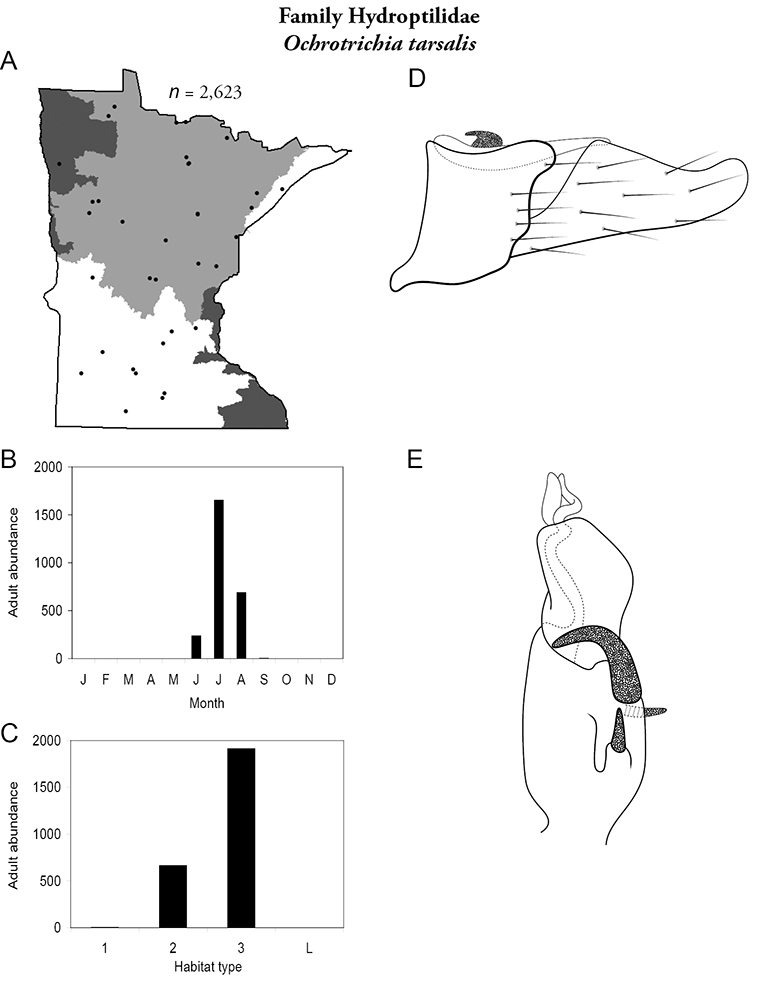
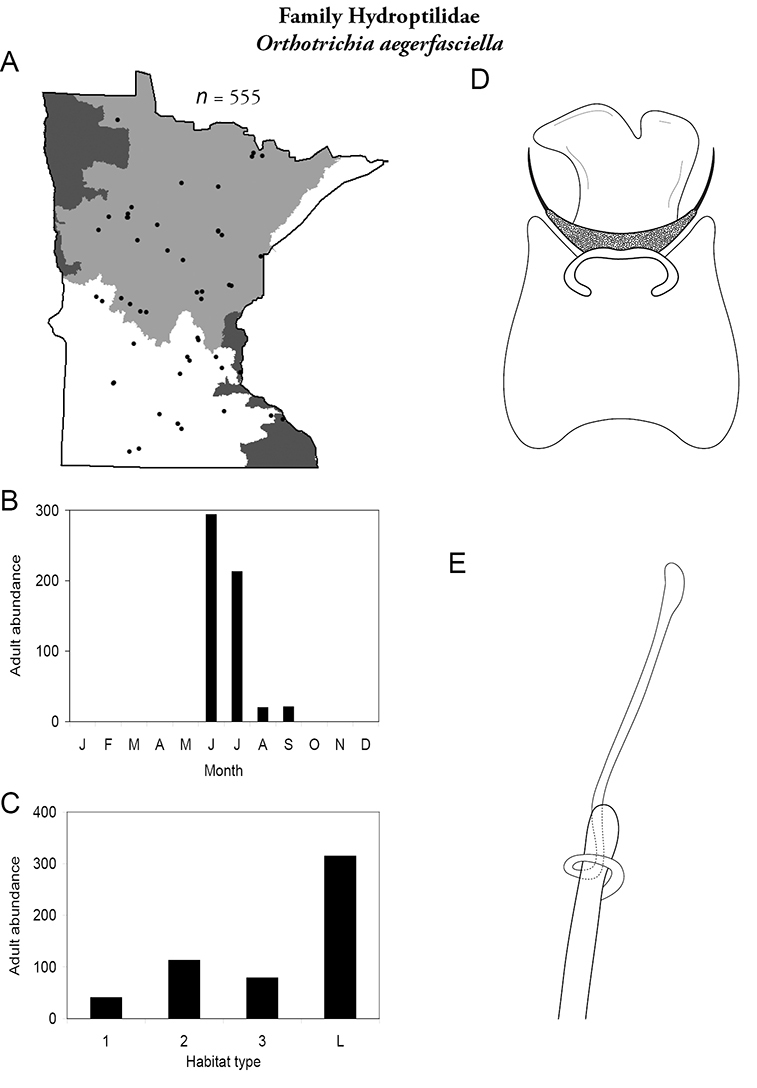
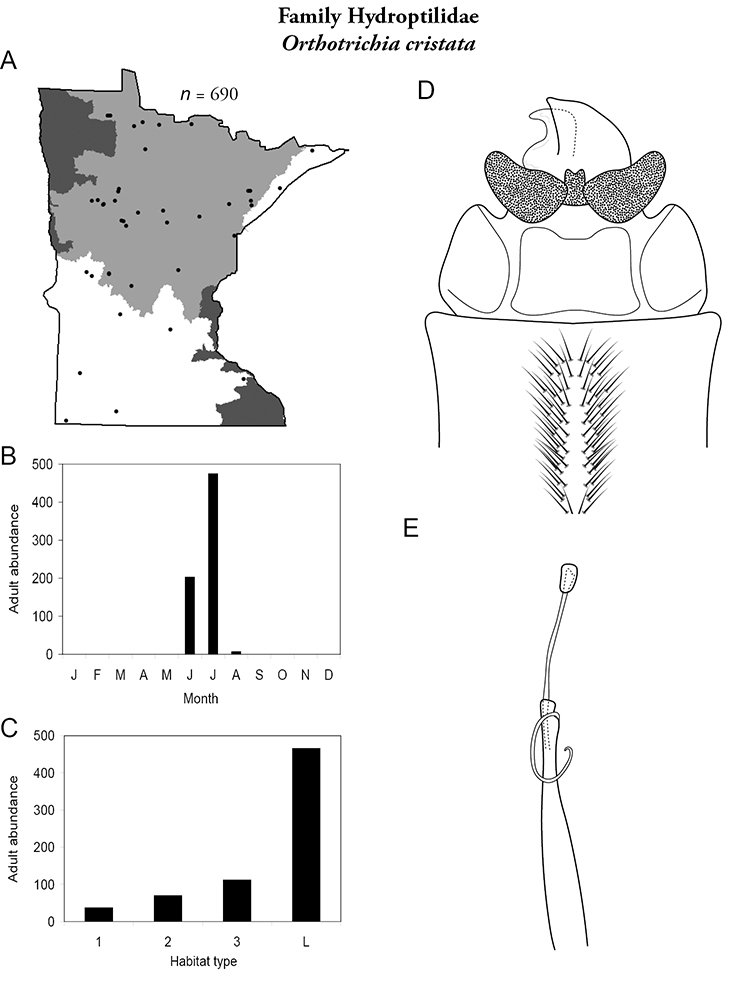
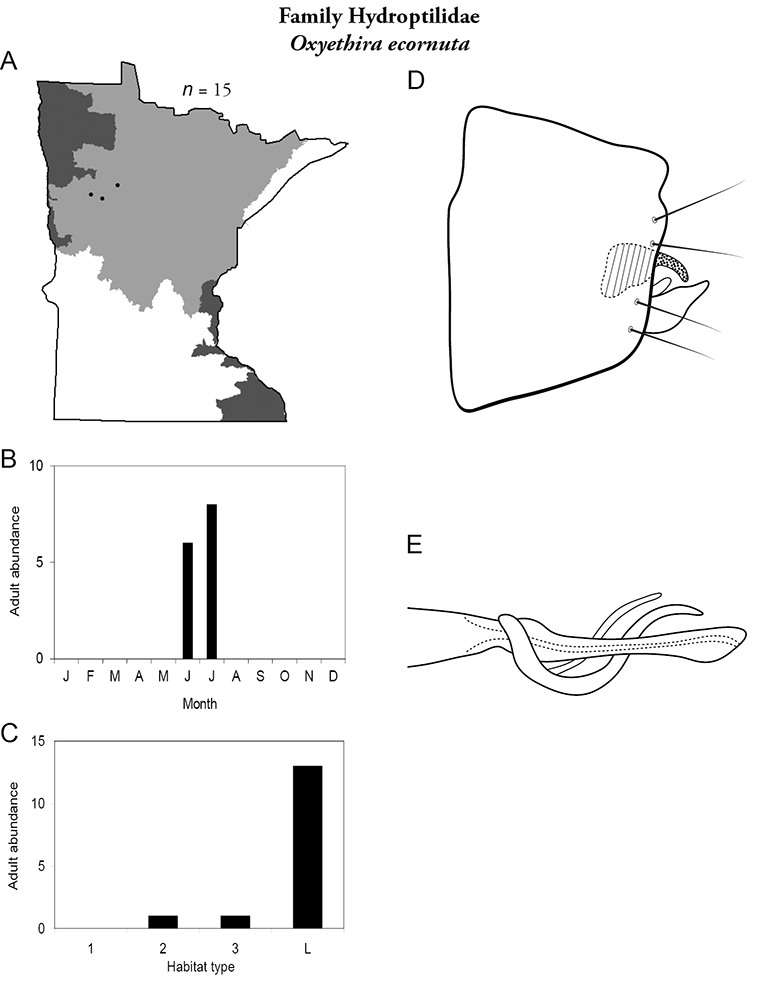
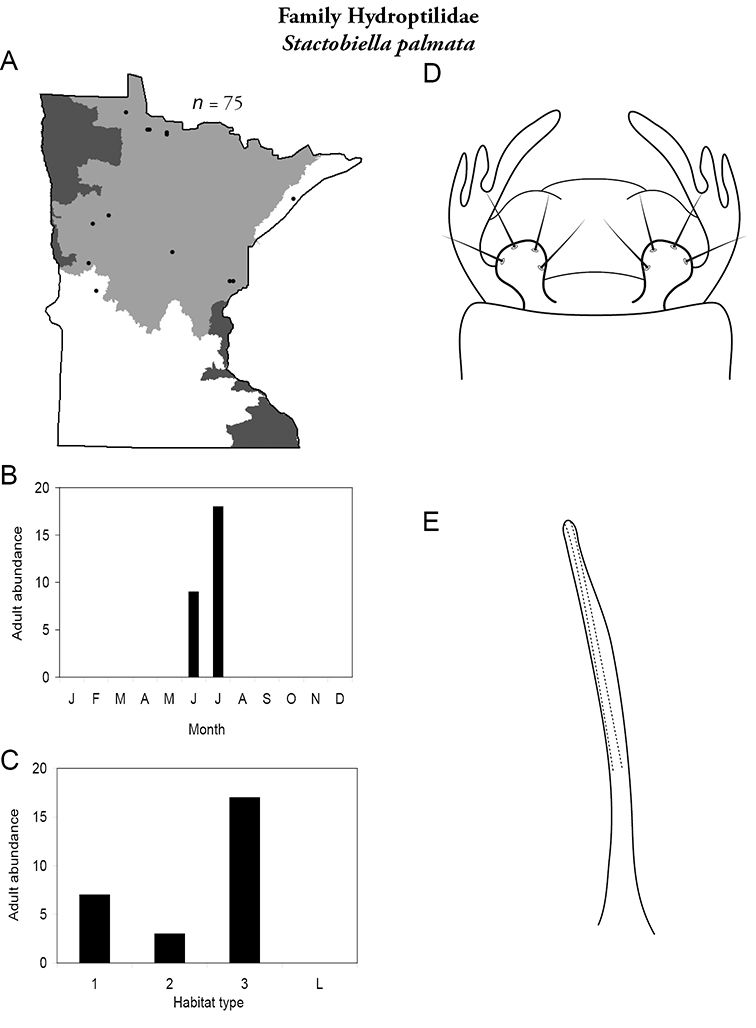
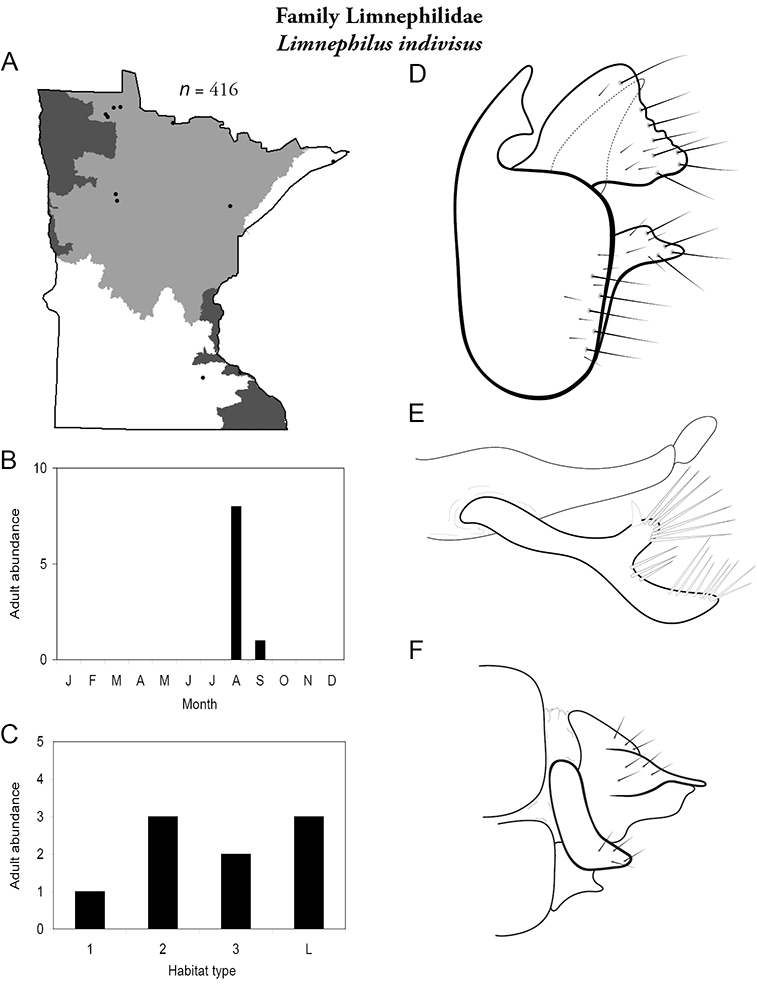
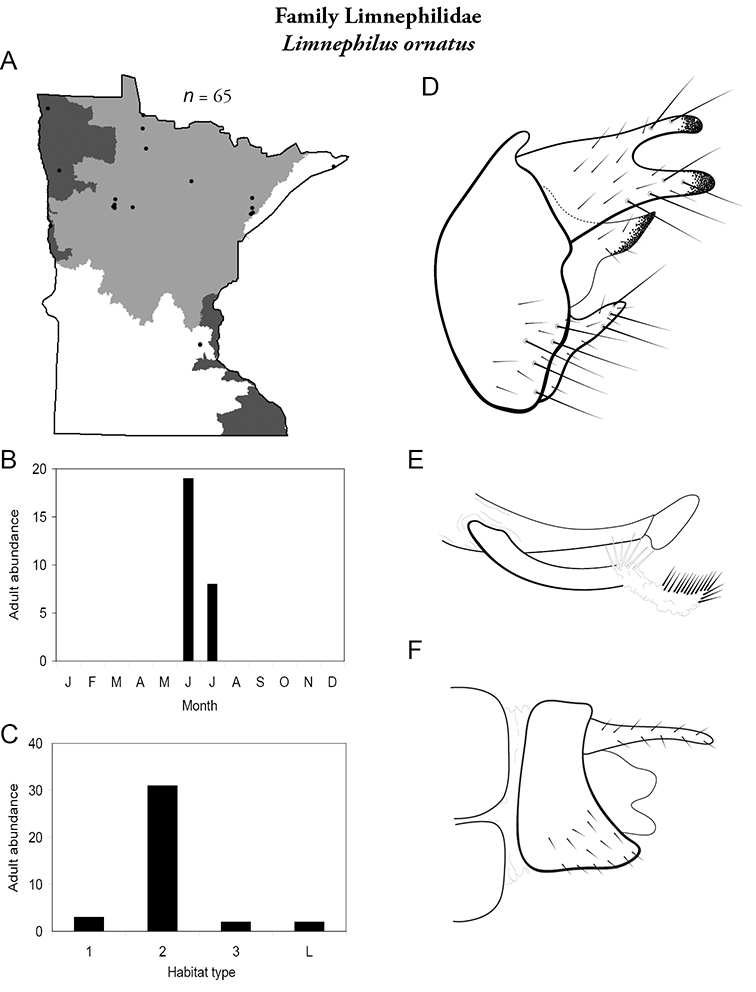
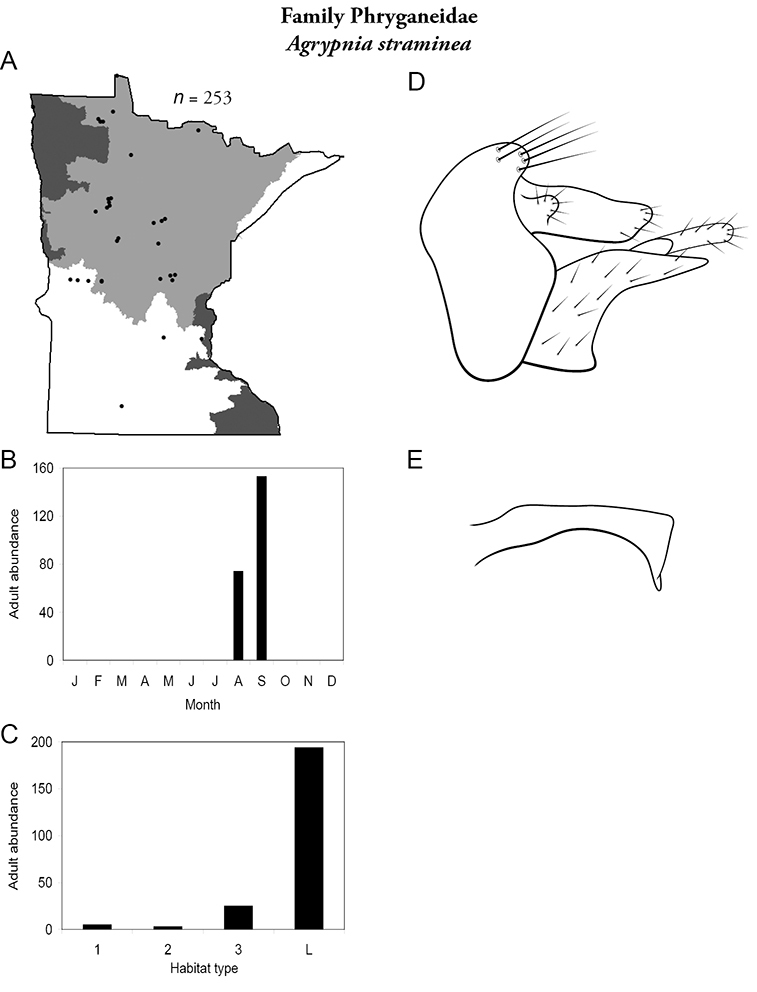
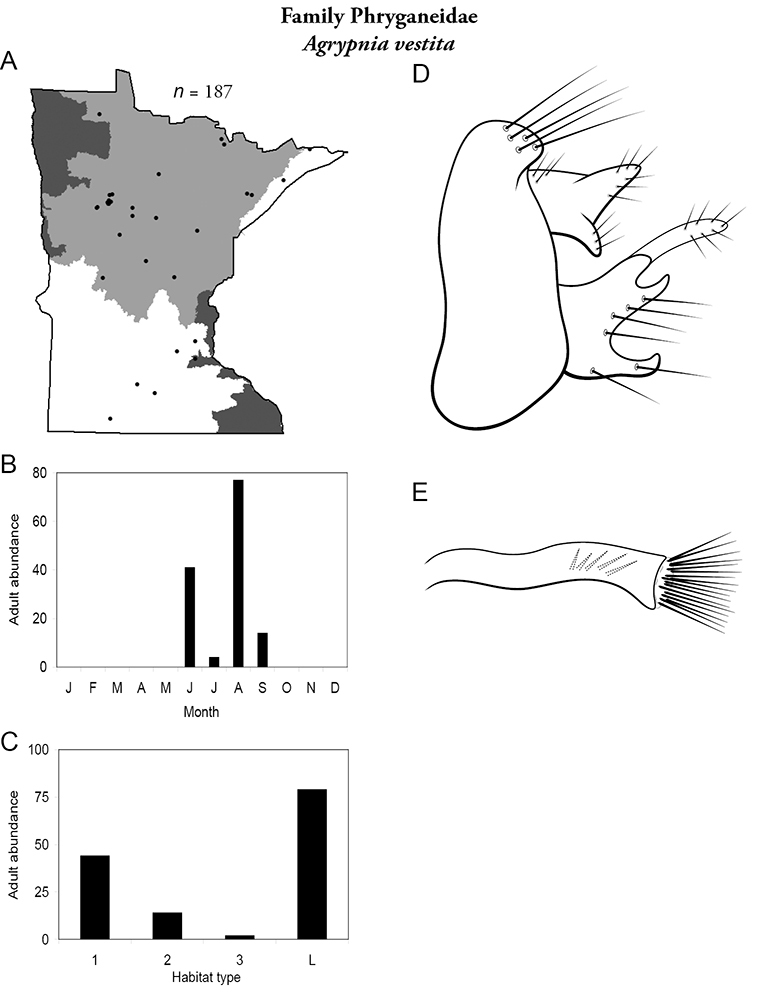
Apatainia zonella A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
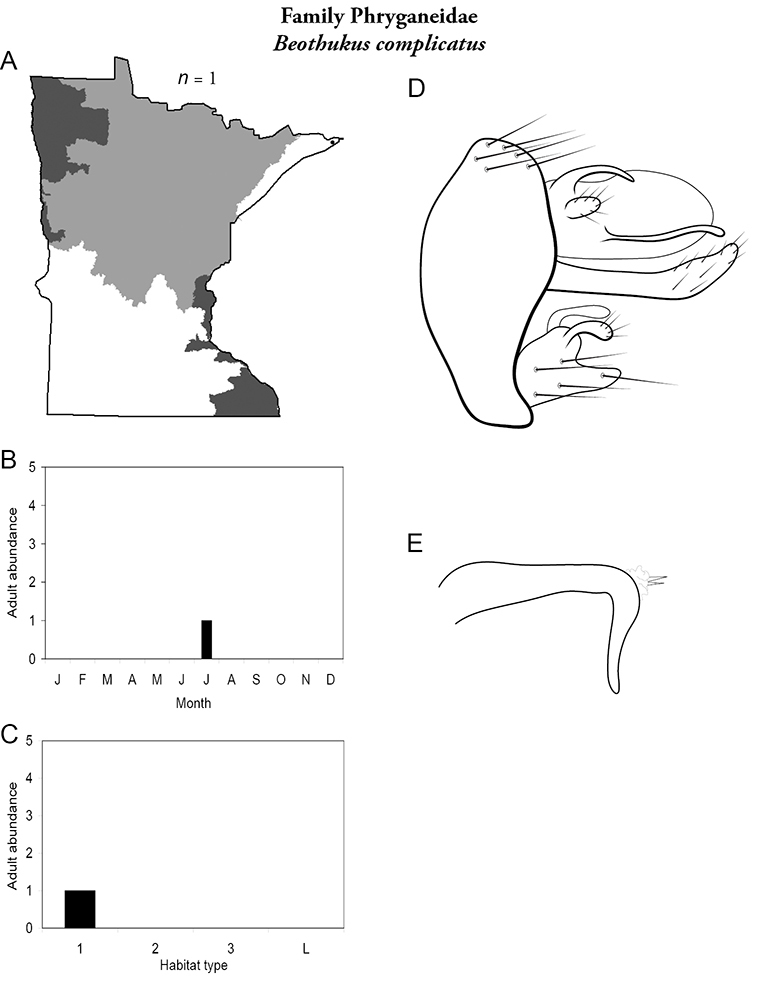
This family, considered a subfamily of the Hydropsychidae in one recent classification (
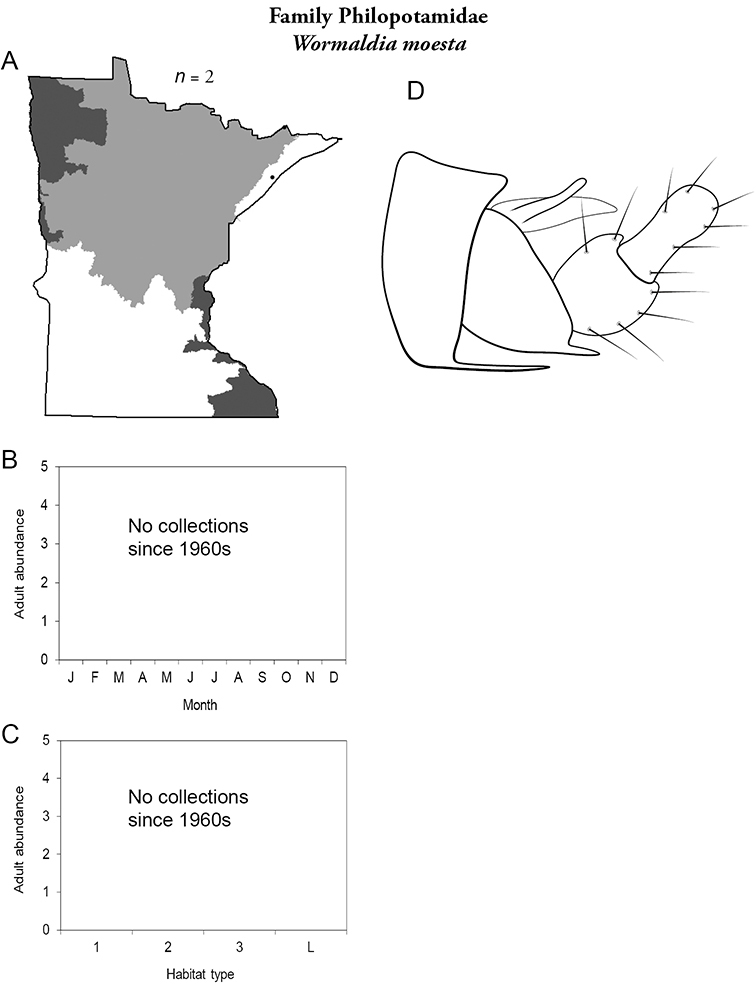
Parapsyche apicalis (Figure 15) is known only from adults collected in May 2001 from Mill Creek, William O’Brien State Park, in the Southeastern Region. The species appears to be at the western edge of its range in Minnesota. Furthermore, Parapsyche apicalis is almost exclusively found in very small (<1 m wide) streams with dense canopy cover (
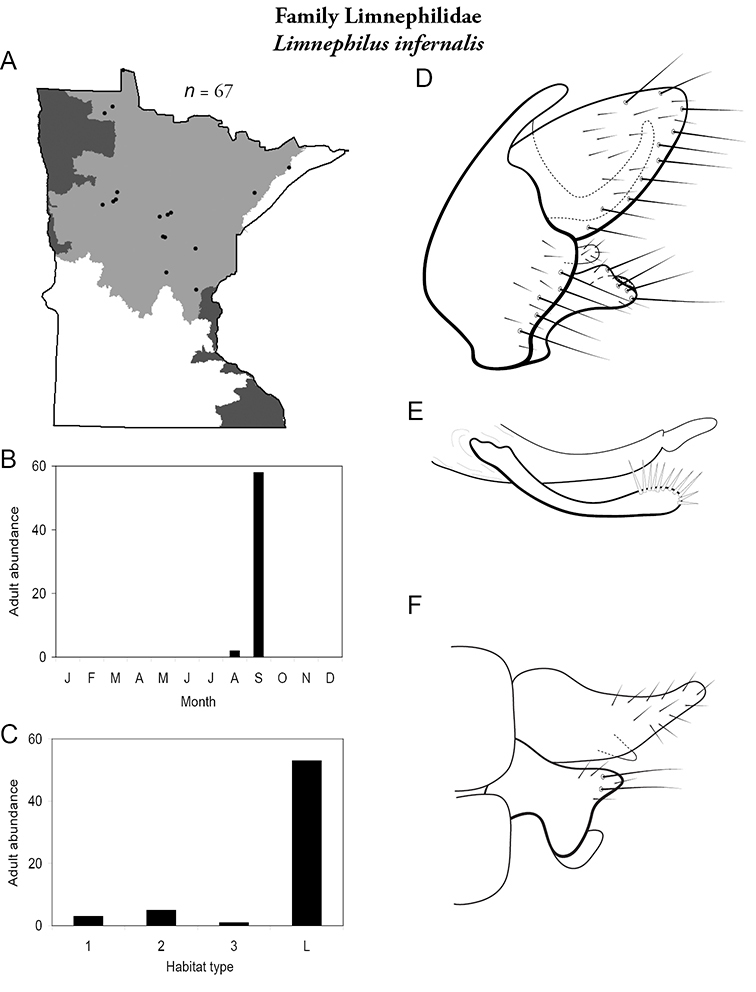
Parapsyche apicalis A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
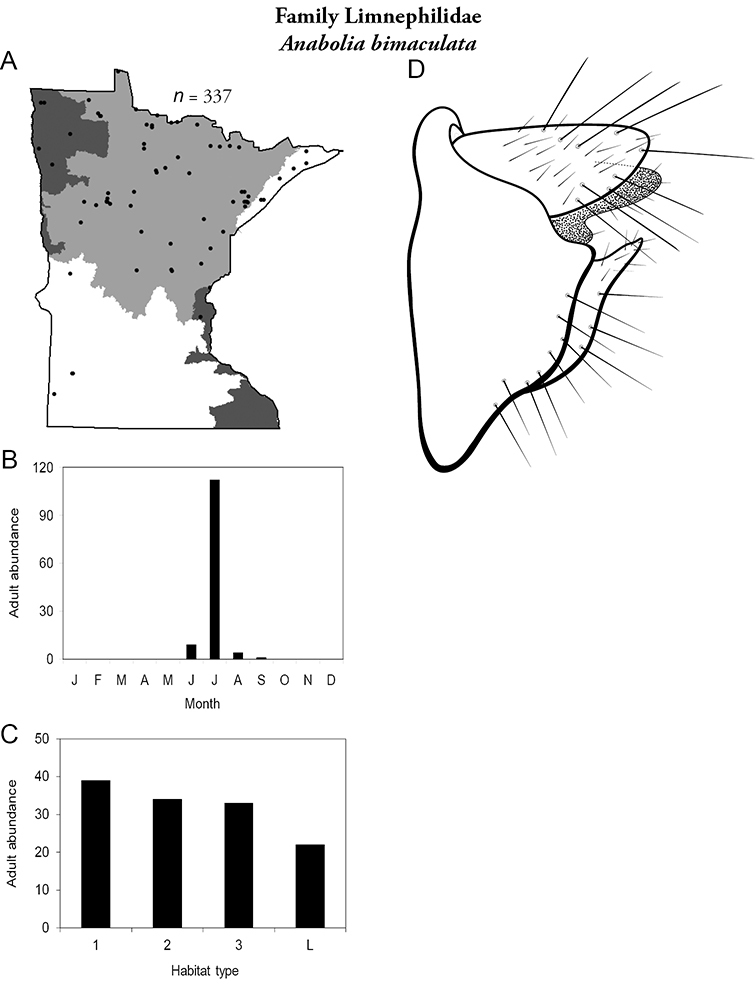
This family contains two genera in Minnesota: Brachycentrus and Micrasema, and a total of 6 species. Both genera are characteristic of running waters; Brachycentrus species tend to inhabit small and medium streams, whereas species of Micrasema prefer medium to large rivers. Both genera contain species that tend to have specific habitat requirements; thus collections can be both sporadic and sometimes containing >1, 000 specimens.
Genus BrachycentrusThe genusBrachycentrus contains 3 species in Minnesota. Two of them are rarely encountered. Larvae typically consume relatively large suspended particulate organic matter that they grasp with their legs (
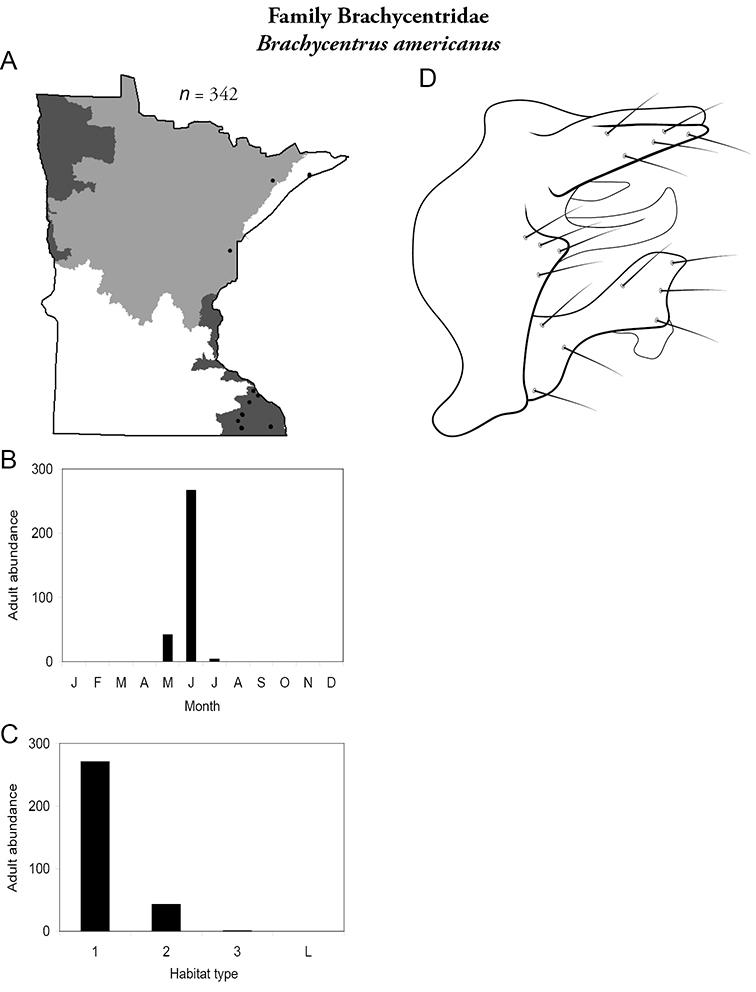
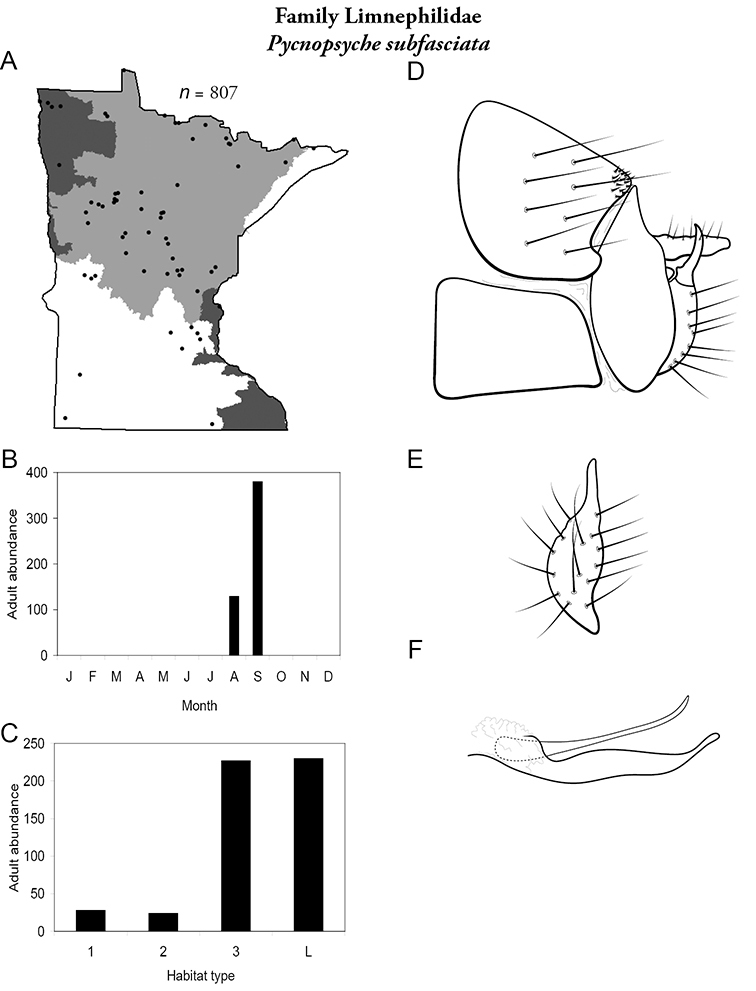
Brachycentrus americanus (Figure 16) was the most abundant species in small streams of the Southeastern Region, representing 38% of all collected specimens (Table 6). It was also the second most abundant species in medium streams of the Southeastern Region. It was also found sporadically in northeastern portion of the state. Adults were present from May to early July. Unlike other reports of Brachycentrus (
Brachycentrus americanus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule.
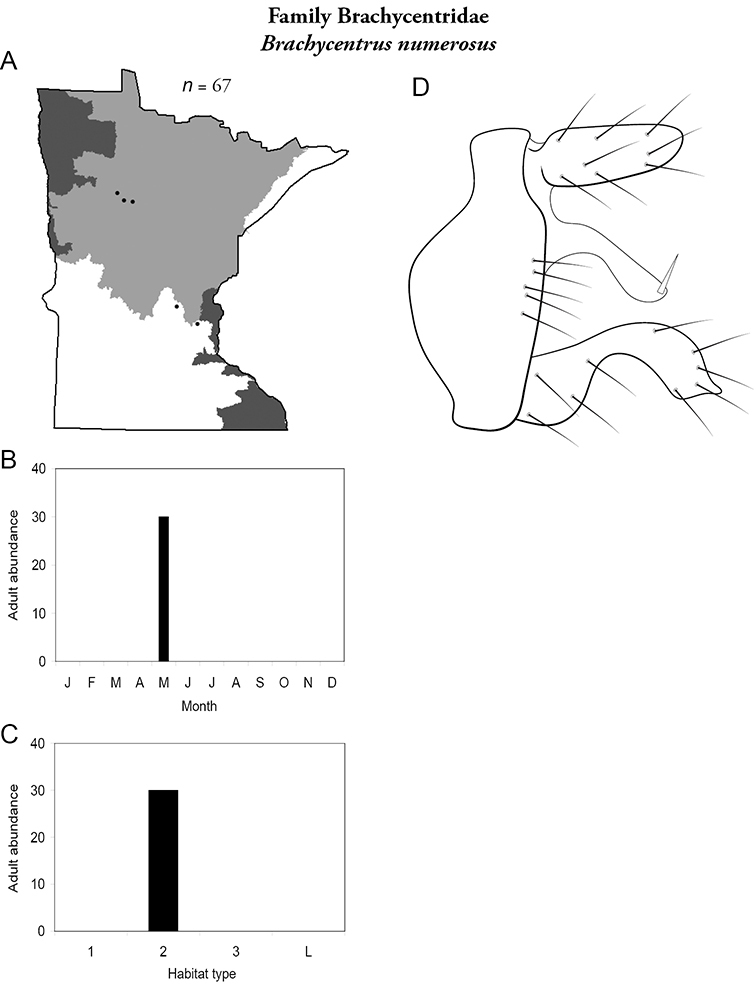
Brachycentrus numerosus (Figure 17) is known mostly from sporadic larval rearing from the Northern and Southern Regions. The only series of adults was collected in May from the Kabekona River, Hubbard County, in the Northern Region using ultraviolet lights.
Brachycentrus numerosus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule.
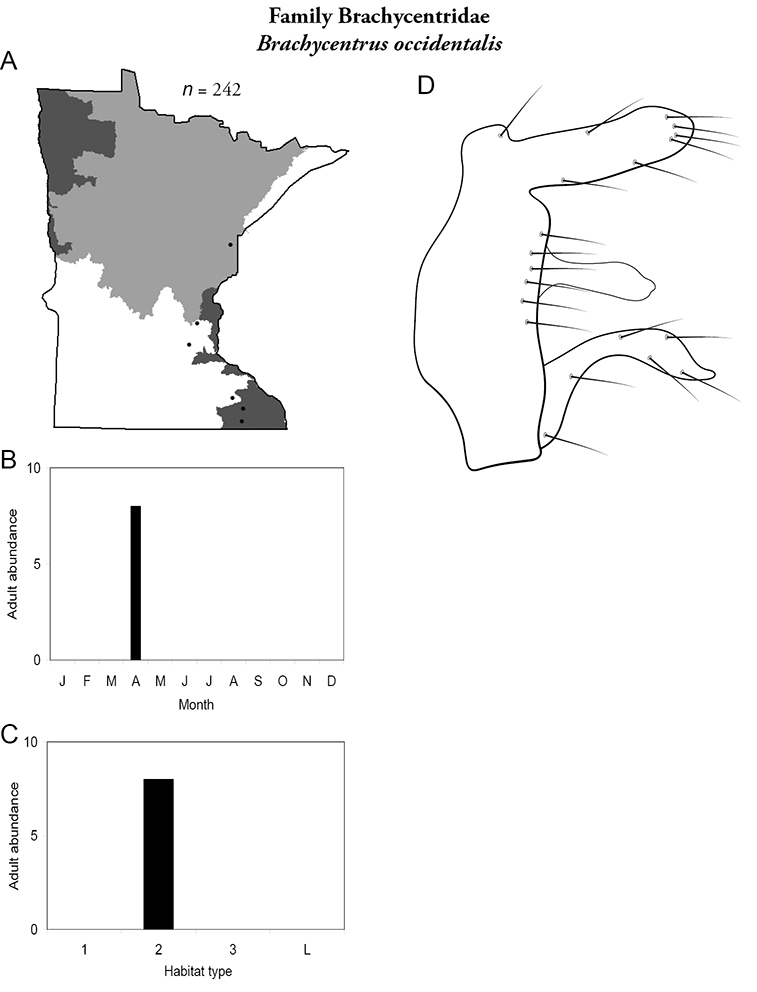
Brachycentrus occidentalis (Figure 18) is known in Minnesota primarily from the Southeastern Region. Nearly all collections have come from reared larvae that emerged in April or May. The only series of adults came from diurnal sweep-netting in April from Forestville State Park in the Southeastern Region.
Brachycentrus occidentalis A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule.
Another Brachycentrus species, Brachycentrus fuliginusos has been reported from Minnesota based on an adult specimen of unknown gender (
The genusMicrasema contains 3 species in Minnesota. For additional species, see
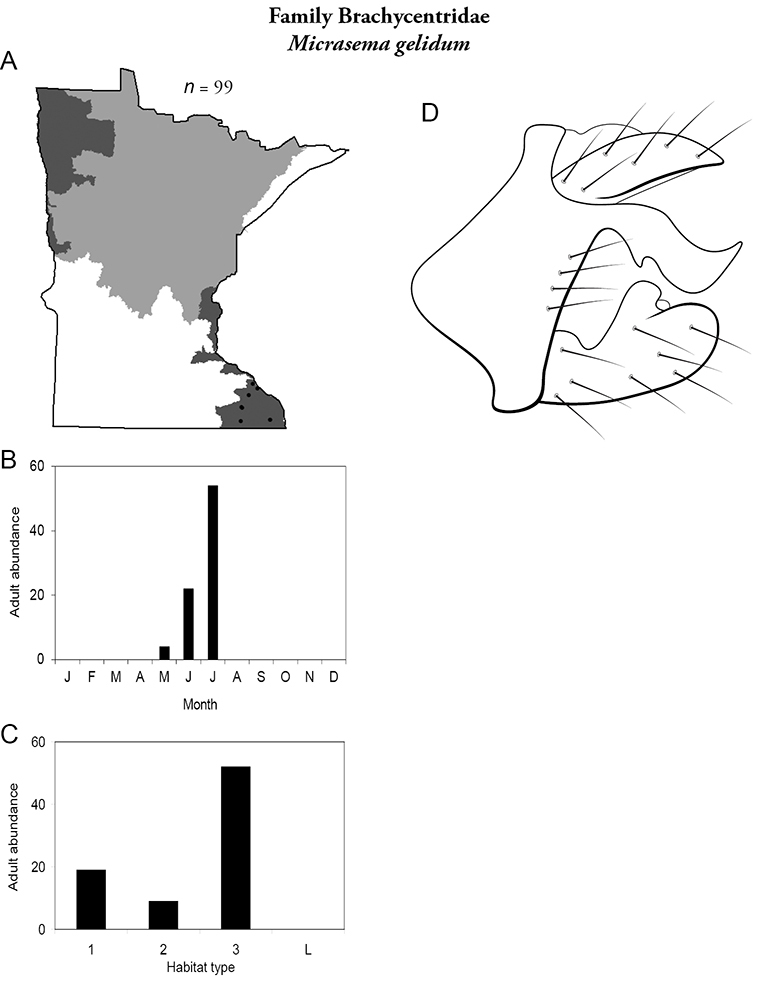
Micrasema gelidum (Figure 19) is known only from the Southeastern Region, where it was most common in all sizes of streams, especially large rivers. Adults were mainly present in June and July.
Micrasema gelidum A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule.
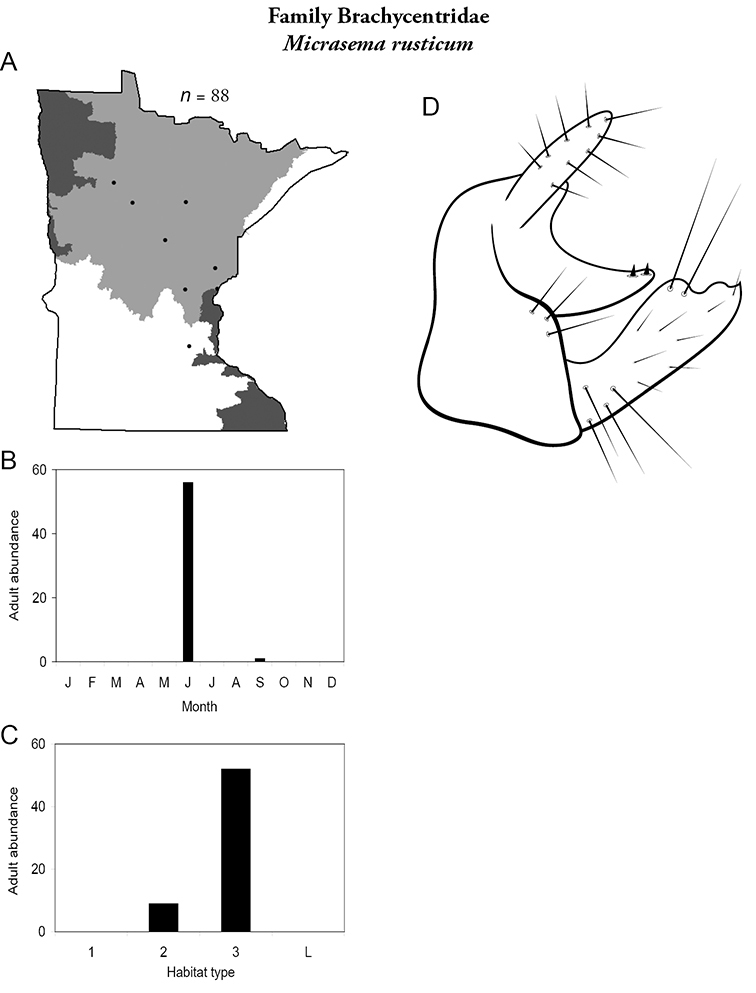
Micrasema rusticum (Figure 20) has been found predominantly in medium and, especially, large rivers of the Northern Region, mostly during June.
Micrasema rusticum A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule.
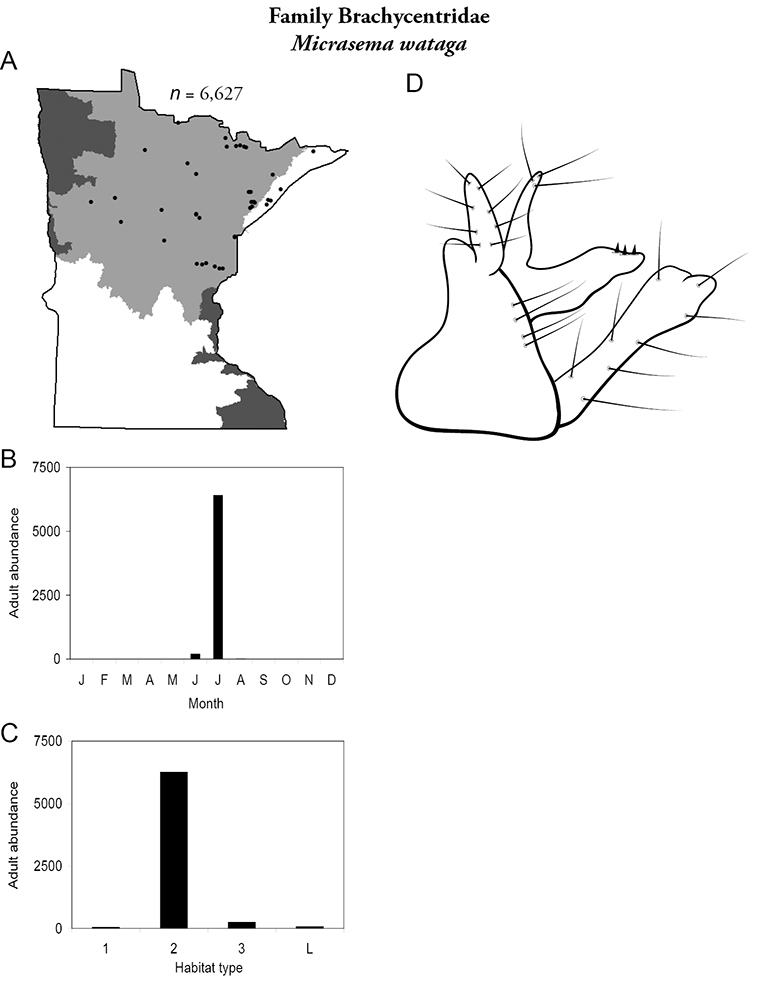
Micrasema wataga (Figure 21) was found throughout the Lake Superior and Northern Regions during June and, especially, July. It was the 10th most abundant species overall in Minnesota (Figure 9); ~90% of this abundance was due to a single collection from the Straight River, Hubbard County, in the Northern Region which yielded >6, 000 specimens.
Micrasema wataga A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule.
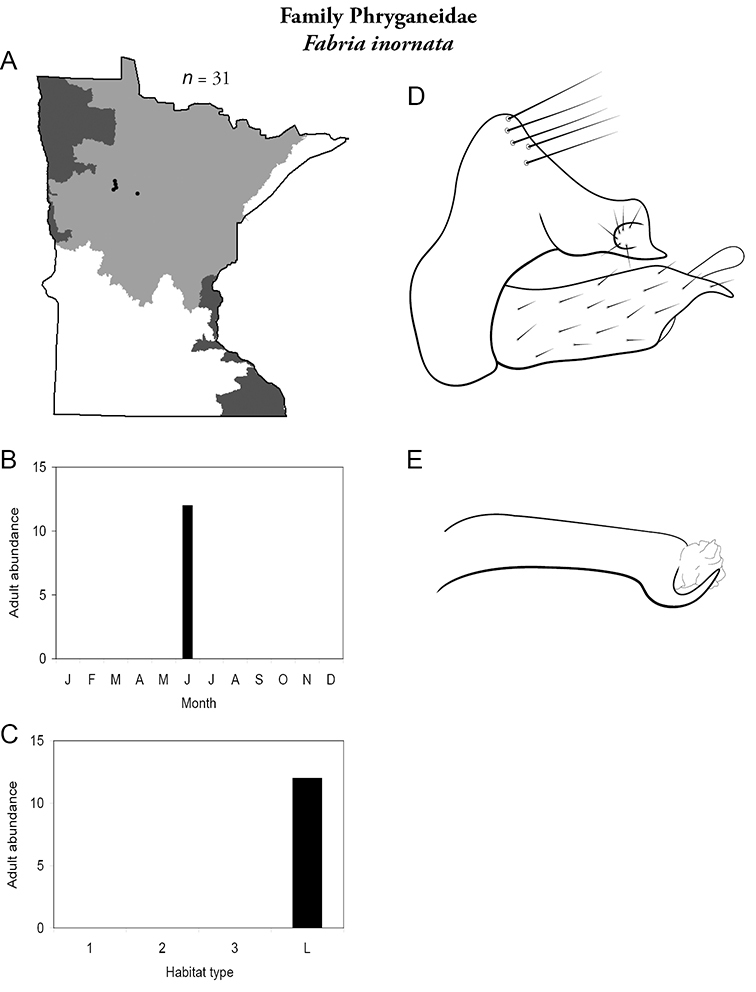
This family contains a single genus in Minnesota, Phylocentropus, and a single species. For additional species, see
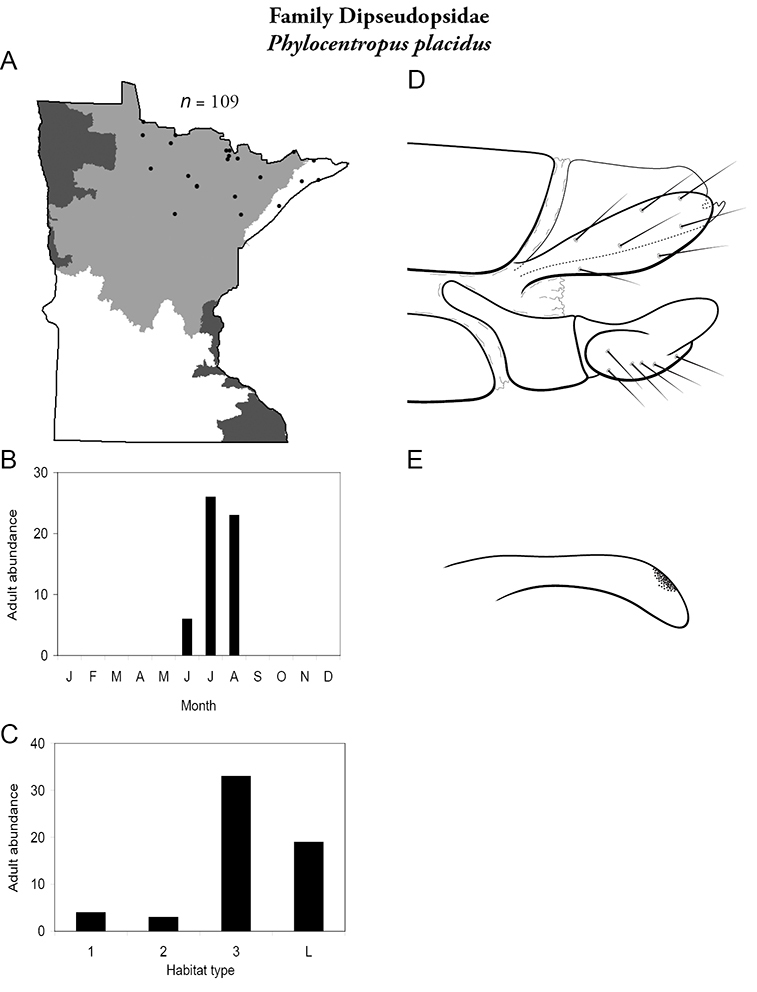
Phylocentropus placidus (Figure 22) was found in the Lake Superior and Northern Regions. It was collected most frequently from large, sandy-bottomed rivers, but was also common in lakes. Adults were most abundant in July and August, with some present in June.
Phylocentropus placidus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
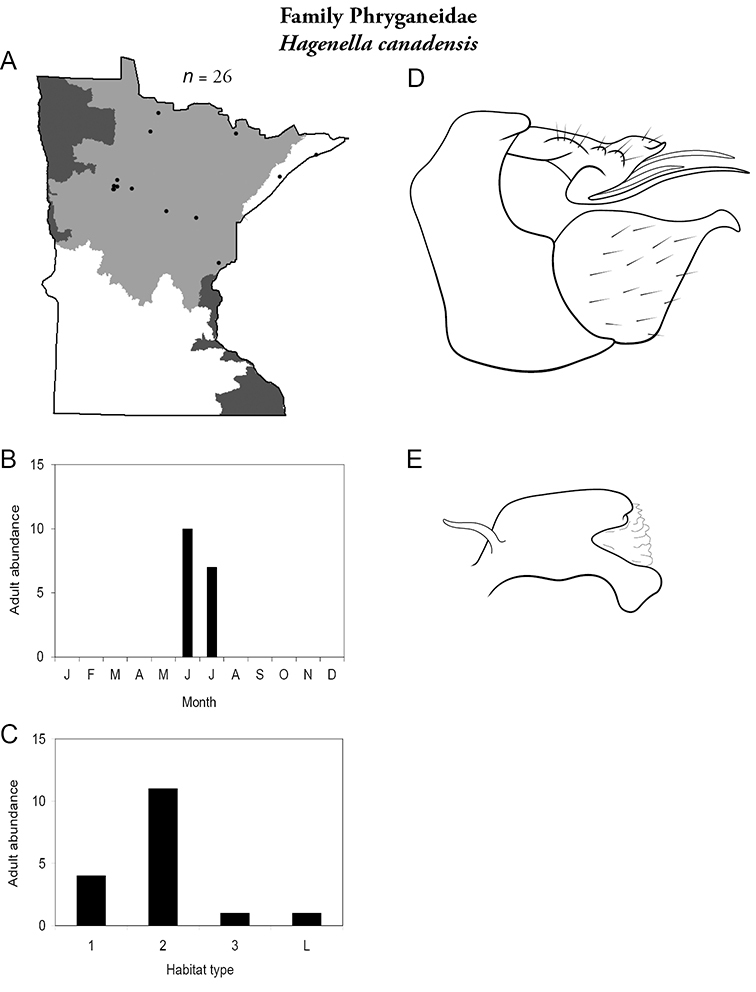
This family contains 3 genera in Minnesota: Agapetus, Glossosoma, and Protoptila, and a total of 7 species. Larvae are found on the surfaces of medium to large rocks in fast-moving current where they graze on algae and diatoms. They construct “saddle” cases that superficially resemble turtle shells (
The genus Agapetus contains 2 species in Minnesota. For additional species see
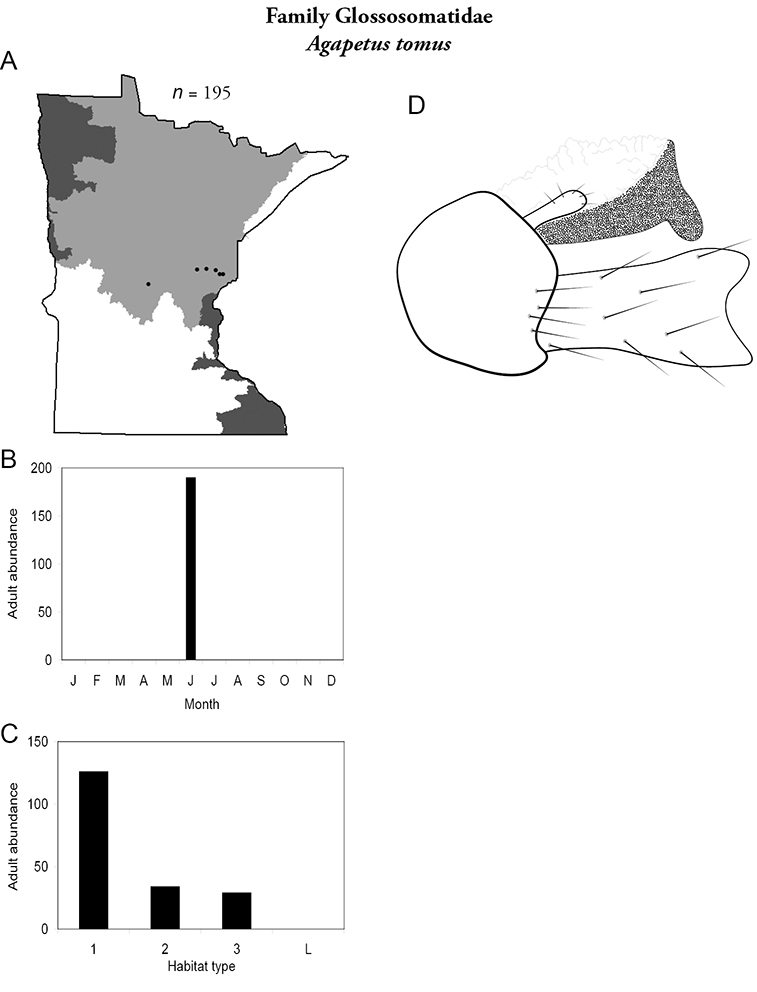
Agapetus tomus (Figure 23) was collected only in June and only from the Northern Region. It was locally abundant in a variety of stream types, especially small streams. Interestingly, all of these streams were in a line approximating the 46° parallel. It is difficult to determine the specific habitat requirements of this species or why it is only found in these specific streams (
Agapetus tomus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule.
Agapetus walkeri (Figure 24) was only found in small and, especially, medium streams of the Lake Superior Region. Adults were collected in July.
Agapetuc walkeri A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule.
Another Agapetus species, A illini was reported from northeastern Minnesota based on a series of specimens (
The genus Glossosoma contains 2 species in Minnesota. For additional species, see
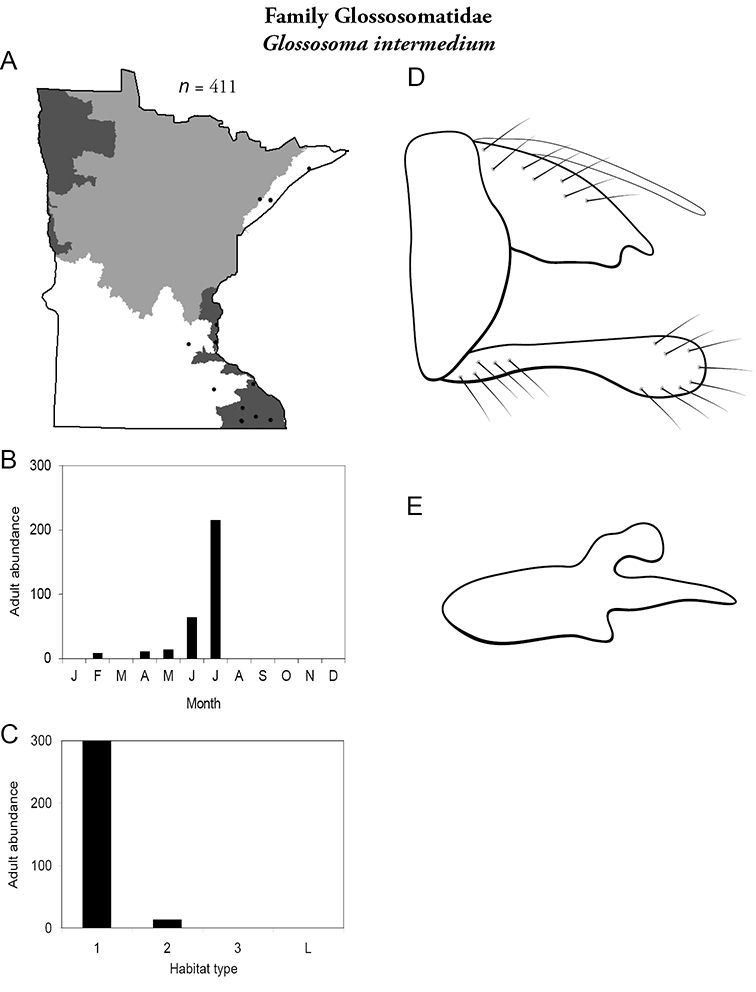
Glossosoma intermedium (Figure 25) was found in small and medium streams, predominately in the Lake Superior and, especially, the Southeastern Regions. The majority of adults were collected during July; however, some were found as early as February. Many others have been reared in the lab from larvae and have emerged in March through May.
Glossosoma intermedium A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
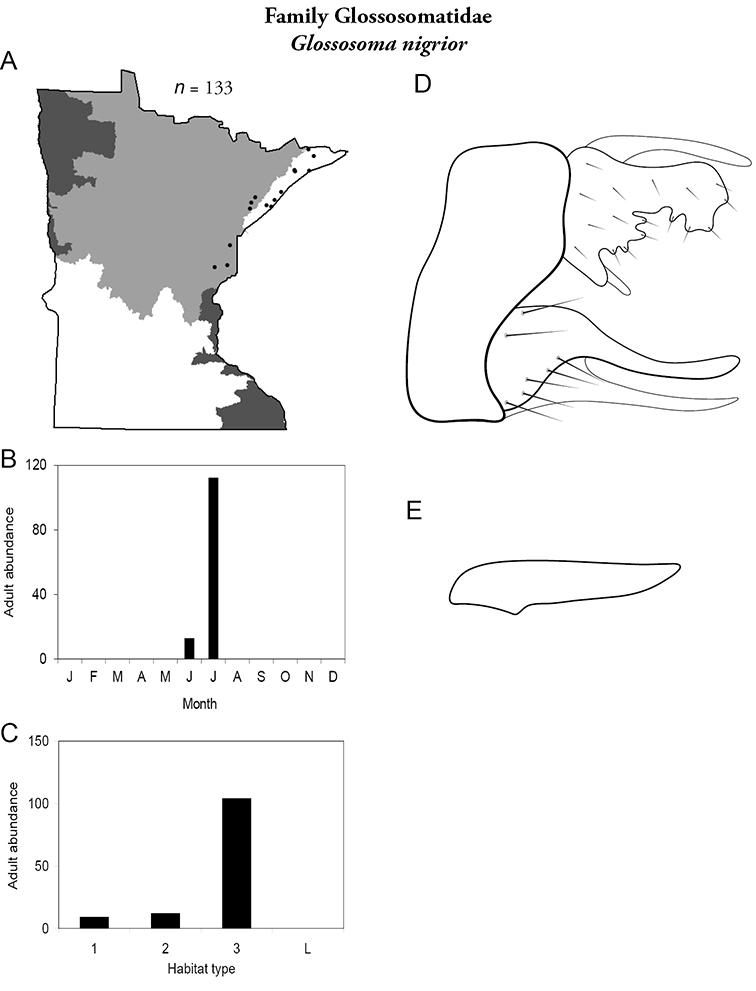
Glossosoma nigrior (Figure 26) is known from the Lake Superior and Northern Regions, predominately from large rivers. It was collected during June and, especially, July.
Glossosoma nigrior A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
The genusProtoptila contains 3 species in Minnesota. For additional species, see
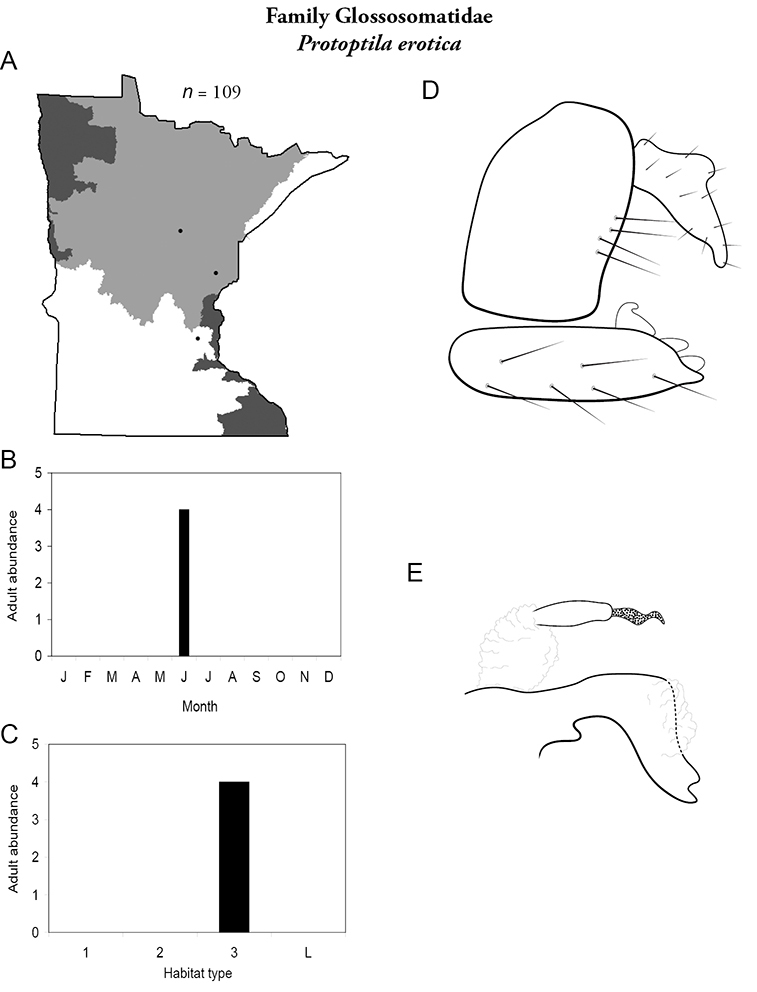
Protoptila erotica (Figure 27) is known from a total of 3 localities in the Northern Region and only 1, the Kettle River in Pine County, since the 1930s. The historical collections yielded >100 specimens. The recent collection yielded only 4. All collections occurred in June. Due to its limited distribution and apparent decrease in abundance since the 1930s, the Minnesota Department of Natural Resources has proposed that Protoptila erotica be listed as “Threatened” (
Protoptila erotica A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
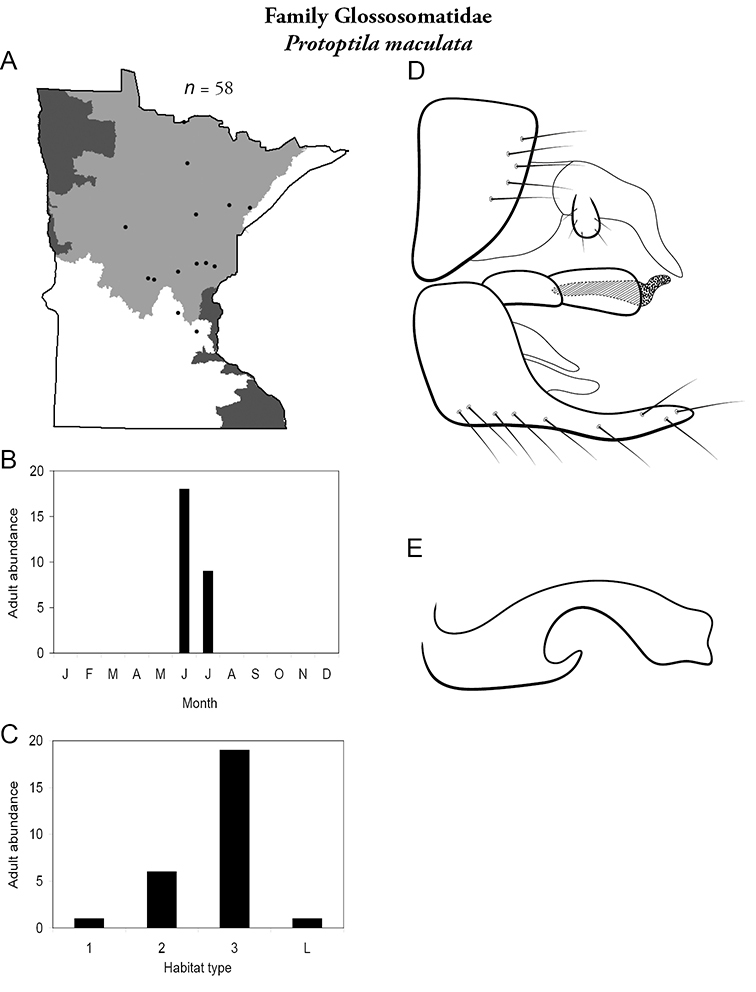
Protoptila maculata (Figure 28) has been found mostly in medium and, especially, large rivers during June and July. It is known predominately from the Northern Region.
Protopila maculata A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
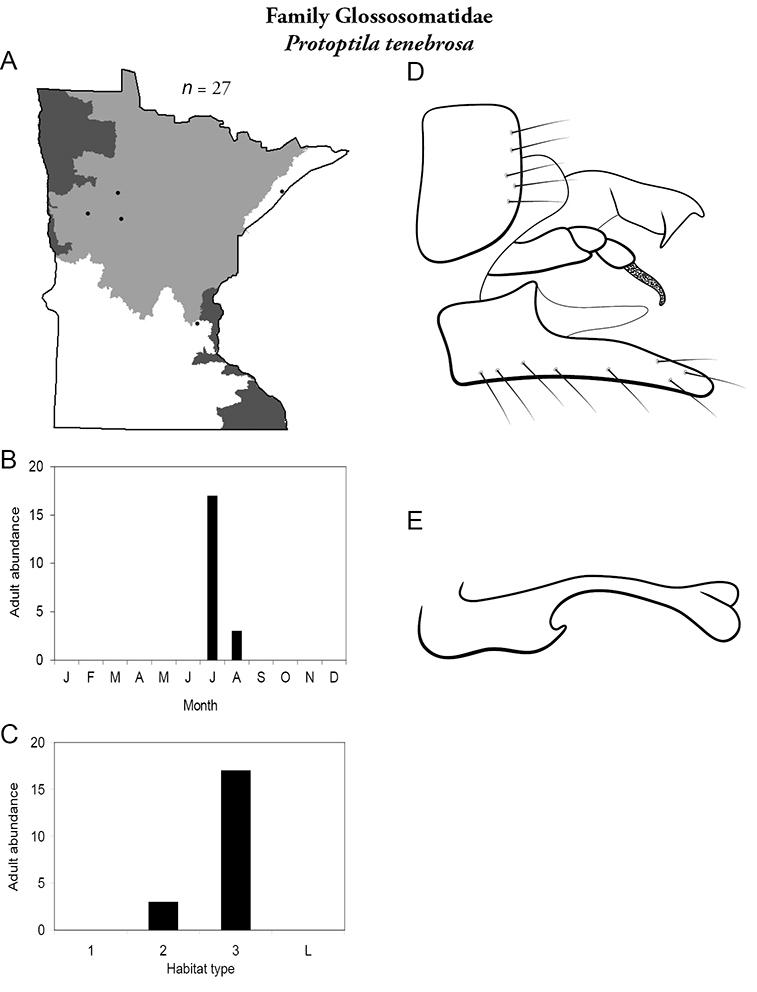
Protoptila tenebrosa (Figure 29) has been collected mostly from large rivers in July. Collecting localities are widely separated from each other.
Protoptila tenebrosa A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
A fourth Protoptila species, Protoptila talola, is known worldwide from a single Minnesota specimen collected in 1941 from an unknown habitat in Pine County. Due to its rarity and Minnesota endemism, the species is listed as “Special Concern” by the Minnesota Department of Natural Resources (
This family contains a single species in Minnesota, Goera, and a single species. Larvae are characteristic of running water where they consume algae and small organic particles from the surfaces of medium and large rocks. Larval cases are constructed of small mineral particles, with larger pebbles used as ballast stones (
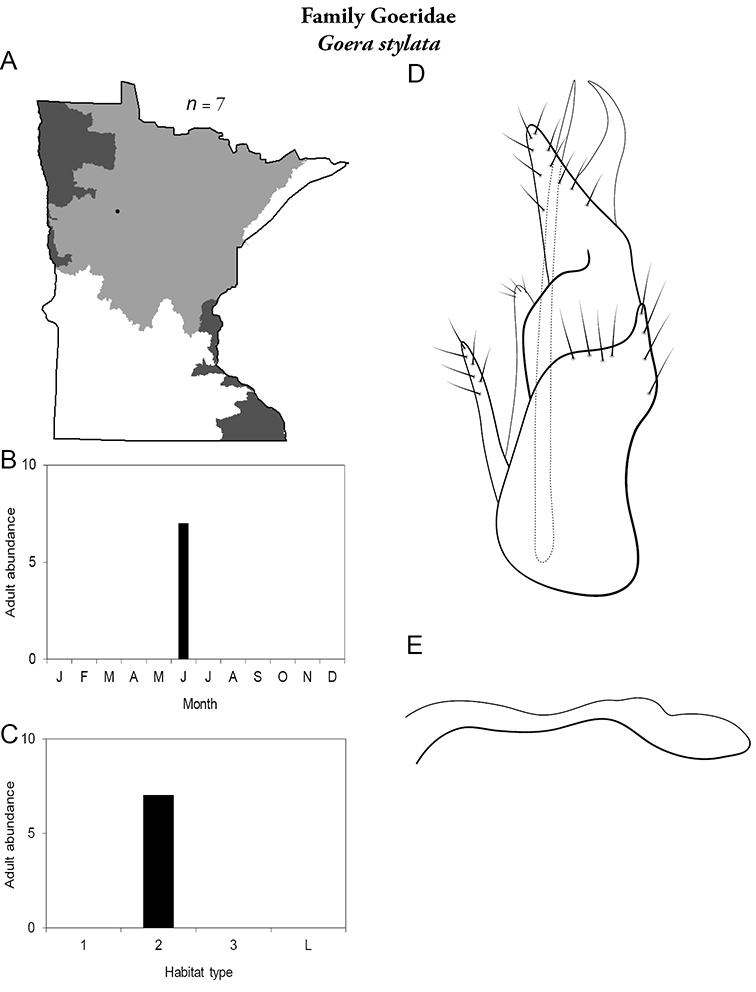
Goera stylata (Figure 30) is known only from LaSalle Creek, Clearwater County, in the Northern Region. Adults were collected during June. The species tends to have specific habitat requirements, and is typically present as an adult only for a brief period of time (
Goera stylata A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule (rotated 90 degrees counter-clockwise) E phallus.
Another Goera species, Goera calcarata, was reported from Minnesota based on a series of larvae (
This family contains 1 genus in Minnesota, Helicopsyche, and a single species. For additional species, see
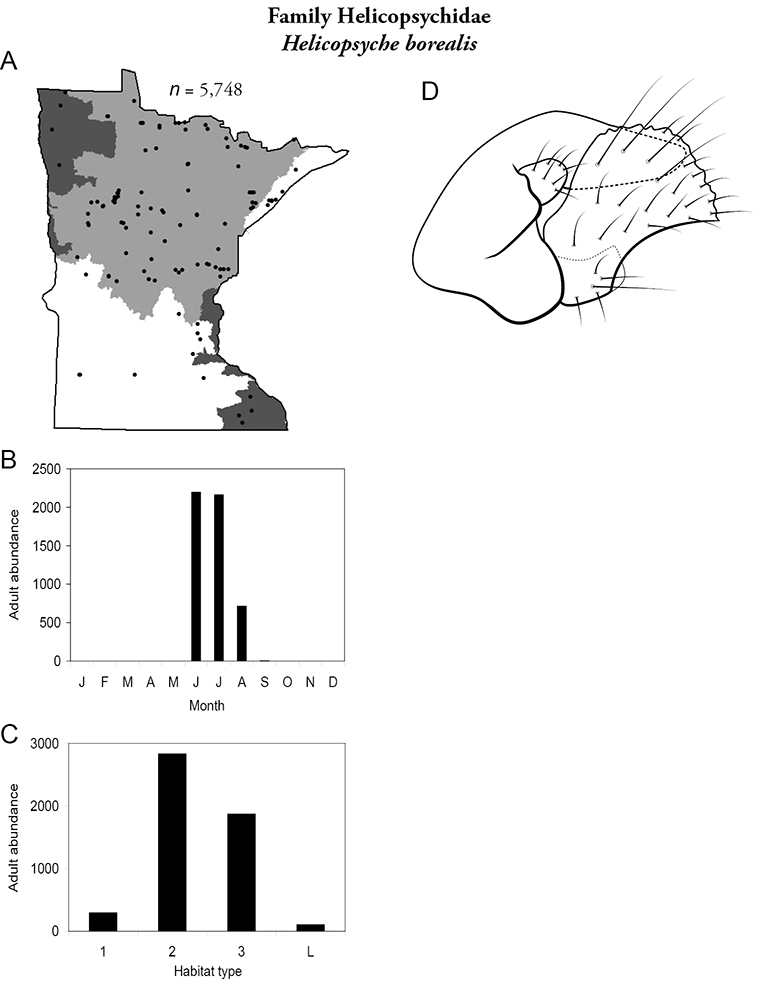
Helicopsyche borealis (Figure 31) was common and abundant throughout Minnesota, especially in medium and large rivers of the Lake Superior and Northern Regions. Adults were present June through August.
Helicopsyche borealis A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule.
This family contains 5 genera in Minnesota: Cheumatopsyche, Diplectrona, Hydropsyche, Macrostemum, and Potamyia, and a total of 29 species. It is the 4th most species-rich family (Figure 6). Larvae are very common and conspicuous members of all types of streams. Indeed, picking up nearly any medium or large rock in the flowing water of virtually any stream is likely to yield larval specimens.
Larvae construct filtering nets of silk that are used to capture suspended particulate organic material in the water column. Different genera and species have nets of different mesh size, thus effectively partitioning the resource (
Adults range 5–18 mm in length. Wings are typically brown with darker reticulations, although some are uniformly brown and 1 species is straw-colored. Specimens can be very abundant in light traps, especially below impoundments with high seston loads. Females are usually much more abundant than males and, unfortunately, not readily identifiable. Thus, hydropsychid species may be considerably more abundant than reported.
The genus Cheumatopsyche contains 11 species in Minnesota. For additional species, see
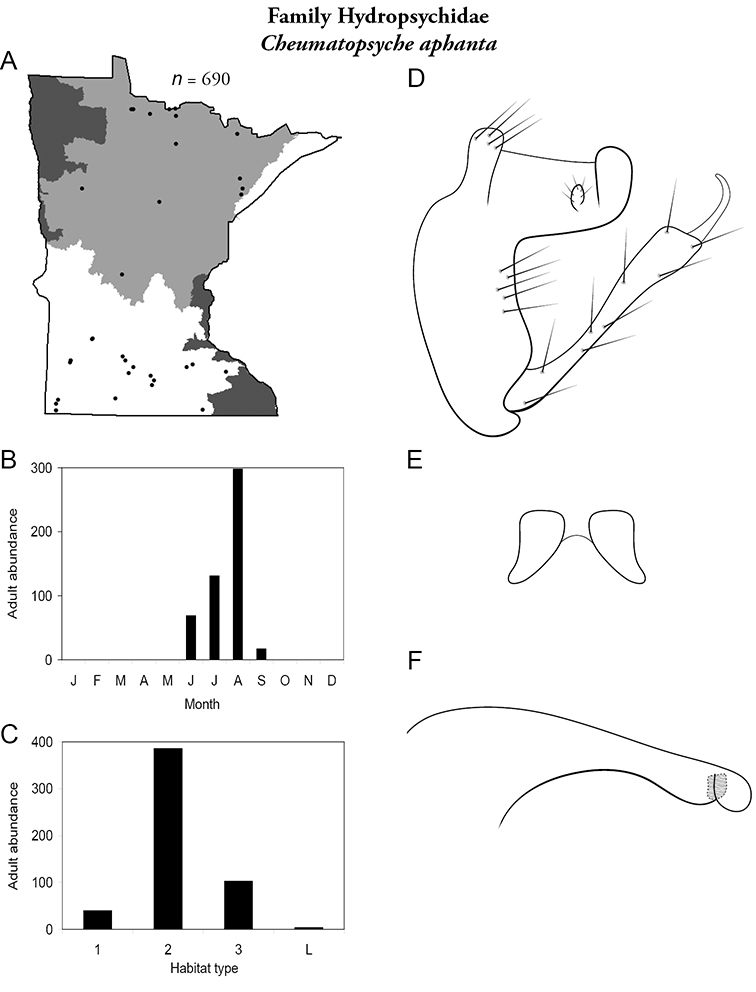
Cheumatopsyche aphanta (Figure 32) has been collected from throughout the Northern and Southern Regions. Adults were present from June to September and especially abundant in August. It was found in all sizes of streams, particularly medium rivers.
Cheumatopsyche aphanta A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E lobes of tergum X (caudal view) F phallus.
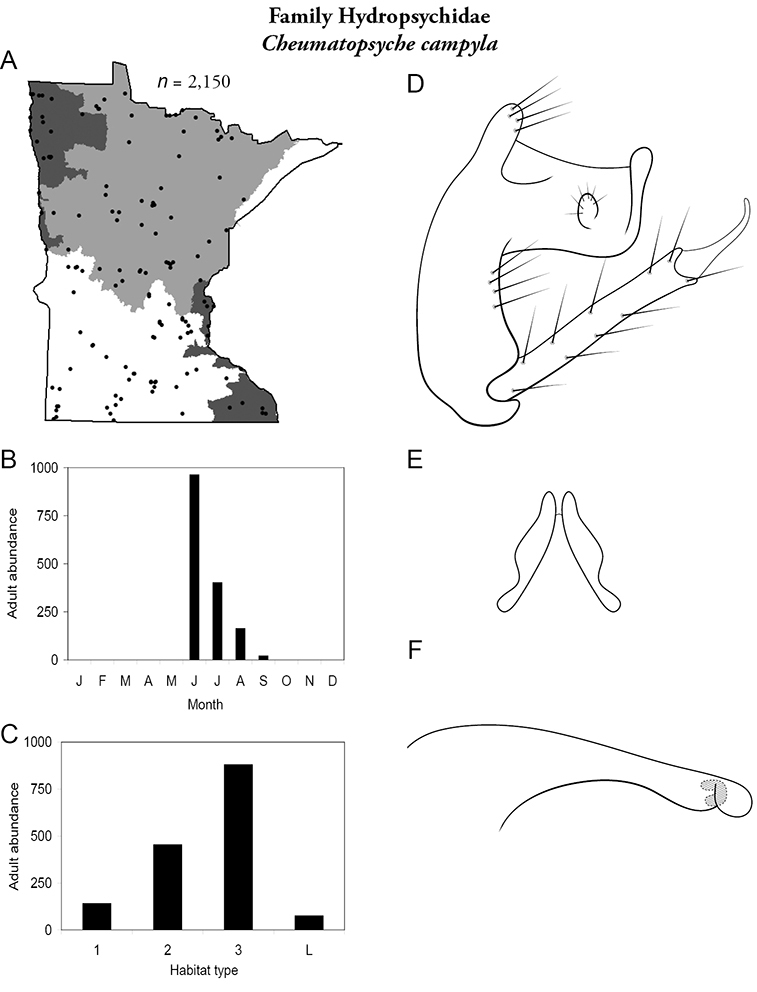
Cheumatopsyche campyla (Figure 33) was common and abundant in all regions except the Lake Superior. Adults were most abundant in June, with decreasing presence through September. Specimens were most abundant in large rivers. In areas of agricultural disturbance, however, Cheumatopsyche campyla greatly increased in abundance, constituting an “indicator species” of disturbed small and medium streams (
Cheumatopsyche campyla A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E lobes of tergum X (caudal view) F phallus.
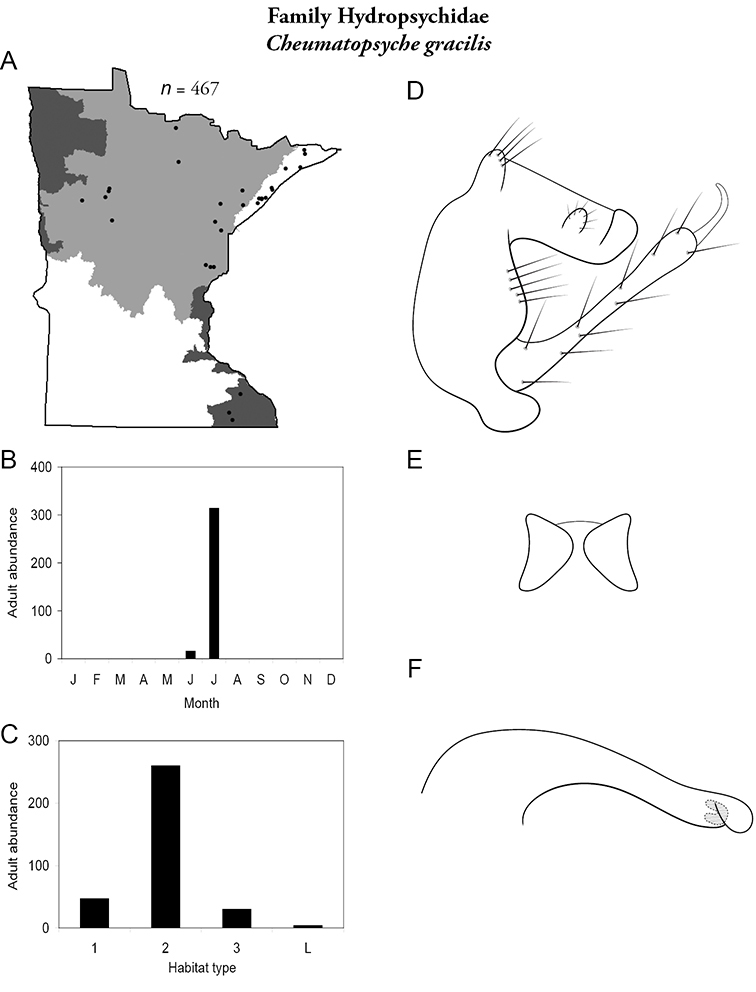
Cheumatopsyche gracilis (Figure 34) was collected in the Lake Superior, Northern, and Southeastern Regions. It was most abundant in medium rivers and found mainly in July.
Cheumatopsyche gracilis A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E lobes of tergum X (caudal view) F phallus.
Cheumatopsyche lasia (Figure 35) is known only from the northwest and southwest corners of the state. It was found mainly in large rivers. Adults were abundant in June, with some present in August.
Cheumatopsyche lasia A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E Lobes of tergum X (caudal view) F phallus.
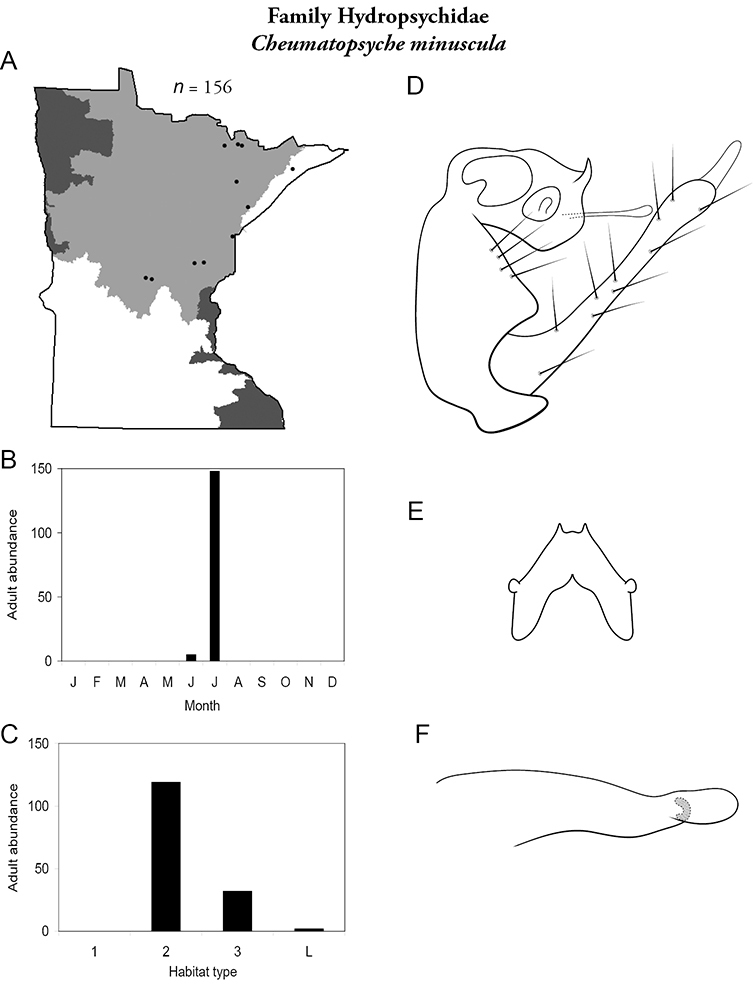
Cheumatopsyche minuscula (Figure 36) has been collected in the Lake Superior and Northern Regions, almost exclusively in July. It was found mostly in medium rivers, with some specimens found in large rivers.
Cheumatopsyche miniscula A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E Lobes of tergum X (caudal view) F phallus.
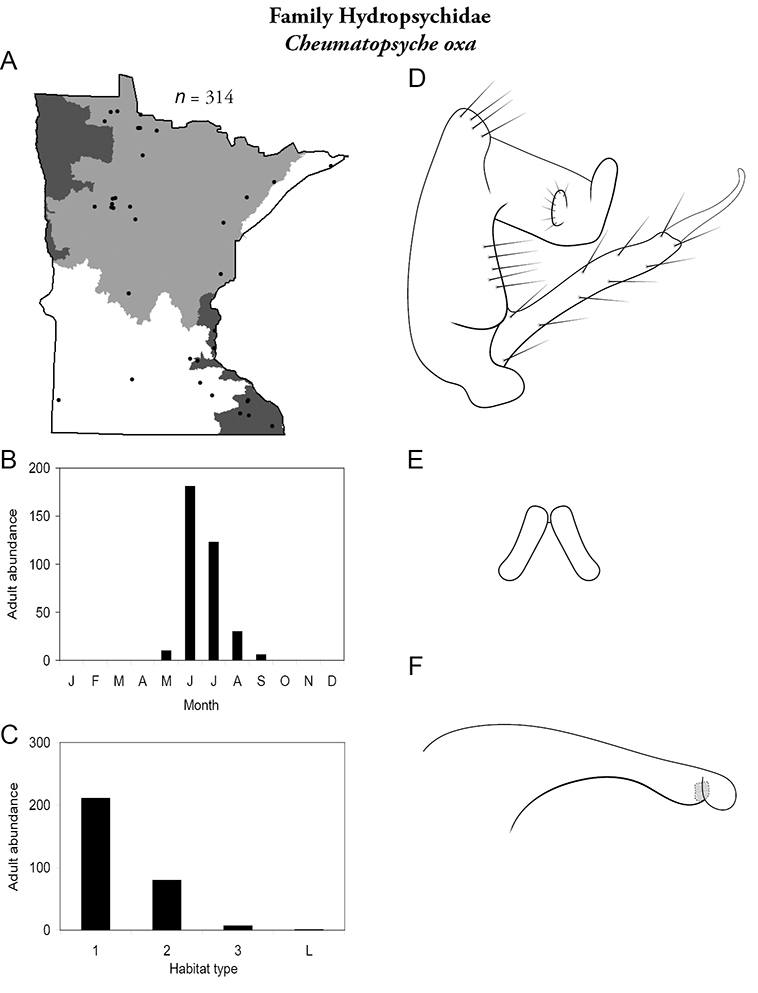
Cheumatopsyche oxa (Figure 37) has been found in all regions except the Northwestern. It was not particularly abundant, however, especially when compared to some of its congeners. Adults were present from May to September, with a greatest abundance in June and July. It was found mainly in small streams and occasionally in medium rivers.
Cheumatopsyche oxa A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E Lobes of tergum X (caudal view) F phallus.
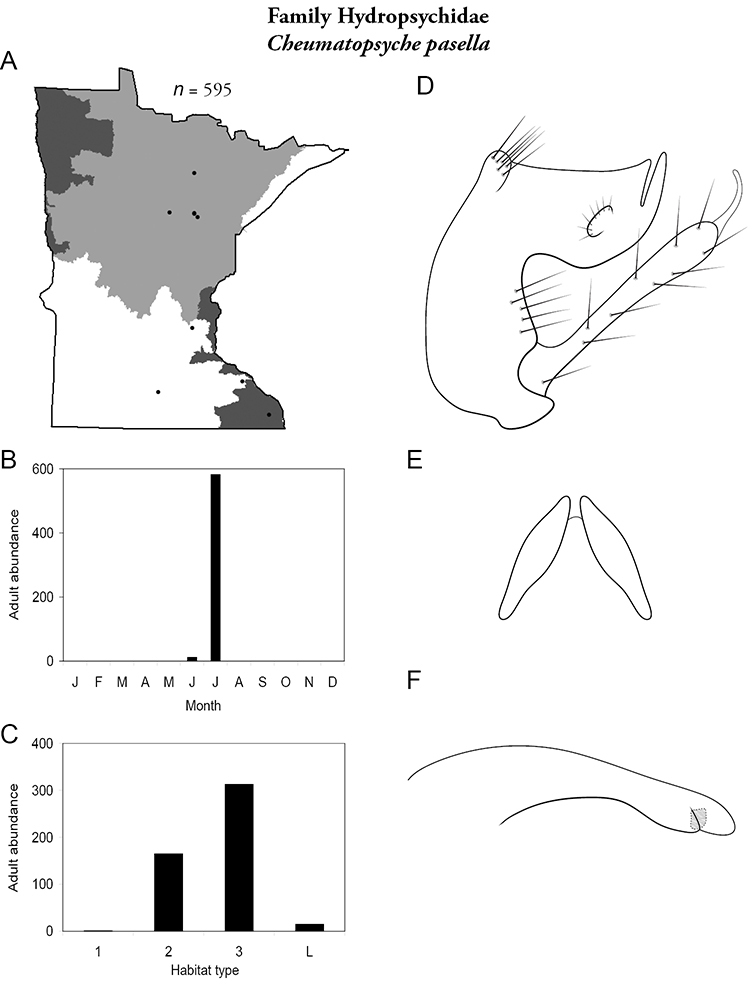
Cheumatopsyche pasella (Figure 38) has been found sporadically in the Northern, Southeastern, and Southern Regions. It was found almost exclusively in July, and mostly in medium and large rivers. It was the 2nd most abundant species of large rivers of the Southeastern Region (Table 6).
Cheumatopsyche pasella A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E Lobes of tergum X (caudal view) F phallus.
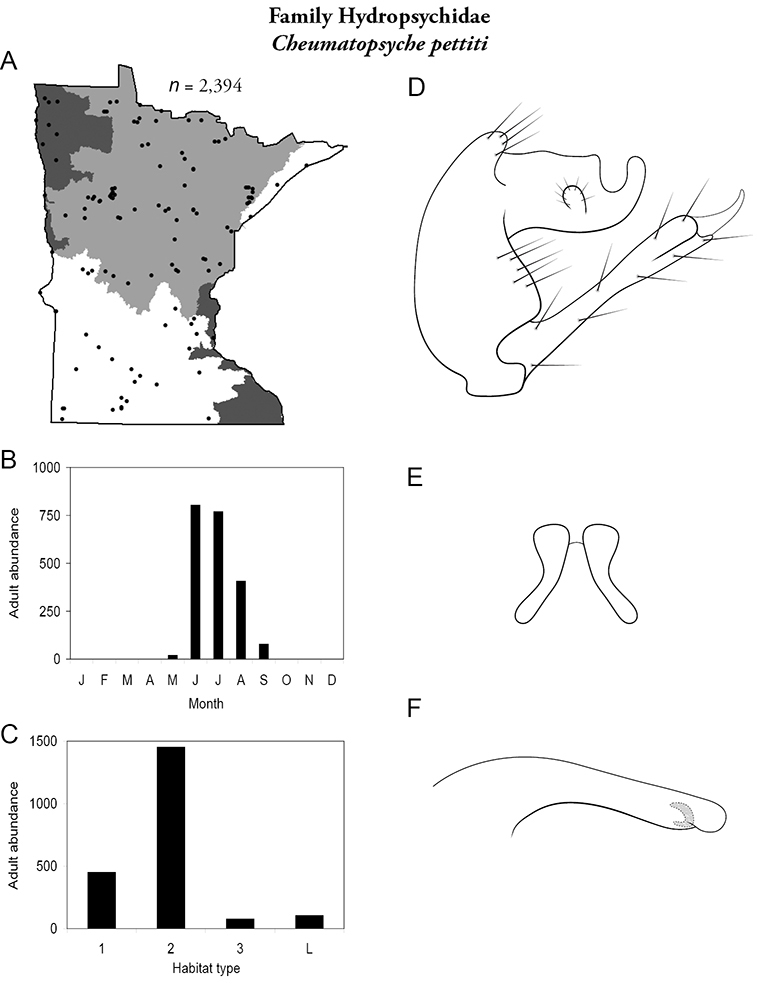
Cheumatopsyche pettiti (Figure 39) was the most widespread Cheumatopsyche species, collected throughout the state from May to September, and abundant from June through August. It was most abundant in small and, especially, medium rivers.
Cheumatopsyche pettiti A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E Lobes of tergum X (caudal view) F phallus.
Cheumatopsyche sordida (Figure 40) is known predominantly from the Northern Region, with occasional collections in the Lake Superior and Northwestern Regions. It was found almost exclusively in medium and large rivers. Adults were present in June and abundant in July.
Cheumatopsyche sordida A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E Lobes of tergum X (caudal view) F phallus.
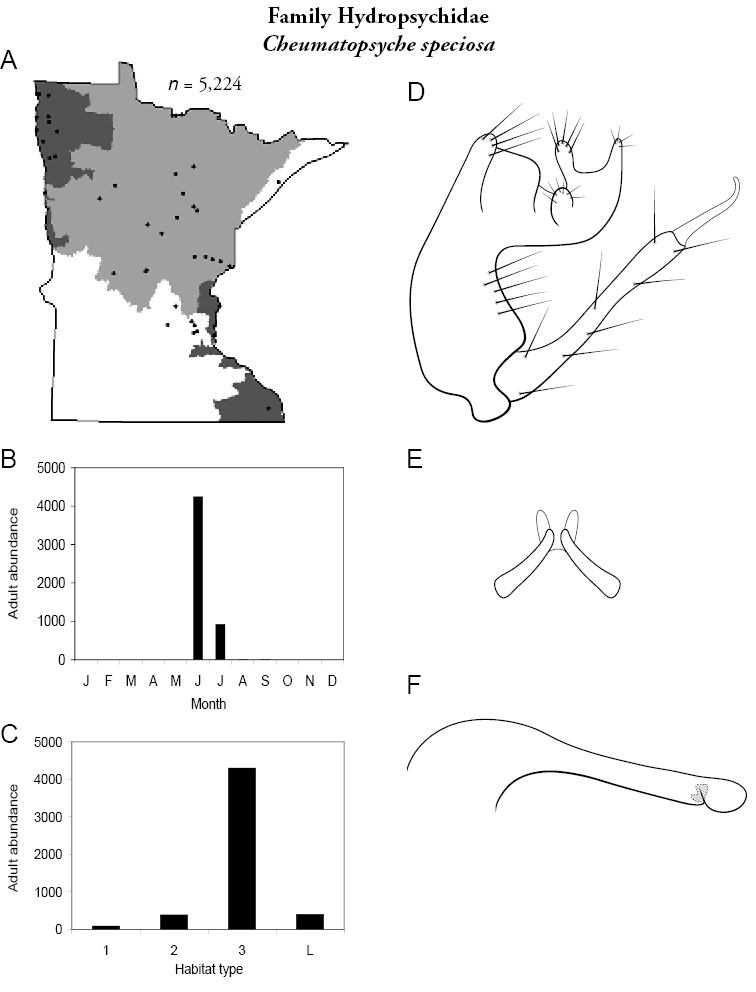
Cheumatopsyche speciosa (Figure 41) has been collected in all regions, but was especially abundant in the Northwestern Region. Overall, the species was most abundant in large rivers. Adults were most abundant in June and present in July. This species is the smallest of the Cheumatopsyche; adults are around 5 mm in length.
Cheumatopsyche speciosa A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E Lobes of tergum X (caudal view) F phallus.
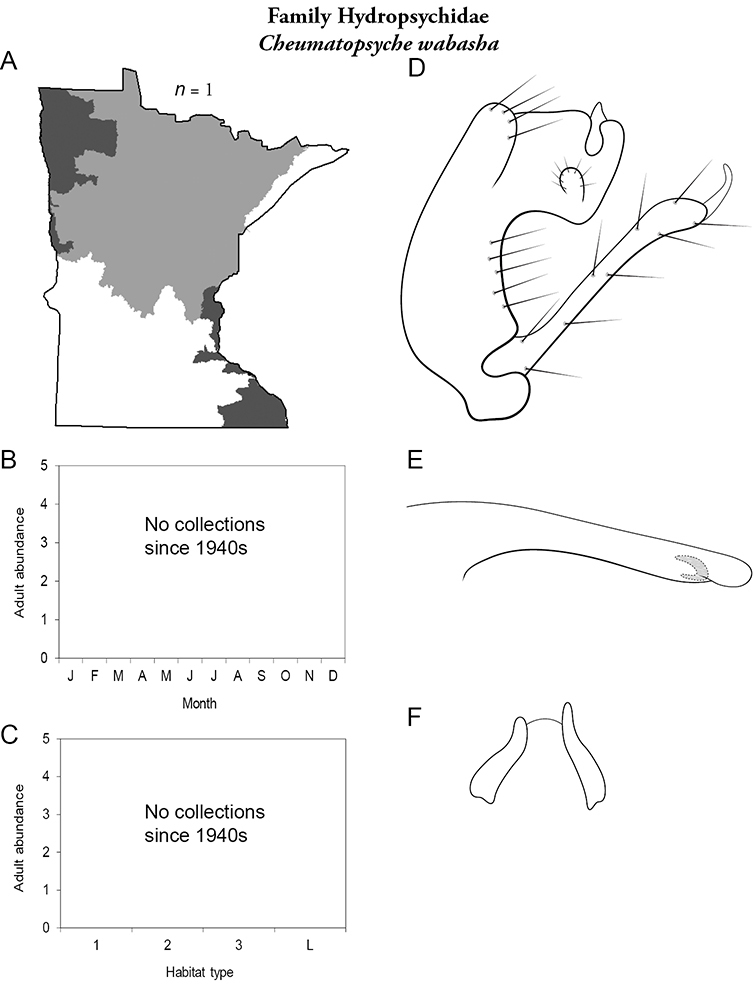
Cheumatopsyche wabasha (Figure 42) was described from a specimen collected in the city of Wabasha during July 1941. The species has not been seen in Minnesota since this holotype collection. It has, however, been collected in Oregon and Tennessee (
Cheumatopsyche wabasha A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E Lobes of tergum X (caudal view) F phallus.
The genus Diplectrona contains a single species in Minnesota. For additional species, see
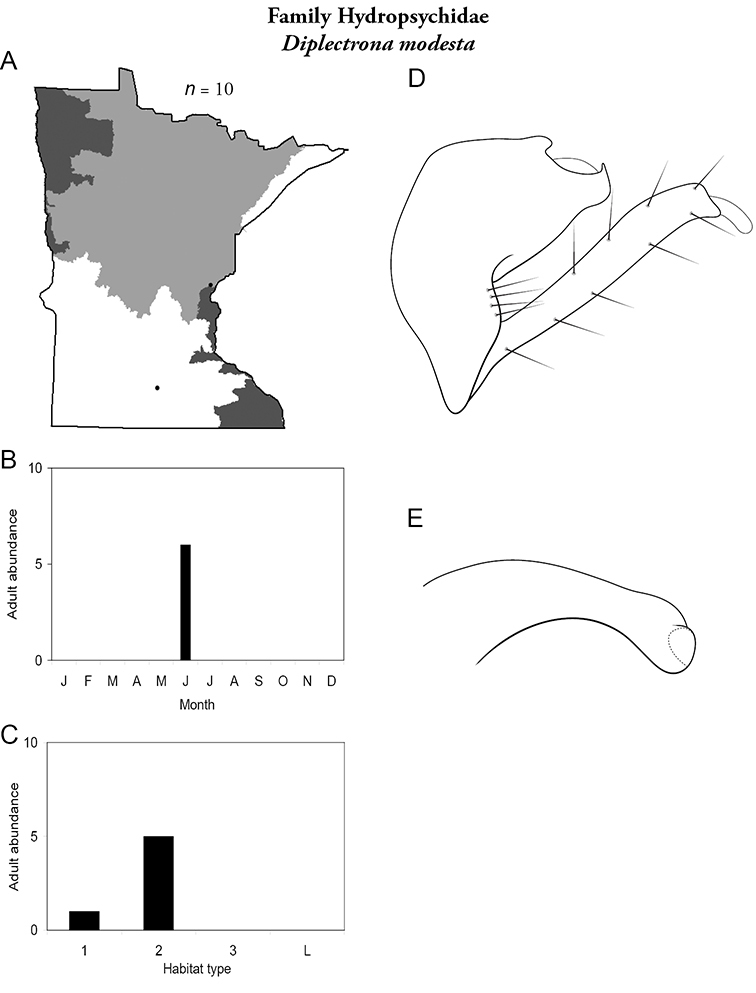
Diplectrona modesta (Figure 43) is known only from a small and medium river in Minneopa State Park in the Southern Region, and from a small unnamed spring in the Northern Region. All specimens were collected during June.
Diplectrona modesta A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
The genus Hydropsyche contains 17 species in Minnesota. It is the 3rd most species-rich genus (Figure 7). For additional species, see
Larvae of the Hydropsyche are very conspicuous on the undersides of medium and large rocks in nearly any stream. Species are more likely to be in smaller streams than Cheumatopsyche, but there are many exceptions. Adults are 8–14 mm in length. Some species have uniformly brown or grey wings. Others include a darker mottled pattern (Figure 290). Separation of males of Hydropsyche bidens, Hydropsyche scalaris, Hydropsyche simulans, and Hydropsyche orris requires very careful examination of the tip of the phallus.
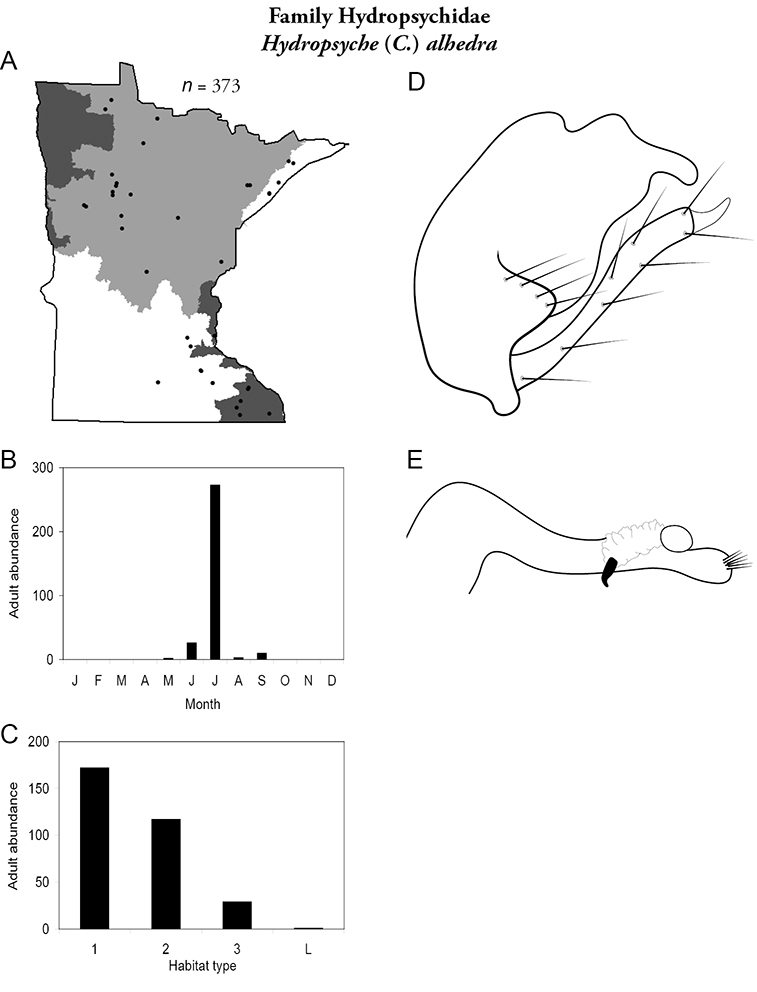
Hydropsyche (C.) alhedra (Figure 44) has been found in all regions except the Northwestern. It was collected from all sizes of streams, especially small and medium streams. Adults were present from May to September, but abundant only in July.
Hydropsyche alhedra A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
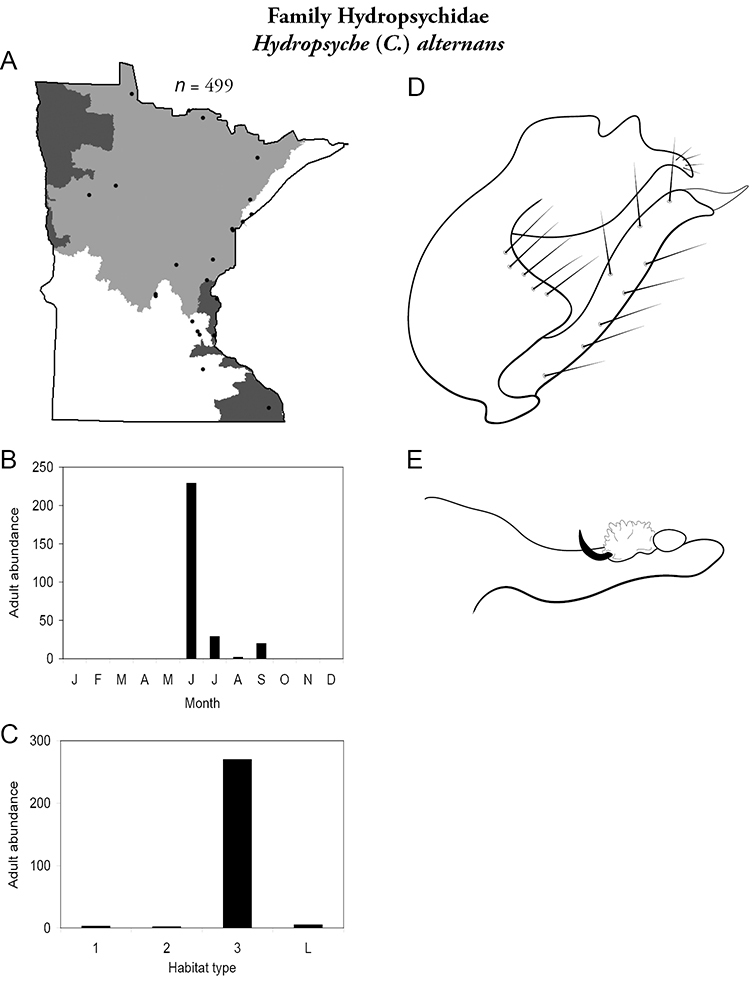
Hydropsyche (C.) alternans (Figure 45) is known from the Northern, Southeastern, and Southern Regions, almost exclusively from large rivers. Adults were abundant in June and present from July to September.
Hydropsyche alternans A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
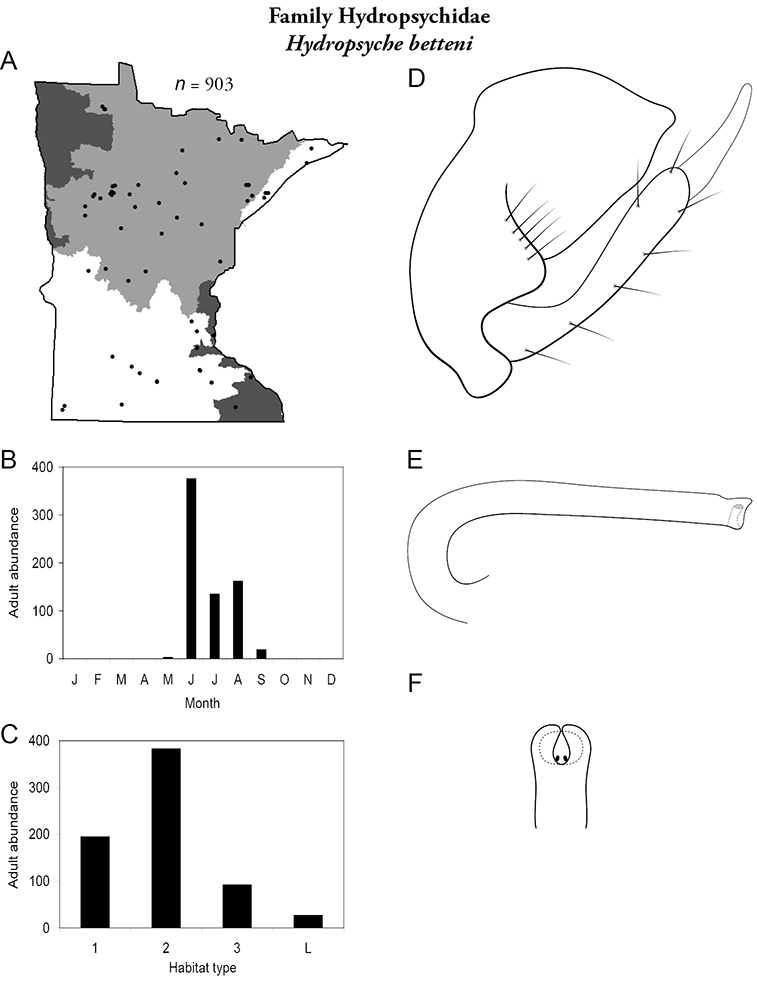
Hydropsyche betteni (Figure 46) has been found in all regions except the Northwestern. It was most abundant in medium rivers, but also found in small streams and large rivers. Some adults were collected in May and September; the majority were found from June through August.
Hydropsyche betteni A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F apical tip of phallus (dorsal view).
Hydropsyche bidens (Figure 47) is known from all regions except the Lake Superior. It was most abundant in medium and, especially, large rivers. It was also, however, the most abundant species in small streams of the Northwestern Region (Table 5). Adults were most abundant in June and also found in July and August.
Hydropsyche bidens A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F apical tip of phallus (dorsal view).
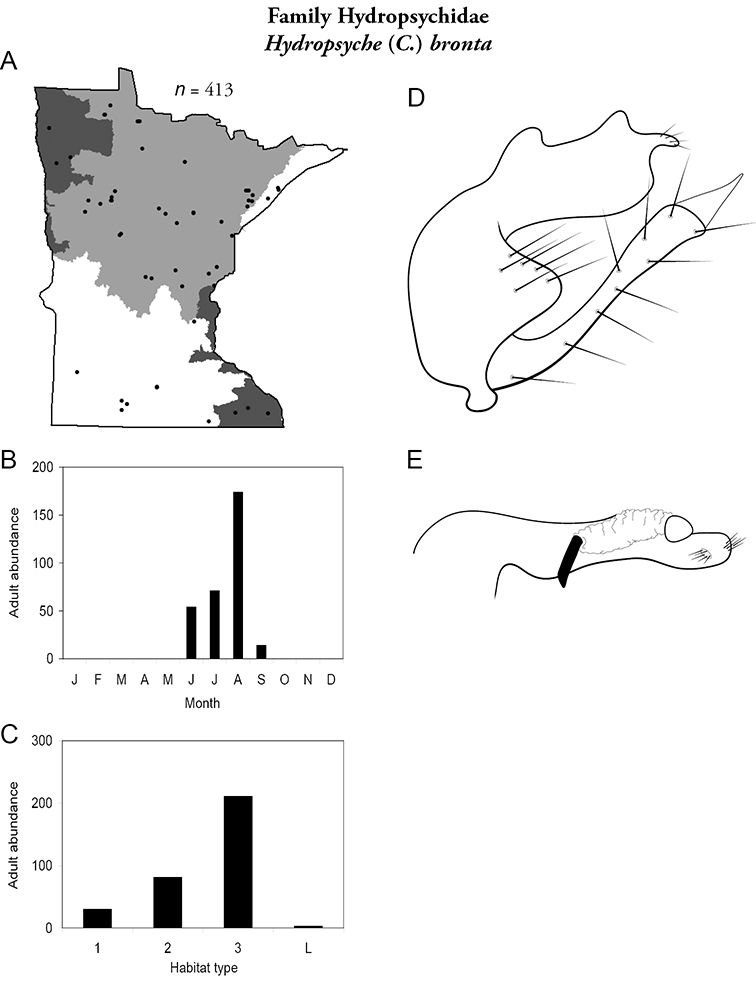
Hydropsyche (C.) bronta (Figure 48) has been collected in all regions, but was not particularly abundant. Adults were most abundant in August, with specimens present June through September. It was found in all sizes of streams, especially large rivers.
Hydropsyche bronta A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
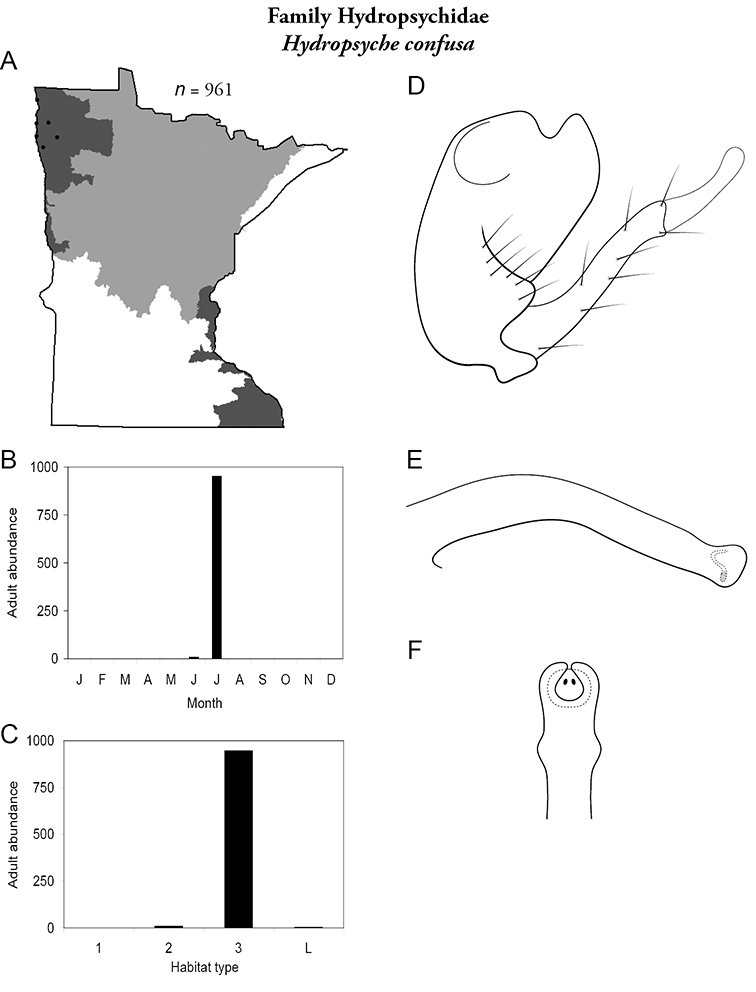
Hydropsyche confusa (Figure 49) is the only species of caddisfly currently found exclusively in the Northwestern Region. Adults were found in July from large rivers. The species was the second most abundant caddisfly in large rivers of the Northwestern Region (Table 5).
Hydropsyche confusa A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F apical tip of phallus (dorsal view).
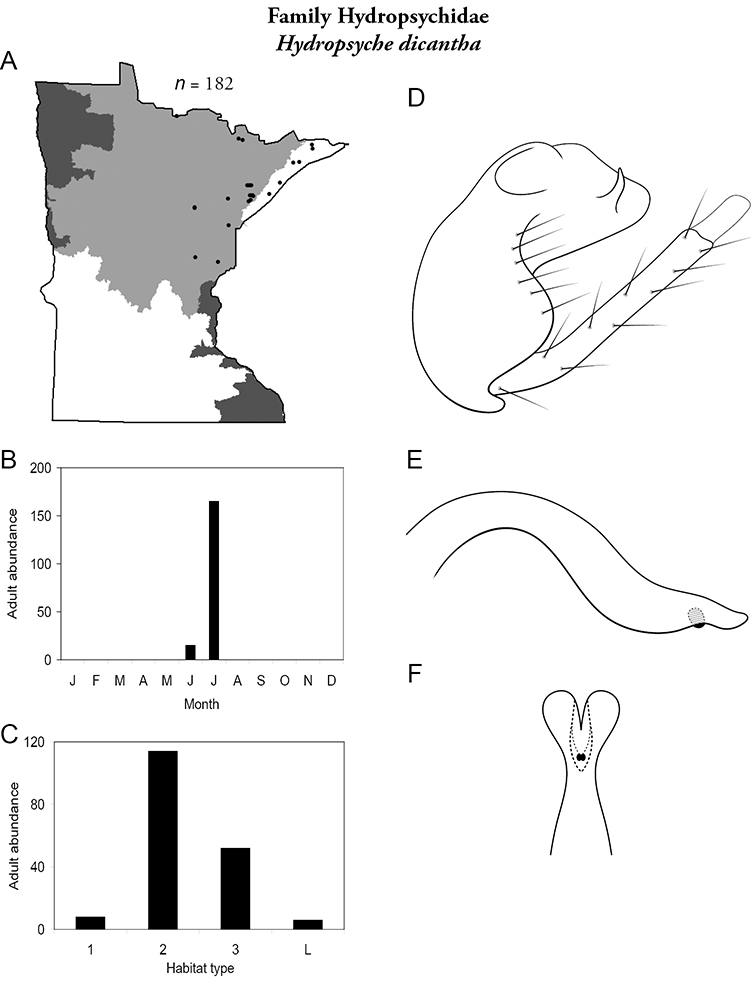
Hydropsyche dicantha (Figure 50) is known from the Lake Superior and Northern regions. It was most abundant in large and, especially, medium rivers. Adults were collected primarily in July.
Hydropsyche dicantha A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F apical tip of phallus (dorsal view).
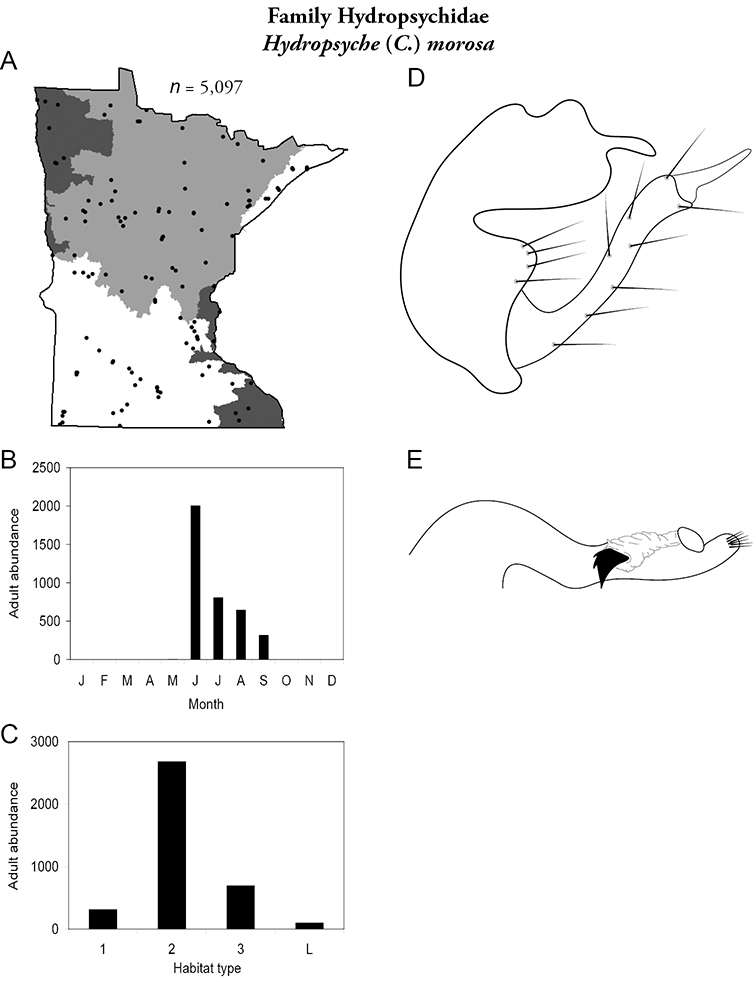
Hydropsyche (C.) morosa (Figure 51) was common throughout the state and found from June through September. It was most abundant in medium rivers.
Hydropsyche morosa A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
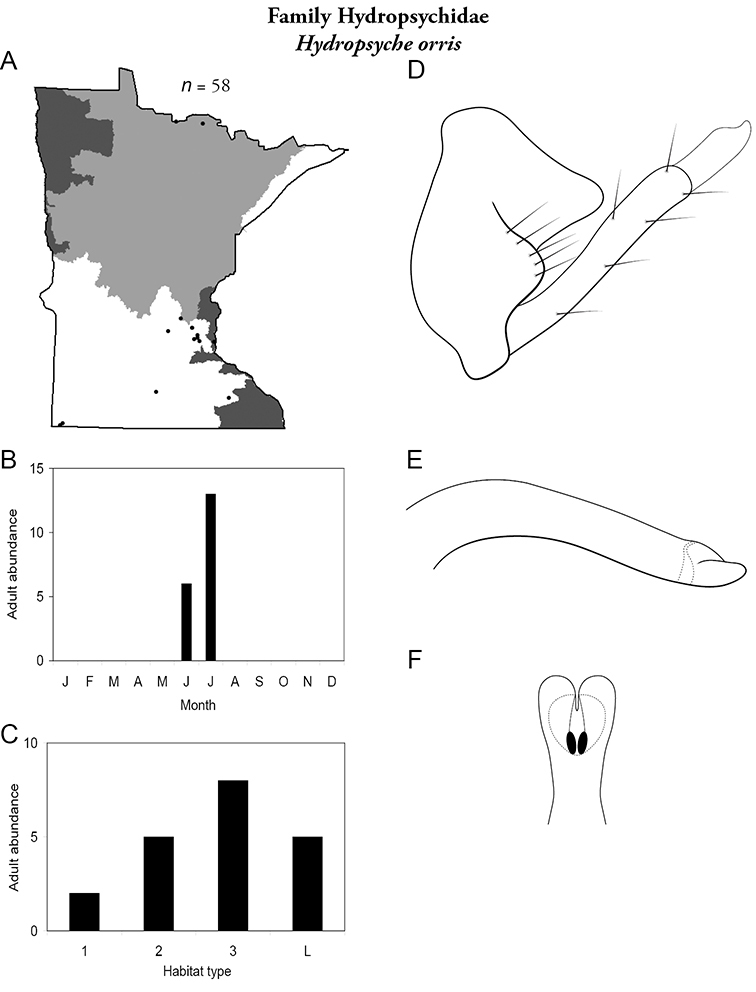
Hydropsyche orris (Figure 52) was collected primarily from the Southern Region, with a couple of collections from large rivers of the Northern Region. Adults were found in June and July from all habitat types, including lakes. Specimens, however, were not particularly abundant.
Hydropsyche orris A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F apical tip of phallus (dorsal view).
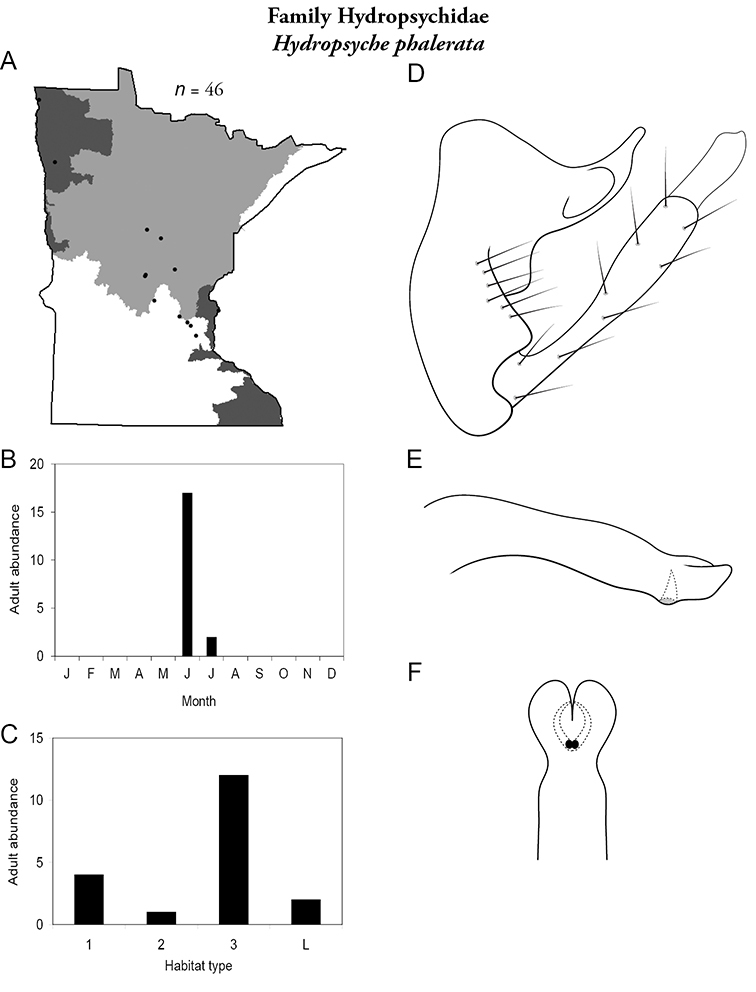
Hydropsyche phalerata (Figure 53) has been found primarily in large rivers sporadically throughout the state, except for in the Lake Superior Region. Nearly all adults were collected in June.
Hydropsyche phalerata A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F apical tip of phallus (dorsal view).
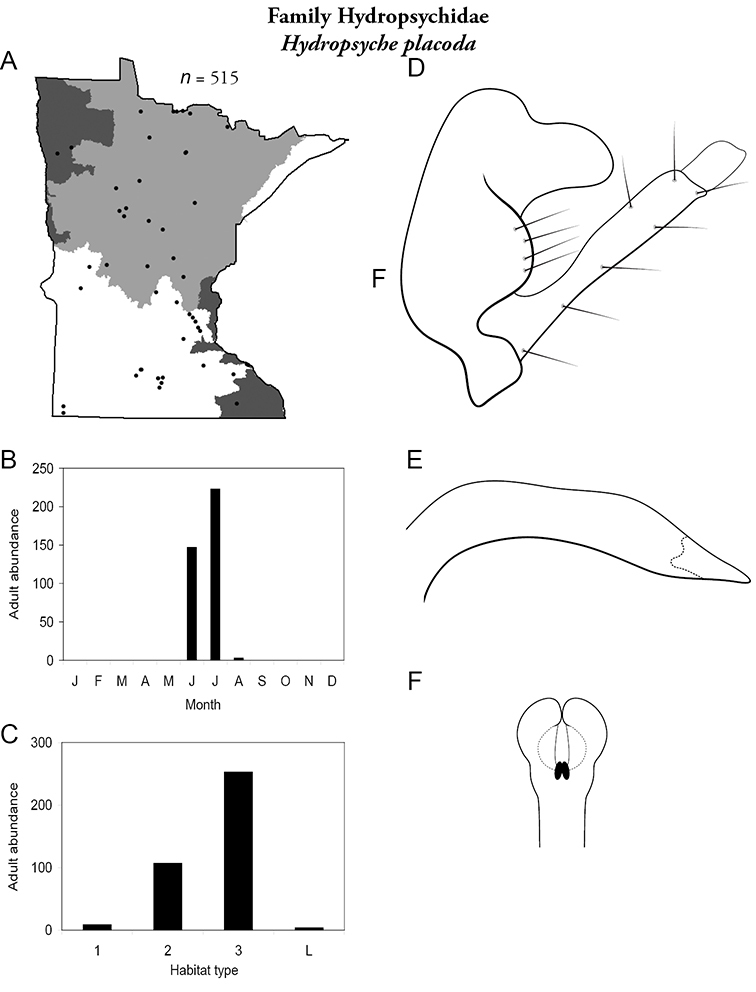
Hydropsyche placoda (Figure 54) is known from all regions except the Lake Superior. It was found primarily in large rivers, with some presence in medium rivers. Adults were found mostly in June and July. In addition to genitalic characteristics, males of this species can be recognized by their enlarged compound eyes.
Hydropsyche placoda A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F apical tip of phallus (dorsal view).
Hydropsyche scalaris (Figure 55) has been collected sporadically throughout the state, mostly during June from medium rivers.
Hydropsyche scalaris A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F apical tip of phallus (dorsal view).
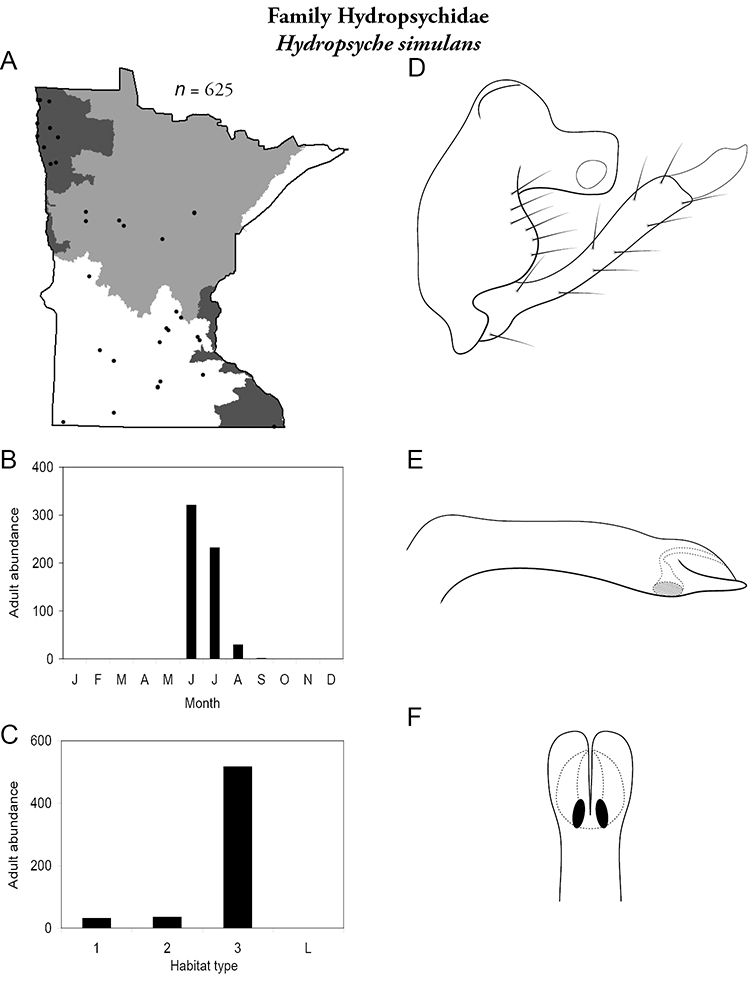
Hydropsyche simulans (Figure 56) has been collected primarily in the Northern, Northwestern, and Southern Regions. Statewide, it was found primarily in large rivers. It was, however, one of the most abundan species in allsizes of stream of the Southern Region due to excess agricultural input (Table 7). It was also determined to be an “indicator species” of habitat disturbance in small and medium streams (
Hydropsyche simulans A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F apical tip of phallus (dorsal view).
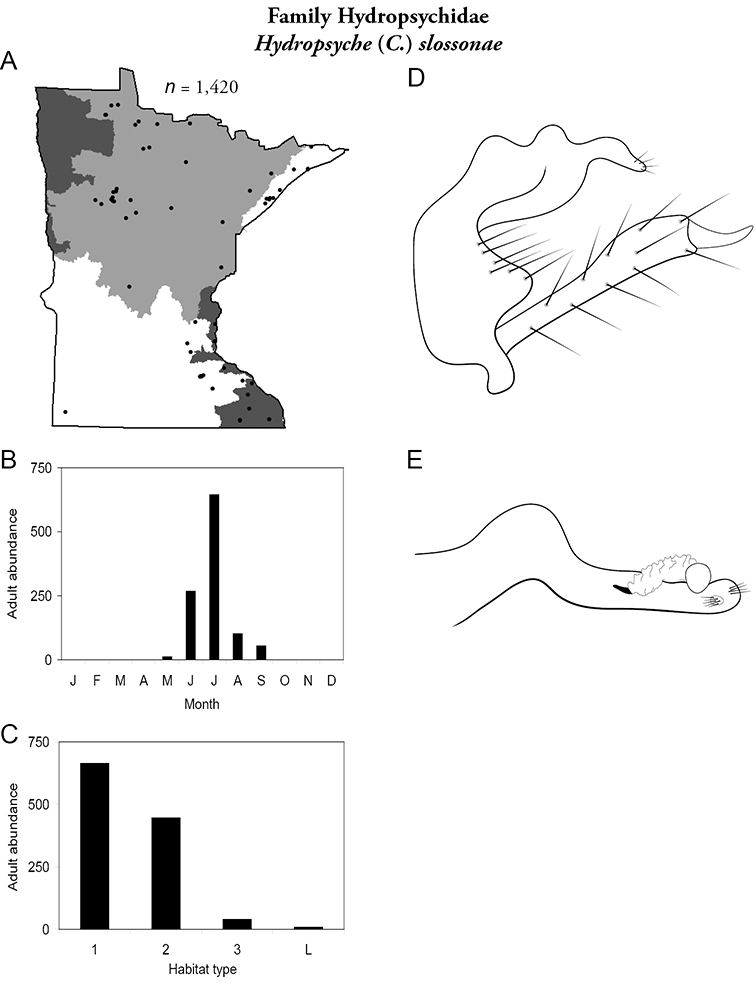
Hydropsyche (C.) slossonae (Figure 57) was found in all regions except the Northwestern. Unlike most hydropsychids, Hydropsyche slossonae was most abundant in small undisturbed streams. Adults were present from May to September, with greatest abundance in July.
Hydropsyche slossonae A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
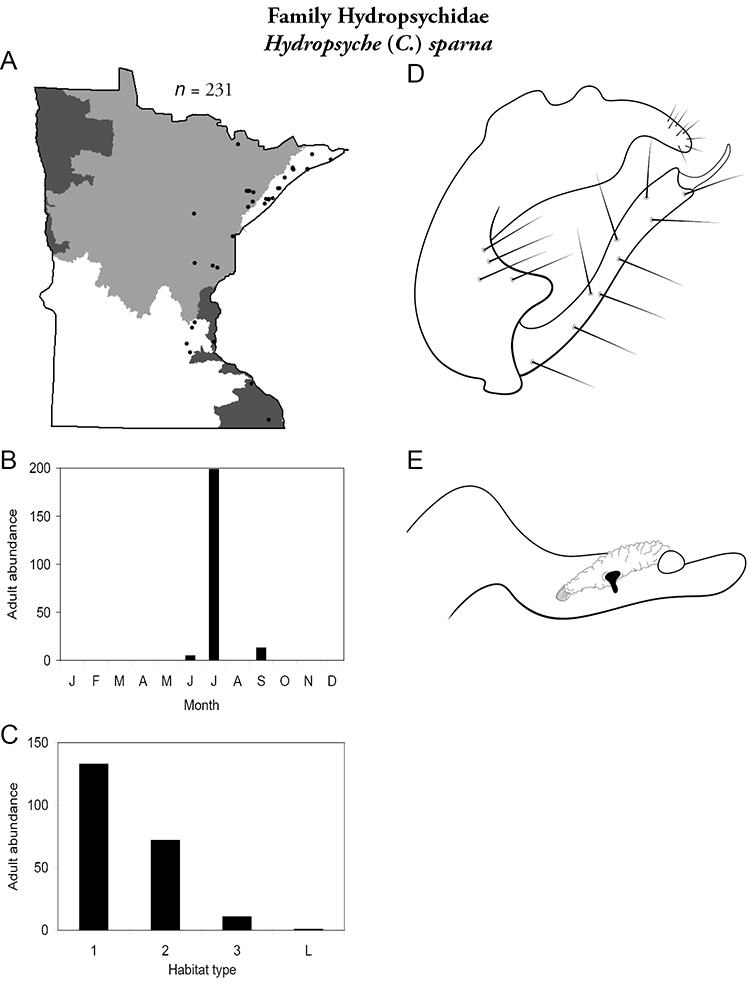
Hydropsyche (C.) sparna (Figure 58) was collected from the eastern third of the state. Similar to Hydropsyche slossonae, Hydropsyche sparna was most abundant in small streams, and fairly abundant in medium rivers. It was the most abundant species of small streams in the Lake Superior Region (Table 3). Nearly all specimens were caught in July.
Hydropsyche sparna A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
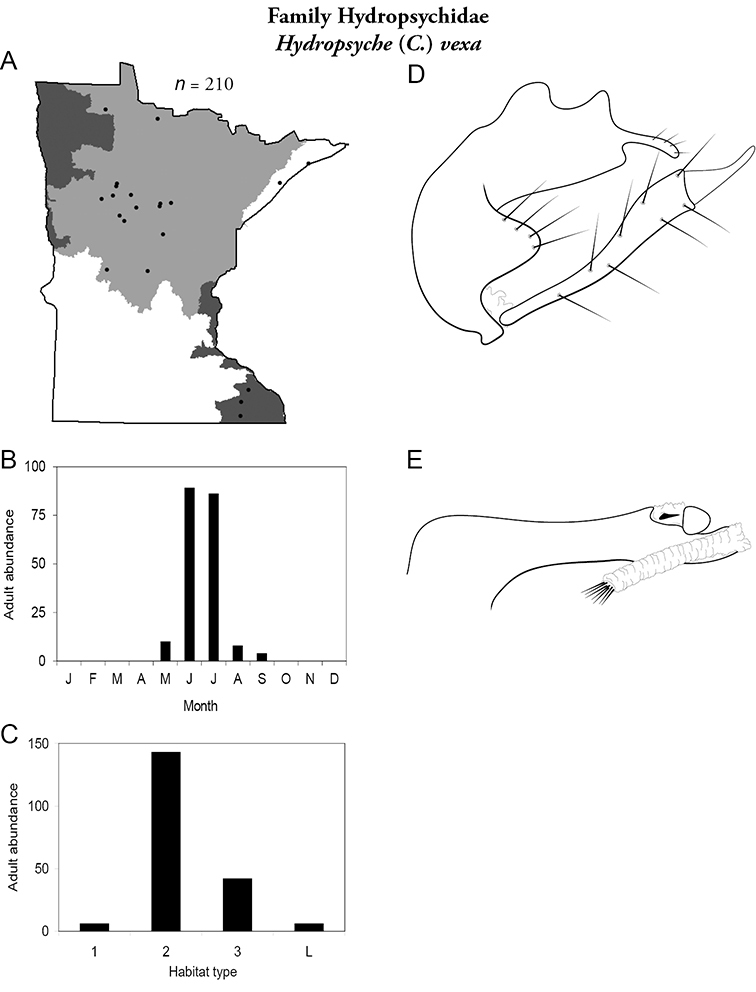
Hydropsyche (C.) vexa (Figure 59) is known from the Lake Superior, Northern, and Southeastern Regions. It was most abundant in large and, especially, medium rivers. Adults were present from May to September and most abundant in June and July.
Hydropsyche vexa A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
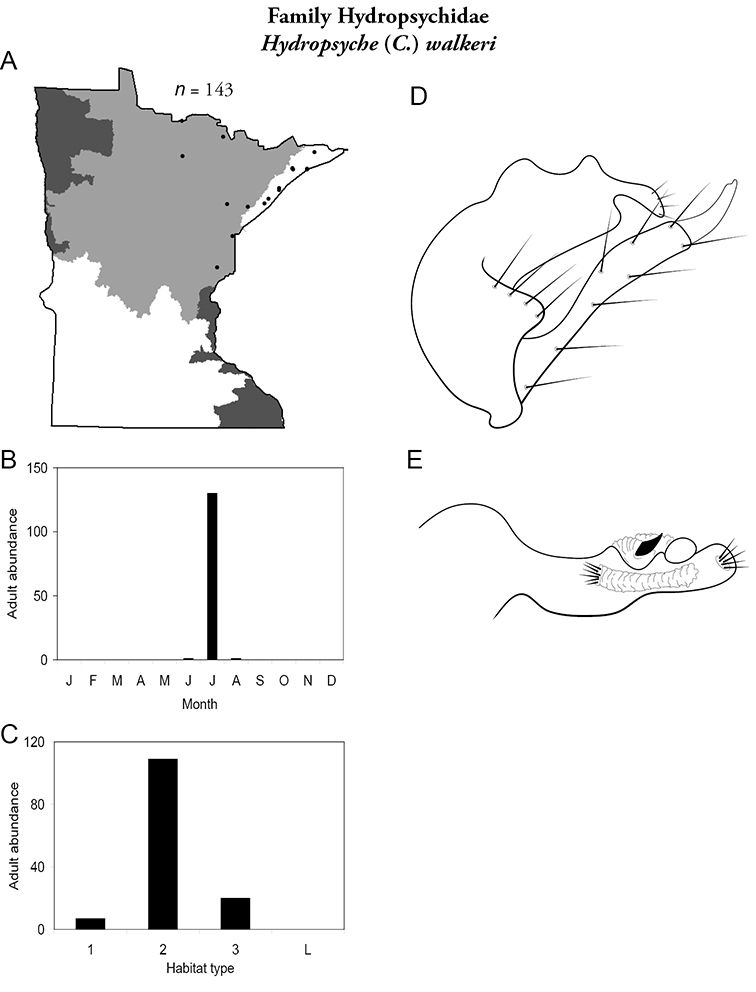
Hydropsyche (C.) walkeri (Figure 60) has been collected from the Lake Superior and Northern Regions, almost exclusively during July. It was most abundant in medium rivers.
Hydropsyche walkeri A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Several other Hydropsyche species: Hydropsyche californica, Hydropsyche cuanis, Hydropsyche hageni, Hydropsyche frisoni, Hydropsyche valanis, and Hydropsyche ventura, have been reported from Minnesota based on larval, female or adult specimens of unknown sex (
The genus Macrostemum contains a single species in Minnesota. For additional species, see
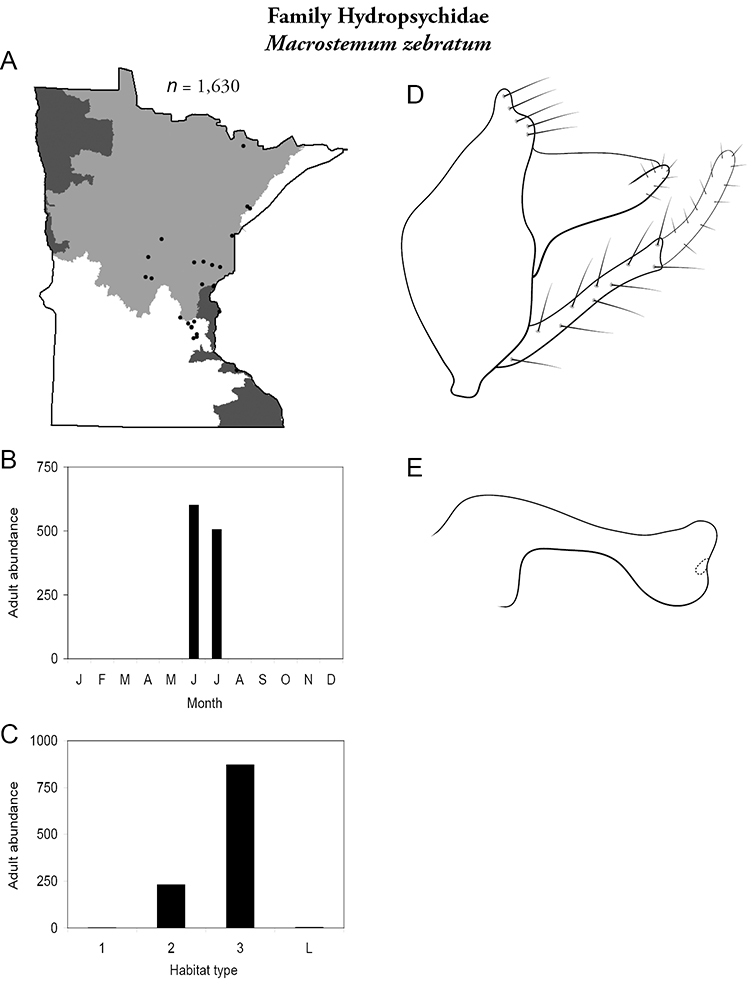
Macrostemum zebratum (Figure 61) has been found in the Northern, Southeastern, and Southern Regions. It was most abundant in large rivers, and found during June and July.
Macrostemum zebratum A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
The genus Potamyia contains a single species in North America and in Minnesota. Larvae are typically found in large rivers, although they can reach a high abundance in smaller streams with high levels of agricultural disturbance. Unlike other hydropsychids, Potamyia adults are straw-colored and have antennae >2x the length of the body (Figure 291). These 2 characteristics render both males and females easy to identify without a microscope.
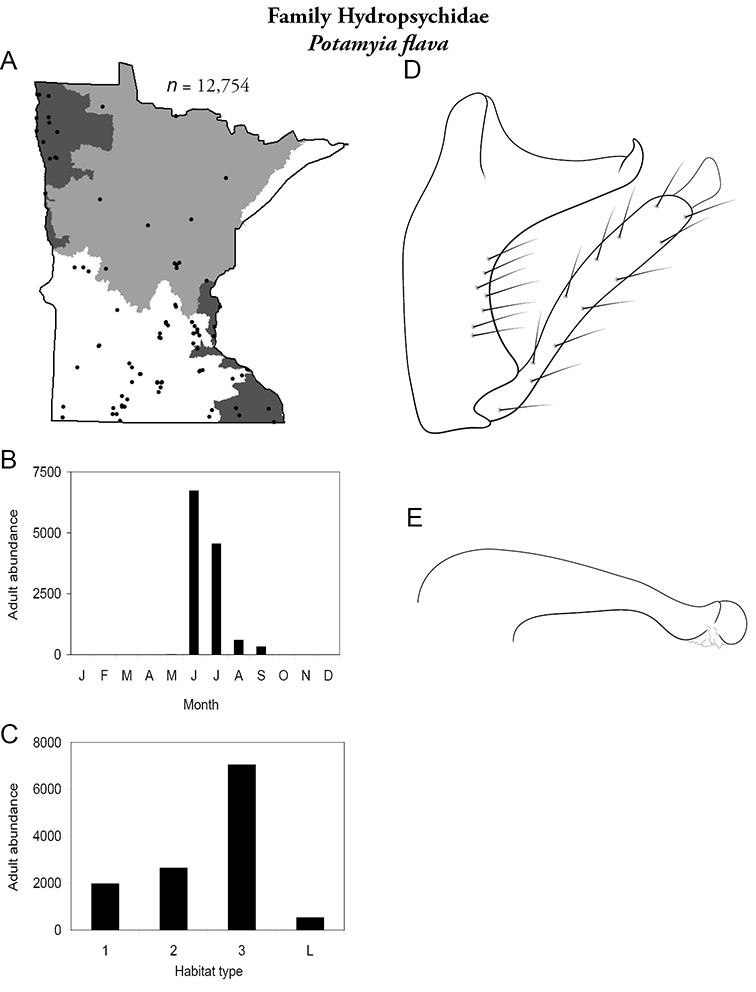
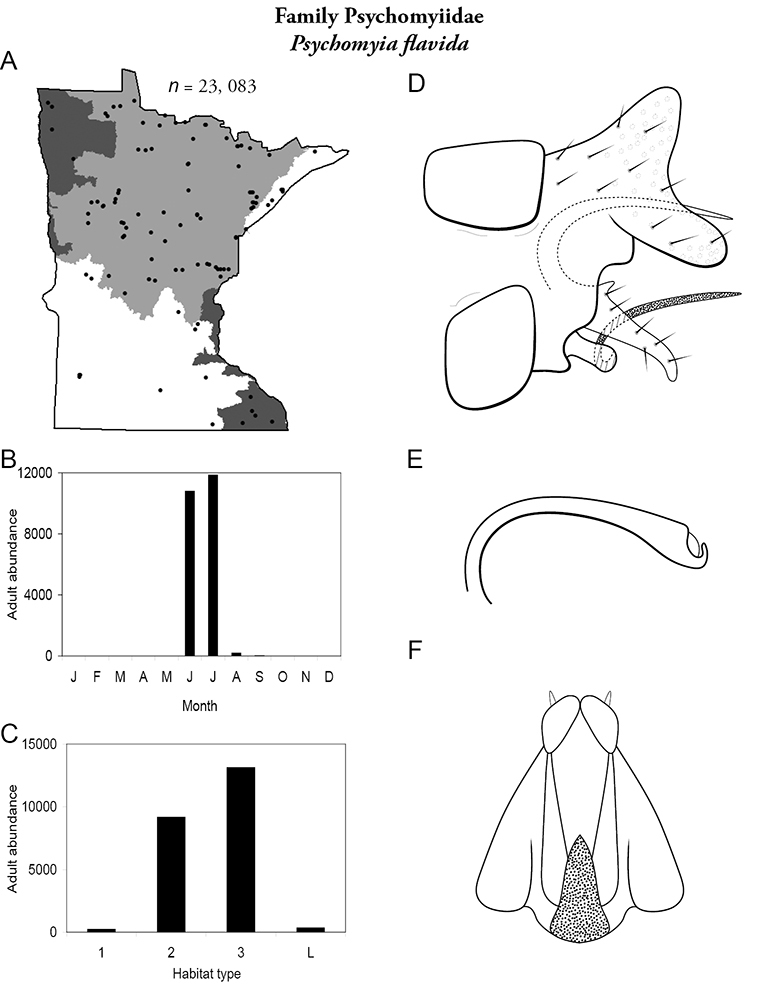
Potamyia flava (Figure 62) was found in all regions except the Lake Superior. It was the most abundant species in all sizes of stream in the Southern Region, and was the most abundant species in medium and large rivers of the Northwestern Region (Table 5). Overall, it was the 6th most abundant species in the state (Figure 9). It alsoexhibited a large increase in abundance in small and medium streams with high levels of organic input, and was determined to be an indicator species of such disturbances (
Potamyia flava A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
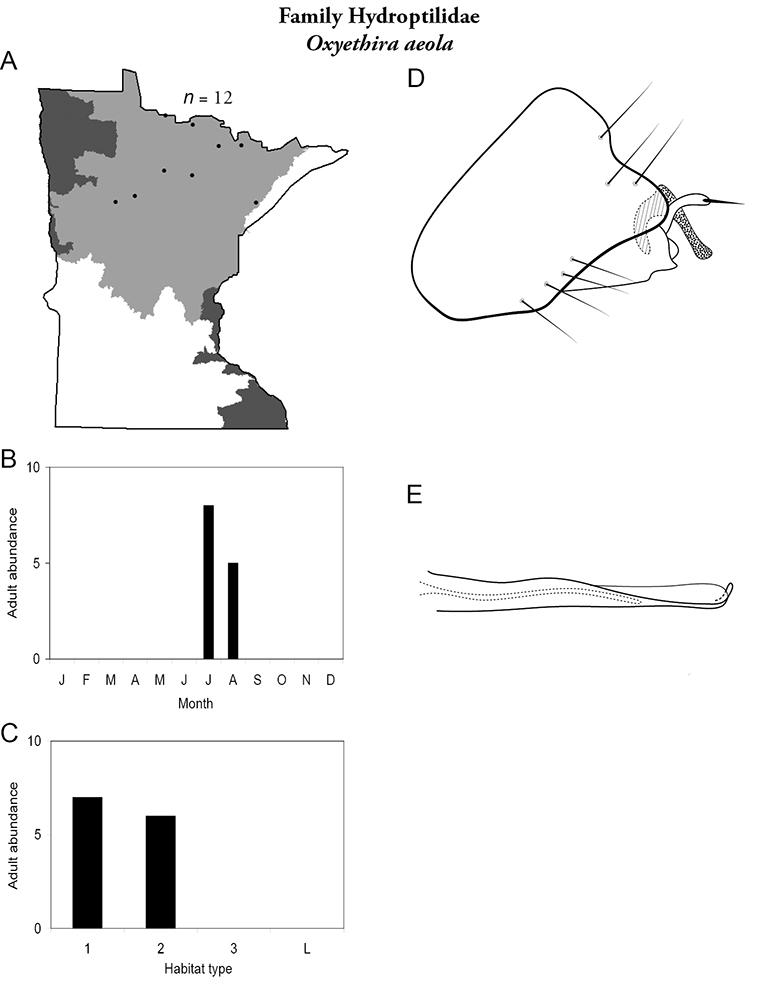
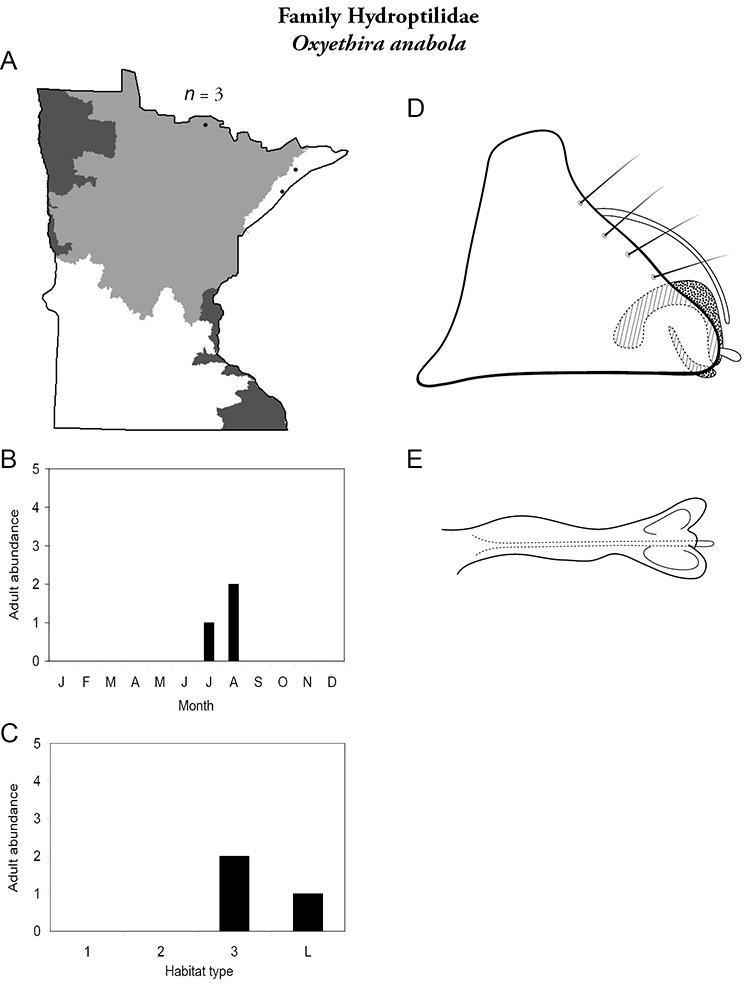
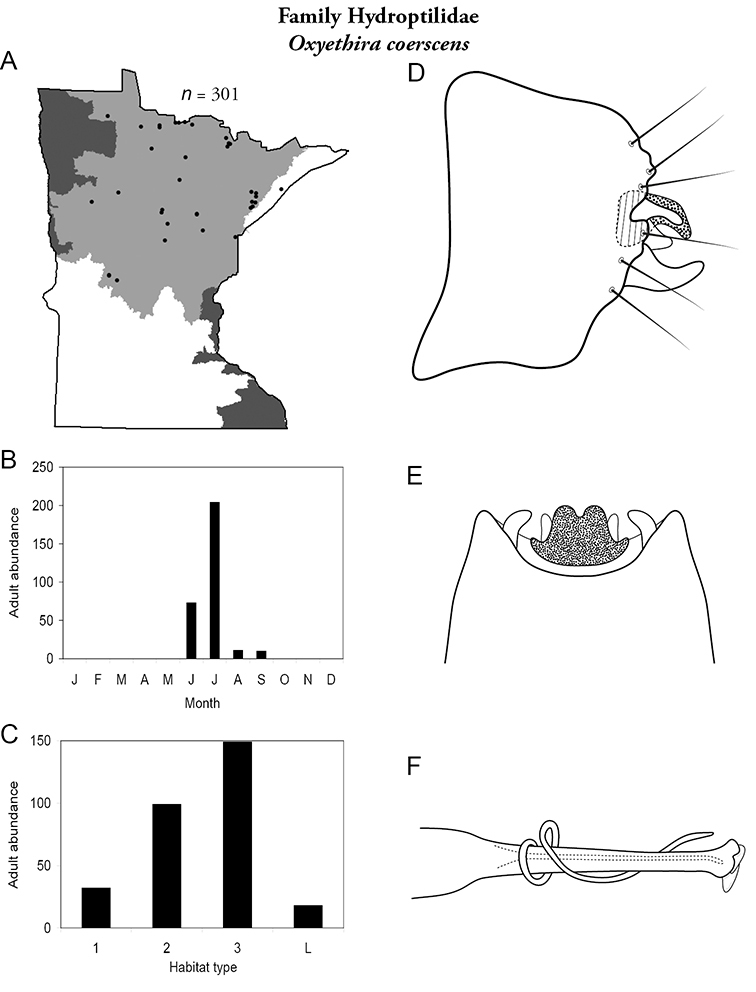
This family contains 10 genera in Minnesota: Agraylea, Hydroptila, Ithytrichia, Leucotrichia, Mayatrichia, Neotrichia, Ochrotrichia, Orthotrichia, Oxyethira, and Stactobiella, and a total of 59 species. It is the most species-rich family in the state. It also contains 2 of the most species-rich genera in the state: Hydroptila and Oxyethira. Members are often referred to as the “microcaddisflies” due to their small size. For additional species of all genera, see
Larvae are found in nearly any type of freshwater habitat, but are usually more abundant in streams. They are unique among caddisflies in their hypermetamorphic life cycle. That is, larvae do not construct cases for the first 4 instars, and are instead free-living. The terminal instar constructs a purse-like case of mainly silk, with occasional algae or small sand grains (
Most adults range 2–3 mm in length. Females of Agraylea may reach 4–5 mm. Wings are usually grey, pointed at their apices, and covered with dense setae. Most genera are macroscopically indistinguishable from each other. Adults can be very abundant in light traps; frequently, more than a dozen species were found together at a single site. Females are typically considerably more abundant than males. Unfortunately, females are not readily identifiable. Thus, species are probably more widespread and abundant than they appear. In fact, due to their small size, even males can be difficult to identify. Specimens must be cleared to have an adequate view of their genitalic structure. Further, the phallus of many species needs to be gently extruded from the genital capsule to obtain a clear view of its structure.
Genus AgrayleaThe genus Agraylea contains a single species in Minnesota. Larvae inhabit lakes and slow-moving areas of streams. They are typically found in the beds of submerged plants upon which they feed (
Agraylea multipunctata (Figure 63) is the largest of the Minnesota hydroptilids, occasionally reaching 5 mm in length. Wings have a distinctive grey and dark brown banding pattern, allowing for easy identification of both males and females with practice (Figure 290). The species is the 8th most widespread caddisfly in Minnesota (Figure 8), found throughout all regions. It was collected from all habitat types. Adults were abundant in June and July, and also present in August and September.
Agraylea multipunctata A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule (ventral view).
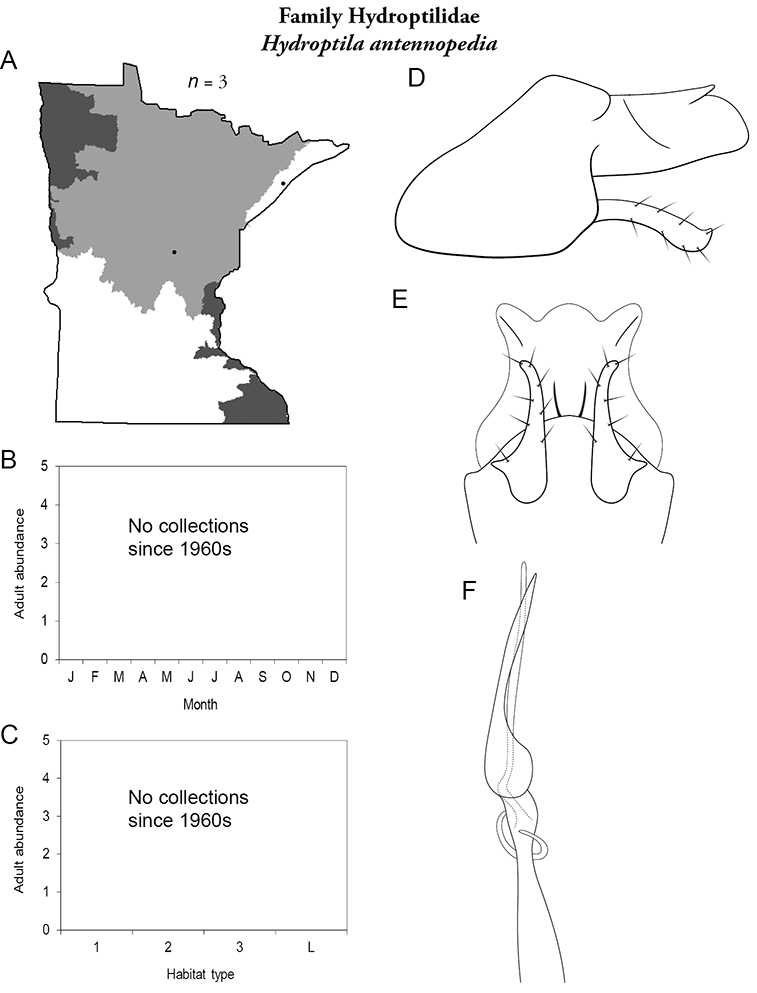
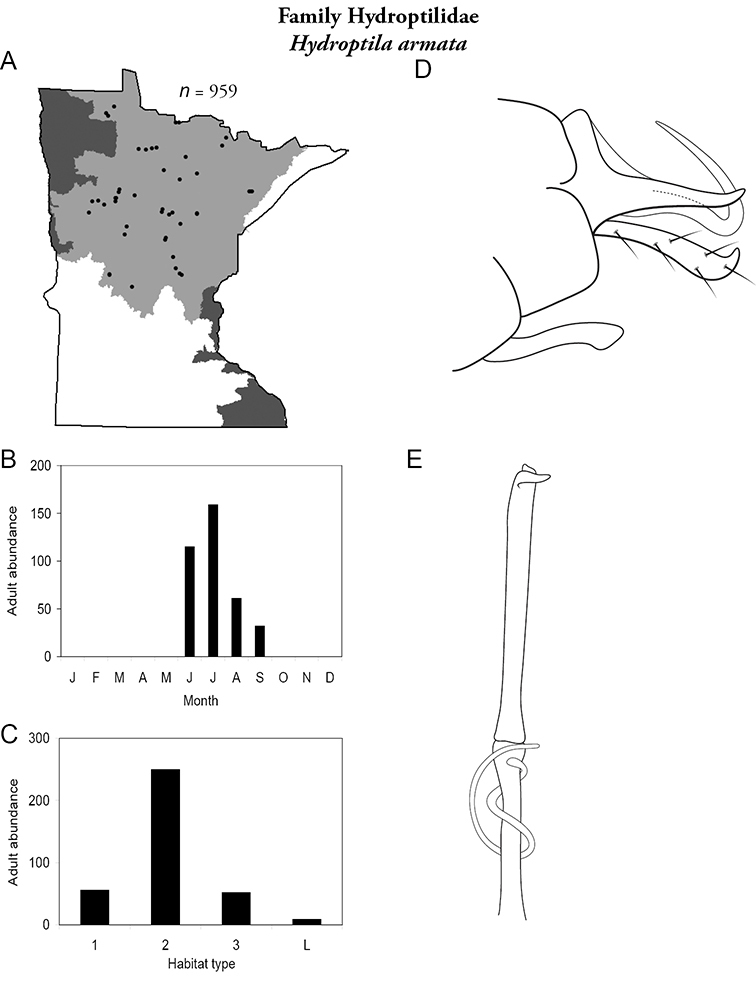
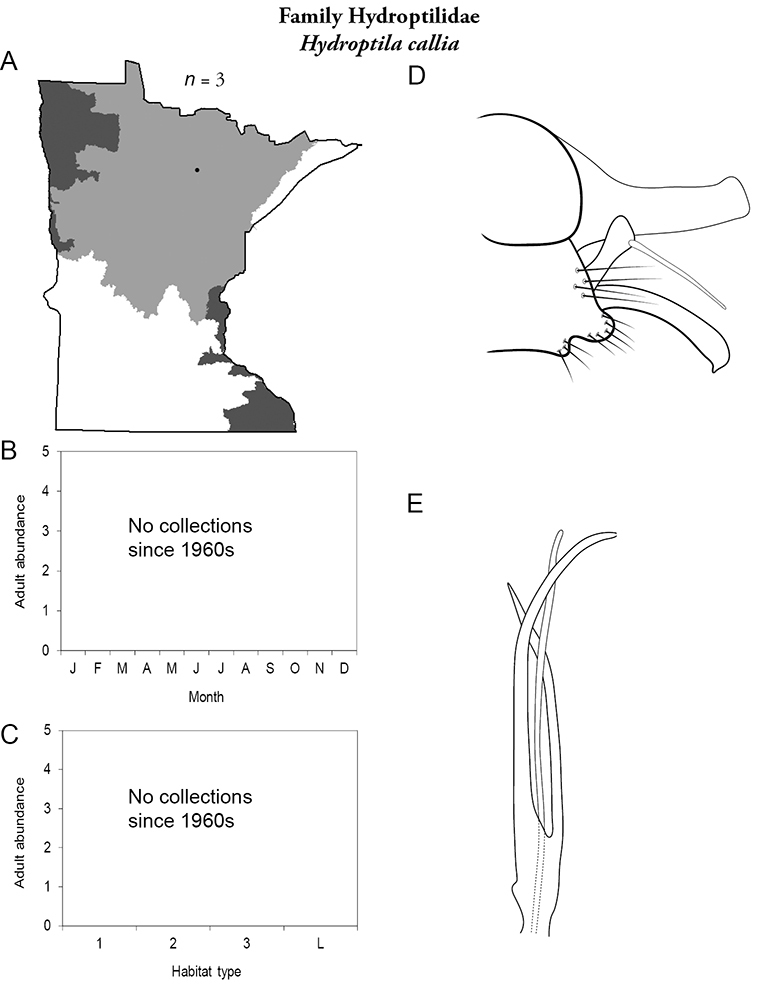
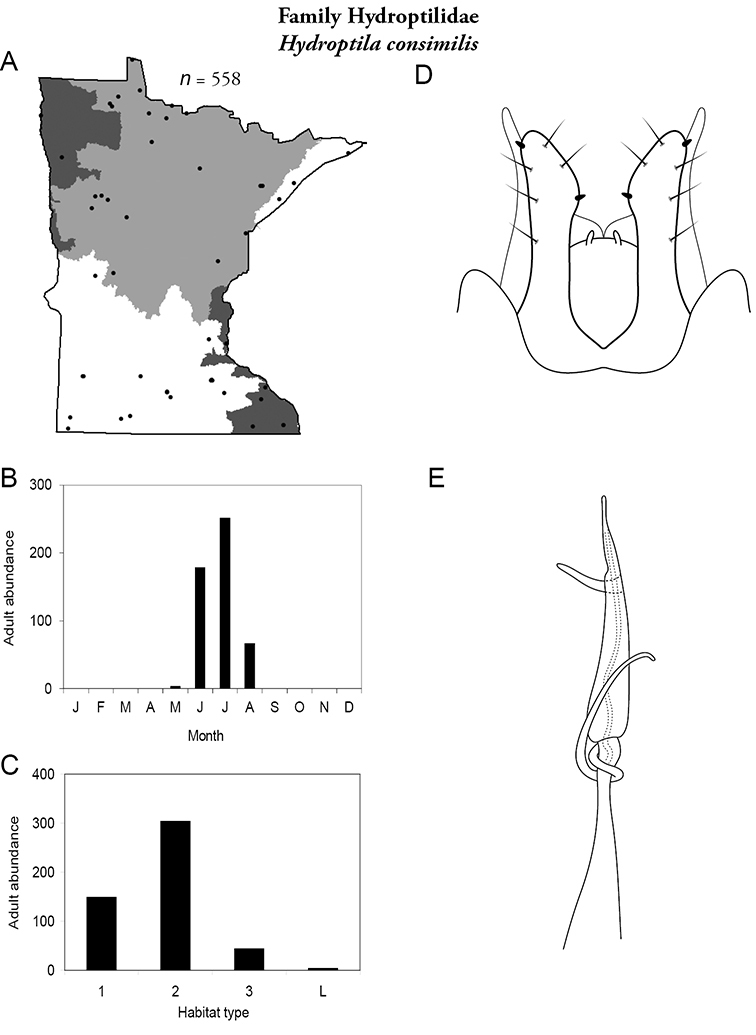
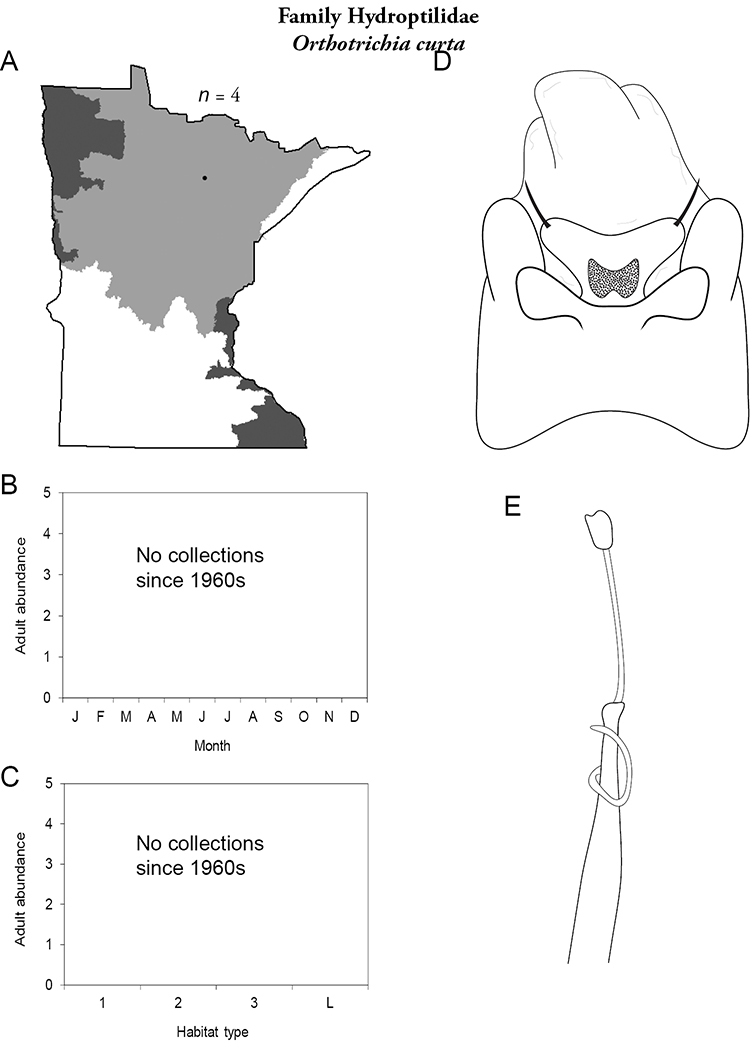
The genus Hydroptila contains 26 species in Minnesota. It is the most species-rich genus in the state. Several of the species, however, have not been collected since the 1960s or earlier. All of these species are known historically from a single or few specimens. Thus, it is difficult to know if they have been extirpated from the state or are just rare and difficult to collect. Several other species are known recently from only a few specimens. Males are rare in collections relative to females, which are not identifiable. Thus, some of the rare species may be more widespread than their known distributions suggest.
Larvae consume the contents of algal cells (
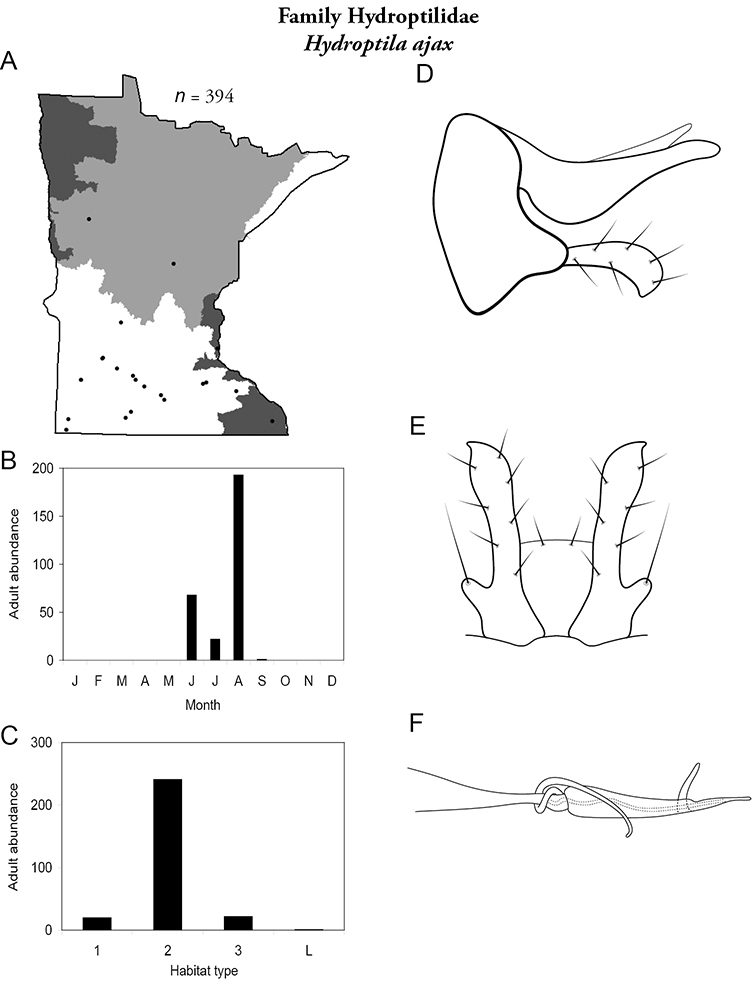
Hydroptila ajax (Figure 64) is known mainly from the Southern Region and sporadically elsewhere. It has been collected mostly from medium rivers and is most abundant in August. Some adults were collected in June, July, and September.
Hydroptila ajax A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E male genital capsule (ventral view) F phallus.
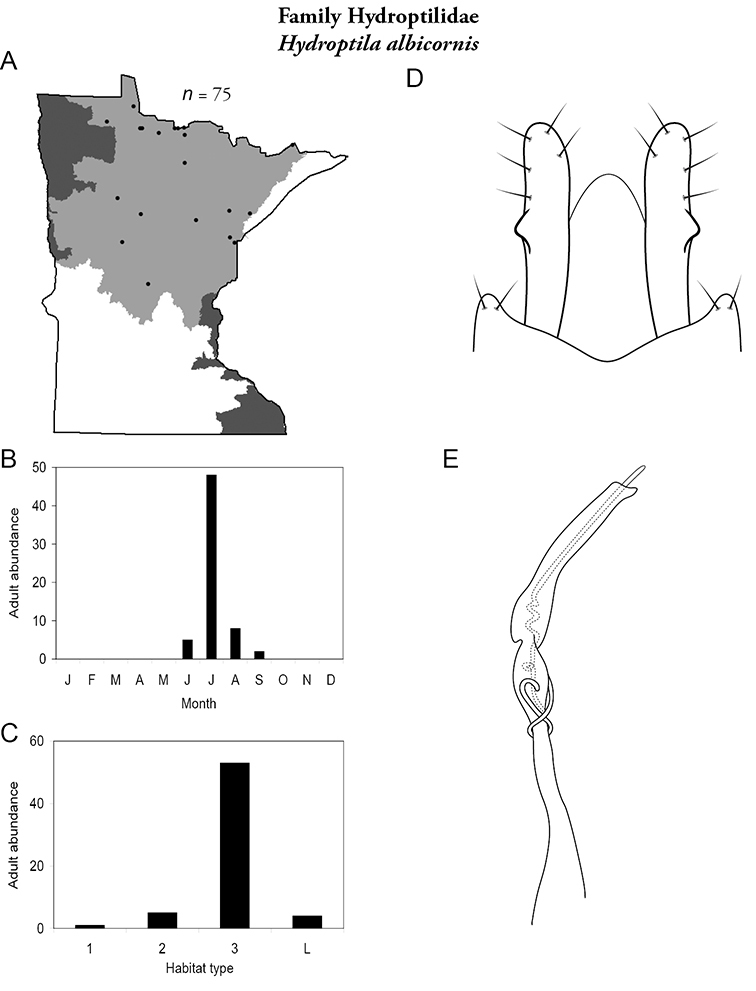
Hydroptila albicornis (Figure 65) is known only from the Northern Region. It was most frequently collected from large rivers during July.
Hydroptila albicornis A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
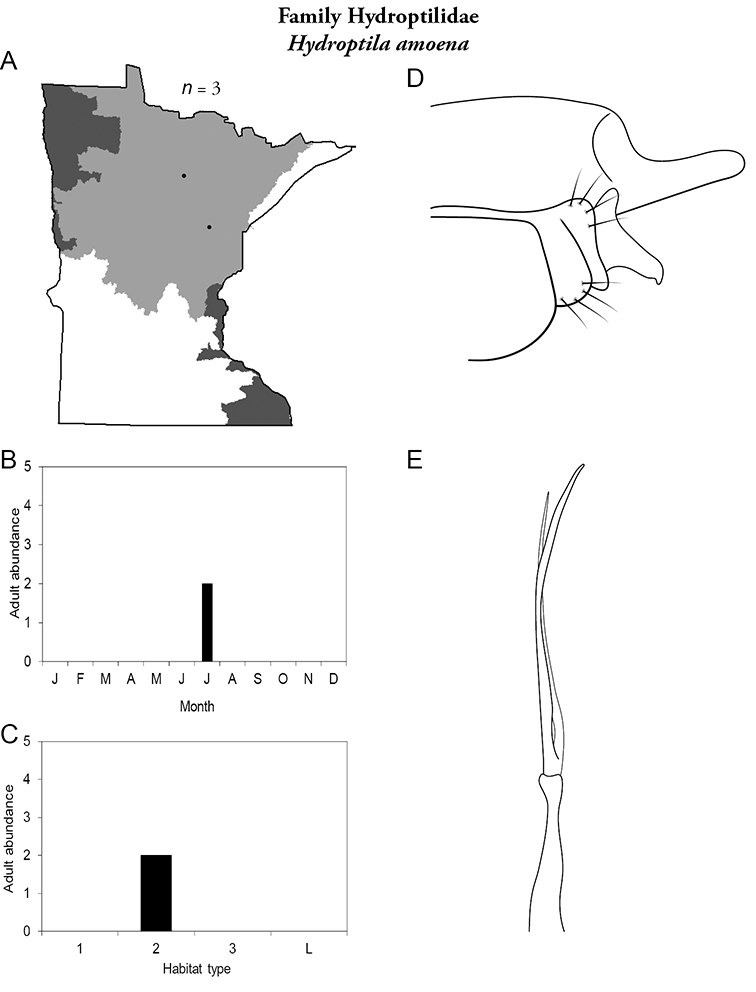
Hydroptila amoena (Figure 66) is known only from a couple of specimens collected from medium rivers of the Northern Region in July.
Hydroptila amoena A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
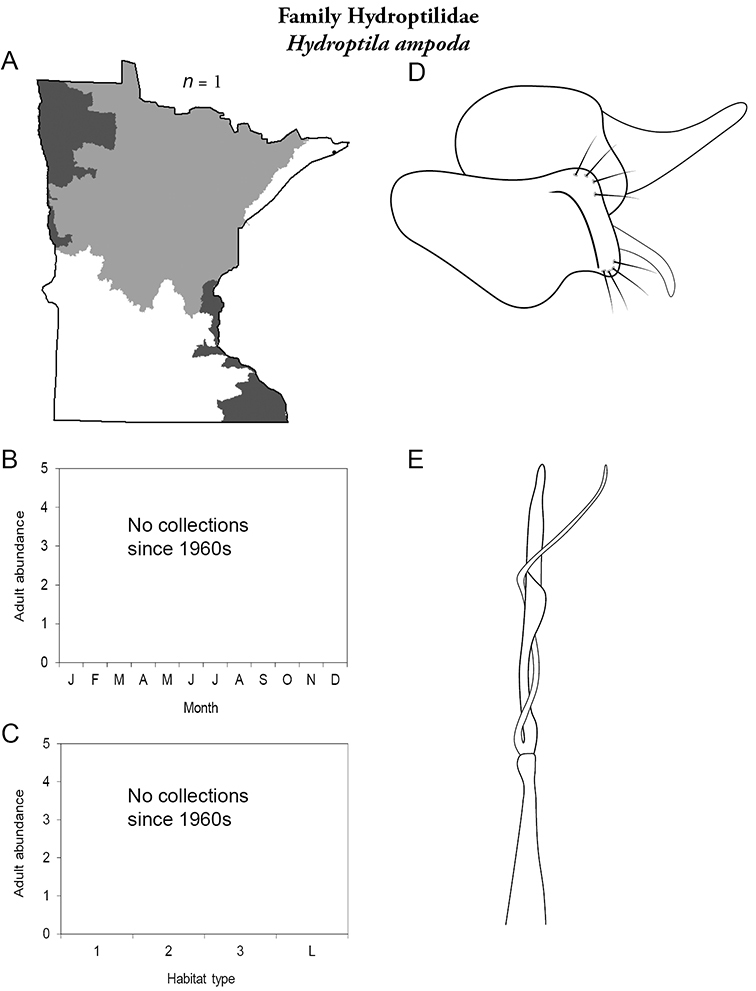
Hydroptila ampoda (Figure 67) has only been collected from the city of Hovland in the 1960s. It has not been collected since.
Hydroptila ampoda A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Hydroptila angusta (Figure 68) has been collected from the Northern and Southern Regions, predominantly from medium and, especially, large rivers. Adults were present from June to September and abundant during July and August.
Hydroptila angusta A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Hydroptila antennopedia (Figure 69) has not been collected since the 1960s. It was found historically from sites in the Lake Superior and Northern Regions.
Hydroptila antennopedia A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E male genital capsule (ventral view) F phallus.
Hydroptila armata (Figure 70) is known exclusively from the Northern Region, from June through September. It was most abundant in medium rivers.
Hydroptila armata A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Hydroptila callia (Figure 71) is known only from a single specimen collected from Link (Lynx) Lake, Itasca County, in the Northern Region during July 1966. It has not been collected since.
Hydroptila calia A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Hydroptila consimilis (Figure 72) was found in all regions, mostly in small and medium streams. Adults were collected mainly June through August.
Hydroptila consimils A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Hydroptila delineata (Figure 73) has been collected only from large rivers within a small area in the southeastern portion of the Northern Region. Adults were present in June, July, and September, but not abundant.
Hydroptila delineata A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule (ventral view).
Hydroptila grandiosa (Figure 74) is primarily known from the Northern and Southern Regions. Interestingly, it was most abundant in both small streams and large rivers, but not medium rivers. Adults were abundant from June through August, and present into September.
Hydroptila grandiosa A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E male genital capsule (ventral view) F phallus.
Hydroptila hamata (Figure 75) is known only from a couple of collections in the Lake Superior and Northern Regions. It has not been collected since the 1960s.
Hydroptila hamata A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F styli of 7th abdominal sternum.
Hydroptila jackmanni (Figure 76) has been collected from the Lake Superior, Northern, and Southeastern Regions. It was most abundant in large rivers and collected almost exclusively during July.
Hydroptila jackmanni A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E male genital capsule (ventral view) F phallus.
Hydroptila metoeca (Figure 77) is known only from a single specimen collected from the city of Garrison, Crow Wing County, in the Northern Region during August 1965. It has not been collected since.
Hydroptila metoeca A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E male genital capsule (ventral view) F phallus.
Hydroptila novicola (Figure 78) is known from the Lake Superior and Northern Regions. It was most abundant in small and medium streams during July. Interestingly, prior to 1999 Hydroptila novicola was known only from a single specimen and listed as “Special Concern” by the Minnesota Department of Natural Resources (
Hydroptila novicola A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule (ventral view) E phallus.
Hydroptila perdita (Figure 79) has been collected sporadically throughout the state, mostly in July. It was most abundant in large rivers.
Hydroptila perdita A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule (ventral view) E phallus.
Hydroptila quinola (Figure 80) is known only from a few small and medium streams in the Lake Superior and Northern Regions. Adults were present in July and September, which probably reflects a lack of collecting in August.
Hydroptila quinola A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule (ventral view) E phallus.
Hydroptila rono (Figure 81) is known only from a single collection from a large waterfall of Minneopa Creek, Minneopa State Park, in the Southern Region during June 2000. The species is typically found in western montane streams, but has also been collected from high gradient rivers in Pennsylvania and Quebec (Morse 2011). Minneopa Creek is, likewise, a high gradient stream, fairly atypical of southern Minnesota. Due to the extreme rarity of the species, and the high degree of habitat disturbance in southern Minnesota, Hydroptila rono has been proposed as “Threatened” by the Minnesota Department of Natural Resources (
Hydroptila rono A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Hydroptila salmo (Figure 82) is known only from a couple of collections from the Lake Superior and Northern Regions. It has not been collected since the 1960s.
Hydroptila salmo A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule (ventral view) E phallus.
Hydroptila scolops (Figure 83) has been collected only from the Southern Region. It was most abundant in small streams, and also found in medium and large rivers. Adults were most abundant in June, and also present July though September.
Hydroptila scolops A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule (ventral view) E phallus.
Hydroptila spatulata (Figure 84) is known from the Lake Superior and, especially, the Northern Regions. It was collected predominantly from medium and large rivers. Adults were most abundant in June, with some presence in July.
Hydroptila spatulata A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule (ventral view) E male genital capsule (ventral view) F phallus.
Hydroptila valhalla (Figure 85) was found in the Lake Superior and Northern Regions, and was locally abundant at several collecting sites. It was found primarily in medium and, especially, large rivers. Adults were present in June and September, but most abundant in July.
Hydroptila valhalla A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Hydroptila waskesia (Figure 86) is known only from the City of Garrison, Crow Wing County, collected in 1964 and 1965, and from Hansen Creek, Roseau County, collected in July of 2000. Both localities are in the Northern Region. Due to the rarity of Hydroptila waskesia and the lack of undisturbed habitats in its range (
Hydroptila waskesia A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule.
Hydroptila waubesiana (Figure 87) has been collected from all regions except the Lake Superior. It was found in all habitat types, and most abundant in medium streams. Adults were present from June to September, reaching highest abundance in June.
Hydroptila waubesiana A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule (ventral view).
Hydroptila wyomyia (Figure 88) is known from the Lake Superior and, especially, the Northern Regions where it was found primarily in streams, particularly medium rivers. It reached highest adult abundance in July and was also present June, August, and September.
Hydroptila wyomyia A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Hydroptila xera (Figure 89) has been collected from and near the Northern Region, predominantly from small and medium streams. Adults were most abundant in June and July, and present in August and September.
Hydroptila xera A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Another Hydroptila species, Hydroptila virgata, was reported from Minnesota based on a female specimen (
The genus Ithytrichia contains a single species in Minnesota. Larvae are typically found on the surface of medium to large rocks where they consume algae and diatoms (
Ithytrichia clavata (Figure 90) has been found sporadically from the northern half of the state during July and August. Surprisingly, it was primarily collected from lakes. Numerous other reports (
Ithytrichia clavata A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule (ventral view) E phallus.
The genus Leucotrichia contains a single species in a Minnesota. For additional species, see
Leucotrichia pictipes (Figure 91) is known from the Lake Superior and Northern Regions, where it appears rare but locally abundant. All specimens have been collected from large rivers. Adults have been collected from May to August. In addition, hundreds of larvae have also been found throughout the same area. Since Leucotrichia pictipes is the only known Leucotrichia species in the eastern U.S., it is assumed that these larvae are all of this species.
Leucotrichia pictipes A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
The genus Mayatrichia contains a single species in a Minnesota. Larvae are typically found in fast-moving areas of medium and large rivers. They appear to consume small organic particles (
Mayatrichia ayama (Figure 92) is known primarily from the Northern and Southern Regions, and sporadically elsewhere. It was found almost exclusively from medium and large rivers, primarily during August.
Mayatrichia ayama A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
The genus Neotrichia contains 5 species in Minnesota. They are the smallest of the microcaddisflies, with adults usually around 2 mm in length. Larvae typically prefer large river habitats (
Neotrichia falca (Figure 93) is known only from large rivers of the Northern Region. It was most abundant in July, with some specimens present in August.
Neotrichia falca A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E male tergum X (dorsal view) F phallus.
Neotrichia halia (Figure 94) has been found in or near the Lake Superior Region, exclusively during July. It was collected almost entirely from large rivers.
Neotrichia halia A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule (ventral view) E phallus.
Neotrichia minutisimella (Figure 95) may be the single smallest caddisfly in North America (
Neotrichia minutisimella A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E male genital capsule (ventral view).
Neotrichia okopa (Figure 96) is the only Neotrichia species in Minnesota that is most abundant in medium rivers, although it was also found in large rivers. Collections have occurred in the Lake Superior, Northern, and Southern Regions during July and August.
Neotrichia okopa A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E male genital capsule (ventral view) F phallus.
Neotrichia vibrans (Figure 97) is known only from the Northern Region, and was found primarily during July. It was typically collected from large rivers.
Neotrichia vibrans A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule (ventral view) E phallus.
The genus Ochrotrichia contains 2 species in Minnesota. Larvae live in a variety of stream types and consume diatoms from rock surfaces (
Ochrotrichia spinosa (Figure 98) is known only from 2 small streams in the Northern and Southeastern Regions. Since 1960, the only collection occurred in Valley Creek, Washington County, in the Southeastern Region during July of 2001. Due to its rarity, and the high degree of habitat disturbance around Valley Creek, the Minnesota Department of Natural Resources has proposed “Endangered” status for the species (
Ochrotrichia spinosa A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E male genital capsule (ventral view).
Ochrotrichia tarsalis (Figure 99) has been found primarily in the Northern and Southern Regions, entirely from medium and large rivers. Adults were most abundant in July and also present in June and August.
Ochrotrichia tarsalis A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E male tergum X (dorsal view).
Two other Ochrotrichia species: Ochrotrichia stylata and Ochrotrichia wojcicky, were reported from Minnesota, both based on adult specimens of unknown sex (
The genus Orthotrichia contains 4 species in Minnesota, 3 of which are common throughout the Northern Region and frequently collected together. Larvae typically inhabit beds of submerged macrophytes in lakes and slow-moving areas of streams where they feed by piercing algal cells (
Orthotrichia aegerfasciella (Figure 100) was collected throughout the Northern and Southern Regions, predominantly from lakes, but also from all sizes of streams. Adults were abundant in June and July, and present in August and September.
Orthotrichia aegerfasciella A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital (ventral view) E phallus.
Orthotrichia balduffi (Figure 101) has been found in or near the Northern Region, mostly during July. Like Ochrotrichia aegerfasciella, it was collected from all habitat types, but most frequently from lakes.
Orthotrichia balduffi A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital (ventral view) E phallus.
Orthotrichia cristata (Figure 102) was the most abundant Orthotrichia species, found throughout the Lake Superior, Northern, and Southern Regions. It, too, was most abundant in lakes and occasionally found in streams. Adults were present primarily during June and July, with a few found in August.
Orthotrichia cristata A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital (ventral view) E phallus.
Orthotrichia curta (Figure 103) is known only from a single specimen collected from Link (Lynx) Lake, Itasca County, in the Northern Region during July 1965. It has not been seen in Minnesota since this collection, and it is difficult to know if the species has been extirpated or is rare and difficult to collect.
Orthotrichia curta A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital (ventral view) E phallus.
The genus Oxyethira contains 16 species in Minnesota. It is the 5th most species-rich genus in the state (Figure 7). Larvae are found in both lakes and a wide variety of streams where they feed on algal cells (
Oxyethira aeola (Figure 104) is known only from the Northern Region. It has been collected during July and August from small and medium streams.
Oxyethira aeola A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Oxyethira anabola (Figure 105) is known only from a few specimens found in the Lake Superior and Northern Regions during July and August. It was collected only from lakes and large rivers.
Oxyethira anabola A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Oxyethira arraya (Figure 106) has been found in the Northern Region from June through August. Specimens were collected from lakes and medium rivers.
Oxyethira arraya A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Oxyethira coerscens (Figure 107) is known primarily from the Northern Region. It was most abundant in medium and, especially, large rivers. Adults were abundant in June and July and also present in August and September.
Oxyethira coerscens A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E male genital capsule (ventral view) F phallus.
Oxyethira ecornuta (Figure 108) is known only from a few collections from lakes of the Northern Region during June and July. These are the only known collections of Oxyethira ecornuta from anywhere in the U.S. Due to its rarity and its exclusive presence in Minnesota, the Minnesota Department of Natural Resources has proposed “Threatened” status for the species.
Oxyethira ecornuta A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Oxyethira forcipata (Figure 109) was the 9th most widespread species overall in Minnesota (Figure 8). It was collected primarily in the Northern Region, and found in nearly every light trap from that region. It is also known sporadically from the Lake Superior, Southeastern, and Southern Regions. It was most abundant in small streams, but found in other habitat types as well. Adults were present from June to September and most abundant in July.
Oxyethira forcipata A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E male genital capsule (ventral view) F phallus.
Oxyethira itascae (Figure 110) was described from Lake Itasca State Park (
Oxyethira itascae A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Oxyethira michiganensis (Figure 111) has been found in the Lake Superior and Northern Regions. It was collected mostly from lakes, with some specimens from medium rivers. Some adults emerged as early as May. The highest abundance, however, was in August, suggesting a possible bivoltine life cycle. Interestingly, in Michigan the species is probably univoltine, with peak emergence throughout June and July (
Oxyethira michiganensis A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Oxyethira obtatus (Figure 112) is known primarily from lakes of the Northern Region. Adults were collected primarily in August, with some present in May, July, and September.
Oxyethira obtatus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Oxyethira pallida (Figure 113) has been collected from the Northern and Southern Regions from all habitat types. Adults were most abundant in September, with some present from June through August.
Oxyethira pallida A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Oxyethira rivicola (Figure 114) has been collected primarily from the Northern Region, with some collections from the Lake Superior and Southern Regions. Adults were present from June through September, and most abundant in small and medium streams.
Oxyethira rivicola A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E male genital capsule (ventral view) F phallus.
Oxyethira rossi (Figure 115) is known only from a couple of collections in the Lake Superior Region. All occurred during July and were from large rivers.
Oxyethira rossi A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Oxyethira serrata (Figure 116) has been found mostly in the Northern Region and sporadically elsewhere. It was found almost exclusively in lakes. Adults were present from May to September, with highest abundance occurring in August.
Oxyethira serrata A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Oxyethira sida (Figure 117) is known from the Lake Superior and Northern Regions, primarily during June and July, from medium rivers.
Oxyethira sida A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E male genital capsule (ventral view) F phallus.
Oxyethira verna (Figure 118) has been collected from and near the Northern Region. It was found in all habitats except large rivers, and present from June to September.
Oxyethira verna A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Oxyethira zeronia (Figure 119) has been found almost exclusively during July from lakes and medium rivers of the Northern Region.
Oxyethira zeronia A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E male genital capsule (ventral view) F phallus.
The genus Stactobiella contains 2 species from Minnesota. Neither are commonly encountered. Larvae usually inhabit fast-moving streams and consume algal cells or diatoms (
Stactobiella delira (Figure 120) has been found in the Lake Superior and Northern Regions during June and July. It was found exclusively in streams, primarily large rivers.
Stactobiella delira A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule (ventral view) E phallus.
Stactobiella palmata (Figure 121) is primarily known from the Northern Region, and sporadically elsewhere. Like Stactobiella delira, it was found primarily in large rivers during June and July.
Stactobiella palmata A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule (ventral view) E phallus.
This family contains a single genus in Minnesota, Lepidostoma, and a total of 10 species. Larvae are shredders and typically inhabit slow-moving areas of woodland streams with considerable canopy cover. Cases are quadrate tubes constructed of small elongate pieces of wood arranged transversely (
The genus Lepidostoma contains 10 species in Minnesota. It is the 9th most species-rich genus (Figure 7). Most of these species are known only from a single or a few collections. Others are rare but locally abundant. Only one species, Lepidostoma togatum, is common. For additional species, see
Lepidostoma americanum (Figure 122) is known only from a single specimen collected during July 2000 from a small unnamed spring near Grand Portage National Monument in the Lake Superior Region.
Lepidostoma americanum A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Lepidostoma bryanti (Figure 123) is known mostly from small streams in the Northern Region, with some collections occurring in the Lake Superior and Southeastern Regions. It was most abundant in June, with a few specimens collected in July and September. Nearly 75% of all specimens were collected from Sucker Creek, Clearwater County, in the Northern Region where the species appears to be locally abundant.
Lepidostoma bryanti A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Lepidostoma cinerum (Figure 124) is known from only 6 specimens collected from a small and a medium stream in and near the Lake Superior Region during September 2000.
Lepidostoma cinereum A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Lepidostoma costale (Figure 125) has been found exclusively in August, mostly from small streams of the Lake Superior and Northern Regions. As with Lepidostoma bryanti, Lepidostoma costale appears locally abundant at Sucker Creek in Clearwater County.
Lepidostoma costale A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Lepidostoma griseum (Figure 126) is known only from a single specimen collected from Mill Creek, William O’Brien State Park, in the Southeastern Region during July 2002.
Lepidostoma griseum A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Lepidostoma libum (Figure 127) is known only from 3 specimens collected from Minneopa Creek, Minneopa State Park, in the Southern Region during June 2000. The species is stenothermic, sensitive to changes in riparian canopy, and typically found in small spring habitats with dense canopy cover (
Lepidostoma libum A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Lepidostoma prominens (Figure 128) is known from only 2 specimens collected from the Temperance River, Lake County, in the Lake Superior Region during July 1991.
Lepidostoma prominens A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Lepidostoma sackeni (Figure 129) is known only from a single specimen collected from the upper Mississippi River, Clearwater County, in the Northern Region during August 1988.
Lepidostoma sackeni A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Lepidostoma togatum (Figure 130) was, by far, the most common and abundant Lepidostoma species. It was found predominantly in medium and large rivers of the Lake Superior and Northern Regions, with some specimens from the Southeastern Region. It was the most abundant species overall in medium rivers of the Lake Superior Region (Table 3). It was most abundant during July, with some specimens in June, August, and September.
Lepidostoma togatum A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Lepidostoma unicolor (Figure 131) was found exclusively in the Lake Superior Region in July and September. The apparent bivoltine life cycle was probably caused by a lack of collecting in August. Specimens were collected from medium streams, including one as it entered into Lake Superior.
Lepidostoma unicolor A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
This family contains 8 genera in Minnesota: Ceraclea, Leptocerus, Mystacides, Nectopsyche, Oecetis, Setodes, Triaenodes, and Ylodes, and a total of 46 species. It is the 3rd most species-rich family (Figure 6).
Larvae construct tubular portable cases usually composed of mineral fragments. They are generally more abundant in lakes, although many individual species are more abundant in streams. Larvae are usually either gathering collectors or shredders; some are predators. Adults range 8–20 mm in length. Most are tan or brown in color, although specimens of Nectopsyche can be bright white, and those of Mystacides sepulchralis are jet black. All adults have antennae >2× longer than their bodies, a useful familial identification characteristic if antennae are intact.
Adults of many leptocerid species were extremely abundant in light traps throughout the state. In fact, of the 10 most abundant species in Minnesota, 6 were leptocerids (Figure 9). Moreover, the top 6 most widespread species overall in the state were all leptocerids (Figure 8).
Genus CeracleaThe genus Ceraclea contains 16 species in Minnesota. It is the 5th most species-rich genus (Figure 7). It contains several species in or near the top 10 of most abundant and widespread species in the state (Figures 8–9). Larvae usually feed on detritus, although some are predatory on freshwater sponges (
Ceraclea alagma (Figure 132) was abundant in lakes of all regions, and found June through August. It was the 2nd most abundant species in lakes of the Northwestern Region, and 3rd most abundant in lakes of the Southeastern Region (Table 5–6).
Ceraclea alagma A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Ceraclea albosticta (Figure 133) is known only from a single specimen collected in July 2000 from an unnamed spring near Grand Portage National Monument in the Lake Superior Region.
Ceraclea albosticta A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus (rotated 90 degrees counter-clockwise).
Ceraclea alces (Figure 134) has been collected during July from lakes and large rivers of the Lake Superior and Northern Regions. Only 2 specimens have been collected since the 1950s.
Ceraclea alces A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Ceraclea ancylus (Figure 135) was found in all regions throughout the state, mostly from lakes. Adults were present in June and, especially, July.
Ceraclea ancylus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Ceraclea annulicornis (Figure 136) has been found in lakes and large rivers of the Lake Superior and Northern Regions. Adults were present in July and September, which probably reflected a lack of collecting in August.
Ceraclea annulicornis A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Ceraclea arielles (Figure 137) is known only from or near the Northern Region. It was found predominantly in medium and large rivers during June and July.
Ceraclea arielles A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Ceraclea cancellata (Figure 138) has historically been found in all regions. Since the 1950s, however, it has been collected predominantly in the Lake Superior and Northern Regions. Overall, it was the 10th most widespread species in the state (Figure 8). It was found in all types of streams, especially medium rivers of the Lake Superior Region (Table 3). Statewide, it was most abundant in lakes. Adults were present from June through August. The presence of this species in medium rivers was determined by
Ceraclea cancellata A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Ceraclea diluta (Figure 139) is known from the Lake Superior and Northern Regions, primarily in lakes. Adults were present during June and July.
Ceraclea diluta A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Ceraclea excisa (Figure 140) was collected in June and July, predominantly in the Northern Region. It was only found in streams, especially medium streams.
Ceraclea excisa A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Ceraclea flava (Figure 141) was collected mostly in the Northern and Northwestern Regions. It was found in streams, particularly larger rivers, during June and July.
Ceraclea flava A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Ceraclea maculata (Figure 142) is known from all regions except the Lake Superior, with adults present mostly in June and July. It was collected mainly from streams, especially large rivers.
Ceraclea maculata A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Ceraclea mentiea (Figure 143) was collected exclusively from medium and large rivers in or near the Northern Region during June and July.
Ceraclea mentiea A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Ceraclea resurgens (Figure 144) is known only from the Northern Region. It was most abundant in medium and large rivers during July, with some adults present in June.
Ceraclea resurgens A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Ceraclea tarsipunctata (Figure 145) was abundant in all habitats except small streams, and was especially abundant in large rivers. It was widespread in all regions throughout the state and was the 4th most widespread species overall (Figure 8). It was also the 7th most abundant species in the state (Figure 9). It was the single most abundant species in both medium and large rivers of the Southeastern Region (Table 6). Adults were most abundant in July, with some specimens present in June, August, and September.
Ceraclea tarsipunctata A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Ceraclea transversa (Figure 146) was historically found in all regions. Since the 1950s, however, it has been collected commonly in the Lake Superior and Northern Regions and found only sporadically elsewhere. It was abundant in all sizes of streams, especially medium and large rivers. It was the most and 2nd most abundant species in large and medium rivers, respectively, of the Lake Superior Region (Table 3). Overall, it was the 5th most widespread species and the 8th most abundant species in the state (Figure 8–9). Adults were most abundant in July, with some specimens present in June, August, and September. Its presence in medium rivers was determined by
Ceraclea transversa A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Ceraclea wetzeli (Figure 147) is known only from the Lake Superior and Northern Regions. Adults were only collected in July, almost exclusively from large rivers.
Ceraclea wetzeli A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Another Ceraclea species, Ceraclea vertreesi, has been previously reported from Minnesota from a series of collections made in 1989 in and around Lake Itasca State Park in the Northern Region (
Another Ceraclea species, Ceraclea brevis, is known worldwide from only a single specimen collected in 1965 from an unknown locality in Crow Wing County in the Northern Region (
Two other Ceraclea species: Ceraclea neffi and Ceraclea nepha, have been reported from Minnesota based on larval records (
The genus Leptocerus contains a single species in North America and in Minnesota. Larvae typically inhabit lakes and slow-moving areas of streams, and can actively swim through beds of aquatic plants. They appear to be generalist feeders on organic particles. Cases are composed of silk only (
Leptocerus americanus (Figure 148) has been commonly collected throughout all regions and was the 6th most widespread species overall in the state (Figure 8). It was also the 2nd most abundant species overall in the state (Figure 9), and was abundant in all habitat types, especially lakes. It was the most abundant species in lakes and small streams of the Northern Region (Table 4). It was also the most abundant species in lakes of the Southern Region, and the 2nd most abundant species in lakes of the Southeastern Region (Table 6). Adults were found during June and July.
Leptocerus americanus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
The genus Mystacides contains 2 species in Minnesota. For additional species, see
Mystacides interjecta (Figure 149) has been collected predominantly from lakes of the Northern and Southern Regions, with sporadic records in other regions and habitat types. Adults were most abundant in June, decreasing in abundance through September.
Mystacides interjecta A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Mystacides sepulchralis (Figure 150) has a similar distribution and flight period as Mystacides interjecta, but was more abundant in medium streams and less abundant in the Southern Region.
Mystacides sepulchralis A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
The genus Nectopsyche contains 4 species in Minnesota. For additional species, see
Nectopsyche candida (Figure 151) was collected mainly from the Northern and Southern Regions, mostly from large rivers in June and July, with a few specimens in August.
Nectopsyche candida A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule.
Nectopsyche diarina (Figure 152) had a similar general distribution and flight period as Nectopsyche candida, but was more common in medium rivers.
Nectopsyche diarina A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule.
Nectopsyche exquisita (Figure 153) was mostly found in the Northern Region, and sporadically elsewhere in the state. It was found in all habitats except small streams, and was most abundant in medium rivers.
Nectopsyche exquisita A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule.
Nectopsyche pavida (Figure 154) was collected from the eastern third of the state during July. It was most abundant in large rivers.
Nectopsyche pavida A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule.
The genus Oecetis contains 10 species in Minnesota. It is the 8th most species-rich genus in the state (Figure 7). It contains several species in or near the top 10 of most abundant and widespread species in the state (Figures 8–9). Larvae are predatory and can be found on the bottom substrates of lakes and slow-moving rivers. Cases are normally composed of mineral particles, but may also include pieces of bark or leaves (
Oecetis avara (Figure 155) was abundant in all regions of Minnesota. Overall, it was the 5th most abundant species in the state (Figure 9). It was the 2nd most abundant species in both medium and large rivers of the Northern Region (Table 4). It was found in all habitat types, but was most abundant overall in large rivers. Adults were present from June to August.
Oecetis avara A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Oecetis cinerascens (Figure 156) was found in all regions of Minnesota. Overall, it was the 3rd most widespread species in the state (Figure 8). It was found in all habitat types, but was most abundant in lakes. Adults were abundant in June and July and present in August and September.
Oecetis cinerascens A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Oecetis disjuncta (Figure 157) has been collected only from small and medium streams in the Lake Superior and Southeastern regions. All streams were cold and fast-moving. Adults were present only in July.
Oecetis disjuncta A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Oecetis ditissa (Figure 158) is known in Minnesota only from a single specimen collected from Minneopa Creek, Minneopa State Park, in the Southern Region during June 2000. The species appears to be at the northwestern edge of its known range. Due to the extreme rarity of Oecetis ditissa in the state and the high degree of habitat degradation in southern Minnesota (
Oecetis ditissa A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E male inferior appendages (ventral view) F phallus.
Oecetis immobilis (Figure 159) has been collected predominantly from the Northern and Southern Regions, but found in all regions. It was found mostly in lakes and medium rivers. Adults were abundant in June and July and also present in August and September.
Oecetis immobilis A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Oecetis inconspicua (Figure 160) was commonly collected throughout all regions of the state. It was, by far, the most widespread species in Minnesota, found in >80% of all collections in the state (Figure 8). It was also the 3rd most abundant species overall (Figure 9). It was abundant in all habitat types, especially lakes and medium rivers. Adults were present from June through September. In short, nearly any ultraviolet light trap set near an aquatic habitat in Minnesota during warm weather will likely yield this species.
Oecetis inconspicua A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E male inferior appendages (ventral view) F phallus.
Oecetis nocturna (Figure 161) was found predominantly in the Northern and Southern Regions of the state. It was most abundant in medium and, especially, large rivers from June through August.
Oecetis nocturna A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E male inferior appendages (ventral view) F phallus.
Oecetis ochracea (Figure 162) was found in the Northern, Northwestern, and Southern Regions. It was abundant in both June and July and found in all habitat types, but was least abundant in medium rivers.
Oecetis ochracea A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Oecetis osteni (Figure 163) was collected mostly from or near the Northern Region and found sporadically elsewhere in the state. It was most abundant in lakes during July, with some specimens found in June and August. It was the 3rd most abundant species in lakes of the Northern Region (Table 4).
Oecetis osteni A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Oecetis persimilis (Figure 164) is known mainly from the Lake Superior and Northern Regions. It was found mostly in medium and large rivers, and was abundant in July with some specimens collected in June and August.
Oecetis persimilis A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
The genus Setodes contains 2 species in Minnesota. For additional species, see
Setodes incertus (Figure 165) was collected predominantly from large rivers of the Northern Region during July.
Setodes incertus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Setodes oligius (Figure 166) was found in lakes and, occasionally, medium streams of the Northern Region during June and July.
Setodes oligius A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
A 3rd Setodes species, Setodes guttatus, has been reported from Minnesota based on a female specimen (e.g.,
The genus Triaenodes contains 9 species in Minnesota. It is the 10th most species-rich genus (Figure 7). For additional species, see
Triaenodes abus (Figure 167) was commonly collected in the Northern Region and sporadically elsewhere. It was found in all types of habitats, especially lakes and medium rivers. Adults were abundant in June and July, with a few specimens present in August.
Triaenodes abus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Triaenodes baris (Figure 168) has been found exclusively in the Northern Region, mostly from medium rivers in June, but also from large rivers and lakes.
Triaenodes baris A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Triaenodes dipsius (Figure 169) was found predominantly in the Northern Region, and sporadically elsewhere. Adults were abundant in both June and July, with a few specimens present in August and September. It was most abundant in streams, especially small streams.
Triaenodes dipsius A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Triaenodes flavescens (Figure 170) is known historically from the Northwestern Region. The species has not been collected in that region since the 1940s, however. The only recent collection occurred in Sucker Creek, Clearwater County, in the Northern Region, during July 1988. Due to its rarity and apperent decrease in distribution, the Minnesota Department of Natural Resources has proposed “Special Concern” status for the species (
Triaenodes flavescens A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Triaenodes ignitus (Figure 171) is known mainly from the Northern Region where it occurred predominantly in medium streams in July.
Triaenodes ignitus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Triaenodes injustus (Figure 172) was abundant in the Lake Superior and Northern Regions and found occasionally in other regions. It was the single most abundant species in lakes of the Lake Superior Region (Table 3), and also found in all sizes of streams. Adults were abundant in June and July and occasionally present in August and September.
Triaenodes injustus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Triaenodes marginata (Figure 173) was abundant in the Northern Region, especially in small and medium streams. It was also found sporadically in other regions. Overall, it was the 9th most abundant species in the state (Figure 9). Adults were abundant in June and July, moderately abundant in August, and occasionally present in September.
Triaenodes marginatus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Triaenodes nox (Figure 174) was abundant in the Northern Region and found sporadically in the other regions. Most adults were collected in July and were abundant in all habitat types except large rivers.
Triaenodes nox A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Triaenodes tarda (Figure 175) was the 2nd most widespread species in Minnesota (Figure 8), although it was not especially abundant. It was found in all regions and in all habitat types. Adults were present from May to September and abundant June through August.
Triaenodes tarda A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Another Triaenodes species, Triaenodes borealis, was described by
The genus Ylodes contains 2 species in Minnesota. For additional species, see
Ylodes frontinalis (Figure 176) is known historically from Glacial Lake State Park in the Southern Region. The only specimens collected since the 1950s came from Hayes Lake, Hayes Lake State Park, in the Northern Region during August 2000. Due to the rarity of this species, and the loss of habitat throughout its known collecting area (
Ylodes frontinalis A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Ylodes reuteri (Figure 177) has been collected from the western third of the state. It was found in all sizes of streams, and present from June to September. Almost 90% of specimens, however, were found prior to 1945, suggesting that the species is decreasing in abundance.
Ylodes reuteri A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
This family contains 19 genera in Minnesota: Anabolia, Arctopora, Asynarchus, Chilostigma, Frenesia, Glyphopsyche, Grammotaulius, Hesperophylax, Hydatophylax, Ironoquia, Lenarchus, Leptophylax, Limnephilus, Nemotaulius, Onocosmoecus, Philarctus, Platycentropus, Pseudostenophylax, and Pycnopsyche, and a total of 50 species. It is the 2nd most species-rich family in the state. Many species, however, have not been collected since the 1960s or earlier.
Larvae are found throughout a wide variety of lotic and lentic habitats. Cases are tubular in shape and constructed out of rock or plant material. Larvae range in size from 15 to 35 mm in length; cases of some species reach 80 mm. Most species are shredders, dependent on allochthonous input for food and case-building material (
Due to their large size and corresponding long lifespan, and due to their dependence on allochthonous debris as a food source, most limnephilids are sensitive to habitat disturbance, especially removal of forest canopy cover. Thus, species in general appear to have been regionally extirpated from their historical habitats at a rate nearly 3× that of species in other families (
Although limnephilids remain widespread throughout the Northern and Lake Superior regions, they are usually not abundant. Furthermore, many species are present as adults during the fall and even during the winter. Thus, they can be difficult to collect. Most Minnesota limnephilid collections have yielded <5 specimens. The exception is Pycnopsyche, which can be very abundant during August and September. Due to this typical lack of abundance, nearly a dozen limnephilid species have been discovered in Minnesota since the 1980s and additional species may remain undiscovered.
Genus AnaboliaThe genus Anabolia contains 4 species in Minnesota. Two of the species are fairly widespread, 1 is listed as “Special Concern” by the Minnesota Department of Natural Resources (
Anabolia bimaculata (Figure 178) is known primarily from the northern half of the state, with occasional collections elsewhere. It was widespread throughout all habitats of the Lake Superior and Northern regions, although it was rarely abundant. Adults were collected mostly during July.
Anabolia bimaculata A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule.
Anabolia consocia (Figure 179) has similar distribution and habitat preference as Anabolia bimaculata; the two species were often collected together. It differs from the latter species in being more frequently collected in the southern half of the state and in having adults commonly collected into September. Overall, it was less abundant that Anabolia bimaculata.
Anabolia consocia A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule.
Anabolia ozburni (Figure 180) is known only from 6 specimens collected in the westcentral portion of the Northern Region during both June and July. It has been collected from both small and medium streams and their lentic headwaters. Due to the rarity of Anabolia ozburni and the vulnerability to riparian disturbance of habitats throughout its known range, the Minnesota Department of Natural Resources has proposed “Special Concern” status for the species (
Anabolia ozburni A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule.
Anabolia sordida (Figure 181) is known historically from 2 sites in Minnesota. Both of them are cities: Crookston and Hallock, in the Northwestern Region, and not associated with any particular habitat type. The species was collected 7 times in June, July, and August over a period from 1935 to 1937. It has not been seen in the state since this time despite extensive collecting in its known range, and appears to be extirpated. The loss of this species almost certainly has occurred due to habitat loss in the Northwestern Region since the 1930s (
Anabolia sordida A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule.
The genus Arctopora contains a single species in Minnesota. Little is known about larval biology except that they usually inhabit lakes and, sometimes, temporary pools (
Arctopora pulchella (Figure 182) is known only from 4 specimens in the Northern Region during July 1965. The species has not been collected since 1965 despite extensive collecting throughout its known range. The area known to support Arctopora pulchella has not been greatly disturbed since the 1960s, and so it is difficult to know if the species has been extirpated or is naturally rare and difficult to collect.
Arctopora pulchella A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F female genital capsule.
The genus Asynarchus contains 3 species in Minnesota. One species appears to have decreased in its range, 1 is listed as “Threatened” by the Minnesota Department of Natural Resources (
Asynarchus montanus (Figure 183) is known historically from the Lake Superior, Northern, and Northwestern Regions. It has not been collected in the last region since the 1930s. Since the 1980s, it has been collected from small and medium streams from June through August.
Asynarchus montanus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Asynarchus mutatus (Figure 184) is known historically from the city of Finland in the Lake Superior Region, and Lake Itasca in the Northern Region. It has not been collected in the state since 1965. The species never appeared to be common, so it is difficult to know if it has been extirpated or is naturally rare and difficult to collect.
Asynarchus mutatus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Asynarchus rossi (Figure 185) is the subject of systematic confusion, and is sometimes placed in the genus Limnephilus (
Asynarchus rossi A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
The genus Chilostigma contains a single species in Minnesota. The larva of the genus has yet to be definitively associated with the adult.The genus contains some of the smallest limnephilids, ranging 10–12 mm in length.
Chilostigma itascae (Figure 186) is known worldwide from only 3 localities in the Northern Region. All three of these localities were either boggy, slow-moving areas of streams or else shallow lentic habitats. The species is unique in its seasonal emergence; all adults were found crawling on the surface of the snow on relatively warm days in winter and early spring. Although the species may be more common than reported due to its winter emergence, the Minnesota specimens remain the only known examples of Chilostigma itascae in the world. Thus, due to its extreme rarity and Minnesota endemism, Chilostigma itascae is listed as “Endangered” by the Minnesota Department of Natural Resources (
Chilostigma itascae A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F female genital capsule.
The genus Frenesia contains a single species in Minnesota. Larvae inhabit cold streams and feed on decaying wood and leaves (
Frenesia missa (Figure 187) is known only from small and medium streams of the Southeastern and Southern Regions. It is unique in its late fall emergence; adults were collected in October and November. It is possible that the species is more common than it appears due to its presence when few collectors are out.
Frenesia missa A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F female genital capsule.
The genus Glyphopsyche contains a single species from Minnesota. For additional species, see
Glyphopsyche irrorata (Figure 188) is known only from Grand Portage Creek, Cook County, in the Lake Superior Region, and Lake Itasca, Clearwater County, in the Northern Region. The former collection occurred in July 2000 and the latter in July 1977. The species is unique in sometimes overwintering in the adult stage (
Glyphopsyche irrorata A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E female genital capsule.
The genus Grammotaulius contains a single species in Minnesota. For additional species, see
Grammotaulius interrogationis (Figure 189) is known only from a single specimen collected from “Cook County” in July 1938. The species has not been collected in Minnesota since then. The majority of Cook County is in the Lake Superior Region, with some in the Northern Region. Minnesota is the most southern collecting locality for the species, which occurs as far north as Greenland (
Grammotaulius interrogationis A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F female genital capsule.
The genus Hesperophylax contains a single species from Minnesota. For additional species, see
Hesperophylax designatus (Figure 190) has been collected from all regions except the Northwestern, but was most abundant in the southeast portion of the state. It is known primarily from medium and, especially, small streams. Adults have been collected from June through August.
Hesperophylax designatus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E female genital capsule.
The genus Hydatophylax contains a single species from Minnesota. For additional species, see
Hydatophylax argus (Figure 191) is known from the Lake Superior and Northern regions. Adults were collected mainly in June and mostly from medium rivers.
Hydatophylax argus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F female genital capsule.
The genus Ironoquia contains 2 species from Minnesota. For additional species, see
Ironoquia lyrata (Figure 192) has been collected from small and medium streams of the Northern and Southern Regions. Adults were present mainly in September.
Ironoquia lyrata A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Ironoquia puncatissima (Figure 193) is only known from small streams, mainly in the Southern Region. Adults were caught in September. Undisturbed small streams are extremely rare in the Southern Region and are vulnerable to urban and agricultural development (
Ironoquia punctatissima A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
The genus Lenarchus contains 2 species from Minnesota. Both are very rare. For additional species, see
Lenarchus crassus (Figure 194) is known only from an unnamed spring near Grand Portage National Monument in the Lake Superior Region collected during July 2000.
Lenarchus crassus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Lenarchus keratus (Figure 195) is known only from the City of Hovland in the Lake Superior Region. A single specimen was collected in 1965. It has not been found in Minnesota since this collection.
Lenarchus keratus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
The genus Leptophylax contains a single species in North America and in Minnesota. Larvae have yet to be positively associated with the adults. Adults are brown in color and 10–14 mm in length.
Leptophylax gracilis (Figure 196) has been collected from all regions except the Lake Superior. All collections except 1, however, occurred in the 1940s or earlier. The most recent collection occurred from an unnamed tributary of the Mississippi River, Hubbard County, in the Northern Region during August 1989.
Leptophylax gracilis A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
The genus Limnephilus contains 19 species from Minnesota. It is the 2nd most species-rich genus in the state. The genus contains around 100 species in North America (
Many Limnephilus species historically common throughout the state are now restricted to the Lake Superior and Northern Regions. Several others are known in Minnesota exclusively from 1 or few specimens collected in the 1960s or earlier. For additional species, see
Limnephilus argenteus (Figure 197) in known exclusively from all sizes of streams in July. It has been found in or near the Lake Superior Region, but was not abundant.
Limnephilus argenteus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F female genital capsule.
Limnephilus canadensis (Figure 198) has been historically collected throughout the Northern and Northwestern Regions. All specimens since the 1930s, however, have come from the Northern Region. Adults were most common in July, and were found primarily in medium and large rivers.
Limnephilus canadensis A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F female genital capsule.
Limnephilus hyalinus (Figure 199) is known primarily from the Northern and Northwestern regions, with sporadic collections from other regions. It is one of the few limnephilids to still be found in the Northwestern Region. Adults were present from July to September. It was collected from all habitat types, especially medium rivers.
Limnephilus hyalinus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F female genital capsule.
Limnephilus indivisus (Figure 200) has been found sporadically in all regions of the state. It has, however, been collected only in the Northern Region since the 1960s. Further, nearly 98% of total specimens were collected prior to the 1970s, suggesting that the species is decreasing in abundance through out the state. The few recent specimens were collected from all habitat types, mainly during August.
Limnephilus indivisus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F female genital capsule.
Limnephilus infernalis (Figure 201) has been collected from throughout the Northern Region, mostly from lakes during September.
Limnephilus infernalis A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F female genital capsule.
Limnephilus janus (Figure 202) has been collected only from Little Elbow Creek, Mahnomen County, in the Northern Region during July 2000. Due to the rarity of this species, the Minnesota Department of Natural Resources has proposed “Threatened” status for Limnephilus janus (
Limnephilus janus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F female genital capsule.
Limnephilus moestus (Figure 203) has been collected only from the Lake Superior and Northern Regions since the 1960s, with some previous collections in the other regions of the state. It was found in all habitat types, exclusively during July.
Limnephilus moestus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F female genital capsule.
Limnephilus ornatus (Figure 204) has been historically found in all regions except the Southeastern. Since the 1960s, however, it is only known from the Lake Superior and Northern Regions. It has been collected mostly from medium rivers in June and July.
Limnephilus ornatus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F female genital capsule.
Limnephilus partitus (Figure 205) is known only from a single specimen collected from an unknown locality on the Kawishiwi River in the Lake Superior Region. The locality on the species’ distribution map is, thus, an approximation. The date of the collection is also unknown. The collector of the specimen, R.L. Knight, accessioned specimens into the UMSP during the 1920s. Thus, it is presumed that the specimen was collected during this period. The species has not been collected since.
Limnephilus partitus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F female genital capsule.
Limnephilus parvulus (Figure 206) is known only from sites in Becker and Clearwater Counties in the Northern Region. Some adults were present in May; most were collected in July. It was found in all habitats except large rivers, and was most abundant in small streams.
Limnephilus parvulus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F female genital capsule.
Limnephilus perpusilis (Figure 207) is only known from the Northern Region since the 1960s, although it has been collected historically from all regions except the Lake Superior. It was found predominantly in small and medium streams. Adults were present mainly in July.
Limnephilus perpusillis A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F female genital capsule.
Limnephilus rhombicius (Figure 208) has been found sporadically from throughout the state, although all collections since the 1960s have occurred in the Lake Superior and Northern Regions. It was present in small and medium streams as well as lakes. Adults were present from June through August.
Limnephilus rhombicius A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F female genital capsule.
Limnephilus sackeni (Figure 209) is known only from 4 specimens collected from Lake of the Woods, Lake of the Woods County, in the Northern Region during September 1999.
Limnephilus sackeni A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F female genital capsule.
Limnephilus secludens (Figure 210) is known historically from the Northern, Northwestern, and Southern Regions. It was particularly abundant in the Northwestern Region and represented in that region by >100 specimens collected from 1935 to 1937. It has not been collected in the Northwestern Region since 1941. Since 1968, it is known only from a single specimen collected from an unnamed spring, Martin County, in the Southern Region during July 1999. Such habitat types are now extremely rare in the Northwestern and Southern Regions (
Limnephilus secludens A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F female genital capsule.
Limnephilus sericeus (Figure 211) has been collected from the Lake Superior and Northern Regions since 1940; it was also collected in the Northwestern Region during the 1930s. It was most abundant in small and, especially, medium streams. Adults were present in July and August, and most abundant in September.
Limnephilus sericeus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F female genital capsule.
Limnephilus sublunatus (Figure 212) is known only from a single specimen collected from the city of Guthrie, Hubbard County, in the Northern Region during July 1965.
Limnephilus sublunatus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F female genital capsule.
Limnephilus submonifer (Figure 213) has historically been found throughout the state. Since the 1960s, however, it has been collected only in the Lake Superior and Northern Regions. It was most abundant in small and, especially, medium stream. Adults were abundant in September and present June through August.
Limnephilus submonifer A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F female genital capsule.
Limnephilus tarsalis (Figure 214) is known only from the cities of Baudette in Lake of the Woods County, and Cotton in Saint Louis County. Both sites are in the Northern Region. It has not been collected since the 1960s, however, and it is difficult to know if it is extirpated or just difficult to collect.
Limnephilus tarsalis A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F female genital capsule.
Limnephilus thorus (Figure 215) is known from a few sites in the Lake Superior and Northern Regions. Adults were present mainly in August and found exclusively in small streams.
Limnephilus thorus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F female genital capsule.
Another Limnephilus species, Limnephilus externus, was reported from Minnesota based on a single pupa (
The genus Nemotaulius contains a single species in Minnesota. For additional species, see
Nemotaulius hostilis (Figure 216) is known mainly from the Northern Region, with some scattered records from the Southern Region. It was found predominantly in medium rivers, but also in lakes and small streams. Most adults were caught in July, with a few in May and August.
Nemotaulius hostilis A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E female genital capsule.
The genus Onocosmoecus contains a single species in Minnesota. For additional species, see
Onocosmoecus unicolor (Figure 217) has been collected only from or near the Lake Superior Region. It was found in small and medium rivers, exclusively during September.
Onocosmoecus unicolor A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F female genital capsule.
The genus Philarctus contains a single species in Minnesota. Larvae typically inhabit lakes and slow-moving areas of streams where they feed mainly on detritus (
Philarctus quaeris (Figure 218) is known in Minnesota only from 3 collections in the 1930s from the City of Crookston, Polk County, in the Northwestern Region. These collections pre-date the majority of habitat destruction in this region (
Philarctus quaeris A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
The genus Platycentropus contains 2 fairly common species in Minnesota that differ mainly in their adult flight period. Larvae live in a wide variety of habitats, from cool streams to warm ponds (
Platycentropus amicus (Figure 219) has been found throughout the Lake Superior and Northern Regions. It was most abundant in lakes and small streams and the interface between them. Adults were most abundant in August and, especially, September.
Platycentropus amicus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Platycentropus radiatus (Figure 220) had a similar distribution and habitat preference as Platycentropus amicus. It differs in its greater prevalence in medium rivers and its greater abundance in June and July.
Platycentropus radiatus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
The genus Pseudostenophylax contains a single species in Minnesota. Larvae are usually found in cold springs or streams. Larval cases are composed of small uniform mineral particles (
Pseudostenophylax sparsus (Figure 221). has been found sporadically from the Lake Superior, Northern, and Southern Regions. It was locally abundant throughout Minneopa State Park in the Southern Region. It was collected mainly from small and, especially, medium streams and present mostly in June.
Pseudostenophylax sparsus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus (dorsal view, reduced 50%).
Another species of Pseudostenophylax, Pseudostenophylax uniformis, was reported from Minnesota by
The genus Pycnopsyche contains 5 species in Minnesota. For additional species, see
Pycnopsyche aglona (Figure 222) is known from the Lake Superior and Northern Regions. It has been collected exclusively from small and medium streams, and is most abundant during September.
Pycnopsyche aglona A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E male inferior appendage (caudal view) F phallus.
Pycnopsyche guttifer (Figure 223) is known mostly from the Lake Superior and Northern Regions where it was, by far, the most abundant of the Pycnopsyche species. It was found in all habitat types, but was most abundant in medium and large rivers. Adults were collected in August and, especially, September.
Pycnopsyche guttifer A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E male inferior appendage (caudal view) F phallus.
Pycnopsyche lepida (Figure 224) is also known mostly from the Lake Superior and Northern region, with scattered records from the other regions. Adults were collected in August and September from all habitat types.
Pycnopsyche lepida A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E male inferior appendage (caudal view) F phallus.
Pycnopsyche limbata (Figure 225) is known from scattered localities in the Lake Superior and Northern Regions. Adults were collected in August and September, almost exclusively from small streams.
Pycnopsyche limbata A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E male inferior appendage (caudal view) F phallus.
Pycnopsyche subfasciata (Figure 226) was the most widespread of the Pycnopsyche species, found in all regions. It was most abundant in lakes and large rivers, and collected during August and September.
Pycnopsyche subfasciata A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E male inferior appendage (caudal view) F phallus.
Another Pycnopsyche species, Pycnopsyche scabripennis, was reported from Minnesota from a single larva (
This family contains one genus in Minnesota, Molanna, and a total of 4 species. Larvae are typically found on rocky substrates where they graze on periphyton (
The genusMolanna contains 4 species in Minnesota. Larvae can be found in both lakes and streams. Individual species often have a strong preference for a particular type of habitat.
Molanna blenda(Figure 227) has only been found in or near the Lake Superior Region during July. It is known only from streams, typically small streams.
Molanna blenda A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule.
Molanna flavicornis (Figure 228) has been collected throughout all regions except the Southeastern. It was found almost exclusively in lakes, and abundant from June through August, with a few specimens collected in September.
Molanna flavicornis A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule.
Molanna tryphena (Figure 229) was found predominately in medium streams and is only known from the Northern Region. Adults have been collected mostly in June and July, with some specimens found in August and September.
Molanna tryphena A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule.
Molanna uniophila (Figure 230) was the most abundant of the Molanna species, found in both lakes and streams, but more commonly in lakes. It was common throughout the Lake Superior and Northern Regions and found occasionally in the Southern Region.
Molanna uniophila A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule.
Another Molanna species, Molanna ulmerina, was reported from a specimen of unknown sex (
This family contains a single genus in Minnesota, Psilotreta, and a single species. For additional species, see
Psilotreta indecisa (Figure 231) is known in Minnesota only from a single set of larval sclerites collected from the Cross River, Cook County, at the eastern edge of the Northern Region during August 2007. The Cross River is a medium-sized, fast-flowing woodland stream. The actual organism had already vacated its case and emerged as an adult before the collection. Thus, adults have not been definitely associated with these specimens and it is possible that they may of a different species. The abandoned sclerites, however, do key to Psilotreta indecisa using
Psilotreta indecisa A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
This family contains 3 genera in Minnesota: Chimarra, Dolophilodes, and Wormaldia, and a total of 6 species. The last genus, however, has not been collected in Minnesota since the 1960s. Larvae, especially those of Chimarra, are common, conspicuous, and sometimes very abundant on the undersides of medium to large rocks in most types of streams. Larvae of all genera are filtering collectors. They construct a sac-like silken net that they use to capture small suspended particulate organic matter from the stream current (
The genus Chimarra contains 4 species in Minnesota. For additional species, see
Chimarra atterima (Figure 232) is known from only 2 locations in the Lake Superior and Northern Regions collected during June. Both localities were large, fast-moving rivers.
Chimarra aterrima A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Chimarra feria (Figure 233) is known mostly from cold small and medium rivers of the Lake Superior and Northern Regions. Specimens were collected in June and July.
Chimarra feria A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Chimarra obscurra (Figure 234) was common and abundant in the Lake Superior, Southern and, especially, the Northern Region. It was most abundant in medium and large rivers. It was the single most abundant species in medium rivers of the Northern Region, and the 3rd most abundant species in large rivers of the region (Table 4). Overall, it was the 4th most abundant species in Minnesota, with many collections approaching or exceeding 1000 specimens (Figure 9). Most adults were caught in July, with a few specimens found in June, August, and September.
Chimarra obscurra A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Chimarra socia (Figure 235) was found in the Lake Superior and Northern Regions. It was most abundant in large rivers, and was the 2nd most abundant species overall in large rivers of the Lake Superior Region (Table 3). Adults were found in June and, especially, July. In one large river, the Rainy River in Koochiching County, a specimen of Chimarra socia was discovered with 2 complete sets of male genitalia, one of the very few known cases of a “supermale” caddisfly (
Chimarra socia A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
The genus Dolophilodes contains a single species in Minnesota. Wings of adults are brown in color with darker brown reticulations. For additional species, see
Dolophilodes distinctus (Figure 236) is known only from or near the Lake Superior Region, where it was common in small streams, and also found in medium and large rivers. It was the 3rd most abundant species in small streams of the region (Table 3). Adults were most abundant in July. Some specimens, including brachypterous females, emerged as early as March, breeding on the surface of the snow. These early emergent specimens were able to remain active for 4 days while contained within a 5°C darkened refrigerator. When placed in a ventilated container at 22° C, the individuals expired within 15 minutes (unpublished data).
Dolophilodes distinctus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule.
The genus Wormaldia contains a single species in Minnesota. For additional species, see
Wormaldia moesta (Figure 237) is known only from 2 specimens from northeastern Minnesota. It has not been collected since the 1960s. It is not clear if the species has been extirpated or is rare and difficult to collect.
Wormaldia moesta A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule.
This family contains 8 genera in Minnesota: Agrypnia, Banksiola, Beothukus, Fabria, Hagenella, Oligostomis, Phryganea, and Ptilostomis, and a total of 18 species. For additional species of all genera, see
Due to their large size and corresponding long lifespan, and also due to their shredder feeding habits, phryganeids appear to be very sensitive to habitat disturbance, especially the modification of the riparian canopy. Since the 1940s, phryganeid species have been extirpated from disturbed habitats in Minnesota, particularly those in the Northwestern and Southern Regions, at nearly 4× the rate of species in other families (
The genusAgrypnia contains 6 species in Minnesota, although 1 is likely extirpated. Larvae can be either shredders or omnivores (
Agrypnia deflata (Figure 238) is known only from 2 specimens collected from a lake and small stream during July in and near the Lake Superior Region.
Agrypnia deflata A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Agrypnia glacialis (Figure 239) was collected several times between 1935 and 1941 in the northwestern portion of the state. It has not been collected in Minnesota since 1941 and is presumed extirpated from the state, likely due to agricultural disturbance throughout its historical range (
Agrypnia glacialis A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Agrypnia improba (Figure 240) was found mainly in small and medium streams and from lakes. It is known only from the Lake Superior and Northern Regions. Adults were collected in June and July.
Agrypnia improba A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Agrypnia macdunnougi (Figure 241) is known only from a lake and medium stream in the Lake Superior Region. Specimens were collected in July and August.
Agrypnia macdunnoughi A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Agrypnia straminea (Figure 242) was the most abundant of the Agrypnia species. It was found mostly in lakes throughout the Northern and Southern Regions and collected during August and September.
Agrypnia straminea A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Agrypnia vestita (Figure 243) was found predominantly in the Northern and Southern Regions, typically in lakes, or less commonly in small and medium streams. Adults were present from June to September.
Agrypnia vestita A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Two other Agrypnia species: Agrypnia colorata and Agrypnia obsoleta have been reported from northeastern Minnesota (
The genusBanksiola contains 3 species in Minnesota. One is common and the other 2 appear extirpated from the state. Larvae inhabit lakes and wetlands as well as slow-moving areas of streams. They typically start out as shredders, but may become predatory in later instars (
Banksiola crotchi (Figure 244) is the only species in the genus to have been collected since the 1950s. It was common and abundant throughout the Lake Superior and Northern Regions and found sporadically elsewhere. Adults were collected during June and July from small and medium streams and, more commonly, from lakes and wetlands. The presence of this species was determined by
Banksiola crotchi A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Banksiola dossuaria (Figure 245) is known historically from and near the Lake Superior Region, but has not been collected since the 1950s. It appears to have always been rare, and so it is not clear if the species is extirpated from the state or merely difficult to collect (
Banksiola dossuaria A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Banksiola smithi (Figure 246) is known only from an unknown site in Lake Itasca State Park in the Northern Region. The date of collection and collector are both unknown, but the condition of the specimen and the type of vial used both appear to indicate the 1930–1940 era. It has not been collected since.
Banksiola smithi A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
The genus Beothukus contains a single species in North America and in Minnesota. Larvae are known from bogs, including a sphagnum pool with a pH of 4.2 (
Beothukus complicatus (Figure 247) is known from only a single specimen collected from a small unnamed spring near Grand Portage National Monument in the Lake Superior region during July 2000.
Beothukus complicatus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
The genus Fabria contains a single species in North America and in Minnesota. Larvae inhabit lakes and slow-moving areas of streams, particularly areas with dense beds of aquatic plants (
Fabria inornata (Figure 248) is known only from lakes in the area around Lake Itasca State Park in the Northern Region. Adults were found only in June.
Fabria inornata A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
The genus Hagenella contains a single species in Minnesota. Larvae live in slow-moving areas of streams. They construct cases of leaf pieces arranged in rings instead of the typical phryganeid spiral pattern (
Hagenella canadensis (Figure 249) was found in the Lake Superior and Northern Regions, mostly in small and medium streams. All collections occurred in June and July.
Hagenella canadensis A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
The genus Oligostomis contains a single species in Minnesota. Larvae inhabit slow-moving areas of cool woodland streams. They eat mostly vascular plant tissue, but also consume algae and small arthropods (
Oligostomis ocelligera (Figure 250) is known in Minnesota only from 2 larval specimens. These specimens have not been associated with an adult, and so it is possible that they may be of a different species. The range and size, however, are consistent with Oligostomis ocelligera, which has also been found in Wisconsin. The Minnesota larvae came from a very small stream of the Black Dog Wildlife Refuge in the Southern Region during December 1989.
Oligostomis ocelligera A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
The genus Phryganea contains 2 species in Minnesota. Larvae are typically found in lakes and slow-moving areas of streams. Specimens have been located 100 m deep in Lake Superior (
Phryganea cinerea (Figure 251) was found throughout all regions except the Southeastern. It was most abundant in lakes, but also found in all sizes of streams. Adults were present June through August.
Phryganea cinerea A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Phryganea sayi (Figure 252) is only known in Minnesota from a single specimen collected from the Sunrise River, Chisago County, in the Southeastern Region during July 2004.
Phryganea sayi A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
The genus Ptilostomis contains 3 species in Minnesota. Two of them are common throughout much of the state and frequently collected together. Larvae can occur in nearly any habitat, from small springs to vernal pools (
Ptilostomis angustipennis (Figure 253) is known only from a single collection from Mill Creek, William O’Brien State Park, in the Southeastern Region during July 2002.
Ptilostomis angustipennis A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Ptilostomis ocellifera (Figure 254) was found in all regions except the Northwestern. It was most common in lakes and small to medium streams. Adults were collected primarily in June and July.
Ptilostomis ocellifera A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Ptilostomis semifasciata (Figure 255) had a similar distribution and flight periodicity as Ptilostomis ocellifera, differing in its greater affinity for larger rivers and its presence in all regions.
Ptilostomis semifasciata A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
This family contains 4 genera in Minnesota: Cyrnellus, Neureclipsis, Nyctiophylax, and Polycentropus, and a total of 25 species. It is the 5th most species-rich family (Figure 6). Larvae produce silken tubular retreats which are affixed to the undersides of rocks, and are typically either predators or detritivores (
The genusCyrnellus contains a single species in North America and in Minnesota. Larvae are most abundant in large rivers, but can occur in nearly any habitat type. Larval retreats are flattened roofs of silk against a depression on the undersides of rocks (
Cyrnellus fraternus (Figure 256) has been collected sporadically, mainly from the Southeastern and Southern Regions. Adults were only found in July and were predominantly from large rivers.
Cyrnellus fraternus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E male inferior appendages (ventral view).
The genus Neureclipsis contains 3 species in Minnesota. Larvae are found in streams of all sizes. They feed mainly on small arthropods caught in their trumpet-shaped capture nets (
Neureclipsis bimaculata (Figure 257) is known mainly from small streams. It has been found in all regions, but was most abundant in the Northern Region. Adults were most abundant in June, with some specimens present in July and August.
Neureclipsis bimaculata A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Neureclipsis crepuscularis (Figure 258) has been found in the northeastern 2/3 of the state, and is thus known from all regions except the Southern. It was found predominantly in streams, especially medium and large rivers. The majority of adults were found in July, with some in June, August, and September.
Neureclipsis crepuscularis A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Neureclipsis valida (Figure 259) has only been collected in the Northern Region. It was found in all habitat types, with large rivers being the most common type. Adults were abundant in July, and present in August and September.
Neureclipsis valida A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
The genus Nyctiophylax contains 4 species in Minnesota. For additional species, see
Nyctiophylax affinis (Figure 260) was the most widespread and abundant Nyctiophylax species, common in all regions. It was most abundant in lakes, although it occurred in all habitat types. Adults were collected mainly in June and July, with some presence in August.
Nyctiophylax affinis A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule.
Nyctiophylax banksi (Figure 261) is known only from 6 specimens collected from Eaglenest Lake, Saint Louis County, in the Northern Region during 1957 and 1959. The specimens were accesioned into the Illinois Natural History Survey where they remain. The identify of the specimens as Nyctiophylax banksi was confirmed during this study. The species has not been found in Minnesota since these collections. It is known from South Carolina to Ontario, but is not widespread in any portion of its range (
Nyctiophylax banksi A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule.
Nyctiophylax celta (Figure 262) was only found in the Northern Region, mostly in July with some presence in June. It was found almost exclusively in rivers, particularly medium and large rivers.
Nyctiophylax celta A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule.
Nyctiophylax moestus (Figure 263) is known from the Lake Superior, Northern, and Southeastern Regions.Adults were mainly present in June and July. They were collected mostly in small and medium rivers.
Nyctiophylax moestus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule.
The genusPolycentropus contains 17 species in Minnesota. It is the 3rd most species-rich genus. Members are generally considered predators, but some of the smaller species may be detritivores (
Polycentropus (Plectrocnemia) albipunctus (Figure 264) is known only from the Lake Superior and Northern regions. It was found in all habitat types, mainly during July, with a few specimens collected in June.
Polycentropus albipunctus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Polycentropus (Plectrocnemia) aureolus (Figure 265) has been collected mainly from the Northern Region. It was found in all habitat types. Adults were most abundant in July, with some specimens in August and September.
Polycentropus aureolus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Polycentropus centralis (Figure 266) has only been collected from the Lake Superior Region during July. It was found in fast-moving small and medium rivers.
Polycentropus centralis A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Polycentropus (Plectrocnemia) cinereus (Figure 267) is the smallest Polycentropus species and also the most abundant. It was the 7th most widespread species in the state overall, and common in all regions (Figure 8). It was found predominantly in lakes and was the 2nd most abundant species in lakes of the Lake Superior Region (Table 3). Adults were abundant from June to August and present in September.
Polycentropus cinereus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Polycentropus (Plectrocnemia) clinei (Figure 268) is known only from 2 specimens collected from Nicollet Creek, Clearwater County, in the Northern Region during June 1989.
Polycentropus clinei A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Polycentropus confusus (Figure 269) has been collected from the Lake Superior and Northern Regions, mainly during July. Some specimens were found in June and August. It was found mainly in streams of all sizes.
Polycentropus confusus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Polycentropus (Plectrocnemia) crassicornis (Figure 270) has been found sporadically in the northern third of the state, in the Lake Superior, Northern, and Northwestern Regions. All specimens were collected in July from small and medium streams.
Polycentropus crassicornis A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Polycentropus (Holocentropus) flavus (Figure 271) has been collected mostly from the Northern Region, with some specimens known historically from the Northwestern and Southern Regions. Adults were found in June and July, mainly from lakes and medium rivers.
Polycentropus flavus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Polycentropus (Holocentropus) glacialis (Figure 272) is known only from 2 collections from Lake Carlos, Lake Carlos State Park, in the Northern Region. Adults were found in June and August; no collection attempt was made in July. Due to the rarity of the species in Minnesota, and the vulnerability of its only known habitat (
Polycentropus glacialis A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Polycentropus (Plectrocnemia) iculus (Figure 273) is known only from specimens collected from 2 small streams in Lake Itasca State Park in the Northern Region during June and July.
Polycentropus iculus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Polycentropus (Holocentropus) interruptus (Figure 274) was found in all regions, particularly the Northern Region. It was most abundant in lakes and medium rivers. Adults were present in June and July.
Polycentropus interruptus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Polycentropus (Holocentropus) melanae (Figure 275) was only collected in the Northern Region in June and, especially, July. It was found almost exclusively in lakes.
Polycentropus melanae A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Polycentropus (Holocentropus) milaca (Figure 276) is known worldwide from only 4 specimens. The holotype was collected in 1965 from Link (Lynx) Lake, Itasca County, in the Northern Region (
Polycentropus milaca A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Polycentropus pentus (Figure 277) has been found sporadically from all regions except the Northwestern. Adults were present in June and July, and found only in small and medium rivers.
Polycentropus pentus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Polycentropus (Holocentropus) picicornis (Figure 278) is known only from 2 specimens; 1 from a large river in the Lake Superior Region, and 1 from a lake in the Southern Region. Both specimens were found in June.
Polycentropus picicornis A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Polycentropus (Plectrocnemia) remotus (Figure 279) has been collected from the Northern, Southeastern, and Southern Regions. Adults were present from May through September. They were found in equal abundance in small and medium rivers, and in lakes.
Polycentropus remotus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Polycentropus (Plectrocnemia) weedi (Figure 280) is known only from theNorthern Region, mostly from medium and large rivers. Adults were present in August, and abundant during June and July.
Polycentropus weedi A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
This family contains 2 genera in Minnesota: Lype and Psychomyia, and a total of 2 species.Both genera are 5–7 mm in length and are often confused with members of the Hydroptilidae due to this small size. Larvae are either scrapers or gathering collectors and typically found in streams (
The genus Lype contains a single species in North America and in Minnesota. Larvae usually inhabit small cool streams where they consume small organic particles and periphyton (
Lype diversa (Figure 281) was sporadically collected, usually from small streams in the Northern Region during June and July. Females were far more abundant than males.
Lype diversa A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F female genital capsule (ventral view).
The genusPsychomyia contains a single species in Minnesota. For additional species, see
Psychomyia flavida (Figure 282) was commonly collected throughout all regions, especially the Northern. It was found primarily in medium and large rivers during June and July, with occasional specimens caught in August and September. Several collections of Psychomyia flavida yielded >1000 specimens, including one of nearly 10, 000 specimens from the White Earth River, Mahnomen County, in the Northern Region and one of nearly 8, 000 specimens from the Redwood River, Lyon County, in the Southern Region. Due in large part to this extreme abundance at specific sites, Psychomyia flavida was the most abundant species in large rivers of the Northern Region, and the single most abundant species overall in Minnesota, with over 23, 000 specimens examined (Table 4, Figure 9). Interestingly, >99% of these specimens were female. In fact, the large collections noted above were both entirely composed of females. Male Psychomyia flavida specimens were only located in the extreme northeastern portion of the Northern Region. Males are far more common in other states and are readily attracted to lights (e.g.,
Psychomyia flavida A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus F female genital capsule (ventral view).
This family contains a single genus, Rhyacophila, in Minnesota, and a total of 3 species. Larvae typically inhabit cold, fast-moving streams. They are unique among caddisflies in that they do not construct a case, instead remaining free-living until pupation (
The genus Rhyacophila contains3 species in Minnesota. All are restricted to the Lake Superior Region and only 1 is frequently encountered there. For additional species, see
Rhyacophila angelita(Figure 283) is known only from a single specimen collected during July 1965 from the city of Hovland in the Lake Superior Region. It has not been collected since, and it is difficult to know if the species has been extirpated or is simply difficult to collect.
Rhyacophila angelita A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Rhyacophila fuscula(Figure 284) was commonly collected throughout the Lake Superior Region from all sizes of streams, especially medium rivers. The majority of adults were present in July.
Rhyacophila fuscula A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
Rhyacophila vibox(Figure 285) is only known from a single specimen collected from Poplar Creek, Cook County, in the Lake Superior Region during June 2000.
Rhyacophila vibox A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
This family contains one genus in Minnesota, Agarodes, and a single species. For additional species, see
The genus Agarodes contains a single species in Minnesota. Adults are grey in color and range 8–12 mm. Males often have unusual secondary sexual characteristics, most notably enlargement and increased setation of the antennal scapes and maxillary palpi.
Agarodes distinctus (Figure 286) has been collected in June and July, mostly from large rivers. It is known only from the Lake Superior and Northern Regions.
Agarodes distinctus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule E phallus.
This family contains a single genus in Minnesota, Neophylax, and a total of 3 species. One recent classification placed Neophylax in the family Thremmatidae (
The genus Neophylax contains 3 species in Minnesota. Due to the difficulty of collecting night flying adults during the typically cool autumn evenings, all 3 are probably more widespread than their known distributions suggest.
Neophylax concinnus (Figure 287) has been collected from the Lake Superior, Northern, and Southeastern Regions. Adults were collected mainly in September and were abundant on warm evenings. It was collected almost exclusively from small streams.
Neophylax concinnus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule (ventral view).
Neophylax fuscus (Figure 288) is mainly known from the Northern Region and exclusively from large rivers. Adults were collected in September.
Neophylax fuscus A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule (ventral view).
Neophylax oligius (Figure 289) is known from the Lake Superior and Northern Regions. Adults were collected only in September and typically from medium rivers.
Neophylax oligius A total specimens collected and all known collecting localities (Figure 4) B monthly adult abundance (1980s to present) C habitat preference (1980s to present) (Table 1) D male genital capsule (ventral view).
Another species of Neophylax, Neophylax nacatus, was reported from Minnesota from a larval specimen (
Adult specimens of A Glossosoma intermedium (Glossosomatidae) B Protoptila maculata (Glossosomatidae) C Agraylea multupunctata (Hydroptilidae) D Hydropsyche simulans (Hydropsychidae) E Hydropsyche betteni (Hydropsychidae) F Oxyethira forcipata (Hydroptilidae).
Adult specimens of A Potamyia flava (Hydropsychidae) B Mystacides sepulchralis (Leptoceridae) C Leptocerus americanus (Leptoceridae) D Mystacides interjecta (Leptoceridae) E Nectopsychidae candida (Leptoceridae) F Nectopsyche exquisita (Leptoceridae).
Adult specimens of A Ceraclea cencellata (Leptoceridae) B Ceraclea transversa (Leptoceridae) C Ceraclea maculata (Leptoceridae) D Oecetis avara (Leptoceridae) E Triaenodes marginata (Leptoceridae) F Triaenodes tarda (Leptoceridae) G Oecetis inconspicua (Leptoceridae).
Adult specimens of A Asynarchus rossi (Limnephilidae) B Frenesia missa (Limnephilidae) C Hesperophylax designatus (Limnephilidae) D Limnephilus submonifer (Limnephilidae) E Limnephilus infernalis (Limnephilidae).
Adult specimens of A Platycentropus radiatus (Limnephilidae) B Molanna uniophila (Molannidae) C Neophylax fuscus (Uenoidae) D Limnephilus indivisus (Limnephilidae) E Pycnopsyche guttifer (Limnephilidae) F Neophylax oligius (Uenoidae).
Adult specimens of A Ptilostomis ocellifera (Phryganeidae) B Agrypnia straminea (Phryganeidae) C Banksiola crotchi (Phryganeidae).
Primary funding for this research came from a U.S. Environmental Protection Agency Science to Achieve Results Fellowship, and substantial support from the Minnesota Nongame Wildlife Tax Checkoff and Minnesota State Park Nature Store Sales through the Minnesota Department of Natural Resources’ (MNDNR) Natural Heritage and Nongame Research Program. Special thanks are due to R.J. Baker, MNDNR, for assistance with the latter funding source and for his continued interest in the project. Support for writing this monograph came from the Hillsdale College (HC) Faculty Development Fund. Further support came from a Doctoral Dissertation Special Grant from the Graduate School, University of Minnesota (UM); several grants from the Dayton and Wilkie Fund, Bell Museum of Natural History, UM; and several grants from the Chiang Travel Fund, Department of Entomology, UM. Support for databasing the University of Minnesota caddisfly collection came from UM Insect Collection grants AES0017029 and AES0017017 to R.W. Holzenthal. Publication costs were supported by the HC Department of Biology, the UM Department of Entomology, and the UM Insect Collection.
I sincerely thank the many workers, from the 1890s to the 2010s, who have collected Minnesota caddisflies and deposited them into the University of Minnesota Insect Collection, especially R.J. Blahnik, D.G. Denning, W. Elkins, D.A. Etnier, A.A. Granovsky, R.W. Holzenthal, J. Huisman, O. Lugger, D.B. MacLean, and M.P. Monson. I further thank D.A. Etnier (University of Tennessee), C. Favret (Illinois Natural History Survey), D.B. MacLean, and O.S. Flint (National Museum of Natural History) for making specimens available for my examination.
I thank G.D. Archibald, M.L. Galatowitsch, K.A. Egerman, C.C. Fenendael, P.A. Gillis, K. Ha, A.S. Haughland, T.J. Ling, E.A. Malcolm, N.J. O’Neil, R.C. Stephen, J.M. Zaspel, and J.L. Zeglin for laboratory assistance; and M. Carroll, D.A. Etnier, E. Etnier, K. Ghandi, and R. Suranyi for field assistance. I thank C. Cialek and, especially, S. Maedor, Minnesota Geospatial Information Office, for GIS assistance. I thank A. Lashaway, HC Department of External Affairs, for other computer assistance. I thank P. Fowler and C. Hoard, HC Department of Biology, for office assistance. A permit to collect in State Park habitats was provided by E. Quinn, MNDNR, Division of Parks and Recreation. Preliminary sketches of many species were prepared by K. Kuda. The valuable comments of S.R. Moulton, R.W. Holzenthal, and 1 anonymous reviewer improved earlier versions of the manuscript.
Lastly and most importantly, I thank R.W. Holzenthal for his advice and assistance throughout this project, and for maintaining a world-class caddisfly collection and database at the University of Minnesota. Having such an infrastructure already in place makes a study like this much more feasible.