(C) 2011 Wolfram Mey. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
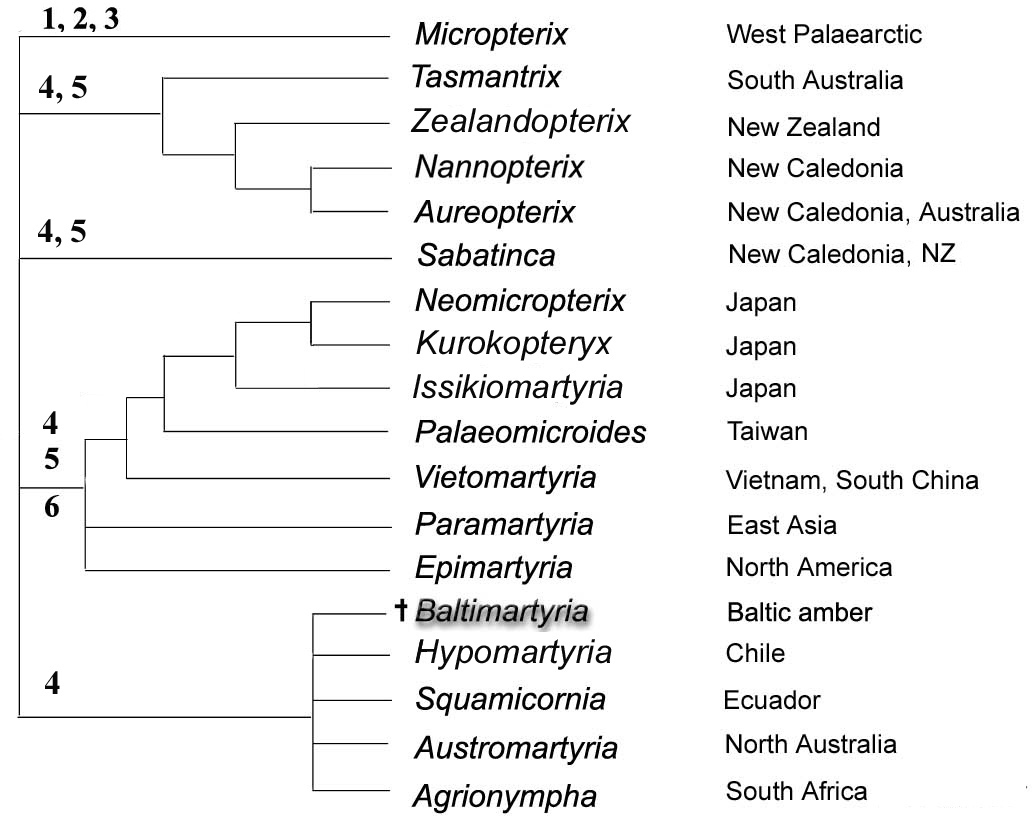
This paper describes a rare case of a male moth in Baltic amber in an excellent position for establishing a species. The moth represents the second species of the genus Baltimartyria Skalski, 1995, described herein as Baltimartyria rasnitsyni sp. n. The detection of this new species prompts research on the systematic position of the genus within the family Micropterigidae. The genus was found to provide none of the apomorphic characters that would allow placement in one of the monophyletic lineages within the family. The genus is provisionally assigned to the “southern sabatincoid group”, a weakly supported assemblage of Southern Hemisphere genera. The sister genus has still to be determined. Baltimartyria is the first North Hemisphere representative in this group. Some general aspects of historical biogeography relevant for the group are briefly discussed.
Insecta, Lepidoptera, Micropterigidae, fossils, Baltic Amber
In comparison with other mega-diverse insect orders preserved in Baltic amber, the Lepidoptera are poorly known (
For many years I had a Baltic amber piece on my desk containing a male moth in a splendid position. The up-held and slightly rubbed wings reveal venational and partial genitalic characters well. The species clearly belongs to Micropterigidae, the most ancestral family of extant Lepidoptera. A number of fossil genera and species were described and assigned to this family (Koslov 1988, Koslov et al. 2002) but their placement in Micropterigidae was rejected by
Records of Micropterigidae described from amber.
| species | origin | first revision | current combination |
|---|---|---|---|
| Micropteryx pervetus Cockerell, 1919 | Burmese amber |
|
Sabatinca s.l. |
| Micropteryx proavitella Rebel, 1936 | Baltic amber |
|
Baltimartyria |
| Parasabatinca aftimacra Whalley, 1978 | Lebanese amber | Parasabatinca | |
| Micropterix gertraudae Kurz & Kurz, 2010 | Baltic amber | Micropterix |
Baltimartyria Skalski, 1995
urn:lsid:zoobank.org:act:1D69948C-B1FF-44BC-B313-2BAA38FA82DF
http://species-id.net/wiki/Baltimartyria_rasnitsyni
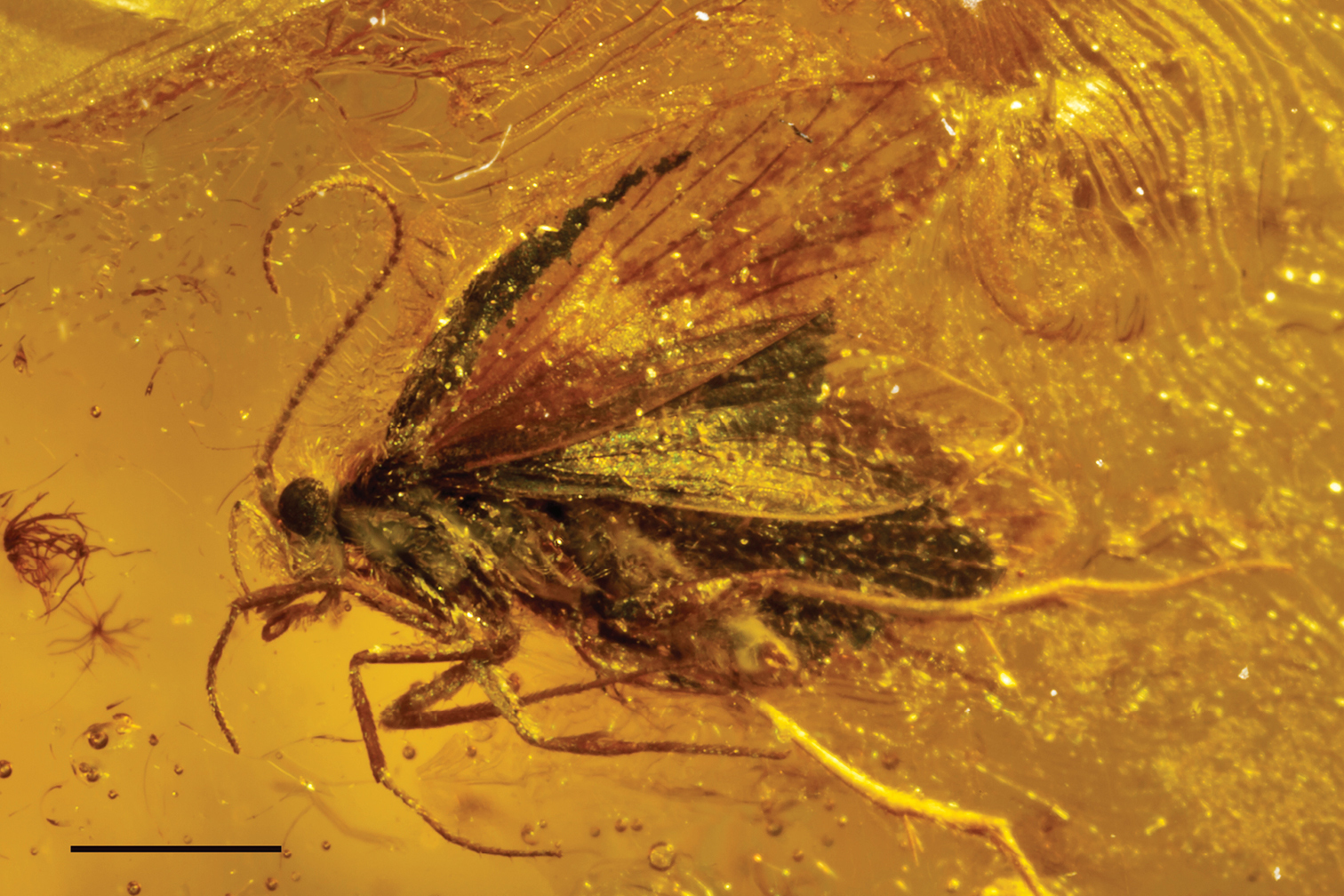
Figs 1–11Holotype male, Baltic Amber, MB.I 5950, deposited in Museum für Naturkunde, Berlin.
The adult moth is completely preserved and clearly visible from a ventro-lateral view. Right maxillary palps and antenna covered by body, dorsal and inner side of genitalia filled by white emulsion.
Named in honour of Alexandr P. Rasnitsyn, the eminent Russian paleoentomologist.
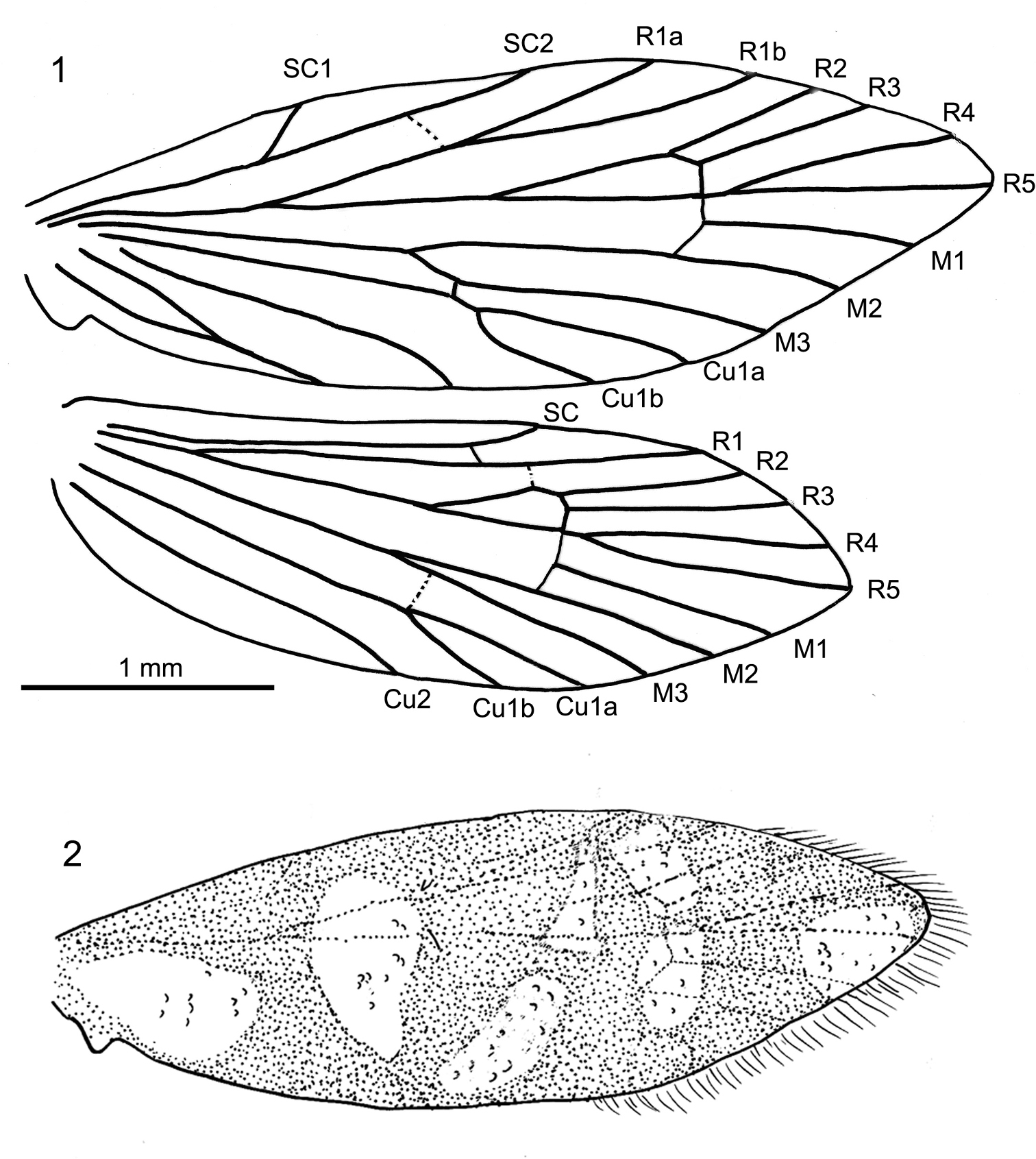
Baltimartyria rasnitsyni sp. n. can be separated from Baltimartyria proavitella by segment four of maxillary palps being as long as third and second segment together, and by shortly stalked R4 and R5 in both fore- and hindwings. In Baltimartyria proavitella, the two terminal segments of the maxillary palps are as long as the third segment, and in the fore- and hindwings all terminal R branches originate separately from the cell.
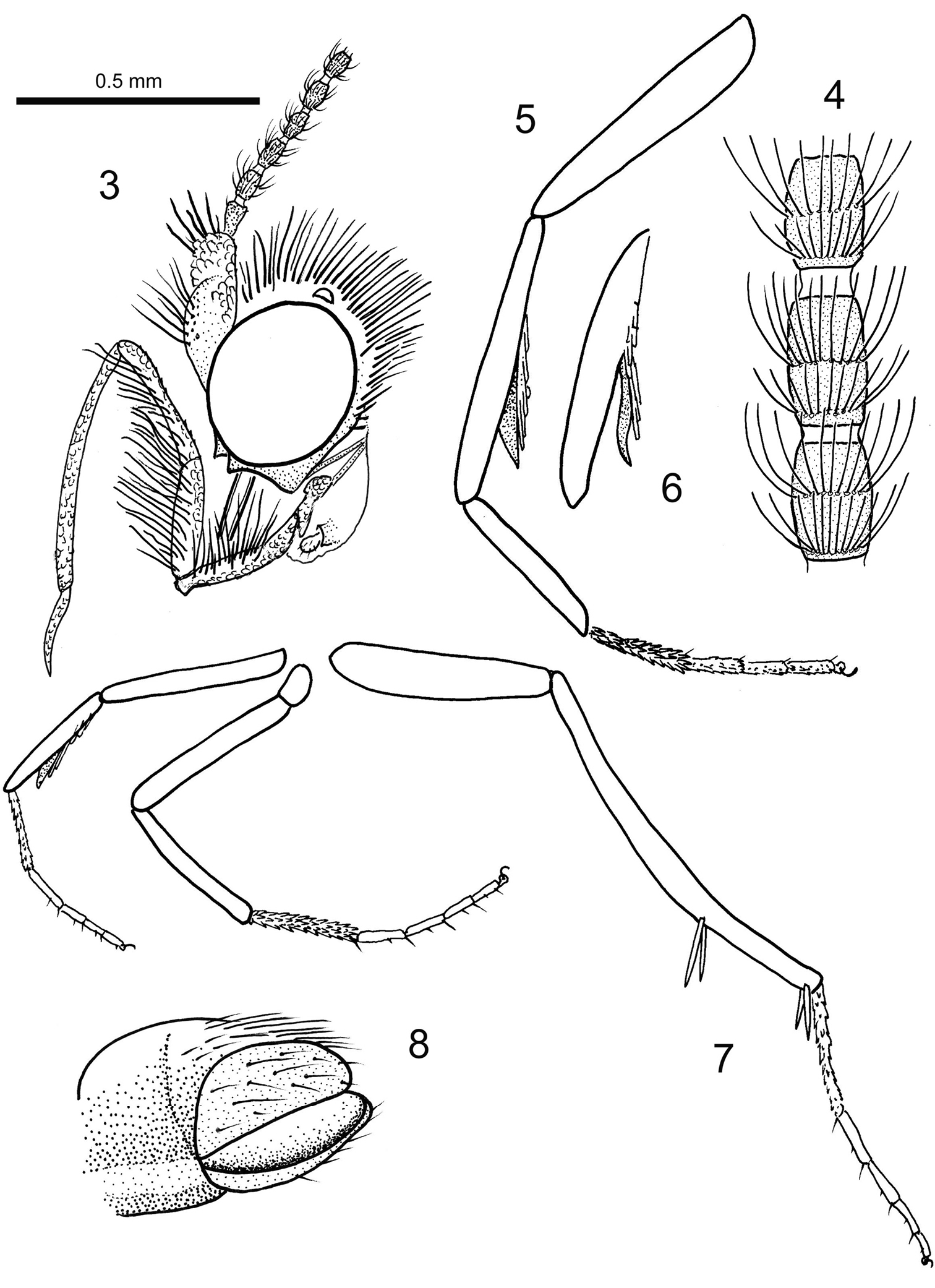
Length of forewing 4 mm, length of body 3 mm; head with erect, piliform scales on vertex, ocelli pale white, scape and pedicel together as long as eye diameter, scaled, 35 barrel-shaped flagellomeres present, basal segments (1–3) with scales, subsequent segments unscaled, each flagellomere with two whorls of long sensilla trichodea, one basal and one at mid-length, ascoids not clearly visible; maxillary palps five segmented, basal segments of equal length, fourth segment longest, terminal segment short, acute and with short bristles; labial palps two-segmented, terminal segment small, rounded; mandibles present (only base visible); fore-tibia with blade-like epiphysis exhibiting an acute tip, spurs 0.0.4, basitarsus of all legs covered with short, acute and semi-erect scales, tarsal segments with terminal, pair of ventral bristles; reconstructed wing pattern in Fig. 2, venation in Fig. 1, R1 with two branches in forewing, R4 and R5 on a short common stalk from cell in both wings, (anal field of hindwing only partially visible); ventral side of abdominal segment VIII membranous (= pale brown), lateral sides sclerotised (= dark brown), vinculum deeply retracted into segment VIII (segmental limits obsolete by milky nebulae); valvae simple, elliptical and spoon-like, outer surface covered by hairs.
Baltimartyria rasnitsyni sp. n. 1 wing venation 2 reconstructed wing pattern.
Baltimartyria rasnitsyni sp. n. 3 head, lateral view 4 flagellomeres from mid-antenna, enlarged 5 foreleg, enlarged 6 tibia of foreleg with epiphysis from different view 7 legs 8 tip of abdomen and genitalia, ventrolateral view.
Male holotype of Baltimartyria rasnitsyni sp. n. Right hand side position, scale bar 1 mm.
Male holotype of Baltimartyria rasnitsyni sp. n. Details of head, scale bar 1 mm.
Male holotype of Baltimartyria rasnitsyni sp. n. Antenna, scale bar 1 mm.
Presumed phylogenetic relationships within Micropterigidae based on
Characters of Micropterigidae observable in Baltimartyria and relevant for its placement.
| number | character | plesiomorphic state | apomorphic state |
|---|---|---|---|
| 1 | R in forewing | all branches to costa | R5 to wing apex |
| 2 | R1 in forewing | forked | simple, unforked |
| 3 | male segment IX | caudal margin simple and straight | caudal margin with processes |
| 4 | male venter VIII | sclerotised | more or less membranous |
| 5 | R in hindwing | complete vein to costal margin | more or less coalescent with Sc |
| 6 | antenna | filiform, flagellomeres barrel-shaped | flagellomeres modified |
Given the uncertainty about the placement of Baltimartyria any discussion on aspects of its historical biogeography is premature. However, the following, general remarks might be useful for considering in future discussions.
According to the geographic distribution of extant Micropterigidae (
However, in Eskov`s discussion on Gondwanan vs. non-Gondwanan origin of taxa the role of drifting terranes is not considered. In South East Asia a series of terranes were identified, which were attached to the Asian continent during the Paleozoic and Mesozoic. They all arrived from the south and had their origin on the northern margin of Gondwana (
There are undoubtedly more undetected micropterigid species, fossil and extant, on the globe. Each discovery provides new information and throws new light on current phylogenetic and biogeographic reconstructions.
The paper was presented at the workshop on Baltic Amber during the DGaaE (= Deutsche Gesellschaft für allgemeine und angewandte Entomolgie) conference in Berlin in March 2011. The organiser of the workshop, W. Wichard, invited me to talk on amber Lepidoptera and proposed to submit the present paper for the Festschrift. C. Neumann took a photo of the inclusion and provided the inventory number. H.C. Zeller-Lukashort gave some important hints. Jason Dunlop corrected the English text. N.P. Kristensen and D.C. Lees reviewed the manuscript and suggested many corrections and improvements. I sincerely thank all of them for their input and help.