(C) 2011 Terry L. Erwin. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Information on the three previously described species of Halocoryza Alluaud is updated and a new species for the genus from Isla Carmen, Sea of Cortés, Baja California Sur, México is described. Halocoryza whiteheadiana sp. n. was found at UV light on a beach of that island. This species does not fit the profile of the other three species, i.e., living on coralline beach sands, or in the Mangrove intertidal zone. Two alternative possibilities as to why this is so are suggested and a study plan for testing these possibilities is proposed.
ResumenInformación de las tres especies de Halocoryza Alluaud previamente descritas se actualiza y una especie nueva para el género se describe de la Isla de Carmen, Mar de Cortés, Baja California Sur, México. Halocoryza whiteheadiana sp. n. se encontró a luz UV en la playa de esa isla. Esta especie no corresponde con los perfiles de las otras tres especies (por ejemplo, viviendo en arenas coralinas de playa o en la zona intermareal en el manglar. Dos posibles alternativas para la razón de esto son sugeridas y un plan de estudio para probar estas posibilidades se propone.
Sea of Cortés, Caribbean Sea, Gulf of México, intertidal zone, sandy beaches, beetles, centipedes, coral reefs, Isla Carmen
Mar de Cortés, Mar Caribe, Golfo de México, zona intermareal, playas, arenosas, escarabajos, ciempiés, arrecifes de coral, Isla Carmen
Halocoryza beetles belong to the subtribe Clivinina and are closely related to the genus Schizogenius
It is named here in honor of Donald R. Whitehead† who had
a great interest in this group of beetles and discovered a lot about
their special place on the sea shore. I have personally collected but
one specimen of this genus on the shore of the Caribbean side of Panamá,
and was amazed that these beetles accept such saline conditions, as
they do. Whitehead pondered whether Halocoryza should be included within Schizogenius, perhaps as a subgenus, as is Listropus Putzeys, a group that
Methods and species concepts follow those previously described (
Included in this study are a total of 12 specimens from
the National Museum of Natural History, Washington, DC (NMNH) in my
charge, and a single paratype from the California Academy of Sciences
(CASC, David H. Kavanaugh, Curator). The habitus images of the adult
beetles portray most of the character states referred to in the key
provided. Illustrations of male genitalia (modified from
Geographical data are presented for species based on all known specimens available at the time of manuscript preparation, including those in the literature. Georeferences have been determined from locality information provided on specimen labels; only those exact georeferences that are provided on the label are placed in quotes, otherwise I have estimated these as closely as possible from places, mileage, etc. listed on the label and searched with Google Earth. Latitude and longitude are reported in decimal degrees. Here, English vernacular names are proposed, as common names are becoming increasingly needed in conservation and/or agricultural and forestry applications, and for the Encyclopedia of Life (www.eol.org).
Accounts of taxa Halocoryza Alluaud, 1919Saline Catarrh Beetles
Halocoryza maindroni
Four
Stable. Adelphotaxon: Schizogenius Putzeys, 1846
Equatorial to Tropic of Cancer; sea coasts and islands of east Africa – Comoros – Mayotte; Djibouti; Madagascar; Mauritius; Saudi Arabia; Somalia; and natural invasive from the Caribbean into west Africa – Cameroon; Ecuador – Galapagos Islands; Barbados; Brazil – Pernambuco; Dominican Republic; Grenada; Guadeloupe; Jamaica; México – BJ, GO, QR, YC; Panamá; Puerto Rico; USA – FL; Virgin Islands – St. John, St. Thomas
Sea beaches and mangrove intertidal zone
The common name, Saline Catarrh Beetles, proposed here follows my principle of translating the scientific name as strictly as possible. In this case, “coryza” comes from the Greek, koryza, meaning cold, catarrh, as in disease. Why Alluaud named the genus so is not known.
Differing in adult attributes from those of its adelphotaxon, Schizogenius Putzeys, 1846, by the following: Pygidium not striate or with very subtly crenulate striae; antennomere 2 pluristose. In addition, mandibles prominent, nearly straight laterally, abruptly angulate near apices; lacinia asetose on outer margin; frontal carinae nearly perfectly regular, parallel, equidistant, and equally raised; frons evenly convex; neck not pitted or punctate dorsally; eyes reduced, bordered laterally by a distinct carina; gula broad; mentum not deeply emarginate at middle, with median tooth obsolete and epilobes short; tarsi short; paramedian carinae of sternum II short, widely spaced and poorly developed; median lobe of male genitalia neither arcuate nor abruptly deflexed in apical third; fused stylus and coxite of the ovipositor with one robust seta (Whitehead, 1966, 1972).
Sea beaches, intertidal lagoons on the edges of mangroves, and island shores of the Atlantic, Indian, and Pacific Oceans, the Caribbean Sea, Sea of Cortés, and the Gulf of México.
The species list below, as well as arrangement of descriptions that follow is ordered alphabetically.
|
Halocoryza acapulcana |
Ecuador; México |
| Halocoryza arenaria (Darlington, 1939) | Barbados; Brazil; Dominican Republic; Grenada; Guadeloupe; Jamaica; México; Panamá; Puerto Rico; USA; Virgin Islands; Africa – Cameroon |
| Halocoryza maindroni Alluaud 1919 | Comoros – Mayotte; Djibouti; Madagascar; Mauritius; Saudi Arabia; Somalia |
| Halocoryza whiteheadiana sp. n. | México |
| 1 | Pronotum without median sulcus, anterior angles acute; Indian Ocean | Halocoryza maindroni Alluaud, 1919 |
| 1’ | Pronotum with median sulcus, anterior angles rounded; Atlantic, Caribbean, or Pacific Oceans | 2 |
| 2(1’) | Form markedly elongate; pronotum without paramedian carinae at margins of sulcus, Sea of Cortés | Halocoryza whiteheadiana sp. n. |
| 2’ | Form moderately elongate; pronotum with paramedian carinae at margins of sulcus | 3 |
| 3(2’) | Smaller species (LE: 1.20 – 1.35mm), elytra sparsely setose, interval 3 with 10 or fewer setae; color pale testaceous; Pacific Ocean | Halocoryza acapulcana Whitehead, 1966 |
| 3’ | Larger species (LE: 1.35 – 1.45mm), elytra densely setose, interval 3 with 10 or more setae; color dark testaceous; Atlantic Ocean and Caribbean Sea | Halocoryza arenaria (Darlington, 1939) |
Acapulco Saline Catarrh Beetle
http://species-id.net/wiki/Halocoryza_acapulcana
Figs 1, 4, 7Native, New World. Ecuador – Galapagos Islands: Rábida (Jervis); México – OA.
Macrohabitat: Lowlands, sea level, in the intertidal zone of beaches. Microhabitat: Adults are ground-dwelling on saline soils. Dispersal abilities: Macropterous, capable of flight; slow runners. Seasonal occurrence: Adults have been found in March and August. Behavior: Nocturnal predators, adults are attracted to lights.
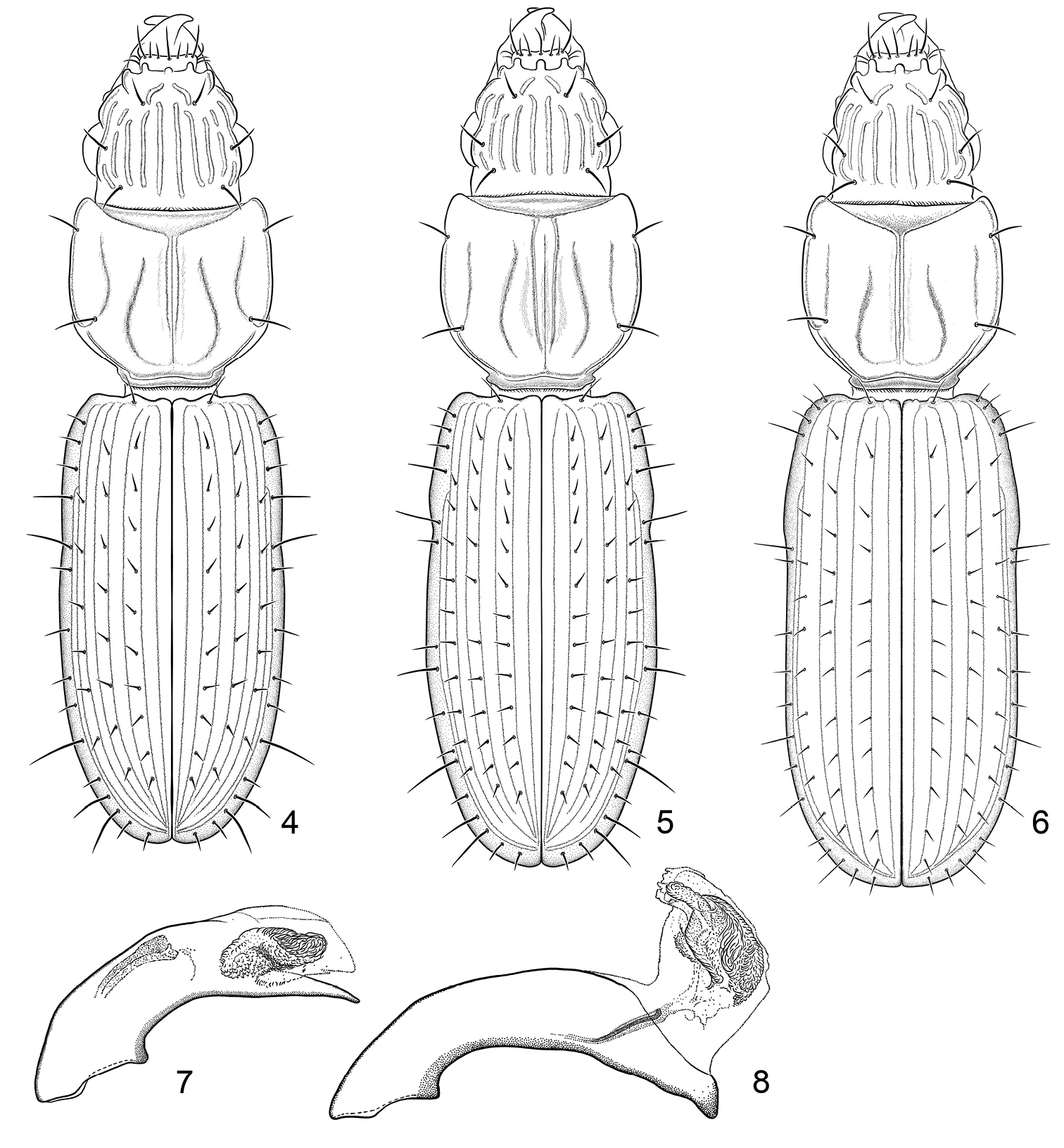
Figure 1–2. 1 Color image, showing habitus of Halocoryza acapulcana Whitehead, 1966, dorsal aspect, ABL = 2.4mm, ADP127171; Acapulco, México 2 Color image, showing habitus of Halocoryza arenaria (Darlington, 1939), dorsal aspect, ABL = 2.9mm, ADP116798; St. Johns, Virgin Islands.
Sand Saline Catarrh Beetle
http://species-id.net/wiki/Halocoryza_arenaria
Figs 2, 5, 8Native, New World. Barbados; Brazil; Dominican Republic; Grenada; Guadeloupe; Jamaica; México – QR, YC; Panamá; Puerto Rico; USA – FL; Virgin Islands – St. John, St. Thomas; natural invasive, Africa – Cameroon.
Macrohabitat: Lowlands, sea level – 1 meter altitude, on sea beaches and in the intertidal area, at or near the high tide line, and in mangrove swamps. Microhabitat: Adults are ground-dwelling on exposed wet substrate consisting of coquina-coral cemented by very fine silt or sand and covered with seaweed mats. Dispersal abilities: Wing-polymorphic: macropterous form probably capable of flight; brachypterous form, consequently flightless thus vagility limited to walking or running; both forms slow runners. Seasonal occurrence: Adults have been found in March – April, July, and October. Behavior: Adults are nocturnal predaceous halobionts and take cover in the sand or under drift and piles of seaweed on the beach. Populations of this species are associated with the centipede Pectiniunguis halirrhytus Crabill. In the northern part of their range, adults overwinter in the substrate; in the southern part, they likely aestivate during the dry season in the substrate.
Color image, showing habitus of Halocoryza whiteheadiana sp. n., 3a, left lateral aspect, 3b, dorsal aspect, ABL = 2.9mm; Holotype: Isla Del Carmen, BJ, México.
4 Line drawing, showing habitus of Halocoryza acapulcana Whitehead, 1966, dorsal aspect, ABL = 2.4mm; Acapulco, México; modified from
Maindron’s Saline Catarrh Beetle
http://species-id.net/wiki/Halocoryza_maindroni
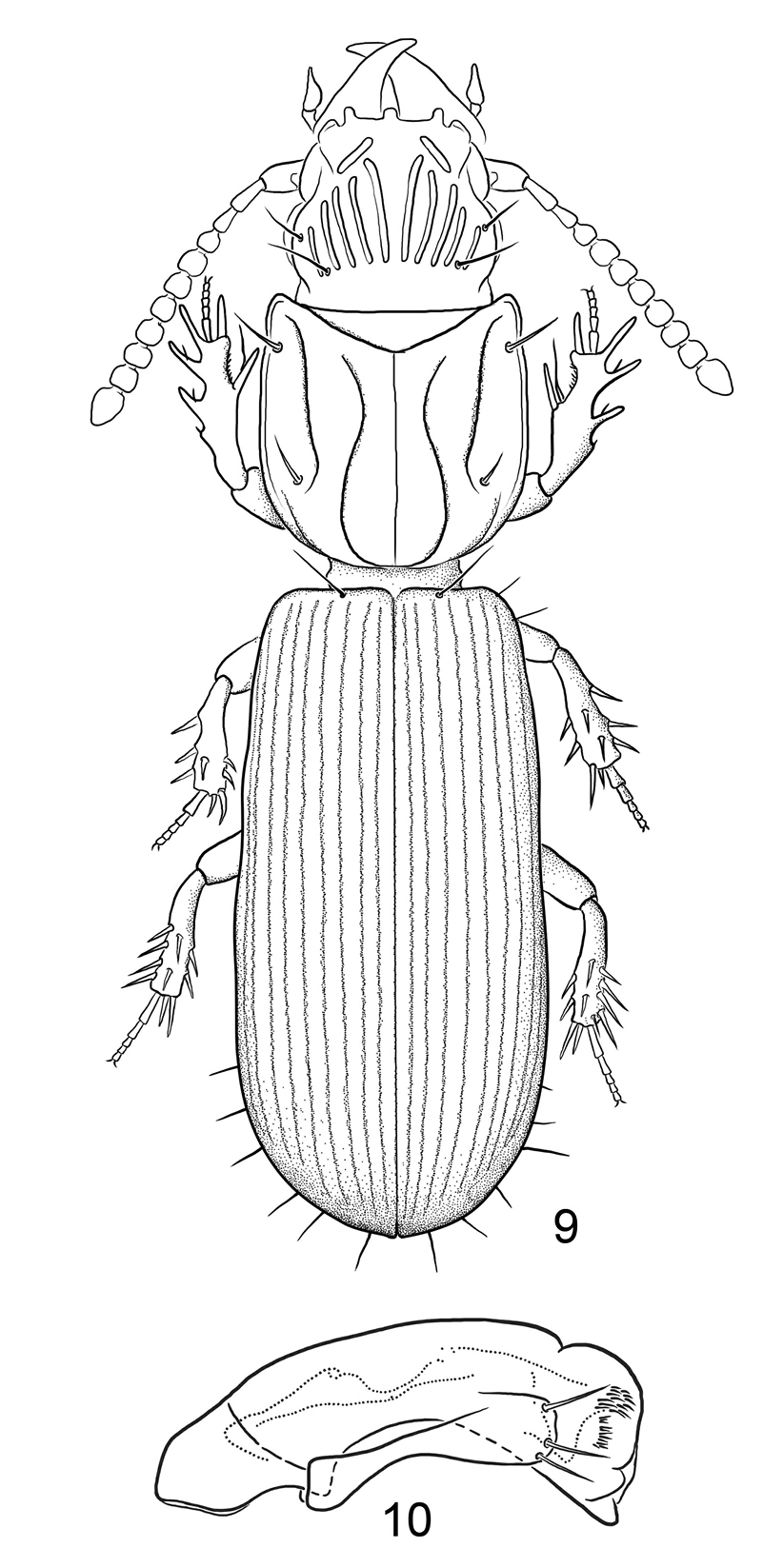
Figs 9, 10Native, Old World. Comoros – Mayotte; Djibouti; Madagascar; Mauritius; Saudi Arabia; Somalia.
Macrohabitat: Lowlands, sea level, in the intertidal zone of sea beaches. Microhabitat: Adults are ground-dwelling on coralline sands in the vicinity of coral reefs. Dispersal abilities: Wing-polymorphic: macropterous form probably capable of flight; brachypterous form, consequently flightless thus vagility limited to walking or running; both forms slow runners. Seasonal occurrence: Adults have been found in January and October. Behavior: Nocturnal predators, adults take cover during the day under dry seaweed just above the high water mark.
9 Line drawing, showing habitus of Halocoryza maindroni
Whitehead’s Saline Catarrh Beetle
urn:lsid:zoobank.org:act:FDB58B5D-FFB1-442F-BBEA-EBD82BC958F8
http://species-id.net/wiki/Halocoryza_whiteheadiana
Figs 3a, 3b, 6Baja California Sur, Isla Carmen, north end, sea level, approximately “26.05°N, 111.1°W, ” 18-19 July 1984 (S.E. Miller) (NMNH: ADP127139, female).
The epithet “whiteheadiana” is an eponym, based on the family name of Donald R. Whitehead†, who had a profound interest in the species of this genus and its adelphotaxon, Schizogenius Putzeys, during his relatively short career.
Whitehead’s Saline Catarrh Beetle.
With the attributes of the genus described by
(Fig. 3). Size: Very small, ABL = 2.8 mm, SBL = 2.52 mm, EW = 0.69 mm, LP = 0.647mm, WP = 0.622mm, LE = 1.476mm. Color: Rufotestaceous throughout. Luster:Shiny throughout. Head: Labrum slightly emarginate apically, six-setose. Frons markedly tri-tuberculate apically, laterally markedly lobate, lobes nearly vertical, bicarinate basally, carinae set on an angle, widest basally. Eyes slightly convex; gena short and flat. Occiput five-carinate each side; rim above eye also carinate. Prothorax: Markedly convex, moderately longer than broad (W/L: 0.96), narrowed basally anteriad of posterior lateral pore; surface smooth, five-sulcate; lateral sulci 2/3 length of pronotum, extended to posterior lateral pore, paramedian sulci sigmoid shaped, not reaching base, median sulcus deep, extended from near apex to base of pronotum, slightly crossing anterior transverse sulcus; anterior and posterior lateral setae present. Pterothorax: Elytra markedly convex (W/L: 0.46), intervals markedly convex, intervals 3, 5, and 7 each with a serial row of setiferous punctures, 10 such in interval 3. Legs: Normal in female. Abdomen: Abdominal sterna III to VI of female with normal ambulatory setae, VII with a pair of setae each side. Male genitalia: Unknown. Female genitalia: Not studied.
These beetles, as represented by the holotype, are macropterous and are capable of flight; they are slow runners, strong burrowers. However, both the Caribbean and Indian Ocean species are wing-polymorphic and perhaps with additional specimens, Halocoryza whiteheadiana may prove to be the same.
Adults of other Halocoryza species are found on coralline sands in the intertidal zone of open beaches and among mangroves; a larva of the Indian Ocean species of this genus was found under dry seaweed just above the high-water mark on coralline sands. The single known specimen of Halocoryza whiteheadiana was collected at UV light on a sandy beach on the north shore of Isla Carmen, Baja California Sur. Adults of Halocoryza whiteheadiana are likely nocturnal halobiont predators, as are members of the other known species of this genus.
None.
According to
Thanks to information provided by my good friend and colleague, Rick Brusca, and his colleagues Markes E. Johnson and Ramon Andres Lopez Perez, I now know that Isla Carmen has fossil coral deposits on it and that corals in the past were more extensive in the Sea of Cortés. Today, they occur in the waters off Isla Carmen, but do not form reefs there. Thus, species of the genus Halocoryza could be indicators of both present and/or past corals in the adjacent seas that are presently contributing, or have in the past, to the sandy mix of the beach on which they are found. Alternatively, Halocoryza whiteheadiana may represent a species that has undergone an ecological shift since the Pleistocene to an existence on another form of calcium carbonate, namely crushed mollusk shells. Determining if they also occur on the rhodolithic sands, (i.e. those of derived from coralline alga which are major contributors of CaCO3 to beaches in the area) will require an additional sampling.
In addition to the museum curator noted above, I also extend hearty thanks to Charyn Micheli (the Resumen, specimen prep, measures, and friendly review of the manuscript) and Karolyn Darrow (images and illustrations), both of the Department of Entomology at the Smithsonian Institution, and to Elizabeth Driscoll (Sonora, CA) for final editorial review of the manuscript. And, a special thanks to Rick Brusca, Markes E. Johnson, and Andres Lopez Perez, for historical information on the corals of the Sea of Cortés. George E. Ball and David H. Kavanaugh provided excellent reviews that added greatly to substance and constancies in style.