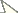(C) 2011 Jéssica P. Gillung. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Information on the three previously described species of Halocoryza Alluaud is updated and a new species for the genus from Isla Carmen, Sea of Cortés, Baja California Sur, México is described. Halocoryza whiteheadiana sp. n. was found at UV light on a beach of that island. This species does not fit the profile of the other three species, i.e., living on coralline beach sands, or in the Mangrove intertidal zone. Two alternative possibilities as to why this is so are suggested and a study plan for testing these possibilities is proposed.
Spider fly, Acroceridae, cybertaxonomy
Spider flies (Diptera: Acroceridae)
are a geographically cosmopolitan group although most species are
relatively rarely collected. Most species feed at flowers and are likely
important specialized pollinators as suggested by their frequently
elongate proboscis (often equal to body length) and nectar feeding
habits, although some species have reduced or even vestigial mouthparts
(
Acroceridae comprise over 520 described species in about 53 genera (
There are approximately 52 species and 14 genera in Philopotinae, both living and fossil, found in all major biogeographical regions. Two morphological groups are easily recognizable in the subfamily based on reduction of wing venation (i.e. number of wing cells and primary veins approximating wing margin). The first group comprises six genera with relatively complete wing venation and includes: Dimacrocolus Schlinger, 1961 (Madagascar), Eulonchiella Meunier, 1912 (Baltic amber), Helle Osten Sacken, 1896 (New Zealand), Megalybus Philippi, 1865(Chile), Parahelle Schlinger, 1961 (Madagascar) and Thyllis Erichson, 1840 (South Africa and Madagascar). The second group comprises eight genera characterized by reduced wing venation such that cells d, bm and even m3 are absent through reduction and loss of crossveins. The wings typically have only major veins radiating from cell br (Figs 1B, D). Genera in this group include Africaterphis Schlinger, 1968 (Africa), Archaeterphis Hauser & Winterton, 2007 (Baltic amber), Oligoneura Bigot, 1878 (Palaearctic), Philopota Wiedemann, 1830 (South and Central America), Prophilopota Hennig, 1966 (Baltic amber), Quasi gen n. (Mexico), Schlingeriella gen n. (New Caledonia) and Terphis Erichson, 1840 (South America).
Eulonchiella eocenica Meunier, 1912 was briefly described and poorly illustrated by
Terminology follows
http://species-id.net/wiki/Eulonchiella
Figs 1E–G, 2Neotype male, Baltic amber (#DB 10-12) (CAS).
Body shape arched; colouration non-metallic brown-black; head spherical, size slightly smaller than thorax width; eye bare; male frons narrowed; eyes contiguous above and below antennal base; posterior margin of eye rounded; proboscis length greater than head length; position of antenna in middle of frons; flagellum shape stylate; palpus present; thorax with postpronotal lobes enlarged, medially contiguous to form collar; legs not greatly elongated, tibial spines absent; pulvilli present; subscutellum slightly enlarged; wing hyaline, markings absent; costa ending in radial field; costal margin straight in both sexes; humeral crossvein present; alula well developed; anal lobe not enlarged; R2+3 present; R4+5 present as single vein; radial veins meeting wing margin before wing apex; cell r4+5 bisected by crossvein 2r-m, narrow elongate; discal cell present, closed apically; medial veins M1, M2 and M3 present; medial veins tapered and faint towards margin; cell m3 absent; CuA1 joining M3 and petiolate, not reaching wing margin; CuA2 fused to A1, not reaching wing margin, petiolate; abdomen smooth, shape rounded, cylindrical, similar width to thorax.
The above diagnosis is based on a neotype male of Eulonchiella eocenica Meunier deposited in the Poinar collection (#DB 10-12) (to be ultimately housed in CAS).
Quasi fisheri gen. et sp. n. A head and postpronotal lobes, anterior B wing C head, lateral. Schlingeriella irwini gen. et sp. n. D wing. Eulonchiella eocenica Meunier E head and postpronotal lobes, anterior F habitus in situ, lateral. Scale line = 0.2 mm.
Eulonchiella eocenica Meunier (Baltic Amber). Body length = ca. 4.5 mm.
urn:lsid:zoobank.org:act:CD9618A4-E458-4B16-A7D6-0D188D77042E
http://species-id.net/wiki/Quasi
Figs 1A–C, 3–5Quasi fisheri sp. n.
Body shape arched; colouration non-metallic pale brown; head width slightly smaller than thorax width; shape hemispherical; postocular ridge and occiput rounded; posterior margin of eye rounded; eyes bare; three ocelli present, medial ocellus slightly smaller; position of antennae on head nearer to mouthparts; eyes contiguous above antennal base, not contiguous below; palpi absent; proboscis much shorter than head length; flagellum shape stylate, apex with terminal seta; thorax with postpronotal lobes enlarged, medially contiguous to form collar; subscutellum not enlarged, barely visible; legs with tibial spines absent; pulvilli present; legs not greatly elongated; wing hyaline, markings absent; costa ending in radial field; costal margin straight; humeral crossvein absent; radial veins meeting wing margin before wing apex; R1 slightly inflated distally at pterostigma; R2+3 present, reaching wing margin; R4+5 present as very short, single vein, not reaching wing margin; medial vein compliment with only one M vein present; discal cell absent; medial vein very short, not reaching wing margin; cell m3 absent; crossvein 2r-m absent; Cu reduced, not reaching wing margin; anal lobe not enlarged; alula well developed; abdomen smooth, shape rounded, cylindrical, similar width to thorax.
Derived from Latin quasi, appearing as if resembling; referring to the likeness of this species to members of Terphis.
This genus is represented by only a single species Quasi fisheri sp. n. from Veracruz, Mexico. It is closely related to Terphis and Philopota based on reduction in wing veins. The position of the antennae, proximate to the reduced mouthparts, reduced wing venation and absence of abdominal tubercles readily differentiates this genus from all other philopotine genera.
Quasi fisheri gen. et sp. n., male, anterolateral view [Morphbank: 693076]. Body length = 6.0 mm.
Quasi fisheri gen. et sp. n., male, dorsal view [Morphbank: 693077]. Body length = 6.0 mm.
Quasi fisheri gen. et sp. n., male, anterior view [Morphbank: 693078]. Body length = 6.0 mm.
urn:lsid:zoobank.org:act:373C7B1F-51DF-4D7C-95FC-F91060433501
Holotype male, MEXICO: Veracruz: Córdoba, 12-25.vii.1964, E. Fisher, D. Verity [18.896, -96.923] (CSCA).
Medium body size (male body: 6.0 mm), male wing almost as long as the body (male wing: 5.3 mm); Head. Eyes, antennae, face and occiput brown, occiput as narrow as the ocellar tubercle, ocelli brown, antennal tubercle brown and smaller than pedicel. Thorax. Postpronotal lobes, mesothorax, scutellum, subscutellum and coxae light brown with darker longitudinal markings, legs and lower calypter yellowish brown, pulvilli yellow, tarsal claws black, haltere yellow, wing hyaline with yellow veins. Abdomen. Tergites brown, with lateral margins yellow, sternites dark brown.
Male genitalia. The genitalia were not dissected because the holotype is the only specimen available. Genitalic dissection is not necessary to diagnose the genus, since it can be differentiated based on external characters.
This species is named in honor of Eric Fisher, the collector of the only known specimen of this unusual species.
urn:lsid:zoobank.org:act:99EAC1BE-4A6F-43E0-B61A-6460BF68694E
http://species-id.net/wiki/Schlingeriella
Figs 1D, 6–7Schlingeriella irwini sp. n.
Body shape arched; colouration non-metallic dark brown; head width much smaller than thorax (female) or slightly smaller than thorax (male); head spherical; postocular ridge and occiput extended posteriorly into slight ridge; posterior margin of eye rounded; eyes bare; position of antennae on head near middle of frons, slightly nearer to mouthparts; eyes contiguous above antennal base, not contiguous below; palpi present; proboscis longer than head; antennal flagellum stylate, apex with terminal seta; thorax with postpronotal lobes enlarged, medially contiguous to form collar; subscutellum enlarged; legs not greatly elongated; tibial spines absent; pulvilli present; wing hyaline, markings absent; costa ending in radial field; costal margin straight in both sexes; humeral crossvein absent; radial veins meeting wing margin before wing apex; R1 inflated distally at pterostigma; R2+3 present; R4+5 present as single vein, slightly curved anteriorly midway; veins M1, M2 and M3 present; discal cell absent; medial veins reaching wing margin (or nearly so); cell m3 absent; crossvein 2r-m absent; Cu reduced, not reaching wing margin; anal lobe not enlarged; alula well developed; abdomen smooth, rounded, cylindrical in shape, similar width to thorax (male) or greatly rounded, inflated (female).
This genus is named in honor of Evert I. Schlinger, not only a collector of specimens of this species, but a foremost expert on world Acroceridae taxonomy and patron of dipterology.
This genus is represented by only a single species (Schlingeriella irwini sp. n.) from New Caledonia.
Schlingeriella irwini gen. et sp. n., male, lateral view [Morphbank: 693079]. Body length = 2.4 mm.
Schlingeriella irwini gen. et sp. n., female, lateral view [Morphbank: 693080]. Body length = 4.4 mm.
urn:lsid:zoobank.org:act:9AF204C7-FA7F-4DBC-B71D-4FFFCE367FB4
AY144402.1, AY140881.1, AF539888.1
Holotype male, New Caledonia: Riviere Bleue, refuse area, 700’, 28.xi.1992 E. & M. Schlinger, at Scaveola flower, prey of green crab spider [-22.112, 166.677] (MNHN) (EIS013911).
New Caledonia: 3 males, 1 female, Riviere
Bleue, same data as holotype (CAS, EIS013912, 013913) (CSCA, 013914,
013915); male, Riviere Bleue, 600’, 19.6 km on Riviere Bleue Road.
M.T., 16-17.xi.1992, E. & M. Schlinger coll. (EIS013910); female,
Riviere Bleue, 700’, Malaise, 6-16.xi.1992, E. & M. Schlinger,
D. Webb; 1 male, 1 female [abdomen only], Mt. Nihgua, Nov. 2000, E.
I. Schlinger & L. J. Boutin [voucher specimens from
Male with small body size (male body: 2.4 mm) and wing as long as the body (male wing: 2.5 mm), female with medium body size (female body: 4.4 mm) and wing longer than the body (female wing: 6.0 mm). Head. Eyes, occiput and ocellar tubercle dark brown, occiput wider than the face; ocelli shining light brown, antennal tubercle shining black, antennae light brown, face black, clypeus shining brown, as long as the antennae, proboscis yellow. Thorax. Uniform dark brown with short whitish pile; coxae yellow, legs dark yellow, femora with darker yellow-brown suffusion, lower calypter and haltere pale yellow, wings hyaline with brown veins. Abdomen. Dark brown; female tergites I-II entirely brown, tergites III-VI with the anterior half yellow and the posterior half brown, sternites brown.
This species is named in honour of Michael E. Irwin.
Key to world genera of living and fossil Philopotinae
| 1 | Wing venation reduced, with only one basal cell (br) present (Fig. 1B, D) | 7 |
| – | Wing venation relatively complete, with additional cells d, bm, cu-p and basal r4+5 present (Fig. 1E) | 2 |
| 2 | Palpi present | 4 |
| – | Palpi absent | 3 |
| 3 | Eyes densely pilose (South Africa and Madagascar) | Thyllis Erichson, 1840 |
| – | Eyes very sparsely pilose or bare (Madagascar) | Parahelle Schlinger, 1961 |
| 4 | Eyes pilose | 6 |
| – | Eyes apilose | 5 |
| 5 | Eyes contiguous below antennae; humeral crossvein present; vein R1 not inflated (Baltic amber) (Figs 1E-G, 2) | Eulonchiella Meunier, 1912 |
| – | Eyes separate below antennae; humeral crossvein absent; vein R1 inflated at pterostigma (New Zealand) | Helle Osten Sacken, 1896 |
| 6 | Legs relatively very long; male with tufted projection at the base of costa (Madagascar) | Dimacrocolus Schlinger, 1961 |
| – | Legs of normal length; male without tufted projection at the base of costa (South America) | Megalybus Philippi, 1865 |
| 7 | Eyes pilose | 12 |
| – | Eyes apilose | 8 |
| 8 | Mouthparts equal to, or longer than head length | 9 |
| – | Mouthparts much shorter than head length | 10 |
| 9 | Wing veins reaching wing margin; M2 not connected to M vein, unsclerotized and discontinuous basally; vein R1 inflated at pterostigma (New Caledonia) (Figs 1D, 6–7) | Schlingeriella gen. n. |
| – | Wing veins not reaching wing margin ( |
Prophilopota Hennig, 1966 |
| 10 | Three pairs of tubercles present on segments II - IV of abdomen; occiput extended posteriorly to form an acute ridge (South America) | Terphis Erichson, 1840 |
| – | Abdomen without tubercles; occiput rounded, not extended posteriorly | 11 |
| 11 | Antenna on ventral side of head, adjacent to mouthparts; abdomen conical (Mexico) (Figs 1A-C, 3–5) | Quasi gen. n. |
| – | Antennae on lower front side of head, but not adjacent to mouthparts; abdomen rounded (Africa) | Africaterphis Schlinger, 1968 |
| 12 | Large hemispherical head; posterior margin of eye emarginate; mouthparts shorter than head; occiput rounded; postpronotal lobes proximate but not contiguous medially (Baltic amber) | Archaeterphis Hauser & Winterton, 2007 |
| – | Head smaller and almost spherical, eye not emarginate posteriorly; mouthparts elongate, longer than head, occiput extended posteriorly to form acute ridge; postpronotal lobes contiguous medially | 13 |
| 13 | Palpi present (Palaearctic) | Oligoneura Bigot, 1878 |
| – | Palpi absent (Neotropical) | Philopota Wiedemann, 1830 |
While two groups can be differentiated within Philopotinae based on reduction of wing venation, three clades have been identified by
Philopota genus group– This genus group comprises Africaterphis from Africa, the Palaearctic Oligoneura, Archaeterphis and Prophilopota, and new world genera Philopota, Megalybus, Quasi gen n. and Terphis. Archaeterphis is a very distinctive genus, closely related to the extant genus Africaterphis (Hauser & Winterton, 2007). Prophilopota is presumably more closely related to Oligoneura, since both genera share the presence of maxillary palpi and similar shape of the antennal tubercle (Schlinger, 1971). Quasi gen n. is closely related to Philopota and in particular, Terphis. This genus shares with Terphis
the insertion of the antennae on the lower side of head, reduced
mouthparts, presence of relatively well-developed subscutellum and
substantial reduction of the wing venation, the latter being less
reduced in Philopota. In addition, both Philopotaand Quasi gen n.lack the abdominal tubercles present in Terphis, and share a conical abdomen, instead of a swollen one that characterizes Terphis. Genera in the Philopota
genus group are found in the northern and southern hemispheres with
greater diversity in the New World (four genera). All genera in this
group have reduced wing venation except Megalybus, the sister genus to the clade (
Helle genus group– Schlingeriella gen n. was included in the study by
Thyllis genus group– This group contains genera with complete wing venation including three Afrotropical genera (Dimacrocolus, Parahelle and Thyllis) and the Palaearctic genus Eulonchiella. Dimacrocolus and Parahelle are endemic to Madagascar while Thyllis is found in both Madagascar and South Africa.
The paper is dedicated to Dr. Evert I. Schlinger not only for his support and patronage of dipterology, but for his substantial work on Acroceridae over the previous decades. Thank you to Chris Grinter for assistance and help with organizing loans of specimens and to Martin Hauser for his assistance during the visit by J.G. to CSCA. Thank you to Norman Woodley and Martin Hauser for their comments on the draft manuscript. This paper is based upon work supported by the National Science Foundation (NSF) under DEB award number 0614213. Any opinions, findings, and conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of NSF.