(C) 2011 J. Ray Fisher. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Trachymolgus purpureus Fisher & Dowling sp. n. is described from the Ozark highlands of North America. A diversity of imaging techniques are used to illustrate the species including low-temperature scanning electron microscopy (LT-SEM), stereomicrography, compound light micrography, and digitally created line drawings. Developmental stages (larva, nymphs, and adult) and morphology are illustrated and discussed, and terminological corrections are suggested. Trachymolgus recki Gomelauri, 1961 is regarded as being described from tritonymphs. A key to Trachymolgus is presented.
LT-SEM, taxonomy, Prostigmata, new species, image diversity
Bdellidae Dugès, 1834 generally have a striated, unsclerotized integument. Exceptions occur in Cytinae Grandjean, 1938, which comprises three of the most distinctive bdelloid genera. Cyta Heyden, 1826 are common mites known for their stocky bodies, massive chelicerae, and unpaired fifth eye. Rigibdella ignea Tseng, 1978 from Taiwan have sclerotized, striated holodorsal shields (
Mites were collected primarily from leaf litter
samples in the Ozark Mountains of Arkansas (U.S.A.), specifically
Buffalo National River and Devil’s Den State Park, and extracted using
Berlese-Tullgren funnels. Approximately half of the specimens were
slide-mounted with Hoyer’s medium (see
An effort is made to implement terminology that is
broadly applicable and well accepted across acariforms despite
conventions used among bdelloid authors. Thus, two terms have been
renamed herein. First, “hypostome” is used by many (
Second, the major idiosomal divisions of bdelloids are regularly referred to as the “propodosoma” and “hysterosoma” (Bdellidae:
With regard to hysterosomal setal notation, we follow the chaetotaxic system of (
Leg chaetotaxy follows Grandjean’s system as reviewed by Norton (1977). However, leg chaetotaxy is poorly studied among Eupodina, and only distal tarsal setae are denoted presently, which has been adopted by other eupodine authors (e.g., Jesionowska 2010). Nevertheless, we believe Grandjean’s system can be employed with other leg setae, and will readdress this in a more detailed forthcoming study.
ImagesMost species descriptions include only a few image types; line drawings are most common in acarology (e.g.,
Line drawings were created digitally with Adobe Illustrator CS5 and a Wacom Cintiq 21UX tablet using procedures outlined in
Low-temperature scanning electron micrographs (LT-SEM) were made using an S-4700 field emission scanning electron microscope (Hitachi High Technologies America, Inc., Pleasanton, Calif.) equipped with a Quorum CryoPrep PP2000 (Energy Bean Sciences, East Grandby, Conn.) cryotransfer system. To prepare specimens, mites were placed on 12 mm diameter ultra smooth carbon double sided adhesive tabs (Electron Microscopy Sciences, Hatfield, PA) which were adhered to flat specimen holders consisting of 16x30mm copper plates that were tacked on the edges to the tabs with a small dot of Tissue Tek (OCT Compound, Ted Pella, Inc., Redding, Calif.), which acted as the cyro-adhesive upon freezing. The samples were frozen conductively, in a Styrofoam box, by placing the plates on the surface of a pre-cooled (-96°C) brass bar whose lower half was submerged in liquid nitrogen (LN2). After 20–30s, the holders containing the frozen samples were transferred to a LN2 Dewar for future use or cryotransferred under vacuum to the cold stage in the pre-chamber of the cryotransfer system. Removal of any surface contamination (condensed water vapor) took place in the cryotransfer system by etching the frozen specimens for 10–15 min by raising the temperature of the stage to -90°C. Following etching, the temperature was lowered below -130°C, and a magnetron sputter head equipped with a platinum target, was used to coat the specimens with a very fine layer of platinum. The specimens were transferred to a pre-cooled (-130°C) cryostage in the SEM for observation. An accelerating voltage of 5kV was used to view the specimens. Images were captured using a 4pi Analysis system (Durham, N.C.). Images were sized and placed together into figures using Adobe® Photoshop 7.0 and CS4.
Taxonomyurn:lsid:zoobank.org:act:E0FAE922-2B81-4FB5-8D50-CE0F93517CD2
Trachymolgus purpureus sp. n. is heavily armored with distinctive integument characteristic of Trachymolgus (Figs 1–2). Like Trachymolgus jesusi, the integument is dark purple, whereas Trachymolgus nigerrimus was described as black. Like Trachymolgus jesusi and Trachymolgus nigerrimus, there are two teeth on the fixed cheliceral digit. Like Trachymolgus nigerrimus, Trachymolgus purpureus has one tooth on the movable digit (Trachymolgus jesusi have three) and a serrated edge proximal to the tooth (undescribed in other species). All stages have two pairs of eyes, unlike the larva, proto- and deutonymphs of Trachymolgus jesusi, which lack eyes (tritonymphs and adults have two pairs). Trachymolgus purpureus pedipalpal basi- and telofemora are only fused dorsally. Trachymolgus jesusi pedipalp femora are completely fused, whereas Trachymolgus nigerrimus are completely divided. Trachymolgus purpureus, like other Trachymolgus, have undivided femora on legs I-II (femora III-IV are divided). All other Bdellidae have divided femora on all legs. Trachymolgus jesusi is the only bdellid reported to have undivided femora on legs II and III. The ontogeny of Trachymolgus purpureus differs markedly from that described for Trachymolgus jesusi, the only other species where ontogeny was investigated. Finally, there are many chaetotaxic differences on the appendages and venter between Trachymolgus purpureus and Trachymolgus jesusi. Most chaetotaxy of Trachymolgus nigerrimus remain to be investigated. See Remarks for discussion of Trachymolgus recki.
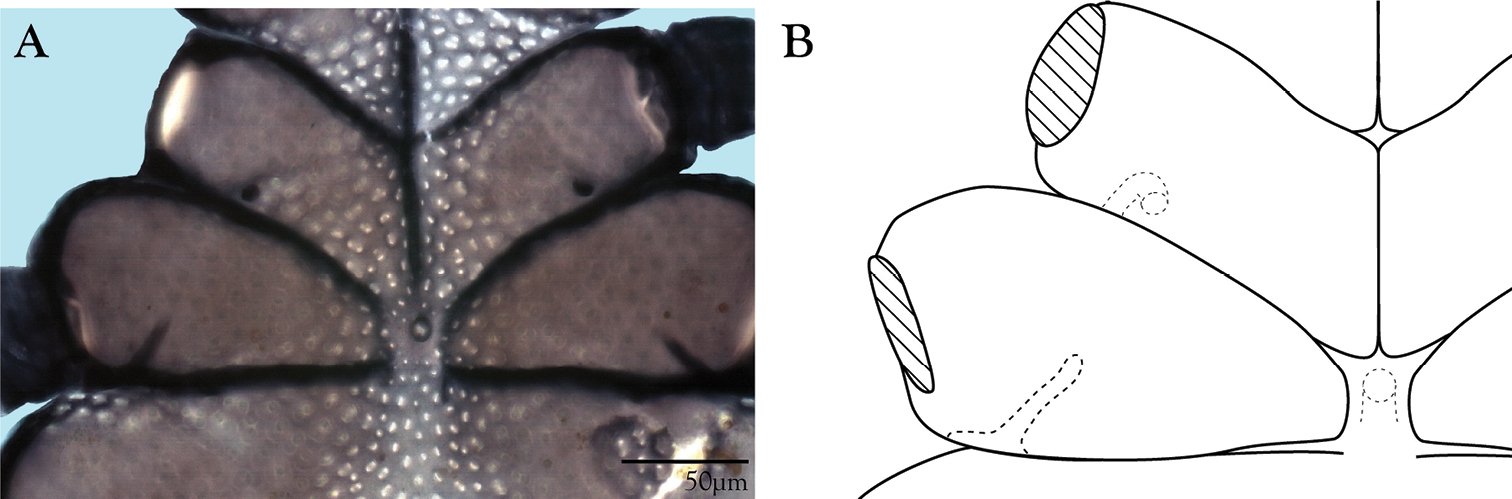
Females and males similar, except for genitalia, size, and chaetotaxic differences noted in Table 1. Color dark purple; occasionally immatures and adults were collected from the Buffalo National River (Arkansas) with an internally green coloration, which rendered the normally purple mite teal; teal specimens returned to purple after a few days in 95% ethanol, and were indistinguishable from normal specimens when slide-mounted (we also collected Penthaleus, a normally black to dark blue mite, from the same habitat exhibiting green internal coloration). Integument divided into heavily armored sclerites with foveolate sculpturing (Fig. 2a). The foveolate indentions (foveolae) are bordered with pits (Fig. 2b). Measurements in Tables 2–5.
Leg chaetotaxy. Female (♀), male (♂), tritonymph (3N), deutonymph (2N), protonymph (1N), larva (L), pedipalp (Pp), legs I-IV (I-IV). Numbers represent setal counts for barbulate setae (undesignated), solenidia (s), and trichobothria (tr). Male setal counts that are not different from the female are denoted with an asterisk (*). Absent characters are denoted with a dash (-). Fused segments are denoted by fused cells. Numbers in parentheses denote occurrences of two solenidia on tarsus II in some specimens.
| Stage | Coxa | Trochanter | Basifemur | Telofemur | Genu | Tibia | Tarsus | |
|---|---|---|---|---|---|---|---|---|
| ♀ | Pp | - | 0 | 8 | 4 | 4; 1s | ||
| I | 7 | 2 | 18 | 4; 2s | 8; 2s; 1tr | 28; 5s | ||
| II | 7 | 2 | 20 | 4; 1s | 9; 1s | 26; 1(2)s | ||
| III | 9 | 2 | 10 | 10 | 5; 1s | 9; 1s | 24; 1tr | |
| IV | 8 | 2 | 10 | 10 | 5; 1s | 9; 1tr | 21; 1s | |
| ♂ | Pp | - | * | * | * | * | ||
| I | 6 | * | * | * | * | * | ||
| II | 6 | * | * | * | * | * | ||
| III | 6 | * | * | * | * | * | * | |
| IV | 10 | * | * | * | * | * | * | |
| 3 N | Pp | - | 0 | 6 | 4 | 4; 1s | ||
| I | 4 | 2 | 18 | 4; 2s | 8; 2s; 1tr | 24; 5s | ||
| II | 4 | 2 | 18 | 4; 1s | 8; 1s | 22; 1s | ||
| III | 4 | 2 | 9 | 9 | 4; 1s | 9; 1s | 20; 1tr | |
| IV | 3 | 1 | 6 | 7 | 4; 1s | 8; 1tr | 19; 1s | |
| 2 N | Pp | - | 0 | 4-5 | 4 | 4; 1s | ||
| I | 4 | 1 | 12 | 4; 2s | 7; 2s; 1tr | 20; 5s | ||
| II | 2 | 1 | 11 | 4; 1s | 6; 1s | 18; 1(2)s | ||
| III | 4 | 1 | 6 | 6 | 4; 1s | 6; 1s | 16; 1tr | |
| IV | 2 | 1 | 2 | 4 | 4; 1s | 6; 1tr | 15; 1s | |
| 1 N | Pp | - | 0 | 4 | 4 | 4; 1s | ||
| I | 2 | 1 | 7 | 4; 2s | 5; 2s | 18; 4s | ||
| II | 1 | 1 | 6 | 4; 1s | 5; 1s | 16; 1s | ||
| III | 1 | 1 | 1 | 4 | 4; 1s | 5; 1s | 12; 1tr | |
| IV | 0 | 0 | 0 | 0 | 1 | 7 | ||
| L | Pp | - | 0 | 2 | 4 | 4; 1s | ||
| I | 3 | 0 | 7 | 4; 2s | 5; 2s | 16; 3s | ||
| II | 1 | 0 | 6 | 4; 1s | 5; 1s | 14; 1s | ||
| III | 2 | 0 | 6 | 4; 1s | 5; 1s | 12; 1tr | ||
| IV | - | - | - | - | - | - | ||
Body measurements. Stage (St), female (♀), male (♂), tritonymph (3N), deutonymph (2N), protonymph (1N), and larva (L), mean (M), standard deviation (S), range (R), number examined (n), idiosomal length (Idi L) and width (Idi W), and lengths of proterosomal shield (Pro), hysterosomal shield (Hys), lateral shield (Lat), subcapitulum (Sub), chelicerae (Chel), pedipalps (Ped), anal shield (Ana), genital shield (Gen), and legs I-IV (L I-IV). Absent characters are denoted with a dash (-). All measurements in micrometers.
| St | Idi L | Idi W | Pro | Hys | Lat | Sub | Chel | Ped | Ana | Gen | I | II | III | IV | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ♀ | M | 791 | 505 | 279 | 512 | 485 | 307 | 283 | 344 | 113 | 171 | 470 | 457 | 526 | 600 |
| S | 24 | 14 | 9 | 16 | 11 | 13 | 7 | 4 | 5 | 9 | 9 | 13 | 6 | 37 | |
| R | 768-838 | 488-525 | 273-298 | 495-540 | 475-503 | 285-323 | 273-295 | 338-350 | 108-120 | 160-190 | 458-483 | 438-478 | 515-533 | 538-665 | |
| n | 7 | 7 | 7 | 7 | 7 | 8 | 8 | 6 | 7 | 8 | 7 | 7 | 7 | 8 | |
| ♂ | M | 753 | 483 | 270 | 483 | 448 | 294 | 277 | 345 | 105 | 165 | 468 | 445 | 518 | 594 |
| S | 52 | 25 | 10 | 45 | 34 | 8 | 9 | 7 | 8 | 9 | 16 | 13 | 21 | 25 | |
| R | 693-825 | 465-500 | 260-283 | 425-545 | 390-475 | 288-305 | 268-293 | 338-355 | 95-118 | 150-175 | 443-483 | 435-465 | 488-540 | 555-628 | |
| n | 6 | 2 | 6 | 6 | 6 | 5 | 6 | 5 | 6 | 6 | 6 | 6 | 6 | 6 | |
| 3N | M | 684 | 452 | 259 | 268 | 134 | 258 | 242 | 301 | 95 | 102 | 393 | 378 | 451 | 509 |
| S | 99 | 70 | 38 | 52 | 10 | 10 | 10 | 12 | 6 | 4 | 23 | 27 | 17 | 43 | |
| R | 588-808 | 350-500 | 230-314 | 220-324 | 126-145 | 250-273 | 228-255 | 288-315 | 88-100 | 98-105 | 363-410 | 353-405 | 438-475 | 453-553 | |
| n | 4 | 4 | 4 | 3 | 3 | 5 | 5 | 5 | 5 | 3 | 4 | 4 | 4 | 4 | |
| 2N | M | 549 | 375 | 213 | 227 | 88 | 225 | 204 | 249 | 77 | 61 | 331 | 318 | 373 | 391 |
| S | 77 | 53 | 26 | 17 | - | 4 | 7 | 3 | 3 | 2 | 5 | 3 | 7 | 8 | |
| R | 500-665 | 330-450 | 191-250 | 205-241 | - | 220-230 | 198-213 | 225-260 | 73-80 | 60-63 | 328-338 | 315-323 | 365-383 | 380-398 | |
| n | 4 | 4 | 4 | 4 | 1 | 4 | 4 | 3 | 4 | 2 | 4 | 4 | 4 | 4 | |
| 1N | M | 508 | 375 | 168 | 325 | - | 171 | 162 | 210 | 65 | 30 | 265 | n/a | 305 | 288 |
| S | - | - | - | - | - | 1 | 2 | 2 | 2 | 2 | 4 | n/a | - | 285-290 | |
| R | - | - | - | - | - | 170-172 | 160-163 | 206-213 | 64-65 | 25-30 | 263-265 | n/a | - | 4 | |
| n | 1 | 1 | 1 | 1 | - | 2 | 2 | 2 | 2 | 2 | 2 | n/a | 1 | 2 | |
| L | M | 323 | 243 | 118 | 88 | - | 137 | 128 | 195 | 48 | - | 210 | 200 | 238 | - |
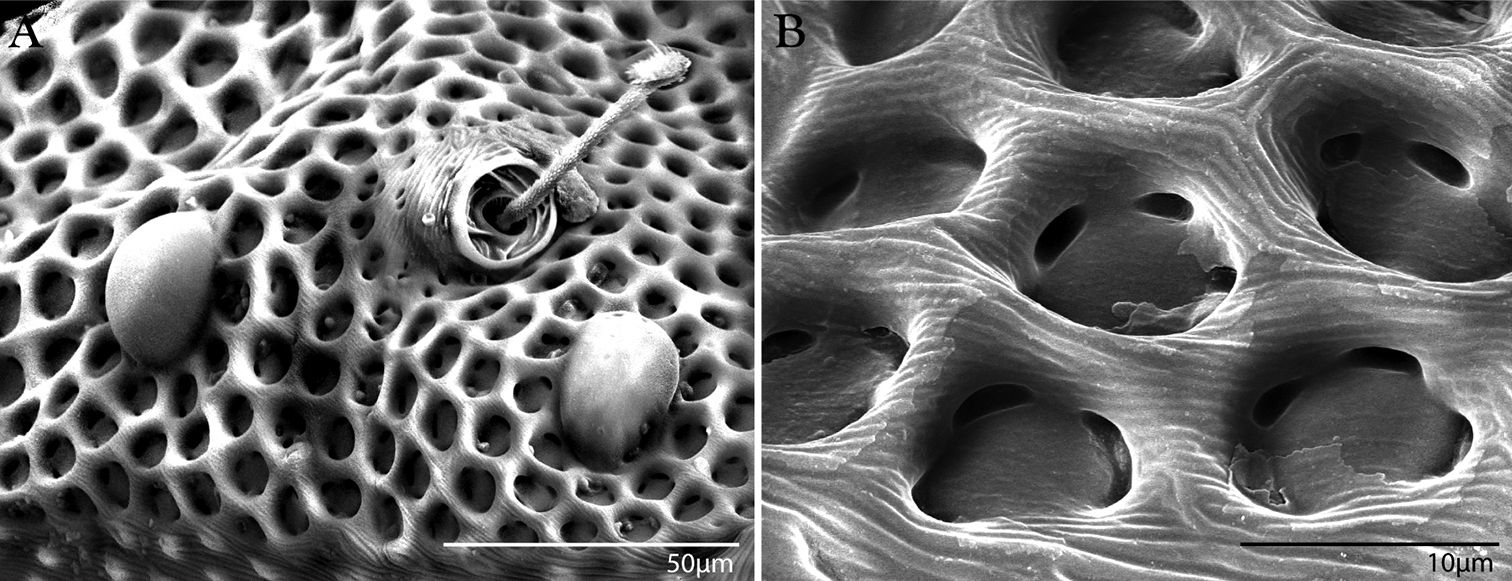
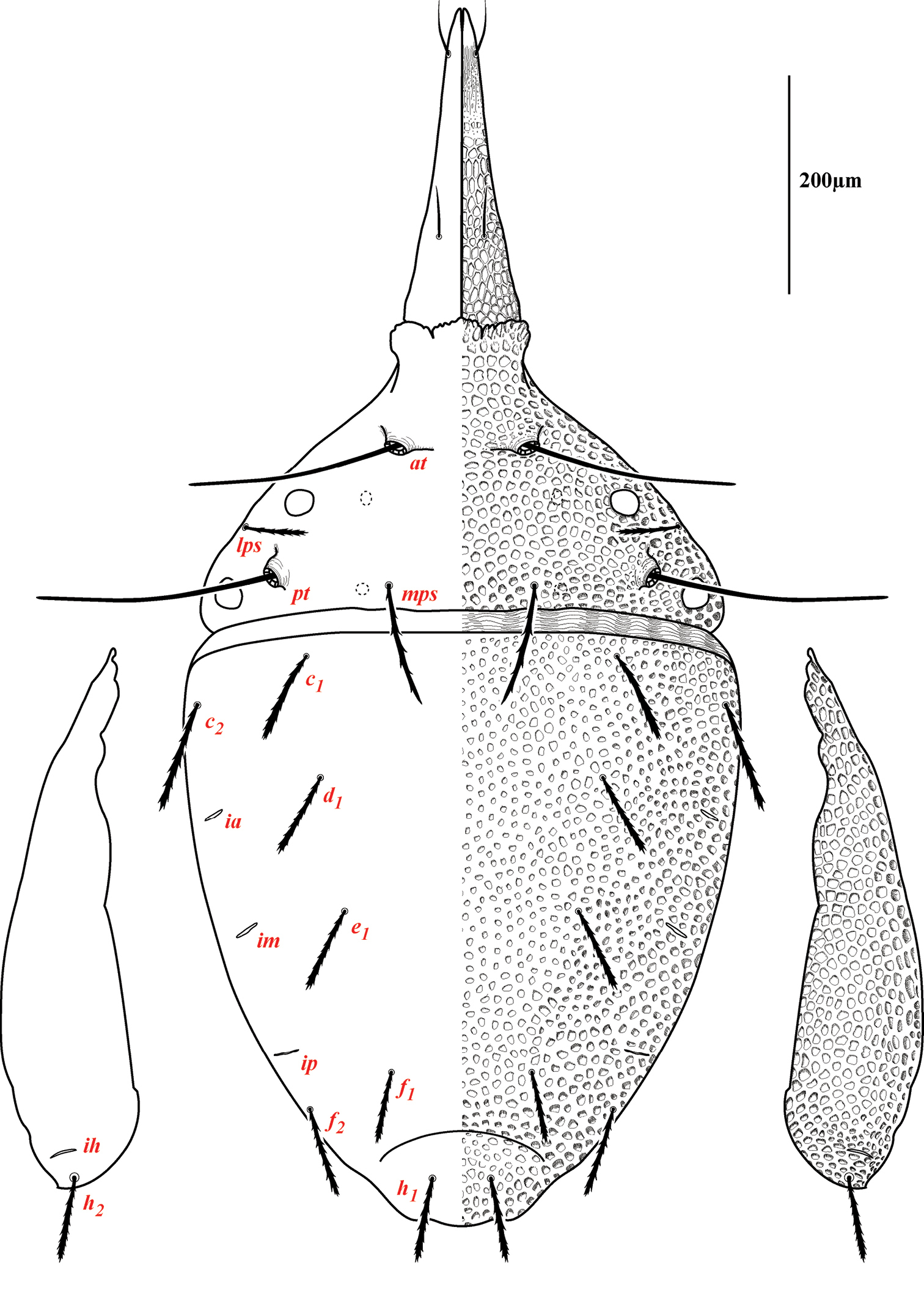
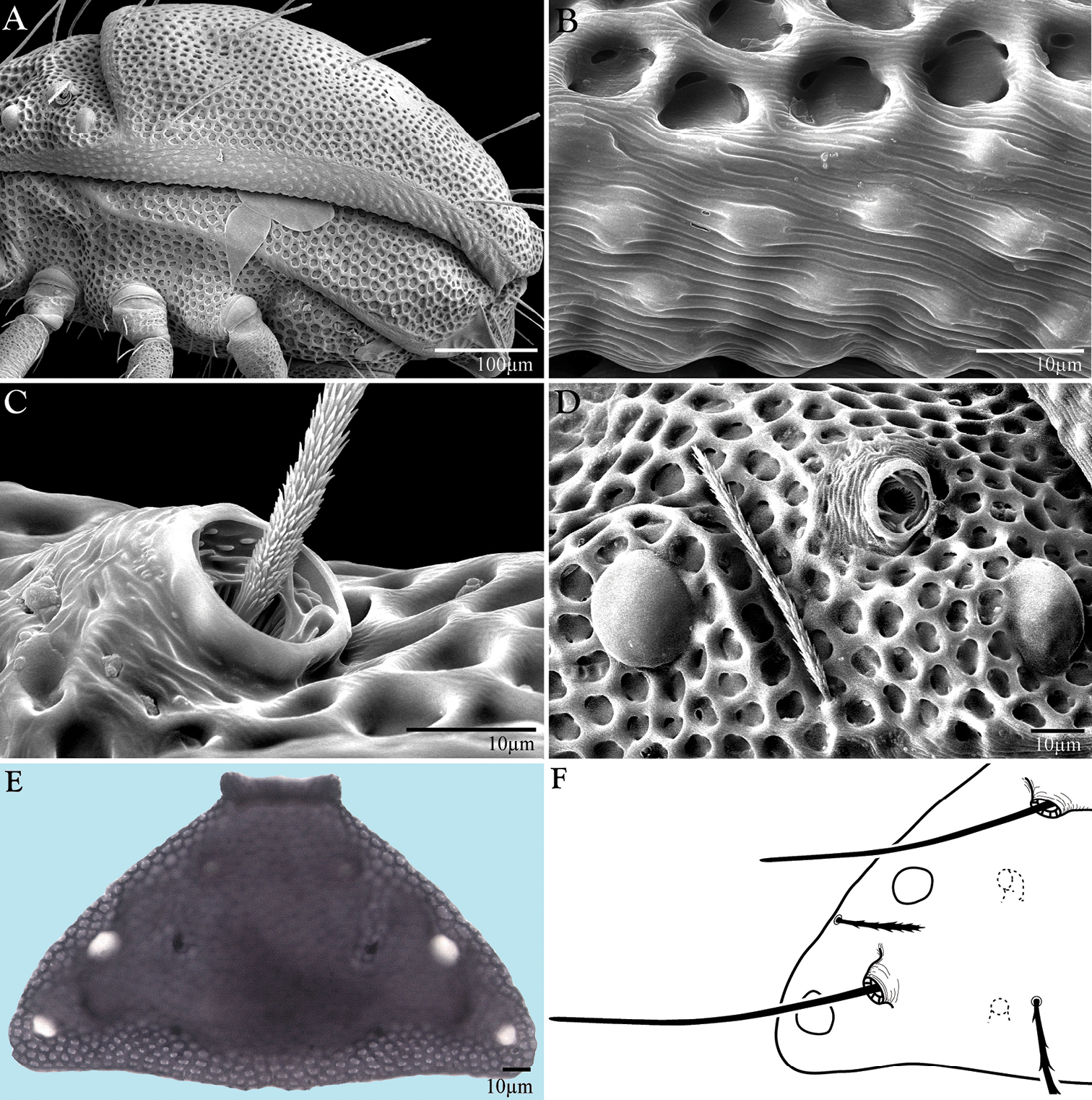
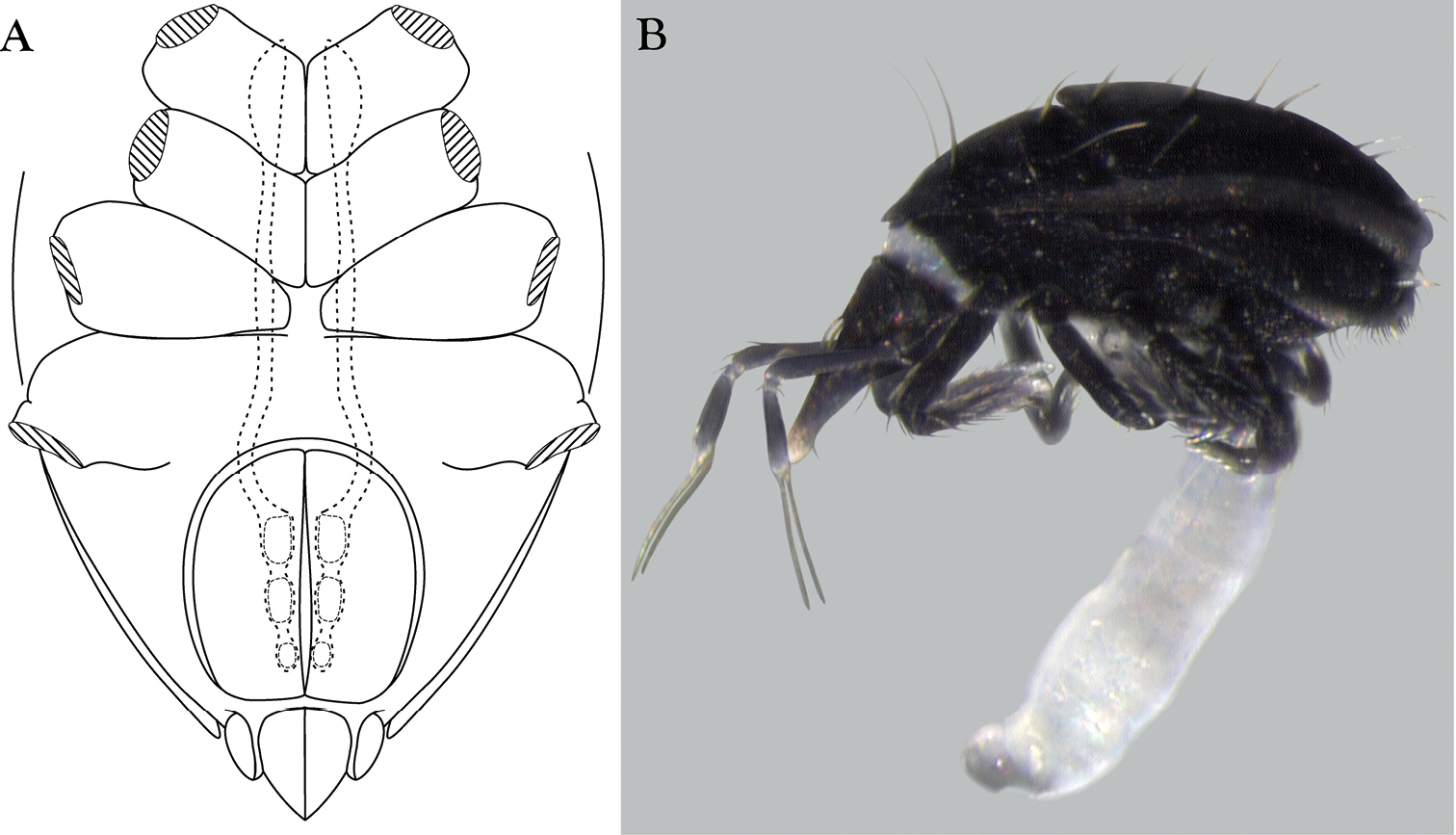
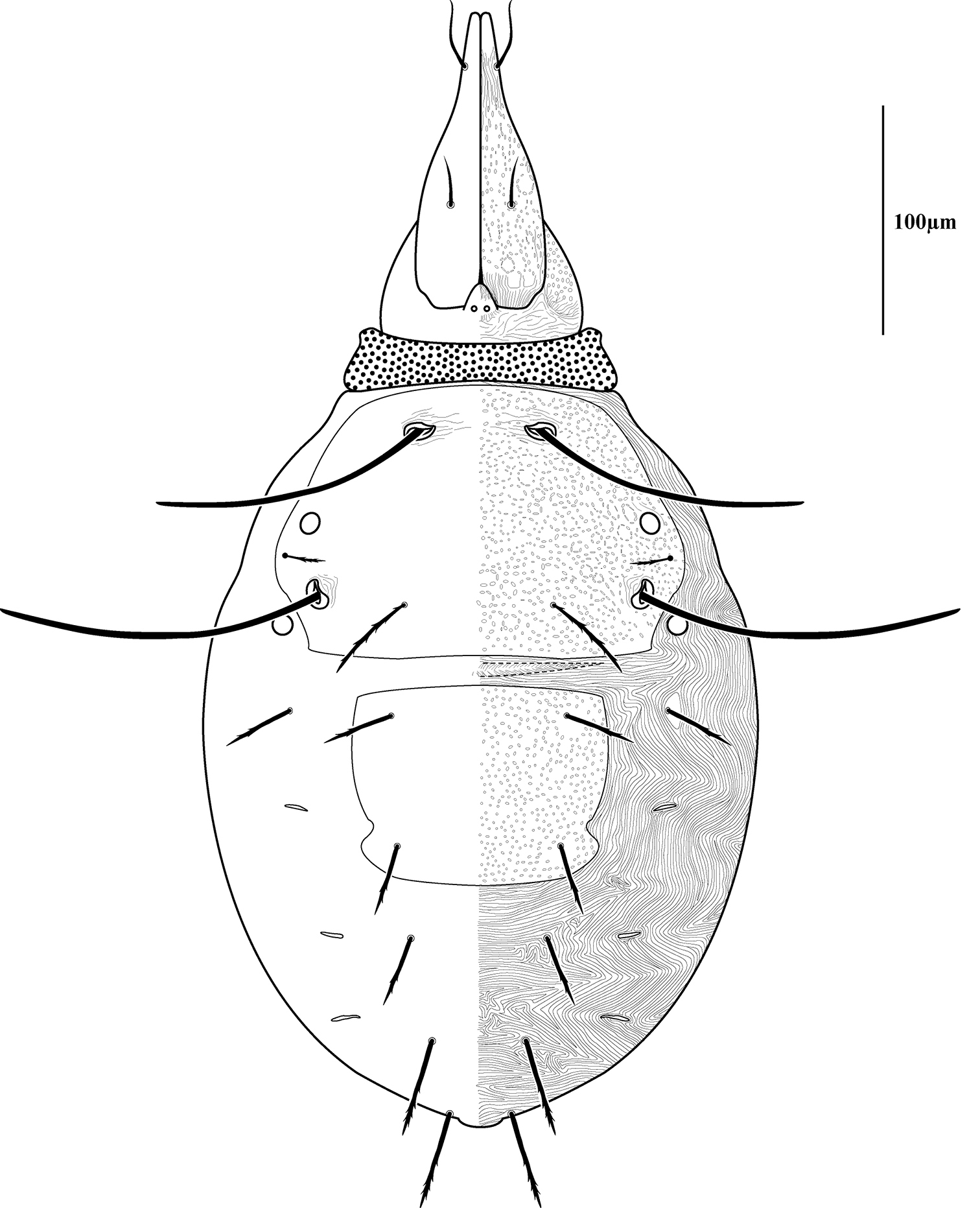
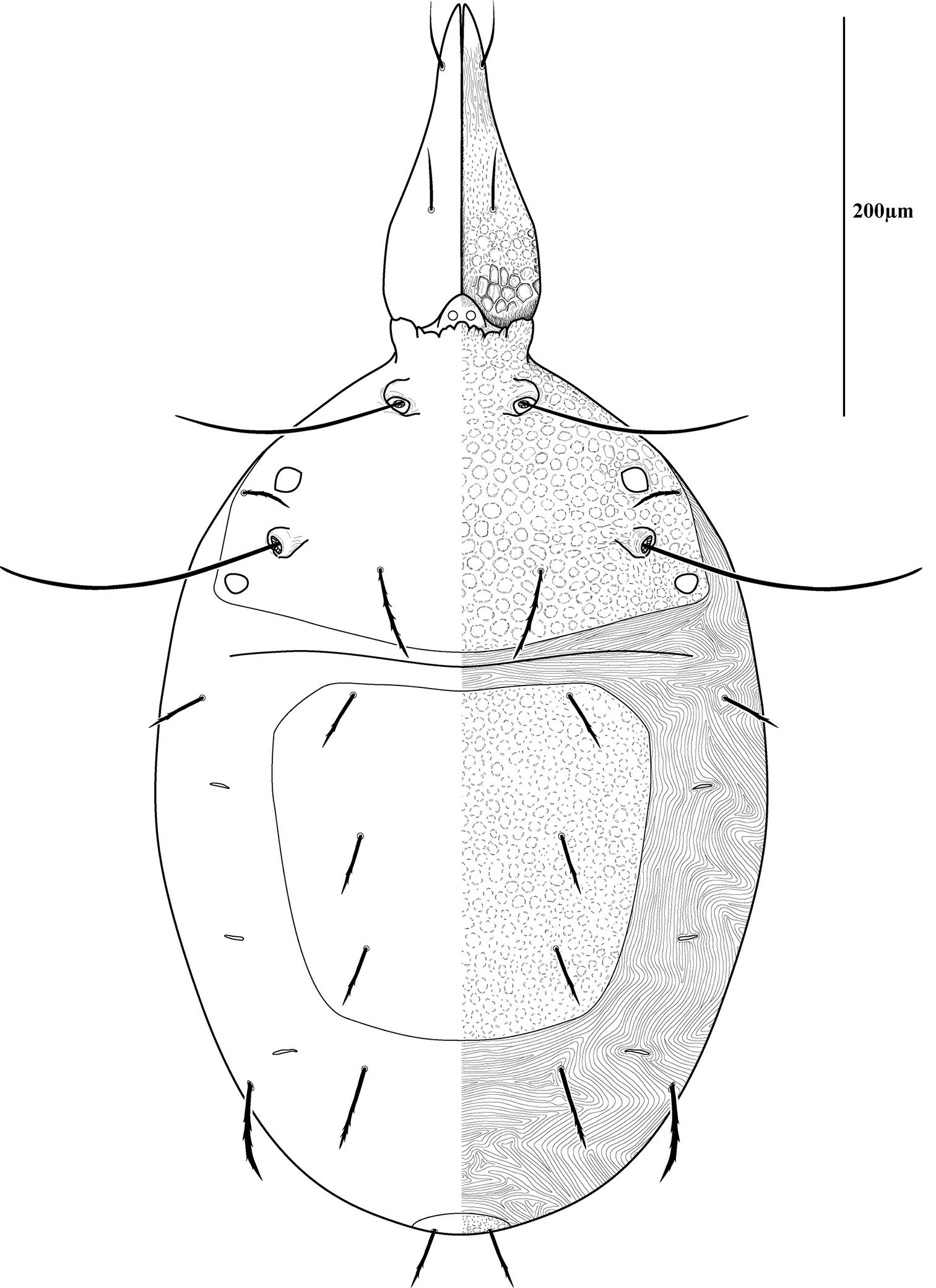
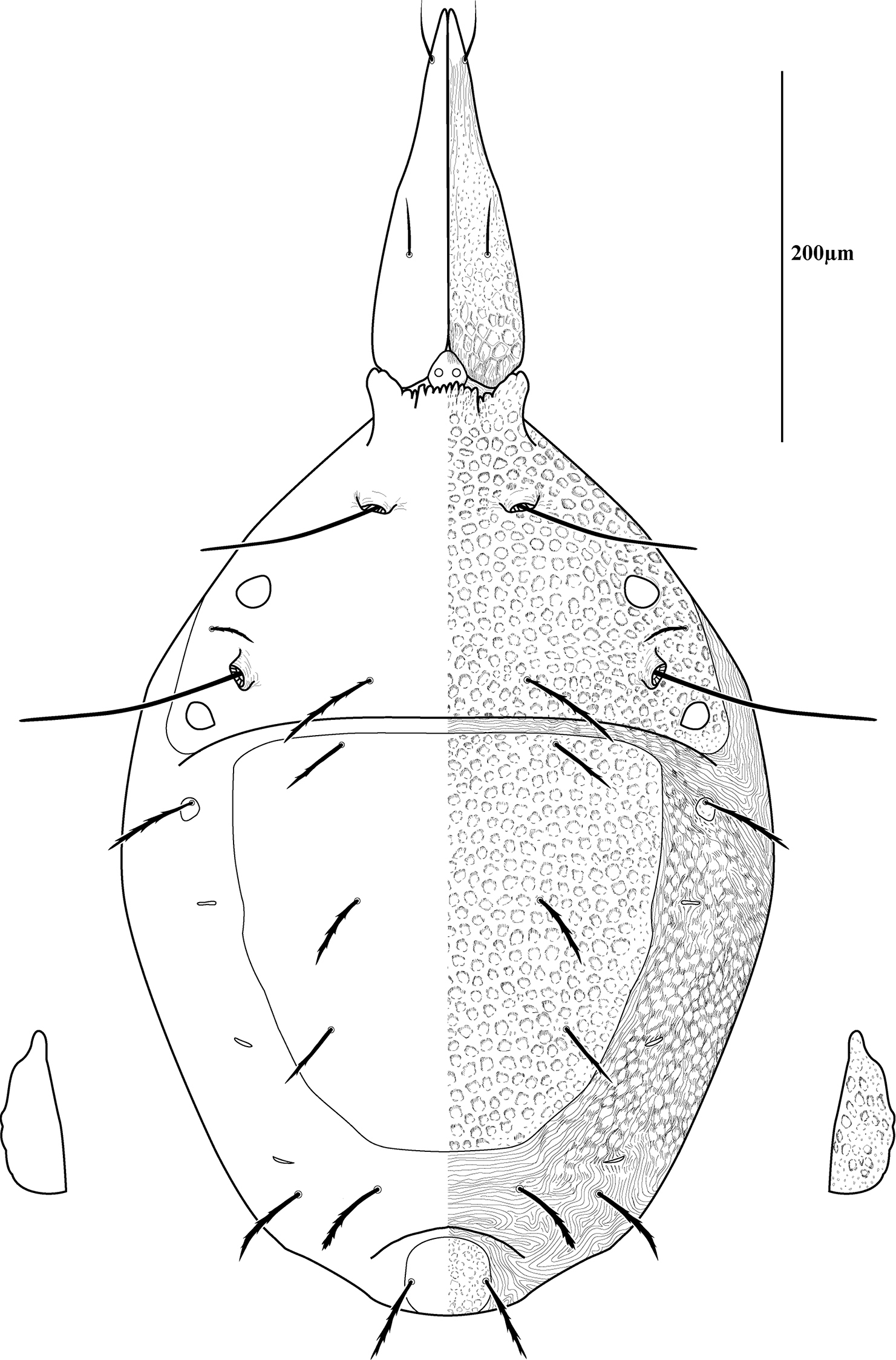
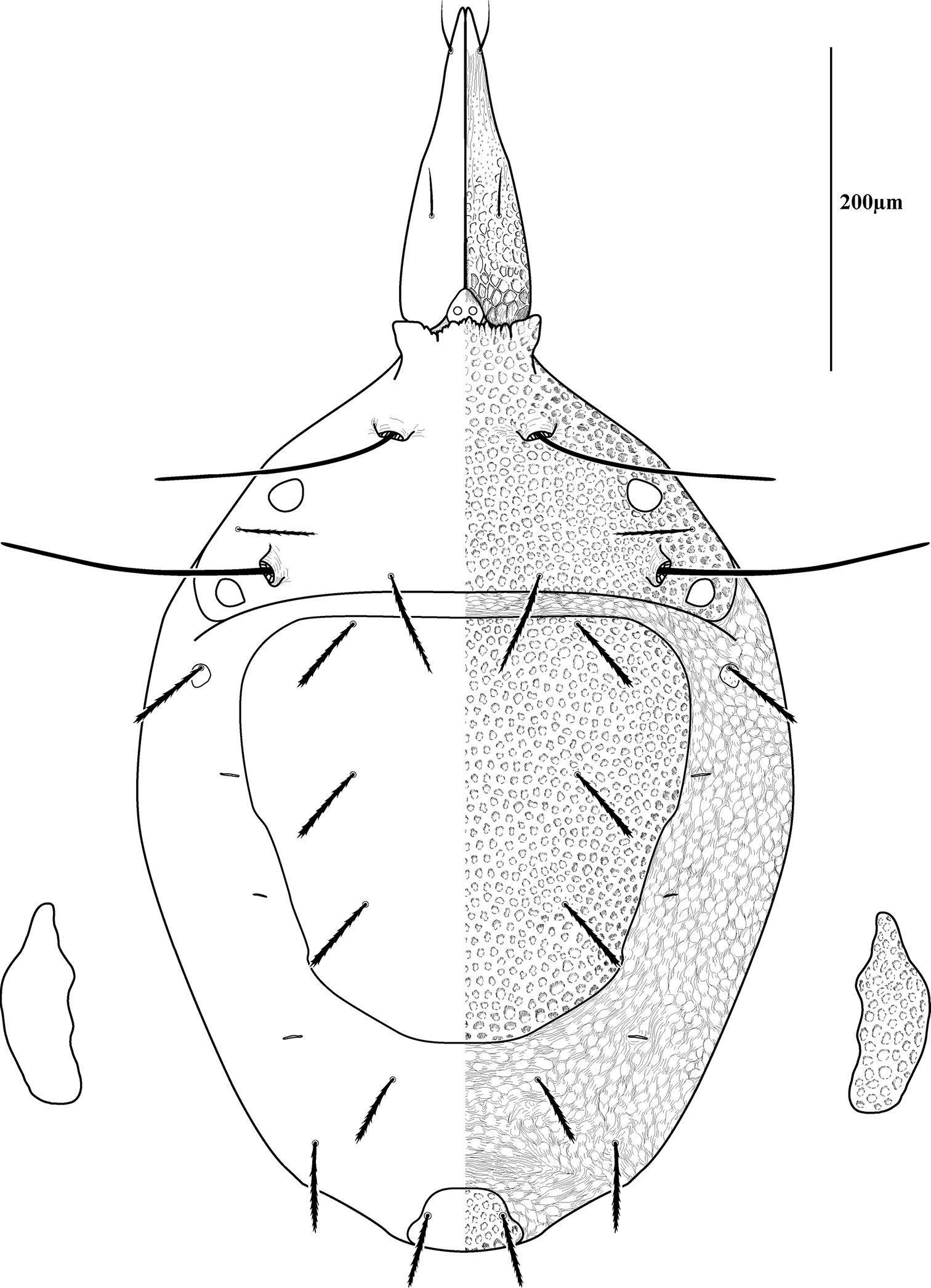
Dorsal idiosoma (Fig. 3). Idiosoma dorsally armored with two large tergites: proterosomal and hysterosomal shields (see Terminology). Dorsal membrane (between proterosomal and hysterosomal shields and between dorsal and lateral shields) striated and accompanied with raised bumps similar in size to the foveolate indentions (Figs 4, 5a-b). Proterosoma ending anteriorly in a crenulated, tri-lobed shelf (crown) covering the stigmata. Two pairs of eyes present. Two pairs of minutely barbulate trichobothria: anterior (at) and posterior trichobothria (pt). Barbules are difficult to discern with light microscopy (Fig. 5c). Two pairs of barbulate setae are present: lateral proterosomal (lps) and median proterosomal setae (mps). Setae lps are oriented dorsomedially and lay in a groove posterior to the first pair of eyes (Fig. 5d); mps are the longest barbulate setae. Two pairs of heavily sclerotized, cylindrical, internally directed structures are apparent (Fig. 5e-f) that we interpret to be apodemes. Hysterosoma folding over posterior, shelf-like portion of proterosoma; with three lyrifissures (ia, im, and ip) and seven barbulate setae as follows: c1, c2, d1, e1, f1, f2, and h1. Posteriorly, the hysterosomal shield folds inward between the f1-2 and h1 forming a curved lateral furrow isolating h1 on a raised area.
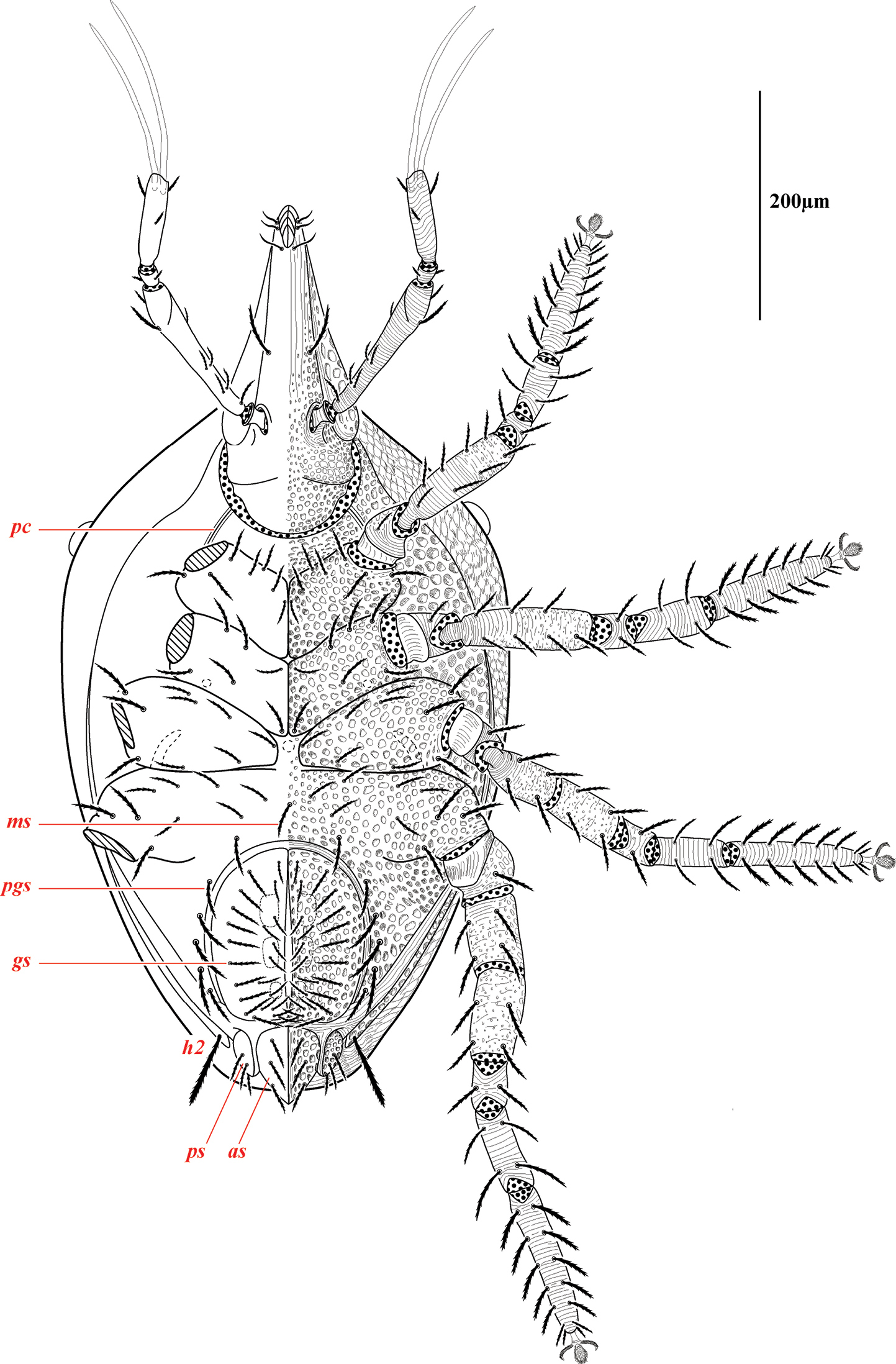
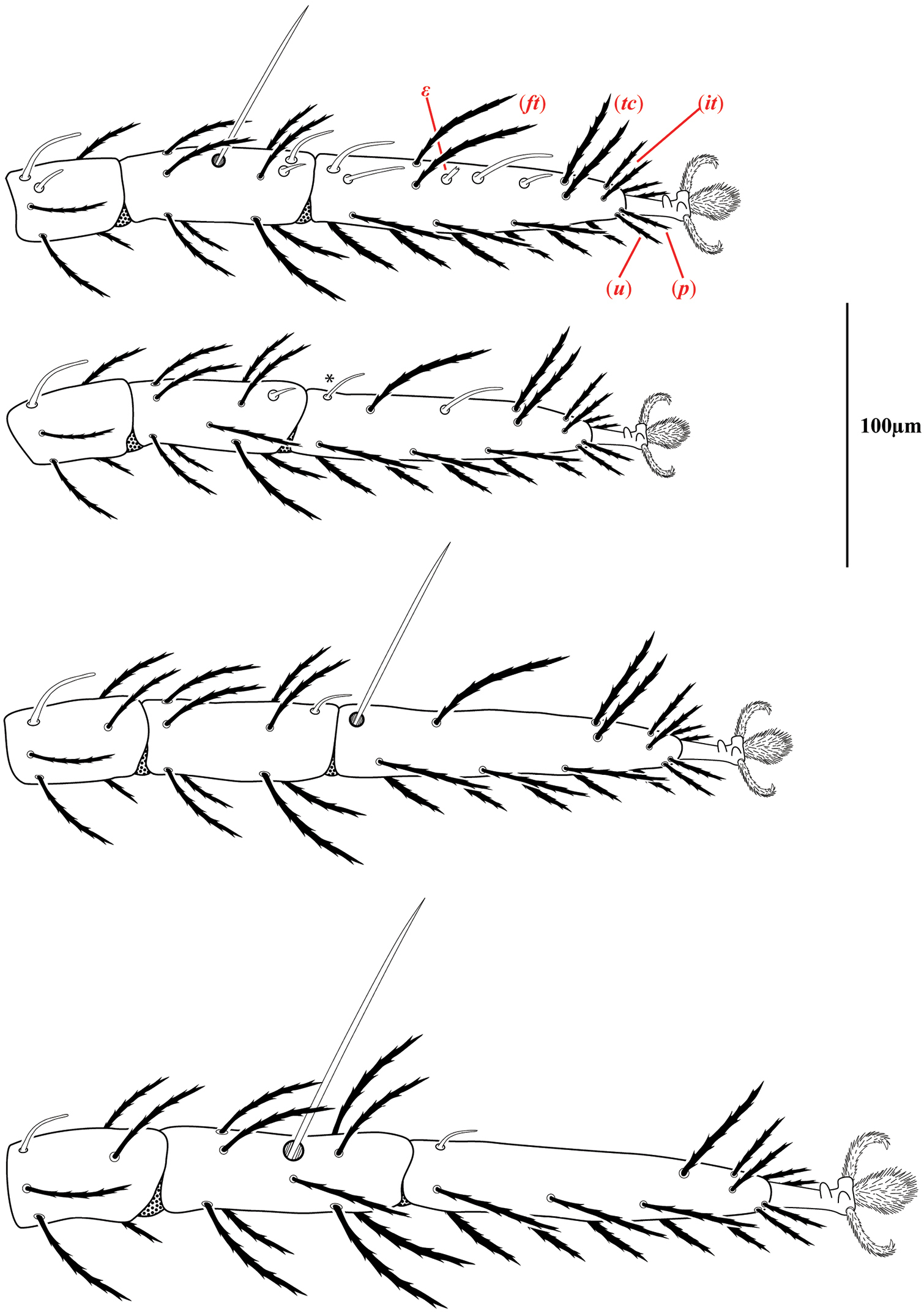
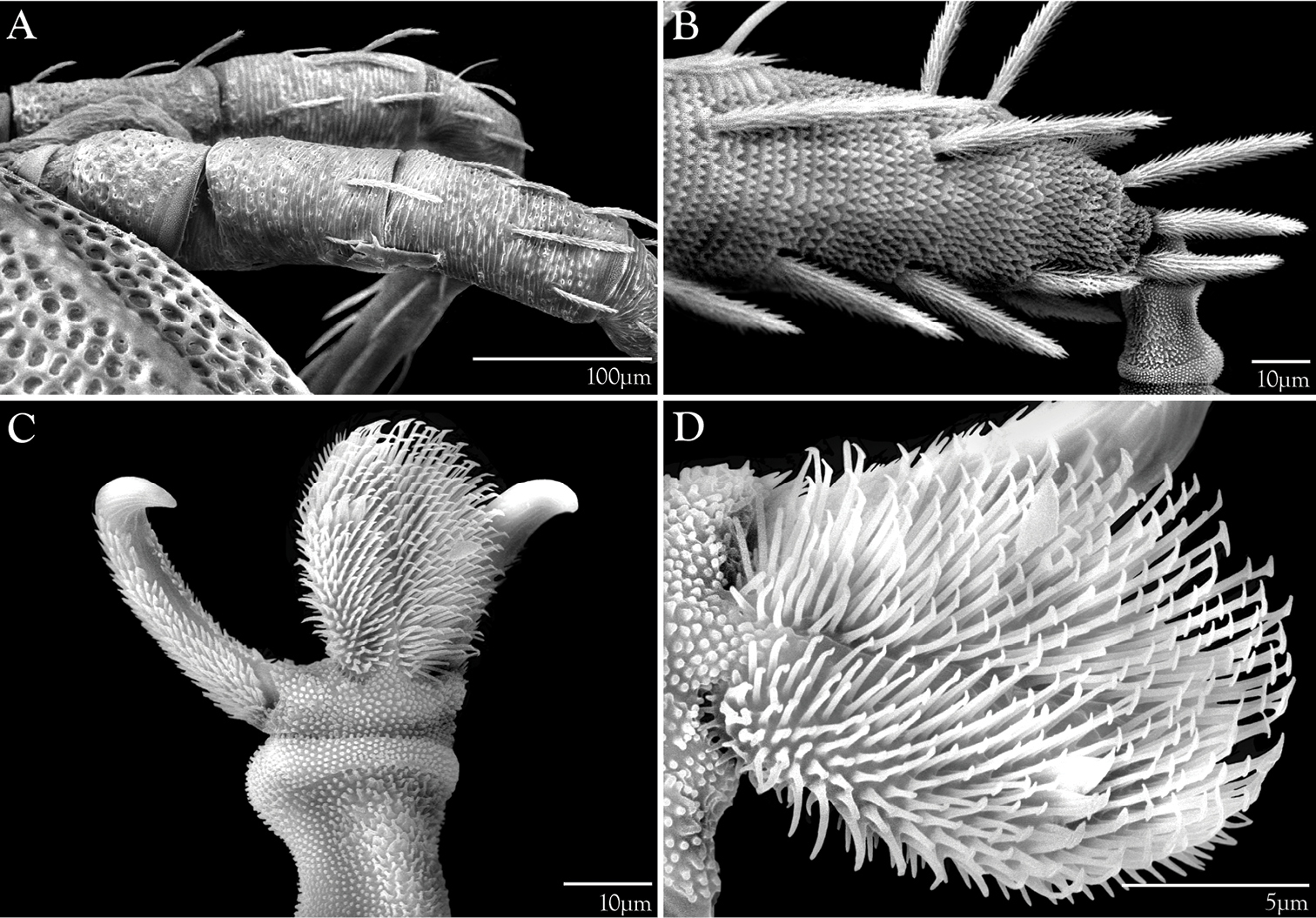
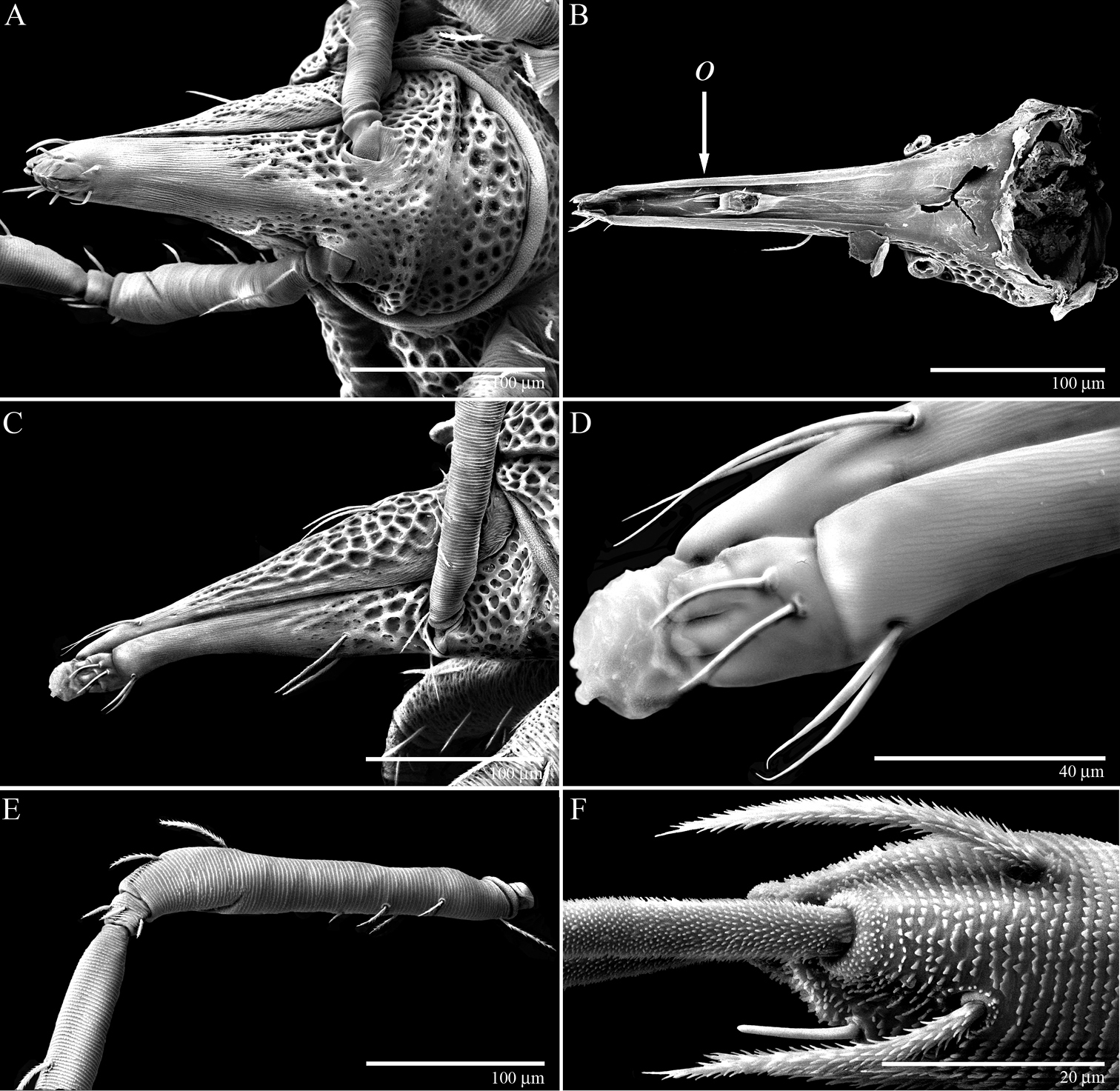
Ventral idiosoma(Fig. 4). Lateral shieldseach posteriorly containing one lyrifissure (ih) and one barbulate seta (h2). Podocephalic canals lead from the posteriolateral edges of the gnathosoma and curve around coxal field I, and are visible externally (Figs 1a, 4). Ventral membrane is striated but lacks bumps. Genital region covered with one pair of genital shields each containing more than 20 barbulate setae. There are six pairs of paragenital setae; one unpaired median seta between coxal field IV; three pairs of genital papillae; one pair of genital tracheae associated with the genital papillae that leads into the body anteriorly from the anterior-most genital papilla, and ending in spoon-shaped platytracheae near coxal field I (Fig. 6a). Female with long, telescoping ovipositor that approaches body length (Fig. 6b); with 16 setae. Male amphoid sclerites each with nine setae. Unpaired median cylindrical structure interpreted as an apodeme between coxal field III (Fig. 8). Anal region with two pairs of sclerites: anal shields and paranal shields, each usually containing three pairs of barbulate setae. Either side of both anal and paranal shields may have one to two extra setae (symmetrically or asymmetrically). Legs(Figs 4, 7): coxal fields I-III distinct, coxal field IV indistinguishably fused medially with venter; sclerotized, inwardly directed cylindrical structures (interpreted here as apodemes) are readily apparent on coxal field II and III (Fig. 8). Trochanters, femora, and genua sclerotized, with pitted, sculptured armor, especially II and III (Figs 4, 9a); other podomeres unsclerotized with papillated striations (Fig. 9b). Podomeres with eight possible setal rows positioned ventrally (unpaired), medioventrally (paired), lateroventrally (paired), laterally (paired), laterodorsally (paired), and dorsally (unpaired). Base of the ambulacrum surrounded with two pairs of setae: prorals (p) and unguinals (u). Proximally, the dorsal setae are as follows: iterals (it), tectals (tc), and fastigials (ft). The tectals are paired on all legs except IV; fastidials are paired only on leg I, and are absent on leg IV. Other setal homologies remain to be investigated. Baculiform solenidia present on genua I-IV (σ), tibiae I and III (γ), and tarsi I, II, and IV (ω); short, ceratiform solenidia present on tibiae I and II (γ); and a short solenidion present on tarsi I that has the appearance of being raggedly broken, interpreted here as the famulus (ε). Trichobothria present on tibiae I and IV, and tarsus III. Apotele with barbulate ungues and pulvilli with tenant hairs (Fig. 9c-d). Leg arthrodial membrane is unsculptured.
Gnathosoma(Fig. 10). Subcapitulum (Fig. 10a) foveolate and armored posteriorly, longitudinally striated anteriorly (Fig. 11a); ventrally with two pairs of smooth adoral setae (ad), one pair of smooth anterior setae (avs), and one pair of barbulate posterior setae (pvs); dorsally with one pair of smooth, thin, straight setae (ds) that are hidden under the chelicerae in life; ending in three pairs of lateral lips (Figs 10a, 11d). Oral opening located midway between ventral setae (Fig. 11b). Gnathosomal membrane unsculptured. Pedipalps (Fig. 10b) entirely striated (Fig. 11e), becoming more papillated-striated distally (Fig. 11f); femora partially fused dorsally; terminal setae (ves and des) finely barbulate (Fig. 11f). Chelicerae (Fig. 10c) with foveolate armoring basally, and longitudinal striation distally (Fig. 11c); with two dorsal barbulate setae. Fixed digit ending in a hook, and with two teeth (one small and one large and triangular); movable digit with one small tooth and a serrated edge proximal to the tooth (Fig. 10d).
Dorsal setal measurements. Female (♀), male (♂), tritonymph (3N), deutonymph (2N), protonymph (1N), and larva (L), mean (M), standard deviation (S), range (R), number examined (n), anterior and posterior trichobothria (at and pt), lateral and medial proterosomal setae (lps and mps). All measurements in micrometers.
| Stage | at | lps | pt | mps | c1 | c2 | d1 | e1 | f1 | f2 | h1 | h2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ♀ | M | 182 | 56 | 214 | 103 | 74 | 90 | 81 | 75 | 70 | 82 | 67 | 69 |
| S | 23 | 11 | 18 | 8 | 6 | 17 | 8 | 3 | 5 | 12 | 3 | 4 | |
| R | 163-208 | 40-75 | 200-238 | 93-113 | 65-83 | 60-115 | 70-90 | 73-78 | 65-75 | 75-105 | 63-70 | 63-75 | |
| n | 3 | 7 | 4 | 7 | 5 | 7 | 4 | 4 | 3 | 6 | 5 | 7 | |
| ♂ | M | 186 | 64 | 214 | 98 | 76 | 83 | 77 | 76 | 66 | 69 | 65 | 68 |
| S | 4 | 7 | 15 | 4 | 1 | 8 | 7 | 5 | 11 | 8 | 7 | 5 | |
| R | 180-190 | 58-75 | 200-230 | 93-100 | 75-78 | 78-95 | 65-85 | 70-83 | 55-78 | 58-78 | 55-78 | 63-75 | |
| n | 4 | 6 | 3 | 5 | 3 | 4 | 5 | 4 | 3 | 6 | 6 | 6 | |
| 3N | M | 166 | 53 | 174 | 74 | 56 | 66 | 53 | 53 | 53 | 63 | 51 | 56 |
| S | 18 | 4 | 2 | 3 | 6 | 6 | 1 | 2 | 4 | 3 | 1 | 7 | |
| R | 155-188 | 48-58 | 173-175 | 70-78 | 50-65 | 60-73 | 53-55 | 50-55 | 48-55 | 60-65 | 50-53 | 48-65 | |
| n | 3 | 5 | 2 | 4 | 5 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | |
| 2N | M | 136 | 39 | 183 | 63 | 41 | 46 | 38 | 40 | 44 | 60 | 47 | 47 |
| S | 2 | 1 | - | 3 | 4 | 4 | 0 | 2 | 4 | 2 | 2 | 3 | |
| R | 135-138 | 38-40 | 175-190 | 60-65 | 38-45 | 40-50 | 38 | 38-43 | 40-50 | 58-63 | 45-50 | 43-50 | |
| n | 2 | 4 | 1 | 3 | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | |
| 1N | M | 111 | 21 | 155 | 48 | 30 | 38 | 30 | 35 | 43 | 51 | 36 | 47 |
| S | 34 | 8 | 49 | - | - | 4 | - | - | 3 | 1 | 1 | 1 | |
| R | 88-135 | 15-26 | 120-190 | - | - | 35-41 | - | - | 41-45 | 50-52 | 35-37 | 46-48 | |
| n | 2 | 2 | 2 | 1 | 1 | 2 | 1 | 1 | 2 | 2 | 2 | 2 | |
| L | M | 105 | 20 | 118 | 43 | 30 | 33 | 33 | 40 | 40 | 43 | 40 | 40 |
Gnathosomal measurements. Female (♀), male (♂), tritonymph (3N), deutonymph (2N), protonymph (1N), and larva (L), mean (M), standard deviation (S), range (R), number examined (n), dorsal subcapitulars (ds), proximoventral subcapitulars (pvs), distoventral subcapitulars (dvs), adorals (ad), dorsal end setae (des), ventral end setae (ves), cheliceral distal seta (cds), and cheliceral proximal seta (cps). All measurements in micrometers.
| Stage | ds | pvs | dvs | ad | des | ves | cds | cps | |
|---|---|---|---|---|---|---|---|---|---|
| ♀ | M | 46 | 52 | 26 | 19 | 196 | 180 | 48 | 51 |
| S | 4 | 6 | 2 | 2 | 10 | 22 | 5 | 5 | |
| R | 43-53 | 43-63 | 23-28 | 15-23 | 175-203 | 130-193 | 40-53 | 43-55 | |
| n | 5 | 7 | 7 | 8 | 7 | 7 | 8 | 7 | |
| ♂ | M | 41 | 44 | 25 | 15 | 199 | 182 | 44 | 46 |
| S | 7 | 6 | 4 | 4 | 16 | 9 | 3 | 6 | |
| R | 33-48 | 38-50 | 18-28 | 10-20 | 185-225 | 173-193 | 40-48 | 40-55 | |
| n | 4 | 3 | 5 | 5 | 5 | 5 | 6 | 5 | |
| 3N | M | 37 | 41 | 27 | 16 | 168 | 158 | 38 | 36 |
| S | 1 | 2 | 4 | 3 | 8 | 5 | 1 | 5 | |
| R | 35-38 | 38-43 | 20-30 | 13-20 | 160-180 | 153-165 | 38-40 | 30-40 | |
| n | 4 | 5 | 5 | 5 | 5 | 5 | 3 | 3 | |
| 2N | M | 31 | 36 | 21 | 14 | 143 | 133 | 37 | 31 |
| S | 2 | 2 | 3 | 1 | 3 | 3 | 3 | 1 | |
| R | 28-33 | 33-38 | 20-25 | 13-15 | 140-145 | 130-135 | 33-40 | 30-33 | |
| n | 4 | 4 | 4 | 4 | 3 | 3 | 4 | 4 | |
| 1N | M | 20 | 30 | 20 | 13 | 121 | 115 | 29 | 32 |
| S | - | - | - | - | 118-125 | 113-118 | - | - | |
| R | - | - | - | - | 5 | 4 | - | - | |
| n | 1 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | |
| L | M | 19 | 30 | 24 | 12 | 100 | 85 | 30 | 34 |
Measurements and chaetotaxy of immatures are given in Tables 1–6. Developmental stages are illustrated in Figures 13–16. Like other mites, developmental stages can be easily recognized by leg number (larvae have six legs) and genital development (Fig. 17). Chaetotaxic differences and femoral divisions are also helpful (Tables 1, 6). All immature stages appear soft bodied (despite dorsal sclerites) and vary in color from light green or purple to yellowish-white (Fig. 12).
Due to the unique armored morphology of Trachymolgus, other interesting developmental changes are present. These are discussed below.
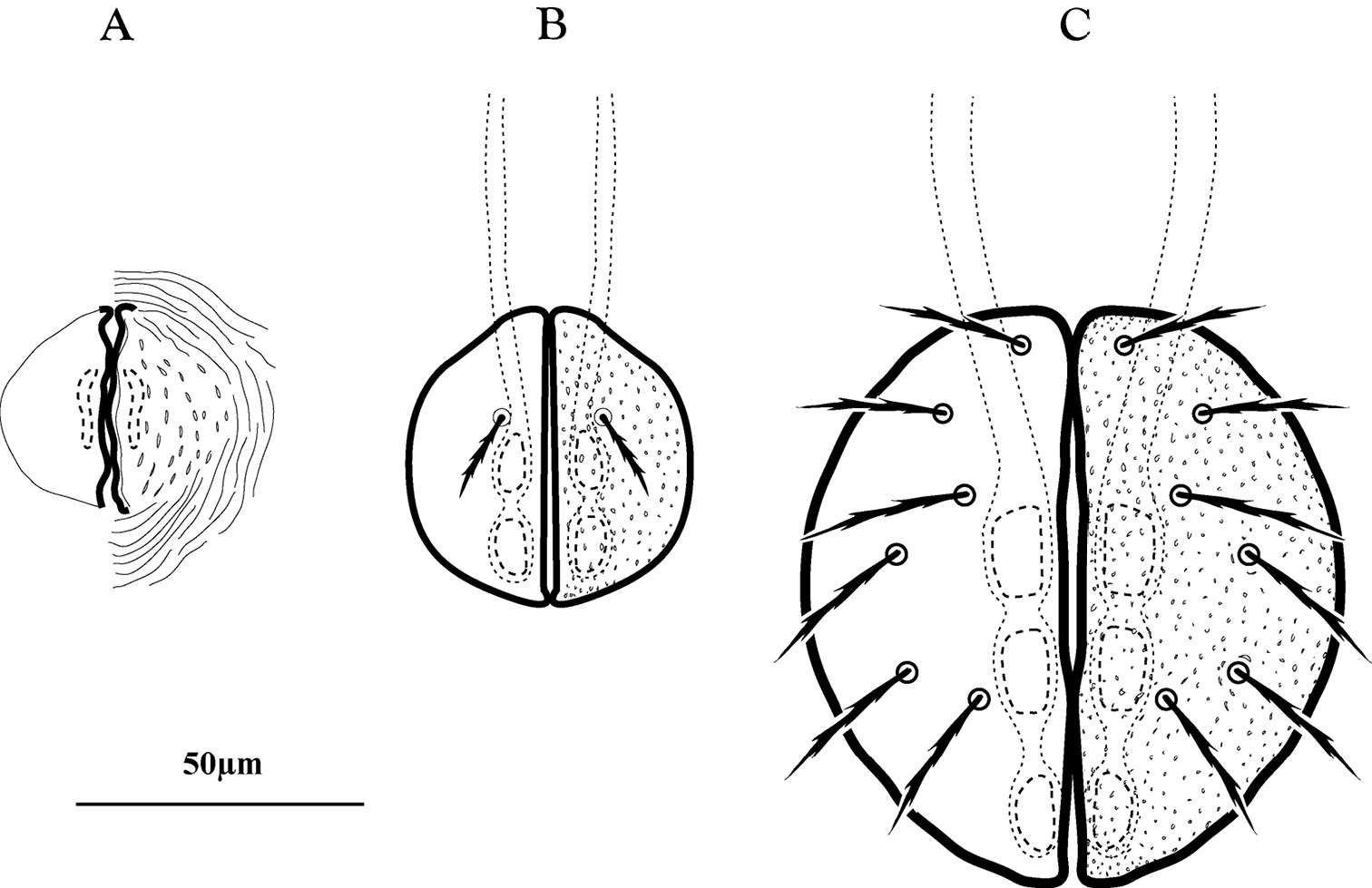
Dorsal sclerites and setae.None of the immature stages of Trachymolgus purpureus have complete dorsal shields as seen in the adult. This is unlike Trachymolgus jesusi, which was described as having an armored tritonymph and unsclerotized proto- and deutonymphs. In Trachymolgus purpureus, all stages have dorsal sclerites. Shield sculpturingis underdeveloped in the larva with foveolate indentions absent but pits present (Fig. 13); protonymphs also lack foveolate indentions, but the pits are more organized, reminiscent of the indentions (Fig. 14); deutonymphs begin to develop foveolate indentions (Fig. 15), which are nearly complete in the tritonymph (Fig. 16). The proterosomal shield of the larva does not encompass the posterior pair of eyes, and the anterior crown is not developed, leaving the gnathosomal membrane appearing as a collar. The protonymph has a well-developed proterosomal shield that encompasses all eyes and has a complete crown. Hysterosomal shield of the larva only encompasses c1 and d1; nymphal stages also encompass e1. Small sclerotized regions containing pits, but not foveolate indentions, are present around c2 in the deuto- and tritonymphs. A posterior shield encompassing h1, contiguous with the hysterosomal shield in adults, is present in nymphal stages, but not larvae. Larvae completely lack f2.
Lateral shields.Lateral shields are present in deuto- and tritonymphs (Figs 15, 16), but do not encompass h2 or ih, as in adults (Fig. 3). Furthermore, in addition to lateral shields, larvae lack h2. Lyrifissure ih was not found in any immature stage.
Pseudotracheae.As described for Trachymolgus jesusi, pseudotracheae are lacking in the larva and protonymph, but are well-developed in the deutonymph (Fig. 17).
Membranes.As discussed above, adult Trachymolgus purpureus striations are accompanied with bumps (Fig. 5b), unlike other bdellid membranes that exhibit fingerprint-like striations. However, larvae and protonymphs lack bumps and have typical fingerprint-like striations (Figs 13, 14). Membrane bumps begin to develop on the deutonymphal dorsum (Fig. 15), and are well developed in the tritonymph (Fig. 16). All stages have normal, fingerprint-like striations on the venter.
Ventral setal measurements. Female (♀), male (♂), tritonymph (3N), deutonymph (2N), protonymph (1N), and larva (L), mean (M), range (R), number examined (n), anal setae (as), paranal setae (ps), genital setae (gs), paragenital setae (ps), unpaired median seta (ums). Absent characters are denoted with a dash (-). All measurements in micrometers.
| Stage | as1 | as2 | as3 | as4 | as5 | ps1 | ps2 | ps3 | long gs | short gs | pgs | ums | |
| ♀ | M | 43 | 43 | 44 | 40 | - | 43 | 52 | 54 | 34 | 17 | 41 | 30 |
| S | 1 | 2 | 3 | - | - | 4 | 3 | 2 | 2 | 2 | 2 | 1 | |
| R | 42-45 | 41-46 | 41-49 | - | - | 39-48 | 47-55 | 52-56 | 31-37 | 14-20 | 38-46 | 29-32 | |
| n | 6 | 6 | 5 | 1 | - | 6 | 5 | 5 | 7 | 6 | 7 | 4 | |
| ♂ | M | 45 | 43 | 42 | 41 | 43 | 45 | 51 | 52 | 35 | 18 | 42 | 37 |
| S | 4 | 7 | 2 | 3 | - | 3 | 1 | 6 | 5 | 4 | 6 | 3 | |
| R | 40-51 | 35-56 | 40-44 | 37-43 | - | 42-50 | 50-52 | 45-62 | 27-44 | 14-21 | 34-48 | 35-40 | |
| n | 6 | 6 | 5 | 4 | 1 | 6 | 5 | 6 | 6 | 3 | 6 | 3 | |
| 3N | M | 37 | 37 | 38 | 36 | - | 42 | 43 | 45 | 27 | 18 | 32 | 26 |
| S | 2 | 3 | 3 | - | - | 2 | 4 | 4 | 2 | 2 | 2 | 1 | |
| R | 35-40 | 35-41 | 35-40 | - | - | 40-44 | 40-47 | 40-49 | 25-30 | 16-20 | 30-35 | 25-27 | |
| n | 4 | 4 | 3 | 1 | - | 4 | 4 | 4 | 4 | 3 | 4 | 3 | |
| 2N | M | 30 | 29 | 29 | 32 | - | 35 | 39 | 38 | 21 | - | 23 | 28 |
| S | 5 | 1 | 2 | 1 | - | 2 | 3 | 2 | 2 | - | 3 | 3 | |
| R | 24-35 | 28-30 | 26-30 | 31-33 | - | 32-36 | 34-42 | 35-40 | 19-23 | - | 21-25 | 25-30 | |
| n | 4 | 4 | 4 | 2 | - | 4 | 4 | 4 | 2 | - | 2 | 3 | |
| 1N | M | 24 | 25 | 22 | - | - | 27 | 32 | 37 | 24 | 19 | 24 | 25 |
| S | 0 | 1 | 2 | - | - | 4 | 3 | 4 | - | - | - | - | |
| R | 24 | 24-25 | 20-23 | - | - | 24-30 | 30-34 | 34-40 | - | - | - | - | |
| n | 2 | 2 | 2 | - | - | 2 | 2 | 2 | 1 | 1 | 1 | 1 | |
| L | M | 20 | 21 | 23 | - | - | - | - | - | - | - | - | - |
Recognizing life stages. Female (♀), male (♂), tritonymph (3N), deutonymph (2N), protonymph (1N), and larva (L). Numbers represent setal counts; those in parentheses denote counts when extra setae are present. Absent characters are denoted with a dash (-).
| Stage | Adoral Setae | Anal Setae | Paranal Setae | Genital Setae | Paragenital Setae | Femora III divided | Femora IV divided |
|---|---|---|---|---|---|---|---|
| ♀ | 2 | 3 (4) | 3 | >20 | 6 | yes | yes |
| ♂ | 2 | 3 (5) | 3 (4) | >20 | 6 | yes | yes |
| 3N | 2 | 3 (4) | 3 | 6 | 5 | yes | yes |
| 2N | 2 | 3 (4) | 3 | 1 | 5 | yes | yes |
| 1N | 2 | 3 | 3 | 0 | 0 | yes | no |
| L | 1 | 3 | 1 | - | - | no | no |
In the early 1980s, Trachymolgus
was collected by Cal Welbourn on a rocky bluff in the Buffalo National
River (Arkansas). John Kethley recollected three specimens from the same
bluff a few years later. Another specimen (one female) was collected by
Evert Lindquist in the St. Lawrence Islands National Park, Canada (
The known distribution of North American Trachymolgus is Mexico (Trachymolgus jesusi), Ozark highlands (Trachymolgus purpureus),
central Ohio (undet. species), and the northern Appalachian mountains
(undet. species). Other groups have a similar distribution, and the
biogeographic affinity between the Ozark and Appalachian mountains, and
between Mexico and the eastern U.S. has been well documented. Examples
include mosses (
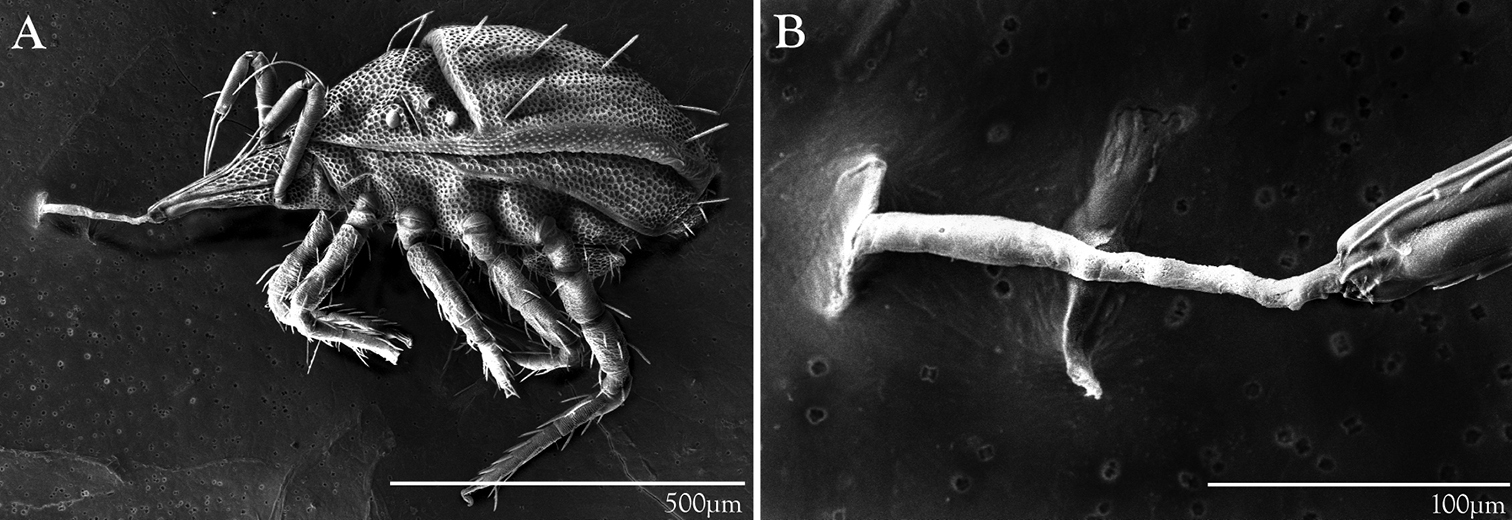
Trachymolgus purpureus seems to have extremely high temperature tolerances. They were found crawling on rock surfaces in direct sunlight during a drought in the hottest and driest time of year (August), and were collected near the surface during the winter. When preparing live specimens for LT-SEM, mites are set atop a metal bar that is subjected to liquid nitrogen fumes which freezes them mid-stride for imaging. When Trachymolgus purpureus was subjected to liquid nitrogen temperatures however, they would simply run, curl their legs, and roll off the plate (see Fig. 18a). This made imaging live specimens very difficult.
LT-SEM imaging illuminated another behavioral characteristic of Trachymolgus purpureus. Though other bdellids have been known to orally produce silk to tether prey (
Feeding behavior.We observed a tritonymph of Trachymolgus purpureus feeding on a small mite approximately 200–250m long. Unfortunately, the prey could not be retrieved for identification. The tritonymph fed with prey elevated from the ground. There seemed to be a droplet surrounding the bite site, interpreted here as silk seen in Figures 11d. We hypothesize that Trachymolgus purpureus uses a drop of silk at the bite site to act as a gasket when sucking prey fluids.
(27 individuals on slides). HOLOTYPE: female, collected from leaf litter, USA, Arkansas, Washington Co., Devil’s Den State Park (35°46.817 N, 94°14.750 W), 23 Sep 2009, by JR Fisher & MJ Skvarla, APGD 09-0923-006.
PARATYPES: Female (n=7): 2 individuals collected from leaf litter on rocky slope, USA, Arkansas, Washington Co., Devil’s Den State Park (35°46'50.1N, 94°14'45.9"W), 28 Aug 2008, by APG Dowling, APGD 08-0828-004 ● 2 individuals collected from leaf litter on rocky slope, USA, Arkansas, Washington Co. Devil’s Den State Park (35°46'50.1"N, 94°14'45.9"W), 30 Aug 2009 by JR Fisher, APGD 09-0830-001 ● 1 individual collected from leaf litter, USA, Arkansas, Newton Co., Buffalo National River, Roark Bluff (36°01'56.2"N, 93°20'01.5"W), 7 Sep 2009 by JR Fisher, APGD 09-0907-005 ● 1 individual collected from American beech leaf litter, USA, Arkansas, Newton Co., Buffalo National River, Boen Gulf (35°52.062 N, 093°24.092 W), 14 Mar 2010 by JR Fisher, APGD 10-0314-019 ● 1 individual collected from litter on rocky bluff, USA, Arkansas, Newton Co., Buffalo National River, Roark Bluff (36°01'56.2"N, 93°20'01.5"W), [date unknown] by Cal Welbourn, OSAL 0061853. Male (n=5): 1 individual collected from rocky overhang, USA, Arkansas, Washington Co., Devil’s Den State Park (35°46'50.1N, 94°14'45.9"W) by APG Dowling, APGD 08-0822-001 ● 1 individual collected from cedar litter, USA, Arkansas, Washington Co., Buffalo National River, Roark Bluff (36°01'56.2"N, 93°20'01.5"W) by JR Fisher, APGD 09-0802-006 ● 1 individual collected from leaf litter on rocky slope, USA, Arkansas, Washington Co. Devil’s Den State Park (35°46'50.1"N, 94°14'45.9"W), 30 Aug 2009 by JR Fisher, APGD 09-0830-001 ● 1 individual collected from leaf litter on rocky slope, USA, Arkansas, Washington Co. Devil’s Den State Park (35°46'50.1"N, 94°14'45.9"W), 30 Aug 2009 by JR Fisher, APGD 09-0830-003 ● 1 individual collected from leaf litter, USA, Arkansas, Newton Co., Buffalo National River, Roark Bluff (36°01'56.2"N, 93°20'01.5"W), 7 Sep 2009 by JR Fisher, APGD 09-0907-005. 1 individual collected from leaf litter, USA, Arkansas, Newton Co., Buffalo National River, Boen Gulf (35°52.062 N, 093°24.092 W), 10 Apr 2010 by APG Dowling. Tritonymph(n=6): 3 individuals collected from cedar litter, USA, Arkansas, Washington Co., Buffalo National River, Roark Bluff (36°01'56.2"N, 93°20'01.5"W) by JR Fisher, APGD 09-0802-006 ● 1 individual collected from oak litter, USA, Arkansas, Washington Co., Buffalo National River, Steel Creek trail (36°01'56.2"N, 93°20'01.5"W) by JR Fisher, APGD 09-0802-001 ● 1 individual collected from leaf litter, USA, Arkansas, Newton Co., Buffalo National River, Roark Bluff (36°01'56.2"N, 93°20'01.5"W), 7 Sep 2009 by JR Fisher, APGD 09-0907-005 ● 1 individual collected from litter on rocky bluff, USA, Arkansas, Newton Co., Buffalo National River, Roark Bluff (36°01'56.2"N, 93°20'01.5"W), [date unknown] by John Kethley, FMNH 2. Deutonymph (n=4): 3 individuals collected from cedar litter, USA, Arkansas, Washington Co., Buffalo National River, Roark Bluff (36°01'56.2"N, 93°20'01.5"W) by JR Fisher, APGD 09-0802-006. 1 individual collected from leaf litter on rocky slope, USA, Arkansas, Washington Co. Devil’s Den State Park (35°46'50.1"N, 94°14'45.9"W), 30 Aug 2009 by JR Fisher, APGD 09-0830-002. Protonymph (n=2): 1 individuals collected from leaf litter on rocky slope, USA, Arkansas, Washington Co., Devil’s Den State Park (35°46'50.1N, 94°14'45.9"W), 28 Aug 2008, by JR Fisher & MJ Skvarla, APGD 08-0828-004 ● 1 individual collected from cedar litter, USA, Arkansas, Washington Co., Buffalo National River, Roark Bluff (36°01'56.2"N, 93°20'01.5"W) by JR Fisher, APGD 09-0802-006. Larva (n=1): 1 individual collected from oak litter, USA, Arkansas, Washington Co., Buffalo National River, Steel Creek trail (36°01'56.2"N, 93°20'01.5"W) by JR Fisher, APGD 09-0802-001.
This species is named for the Latin "purpureus", meaning purple.
Trachymolgus recki was described from two specimens from Georgia (
We have found that purple Trachymolgus purpureus immatures lose color more readily than adults when slide-mounted, and some immatures are yellowish-white in life (Fig. 12b). Also, though deuto- and tritonymphal shields are foveolate, they are not as heavily sclerotized as in adults, which could give the appearance of being un-armored. Adults of Trachymolgus purpureus and Trachymolgus jesusi have higher setal counts with >20 and 10 genital setae, respectively, and coxal field setae 7-7-9-8 (female Trachymolgus purpureus), 6-6-6-10 (male Trachymolgus purpureus), and 8-5-11-10 (Trachymolgus jesusi). Larvae, proto- and deutonymphs of Trachymolgus jesusi were described as having highly reduced eyes. Gomelauri observed only one pair eyes in Trachymolgus recki. This offers significant evidence to suggest the specimens used to describe Trachymolgus recki were immature. Since these specimens were said to have three pairs of genital papillae, we suggest the description of Trachymolgus recki was based on tritonymphs. Therefore, Trachymolgus recki is excluded from the key below.
Notes on Trachymolgus jesusi, Mejia-Recamier & Palacios-Vargas, 1999Aspects of the morphology and development described for Trachymolgus jesusi (Mejia-Recamier & Palacios-Vargas 1999), suggest major deviations from what is known from other Bdellidae. Unfortunately, we were unable to obtain type specimens of this species.
Key to adult Trachymolgus Berlese (excluding Trachymolgus recki, likely a tritonymph – see above)| 1 | Movable digit with 3 teeth; pedipalp basi- and telofemur completely fused; leg basi- and telofemur III-IV completely fused; dark purple; Mexico | Trachymolgus jesusi |
| – | Movable digit with 1 tooth; pedipalp basi- and telofemur either divided or only partially fused dorsally; leg basi- and telofemur divided; dark purple to black | 2 |
| 2 | Pedipalp basi- and telofemur divided; black; Palaearctic | Trachymolgus nigerrimus |
| – | Pedipalp basi- and telofemur fused dorsally; dark purple; U.S.A. | Trachymolgus purpureus |
We thank Cal Welbourn, for collecting advice and history behind this elusive mite; Fábio Hernandes for an extensive and invaluable digital collection of bdelloid literature; Dave Walter and Roy Norton for their helpful comments on mite morphology; Danielle Keeler (University of Arkansas) for assisting in field collection; Natasha Wright (University of Arkansas) and Chris Pooley (USDA-ARS) for her help with editing photographs. Our thanks to Buffalo National River and Devil’s Den State Park personnel and finally to our friends and families who shoulder the burden of supporting biologists with a smile. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA; USDA is an equal opportunity provider and employer.
Trachymolgus purpureus sp. n. A Lateral view of proterosoma, LT-SEM; B–C Stereomicrographs of live specimens.
Integument of Trachymolgus purpureus sp. n. LT-SEM. A Lateral view of eyes and pt showing foveolate indentions B Magnified view of foveolae and pits.
Dorsum of Trachymolgus purpureus sp. n. Lateral plates removed and displayed laterally. See text for abbreviations.
Venter of Trachymolgus purpureus sp. n. Podocephalic canal (pc), median seta (ms), paragenital setae (pgs), genital shield/setae (gs), paranal shield/setae (ps), anal shield/setae (as). Stippling denotes unstriated membrane.
Morphological aspects of Trachymolgus purpureus sp. n. A LT-SEM of lateral view B enlargement of lateral membrane showing striations accompanied with bumps C Base of pt showing minute barbules D Left lateral view of lps in a groove above anterior eye, pt removed E Compound light micrograph of proterosomal shield with apodemes in focus, appearing as four dark spots F Line drawing of proterosomal shield showing apodemes.
Ventral aspects of Trachymolgus purpureus sp. n. A Venter showing pseudotracheae, legs removed B Stereomicrograph showing extruded ovipositor.
Legs of Trachymolgus purpureus sp. n. Laterodorsal view of distal podomeres. Fastigials (ft), iterals (it), prorals (p), tectals (tc), unguinals (u), and famulus (ε). Stippling denotes unstriated membrane. Asterisk (*) denotes solenidion found in only a few specimens.
Coxal fields of Trachymolgus purpureus sp. n. A Compound light micrograph of venter showing apodemes on coxae II & III B Line drawing with emphasis on apodemes.
Legs of Trachymolgus purpureus sp. n. LT-SEM. A Leg II, showing sclerotized, pitted sculpturing on telofemur and genu B Tarsus I showing papillated striations C Apotele II showing barbulate ungues D Enlargement showing tenant hairs.
Gnathosoma of Trachymolgus purpureus sp. n. A Subcapitulum B Pedipalp C Chelicera D Chela enlarged. Ventral end seta (ves), dorsal end seta (des), lateral lips (l), adorals (ad), anterioventral subcapitular setae (avs), oral opening (o), posterioventral subcapitular setae (pvs), dorsal subcapitular setae (ds). Stippling denotes unstriated membrane.
Gnathosoma of Trachymolgus purpureus sp. n. LT-SEM. A Ventral view of gnathosoma showing subcapitular sculpturing B Dorsal view of subcapitulum showing position of oral opening (o) C Lateral view of gnathosoma showing cheliceral sculpturing D Magnified view of distal gnathosoma showing lateral lips and silk charge E Dorsolateral view of removed pedipalp showing striations F Ventrodistal view of right pedipalp showing papillated striations, finely barbulate ves, and solenidion.
Nymphs of Trachymolgus purpureus sp. n.A Stereomicrograph showing greenish nymph, deutonymph shown B Stereomicrograph showing yellowish-white nymph,
Larva of Trachymolgus purpureus sp. n. See dorsal illustration (Fig. 3) for labeling. Stippling denotes unstriated membrane. Note f2 is lacking.
Protonymph of Trachymolgus purpureus sp. n. See Fig. 3 for labeling.
Deutonymph of Trachymolgus purpureus sp. n. Lateral plates removed and shown laterally. See Fig. 3 for labeling.
Tritonymph of Trachymolgus purpureus sp. n. Lateral plates removed and shown laterally. See Fig. 3 for labeling.
Genital development of immature Trachymolgus purpureus. A Protonymph (note weak sclerotization) B Deutonymph C Tritonymph.
LT-SEM of silk production in Trachymolgus purpureus sp. n. A Lateral habitus showing frozen mite with legs curled, attached to LT-SEM plate with silk tether B Enlargement of anterior gnathosoma and silk tether.