(C) 2011 Michael G. Rix. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The Assassin Spiders of the family Archaeidae are an ancient and iconic lineage of basal araneomorph spiders, characterised by a specialised araneophagic ecology and unique, ‘pelican-like’ cephalic morphology. Found throughout the rainforests, wet sclerophyll forests and mesic heathlands of south-western, south-eastern and north-eastern Australia, the genus Austrarchaea Forster & Platnick, 1984 includes a diverse assemblage of relictual, largely short-range endemic species. With recent dedicated field surveys and significant advances in our understanding of archaeid biology and ecology, numerous new species of assassin spiders have been discovered in the montane sub-tropical and warm-temperate closed forests of mid-eastern Australia, including several rare or enigmatic taxa and species of conservation concern. This fauna is revised and 17 new species are described from south-eastern Queensland and eastern New South Wales: Austrarchaea alani sp. n., Austrarchaea aleenae sp. n., Austrarchaea binfordae sp. n., Austrarchaea christopheri sp. n., Austrarchaea clyneae sp. n., Austrarchaea cunninghami sp. n., Austrarchaea dianneae sp. n., Austrarchaea harmsi sp. n., Austrarchaea helenae sp. n., Austrarchaea judyae sp. n., Austrarchaea mascordi sp. n., Austrarchaea mcguiganae sp. n., Austrarchaea milledgei sp. n., Austrarchaea monteithi sp. n., Austrarchaea platnickorum sp. n., Austrarchaea raveni sp. n. and Austrarchaea smithae sp. n. Adult specimens of the type species, Austrarchaea nodosa (Forster, 1956) are redescribed from the Lamington Plateau, south-eastern Queensland, and distinguished from the sympatric species Austrarchaea dianneae sp. n. A key to species and a molecular phylogenetic analysis of COI and COII mtDNA sequences complement the species-level taxonomy, with maps, habitat photos, natural history information and conservation assessments provided for all species.
new species, taxonomy, rainforest, conservation, cytochrome c oxidase, mitochondrial DNA, Palpimanoidea
The ‘assassin spiders’ of the family Archaeidae
are an ancient and iconic lineage of basal araneomorph spiders,
characterised by a remarkable cephalic morphology and specialised
araneophagic ecology. Archaeid spiders are obligate predators of other
spiders, and all possess a grossly-elevated, ‘pelican-like’
cephalothorax and long chelicerae (Figs 1, 4A-C) which are used to hunt and capture their spider prey (
Assassin spiders are iconic among arachnids due to the
extraordinary history of their discovery, their remarkable appearance
and antiquity, their limited distribution on the southern continents,
their extreme endemism, and their highly specialised araneophagic
biology (
The Recent archaeid fauna consists of 37 described species in three genera (
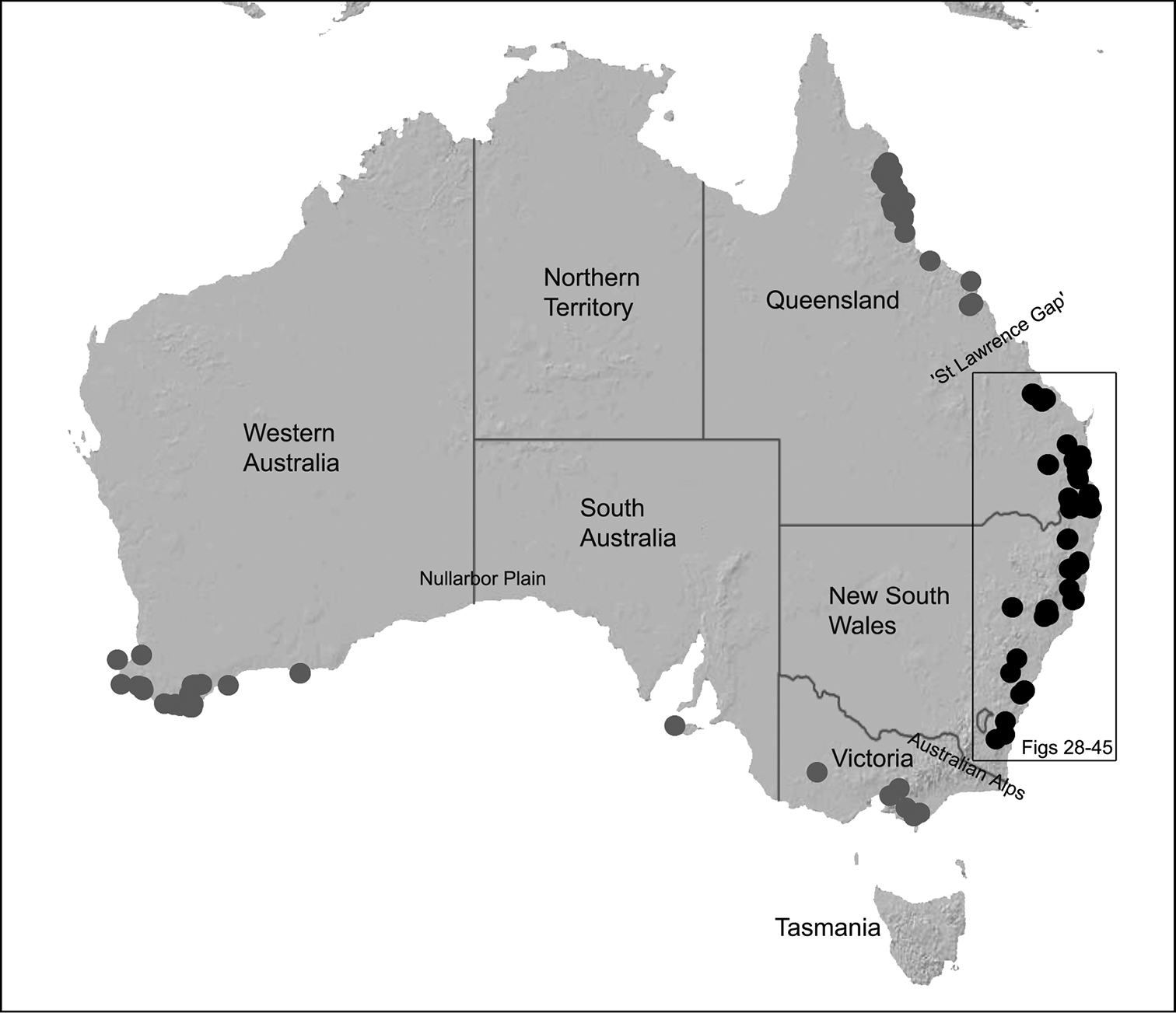
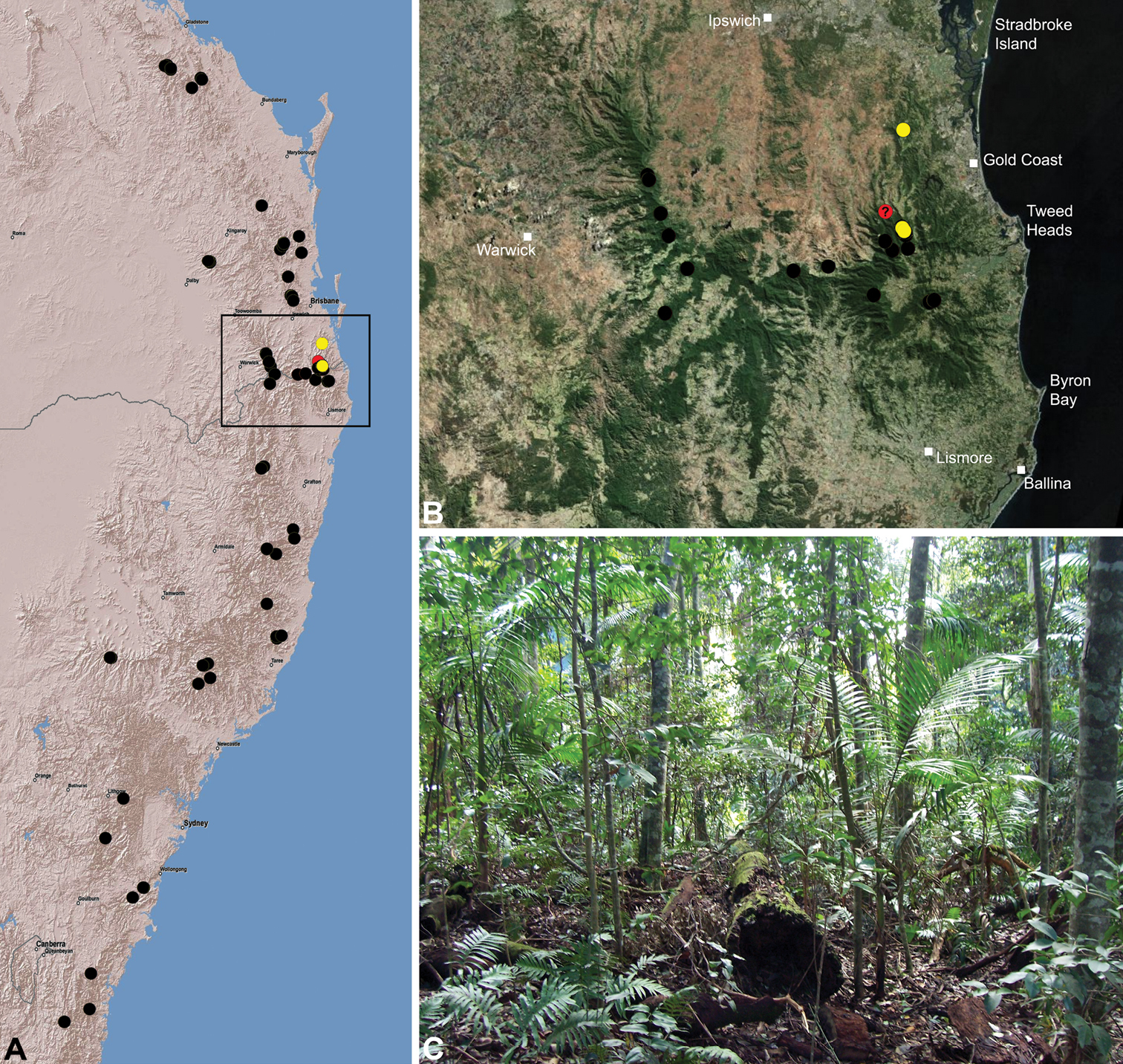
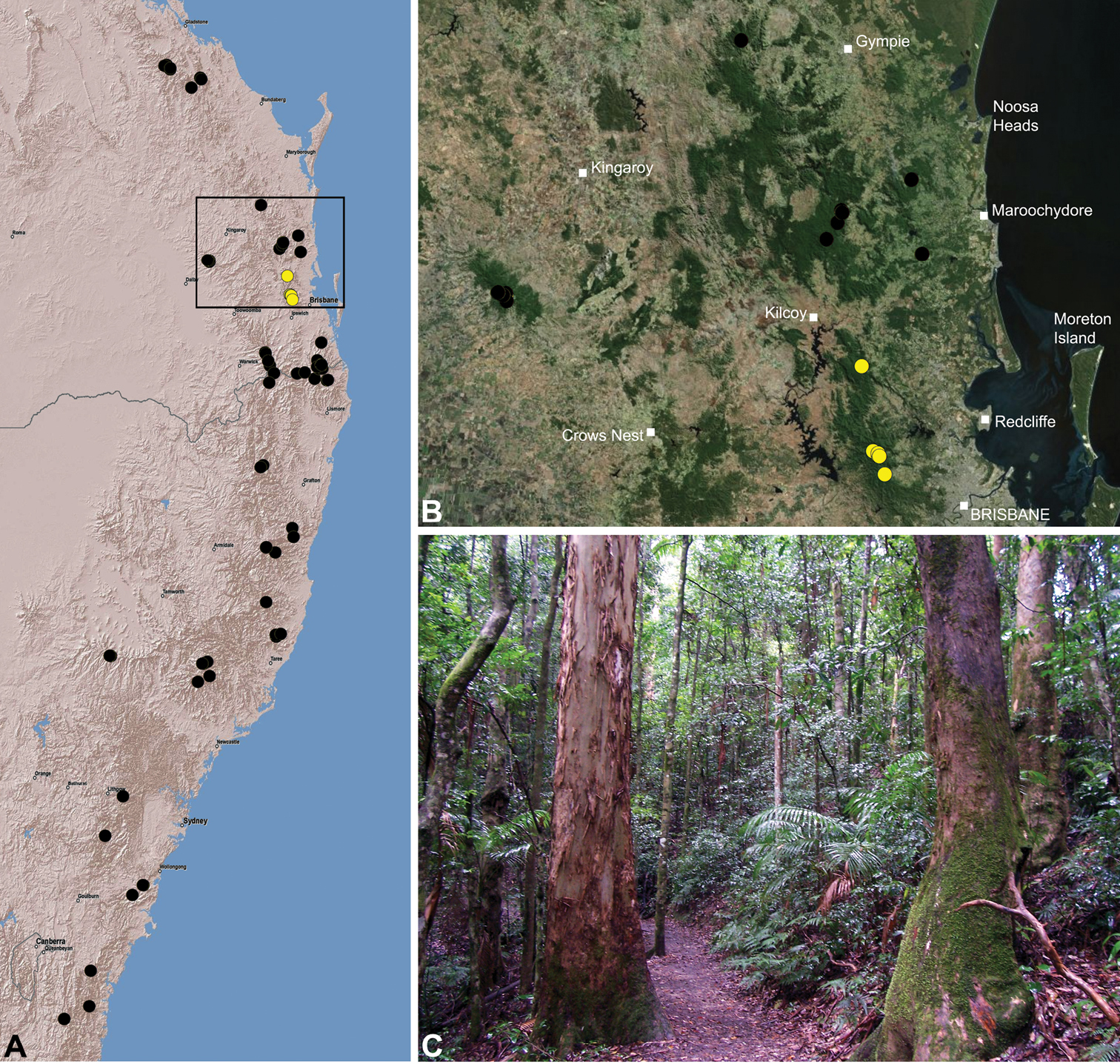
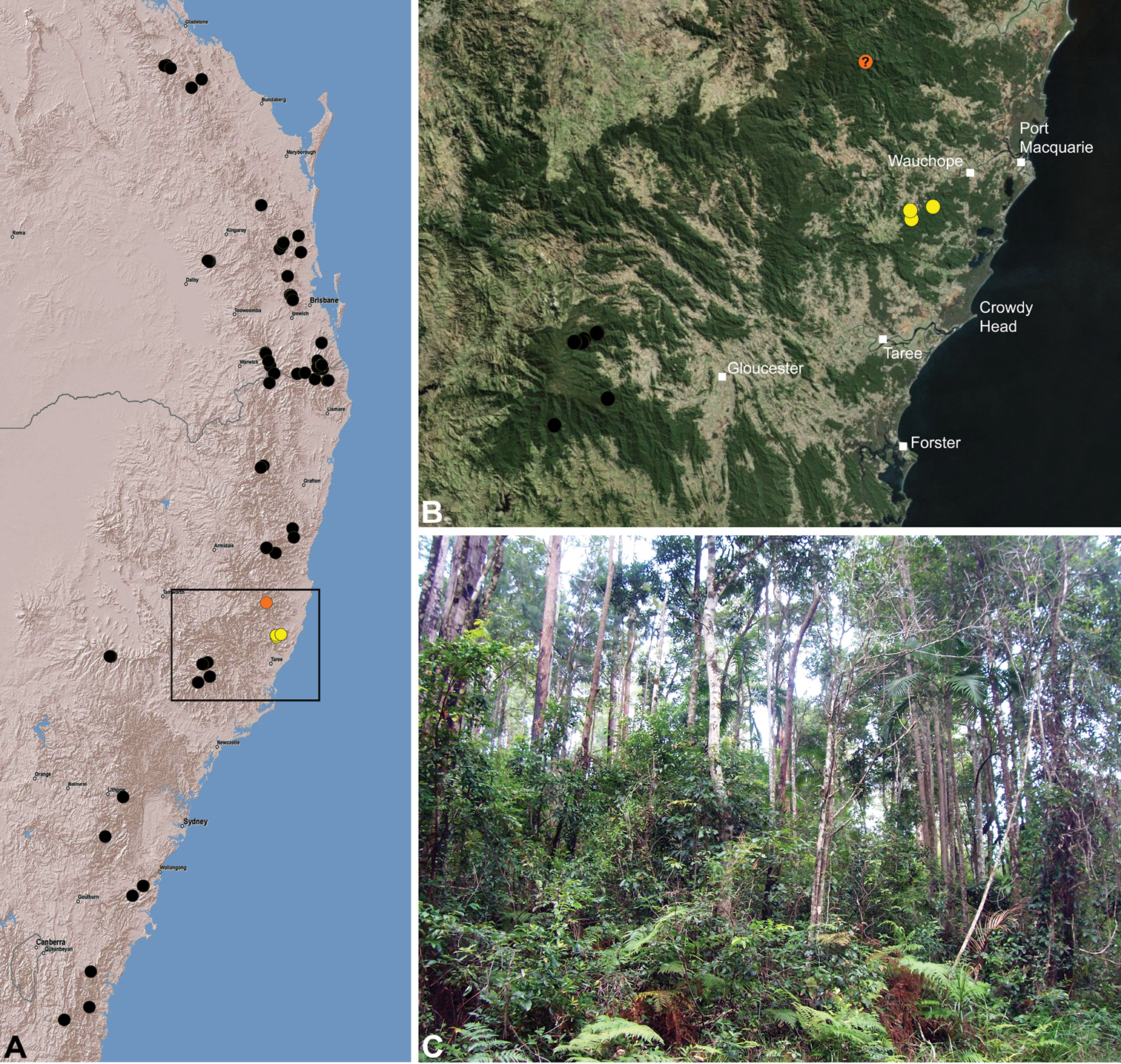
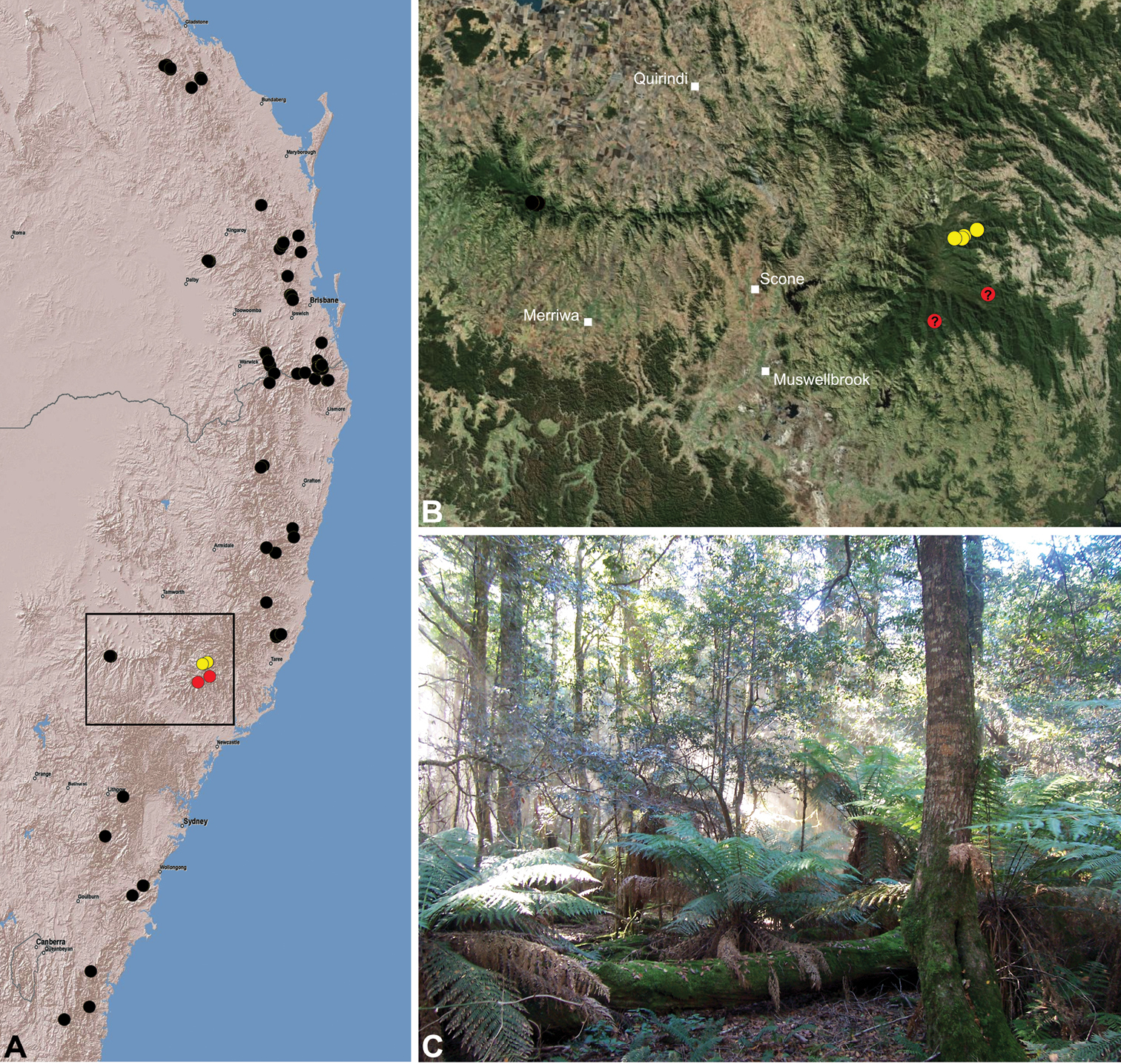
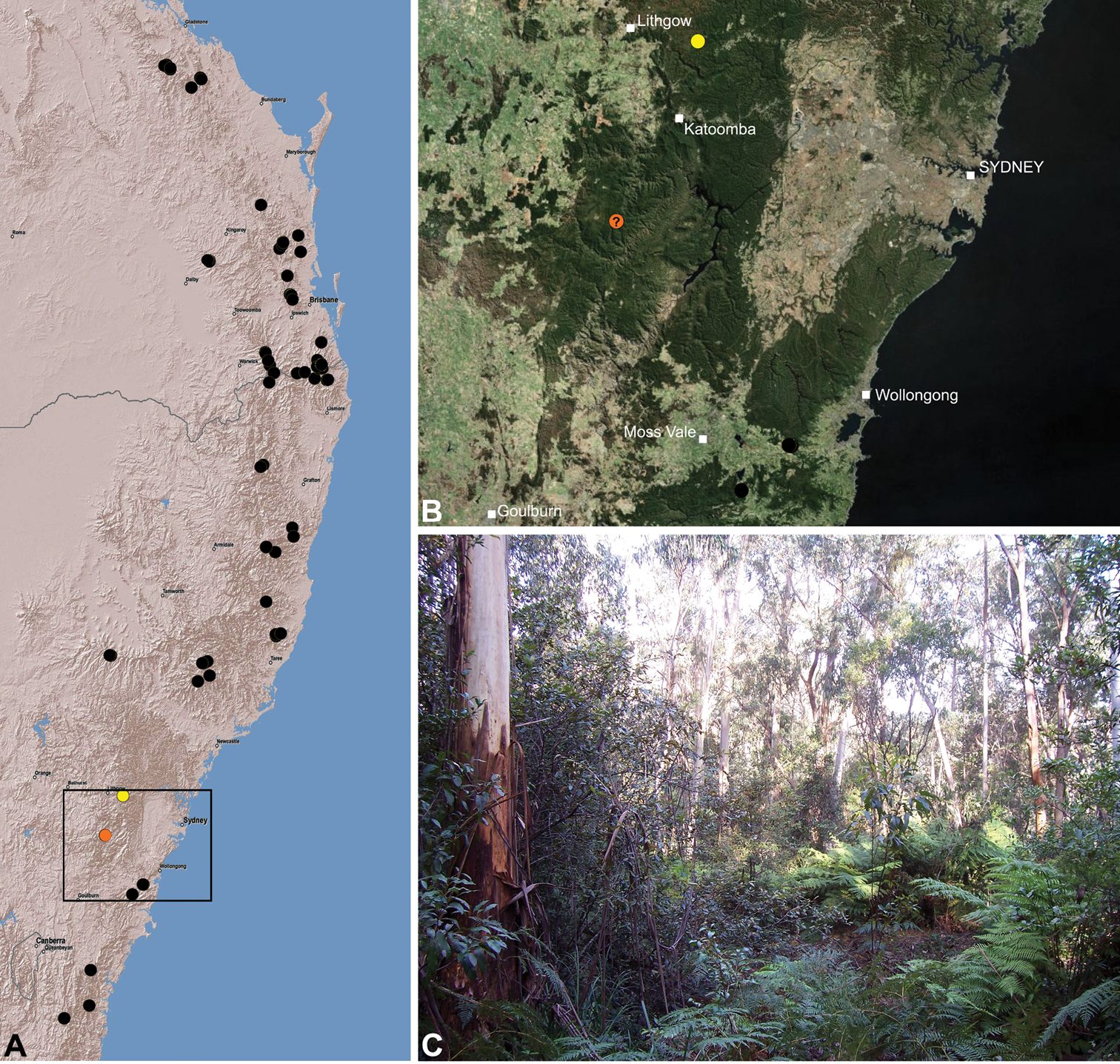
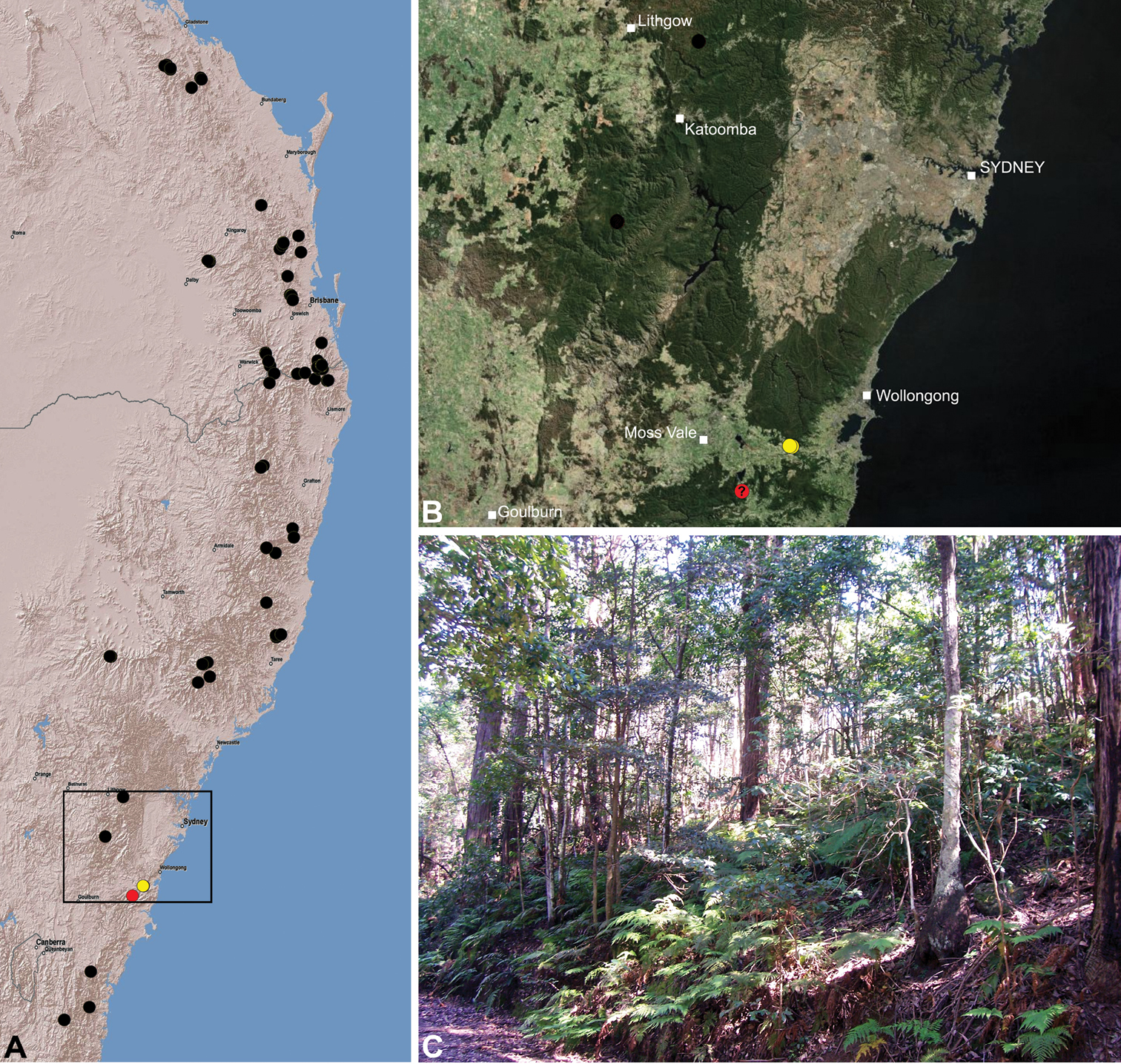
The current paper is thus a taxonomic revision of the species of Archaeidae known from ‘mid-eastern’ Australia, including those from south-eastern Queensland and eastern New South Wales, north of the Australian Alps (Fig. 2). The type species, Austrarchaea nodosa, is redescribed from the Lamington Plateau, south-eastern Queensland, and an additional 17 new species are described from habitats between Kroombit Tops National Park in Queensland and the Badja State Forest in southern New South Wales. These 18 species were found to form a monophyletic clade in a molecular phylogenetic analysis (Fig. 3B), and the remaining Australian Archaeidae will be described in forthcoming monographic treatments.
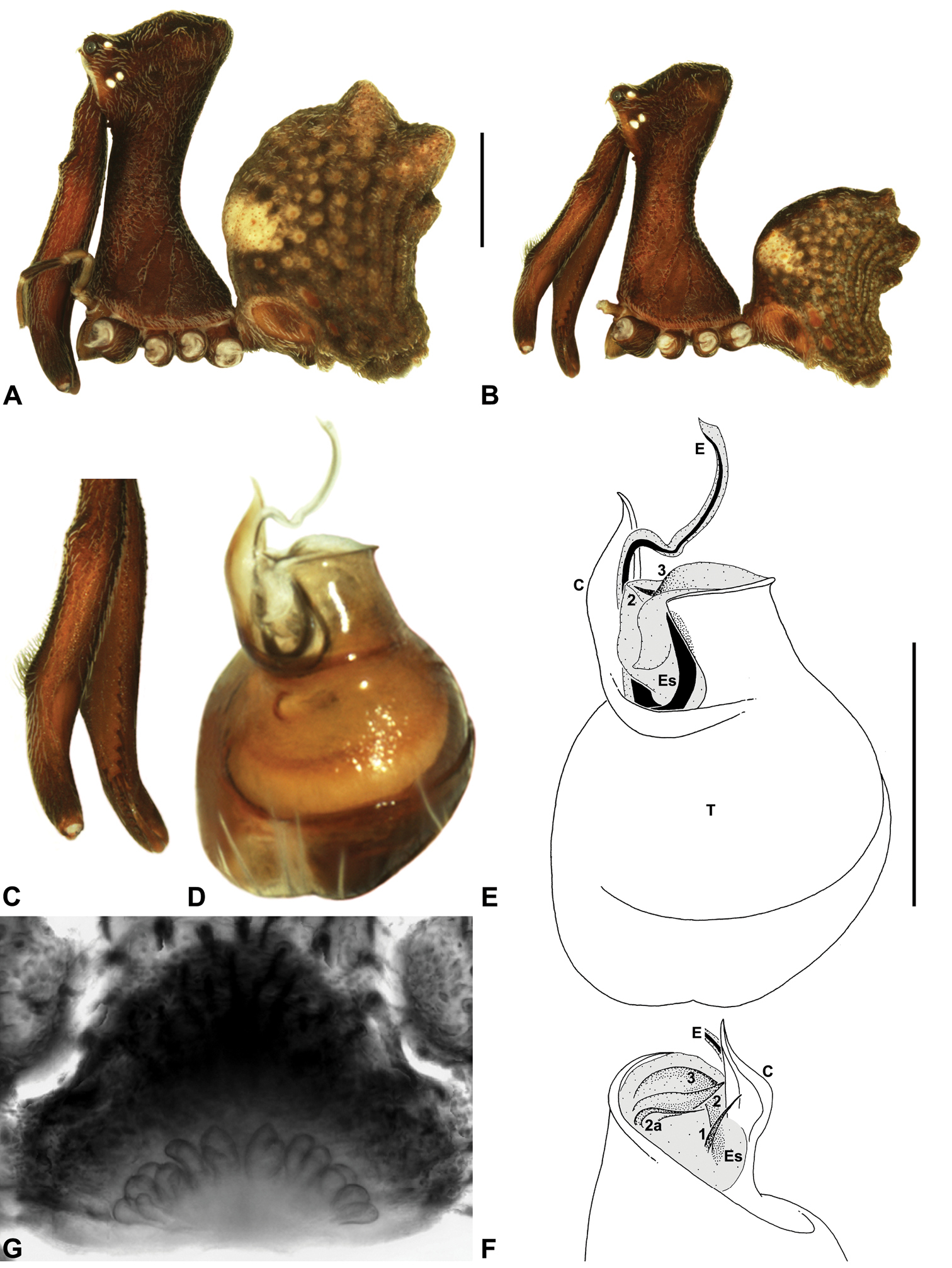
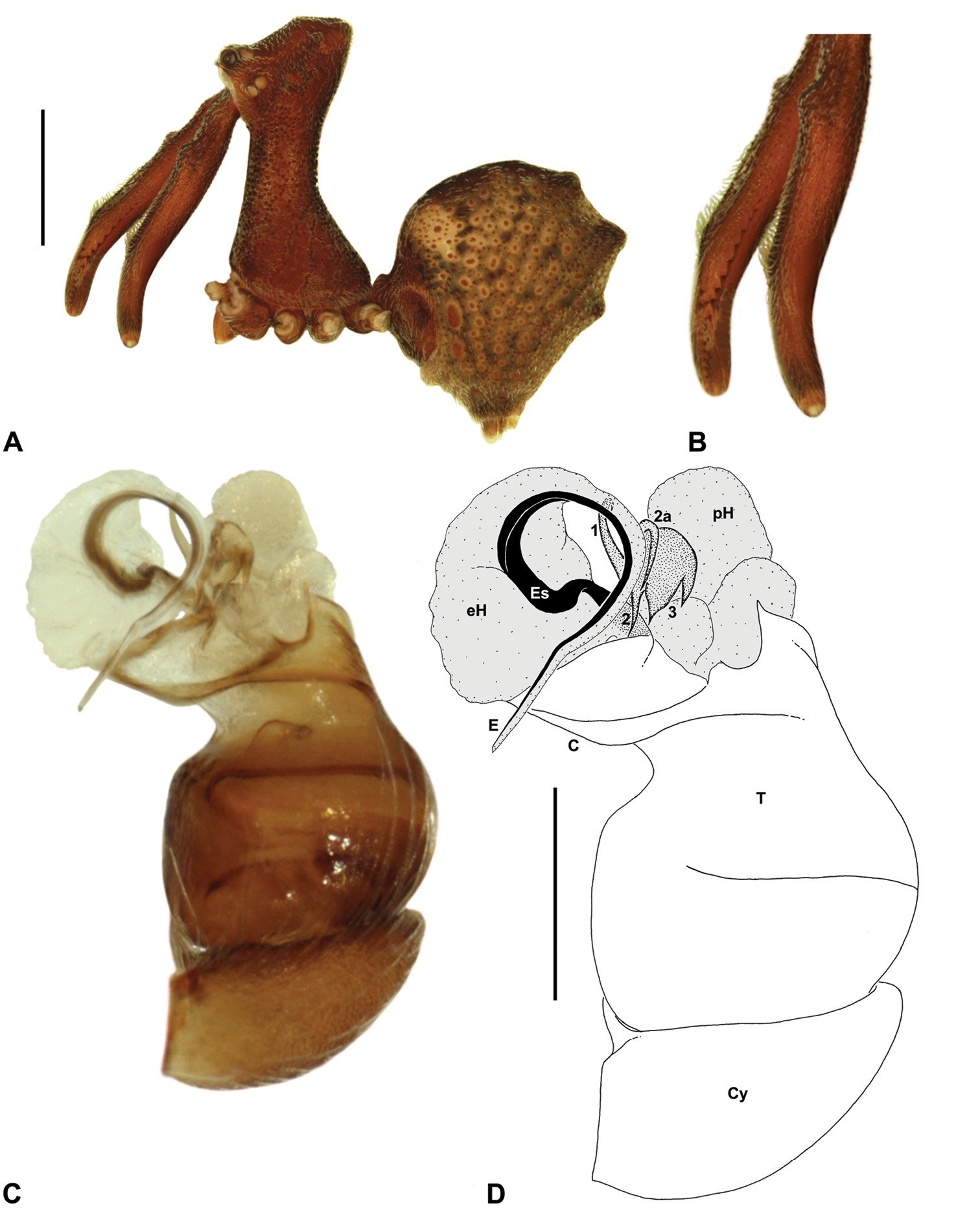
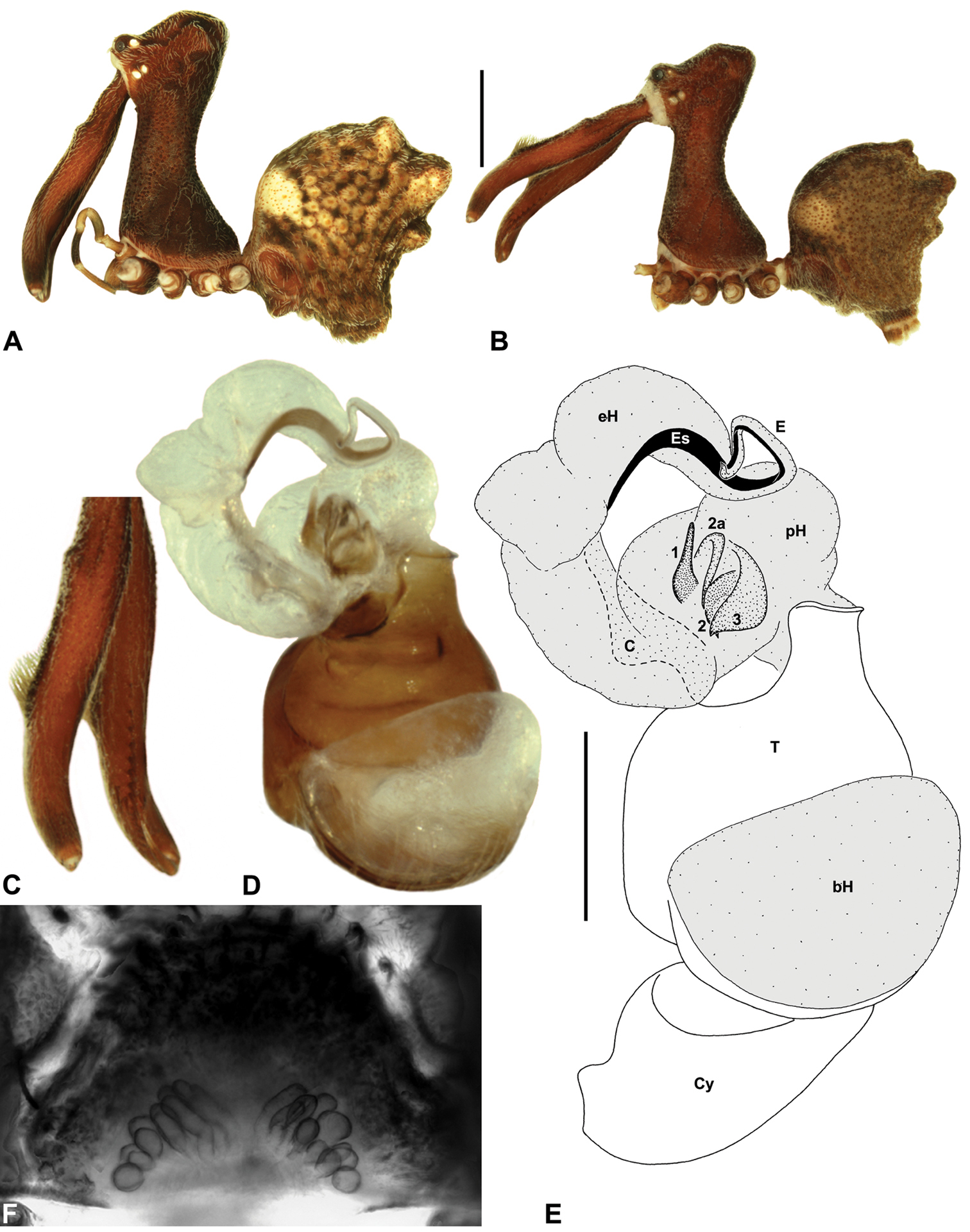
Material and methodsAll taxa were described and illustrated from specimens stored in 75% or 95% ethanol. Digital images were taken using a Leica MZ16A binocular microscope and a Leica DM2500 compound microscope, with auto-montage images captured using Leica DFC500 mounted cameras with Leica Application Suite Version 3.6.0 software. Male left pedipalps were dissected prior to imaging and bulbs were aligned for standardised comparison in the retrolateral and pro-distal positions illustrated; expanded pedipalps were illustrated in a retro-ventral position. Female genitalia were dissected and cleared in a 10% lactic acid plus 90% glycerol solution, prior to mounting on temporary glass slides. Illustrations were made on Utoplex tracing paper, using printed template auto-montage images.
Measurements. Measurements are in millimetres (rounded to the nearest hundredth of a millimetre) and were taken using an ocular graticule on a Leica M80 binocular microscope. Left legs were removed from specimens prior to taking measurements and imaging lateral body profiles. Lateral profile images were standardised for inter-specific comparison by vertically aligning the centre of each left anterior median eye with the lower anterior margin of the carapace (above the labrum) (Fig. 6). Carapace height was measured in lateral view, from the margin of the pars thoracica above coxa II to the highest point of the pars cephalica (Fig. 6). Carapace length was measured from the lower anterior margin of the carapace (above the labrum) to the posterior margin of the pars thoracica (above the pedicel) (Fig. 6). ‘Neck’ width was measured in lateral view, at the narrowest point of the carapace, with total length, carapace width, abdomen length and abdomen width all measured in dorsal view.
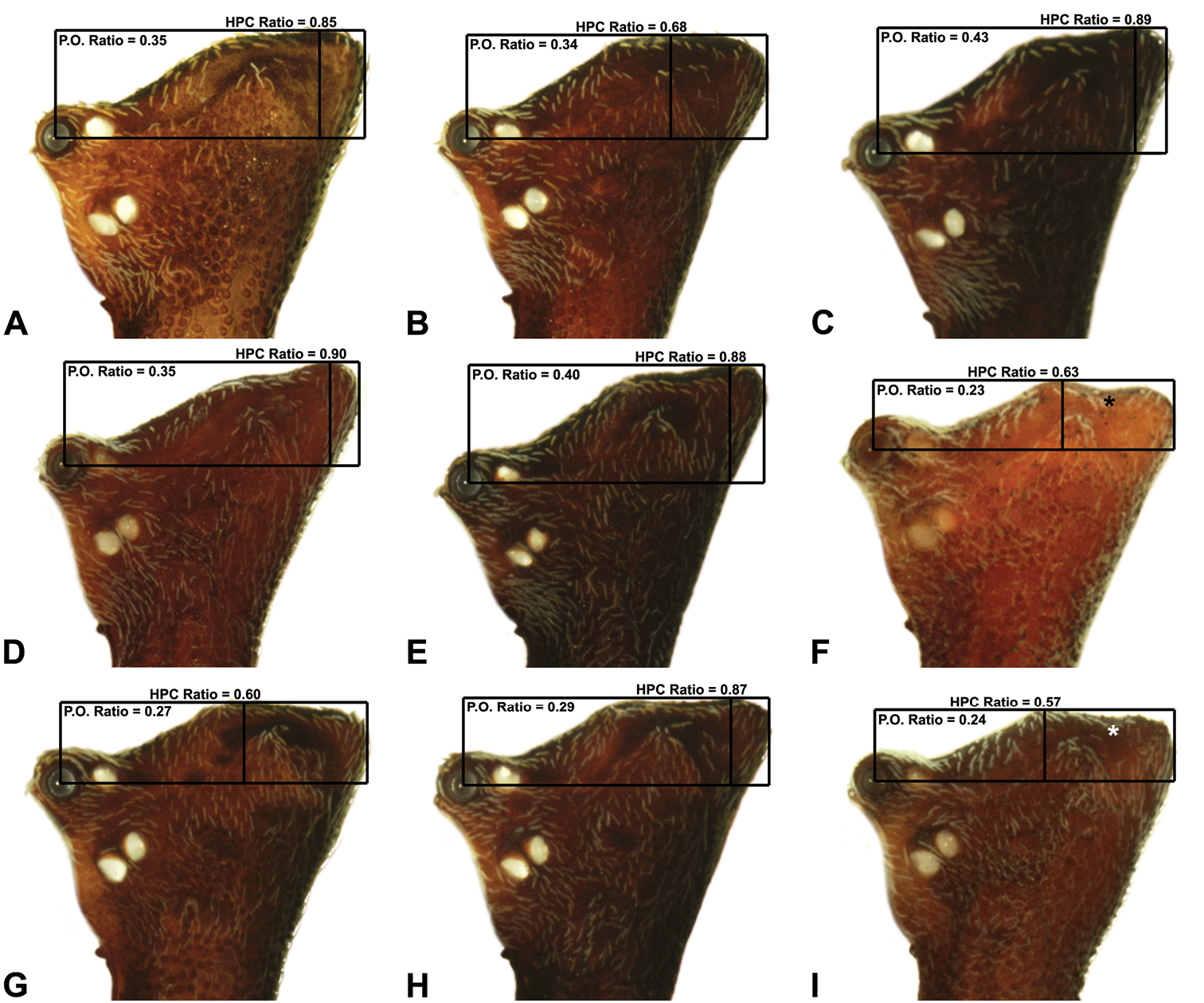
Morphometrics. To quantify inter-specific
variation in the shape of the cephalothorax and ‘head’, three
morphometric ratios were derived from lateral profile images (see Figs 6-9). The carapace height to carapace length (CH/CL) ratio, used extensively by
Molecular and laboratory methods. For molecular
analyses, specimens were preserved in 95% ethanol and stored at 4°C.
Between two and seven legs of each individual were removed for DNA
extractions and whole genomic DNA was extracted from leg tissue samples
using the Qiagen DNeasy Blood and Tissue Kit protocol for animal
tissues. Polymerase chain reaction (PCR) amplification of target gene
regions was achieved using Invitrogen Platinum Taq Polymerase
chemistry, in an Eppendorf Mastercycler ep gradient S thermal cycler.
For each PCR reaction, 2 µl of extracted DNA, 0.25 µl of Platinum Taq
(at 5 u/µl), 2 µl of MgCl2 (at 50 mM), 2.5 µl of 10x PCR buffer, 5
µl of dNTPs (at 1 mM) and 5 µl of each primer (at 2 µM) were used in
every 25 µl reaction. For most taxa, 1071 bp of the mitochondrial
cytochrome c oxidase subunit I (COI) gene, along with 535–541 bp
of the adjacent COII gene (~1609 bp in total), were amplified using
the primers ArCO1 (newly-designed for this study) and C2-N-3661b
(modified from
Conventions. Throughout this paper the term ‘Border Ranges’ is used to denote the mountainous geographic border region between south-eastern Queensland and northern New South Wales (see Fig. 28B), including the McPherson Range and Main Range, and encompassing the World Heritage-listed rainforests of the Mount Warning, Border Ranges, Springbrook, Lamington, Mount Chinghee, Mount Barney, Mount Nothofagus, Mount Clunie, Koreelah and Main Range National Parks.
For Material Examined sections, specimens of
indeterminate identification (usually juveniles) are included for
mapping purposes, and tentatively linked to named species according to
their geographic proximity to type localities (or in the case of
genotyped juvenile specimens, according to their molecular phylogenetic
affinity); such specimens are highlighted in Figures 28–45,
and individually listed for each species. Specimens not examined for
the current revision, but currently housed at the California Academy of
Sciences (due to ongoing research) are also listed separately (with
identifications confirmed by H. Wood), along with unexamined juvenile
specimens recently accessioned. Specimens sequenced for the molecular
analysis are denoted by superscript codes, which correspond to specimen
codes as shown in Figure 3B and Table 2. For species Diagnoses, molecular autapomorphies (e.g. see
Abbreviations used in the text are as follows:
ALE Anterior lateral eye/s
AME Anterior median eye/s
CH/CL Carapace height (CH) to carapace length (CL) ratio
F1/CL Femur I length (F1) to carapace length (CL) ratio
HPC Highest point of pars cephalica
HT 1–6 Abdominal hump-like tubercles 1–6
PME Posterior median eye/s
TS 1–3 Tegular sclerites 1–3
Specimens described in this study are lodged at the following institutions:
AMS Australian Museum, Sydney (G. Milledge)
ANIC Australian National Insect Collection, Canberra (B. Halliday)
CASENT California Academy of Sciences, San Francisco (C. Griswold, D. Ubick)
MACN Museo Argentino de Ciencias Naturales, Buenos Aires (M. Ramírez)
QMB Queensland Museum, Brisbane (R. Raven, O. Seeman)
WAM Western Australian Museum, Perth (MSH, J. Waldock)
Phylogenetic analysisTo complement and inform the morphological hypotheses presented for the species-level taxonomy (see below), and to provide molecular autapomorphies useful for distinguishing species of Austrarchaea from mid-eastern Australia, a molecular taxonomic approach was employed using mitochondrial DNA nucleotide sequences. A 1071 bp fragment of the cytochrome c oxidase subunit I (COI) gene, along with a 535–541 bp fragment of the adjacent COII gene (Fig. 3A, Table 3), were amplified in species of Austrarchaea (and outgroups) for analysis under a Bayesian framework. These data were generated and aligned as described in the Methods (above), and the resulting nexus file (see Appendix I) was analysed as highlighted (below).
Taxa. Specimens of Archaeidae
were collected throughout mid-eastern Australia in March-May 2010, for
use in molecular analyses. At least three specimens from each major
population were sequenced for COI and COII; for some populations, fewer
specimens were available. Most populations of Archaeidae
previously known from mid-eastern Australia were successfully sampled
and sequenced for the molecular analysis (see superscript DNA codes in
the Material Examined sections, below), with numerous newly discovered
populations also included. In total, sequences from 94 taxa were added
to the final alignment (see Table 2), including 79 Austrarchaea from mid-eastern Australia, one archaeid specimen from north-eastern Queensland and eight Archaeidae from Victoria and Western Australia. A specimen of the Madagascan species Eriauchenius workmani O.P.-Cambridge, 1881 was also included, along with three other Palpimanoidea in the families Mecysmaucheniidae and Palpimanidae. The tree was rooted with the outgroups Hickmania troglodytes (Higgins & Petterd, 1883) (Austrochilidae) and Tarlina smithersi Gray, 1987 (Gradungulidae) (both in the superfamily Austrochiloidea), shown to be sister or basal to the Palpimanoidea in previous analyses (see
Analysis. To infer phylogenetic relationships among sequenced specimens of Archaeidae
from mid-eastern Australia, a combined, gene-partitioned Bayesian
phylogenetic analysis was executed in MrBayes Version 3.1.2 (
Results and discussion. The summary phylogenetic tree resulting from Bayesian analysis of the COI and COII data is presented in Figure 3B. The family Archaeidae and the genus Austrarchaea (as currently defined) were both monophyletic and strongly supported, with all mid-eastern Australian taxa similarly united in a monophyletic (although weakly supported) clade (highlighted green in Fig. 3B). Within this mid-eastern Australian lineage, evidence for at least 17 morphological species was supported by 17 equivalently-monophyletic and strongly supported molecular clades; inter-specific (i.e. sister-species) pairwise divergences for the combined (COI + COII) dataset ranged from 8–10%, with intra-specific divergences ranging from 0–6%. Three monophyletic clades from populations known only by juveniles (from the Kanangra-Boyd National Park, Willi Willi National Park and Badja State Forest) had sequence divergences in the range 8–9% (relative to sister-clades), suggesting that these populations may represent distinct species. Deeper species-group lineages were generally poorly supported by the COI and COII data, although Austrarchaea monteithi sp. n. was clearly inferred as a basal taxon, sister to all other species from mid-eastern Australia (Fig. 3B).
The results of the molecular phylogenetic analysis highlight the utility of comparing molecular and morphological taxonomic techniques, and provide a first insight into the possible phylogenetic relationships among Australian Archaeidae. Despite their exaggerated morphology and specialised ecology, species of Austrarchaea are otherwise morphologically conservative haplogyne spiders, with only relatively subtle inter-specific somatic and genitalic differences between adults, and a diagnostic requirement in most species for adult male specimens. This morphological conservatism, combined with the general paucity of specimens in collections, the relative over-representation of juveniles in collections and in the field, along with the difficulties associated with collecting adult males, renders the identification of species of Austrarchaea difficult based on morphology alone. By sequencing juveniles and adults from across mid-eastern Australia, a much clearer picture of the distribution and limits of each species has been achieved; populations known only from juveniles and females could be confidently linked to type localities, and newly-collected juvenile specimens could for the first time be associated with conspecific adult specimens. In the case of collections made at Binna Burra (Lamington National Park) in April 2010, juvenile specimens of two sympatric species were successfully genotyped to determine their identification, and to test whether Austrarchaea nodosa and Austrarchaea dianneae sp. n. were truly sympatric on the Lamington Plateau (see Nomenclatural Remarks for Austrarchaea nodosa, below).
The phylogenetic relationships inferred for Australian species of Archaeidae remain highly preliminary in the absence of additional genes and a greater taxon sample from southern and north-eastern Australia (M. Rix, unpublished data), however several key results are worthy of discussion. Firstly, the enigmatic Austrarchaea monteithi sp. n., from the Gibraltar Range National Park (Fig. 19), was clearly inferred as a basal sister-species to all other Archaeidae from mid-eastern Australia, which together formed a monophyletic (although weakly supported) mid-eastern Australian clade (highlighted green in Fig. 3B) sister to an undescribed species from north-eastern Queensland. This result is congruent with morphology, in that the linear gradation seen in the number of dorsal hump-like tubercles on the abdomen (four in north-eastern Queensland taxa; five in Austrarchaea monteithi sp. n.; six in all other mid-eastern Australian taxa; Figs 5E-G) matches the inferred gradation of clades in Figure 3B. Similarly, the observed gap in the distribution of archaeid species in central Queensland, roughly consistent with the ‘St Lawrence Gap’ (Webb and Tracey 1981) between Gladstone and Mackay (Fig. 2), seems to reflect a genuine phylogenetic barrier, rather than a collecting artefact. The other major gap in the distribution of Archaeidae in mesic eastern Australia, roughly consistent with the mountainous Australian alpine zone bordering New South Wales and Victoria (Fig. 2), seems to also reflect a second major phylogenetic barrier between a divergent clade of southern Australian taxa (highlighted blue in Fig. 3B) and all other Australian Archaeidae.
Clearly, applying molecular taxonomic methods to a morphological taxonomy is of great utility for species of Austrarchaea.
For the current revision, molecular data are clearly linked to
specimens and to museum registration numbers by using DNA taxon codes in
Material Examined sections, each of which corresponds to an equivalent
code in Table 2, and to branch terminals in Figure 3B.
To fully integrate the molecular data with the morphological taxonomic
hypotheses presented (below), species are also diagnosed (where
possible) with unique molecular autapomorphies, in addition to standard
morphological characters (see Conventions, above). This approach will
facilitate the molecular identification of specimens in the future (as
advocated by numerous authors, e.g.
Primers used to amplify and sequence COI and COII genes for the molecular analysis. Underlined letters denote nucleotide modifications.
| Name | Sequence (5’-3’) | Type (Gene) | References |
|---|---|---|---|
| PCR PRIMERS | |||
| ArCO1 | CATTTAGCTGGTGCTTCTTCTATT | Forward (COI) | |
| ArCO1a1 | CATTTAGCTGGTGCTTCATCTATT | Forward (COI) | |
| ArCO1c2 | CATTTGGCTGGGGCGTCATCAATT | Forward (COI) | |
| ZrCO13 | TCTTTACATTTAGCTGGTGCTTCTT | Forward (COI) | |
| C2-N-3661 | CACAAATTTCTGAACATTGACCA | Reverse (COII) |
|
| C2-N-3661a4 | CACAAATTTCAGAACATTGACCA | Reverse (COII) |
|
| C2-N-3661b5 | CACAAATTTCAGAACATTGACCT | Reverse (COII) |
|
| SEQUENCING/PCR PRIMERS* | |||
| SeqF2a | TYCATTATGTWTTAAGAATAGG | Forward (COI) | |
| SeqF2a1 | CATTTYCATTATGTDTTRAGAATRGG | Forward (COI) | |
| SeqR1 | CATCAGGATAATCWGAATAHCG | Reverse (COI) | |
| SeqR1a | CATCWGGRTARTCHGAATAHCGACG | Reverse (COI) | |
* Used as PCR primers in certain taxa (see Table 2)
1 Used for Victorian and Stirling Range National Park Austrarchaea spp.
2 Used for Otiothops birabeni
3 Used for Zearchaea sp. 2
4 Used for outgroups and Western Australian/Victorian Austrarchaea spp.
5 Used for most south-eastern Australian Austrarchaea spp.
Specimens sequenced for the molecular analysis. Primer sequences are further listed in Table 1.
| Species/Museum No. | SpecimenCode | GenBankAccession | Forward PCRPrimer/s | Reverse PCRPrimer/s |
|---|---|---|---|---|
| OUTGROUPS | ||||
| Hickmania troglodytes (Higgins & Petterd, 1883) (Bubs Hill Karst, TAS): | ||||
| WAM T79989 | N.A. | JF909360 | ArCO1 | C2-N-3661a |
| Tarlina smithersi Gray, 1987 in Forster et al., 1987 (Willi Willi National Park, NSW): | ||||
| WAM T112581 | N.A. | JF909361 | ArCOI/SeqF2a | SeqR1/C2-N-3661a |
| Otiothops birabeni Mello-Leitão, 1945 (Parque Nacional El Palmar, Argentina): | ||||
| MACN Ar11491 | N.A. | JF909362 | ArCO1c/SeqF2a | SeqR1/C2-N-3661a |
| Zearchaea sp. 1 (Lewis Pass, New Zealand): | ||||
| WAM T79990 | N.A. | JF909363 | ArCO1/SeqF2a | SeqR1/C2-N-3661a |
| Zearchaea sp. 2 (Milford Sound, New Zealand): | ||||
| WAM T112582 | N.A. | JF909364 | ZrCO1 | C2-N-3661a |
| Eriauchenius workmani O.P.-Cambridge, 1881 (Ranomafana, Madagascar): | ||||
| CASENT 9018984 | N.A. | JF909365 | ArCO1 | C2-N-3661a |
| OTHER AUSTRARCHAEA SPP. | ||||
| Austrarchaea sp. (Acheron Gap, VIC): | ||||
| WAM T112583 | Ar14-49-F | JF909366 | ArCO1a | C2-N-3661a |
| WAM T112583 | Ar14-134-J | JF909367 | ArCO1a | C2-N-3661a |
| Austrarchaea mainae Platnick, 1991b (Albany, SW. WA): | ||||
| WAM T89572 | WF-9-F | JF909368 | ArCO1 | C2-N-3661a |
| WAM T89578 | GR-17-J | JF909369 | ArCO1 | C2-N-3661a |
| Austrarchaea robinsi Harvey, 2002a (Stirling Range, SW. WA): | ||||
| WAM T89558 | EP-40-J | JF909370 | ArCO1a | C2-N-3661a |
| WAM T89558 | EP-41-J | JF909371 | ArCO1a | C2-N-3661a |
| Austrarchaea sp. (Karri Valley, SW. WA): | ||||
| WAM T89565 | KV-38-J | JF909372 | ArCO1 | C2-N-3661a |
| Austrarchaea sp. (Wellington National Park, SW. WA): | ||||
| WAM T112584 | CO-158-F | JF909373 | ArCO1 | C2-N-3661a |
| Austrarchaea sp. (Daintree National Park, NE. QLD): | ||||
| WAM T97462 | MG-45-J | JF909374 | ArCO1 | C2-N-3661 |
| MID-EASTERN AUSTRALIAN AUSTRARCHAEA SPP. | ||||
| Austrarchaea nodosa (Forster, 1956): | ||||
| WAM T89592 | LAM-51-J | JF909375 | ArCO1/SeqF2a1 | SeqR1a/C2-N-3661 |
| WAM T112571 | Ar56-58-J | JF909376 | ArCO1 | C2-N-3661b |
| WAM T112572 | Ar57-46-J | JF909377 | ArCO1 | C2-N-3661b |
| WAM T112573 | Ar58-53-J | JF909378 | ArCO1 | C2-N-3661b |
| Austrarchaea alani sp. n.: | ||||
| WAM T112550 | KT-63-F | JF909379 | ArCO1 | C2-N-3661b |
| WAM T112550 | KT-64-J | JF909380 | ArCO1 | C2-N-3661b |
| WAM T112550 | KT-65-J | JF909381 | ArCO1 | C2-N-3661b |
| WAM T112551 | KT-66-M | JF909382 | ArCO1 | C2-N-3661b |
| WAM T112551 | KT-67-J | JF909383 | ArCO1 | C2-N-3661b |
| Austrarchaea aleenae sp. n.: | ||||
| WAM T112552 | BUL-68-M | JF909384 | ArCO1/SeqF2a1 | SeqR1a/C2-N-3661b |
| WAM T112552 | BUL-69-J | JF909385 | ArCO1/SeqF2a1 | SeqR1a/C2-N-3661b |
| WAM T112552 | BUL-70-J | JF909386 | ArCO1/SeqF2a1 | SeqR1a/C2-N-3661b |
| Austrarchaea binfordae sp. n.: | ||||
| AMS KS114969 | Ar46-106-M | JF909402 | ArCO1 | C2-N-3661b |
| Austrarchaea christopheri sp. n.: | ||||
| AMS KS114968 | Ar49-95-M | JF909387 | ArCO1 | C2-N-3661b |
| WAM T112554 | Ar49-96-J | JF909388 | ArCO1 | C2-N-3661b |
| WAM T112554 | Ar49-97-J | JF909389 | ArCO1 | C2-N-3661b |
| WAM T112553 | Ar50-98-J | JF909390 | ArCO1 | C2-N-3661b |
| WAM T112553 | Ar50-99-J | JF909391 | ArCO1 | C2-N-3661b |
| WAM T112553 | Ar50-100-J | JF909392 | ArCO1 | C2-N-3661b |
| Austrarchaea cunninghami sp. n.: | ||||
| WAM T112555 | Ar55-89-F | JF909393 | ArCO1 | C2-N-3661b |
| WAM T112555 | Ar55-90-J | JF909394 | ArCO1 | C2-N-3661b |
| WAM T112555 | Ar55-91-J | JF909395 | ArCO1 | C2-N-3661b |
| Austrarchaea dianneae sp. n.: | ||||
| WAM T112557 | Ar59-60-M | JF909396 | ArCO1 | C2-N-3661b |
| WAM T112557 | Ar59-61-J | JF909397 | ArCO1 | C2-N-3661b |
| WAM T112557 | Ar59-62-J | JF909398 | ArCO1 | C2-N-3661b |
| WAM T112556 | Ar56-54-M | JF909399 | ArCO1 | C2-N-3661b |
| WAM T112556 | Ar56-55-J | JF909400 | ArCO1 | C2-N-3661b |
| WAM T112556 | Ar56-56-J | JF909401 | ArCO1 | C2-N-3661b |
| Austrarchaea harmsi sp. n.: | ||||
| WAM T112559 | Ar70-73-M | JF909406 | ArCO1/SeqF2a1 | SeqR1a/C2-N-3661b |
| WAM T112559 | Ar70-74-J | JF909407 | ArCO1/SeqF2a1 | SeqR1a/C2-N-3661b |
| WAM T112559 | Ar70-75-J | JF909408 | ArCO1/SeqF2a1 | SeqR1a/C2-N-3661b |
| WAM T112560 | Ar71-71-J | JF909409 | ArCO1/SeqF2a1 | SeqR1a/C2-N-3661b |
| WAM T112560 | Ar71-72-J | JF909410 | ArCO1/SeqF2a1 | SeqR1a/C2-N-3661b |
| Austrarchaea helenae sp. n.: | ||||
| WAM T112561 | Ar30-124-J | JF909411 | ArCO1 | C2-N-3661b |
| WAM T112561 | Ar30-125-J | JF909412 | ArCO1 | C2-N-3661b |
| WAM T112561 | Ar30-126-J | JF909413 | ArCO1 | C2-N-3661b |
| Austrarchaea judyae sp. n.: | ||||
| WAM T112563 | Ar67-76-F | JF909414 | ArCO1 | C2-N-3661b |
| WAM T112563 | Ar67-78-J | JF909415 | ArCO1 | C2-N-3661b |
| WAM T112562 | Ar66-79-J | JF909416 | ArCO1 | C2-N-3661b |
| WAM T112564 | Ar68-80-M | JF909417 | ArCO1 | C2-N-3661b |
| WAM T112564 | Ar68-81-J | JF909418 | ArCO1 | C2-N-3661b |
| WAM T112564 | Ar68-82-J | JF909419 | ArCO1 | C2-N-3661b |
| Austrarchaea mascordi sp. n.: | ||||
| AMS KS114973 | Ar41-48-F | JF909420 | ArCO1 | C2-N-3661b |
| WAM T112566 | Ar41-113-J | JF909421 | ArCO1 | C2-N-3661b |
| WAM T112566 | Ar41-114-J | JF909422 | ArCO1 | C2-N-3661b |
| WAM T112565 | Ar40-115-M | JF909423 | ArCO1 | C2-N-3661b |
| Austrarchaea mcguiganae sp. n.: | ||||
| WAM T112567 | Ar28-47-J | JF909424 | ArCO1 | C2-N-3661 |
| WAM T112567 | Ar28-128-J | JF909425 | ArCO1 | SeqR1A/C2-N-3661 |
| Austrarchaea milledgei sp. n.: | ||||
| WAM T112568 | Ar43-107-F | JF909426 | ArCO1 | C2-N-3661b |
| WAM T112568 | Ar43-108-J | JF909427 | ArCO1 | C2-N-3661b |
| WAM T112568 | Ar43-109-J | JF909428 | ArCO1 | C2-N-3661b |
| WAM T112569 | Ar42-110-J | JF909429 | ArCO1 | C2-N-3661b |
| WAM T112569 | Ar42-111-J | JF909430 | ArCO1 | C2-N-3661b |
| WAM T112569 | Ar42-112-J | JF909431 | ArCO1 | C2-N-3661b |
| Austrarchaea monteithi sp. n.: | ||||
| AMS KS114976 | Ar52-92-F | JF909432 | ArCO1 | C2-N-3661b |
| WAM T112570 | Ar52-93-J | JF909433 | ArCO1 | C2-N-3661b |
| WAM T112570 | Ar52-94-J | JF909434 | ArCO1 | C2-N-3661b |
| Austrarchaea platnickorum sp. n.: | ||||
| WAM T112558 | Ar51-101-M | JF909403 | ArCO1 | C2-N-3661b |
| WAM T112558 | Ar51-102-F | JF909404 | ArCO1 | C2-N-3661b |
| WAM T112558 | Ar51-103-J | JF909405 | ArCO1 | C2-N-3661b |
| Austrarchaea raveni sp. n.: | ||||
| QMB S90192 | Ar73-83-F | JF909435 | ArCO1/SeqF2a | SeqR1/C2-N-3661b |
| WAM T112574 | Ar73-84-J | JF909436 | ArCO1/SeqF2a | SeqR1/C2-N-3661b |
| WAM T112574 | Ar73-85-J | JF909437 | ArCO1/SeqF2a | SeqR1/C2-N-3661b |
| WAM T112575 | Ar69-86-M | JF909438 | ArCO1/SeqF2a | SeqR1/C2-N-3661b |
| WAM T112575 | Ar69-87-J | JF909439 | ArCO1/SeqF2a | SeqR1/C2-N-3661b |
| WAM T112575 | Ar69-88-J | JF909440 | ArCO1/SeqF2a | SeqR1/C2-N-3661b |
| Austrarchaea smithae sp. n.: | ||||
| WAM T112576 | Ar32-116-F | JF909441 | ArCO1 | C2-N-3661b |
| WAM T112576 | Ar32-117-J | JF909442 | ArCO1 | C2-N-3661b |
| WAM T112576 | Ar32-118-J | JF909443 | ArCO1 | C2-N-3661b |
| Austrarchaea sp. indet. (Willi Willi National Park, NSW): | ||||
| WAM T112580 | Ar47-104-J | JF909444 | ArCO1 | C2-N-3661b |
| WAM T112580 | Ar47-105-J | JF909445 | ArCO1 | C2-N-3661b |
| Austrarchaea sp. indet. (Kanangra-Boyd National Park, NSW): | ||||
| WAM T112578 | Ar33-119-J | JF909446 | ArCO1 | C2-N-3661b |
| WAM T112578 | Ar33-120-J | JF909447 | ArCO1 | C2-N-3661b |
| WAM T112578 | Ar33-121-J | JF909448 | ArCO1 | C2-N-3661b |
| WAM T112579 | Ar34-122-J | JF909449 | ArCO1 | C2-N-3661b |
| WAM T112579 | Ar34-123-J | JF909450 | ArCO1 | C2-N-3661b |
| Austrarchaea sp. indet. (Badja State Forest, NSW): | ||||
| WAM T112577 | Ar27-129-J | JF909451 | ArCO1 | C2-N-3661b |
| WAM T112577 | Ar27-130-J | JF909452 | ArCO1 | C2-N-3661b |
| WAM T112577 | Ar27-131-J | JF909453 | ArCO1 | C2-N-3661b |
Mitochondrial COI-COII DNA sequence of juvenile Austrarchaea nodosa (Forster, 1956) (WAM T112571), showing the nucleotide numbering system (1-1609) used to designate molecular autapomorphies for species diagnoses. Underlined nucleotides denote stop and initiation codons for COI and COII, respectively.
| COI mtDNA (nucleotides 1-1071) |
|---|
| ATAGGTGCTGTAAATTTTATTTCTACTATTTTGAATATACGATCTTATGGAATGAGAATAGATAAAGTTCCTTTGTTTGTTTGGTCTGTATTAATTACAGCTATTTTATTACTATTATCTTTGCCTGTTTTAGCTGGGGCAATTACAATATTGTTAACAGATCGAAATTTTAATACTTCTTTCTTTGATCCTGCGGGAGGTGGGGATCCTATTTTATTTCAACATTTATTTTGATTTTTTGGTCACCCTGAAGTTTATATTTTAATTTTACCTGGTTTTGGTATTGTTTCTCATGTTATTAGAGGATCAGTAGGTAAGCGTGAGCCTTTTGGTAGATTGGGGATGATTTATGCTATAGTTGGAATTGGTGGGATAGGGTTTGTTGTATGAGCCCATCATATATTTTCTGTTGGAATGGATGTGGATACTCGGGCGTATTTTACTGCTGCTACTATAATTATTGCAGTTCCCACTGGAATTAGGGTATTTAGATGGATAGCTACTTTATATGGGTCTTATTTTAAATTGGAAGCTCCATTATTATGATGTGTGGGATTTGTGTTTTTATTTACTTTAGGCGGGGTTACAGGAGTAGTTTTAGCTAATTCTTCTTTAGATATTGTTTTACATGATACTTACTATGTGGTTGCTCATTTTCATTATGTGTTAAGTATAGGAGCTGTATTTGCTATTTTGGCTGGTATTACTTATTGATTTCCTTTGTTTTTTGGGGTAGTTCTGAATTCAAGGAATTCTAATTTACAATTTTTTATTATATTTATTGGAGTGAATTTAACTTTTTTTCCTCAACATTTTTTGGGGTTAAATGGTATACCACGTCGTTATTCTGATTATCCTGACGCTTTTATTTACTGAAATATAGTTTCTTCTTTAGGGTCTTTATTATCTTTATTAGGAATTTTATTTTTTATATTAATCATTTGAGATGGATTTATTTCAAAAAATTTAGGATTTTCGAATTATTATATATATTCTTCGTTGGAGTGAAATAATGGAGTTCCCCCATTAGATCATACATTTAATCAGTTAGGACAATTGAATATTTAATTT |
| COII mtDNA (nucleotides 1072-1609) |
| TTGCCAACTTGAGGTTCATTGTATTTTCAAAATAGTTCTTCTTTTGTTATGGAGCAGTTAATTTTTTTTCATGATTATACAATGGTAATTTTGATTATGATTATAGTTATTGTGGGGTATTTATTAGTGAATTCTTGTTATGAAAATTATTATAATCACATGTTAAATGAGGGTCAAGAGTTAGAGAGAATTTGGACTGTTCTTCCAGCTTTATTTTTGTTATTAATTGCTTTTCCTTCTTTACAATTGTTATATTTAATAGAGGAAATAGAATTTCCTGAATTAACTATTAAAATTTTAGGTCATCAGTGATATTGATCTTATGAGTATAGAGATATAGGTTTAGATTCGTTTGAGTCTTATATAATCAGAGGGGGGAGTGTACTTTTACGGCTTTTAGAGGTTGATAATAATTTAGTGATCCCTTATAATTCTATTACTCGTATAATTATTTCTAGAAGAGATGTTATTCATTCTTGAACTATTCCGTCTTTAGGTGTAAAAATAGATGCTATTCCAGGTCGATTAAACCAAATTT |
Family Archaeidae Koch & Berendt, 1854
http://species-id.net/wiki/Austrarchaea
Archaea nodosa Forster, 1956, by original designation.
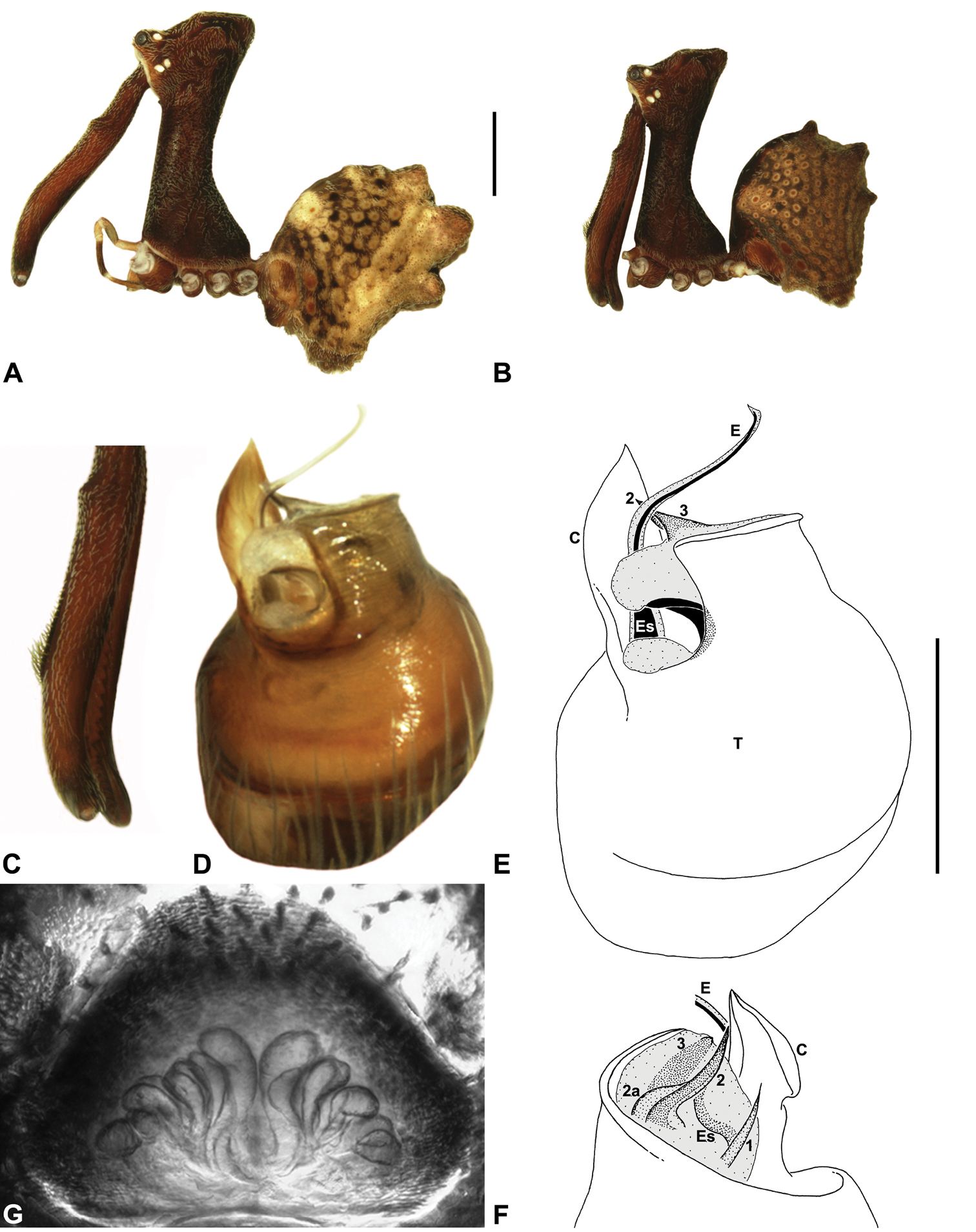
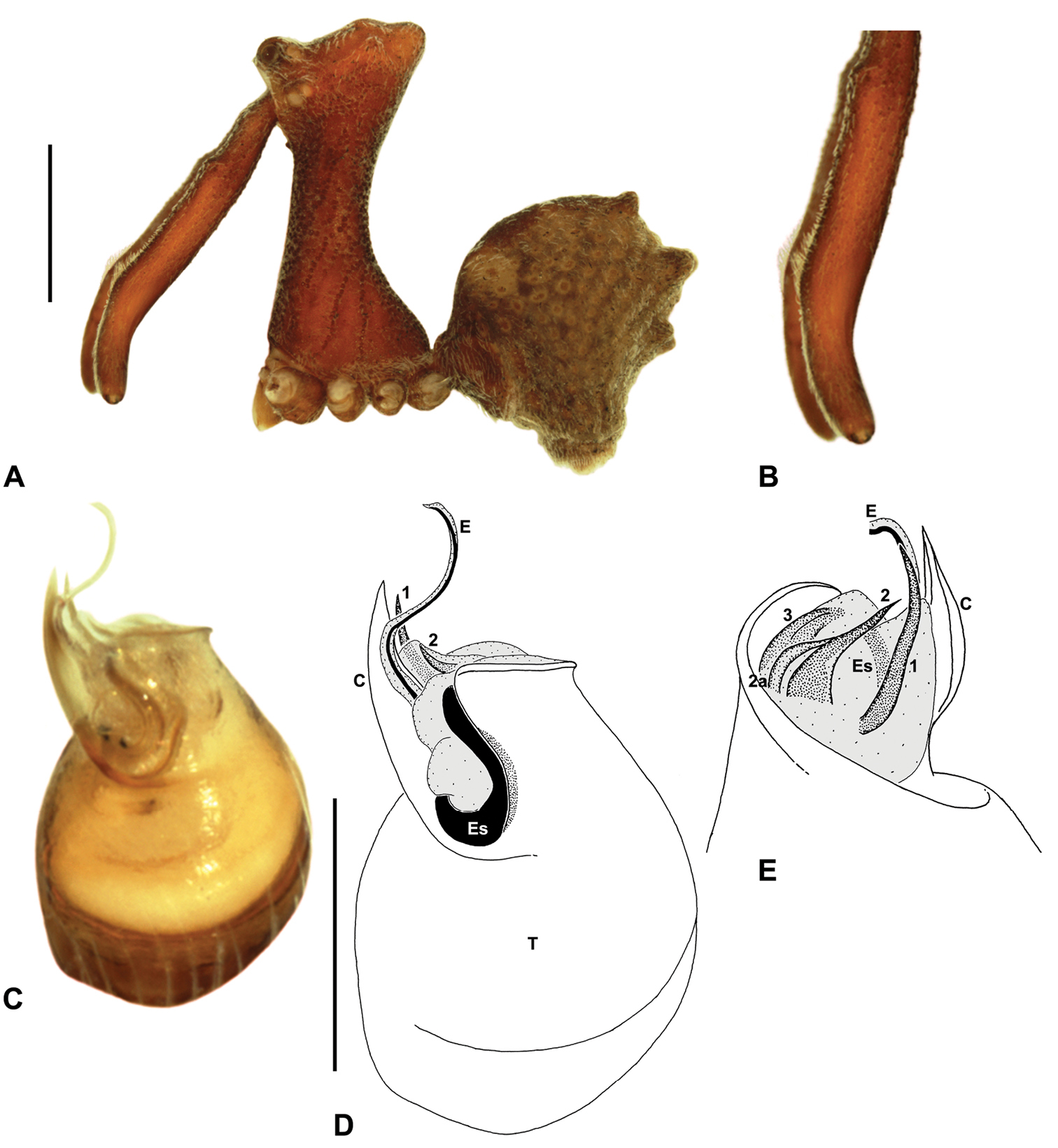
Species of Austrarchaea can be distinguished from all other extant Archaeidae (i.e. Malagasy and African species of Eriauchenius and Afrarchaea) by the presence of numerous, clustered spermathecae in females (Figs 5D, 10G, 14G) and by the presence of a long, wiry embolus on the pedipalp of males (Figs 10E, 15E, 27E) (
Small, haplogyne, araneomorph spiders; total length 2.5 to 5.0.
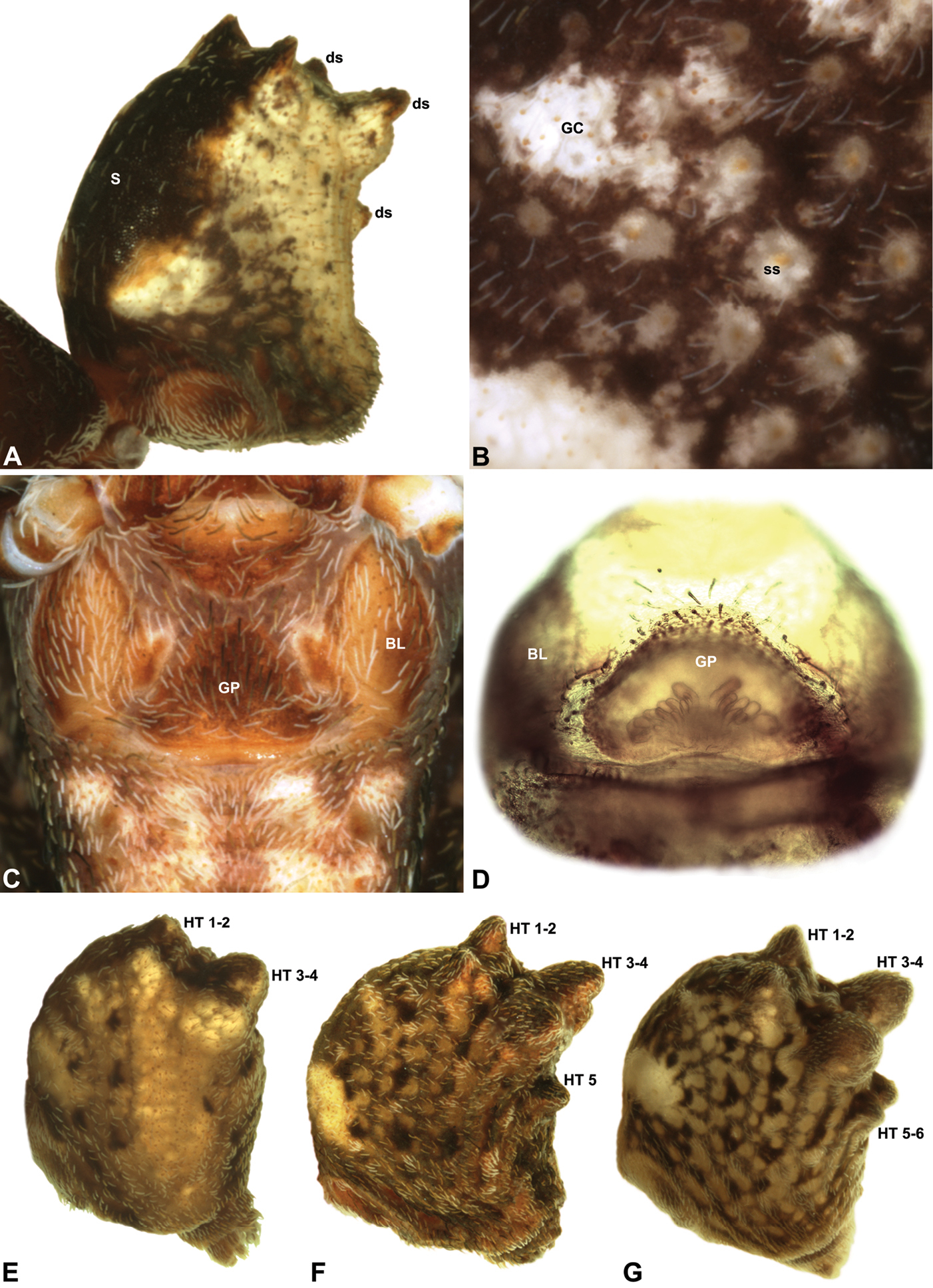
Colouration: Body colouration cryptic and relatively uniform across species, usually with only subtle intraspecific variation in abdominal patterning; carapace, sternum and chelicerae tan brown to dark reddish-brown, interspersed with darker regions of granulate cuticle (Fig. 5), covered in highly reflective setae; legs tan-brown to darker reddish-brown, with pattern of darker annulations on distal segments; abdomen mottled with beige and variable hues of grey-brown (Figs 5E-G), with darker sclerites, scutes and sclerotic spots (Figs 5A-B); paler beige markings due to reflective, subcuticular guanine crystals (Fig. 5B); antero-lateral face of abdomen always with large, humeral patch of reflective guanine crystals (Figs 5A, 5E-G).
Cephalothorax: Carapace greatly elevated anteriorly (CH/CL ratio usually 2.0–2.4; Fig. 6), with raised, highly modified pars cephalica forming ‘neck’ and bulbous ‘head’ (see
Legs and female pedipalp: Legs (longest to shortest) 1–4–2–3, covered with short plumose setae; spines absent; patella I long, greater than one-third length of femur I. Trichobothria present on tibiae and metatarsi of legs; tibiae I-IV each with two trichobothria; metatarsi I-IV each with single trichobothrium; bothrial bases with strongly ridged hood. Tarsi shorter than metatarsi, with capsulate tarsal organ and three claws; tarsi, metatarsi and distal tibiae of legs I-II usually with ventral and pro-ventral rows of moveable, spatulate setae. Female pedipalp with long, porrect trochanter and small tarsal claw; tibia with two dorsal trichobothria.
Abdomen: Abdomen arched anteriorly, rounded-subtriangular in lateral view, usually with four to six large hump-like tubercles on dorsal surface (Figs 5A, 5E-G); cuticle covered with short plumose setae and numerous sclerotic spots (Figs 5A-B). Epigastric region with sclerotised (setose) book lung covers and dorsal and ventral plates surrounding pedicel (Fig. 5C) (plates fused in males); dorsal pedicel plate with transverse ridges; females with median genital plate and sclerotised lateral sigillae (Figs 5C-D); males with broad dorsal scute fused anteriorly to epigastric sclerites, with or without additional paired sclerites associated with hump-like tubercles (Fig. 5A). Six spinnerets, surrounded by thickened cuticle; ALS largest, PMS smallest; colulus absent. Posterior pair of divided tracheal spiracles situated anterior to spinnerets; males also with transverse row of epiandrous gland spigots situated closely anterior to epigastric furrow.
Genitalia: Female genitalia haplogyne, with sclerotised, strongly arched genital plate anterior to epigastric furrow (Figs 5C-D); internally with gonopore leading to large, spherical membranous bursa (Fig. 17G; see also
As noted by
Assassin spiders occur in mesic habitats throughout south-eastern, south-western and north-eastern mainland Australia (Fig. 2), usually in montane rainforests (Figs 30C, 38C, 41C) and wet eucalypt forests (Figs 39C, 42C, 45C), but occasionally in temperate heathlands or lowland rainforests (Fig. 40C). In south-eastern Australia they occur on Kangaroo Island (South Australia) and along the Great Dividing Range, from Grampians National Park in south-western Victoria north to Kroombit Tops National Park in south-eastern Queensland. In south-western Western Australia they occur from the Leeuwin-Naturaliste National Park east to Cape Le Grand National Park, with outlying populations in the Porongurup and Stirling Range National Parks. In north-eastern Queensland archaeids occur along the Great Dividing Range, from Eungella National Park near Mackay north to the Mount Finnigan Uplands, near Cooktown. Although this distribution is markedly concordant with the distribution of closed and tall open forests in Australia’s east and extreme south-west (see Specht 1981), assassin spiders appear to be notably absent from Tasmania, from the Australian Alps and from the ‘St Lawrence Gap’ (Webb and Tracey 1981) (Fig. 2), as evidenced by the lack of museum specimens and despite targeted searches by the senior author.
Five described species – Austrarchaea daviesae Forster & Platnick, 1984, Austrarchaea hickmani (Butler, 1929), Austrarchaea mainae Platnick, 1991b, Austrarchaea nodosa (Forster, 1956) and Austrarchaea robinsi Harvey, 2002a – and the 17 new species from mid-eastern Australia: Austrarchaea alani sp. n., Austrarchaea aleenae sp. n., Austrarchaea binfordae sp. n., Austrarchaea christopheri sp. n., Austrarchaea clyneae sp. n., Austrarchaea cunninghami sp. n., Austrarchaea dianneae sp. n., Austrarchaea harmsi sp. n., Austrarchaea helenae sp. n., Austrarchaea judyae sp. n., Austrarchaea mascordi sp. n., Austrarchaea mcguiganae sp. n., Austrarchaea milledgei sp. n., Austrarchaea monteithi sp. n., Austrarchaea platnickorum sp. n., Austrarchaea raveni sp. n. and Austrarchaea smithae sp. n.
At least three clades of Archaeidae can be recognised in Australia (Fig. 3B; see also
| 1 | Abdomen with five dorsal hump-like tubercles (Fig. 5F) | Austrarchaea monteithi sp. n. |
| – | Abdomen with six dorsal hump-like tubercles, in three pairs (Fig. 5G) | 2 |
| 2 | Male chelicerae with dense tuft of accessory setae on anterior face of paturon (Figs 16C, 17C, 23C) | 3 |
| – | Male chelicerae with uniform brush (Figs 12C, 19C, 22C) or comb (Figs 14C, 18C) of accessory setae on anterior face of paturon | 5 |
| 3 | Tuft of accessory setae on anterior face of male paturon very strong, dorsally-directed, with ‘pick-like’ profile in lateral view (Fig. 16C) | Austrarchaea harmsi sp. n. |
| – | Tuft of accessory setae on anterior face of male paturon less pronounced, with shorter, densely-bunched profile in lateral view (Figs 17C, 23C) | 4 |
| 4 | Tegular sclerite 3 (TS 3) very large, porrect (Figs 17D-F); TS 2 thin, spiniform (Fig. 17F) | Austrarchaea aleenae sp. n. |
| – | Tegular sclerite 3 (TS 3) not enlarged, rounded-rectangular (Fig. 23E); TS 2 spur-like, not spiniform (Fig. 23E) | Austrarchaea milledgei sp. n. |
| 5 | Tegular sclerite 1 (TS 1) very long, rod-like, visible in retrolateral view, reaching to near distal apex of conductor, with broadly-rounded apex (Figs 20C-E) | Austrarchaea christopheri sp. n. |
| – | Tegular sclerite 1 (TS 1) usually relatively short, obscured by conductor in retrolateral view (Figs 18F, 25F); if TS 1 long, never with broadly-rounded apex (Figs 10F, 22F) | 6 |
| 6 | Highest point of male pars cephalica (HPC) near posterior margin of ‘head’ (with carapace sometimes almost horizontal anterior to HPC; Figs 8A, 8H), ratio of HPC to post-ocular length = 0.84 (Figs 8A, 8C-D, 8H, 9D) | 7 |
| – | Highest point of male pars cephalica (HPC) closer to middle of ‘head’, ratio of HPC to post-ocular length < 0.75 (Figs 8F-G, 8I, 9C, 9F-I) | 11 |
| 7 | Male chelicerae with short comb of accessory setae on anterior face of paturon (Figs 14C, 18C) | 8 |
| – | Male chelicerae with longer brush of accessory setae on anterior face of paturon (Figs 11C, 15C) | 9 |
| 8 | Conductor ‘ear-shaped’, with large proximal lobe (Figs 14D-F); tegular sclerite 3 (TS 3) triangular, with pointed apex (Fig. 14E) | Austrarchaea raveni sp. n. |
| – | Conductor foliate, obliquely-angled (Figs 18D-E); tegular sclerite 3 (TS 3) very large, porrect, with broadly-pointed rectangular apex (Figs 18D-F) | Austrarchaea alani sp. n. |
| 9 | Tegular sclerite 1 (TS 1) very long, spiniform, visible in retrolateral view, reaching to near distal apex of conductor, with sharply-pointed apex (Figs 22E-F) | Austrarchaea binfordae sp. n. |
| – | Tegular sclerite 1 (TS 1) relatively short, shorter than TS 2, obscured by conductor in retrolateral view (Figs 11F, 15F) | 10 |
| 10 | Conductor ‘spade-shaped’, with sharply-incised proximal margin (Figs 15D-F); male ‘head’ strongly elevated postero-dorsally, post-ocular ratio > 0.40 (Fig. 8C) | Austrarchaea judyae sp. n. |
| – | Conductor foliate, without sharply-incised proximal margin (Figs 11D-E); male ‘head’ not strongly elevated dorsally, post-ocular ratio < 0.30 (Fig. 8H) | Austrarchaea dianneae sp. n. |
| 11 | Tegular sclerite 1 (TS 1) very thin, filiform (Figs 10F, 24F) | 12 |
| – | Tegular sclerite 1 (TS 1) broader, spiniform or rod-like (Figs 12F, 13E, 21F, 25F) | 13 |
| 12 | Proximal portion of embolic sclerite very broad, flanged, overlying proximal conductor (Figs 10D-E). | Austrarchaea nodosa (Forster, 1956) |
| – | Proximal portion of embolic sclerite not flanged, fully-embraced by conductor (Figs 24D-E) | Austrarchaea mascordi sp. n. |
| 13 | Tegular sclerite 1 (TS 1) rod-like, without sharply-pointed apex (Fig. 27E) | Austrarchaea mcguiganae sp. n. |
| – | Tegular sclerite 1 (TS 1) usually spiniform, with sharply-pointed apex (Figs 12F, 13E, 21F, 25F, 26D) | 14 |
| 14 | Male ‘head’ strongly elevated dorsally, post-ocular ratio > 0.38 (Fig. 9G); highest point of pars cephalica (HPC) approaching posterior quarter of ‘head’, ratio of HPC to post-ocular length ~0.70 (Fig. 9G) | Austrarchaea smithae sp. n. |
| – | Male ‘head’ not strongly elevated dorsally, post-ocular ratio = 0.35 (Figs 8F-G, 9C, 9I); highest point of pars cephalica (HPC) near middle of ‘head’, ratio of HPC to post-ocular length < 0.65 (Figs 8F-G, 9C, 9I) | 15 |
| 15 | Male ‘head’ with concave depression near posterior margin (Fig. 8F) | Austrarchaea clyneae sp. n. |
| – | Male ‘head’ without concave depression near posterior margin (Figs 8G, 9C, 9I) | 16 |
| 16 | Tegular sclerite 1 (TS 1) spiniform, with long, gently-tapered apex (Figs 21F, 26D) | 17 |
| – | Tegular sclerite 1 (TS 1) relatively short, with rectangular base and sharply-tapered apex (Fig. 12F) | Austrarchaea cunninghami sp. n. |
| 17 | Tegular sclerite 1 (TS 1) with curled distal tip (Fig. 26D) | Austrarchaea helenae sp. n. |
| – | Tegular sclerite 1 (TS 1) straight, without curled distal tip (Fig. 21F) | Austrarchaea platnickorum sp. n. |
McPherson Range Assassin Spider
http://species-id.net/wiki/Austrarchaea_nodosa
Figs 1A-B, 5D, 7I, 8I, 10, 28Holotype juvenile: Lamington National Park, Tullawallal [Antarctic Beech forest], south of Binna Burra, Queensland, Australia, [28°12'20"S, 153°11'20"E], from moss, 31.X.1955, T. Woodward (QMB W1955).
AUSTRALIA: Queensland: Lamington National Park: Binna Burra, track to Tullawallal Antarctic Beech forest, 28°12'20"S, 153°11'20"E, sifting and teasing low vegetation, 7.IV.2006, M. & A. Rix, 1♂, 2 juveniles (WAM T89592DNA: LAM-51-J); Binna Burra, 11.II.1971, Y. Lubin, R. Raven, V. Davies, 1♀ (QMB S73925); Binna Burra, Ships Stern Circuit track, 28°11'51"S, 153°11'28"E, sifting elevated leaf litter, subtropical rainforest, 764 m, 25.IV.2010, M. & A. Rix, D. & S. Harms, J. Wojcieszek, 1 juvenile (WAM T112571DNA: Ar56-58-J); IBISCA Plot IQ-1100-A, 28°15'29"S, 153°09'32"E, bark spray, 1141 m, 11.III.2007, G. Thompson, A. Marcora, 1♂, 1♀, 1 juvenile (QMB S75416). New South Wales: Border Ranges National Park: Upper Brindle Creek, Wiangarie, 28°23'S, 153°06'E, pyrethrum, Nothofagus rainforest, 840 m, 15.XII.2008, G. Monteith, 1♀ (QMB S87983). Mount Warning National Park: 1975–1976, G. & S. Monteith, 1 juvenile (QMB S20426); Mount Warning, track to summit, 28°24'08"S, 153°16'27"E, sifting elevated leaf litter under Xanthorrhoea, wet eucalypt forest bordering subtropical rainforest, 728 m, 26.IV.2010, M. Rix, 1 juvenile (WAM T112572DNA: Ar57-46-J); off Mount Warning Road, 28°23'51"S, 153°17'20"E, sifting elevated leaf litter, subtropical rainforest, 348 m, 26.IV.2010, D. Harms, 1 juvenile (WAM T112573DNA: Ar58-53-J).
AUSTRALIA: Queensland: Lamington National Park: Mount Hobwee, in moss, 3.IV.1976, R. Raven, 1 juvenile (QMB S30827); Nagarigoon, 8.IV.1976, 1 juvenile (QMB S30817). Mount Chinghee National Park: QM Berlesate, stick brushing, 17.XII.1982, G. Monteith, D. Yeates, G. Thompson, 1 juvenile (QMB S30804). New South Wales: Border Ranges National Park: Border Fence, Levers Plateau, via Rathdowney, pitfall trap, 670 m, 22.V.–IX.1976, G. & S. Monteith, 1 juvenile (QMB S30823).
AUSTRALIA: Queensland: Lamington National Park: Tullawallal Antarctic Beech forest, south of Binna Burra, 28°12'39"S, 153°11'32"E, Nothofagus rainforest, 900 m, 21.III.2006, C. Griswold, D. Silva, R. Raven, B. Baehr, M. Ramírez, 1♂ (CASENT 9018966); Binna Burra, 27.III.1976, R. Raven, V. Davies, 1♀, 1 juvenile (QMB S30820); Binna Burra, 28°11'38"S, 153°11'13"E, rainforest, 790 m, 21-23.III.2006, C. Griswold, D. Silva, R. Raven, B. Baehr, M. Ramírez, 1 juvenile (CASENT 9018963); Binna Burra, 28°11'38"S, 153°11'13"E, Berlese of leaf litter, rainforest, 790 m, 23.III.2006, C. Griswold, D. Silva, R. Raven, B. Baehr, M. Ramírez, 1 juvenile (CASENT 9018964); Binna Burra, along Border Track, 28°11'56"S, 153°11'15"E, beating vegetation, 900 m, 29–30.IV.2009, H. Wood, 1♂ (CASENT 9028426); same data, 1♂ (CASENT 9028388); O'Reillys, 25-26.IX.1986, J. Gallon, R. Raven, 1♀ (QMB S30814).
Austrarchaea nodosa can be distinguished from all other Archaeidae from mid-eastern Australia by the broad, flanged proximal portion of the embolic sclerite (Figs 10D-E; see also
This species can also be distinguished from other genotyped taxa from mid-eastern Australia (see Fig. 3B) by the following seven unique nucleotide substitutions for COI (n = 4): A(42), C(393), C(639), C(939), A(960), A(1038), A(1053).
Male (QMB S30817): Total length 3.18; leg I femur 3.01; F1/CL ratio 2.70. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen mottled grey-brown and beige, with darker reddish-brown dorsal scute and sclerites (Fig. 10B). Carapace very tall (CH/CL ratio 2.30); 1.12 long, 2.56 high, 1.08 wide; ‘neck’ 0.56 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near middle of ‘head’ (ratio of HPC to post-ocular length 0.57), carapace with shallow concave depression posterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.24) (Fig. 8I). Chelicerae with short brush of accessory setae on anterior face of paturon (Fig. 10C). Abdomen 1.64 long, 1.13 wide; with three pairs of dorsal hump-like tubercles (HT 1–6); dorsal scute fused anteriorly to epigastric sclerites, extending posteriorly to first pair of hump-like tubercles; HT 3–6 each covered by separate dorsal sclerites. Unexpanded pedipalp (WAM T89592) (Figs 10D-F) with thin, pointed conductor, gently-tapered and slightly bent along distal half; embolic sclerite with broad, flanged proximal portion overlying proximal conductor; tegular sclerite 1 (TS 1) long, filiform, with sinuous distal tip, visible in retrolateral view; TS 2 spiniform, shorter than TS 1; TS 2a sinuous, largely obscured by TS 2; TS 3 indistinct, embedded within distal haematodocha, barely visible beyond retro-distal rim of tegulum.
Female (QMB S30817): Total length 3.54; leg I femur 3.01; F1/CL ratio 2.40. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen bi-coloured grey-brown and beige, palest posteriorly (Fig. 10A). Carapace tall (CH/CL ratio 2.12); 1.26 long, 2.67 high, 1.15 wide; ‘neck’ 0.64 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near posterior third of ‘head’ (ratio of HPC to post-ocular length 0.63), carapace with shallow concave depression posterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.23) (Fig. 7I). Chelicerae without accessory setae on anterior face of paturon. Abdomen 2.15 long, 1.64 wide; with three pairs of dorsal hump-like tubercles (HT 1–6). Internal genitalia with cluster of ≤ 12 variably shaped spermathecae on either side of gonopore, clusters meeting near midline of genital plate (Figs 5D, 10G); innermost (anterior) spermathecae longest, sausage-shaped, curved antero-laterally; outermost (posterior) spermathecae bulbous; other spermathecae variably pyriform, straight, directed antero-laterally.
Variation: Males (n=2): total length 2.97–3.18; carapace length 1.12–1.13; carapace height 2.56–2.67; CH/CL ratio 2.30–2.36. Females (n=3): total length 3.54–4.00; carapace length 1.21–1.33; carapace height 2.49–2.87; CH/CL ratio 2.06–2.15.
Austrarchaea nodosa is known from rainforest habitats along the McPherson Range and ‘scenic rim’ of extreme south-eastern Queensland and north-eastern New South Wales, in the Lamington, Border Ranges and Mount Warning National Parks (Fig. 28). At Binna Burra (Lamington National Park) it has been found in sympatry with Austrarchaea dianneae sp. n., in the only known example of two-species sympatry among Australian archaeids (see Nomenclatural Remarks, below).
This species has a relatively widespread distribution in several National Parks protected under World Heritage legislation, and is not considered to be of conservation concern.
The holotype specimen of Austrarchaea nodosa, described by
‘Tullawallal’ – the type locality cited by
Secondly, female specimens of both species possess a distinctive ‘head’ morphology; females of Austrarchaea nodosa (as here recognised) are characterised by a shallow concave depression posterior to the highest point of the pars cephalica (HPC) (Fig. 7I), whereas females of Austrarchaea dianneae sp. n. have no such depression and a significantly more pronounced posterior margin of the ‘head’ (Fig. 7H). The holotype juvenile specimen of Austrarchaea nodosa has a clear concave depression posterior to the HPC, and ‘head’ proportions otherwise very similar to the female illustrated in Figure 7I. In contrast, the only known penultimate female specimen of Austrarchaea dianneae sp. n., collected from near Binna Burra (WAM T112556), does not have a concave depression posterior to the HPC, and ‘head’ proportions otherwise similar to the allotype female Austrarchaea dianneae sp. n. illustrated in Figure 7H.
Clearly, given the identification of specimens collected from the type locality and similar nearby habitats, and the morphology of the holotype juvenile specimen, we are as confident as possible in newly-diagnosing Austrarchaea nodosa as the species described above, given an otherwise highly precarious nomenclatural situation.
Gold Coast Hinterland Assassin Spider
urn:lsid:zoobank.org:act:C0149F76-0A44-4DB4-8DAF-088B17161900
http://species-id.net/wiki/Austrarchaea_dianneae
Figs 7H, 8H, 11, 29Holotype male: Tamborine National Park, Joalah section, track to Curtis Falls, Queensland, Australia, 27°55'33"S, 153°11'35"E, sifting elevated leaf litter and hand collecting at night, subtropical rainforest, 313 m, 26.IV.2010, M. Rix, D. Harms (QMB S90185).
Paratypes: Allotype female, same data as holotype (QMB S90186); 2 males and 7 juveniles, same data as holotype (WAM T112557DNA: Ar59-60-M/Ar59-61-J/Ar59-62-J).
AUSTRALIA: Queensland: Lamington National Park: Binna Burra, Ships Stern Circuit track, 28°11'51"S, 153°11'28"E, sifting elevated leaf litter, subtropical rainforest, 764 m, 25.IV.2010, M. & A. Rix, D. & S. Harms, J. Wojcieszek, 2♂, 4 juveniles (WAM T112556DNA: Ar56-54-M/Ar56-55-J/ Ar56-56-J); Wojigumal Creek, 28°12'29"S, 153°11'56"E, pyrethrum, 570 m, 19.III.2008, A. Nakamura, 1♀ (QMB S87980).
AUSTRALIA: Queensland: Lamington National Park: IBISCA Plot IQ-300-C, 28°09'04"S, 153°08'17"E, pitfall trap, 260 m, 23.I.2007, K. Staunton, 1 juvenile (QMB S90181).
The specific epithet is a patronym in honour of the late Dianne Wojcieszek (1962–2003), for her love of the Mount Tamborine Hinterland.
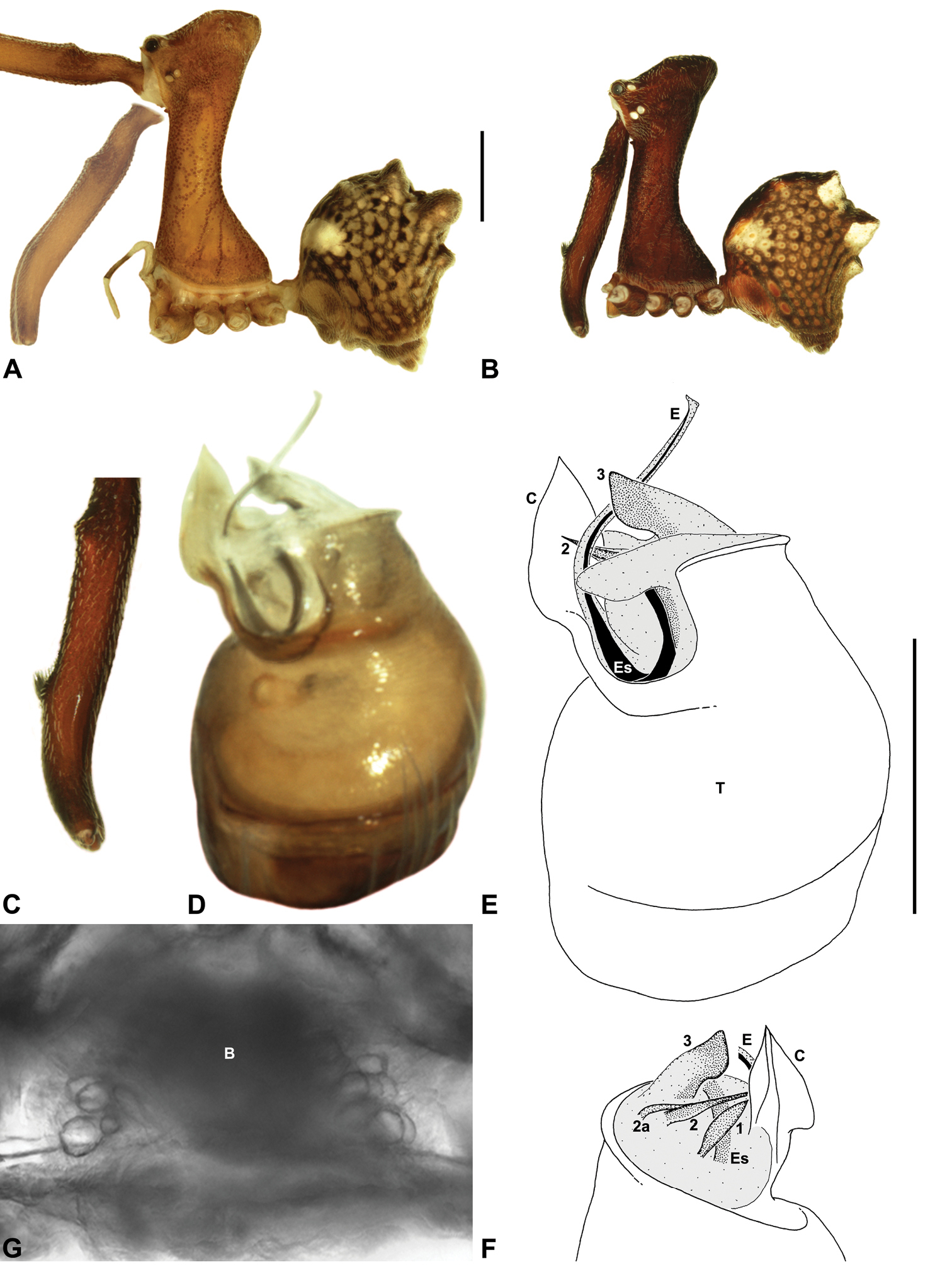
Austrarchaea dianneae can be distinguished from all other Archaeidae from mid-eastern Australia except Austrarchaea cunninghami sp. n. by the shape of the conductor (Figs 11D-E), which is broad, foliate and curved laterally, with a triangular apex; and from Austrarchaea cunninghami sp. n. by the longer, spiniform tegular sclerite 1 (TS 1) (Fig. 11F) and by the more conical, posteriorly elevated shape of the male ‘head’ (Fig. 8H).
This species can also be distinguished from other genotyped taxa from mid-eastern Australia (see Fig. 3B) by the following three unique nucleotide substitutions for COI (n = 6): T(303), G(798), A(1065).
Holotype male: Total length 3.03; leg I femur 3.12; F1/CL ratio 2.83. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen mottled grey-brown and beige, with darker reddish-brown dorsal scute and sclerites (Fig. 11B). Carapace very tall (CH/CL ratio 2.37); 1.10 long, 2.62 high, 1.02 wide; ‘neck’ 0.51 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near posterior margin of ‘head’ (ratio of HPC to post-ocular length 0.87), carapace gently sloping and almost horizontal anterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.29) (Fig. 8H). Chelicerae with brush of accessory setae on anterior face of paturon (Fig. 11C). Abdomen 1.69 long, 1.33 wide; with three pairs of dorsal hump-like tubercles (HT 1–6); dorsal scute fused anteriorly to epigastric sclerites, extending posteriorly to first pair of hump-like tubercles; HT 3–6 each covered by separate dorsal sclerites. Unexpanded pedipalp (Figs 11D-F) with broad, foliate conductor, curved laterally with triangular apex; tegular sclerite 1 (TS 1) spiniform, obscured by conductor in retrolateral view; TS 2 spur-like, longer than TS 1; TS 2a sinuous, largely obscured by TS 2; TS 3 embedded proximally within distal haematodocha, with sharply-pointed, triangular apex projecting beyond retro-distal rim of tegulum.
Allotype female: Total length 3.74; leg I femur 3.18; F1/CL ratio 2.43. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen mottled grey-brown and beige, palest posteriorly (Fig. 11A). Carapace very tall (CH/CL ratio 2.28); 1.31 long, 2.97 high, 1.21 wide; ‘neck’ 0.65 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near middle of ‘head’ (ratio of HPC to post-ocular length 0.55), carapace gently sloping posterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.25) (Fig. 7H). Chelicerae without accessory setae on anterior face of paturon. Abdomen 2.15 long, 1.64 wide; with three pairs of dorsal hump-like tubercles (HT 1–6). Internal genitalia with cluster of ≤ 12 variably shaped spermathecae on either side of gonopore, clusters meeting near midline of genital plate (Fig. 11G); innermost (anterior) spermathecae longest, sausage-shaped, curved antero-laterally; other spermathecae variably pyriform, curved, directed laterally.
Variation: Males (n=5): total length 2.73–3.21; carapace length 1.09–1.13; carapace height 2.53–2.62; CH/CL ratio 2.24–2.39. Females (n=2): total length 3.64–3.74; carapace length 1.31 (invariable); carapace height 2.87–2.97; CH/CL ratio 2.20–2.28.
Austrarchaea dianneae is known only from subtropical rainforest habitats in the Tamborine and Lamington National Parks south of Brisbane, south-eastern Queensland (Fig. 29). At Binna Burra (Lamington National Park) it has been found in sympatry with Austrarchaea nodosa, in the only known example of two-species sympatry among Australian archaeids (see Nomenclatural Remarks for Austrarchaea nodosa, above).
This species is a short-range endemic taxon (
Main Range Assassin Spider
urn:lsid:zoobank.org:act:EFE94CB8-B85A-4573-B181-E6279995D9B2
http://species-id.net/wiki/Austrarchaea_cunninghami
Figs 7G, 8G, 12, 30Holotype male: Main Range National Park, Cunningham's Gap, track to Mount Mitchell, Queensland, Australia, 28°03'05"S, 152°23'41"E, sifting elevated leaf litter, subtropical rainforest and adjacent transitional eucalypt forest, 805 m, 23.IV.2010, M. Rix, D. Harms (QMB S90184).
Paratypes: Allotype female, same data as holotype (QMB S90183); 1 female and 14 juveniles, same data as holotype (WAM T112555DNA: Ar55-89-F/Ar55-90-J/Ar55-91-J).
AUSTRALIA: Queensland: Main Range National Park: Mount Mitchell, pitfall, 1060 m, 1.III.1992, D. Cook, 1 juvenile (QMB S25714).
AUSTRALIA: Queensland: Main Range National Park: Mount Superbus, summit, pyrethrum, trees and logs, 1300 m, 8-9.II.1990, G. Monteith, G. Thompson, H. Janetski, 2 juveniles (QMB S38509); Mount Asplenium, pyrethrum, trees and logs, 1290 m, 30.I.1993, G. Monteith, 1 juvenile (QMB S90179).
The specific epithet is a patronym in honour of British botanist and explorer Allan Cunningham (1791–1839), after whom the type locality of this species – Cunningham’s Gap in the Main Range National Park – is named.
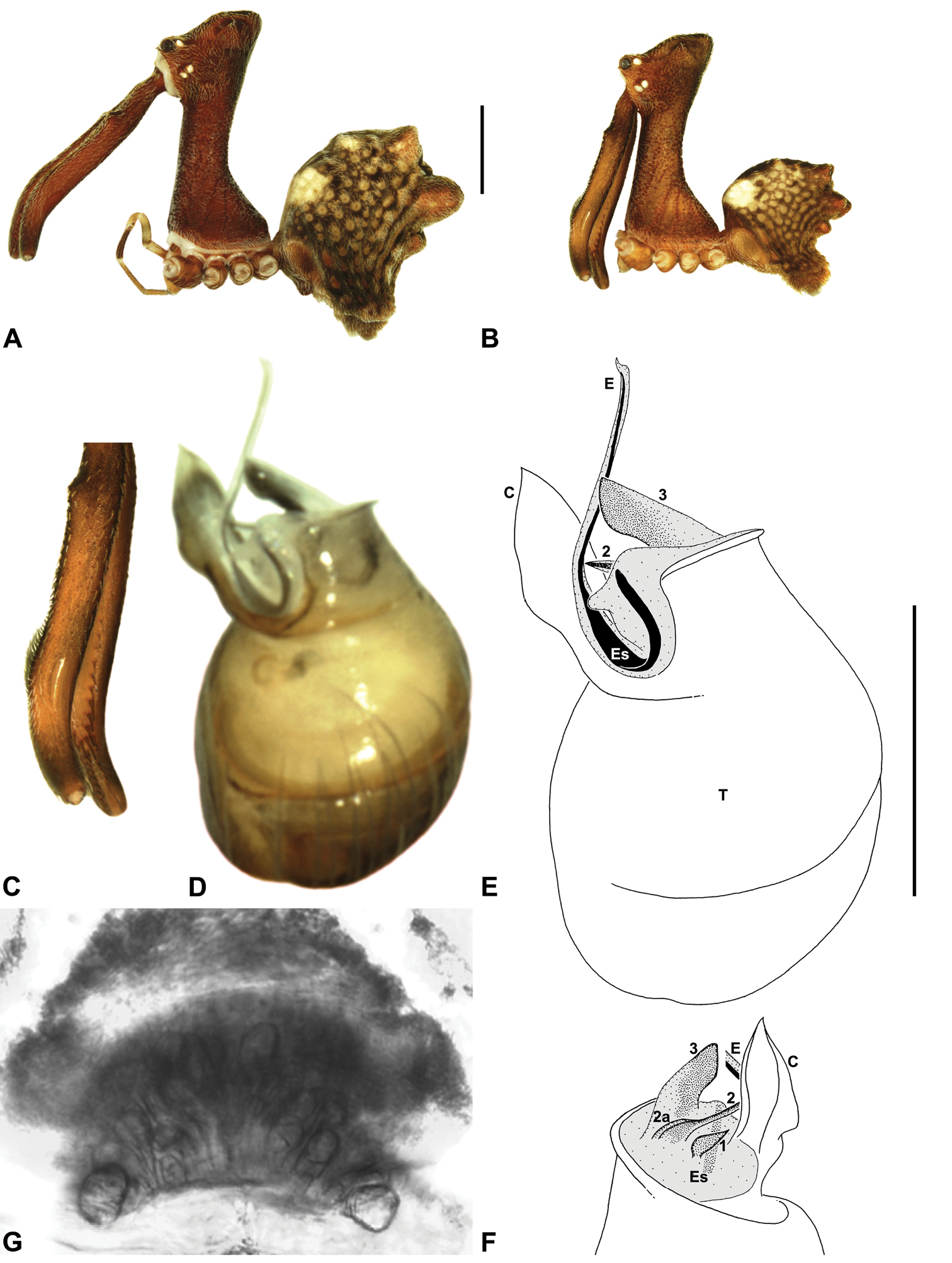
Austrarchaea cunninghami can be distinguished from all other Archaeidae from mid-eastern Australia except Austrarchaea dianneae by the shape of the conductor (Figs 12D-E), which is broad, foliate and curved laterally, with a triangular apex; and from Austrarchaea dianneae by the shorter, sharply-tapered tegular sclerite 1 (TS 1) (Fig. 12F) and by the more rounded, less conical shape of the male ‘head’ (Fig. 8G).
This species can also be distinguished from other genotyped taxa from mid-eastern Australia (see Fig. 3B) by the following four unique nucleotide substitutions for COI and COII (n = 3): C(769), C(981), C(1140), G(1152).
Holotype male: Total length 2.82; leg I femur 3.01; F1/CL ratio 2.70. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen mottled grey-brown and beige, with darker brown dorsal scute and sclerites (Fig. 12B). Carapace very tall (CH/CL ratio 2.21); 1.12 long, 2.46 high, 1.05 wide; ‘neck’ 0.56 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near middle of ‘head’ (ratio of HPC to post-ocular length 0.60), carapace gently sloping posterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.27) (Fig. 8G). Chelicerae with brush of accessory setae on anterior face of paturon (Fig. 12C). Abdomen 1.46 long, 0.97 wide; with three pairs of dorsal hump-like tubercles (HT 1–6); dorsal scute fused anteriorly to epigastric sclerites, extending posteriorly to first pair of hump-like tubercles; HT 3–6 each covered by separate dorsal sclerites. Unexpanded pedipalp (Figs 12D-F) with broad, foliate conductor, strongly curved laterally with triangular, evenly-tapered apex; tegular sclerite 1 (TS 1) relatively short, with rectangular base and sharply-tapered apex, obscured by conductor in retrolateral view; TS 2 spur-like, longer than TS 1; TS 2a sinuous, filiform, exposed distally; TS 3 embedded proximally within distal haematodocha, with sharply-pointed apex projecting beyond retro-distal rim of tegulum.
Allotype female: Total length 3.54; leg I femur 3.24; F1/CL ratio 2.30. Cephalothorax brown; legs tan-brown with darker annulations; abdomen mottled grey-brown and beige (Fig. 12A). Carapace tall (CH/CL ratio 2.20); 1.41 long, 3.10 high, 1.28 wide; ‘neck’ 0.76 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near middle of ‘head’ (ratio of HPC to post-ocular length 0.57), carapace gently sloping posterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.23) (Fig. 7G). Chelicerae without accessory setae on anterior face of paturon. Abdomen 1.90 long, 1.41 wide; with three pairs of dorsal hump-like tubercles (HT 1–6). Internal genitalia with cluster of ≤ 10 variably shaped spermathecae on either side of gonopore, clusters marginally separated near midline of genital plate (Fig. 12G); innermost (anterior) spermathecae longest, sausage-shaped, bent laterally; other spermathecae variably pyriform, curved, directed laterally.
Variation: Females (n=2): total length 3.44–3.54; carapace length 1.38–1.41; carapace height 2.97–3.10; CH/CL ratio 2.15–2.20.
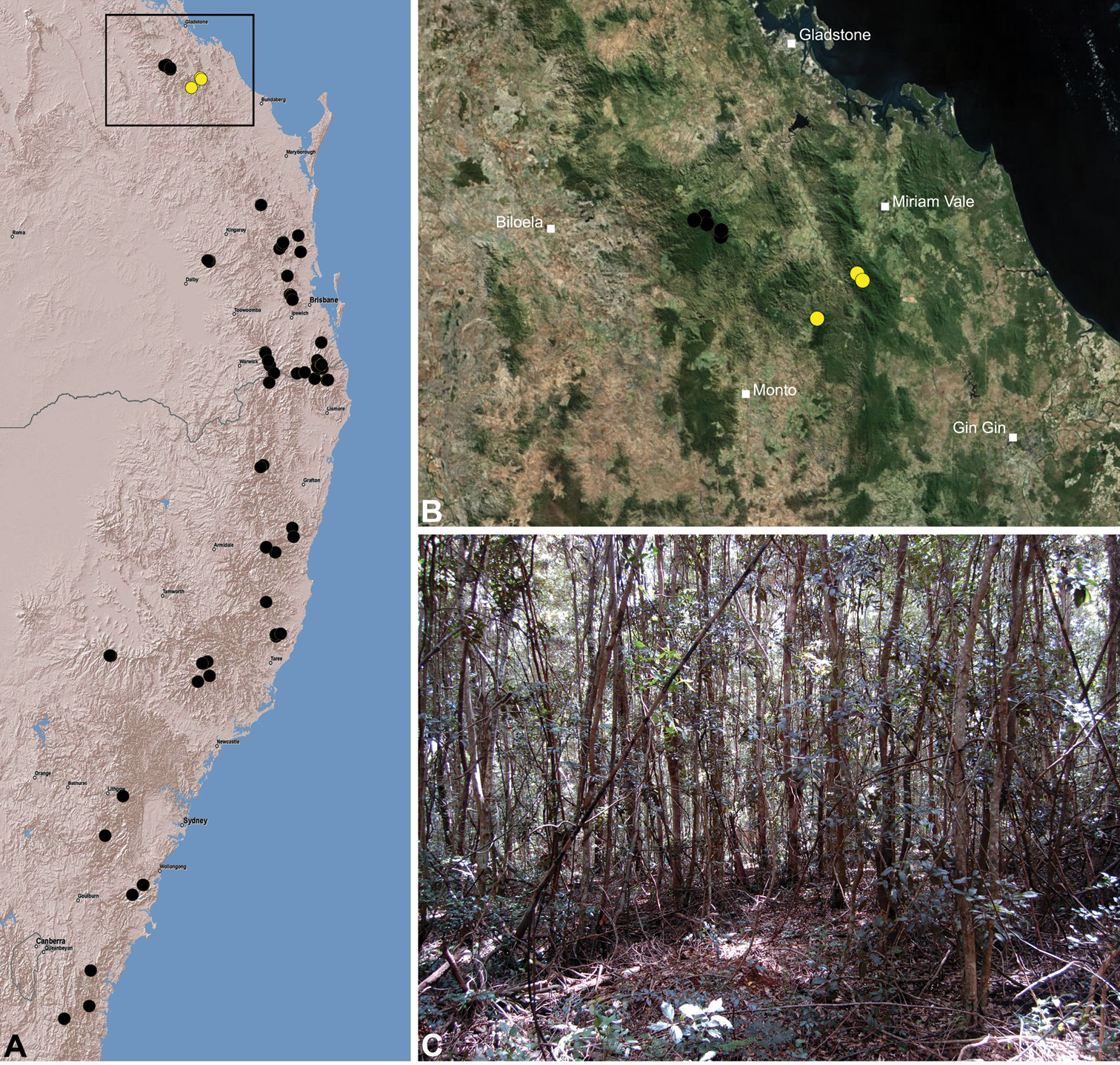
Austrarchaea cunninghami is known only from rainforest habitats in the Main Range National Park of extreme south-eastern Queensland (Fig. 30).
This species is a short-range endemic taxon (
Mount Clunie Assassin Spider
urn:lsid:zoobank.org:act:3F559C8C-005F-442A-84D1-0676D6EB56A6
http://species-id.net/wiki/Austrarchaea_clyneae
Figs 8F, 13, 31Holotype male: Mount Clunie National Park, Mount Clunie, via Woodenbong, Queensland, Australia, 1975–1976, G. & S. Monteith (QMB S20425).
AUSTRALIA: New South Wales: Mount Clunie National Park: Mount Clunie, via Woodenbong, pitfall trap, 670 m, 8.V.–15.VIII.1976, G. & S. Monteith, 2 juveniles (QMB S69811).
AUSTRALIA: New South Wales: Tooloom National Park: “Beaury State Forest", north along Wallaby Creek, 28°26'S, 152°27'E, 830 m, 9.IV.1993, M. Gray, G. Cassis, 1 juvenile (AMS KS37854).
The specific epithet is a patronym in honour of Australian naturalist, zoologist, conservationist, author, wildlife photographer and documentary film-maker Densey Clyne, for her landmark contributions to Australian natural history, and for having such a profound impact on the senior author during his formative childhood years.
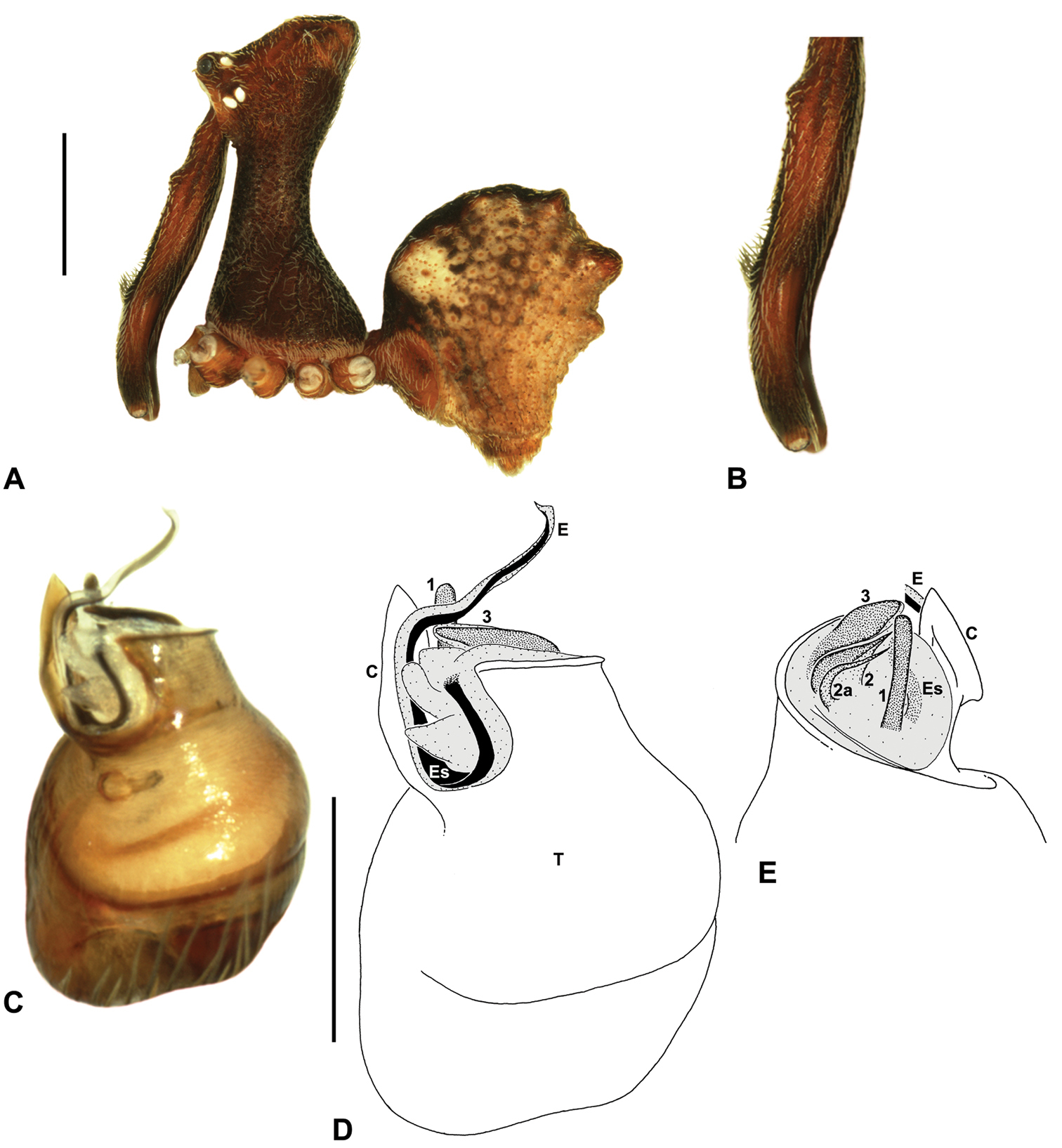
Austrarchaea clyneae can be distinguished from all other Archaeidae from mid-eastern Australia by the very long, spiniform tegular sclerite 1 (TS 1) (Fig. 13E) combined with the unique shape of the conductor (Figs 13C-D), which is thin, gently-curved laterally and pointed distally.
Holotype male: Total length 2.87; leg I femur 2.72; F1/CL ratio 2.62. Cephalothorax reddish-brown; legs tan-brown with darker annulations; abdomen mottled grey-brown and dark beige, with darker reddish-brown dorsal scute and sclerites (Fig. 13A). Carapace very tall (CH/CL ratio 2.22); 1.04 long, 2.31 high, 0.99 wide; ‘neck’ 0.51 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near posterior third of ‘head’ (ratio of HPC to post-ocular length 0.63), carapace with concave depression posterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.23) (Fig. 8F). Chelicerae with short brush of accessory setae on anterior face of paturon (Fig. 13B). Abdomen 1.54 long, 1.18 wide; with three pairs of dorsal hump-like tubercles (HT 1–6); dorsal scute fused anteriorly to epigastric sclerites, extending posteriorly to first pair of hump-like tubercles; HT 3–6 each covered by separate dorsal sclerites. Unexpanded pedipalp (Figs 13C-E) with thin, gently-curved, pointed conductor; tegular sclerite 1 (TS 1) very long, spiniform, reaching to near distal tip of conductor, visible in retrolateral view; TS 2 spur-like, shorter than TS 1; TS 2a sinuous, largely obscured by TS 2; TS 3 indistinct, embedded within distal haematodocha, barely visible beyond retro-distal rim of tegulum.
Female: Unknown.
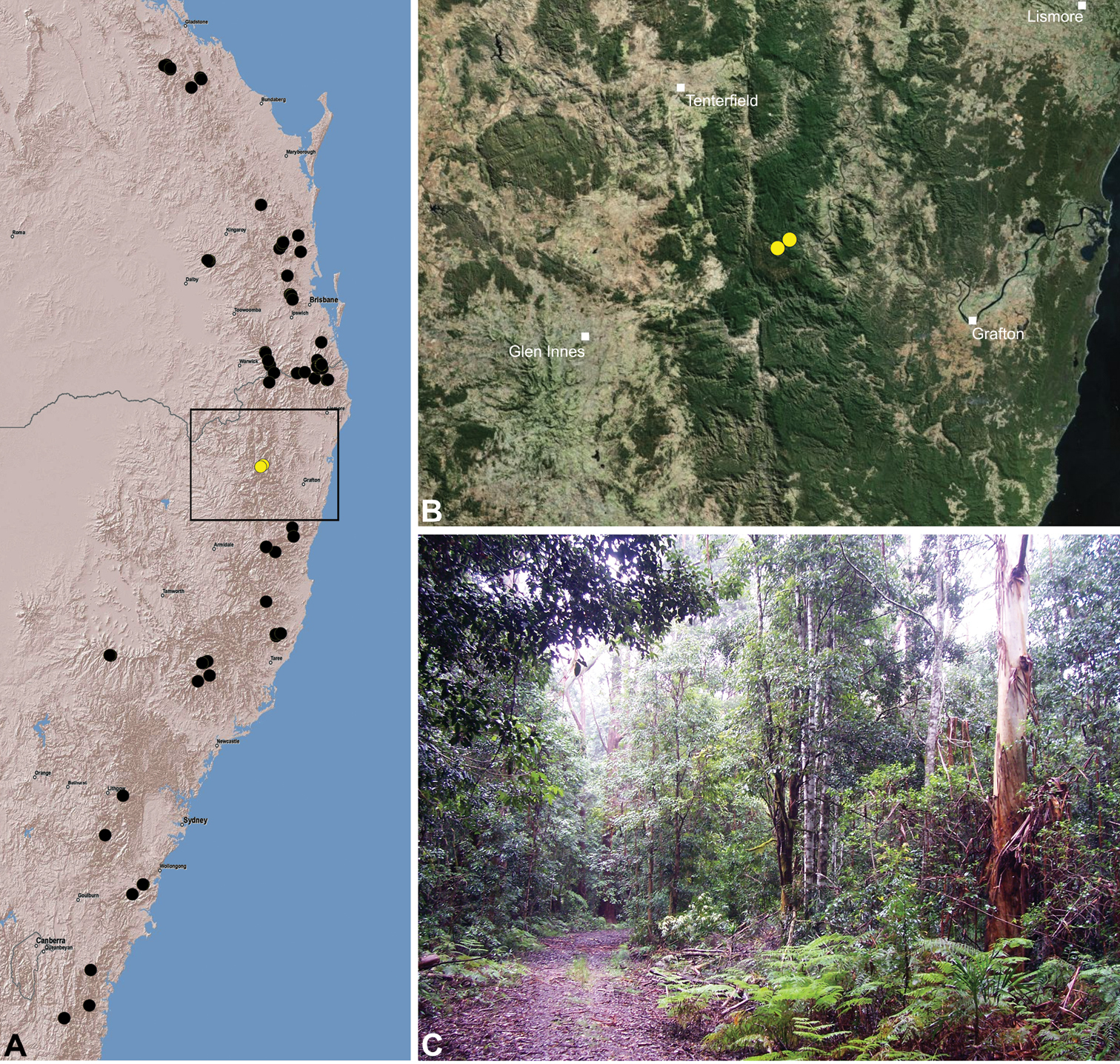
Austrarchaea clyneae is known only from rainforest habitats in the Mount Clunie National Park of extreme north-eastern New South Wales (Fig. 31). A juvenile specimen from Tooloom National Park (near Urbenville) may also belong to this species based on proximity.
This species appears to be a short-range endemic taxon (
D’Aguilar Range Assassin Spider
urn:lsid:zoobank.org:act:8EFF87A1-A86D-452C-8F69-0A4C5191CACA
Figs 1E-F, 7D, 8D, 14, 32Holotype male: Mount Nebo, D'Aguilar Range, Queensland, Australia, 15.VIII.1990, [inside hollow log], M. Harvey, T. Churchill (QMB S90193).
Paratype: Allotype female, D'Aguilar National Park, Mount Glorious, Maiala section, track to Greene's Falls, Queensland, Australia, 27°19'57"S, 152°45'47"E, sifting elevated leaf litter, subtropical rainforest, 633 m, 4.V.2010, M. Rix, D. Harms (QMB S90192DNA: Ar73-83-F).
AUSTRALIA: Queensland: D'Aguilar National Park: Mount Glorious, Maiala section, track to Greene's Falls, 27°19'30"S, 152°45'45"E, hand collected at night from maternal web at base of fallen log, 3.III.2001, M. & A. Rix, 1♀, 1 juvenile (WAM T94092); Mount Glorious, Maiala section, track to Greene's Falls, 27°19'57"S, 152°45'47"E, sifting elevated leaf litter, subtropical rainforest, 633 m, 4.V.2010, M. Rix, D. Harms, 6 juveniles (WAM T112574DNA: Ar73-84-J/Ar73-85-J); Mount Glorious, Maiala section, ANIC Berlesate, ~635 m, 13.III.1973, R. Taylor, 1 juvenile (ANIC). Mount Glorious: “Mount Glorious", spraying logs with Mortein, 26.IV.1988, P. Blus, V. Davies, 1 juvenile (QMB S30810); Hiller Family's Property, 20.I.– 26.VI.1978, G. Monteith, 1 juvenile (QMB S30807). Mount Mee Forest Reserve: The Mill Rainforest Walk, 27°04'57"S, 152°42'39"E, sifting elevated leaf litter, subtropical rainforest, 271 m, 1.V.2010, M. Rix, D. Harms, 1♂, 3 juveniles (WAM T112575DNA: Ar69-86-M/Ar69-87-J/Ar69-88-J).
AUSTRALIA: Queensland: Brisbane Forest Park: Mount Glorious, Lawton Road section of Westside Track, NW. of Maiala, 27°19'07"S, 152°44'50"E, beating ferns ~20 cm from ground, rainforest, 1.I.2010, G. Anderson, 1 juvenile (QMB).
The specific epithet is a patronym in honour of Dr Robert Raven, for his extraordinary contributions to arachnology, and for his ongoing efforts documenting the diverse spider fauna of south-eastern Queensland.
Austrarchaea raveni can be distinguished from all other Archaeidae from mid-eastern Australia by the very short, barely differentiated comb of accessory setae on the male chelicerae (Fig. 14C) combined with the unique shape of the conductor (Figs 14D-E), which is ‘ear-shaped’ with a large proximal lobe.
This species can also be distinguished from other genotyped taxa from mid-eastern Australia (see Fig. 3B) by the following three unique nucleotide substitutions for COI and COII (n = 6): G(9), G(843), T(1408).
Holotype male: Total length 2.90; leg I femur 3.10; F1/CL ratio 2.88. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen mottled grey-brown and beige, with darker reddish-brown dorsal scute and sclerites (Fig. 14B). Carapace very tall (CH/CL ratio 2.41); 1.08 long, 2.59 high, 0.98 wide; ‘neck’ 0.51 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near posterior margin of ‘head’ (ratio of HPC to post-ocular length 0.90), carapace slightly concave anterior to HPC; ‘head’ moderately elevated postero-dorsally (post-ocular ratio 0.35) (Fig. 8D). Chelicerae with short, barely differentiated comb of accessory setae on anterior face of paturon (Fig. 14C). Abdomen 1.59 long, 1.18 wide; with three pairs of dorsal hump-like tubercles (HT 1–6); dorsal scute fused anteriorly to epigastric sclerites, extending posteriorly to first pair of hump-like tubercles; HT 3–6 each covered by separate dorsal sclerites. Partially expanded pedipalp (Figs 14D-F) with lobed, ‘ear-shaped’ conductor; tegular sclerite 1 (TS 1) spiniform, obscured by conductor in retrolateral view; TS 2 spiniform, longer than TS 1, directed across proximal lobe of conductor; TS 2a sinuous, largely obscured by TS 2; TS 3 embedded proximally within distal haematodocha, with sharply-pointed, broadly triangular apex directed retro-ventrally across conductor.
Allotype female: Total length 3.05; leg I femur 3.14; F1/CL ratio 2.58. Cephalothorax brown; legs pale tan-brown with darker annulations; abdomen mottled grey-brown and beige, palest posteriorly (Fig. 14A). Carapace very tall (CH/CL ratio 2.34); 1.22 long, 2.85 high, 1.10 wide; ‘neck’ 0.60 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near posterior margin of ‘head’ (ratio of HPC to post-ocular length 0.83), carapace gently sloping anterior to HPC; ‘head’ moderately elevated postero-dorsally (post-ocular ratio 0.33) (Fig. 7D). Chelicerae without accessory setae on anterior face of paturon. Abdomen 1.59 long, 1.03 wide; with three pairs of dorsal hump-like tubercles (HT 1–6). Internal genitalia with dense cluster of ≤ 15 variably shaped spermathecae on either side of gonopore, clusters meeting near midline of genital plate (Fig. 14G); innermost (anterior) spermathecae sausage-shaped, curved antero-laterally; other spermathecae variably aciniform, straight, directed antero-laterally.
Variation: Males (n=2): total length 2.64–2.90; carapace length 1.06–1.08; carapace height 2.46–2.59; CH/CL ratio 2.31–2.41. Females (n=2): total length 3.05–3.46; carapace length 1.22–1.30; carapace height 2.85–3.10; CH/CL ratio 2.34–2.38.
Austrarchaea raveni is known only from rainforest habitats at Mount Glorious, Mount Nebo and Mount Mee, on the D’Aguilar Range north-west of Brisbane, south-eastern Queensland (Fig. 32).
This species is a short-range endemic taxon (
Sunshine Hinterland Assassin Spider
urn:lsid:zoobank.org:act:190BA8C6-1373-45DB-AA36-813FC8DBCA59
http://species-id.net/wiki/Austrarchaea_judyae
Figs 4F-G, 5A-C, 7C, 8C, 15, 33Holotype male: Conondale National Park, walking trail from Booloumba Creek Day Use Area No. 2, Queensland, Australia, 26°38'38"S, 152°38'50"E, sifting elevated leaf litter, subtropical rainforest, 187 m, 30.IV.2010, M. Rix, D. Harms (QMB S90190).
Paratypes: Allotype female, same data as holotype (QMB S90191); 2 females and 2 juveniles, same data as holotype (WAM T112563DNA: Ar67-76-F/Ar67-78-J).
AUSTRALIA: Queensland: Conondale National Park: off Booloumba Creek Forest Drive, 26°40'45"S, 152°38'06"E, sifting elevated leaf litter, subtropical rainforest, 351 m, 30.IV.2010, M. Rix, D. Harms, 1 juvenile (WAM T112562DNA: Ar66-79-J); Booloumba Creek, leaf litter, 13-18.IV.1976, R. Raven, 1 juvenile (QMB S30822); “Conondale Range", rainforest, 1-3.V.1976, R. Raven, 1 juvenile (QMB S29324); “Conondale National Park", 26°43'30"S, 152°36'00"E, canopy fogging, 26.I.1998, R. Kitching, 1 juvenile (ANIC). Maleny: 7 km SE. of Maleny, rainforest, 900 m, 18.VI.–15.VIII.1982, S. & J. Peck, 2♂ (ANIC). Mapleton Forest Reserve: Bonyee Walk, off Mapleton Forest Drive, 26°33'28"S, 152°51'58"E, sifting elevated leaf litter, subtropical rainforest, 175 m, 1.V.2010, M. Rix, D. Harms, 1♂, 8 juveniles (WAM T112564DNA: Ar68-80-M/Ar68-81-J/Ar68-82-J).
AUSTRALIA: Queensland: Oakview State Forest: “summit”, 26°10’S, 152°20’E, pyrethrum on trees, rainforest, 600 m, 26.V.2002, G. Monteith, 1 juvenile (QMB S90180).
The specific epithet is a patronym in honour of Judy Rix, for her love of the Sunshine Coast hinterland, and for a lifetime of generosity and support to the senior author.
Austrarchaea judyae can be distinguished from all other Archaeidae from mid-eastern Australia by the small body size of males and females (Fig. 6) and by the unique shape of the conductor (Figs 15D-E), which is ‘spade-shaped’ and laterally incised.
This species cannot be distinguished from other genotyped taxa from mid-eastern Australia on the basis of unique nucleotide substitutions, but can be distinguished from all other genotyped taxa from south-eastern Queensland (see Fig. 3B) by the following three nucleotide substitutions for COI and COII (n = 6): G(1010), A(1413), T(1560).
Holotype male: Total length 2.44; leg I femur 2.69; F1/CL ratio 2.92. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen mottled grey-brown and beige, palest posteriorly, with darker reddish-brown dorsal scute and sclerites (Fig. 15B). Carapace very tall (CH/CL ratio 2.38); 0.92 long, 2.19 high, 0.85 wide; ‘neck’ 0.42 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near posterior margin of ‘head’ (ratio of HPC to post-ocular length 0.89), carapace slightly concave anterior to HPC; ‘head’ strongly elevated postero-dorsally (post-ocular ratio 0.43) (Fig. 8C). Chelicerae with short brush of accessory setae on anterior face of paturon (Figs 4F, 15C). Abdomen 1.28 long, 0.97 wide; with three pairs of dorsal hump-like tubercles (HT 1–6); dorsal scute fused anteriorly to epigastric sclerites, extending posteriorly to first pair of hump-like tubercles; HT 3–6 each covered by separate dorsal sclerites (Fig. 5A). Unexpanded pedipalp (Figs 15D-F) with laterally incised, ‘spade-shaped’ conductor; tegular sclerite 1 (TS 1) spiniform, obscured by conductor in retrolateral view; TS 2 spiniform, longer than TS 1; TS 2a sinuous, largely obscured by TS 2; TS 3 embedded proximally within distal haematodocha, with broadly-pointed apex projecting beyond retro-distal rim of tegulum.
Allotype female: Total length 3.08; leg I femur 2.97; F1/CL ratio 2.70. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen mottled grey-brown and beige, palest behind hump-like tubercles (Fig. 15A). Carapace very tall (CH/CL ratio 2.41); 1.10 long, 2.65 high, 0.97 wide; ‘neck’ 0.54 wide; bearing two pairs of rudimentary horns; dual highest points of pars cephalica (HPC1–2) near posterior third of ‘head’(ratio of HPC1 to post-ocular length 0.68) and near posterior margin of ‘head’ (ratio of HPC2 to post-ocular length 0.88), carapace slightly concave between HPC1 and HPC2; ‘head’ strongly elevated postero-dorsally (post-ocular ratio 0.46) (Fig. 7C). Chelicerae without accessory setae on anterior face of paturon. Abdomen 1.90 long, 1.54 wide; with three pairs of dorsal hump-like tubercles (HT 1–6). Internal genitalia with dense cluster of ≤ 15 variably shaped spermathecae on either side of gonopore, clusters meeting near midline of genital plate (Fig. 15G); innermost (anterior) spermathecae longest, sausage-shaped, curved antero-laterally; outermost (posterior) spermathecae bulbous; other spermathecae variably pyriform, straight, directed antero-laterally.
Variation: Males (n=4): total length 2.44–2.51; carapace length 0.92–0.95; carapace height 2.19–2.31; CH/CL ratio 2.37–2.43. Females (n=3): total length 2.67–3.08; carapace length 1.03–1.10; carapace height 2.46–2.65; CH/CL ratio 2.30–2.41. Two male specimens from near Maleny (ANIC) are in poor condition, but seem to have a slightly broader, less markedly incised conductor, suggesting that there may be some population-level variation in the shape of the conductor in this species.
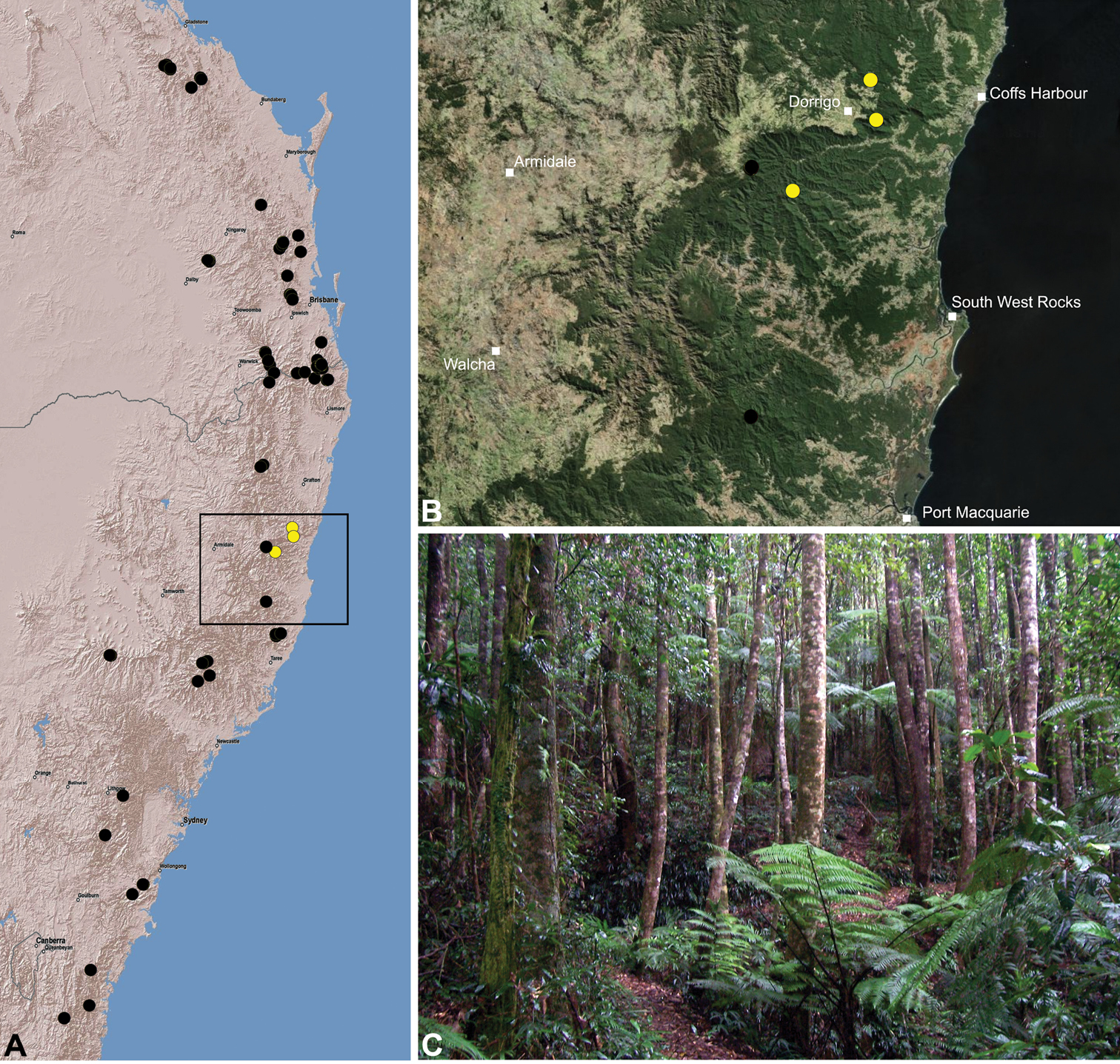
Austrarchaea judyae is known from rainforest habitats on the Blackall and Conondale Ranges of south-eastern Queensland, in the Conondale National Park, Mapleton Forest Reserve and in the region surrounding Maleny/Montville (Fig. 33). A juvenile specimen from Oakview State Forest (near Gympie) may also belong to this species based on proximity.
This species has a relatively widespread distribution in several National Parks and Forest Reserves, and is not considered to be of conservation concern.
Bunya Mountains Assassin Spider
urn:lsid:zoobank.org:act:E8650140-16BF-40B4-AC61-F787040DD692
http://species-id.net/wiki/Austrarchaea_harmsi
Figs 7E, 8E, 16, 34Holotype male, Bunya Mountains National Park, Dandabah, Scenic Circuit track, ~400 m from entrance, Queensland, Australia, 26°52'43"S, 151°35'53"E, sifting elevated leaf litter, subtropical araucarian rainforest, 959 m, 2.V.2010, M. Rix, D. Harms (QMB S90189).
Paratypes: Allotype female, Bunya Mountains National Park, Dandabah, off Bunya Mountains Road, Queensland, Australia, 26°53'07"S, 151°35'38"E, sifting elevated leaf litter, subtropical araucarian rainforest, 1030 m, 2.V.2010, M. Rix, D. Harms (QMB S90187); 1 male, same data as holotype (QMB S90188); 2 males and 3 juveniles, same data as holotype (WAM T112559DNA: Ar70-73-M/Ar70-74-J/Ar70-75-J).
AUSTRALIA: Queensland: Bunya Mountains National Park: Dandabah, on tree trunk at night, 3.III.1976, 1♂ (QMB S1095); off Bunya Mountains Road, 26°53'00"S, 151°35'20"E, sifting elevated leaf litter, subtropical araucarian rainforest, 917 m, 2.V.2010, M. Rix, D. Harms, 3 juveniles (WAM T112560DNA: Ar71-71-J/Ar71-72-J); adjacent to Stirling Family's Property, ~1.5 km SE. of Dandabah, beating low-hanging Bunya Pine branch in rainforest, 7-10.XI.2005, M. Rix, 1 juvenile (WAM T94093); Marlaybrook, 1.III.1976, V. Davies, R. Raven, 1 juvenile (QMB S30826).
AUSTRALIA: Queensland: Bunya Mountains National Park: track starting from Paradise carpark going towards Westcliff lookout, 26°52'33"S, 151°34'24"E, shaking dense mats of grass, transition zone between araucarian rainforest and grasslands, 1040 m, 3.V.2009, H. Wood, 1♂ (CASENT 9028427); same data, 1♂ (CASENT 9034524); same data, 2♀ (CASENT 9028386).
The specific epithet is a patronym in honour of Danilo Harms, for his contributions to arachnology, and his invaluable assistance to the senior author during field work in south-eastern Australia.
Austrarchaea harmsi can be distinguished from all other Archaeidae from mid-eastern Australia by the dense, pick-like tuft of accessory setae on the male chelicerae (Fig. 16C) and by the unique shape of the conductor (Figs 16D-E), which is ‘shield-shaped’ and twisted proximally.
This species can also be distinguished from other genotyped taxa from mid-eastern Australia (see Fig. 3B) by the following eight unique nucleotide substitutions for COI and COII (n = 5): C(57), A(756), A(798), C(1061), C(1191), A(1294), T(1465), A(1467).
Holotype male: Total length 2.67; leg I femur 2.67; F1/CL ratio 2.57. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen mottled grey-brown brown and beige, with darker reddish-brown dorsal scute and sclerites (Fig. 16B). Carapace tall (CH/CL ratio 2.12); 1.04 long, 2.21 high, 0.97 wide; ‘neck’ 0.46 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near posterior margin of ‘head’ (ratio of HPC to post-ocular length 0.88), carapace slightly concave anterior to HPC; ‘head’ strongly elevated postero-dorsally (post-ocular ratio 0.40) (Fig. 8E). Chelicerae with dense, pick-like tuft of accessory setae on anterior face of paturon (Fig. 16C). Abdomen 1.44 long, 1.05 wide; with three pairs of dorsal hump-like tubercles (HT 1–6); dorsal scute fused anteriorly to epigastric sclerites, extending posteriorly to first pair of hump-like tubercles; HT 3–6 each covered by separate dorsal sclerites. Unexpanded pedipalp (Figs 16D-F) with twisted, ‘shield-shaped’ conductor; tegular sclerite 1 (TS 1) relatively short, spiniform, obscured by conductor in retrolateral view; TS 2 spur-like, sinuous, longer than TS 1; TS 2a sinuous, largely obscured by TS 2; TS 3 porrect, spur-like, with sharply-pointed apex mostly obscured in retrolateral view by haematodochal membranes and retro-distal rim of tegulum.
Allotype female: Total length 3.28; leg I femur 2.72; F1/CL ratio 2.28. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen mottled grey-brown and beige (Fig. 16A). Carapace tall (CH/CL ratio 2.09); 1.19 long, 2.49 high, 1.08 wide; ‘neck’ 0.56 wide; bearing two pairs of rudimentary horns (lateral pair asymmetrically reduced); highest point of pars cephalica (HPC) near middle of ‘head’ (ratio of HPC to post-ocular length 0.60), carapace gently sloping posterior to HPC; ‘head’ moderately elevated postero-dorsally (post-ocular ratio 0.36) (Fig. 7E). Chelicerae without accessory setae on anterior face of paturon. Abdomen 1.90 long, 1.44 wide; with three pairs of dorsal hump-like tubercles (HT 1–6). Internal genitalia with dense cluster of ≤ 15 variably shaped spermathecae on either side of gonopore, clusters meeting near midline of genital plate (Fig. 16G); innermost (anterior) spermathecae longest, sausage-shaped, curved antero-laterally; other spermathecae variably pyriform, straight, directed antero-laterally.
Variation: Males (n=5): total length 2.64–3.05; carapace length 1.04–1.08; carapace height 2.15–2.24; CH/CL ratio 2.08–2.14.
Austrarchaea harmsi is known only from araucarian rainforest habitats in the Bunya Mountains National Park of south-eastern Queensland (Fig. 34).
This species is a short-range endemic taxon (
Bulburin Assassin Spider
urn:lsid:zoobank.org:act:B80C6FF2-DD73-44D8-BD1C-5153A64E125F
http://species-id.net/wiki/Austrarchaea_aleenae
Figs 5G, 7B, 8B, 17, 35Holotype male: Bulburin National Park, via Builyan, off Bulburin Forest Road, Queensland, Australia, 24°31'17"S, 151°28'02"E, sifting elevated leaf litter, subtropical vine rainforest, 618 m, 25.X.2010, M. & A. Rix (QMB S90182).
Paratypes: Allotype female, Bulburin National Park (written “Bulburin State Forest"), Queensland, Australia, 25.II.–8.III.1977, R. Raven, V. Davies (QMB S1094); 1 male and 4 juveniles, same data as holotype (WAM T112552DNA: BUL-68-M/BUL-69-J/BUL-70-J).
AUSTRALIA: Queensland: Bulburin National Park: “Bulburin State Forest", 19.III.1975, 1♂, 2 juveniles (QMB S1099); “Bulburin Forestry Nursery", NW. of Bundaberg, under rock in log, rainforest, 580 m, III.1975, M. Gray, C. Horseman, 2♀, 4 juveniles (AMS KS6776); same data, 2 juveniles (AMS KS87). Kalpowar State Forest: Mount Fort William, via Kalpowar, pyrethrum, logs, 18.I.1990, G. Monteith, 1♀, 2 juveniles (QMB S25803); Mount Fort William, 6 km NE. of Kalpowar, pyrethrum in rainforest, 700 m, 18.IX.1989, G. Monteith, 1 juvenile (QMB S31311).
The specific epithet is a patronym in honour of Aleena Wojcieszek, for her love of assassin spiders, and for her support of the senior author over many years.
Austrarchaea aleenae can be distinguished from all other Archaeidae from mid-eastern Australia except Austrarchaea alani sp. n. by the very large, porrect tegular sclerite 3 (TS 3) (Figs 17D-F); and from Austrarchaea alani sp. n. by the dense tuft of accessory setae on the male chelicerae (Fig. 17C).
This species can also be distinguished from other genotyped taxa from mid-eastern Australia (see Fig. 3B) by the following unique nucleotide substitution for COI (n = 3): A(429). The COI and COII substitutions G(363), A(552), G(627), T(897), G(1020), G(1029), G(1317) and T(1422) further distinguish this species from all other south-eastern Queensland species.
Holotype male: Total length 3.10; leg I femur 3.05; F1/CL ratio 2.77. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen mottled grey-brown and beige, palest behind hump-like tubercles, with darker reddish-brown dorsal scute and sclerites (Fig. 17B). Carapace very tall (CH/CL ratio 2.38); 1.10 long, 2.63 high, 1.03 wide; ‘neck’ 0.56 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near posterior third of ‘head’ (ratio of HPC to post-ocular length 0.68), carapace gently sloping and almost horizontal anterior and posterior to HPC; ‘head’ moderately elevated postero-dorsally (post-ocular ratio 0.34) (Fig. 8B). Chelicerae with dense tuft of accessory setae on anterior face of paturon (Fig. 17C). Abdomen 1.67 long, 1.23 wide; with three pairs of dorsal hump-like tubercles (HT 1–6); dorsal scute fused anteriorly to epigastric sclerites, extending posteriorly to first pair of hump-like tubercles; HT 3–6 each covered by separate dorsal sclerites. Unexpanded pedipalp (Figs 17D-F) with broad, distally-directed foliate conductor; tegular sclerite 1 (TS 1) spiniform, widest across middle, obscured by conductor in retrolateral view; TS 2 thin, spiniform, longer than TS 1; TS 2a sinuous, largely obscured by TS 2; TS 3 very large, porrect, with broadly-pointed rectangular apex projecting well beyond retro-distal rim of tegulum.
Allotype female: Total length 3.62; leg I femur 3.17; F1/CL ratio 2.40. Cephalothorax tan-brown; legs pale tan-brown with darker annulations; abdomen mottled grey-brown and beige (Figs 5G, 17A). Carapace very tall (CH/CL ratio 2.25); 1.32 long, 2.97 high, 1.18 wide; ‘neck’ 0.62 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near posterior third of ‘head’ (ratio of HPC to post-ocular length 0.64), carapace gently sloping posterior to HPC; ‘head’ moderately elevated postero-dorsally (post-ocular ratio 0.33) (Fig. 7B). Chelicerae without accessory setae on anterior face of paturon. Abdomen 1.85 long, 1.28 wide; with three pairs of dorsal hump-like tubercles (HT 1–6) (Fig. 5G). Internal genitalia with dense cluster of ≤ 15 variably shaped spermathecae on either side of gonopore, clusters meeting near midline of genital plate (Fig. 17G); innermost (anterior) spermathecae longest, sausage-shaped, curved antero-laterally; outermost (posterior) spermathecae bulbous; other spermathecae variably pyriform, straight, directed antero-laterally.
Variation: Males (n=3): total length 2.82–3.10; carapace length 1.03–1.10; carapace height 2.35–2.63; CH/CL ratio 2.27–2.44. Females (n=4): total length 3.13–3.62; carapace length 1.26–1.32; carapace height 2.82–2.97; CH/CL ratio 2.25–2.26.
Austrarchaea aleenae is known only from rainforest habitats in the Kalpowar-Builyan region of south-eastern Queensland, in the Bulburin National Park and nearby Kalpowar State Forest (Fig. 35).
This species appears to be a short-range endemic taxon (
Kroombit Tops Assassin Spider
urn:lsid:zoobank.org:act:C227AAB1-B201-40F7-A94D-4390A30C8518
http://species-id.net/wiki/Austrarchaea_alani
Figs 4A-E, 7A, 8A, 18, 36Holotype male: Kroombit Tops National Park, creek crossing off Tablelands Road, Queensland, Australia, 24°22'40"S, 150°59'46"E, sifting elevated leaf litter, subtropical rainforest with emergent eucalypts, 799 m, 26.X.2010, M. & A. Rix (QMB S90195).
Paratypes: Allotype female, same data as holotype (QMB S90194); 1 female, same data as holotype (QMB S90196); 2 females and 3 juveniles, same data as holotype (WAM T112550DNA: KT-63-F/KT-64-J/KT-65-J); 1 male and 2 juveniles, Kroombit Tops National Park, Rainforest Walk off Tablelands Road, near Munholme Creek, Queensland, Australia, 24°24'47"S, 151°02'22"E, sifting elevated leaf litter, subtropical rainforest, 753 m, 26.X.2010, M. & A. Rix (WAM T112551DNA: KT-66-M/KT-67-J).
AUSTRALIA: Queensland: Kroombit Tops National Park: Lower Dry Creek, pitfall trap, rainforest, 13-18.XII.1983, G. Monteith, V. Davies, J. Gallon, G. Thompson, 1♂ (QMB S30812); Three Moon Creek, rainforest, 9-19.XII.1983, V. Davies, J. Gallon, 2♀ (QMB S30816); Beauty Spot 98, rainforest, 9-19.XII.1983, V. Davies, J. Gallon, 1♀ (QMB S30803).
The specific epithet is a patronym in honour of Alan Rix, for his great assistance in helping to collect this species, and for a lifetime of generosity and support to the senior author.
Austrarchaea alani can be distinguished from all other Archaeidae from mid-eastern Australia except Austrarchaea aleenae by the very large, porrect tegular sclerite 3 (TS 3) (Figs 18D-F); and from Austrarchaea aleenae by the short comb of accessory setae on the male chelicerae (Fig. 18C).
This species can also be distinguished from other genotyped taxa from mid-eastern Australia (see Fig. 3B) by the following three unique nucleotide substitutions for COI and COII (n = 5): T(684), A(1218), C(1347).
Holotype male: Total length 2.69; leg I femur 2.83; F1/CL ratio 2.68. Cephalothorax reddish-brown; legs tan-brown with darker annulations; abdomen mottled grey-brown and beige, with darker reddish-brown dorsal scute and sclerites (Fig. 18B). Carapace very tall (CH/CL ratio 2.28); 1.06 long, 2.41 high, 0.97 wide; ‘neck’ 0.49 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near posterior margin of ‘head’ (ratio of HPC to post-ocular length 0.85), carapace gently sloping anterior to HPC; ‘head’ moderately elevated postero-dorsally (post-ocular ratio 0.35) (Fig. 8A). Chelicerae with short comb of accessory setae on anterior face of paturon (Fig. 18C). Abdomen 1.38 long, 0.92 wide; with three pairs of dorsal hump-like tubercles (HT 1–6); dorsal scute fused anteriorly to epigastric sclerites, extending posteriorly to first pair of hump-like tubercles; HT 3–6 each covered by separate dorsal sclerites. Unexpanded pedipalp (Figs 18D-F) with broad, obliquely-angled foliate conductor; tegular sclerite 1 (TS 1) relatively short, almost triangular, obscured by conductor in retrolateral view; TS 2 thin, spiniform, longer than TS 1; TS 2a sinuous, largely obscured by TS 2; TS 3 very large, porrect, with broadly-pointed rectangular apex projecting well beyond retro-distal rim of tegulum.
Allotype female: Total length 3.41; leg I femur 3.06; F1/CL ratio 2.66. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen mottled grey-brown and beige (Fig. 18A). Carapace very tall (CH/CL ratio 2.30); 1.15 long, 2.65 high, 1.04 wide; ‘neck’ 0.56 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near posterior margin of ‘head’ (ratio of HPC to post-ocular length 0.81), carapace sloping gently anterior to HPC; ‘head’ strongly elevated postero-dorsally (post-ocular ratio 0.38) (Fig. 7A). Chelicerae without accessory setae on anterior face of paturon. Abdomen 1.97 long, 1.38 wide; with three pairs of dorsal hump-like tubercles (HT 1–6). Internal genitalia with dense cluster of ≤ 15 variably shaped spermathecae on either side of gonopore, clusters meeting near midline of genital plate (Fig. 18G); innermost (anterior) spermathecae longest, sausage-shaped, curved antero-laterally; outermost (posterior) spermathecae bulbous; other spermathecae variably pyriform, straight, directed antero-laterally.
Variation: Males (n=2): total length 2.26–2.69; carapace length 1.03–1.06; carapace height 2.33–2.41; CH/CL ratio 2.28 (invariable). Females (n=4): total length 3.18–3.44; carapace length 1.15–1.18; carapace height 2.58–2.82; CH/CL ratio 2.23–2.39.
Distribution and habitat. Austrarchaea alani is known only from rainforest habitats in the Kroombit Tops National Park of south-eastern Queensland (Fig. 36).
Conservation status. This species appears to be a short-range endemic taxon (
Gibraltar Range Assassin Spider
urn:lsid:zoobank.org:act:BC7A50E4-6220-4CFC-9A80-7D1EFC20E555
http://species-id.net/wiki/Austrarchaea_monteithi
Figs 5F, 7F, 9A, 19, 37Holotype male: Gibraltar Range National Park, World Heritage Walk, off Gwydir Highway, near Richardsons Creek, New South Wales, Australia, 29°29'23"S, 152°19'47, sifting elevated leaf litter, subtropical rainforest, 1061 m, 20.IV.2010, M. Rix, D. Harms (AMS KS114977).
Paratype: Allotype female, same data as holotype (AMS KS114976DNA: Ar52-92-F).
AUSTRALIA: New South Wales: Gibraltar Range National Park: same data as holotype, 2 juveniles (WAM T112570DNA: Ar52-93-J/Ar52-94-J); “Gibraltar Range", pyrethrum, rainforest, 30.III.1980, G. Monteith, 1 juvenile (QMB S30824).
The specific epithet is a patronym in honour of Dr Geoff Monteith, for first discovering this species in the Gibraltar Range National Park.
Austrarchaea monteithi can be distinguished from all other Archaeidae from mid-eastern Australia by the presence of only five dorsal hump-like tubercles on the abdomen (Fig. 5F).
This species can also be distinguished from other genotyped taxa from mid-eastern Australia (see Fig. 3B) by the following 29 unique nucleotide substitutions for COI and COII (n = 3): G(81), T(93), A(243), C(300), C(360), C(396), A(597), A(957), C(993), G(1008), C(1115), A(1212), G(1216), T(1217), T(1220), A(1221), G(1229), G(1231), T(1233), G(1275), C(1369), A(1390), G(1391), G(1414), T(1453), G(1509), A(1525), G(1526), G(1554).
Holotype male: Total length 3.13; leg I femur 2.91; F1/CL ratio 2.58. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen mottled grey-brown and beige, with darker reddish-brown dorsal scute and sclerites (Fig. 19B). Carapace tall (CH/CL ratio 2.07); 1.13 long, 2.33 high, 1.05 wide; ‘neck’ 0.53 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near middle of ‘head’ (ratio of HPC to post-ocular length 0.54), carapace with concave depression posterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.25) (Fig. 9A). Chelicerae with short brush of accessory setae on anterior face of paturon (Fig. 19C). Abdomen 1.69 long, 1.10 wide; with five dorsal hump-like tubercles (HT 1–5), HT1–4 arranged in two pairs; dorsal scute fused anteriorly to epigastric sclerites, extending posteriorly to first pair of hump-like tubercles; HT 3–5 each covered by separate dorsal sclerites. Unexpanded pedipalp (Figs 19D-F) with stout, almost spherical bulb and thin, strongly hooked conductor; embolic sclerite with broad, looped proximal portion extending for entire length of conductor; tegular sclerite 1 (TS 1) relatively short, filiform, obscured by conductor in retrolateral view; TS 2 spur-like, longer than TS 1; TS 2a sinuous, largely obscured by TS 2; TS 3 embedded proximally within distal haematodocha, with sharply-pointed apex projecting ventrally beyond retro-distal rim of tegulum.
Allotype female: Total length 3.38; leg I femur 3.08; F1/CL ratio 2.31. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen mottled grey-brown and beige (Fig. 19A). Carapace tall (CH/CL ratio 2.12); 1.33 long, 2.82 high, 1.21 wide; ‘neck’ 0.66 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near middle of ‘head’ (ratio of HPC to post-ocular length 0.55), carapace with concave depression posterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.23) (Fig. 7F). Chelicerae without accessory setae on anterior face of paturon. Abdomen 1.85 long, 1.44 wide; with five dorsal hump-like tubercles (HT 1–5), HT1–4 arranged in two pairs (Fig. 5F). Internal genitalia with cluster of ≤ 12 variably shaped spermathecae on either side of gonopore, clusters widely separated along midline of genital plate (Fig. 19G); innermost (anterior) spermathecae longest; other spermathecae variably pyriform, straight, directed antero-laterally.
Austrarchaea monteithi is known only from subtropical rainforest habitats in the Gibraltar Range National Park of north-eastern New South Wales (Fig. 37).
This enigmatic species has an imperfectly known distribution, and although potentially restricted, appears to be relatively abundant within the World Heritage-listed Gibraltar Range National Park near Richardsons Creek (M. Rix, pers. obs.). It is not considered to be of conservation concern.
Dorrigo Assassin Spider
urn:lsid:zoobank.org:act:EF9D764D-FA0B-4473-BBD3-B31D30AF251D
http://species-id.net/wiki/Austrarchaea_christopheri
Figs 9B, 20, 38Holotype male: Dorrigo National Park, Rosewood Creek Circuit track from The Never Never Picnic Area, New South Wales, Australia, 30°21'42"S, 152°47'55"E, sifting elevated leaf litter, subtropical rainforest, 1092 m, 17.IV.2010, M. Rix, D. Harms (AMS KS114968DNA: Ar49-95-M).
Paratypes: 2 males and 4 juveniles, same data as holotype (WAM T112554DNA: Ar49-96-J/Ar49-97-J).
AUSTRALIA: New South Wales: Dorrigo National Park: The Never Never, III.–12.XI.1980, G. Monteith, 1♂ (QMB S30806). Cascades National Park: off Briggsvale Road, N. of Megan, 30°15'11"S, 152°46'52"E, sifting elevated leaf litter, subtropical rainforest, 848 m, 17.IV.2010, M. Rix, D. Harms, 3 juveniles (WAM T112553DNA: Ar50-98-J/Ar50-99-J/Ar50-100-J). New England National Park: “Oakes State Forest", Horseshoe Road, ~1.2 km S. of Killiekrankie Mountain, 30°33'10"S, 152°32'15"E, pitfall trap, 11-24.XI.1999, M. Gray, G. Milledge, H. Smith, 1♂ (AMS KS61544).
The specific epithet is a patronym in honour of Christopher Rix, for his close association with the Dorrigo region, and for his great achievements, both personal and professional.
Austrarchaea christopheri can be distinguished from all other Archaeidae from mid-eastern Australia by the very long, uniquely rod-like tegular sclerite 1 (TS 1) (Figs 20D-E).
This species can also be distinguished from other genotyped taxa from mid-eastern Australia (see Fig. 3B) by the following four unique nucleotide substitutions for COI and COII (n = 6): A(63), C(801), A(1070), G(1332).
Holotype male: Total length 3.17; leg I femur 2.96; F1/CL ratio 2.57. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen mottled grey-brown and beige, with darker reddish-brown dorsal scute and sclerites (Fig. 20A). Carapace tall (CH/CL ratio 2.10); 1.15 long, 2.42 high, 1.08 wide; ‘neck’ 0.54 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near posterior margin of ‘head’ (ratio of HPC to post-ocular length 0.86), carapace gently sloping and almost horizontal anterior to HPC; ‘head’ moderately elevated postero-dorsally (post-ocular ratio 0.33) (Fig. 9B). Chelicerae with brush of accessory setae on anterior face of paturon (Fig. 20B). Abdomen 1.64 long, 1.17 wide; with three pairs of dorsal hump-like tubercles (HT 1–6); dorsal scute fused anteriorly to epigastric sclerites, extending posteriorly to first pair of hump-like tubercles; HT 3–6 each covered by separate dorsal sclerites. Unexpanded pedipalp (Figs 20C-E) with thin, broadly-tapered foliate conductor; tegular sclerite 1 (TS 1) very long, rod-like, reaching to near distal tip of conductor, visible in retrolateral view; TS 2 spur-like, shorter than TS 1; TS 2a sinuous, filiform, exposed distally; TS 3 embedded proximally within distal haematodocha, with prominent, pointed apex projecting beyond retro-distal rim of tegulum.
Female: Unknown.
Variation: Males (n=5): total length 2.72–3.23; carapace length 1.09–1.15; carapace height 2.31–2.46; CH/CL ratio 2.07–2.18.
Austrarchaea christopheri is known from rainforest habitats throughout the Dorrigo and New England hinterland of north-eastern New South Wales (west and south-west of Coffs Harbour), in the Dorrigo, Cascades and New England National Parks (Fig. 38).
This species has a relatively widespread distribution in several National Parks protected under World Heritage legislation, and is not considered to be of conservation concern.
New England Assassin Spider
urn:lsid:zoobank.org:act:95A5C68E-D47C-44B0-BE6A-26559C286359
http://species-id.net/wiki/Austrarchaea_platnickorum
Figs 7J, 9C, 21, 39Holotype male: New England National Park, Banksia Point, Beech Forest and start of Lyrebird Track, New South Wales, Australia, 30°29'29"S, 152°24'22"E, sifting elevated leaf litter under tussocky snow grass, Nothofagus rainforest and adjacent snow gum woodland, 1491 m, 18.IV.2010, M. Rix, D. Harms (AMS KS114971).
Paratypes: Allotype female, same data as holotype (AMS KS114970); 3 males, 2 females and 5 juveniles, same data as holotype (WAM T112558DNA: Ar51-101-M/Ar51-102-F/Ar51-103-J).
AUSTRALIA: New South Wales: New England National Park: Banksia Point, ex pan traps, 2-15.X.1984, I. Naumann, J. Cardale, 1 juvenile (ANIC); Point Lookout, 22.III.1980 – 16.III.1981, G. Monteith, 1 juvenile (QMB S30819).
The specific epithet is a patronym in honour of Dr Norman Platnick and his wife Nancy. Dr Platnick’s pioneering research into many different spider lineages – including Archaeidae – has inspired a generation of arachnologists.
Austrarchaea platnickorum can be distinguished from all other Archaeidae from mid-eastern Australia by the very long, spiniform tegular sclerite 1 (TS 1) (Fig. 21F) combined with the unique shape of the conductor (Figs 21D-E), which is thin and ‘arrow-shaped’, with a long triangular apex.
This species can also be distinguished from other genotyped taxa from mid-eastern Australia (see Fig. 3B) by the following eight unique nucleotide substitutions for COI and COII (n = 3): A(354), A(573), A(624), T(986), G(1061), G(1077), C(1110), T(1533).
Holotype male: Total length 3.28; leg I femur 2.67; F1/CL ratio 2.31. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen mottled grey-brown and beige, with darker reddish-brown dorsal scute and sclerites (Fig. 21B). Carapace tall (CH/CL ratio 2.07); 1.15 long, 2.38 high, 1.08 wide; ‘neck’ 0.59 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near middle of ‘head’ (ratio of HPC to post-ocular length 0.59), carapace gently sloping posterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.26) (Fig. 9C). Chelicerae with brush of accessory setae on anterior face of paturon (Fig. 21C). Abdomen 1.85 long, 1.41 wide; with three pairs of dorsal hump-like tubercles (HT 1–6); dorsal scute fused anteriorly to epigastric sclerites, extending posteriorly to first pair of hump-like tubercles; HT 3–6 each covered by separate dorsal sclerites. Unexpanded pedipalp (Figs 21D-F) with thin, triangular ‘arrow-shaped’ conductor; tegular sclerite 1 (TS 1) very long, spiniform, visible in retrolateral view (TS 1 broken, rod-like on left pedipalp; Fig. 21F); TS 2 spur-like, poorly-sclerotised, longer than TS 1; TS 2a sinuous, largely obscured by TS 2; TS 3 indistinct, embedded within distal haematodocha, barely visible beyond retro-distal rim of tegulum.
Allotype female: Total length 4.31; leg I femur 2.79; F1/CL ratio 2.14. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen mottled grey-brown and beige (Fig. 21A). Carapace tall (CH/CL ratio 2.04); 1.31 long, 2.67 high, 1.21 wide; ‘neck’ 0.69 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near middle of ‘head’ (ratio of HPC to post-ocular length 0.60), carapace gently sloping posterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.27) (Fig. 7J). Chelicerae without accessory setae on anterior face of paturon. Abdomen 2.72 long, 1.95 wide; with three pairs of dorsal hump-like tubercles (HT 1–6). Internal genitalia with dense cluster of ≤ 20 variably shaped spermathecae on either side of gonopore, clusters meeting near midline of genital plate (Fig. 21G); innermost (anterior) spermathecae longest, sausage-shaped, curved antero-laterally; other spermathecae variably aciniform, mostly straight, directed antero-laterally.
Variation: Males (n=4): total length 2.97–3.28; carapace length 1.10–1.15; carapace height 2.21–2.38; CH/CL ratio 2.00–2.07. Females (n=3): total length 3.79–4.62; carapace length 1.26–1.31; carapace height 2.54–2.67; CH/CL ratio 2.02–2.12. The holotype male and an additional paratype male (WAM T112558) of this species have a shorter, partially broken tegular sclerite 1 (TS 1) on each left pedipalp (Fig. 21F).
Austrarchaea platnickorum is known only from rainforest and mesic closed forest habitats in the New England National Park of north-eastern New South Wales (Fig. 39).
This species has an imperfectly known distribution, and although potentially restricted, appears to be abundant within the World Heritage-listed New England National Park near Point Lookout (M. Rix, pers. obs.). It is not considered to be of conservation concern.
Kerewong Assassin Spider
urn:lsid:zoobank.org:act:0AE5EF85-E215-48F7-B443-B0B4E144238F
http://species-id.net/wiki/Austrarchaea_binfordae
Figs 7K, 9D, 22, 40Holotype male: Kerewong State Forest, off McLeods Creek Road, New South Wales, Australia, 31°33'39"S, 152°34'44"E, sifting elevated leaf litter, subtropical rainforest, 15.IV.2010, M. Rix, D. Harms (AMS KS114969DNA: Ar46-106-M).
Paratypes: Allotype female, Kerewong State Forest, New South Wales, Australia, 28.XI.1978, D. Milledge (AMS KS13891); 1 male, same data (AMS KS13891).
AUSTRALIA: New South Wales: Lorne State Forest: “Lorne State Forest", pitfall trap, 4.XI.1979, D. Milledge, 1 juvenile (AMS KS5624); same data except 5.VII.1979, D. Milledge, 1 juvenile (AMS KS5390).
AUSTRALIA. New South Wales: Willi Willi National Park: Banda Banda Antarctic Beech Forest, off Banda Road, 31°09'47"S, 152°24'23"E, sifting elevated leaf litter, Nothofagus rainforest, 1045 m, 16.IV.2010, M. Rix, D. Harms, 2 juveniles (WAM T112580DNA: Ar47-104-J/Ar47-105-J).
The specific epithet is a patronym in honour of Dr Greta Binford, for her pioneering research on spider venoms and for contributing to a highly successful basal clades tour.
Austrarchaea binfordae can be distinguished from all other Archaeidae from mid-eastern Australia by the very long, spiniform tegular sclerite 1 (TS 1) (Fig. 22F) combined with the unique shape of the conductor (Figs 22D-E), which is thin and slightly curved laterally, with a ridged ventral margin.
This species can also be distinguished from other genotyped taxa from mid-eastern Australia (see Fig. 3B) by the following 14 unique nucleotide substitutions for COI and COII (n = 1): C(291), C(369), C(489), C(720), C(807), T(1013), A(1014), A(1018), C(1019), A(1177), G(1214), C(1294), C(1312), G(1563).
Holotype male: Total length 2.64; leg I femur 2.63; F1/CL ratio 2.70. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen mottled grey-brown and beige, with darker reddish-brown dorsal scute and sclerites (Fig. 22B). Carapace tall (CH/CL ratio 2.16); 0.97 long, 2.10 high, 0.92 wide; ‘neck’ 0.44 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near posterior margin of ‘head’ (ratio of HPC to post-ocular length 0.84), carapace gently sloping and almost horizontal anterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.31) (Fig. 9D). Chelicerae with brush of accessory setae on anterior face of paturon (Fig. 22C). Abdomen 1.38 long, 1.05 wide; with three pairs of dorsal hump-like tubercles (HT 1–6); dorsal scute fused anteriorly to epigastric sclerites, extending posteriorly to first pair of hump-like tubercles; HT 3–6 each covered by separate dorsal sclerites. Unexpanded pedipalp (Figs 22D-F) with thin, slightly curved conductor with ridged ventral margin; tegular sclerite 1 (TS 1) very long, spiniform, visible in retrolateral view; TS 2 spur-like, poorly-sclerotised, shorter than TS 1; TS 2a sinuous, largely obscured by TS 2; TS 3 indistinct, embedded within distal haematodocha, barely visible beyond retro-distal rim of tegulum.
Allotype female: Total length 4.15; leg I femur 2.82; F1/CL ratio 2.39. Cephalothorax reddish-brown; legs tan-brown with darker annulations; abdomen mottled grey-brown and beige (Fig. 22A). Carapace tall (CH/CL ratio 2.19); 1.18 long, 2.58 high, 1.08 wide; ‘neck’ 0.59 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near posterior third of ‘head’ (ratio of HPC to post-ocular length 0.64), carapace gently sloping posterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.28) (Fig. 7K). Chelicerae without accessory setae on anterior face of paturon. Abdomen 2.62 long, 2.21 wide; with three pairs of dorsal hump-like tubercles (HT 1–6). Internal genitalia with cluster of ≤ 12 variably shaped spermathecae on either side of gonopore, clusters meeting near midline of genital plate (Fig. 21G); innermost (anterior) spermathecae longest, sausage-shaped, curved antero-laterally; other spermathecae variably pyriform, straight, directed antero-laterally.
Variation: Males (n=2): total length 2.64–2.92; carapace length 0.97–1.03; carapace height 2.10–2.18; CH/CL ratio 2.13–2.16.
Austrarchaea binfordae is known only from lowland subtropical rainforest habitats in the Kerewong and Lorne State Forests, near Wauchope, New South Wales (Fig. 40). Two juvenile specimens from Mount Banda Banda (Willi Willi National Park) may also belong to this species, but possess divergent mtDNA sequences indicative of possible speciation (Fig. 3B).
This species appears to be a rare short-range endemic taxon (
Barrington Tops Assassin Spider
urn:lsid:zoobank.org:act:BE4BD907-11B1-4CCA-AD3A-590B2FC9F3AE
http://species-id.net/wiki/Austrarchaea_milledgei
Figs 7L, 9E, 23, 41Holotype male: Barrington Tops State Forest, 0.8 km E. of Moppy Picnic Area, New South Wales, Australia, 31°53'22"S, 151°33'57"E, pitfall trap, Nothofagus rainforest, 1250 m, 18.III.–30.IV.2008, G. Milledge, A. Hegedus (AMS KS103905).
AUSTRALIA: New South Wales: Barrington Tops State Forest: same data as holotype except 31.I.–18.III.2008, 1 juvenile (AMS KS103409); same data as holotype except 18.XII.2007 – 31.I.2008, 1 juvenile (AMS KS102056); same data as holotype except 14.XI.–18.XII.2007, G. Milledge, H. Smith, 1 juvenile (AMS KS104681); same data, 1 juvenile (AMS KS104696); off Barrington Tops Forest Road, 31°54'45"S, 151°29'45, sifting elevated leaf litter under tree ferns, Nothofagus rainforest, 1400 m, 14.IV.2010, M. Rix, D. Harms, 8 juveniles (WAM T112569DNA: Ar42-110-J/Ar42-111-J/Ar42-112-J). Barrington Tops National Park: Quarry Road turnoff, 31°54'45"S, 151°31'10"E, pitfall trap, Nothofagus rainforest, 1230 m, 31.I.–18.III.2008, G. Milledge, A. Hegedus, 1♂, 1 juvenile (AMS KS103340); same data except 18.XII.2007 – 31.I.2008, 1 juvenile (AMS KS102013); off Barrington Tops Forest Road, 31°54'22"S, 151°31'32, sifting elevated leaf litter, complex eucalypt forest with thick understory, 1376 m, 14.IV.2010, M. Rix, D. Harms, 1♀, 3 juveniles (WAM T112568DNA: Ar43-107-F/Ar43-108-J/Ar43-109-J).
AUSTRALIA: New South Wales: Barrington Tops National Park: Gloucester Tops Road, 12.1 km W. of Gloucester River campground, 32°03'45"S, 151°36'02"E, pitfall trap, Nothofagus rainforest, 1260 m, 13.XI.–19.XII.2007, G. Milledge, H. Smith, 1♀ (AMS KS102950). Chichester State Forest: Bungari Road, 1 km from Mount Allyn Road, 32°08'S, 151°26'E, 940 m, 4.II–9.IV.1993, M. Gray, G. Cassis, 1 juvenile (AMS KS38978).
The specific epithet is a patronym in honour of Graham Milledge, for collecting most of the specimens of this species, and for his efforts in documenting the remarkable spider fauna of the Barrington Tops region of New South Wales.
Austrarchaea milledgei can be distinguished from all other Archaeidae from mid-eastern Australia except Austrarchaea aleenae by the short, dense tuft of accessory setae on the male chelicerae (Fig. 23C); and from Austrarchaea aleenae by the broader, spur-like tegular sclerite 2 (TS 2) and much smaller TS 3 (Fig. 23E).
This species can also be distinguished from other genotyped taxa from mid-eastern Australia (see Fig. 3B) by the following unique nucleotide substitution for COII (n = 6): C(1490). The COII substitution T(1511) further distinguishes this species from all other mid-eastern Australian taxa except Austrarchaea monteithi.
Holotype male: Total length 3.08; leg I femur 2.73; F1/CL ratio 2.51. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen mottled grey-brown and beige, palest posteriorly, with darker reddish-brown dorsal scute and sclerites (Fig. 23B). Carapace tall (CH/CL ratio 2.12); 1.09 long, 2.31 high, 1.05 wide; ‘neck’ 0.53 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near posterior margin of ‘head’ (ratio of HPC to post-ocular length 0.87), carapace gently sloping and almost horizontal anterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.26) (Fig. 9E). Chelicerae with dense tuft of accessory setae on anterior face of paturon (Fig. 23C). Abdomen 1.54 long, 1.13 wide; with three pairs of dorsal hump-like tubercles (HT 1–6); dorsal scute fused anteriorly to epigastric sclerites, extending posteriorly to first pair of hump-like tubercles; HT 3–6 each covered by separate dorsal sclerites. Partially expanded pedipalp (Figs 23D-E) with rounded-rectangular, broadly-tapered conductor; tegular sclerite 1 (TS 1) long, spiniform, visible in retro-ventral view; TS 2 spur-like, shorter than TS 1, partially obscured by TS 3; TS 2a sinuous, filiform, exposed distally; TS 3 rectangular, embedded within distal haematodocha, overlying proximal embolic sclerite and TS 2.
Female (WAM T112568): Total length 3.74; leg I femur 2.96; F1/CL ratio 2.24. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen mottled grey-brown and beige (Fig. 23A). Carapace tall (CH/CL ratio 2.02); 1.32 long, 2.67 high, 1.23 wide; ‘neck’ 0.65 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near middle of ‘head’ (ratio of HPC to post-ocular length 0.59), carapace gently sloping posterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.21) (Fig. 7L). Chelicerae without accessory setae on anterior face of paturon. Abdomen 2.10 long, 1.41 wide; with three pairs of dorsal hump-like tubercles (HT 1–6). Internal genitalia with dense cluster of ≤ 15 variably shaped spermathecae on either side of gonopore, clusters meeting near midline of genital plate (Fig. 23F); innermost (anterior) spermathecae longest, sausage-shaped, curved antero-laterally; outermost (posterior) spermathecae bulbous; other spermathecae variably pyriform, mostly straight, directed antero-laterally.
Variation: Males (n=2): total length 3.08–3.23; carapace length 1.09–1.10; carapace height 2.21–2.31; CH/CL ratio 2.00–2.12. For female variation see Remarks (below).
Austrarchaea milledgei is known only from rainforest and mesic closed forest habitats on the Barrington Tops Plateau, in the Barrington Tops National Park and Barrington Tops State Forest, New South Wales (Fig. 41). A juvenile specimen from Chichester State Forest and a single female specimen from Gloucester Tops may also belong to this species (see Remarks, below).
This species is a short-range endemic taxon (
Specimens of Austrarchaea from the Barrington Tops Plateaux (i.e. Barrington Tops, Gloucester Tops and Chichester State Forest) seem to exhibit a larger than normal amount of morphological and molecular variation, as highlighted by the deep (~6%) COI and COII sequence divergences observed among specimens collected along the Barrington Tops Forest Road in April 2010 (Fig. 3A), and the incongruous CH/CL ratio of the single female (AMS KS102950) from Gloucester Tops relative to the only known female from Barrington Tops (WAM T112568) (see Fig. 6). It is possible that two cryptic species may occur in sympatry on the various mountains and plateaux that make up the Barrington Tops region, although the current paucity of specimens makes this difficult to ascertain. For the purposes of this revision, specimens from the northern Barrington Tops National Park (and State Forest) are recognised as conspecific with the holotype of Austrarchaea milledgei, but further work is required to compare males from across the region, and to confirm the identification of the Gloucester Tops and Chichester State Forest populations.
Coolah Tops Assassin Spider
urn:lsid:zoobank.org:act:BB4D8025-E50A-4D77-AAC4-1BE65F2AF9DC
http://species-id.net/wiki/Austrarchaea_mascordi
Figs 1C-D, 7M, 9F, 24, 42Holotype male: Coolah Tops National Park, off Gemini Road Loop, New South Wales, Australia, 31°48'59"S, 150°10'31"E, sifting elevated leaf litter under tree ferns, open eucalypt forest with ferny understory, 1159 m, 12-13.IV.2010, M. Rix, D. Harms (AMS KS114972).
Paratypes: Allotype female, same data as holotype (AMS KS114974); 1 female, same data as holotype (AMS KS114973DNA: Ar41-48-F); 3 females and 6 juveniles, same data as holotype (WAM T112566DNA: Ar41-113-J/Ar41-114-J).
AUSTRALIA: New South Wales: Coolah Tops National Park: Breeza Lookout, 31°49'08"S, 150°11'38"E, sifting elevated leaf litter under bracken ferns, open eucalypt forest with ferny understory, 1128 m, 12-13.IV.2010, M. Rix, D. Harms, 1♂ (WAM T112565DNA: Ar40-115-M); Breeza Lookout, 31°49'17"S, 150°11'28"E, pitfall trap, 7-25.XI.2001, M. Gray, G. Milledge, H. Smith, 1♂ (AMS KS75412).
The specific epithet is a patronym in honour
of the late Ramon Mascord (1913–1983), for his pioneering contributions
to arachnology and natural history, and for writing two of the most
influential popular books on Australian spiders (see
Austrarchaea mascordi can be distinguished from all other Archaeidae from mid-eastern Australia by the relatively short, filiform tegular sclerite 1 (TS 1) (Fig. 24F) combined with the unique shape of the conductor (Figs 24D-E), which is thin and slightly twisted, with a sharply-tapered apex.
This species can also be distinguished from other genotyped taxa from mid-eastern Australia (see Fig. 3B) by the following six unique nucleotide substitutions for COI and COII (n = 4): C(348), G(468), C(651), C(957), A(967), G(1364).
Holotype male: Total length 2.83; leg I femur 2.67; F1/CL ratio 2.45. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen mottled grey-brown and beige, with darker reddish-brown dorsal scute and sclerites (Fig. 24B). Carapace tall (CH/CL ratio 2.14); 1.09 long, 2.33 high, 1.02 wide; ‘neck’ 0.54 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) approaching posterior third of ‘head’ (ratio of HPC to post-ocular length 0.62), carapace gently sloping posterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.28) (Fig. 9F). Chelicerae with brush of accessory setae on anterior face of paturon (Fig. 24C). Abdomen 1.44 long, 1.08 wide; with three pairs of dorsal hump-like tubercles (HT 1–6); dorsal scute fused anteriorly to epigastric sclerites, extending posteriorly to first pair of hump-like tubercles; HT 3–6 each covered by separate dorsal sclerites. Unexpanded pedipalp (Figs 24D-F) with thin, sharply-tapered, slightly twisted conductor; tegular sclerite 1 (TS 1) filiform, obscured by conductor in retrolateral view; TS 2 spur-like, longer than TS 1; TS 2a sinuous, filiform, exposed distally; TS 3 embedded proximally within distal haematodocha, with arched dorsal margin and sharply-pointed apex projecting ventrally beyond retro-distal rim of tegulum.
Allotype female: Total length 3.28; leg I femur 2.87; F1/CL ratio 2.29. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen mottled grey-brown and beige (Fig. 24A). Carapace tall (CH/CL ratio 2.11); 1.26 long, 2.65 high, 1.13 wide; ‘neck’ 0.62 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near middle of ‘head’ (ratio of HPC to post-ocular length 0.60), carapace gently sloping posterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.26) (Fig. 7M). Chelicerae without accessory setae on anterior face of paturon. Abdomen 1.90 long, 1.54 wide; with three pairs of dorsal hump-like tubercles (HT 1–6). Internal genitalia with cluster of ≤ 10 variably shaped spermathecae on either side of gonopore, clusters meeting near midline of genital plate (Fig. 21G); innermost (anterior) spermathecae longest, bilobed, curved antero-laterally; other spermathecae variably pyriform, mostly straight, directed antero-laterally.
Variation: Males (n=3): total length 2.83–2.92; carapace length 1.09–1.10; carapace height 2.33–2.37; CH/CL ratio 2.14–2.17. Females (n=5): total length 2.97–3.49; carapace length 1.21–1.26; carapace height 2.49–2.65; CH/CL ratio 2.05–2.11.
Austrarchaea mascordi is known only from snow gum and wet eucalypt forest habitats in the Coolah Tops National Park, west of Scone, New South Wales (Fig. 42).
This species is a short-range endemic taxon (
Blue Mountains Assassin Spider
urn:lsid:zoobank.org:act:A7583511-45FB-47A5-805F-1AA36E168237
http://species-id.net/wiki/Austrarchaea_smithae
Figs 7N, 9G, 25, 43Holotype male: Blue Mountains National Park, Mount Wilson, off Mount Wilson Road, New South Wales, Australia, 33°30'56"S, 150°21'54"E, sifting elevated leaf litter under Xanthorrhoea, complex eucalypt forest with thick understory bordering rainforest, 948 m, 9.IV.2010, M. Rix, D. Harms (AMS KS114978).
Paratypes: Allotype female, same data as holotype (AMS KS114979); 2 females and 2 juveniles, same data as holotype (WAM T112576DNA: Ar32-116-F/Ar32-117-J/Ar32-118-J).
AUSTRALIA. New South Wales: Kanangra-Boyd National Park: Kanangra Walls, near lookout, 33°59'08"S, 150°06'40"E, sifting elevated leaf litter under Xanthorrhoea, montane sclerophyll forest/heathland with thick understory, 1091 m, 10.IV.2010, M. Rix, D. Harms, 4 juveniles (WAM T112578DNA: Ar33-119-J/Ar33-120-J/Ar33-121-J); Kanangra Waterfall, near Kanangra Walls, 33°59'04"S, 150°06'33"E, sifting elevated leaf litter under ferns, streamside vegetation next to Kanangra Brook, 1069 m, 10.IV.2010, M. Rix, D. Harms, 2 juveniles (WAM T112579DNA: Ar34-122-J/Ar34-123-J).
AUSTRALIA: New South Wales: Kanangra-Boyd National Park: Kanangra Walls, near lookout, 33°59'08"S, 150°06'40"E, sifting elevated leaf litter under Xanthorrhoea, montane sclerophyll forest/heathland with thick understory, 6.XI.2008, H. Smith, 2 juveniles (AMS KS110500).
The specific epithet is a patronym in honour of Dr Helen Smith, for first discovering archaeids in the Blue Mountains region west of Sydney.
Austrarchaea smithae can be distinguished from all other Archaeidae from mid-eastern Australia by the unique shape of the male ‘head’ (Fig. 9G), which is strongly elevated dorsally (post-ocular ratio 0.39), with the highest point of the pars cephalica (HPC) closer to the middle of the ‘head’ than to the posterior margin of the carapace (ratio of HPC to post-ocular length < 0.75).
This species can also be distinguished from other genotyped taxa from mid-eastern Australia (see Fig. 3B) by the following 12 unique nucleotide substitutions for COI and COII (n = 3): C(42), A(45), A(81), G(120), G(288), G(456), A(708), G(758), G(1269), A(1346), C(1368), C(1590).
Holotype male: Total length 2.97; leg I femur 2.79; F1/CL ratio 2.48. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen mottled grey-brown and beige, with darker reddish-brown dorsal scute and sclerites (Fig. 25B). Carapace tall (CH/CL ratio 2.14); 1.13 long, 2.41 high, 1.05 wide; ‘neck’ 0.51 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) approaching posterior quarter of ‘head’ (ratio of HPC to post-ocular length 0.71), carapace gently sloping posterior to HPC; ‘head’ strongly elevated dorsally (post-ocular ratio 0.39) (Fig. 9G). Chelicerae with short brush of accessory setae on anterior face of paturon (Fig. 25C). Abdomen 1.59 long, 1.27 wide; with three pairs of dorsal hump-like tubercles (HT 1–6); dorsal scute fused anteriorly to epigastric sclerites, extending posteriorly to first pair of hump-like tubercles; HT 3–6 each covered by separate dorsal sclerites. Unexpanded pedipalp (Figs 25D-F) with thin, slightly-tapered, rectangular conductor; tegular sclerite 1 (TS 1) long, spiniform, obscured by conductor in retrolateral view; TS 2 spur-like, shorter than TS 1; TS 2a sinuous, largely obscured by TS 2; TS 3 embedded proximally within distal haematodocha, with arched dorsal margin and bluntly-pointed apex projecting ventrally beyond retro-distal rim of tegulum.
Allotype female: Total length 4.00; leg I femur 3.09; F1/CL ratio 2.30. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen mottled grey-brown and beige (Fig. 25A). Carapace tall (CH/CL ratio 2.13); 1.35 long, 2.87 high, 1.23 wide; ‘neck’ 0.65 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near posterior third of ‘head’ (ratio of HPC to post-ocular length 0.66), carapace gently sloping posterior to HPC; ‘head’ moderately elevated dorsally (post-ocular ratio 0.34) (Fig. 7N). Chelicerae without accessory setae on anterior face of paturon. Abdomen 2.56 long, 1.96 wide; with three pairs of dorsal hump-like tubercles (HT 1–6). Internal genitalia with cluster of ≤ 12 variably shaped spermathecae on either side of gonopore, clusters meeting near midline of genital plate (Fig. 25G); innermost (anterior) spermathecae longest, sausage-shaped, curved antero-laterally; outermost (posterior) spermathecae bulbous; other spermathecae variably pyriform, straight, directed antero-laterally.
Variation: Females (n=3): total length 3.74–4.00; carapace length 1.28–1.35; carapace height 2.62–2.92; CH/CL ratio 2.04–2.19.
Austrarchaea smithae is known only from wet eucalypt forest habitats in the Blue Mountains National Park west of Sydney, New South Wales (Fig. 43). Numerous juvenile specimens from the Kanangra Walls Plateau (Kanangra-Boyd National Park) may also belong to this species, but possess divergent mtDNA sequences indicative of possible speciation (Fig. 3B).
This species has an imperfectly known distribution, and although potentially restricted, appears to be relatively abundant within the World Heritage-listed Blue Mountains National Park near Mount Wilson (M. Rix, pers. obs.). It is not considered to be of conservation concern.
Illawarra Assassin Spider
urn:lsid:zoobank.org:act:278EE43B-C8F7-4069-9F60-4B343BB6FF6E
http://species-id.net/wiki/Austrarchaea_helenae
Figs 9I, 26, 44Holotype male: Macquarie Pass National Park, Macquarie Pass, New South Wales, Australia, 34°34’S, 150°39’E, pitfall trap, 12-26.IX.1999, M. Gray, G. Milledge, H. Smith (AMS KS62774).
AUSTRALIA: New South Wales: Macquarie Pass National Park: Macquarie Pass, off Clover Hill Road, 34°34'05"S, 150°39'25"E, sifting elevated leaf litter, subtropical rainforest, 828 m, 8.IV.2010, M. Rix, D. Harms, 3 juveniles (WAM T112561DNA: Ar30-124-J/Ar30-125-J/Ar30-126-J).
AUSTRALIA: New South Wales: Morton National Park: Barrengarry Mountain, ANIC Berlesate, rainforest, 460 m, 20.XII.1967, R. Taylor, C. Brooks, 1 juvenile (ANIC).
The specific epithet is a patronym in honour of Helen Rix, for her love of the Illawarra Escarpment, and for her hospitality to the senior author during field work in eastern Australia.
Austrarchaea helenae can be distinguished from all other Archaeidae from mid-eastern Australia by the long, spiniform tegular sclerite 1 (TS 1) with a curled distal tip (Fig. 26D) combined with the bifurcate, plate-like TS 3 (Fig. 26D).
This species can also be distinguished from other genotyped taxa from mid-eastern Australia (see Fig. 3B) by the following 14 unique nucleotide substitutions for COI and COII (n = 3): G(243), A(291), T(555), C(654), C(843), G(849), C(901), T(903), T(990), A(1206), C(1209), C(1401), C(1500), C(1548).
Holotype male: Total length 3.13; leg I femur 2.69; F1/CL ratio 2.50. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen mottled grey-brown and beige, with darker reddish-brown dorsal scute and sclerites (Fig. 26A). Carapace tall (CH/CL ratio 2.01); 1.08 long, 2.17 high, 1.02 wide; ‘neck’ 0.53 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near middle of ‘head’ (ratio of HPC to post-ocular length 0.57), carapace gently sloping and almost horizontal posterior to HPC; ‘head’ moderately elevated postero-dorsally (post-ocular ratio 0.35) (Fig. 9I). Chelicerae with short brush of accessory setae on anterior face of paturon (Fig. 26B). Abdomen 1.79 long, 1.28 wide; with three pairs of dorsal hump-like tubercles (HT 1–6); dorsal scute fused anteriorly to epigastric sclerites, extending posteriorly to first pair of hump-like tubercles; HT 3–6 each covered by separate dorsal sclerites. Expanded pedipalp (Figs 26C-D) with broadly-triangular, pointed conductor; tegular sclerite 1 (TS 1) long, spiniform, with curled distal tip, visible in retro-ventral view; TS 2 spiniform, shorter than TS 1, largely obscured by embolus and TS 3; TS 2a sinuous, filiform, exposed distally; TS 3 exposed, plate-like, overlying TS 2, with bifurcate, triangular apex directed toward proximal conductor.
Female: Unknown.
Austrarchaea helenae is known only from rainforest habitats in the Macquarie Pass National Park, on the Illawarra Escarpment of south-eastern New South Wales (Fig. 44). A juvenile specimen from Barrengarry Mountain (Morton National Park) may also belong to this species based on proximity.
This species appears to be a rare short-range endemic taxon (
Southern Highlands Assassin Spider
urn:lsid:zoobank.org:act:9926676F-6892-4486-8853-77B3F767AB60
http://species-id.net/wiki/Austrarchaea_mcguiganae
Figs 7O, 9H, 27, 45Holotype male: Monga National Park, Link Road, New South Wales, Australia, 35°34'04"S, 149°54'14"E, 16.III.1999, L. Wilkie, R. Harris, H. Smith (AMS KS62790).
Paratypes: Allotype female, Monga National Park, off Link Road, New South Wales, Australia, 35°34'03"S, 149°54'15"E, sifting elevated leaf litter, complex eucalypt forest with thick understory near tree fern gully, 864 m, 6.IV.2010, M. Rix, D. Harms (AMS KS114975); 1 female and 5 juveniles, same data (WAM T112567DNA: Ar28-47-J/Ar28-128-J).
AUSTRALIA: New South Wales: Deua National Park: Coondella Fire Trail, 35°58'44"S, 149°53'05"E, 11.III.1999, J. Tarnawski, S. Lassau, 1♀ (AMS KS62791). Badja State Forest: Badja Fire Trail, 36°07'30"S, 149°31'37"E, 13.III.1999, J. Tarnawski, S. Lassau, 1 juvenile (AMS KS62792); off Peters Road, near junction with Badja Forest Road, 36°07'38"S, 149°31'36"E, sifting elevated leaf litter, complex eucalypt forest with thick understory, 1075 m, 5.IV.2010, M. Rix, D. Harms, 14 juveniles (WAM T112577DNA: Ar27-129-J/Ar27-130-J/Ar27-131-J).
The specific epithet is a patronym in honour of the late Margaret McGuigan (1920–2010), for her love of the Southern Highlands, and for a lifetime of kindness and support to the senior author.
Austrarchaea mcguiganae can be distinguished from all other Archaeidae from mid-eastern Australia by the relatively short, rod-like, proximally-widened tegular sclerite 1 (TS 1) (Figs 27D-E) combined with the long brush of accessory setae on the male chelicerae (Fig. 27C).
This species can also be distinguished from other genotyped taxa from mid-eastern Australia (see Fig. 3B) by the following seven unique nucleotide substitutions for COI and COII (n = 2): T(57), C(144), T(156), G(465), G(504), C(798), G(1548).
Holotype male: Total length 3.17; leg I femur 2.81; F1/CL ratio 2.49. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen mottled grey-brown and beige, with darker reddish-brown dorsal scute and sclerites (Fig. 27B). Carapace tall (CH/CL ratio 2.00); 1.13 long, 2.26 high, 1.06 wide; ‘neck’ 0.54 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near posterior third of ‘head’ (ratio of HPC to post-ocular length 0.63), carapace gently sloping posterior to HPC; ‘head’ moderately elevated postero-dorsally (post-ocular ratio 0.32) (Fig. 9H). Chelicerae with long brush of accessory setae on anterior face of paturon (Fig. 27C). Abdomen 1.64 long, 1.10 wide; with three pairs of dorsal hump-like tubercles (HT 1–6); dorsal scute fused anteriorly to epigastric sclerites, extending posteriorly to first pair of hump-like tubercles; HT 3–6 each covered by separate dorsal sclerites. Fully expanded pedipalp (Figs 27D-E) with conductor hinged to, and obscured by, embolic haematodocha; tegular sclerite 1 (TS 1) rod-like, bluntly-pointed, visible in retro-ventral view; TS 2 spur-like, slightly longer than TS 1, largely obscured by TS 3; TS 2a sinuous, filiform, exposed distally; TS 3 exposed, plate-like, overlying TS 2, with curved, triangular apex directed toward proximal conductor.
Allotype female: Total length 3.49; leg I femur 2.86; F1/CL ratio 2.28. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen mottled grey-brown and beige (Fig. 27A). Carapace tall (CH/CL ratio 2.04); 1.26 long, 2.56 high, 1.18 wide; ‘neck’ 0.64 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near middle of ‘head’ (ratio of HPC to post-ocular length 0.60), carapace gently sloping posterior to HPC; ‘head’ moderately elevated dorsally (post-ocular ratio 0.33) (Fig. 7O). Chelicerae without accessory setae on anterior face of paturon. Abdomen 2.00 long, 1.41 wide; with three pairs of dorsal hump-like tubercles (HT 1–6). Internal genitalia with cluster of ≤ 12 variably shaped spermathecae on either side of gonopore, clusters meeting near midline of genital plate (Fig. 27F); innermost (anterior) spermathecae longest, sausage-shaped, curved antero-laterally; other spermathecae variably aciniform, straight, directed antero-laterally.
Variation: Females (n=2): total length 3.38–3.49; carapace length 1.26 (invariable); carapace height 2.56–2.62; CH/CL ratio 2.04–2.08.
Austrarchaea mcguiganae is known only from mesic closed forest habitats in the Monga National Park of southern New South Wales (Fig. 45). A female specimen from Deua National Park may also belong to this species based on proximity, and numerous juvenile specimens from the Badja State Forest possess divergent mtDNA sequences indicative of possible speciation (Fig. 3B).
This species appears to be a short-range endemic taxon (
This research was made possible by an Australian Biological Resources Study (ABRS) taxonomy research grant to M. Harvey and M. Rix (Grant No. 209–08), with critical initial support provided by Roy Teale (Biota Environmental Sciences) and significant additional funding provided by Cliffs Natural Resources and Verve Energy. Specimens of Archaeidae were graciously and promptly loaned by various curators, and the authors would like to thank Graham Milledge (Australian Museum), Owen Seeman, Robert Raven (both Queensland Museum) and Bruce Halliday (Australian National Insect Collection) for their curatorial assistance throughout this study. Numerous friends, family and colleagues assisted in the collection of assassin spiders in the field, including Danilo Harms, Stephanie Harms, Graham Milledge, Alan Rix, Helen Smith and Janine Wojcieszek. Special thanks are due to Danilo Harms (University of Western Australia) for all of his invaluable assistance to the senior author during extensive field work throughout south-eastern Australia in March-May 2010, and to Alan Rix for his herculean efforts in accessing the Bulburin and Kroombit Tops National Parks in October 2010. Thanks also to Christine Lambkin and Robert Raven (Queensland Museum) for their significant eleventh hour assistance with permits in Queensland, and to Michael Sharp (New South Wales Parks and Wildlife Service) for helping the senior author access the eastern Coolah Tops National Park in April 2010. Specimens were collected under permit throughout Queensland (Permit No. WITK5498008) and New South Wales (Permit Nos. S13035, XX48918). The molecular component of this research was made possible by facilities in the School of Animal Biology at the University of Western Australia, and special thanks are due to Janine Wojcieszek for her considerable help in processing molecular samples. Finally, thanks to Hannah Wood and Charles Griswold (California Academy of Sciences) for their ongoing support, discussion and collaboration on all things ‘archaeid’, and special thanks to Hannah for providing data on Australian specimens housed at the CAS, and for sending DNA extractions of Malagasy and African taxa for use in molecular analyses. Hannah Wood, Jeremy Miller and an anonymous reviewer provided helpful comments on an earlier draft of the manuscript.
Habitus images of live Archaeidae from mid-eastern Australia: A–B, female Austrarchaea nodosa (Forster, 1956) from Binna Burra, Lamington National Park, Queensland; C–D, female A. mascordi sp. n. from Coolah Tops National Park, New South Wales; E–F, juvenile A. raveni sp. n. from Mount Glorious, Queensland. Images A–D by M. Rix; images E–F by Greg Anderson, used with permission.
Map showing the known distribution of Archaeidae in Australia, with mid-eastern Australian localities highlighted in black. Note the absence of Archaeidae in central-eastern Queensland, the Australian Alps and Tasmania.
Molecular phylogenetic data analysed as part of this study. A, Schematic map of the mitochondrial cytochrome c oxidase subunit I–II (COI–COII) gene complex in Archaeidae and other basal Araneomorphae, showing (i) the position of primers used to amplify and sequence 1.6 kilobases of mtDNA, and (ii) the inferred stop and initiation codons for COI and COII, respectively. Note the centralised, overlapping position of the two internal sequencing primer sites (SeqF2a/SeqR1), and the TTG initiation codon for COII, present in all but one of the spider species sequenced for this study. B, Majority-rule consensus tree with re-estimated branch lengths, resulting from a combined, gene-partitioned Bayesian analysis of the COI–COII mtDNA data. Thickened branches represent clades with >95% posterior probability support, and individual support values are shown above other nodes.
Carapace morphology of Austrarchaea species. A–E, A. alani sp. n.: A, male pars cephalica, frontal view, showing dorsal ‘head’ region, posterior horns (H) and cheliceral foramen (CF); B, female pars cephalica, antero-lateral view, showing ocular bulge (OB), cheliceral foramen (CF) and division of pars cephalica into ‘head’ and ‘neck’ regions; C, male pars thoracica, ‘neck’ and fused cheliceral diastema (fCD), antero-lateral view; D, female chelicerae and peg teeth, frontal view; E, male ‘neck’, lateral view, showing setose tubercles (sT). F–G, A. judyae sp. n.: F, male chelicerae, lateral view, showing accessory setae (AS) and ectal stridulatory file (SF); G, detail of female posterior pars cephalica, lateral view, showing field of densely granulate cuticle.
Abdominal morphology of Austrarchaea species. A–C, A. judyae sp. n.: A, male abdomen, antero-lateral view, showing dorsal scute (S) and additional dorsal sclerites (ds); B, detail of female abdomen, lateral view, showing subcuticular guanine crystals (GC) and concentric arrangements of setae around sclerotic spots (ss); C, female epigastric region, ventral view, showing setose book lung covers (BL) and genital plate (GP). D, Cleared epigastric region of female A. nodosa (Forster), postero-ventral view, showing position of clustered spermathecae under posterior rim of genital plate. E–G, Female abdomens, postero-lateral view, showing arrangement of dorsal hump-like tubercles (HT) in different taxa: E, A. sp. nr. daviesae (QMB S72989, from Mount Bartle Frere, NE. Queensland); F, A. monteithi sp. n.; G, A. aleenae sp. n. Note the presence of only a single posterior hump-like tubercle (HT 5) in A. monteithi.
Graphs depicting the relationship between carapace length (CL) and carapace height (CH) for species of Austrarchaea from mid-eastern Australia. Overall body size variation is quantified by the relative lengths of the carapace, whereas carapace shape variation is reflected by the CH/CL ratio; taxa with a very tall, greatly elevated pars cephalica have a CH/CL ratio > 2.20. Circles ● denote New South Wales and southern Queensland species; and triangles ▲ denote Queensland species (from north of the Border Ranges). Note the relatively small body sizes of A. judyae sp. n., A. binfordae sp. n. and A. alani sp. n., and the relatively tall carapaces of most Queensland taxa. Note also the smaller body sizes and lower variance in carapace length among males relative to females.
Lateral ‘head’ profiles of females of species of Austrarchaea from mid-eastern Australia, showing variation in carapace shape as quantified by the post-ocular ratio (P.O. Ratio) and ratio of highest point of carapace relative to post-ocular length (HPC Ratio): A, allotype A. alani sp. n.; B, allotype A. aleenae sp. n.; C, allotype A. judyae sp. n.; D, allotype A. raveni sp. n.; E, allotype A. harmsi sp. n.; F, allotype A. monteithi sp. n.; G, allotype A. cunninghami sp. n.; H, allotype A. dianneae sp. n.; I, A. nodosa (Forster, 1956); J, allotype A. platnickorum sp. n.; K, allotype A. binfordae sp. n.; L, A. milledgei sp. n. (WAM T112568); M, allotype A. mascordi sp. n.; N, allotype A. smithae sp. n.; O, allotype A. mcguiganae sp. n. Asterisks (*) denote concave depressions.
Lateral ‘head’ profiles of males of species of Austrarchaea from south-eastern Queensland and extreme north-eastern New South Wales (including the Border Ranges), showing variation in carapace shape as quantified by the post-ocular ratio (P.O. Ratio) and ratio of highest point of carapace relative to post-ocular length (HPC Ratio): A, holotype A. alani sp. n.; B, holotype A. aleenae sp. n.; C, holotype A. judyae sp. n.; D, holotype A. raveni sp. n.; E, holotype A. harmsi sp. n.; F, holotype A. clyneae sp. n.; G, holotype A. cunninghami sp. n.; H, holotype A. dianneae sp. n.; I, A. nodosa (Forster, 1956) (QMB S75416). Asterisks (*) denote concave depressions.
Lateral ‘head’ profiles of males of species of Austrarchaea from New South Wales (excluding the Border Ranges), showing variation in carapace shape as quantified by the post-ocular ratio (P.O. Ratio) and ratio of highest point of carapace relative to post-ocular length (HPC Ratio): A, holotype A. monteithi sp. n.; B, holotype A. christopheri sp. n.; C, holotype A. platnickorum sp. n.; D, holotype A. binfordae sp. n.; E, holotype A. milledgei sp. n.; F, holotype A. mascordi sp. n.; G, holotype A. smithae sp. n.; H, holotype A. mcguiganae sp. n.; I, holotype A. helenae sp. n. Asterisks (*) denote concave depressions.
Austrarchaea nodosa (Forster, 1956). A–B, Cephalothorax and abdomen, lateral view: A, female (QMB S75416) from Lamington National Park, Queensland; B, male (QMB S75416) from Lamington National Park, Queensland. C, Male chelicerae, lateral view, showing accessory setae. D–F, Male (WAM T89592) pedipalp: D–E, bulb, retrolateral view; F, detail of distal tegular sclerites, prodistal view. G, Female (QMB S75416) internal genitalia, dorsal view. C = conductor; E = embolus; Es = embolic sclerite; T = tegulum; (TS)1–3 = tegular sclerites 1–3. Scale bars: A–B = 1.0 mm; E = 0.2 mm.
Austrarchaea dianneae sp. n. A–B, Cephalothorax and abdomen, lateral view: A, allotype female (QMB S90186) from Tamborine National Park, Queensland; B, holotype male (QMB S90185) from Tamborine National Park, Queensland. C, Holotype male chelicerae, lateral view, showing accessory setae. D–F, Holotype male pedipalp: D–E, bulb, retrolateral view; F, detail of distal tegular sclerites, prodistal view. G, Allotype female internal genitalia, dorsal view. C = conductor; E = embolus; Es = embolic sclerite; T = tegulum; (TS)1–3 = tegular sclerites 1–3. Scale bars: A–B = 1.0 mm; E = 0.2 mm.
Austrarchaea cunninghami sp. n. A–B, Cephalothorax and abdomen, lateral view: A, allotype female (QMB S90183) from Main Range National Park, Queensland; B, holotype male (QMB S90184) from Main Range National Park, Queensland. C, Holotype male chelicerae, lateral view, showing accessory setae. D–F, Holotype male right pedipalp (flipped horizontal for inter-specific comparison): D–E, bulb, retrolateral view; F, detail of distal tegular sclerites, prodistal view. G, Allotype female internal genitalia, dorsal view. C = conductor; E = embolus; Es = embolic sclerite; T = tegulum; (TS)1–3 = tegular sclerites 1–3. Scale bars: A–B = 1.0 mm; E = 0.2 mm.
Austrarchaea clyneae sp. n. A–E, Holotype male (QMB S20425) from Mount Clunie National Park, New South Wales: A, cephalothorax and abdomen, lateral view; B, chelicerae, lateral view, showing accessory setae; C–D, pedipalpal bulb, retrolateral view; E, detail of distal tegular sclerites, prodistal view. C = conductor; E = embolus; Es = embolic sclerite; T = tegulum; (TS)1–3 = tegular sclerites 1–3. Scale bars: A = 1.0 mm; D = 0.2 mm.
Austrarchaea raveni sp. n. A–B, Cephalothorax and abdomen, lateral view: A, allotype female (QMB S90192) from D’Aguilar National Park, Queensland; B, holotype male (QMB S90193) from D’Aguilar National Park, Queensland. C, Holotype male chelicerae, lateral view, showing accessory setae. D–F, Holotype male pedipalp (partially expanded): D–E, bulb, retrolateral view (inset shows conductor and embolus on unexpanded pedipalp of male from Mt Mee Forest Reserve, Queensland); F, detail of distal tegular sclerites, prodistal view. G, Allotype female internal genitalia, dorsal view. C = conductor; E = embolus; Es = embolic sclerite; T = tegulum; (TS)1–3 = tegular sclerites 1–3. Scale bars: A–B = 1.0 mm; E = 0.2 mm.
Austrarchaea judyae sp. n. A–B, Cephalothorax and abdomen, lateral view: A, allotype female (QMB S90191) from Conondale National Park, Queensland; B, holotype male (QMB S90190) from Conondale National Park, Queensland. C, Holotype male chelicerae, lateral view, showing accessory setae. D–F, Holotype male pedipalp: D–E, bulb, retrolateral view; F, detail of distal tegular sclerites, prodistal view. G, Allotype female internal genitalia, dorsal view. C = conductor; E = embolus; Es = embolic sclerite; T = tegulum; (TS)1–3 = tegular sclerites 1–3. Scale bars: A–B = 1.0 mm; E = 0.2 mm.
Austrarchaea harmsi sp. n. A–B, Cephalothorax and abdomen, lateral view: A, allotype female (QMB S90187) from Bunya Mountains National Park, Queensland; B, holotype male (QMB S90189) from Bunya Mountains National Park, Queensland. C, Holotype male chelicerae, lateral view, showing accessory setae. D–F, Holotype male pedipalp: D–E, bulb, retrolateral view; F, detail of distal tegular sclerites, prodistal view. G, Allotype female internal genitalia, dorsal view. C = conductor; E = embolus; Es = embolic sclerite; T = tegulum; (TS)1–3 = tegular sclerites 1–3. Scale bars: A–B = 1.0 mm; E = 0.2 mm.
Austrarchaea aleenae sp. n. A–B, Cephalothorax and abdomen, lateral view: A, allotype female (QMB S1094) from Bulburin National Park, Queensland; B, holotype male (QMB S90182) from Bulburin National Park, Queensland. C, Holotype male chelicerae, lateral view, showing accessory setae. D–F, Holotype male pedipalp: D–E, bulb, retrolateral view; F, detail of distal tegular sclerites, prodistal view. G, Allotype female internal genitalia, dorsal view, showing membranous bursa overlying clustered spermathecae. B = bursa; C = conductor; E = embolus; Es = embolic sclerite; T = tegulum; (TS)1–3 = tegular sclerites 1–3. Scale bars: A–B = 1.0 mm; E = 0.2 mm.
Austrarchaea alani sp. n. A–B, Cephalothorax and abdomen, lateral view: A, allotype female (QMB S90194) from Kroombit Tops National Park, Queensland; B, holotype male (QMB S90195) from Kroombit Tops National Park, Queensland. C, Holotype male chelicerae, lateral view, showing accessory setae. D–F, Holotype male pedipalp: D–E, bulb, retrolateral view; F, detail of distal tegular sclerites, prodistal view. G, Allotype female internal genitalia, dorsal view. C = conductor; E = embolus; Es = embolic sclerite; T = tegulum; (TS)1–3 = tegular sclerites 1–3. Scale bars: A–B = 1.0 mm; E = 0.2 mm.
Austrarchaea monteithi sp. n. A–B, Cephalothorax and abdomen, lateral view: A, allotype female (AMS KS114976) from Gibralter Range National Park, New South Wales; B, holotype male (AMS KS114977) from Gibralter Range National Park, New South Wales. C, Holotype male chelicerae, lateral view, showing accessory setae. D–F, Holotype male pedipalp: D–E, bulb, retrolateral view; F, detail of distal tegular sclerites, prodistal view. G, Allotype female internal genitalia, dorsal view. C = conductor; E = embolus; Es = embolic sclerite; T = tegulum; (TS)1–3 = tegular sclerites 1–3. Scale bars: A–B = 1.0 mm; E = 0.2 mm.
Austrarchaea christopheri sp. n. A–E, Holotype male (AMS KS114968) from Dorrigo National Park, New South Wales: A, cephalothorax and abdomen, lateral view; B, chelicerae, lateral view, showing accessory setae; C–D, pedipalpal bulb, retrolateral view; E, detail of distal tegular sclerites, prodistal view. C = conductor; E = embolus; Es = embolic sclerite; T = tegulum; (TS)1–3 = tegular sclerites 1–3. Scale bars: A = 1.0 mm; D = 0.2 mm.
Austrarchaea platnickorum sp. n. A–B, Cephalothorax and abdomen, lateral view: A, allotype female (AMS KS114970) from New England National Park, New South Wales; B, holotype male (AMS KS114971) from New England National Park, New South Wales. C, Holotype male chelicerae, lateral view, showing accessory setae. D–F, Holotype male pedipalp: D–E, bulb, retrolateral view; F, detail of distal tegular sclerites, prodistal view. G, Allotype female internal genitalia, dorsal view. Note the broken left tegular sclerite 1 (TS 1) in (F), highlighted (*) at the point of breakage, compared to the long, sharply-pointed right TS 1 (see inset). C = conductor; E = embolus; Es = embolic sclerite; T = tegulum; (TS)1–3 = tegular sclerites 1–3. Scale bars: A–B = 1.0 mm; E = 0.2 mm.
Austrarchaea binfordae sp. n. A–B, Cephalothorax and abdomen, lateral view: A, allotype female (AMS KS13891) from Kerewong State Forest, New South Wales; B, holotype male (AMS KS114969) from Kerewong State Forest, New South Wales. C, Holotype male chelicerae, lateral view, showing accessory setae. D–F, Holotype male pedipalp: D–E, bulb, retrolateral view; F, detail of distal tegular sclerites, prodistal view. G, Allotype female internal genitalia, dorsal view. C = conductor; E = embolus; Es = embolic sclerite; T = tegulum; (TS)1–3 = tegular sclerites 1–3. Scale bars: A–B = 1.0 mm; E = 0.2 mm.
Austrarchaea milledgei sp. n. A–B, Cephalothorax and abdomen, lateral view: A, female (WAM T112568) from Barrington Tops National Park, New South Wales; B, holotype male (AMS KS103905) from Barrington Tops State Forest, New South Wales. C, Holotype male chelicerae, lateral view, showing accessory setae. D–E, Holotype male pedipalpal bulb (expanded), retro-ventral view. F, Female (WAM T112568) internal genitalia, dorsal view. bH = basal haematodocha; C = conductor; Cy = cymbium; E = embolus; Es = embolic sclerite; T = tegulum; (TS)1–3 = tegular sclerites 1–3. Scale bars: A–B = 1.0 mm; E = 0.2 mm.
Austrarchaea mascordi sp. n. A–B, Cephalothorax and abdomen, lateral view: A, allotype female (AMS KS114974) from Coolah Tops National Park, New South Wales; B, holotype male (AMS KS114972) from Coolah Tops National Park, New South Wales. C, Holotype male chelicerae, lateral view, showing accessory setae. D–F, Holotype male pedipalp: D–E, bulb, retrolateral view; F, detail of distal tegular sclerites, prodistal view. G, Allotype female internal genitalia, dorsal view. C = conductor; E = embolus; Es = embolic sclerite; T = tegulum; (TS)1–3 = tegular sclerites 1–3. Scale bars: A–B = 1.0 mm; E = 0.2 mm.
Austrarchaea smithae sp. n. A–B, Cephalothorax and abdomen, lateral view: A, allotype female (AMS KS114979) from Blue Mountains National Park, New South Wales; B, holotype male (AMS KS114978) from Blue Mountains National Park, New South Wales. C, Holotype male chelicerae, lateral view, showing accessory setae. D–F, Holotype male pedipalp: D–E, bulb, retrolateral view; F, detail of distal tegular sclerites, prodistal view. G, Allotype female internal genitalia, dorsal view. C = conductor; E = embolus; Es = embolic sclerite; T = tegulum; (TS)1–3 = tegular sclerites 1–3. Scale bars: A–B = 1.0 mm; E = 0.2 mm.
Austrarchaea helenae sp. n. A–D, Holotype male (AMS KS62774) from Macquarie Pass National Park, New South Wales: A, cephalothorax and abdomen, lateral view; B, chelicerae, lateral view, showing accessory setae; C–D, pedipalpal bulb (expanded), retro-ventral view. C = conductor; Cy = cymbium; E = embolus; eH = embolic portion of distal haematodocha; Es = embolic sclerite; pH = proximal portion of distal haematodocha; T = tegulum; (TS)1–3 = tegular sclerites 1–3. Scale bars: A = 1.0 mm; D = 0.2 mm.
Austrarchaea mcguiganae sp. n. A–B, Cephalothorax and abdomen, lateral view: A, allotype female (AMS KS114975) from Monga National Park, New South Wales; B, holotype male (AMS KS62790) from Monga National Park, New South Wales. C, Holotype male chelicerae, lateral view, showing accessory setae. D–E, Holotype male pedipalpal bulb (fully expanded), retro-ventral view. G, Allotype female internal genitalia, dorsal view. bH = basal haematodocha; C = conductor; Cy = cymbium; E = embolus; eH = embolic portion of distal haematodocha; Es = embolic sclerite; pH = proximal portion of distal haematodocha; T = tegulum; (TS)1–3 = tegular sclerites 1–3. Scale bars: A–B = 1.0 mm; E = 0.2 mm.
Austrarchaea nodosa (Forster, 1956), distribution and habitat: A, topographic map showing the known distribution of Archaeidae in south-eastern Queensland and eastern New South Wales, with collection localities for A. nodosa highlighted in yellow (red highlighted localities denote juvenile specimens of tentative identification); B, satellite image showing detail of inset (A); C, subtropical rainforest near the type locality – Binna Burra, Lamington National Park, Queensland (April 2010). Image (C) by M. Rix.
Austrarchaea dianneae sp. n., distribution and habitat: A, topographic map showing the known distribution of Archaeidae in south-eastern Queensland and eastern New South Wales, with collection localities for A. dianneae highlighted in yellow; B, satellite image showing detail of inset (A); C, subtropical rainforest at the type locality – Joalah Section, Tamborine National Park, Queensland (June 2009). Note the sympatric occurrence of this species with A. nodosa on the ‘scenic rim’ at Binna Burra, Lamington National Park. Image (C) by M. Rix.
Austrarchaea cunninghami sp. n., distribution and habitat: A, topographic map showing the known distribution of Archaeidae in south-eastern Queensland and eastern New South Wales, with collection localities for A. cunninghami highlighted in yellow (red highlighted localities denote juvenile specimens of tentative identification); B, satellite image showing detail of inset (A); C, subtropical rainforest at the type locality – Cunningham’s Gap, Main Range National Park, Queensland (April 2010). Image (C) by M. Rix.
Austrarchaea clyneae sp. n., distribution and habitat: A, topographic map showing the known distribution of Archaeidae in south-eastern Queensland and eastern New South Wales, with collection localities for A. clyneae highlighted in yellow (red highlighted localities denote juvenile specimens of tentative identification); B, satellite image showing detail of inset (A); C, subtropical rainforest at the type locality – Mount Clunie National Park, Queensland (June 2008). Image (C) by A. Rix, used with permission.
Austrarchaea raveni sp. n., distribution and habitat: A, topographic map showing the known distribution of Archaeidae in south-eastern Queensland and eastern New South Wales, with collection localities for A. raveni highlighted in yellow; B, satellite image showing detail of inset (A); C, subtropical rainforest near the type locality – Mount Glorious, D’Aguilar National Park, Queensland (May 2010). Image (C) by M. Rix.
Austrarchaea judyae sp. n., distribution and habitat: A, topographic map showing the known distribution of Archaeidae in south-eastern Queensland and eastern New South Wales, with collection localities for A. judyae highlighted in yellow (red highlighted localities denote juvenile specimens of tentative identification); B, satellite image showing detail of inset (A); C, subtropical rainforest at the type locality – near Booloumba Creek, Conondale National Park, Queensland (May 2010). Image (C) by M. Rix.
Austrarchaea harmsi sp. n., distribution and habitat: A, topographic map showing the known distribution of Archaeidae in south-eastern Queensland and eastern New South Wales, with collection localities for A. harmsi highlighted in yellow; B, satellite image showing detail of inset (A); C, subtropical araucarian (Araucaria bidwillii) rainforest at the type locality – Dandabah, Bunya Mountains National Park, Queensland (May 2010). Image (C) by M. Rix.
Austrarchaea aleenae sp. n., distribution and habitat: A, topographic map showing the known distribution of Archaeidae in south-eastern Queensland and eastern New South Wales, with collection localities for A. aleenae highlighted in yellow; B, satellite image showing detail of inset (A); C, subtropical vine rainforest at the type locality – Bulburin Forest Road, Bulburin National Park, Queensland (October 2010). Image (C) by A. Rix, used with permission.
Austrarchaea alani sp. n., distribution and habitat: A, topographic map showing the known distribution of Archaeidae in south-eastern Queensland and eastern New South Wales, with collection localities for A. alani highlighted in yellow; B, satellite image showing detail of inset (A); C, subtropical rainforest near the type locality – Tablelands Road, Kroombit Tops National Park, Queensland (October 2010). Image (C) by M. Rix.
Austrarchaea monteithi sp. n., distribution and habitat: A, topographic map showing the known distribution of Archaeidae in south-eastern Queensland and eastern New South Wales, with collection localities for A. monteithi highlighted in yellow; B, satellite image showing detail of inset (A); C, subtropical rainforest at the type locality – near Richardsons Creek, Gibralter Range National Park, New South Wales (April 2010). Image (C) by M. Rix.
Austrarchaea christopheri sp. n., distribution and habitat: A, topographic map showing the known distribution of Archaeidae in south-eastern Queensland and eastern New South Wales, with collection localities for A. christopheri highlighted in yellow; B, satellite image showing detail of inset (A); C, subtropical rainforest at the type locality – The Never Never, Dorrigo National Park, New South Wales (April 2010). Image (C) by M. Rix.
Austrarchaea platnickorum sp. n., distribution and habitat: A, topographic map showing the known distribution of Archaeidae in south-eastern Queensland and eastern New South Wales, with collection localities for A. platnickorum highlighted in yellow; B, satellite image showing detail of inset (A); C, snow gum woodland adjacent to cool-temperate Nothofagus moorei rainforest at the type locality – Banksia Point, New England National Park, New South Wales (April 2010). Image (C) by M. Rix.
Austrarchaea binfordae sp. n., distribution and habitat: A, topographic map showing the known distribution of Archaeidae in south-eastern Queensland and eastern New South Wales, with collection localities for A. binfordae highlighted in yellow (orange localities denote genotyped juvenile specimens of tentative identification); B, satellite image showing detail of inset (A); C, lowland subtropical rainforest at the type locality – McLeods Creek Road, Kerewong State Forest, New South Wales (April 2010). Image (C) by M. Rix.
Austrarchaea milledgei sp. n., distribution and habitat: A, topographic map showing the known distribution of Archaeidae in south-eastern Queensland and eastern New South Wales, with collection localities for A. milledgei highlighted in yellow (red highlighted localities denote specimens of tentative identification); B, satellite image showing detail of inset (A); C, cool-temperate Nothofagus moorei rainforest near the type locality – Barrington Tops National Park, New South Wales (April 2010). Image (C) by M. Rix.
Austrarchaea mascordi sp. n., distribution and habitat: A, topographic map showing the known distribution of Archaeidae in south-eastern Queensland and eastern New South Wales, with collection localities for A. mascordi highlighted in yellow; B, satellite image showing detail of inset (A); C, open eucalypt forest near the type locality – Breeza Lookout, Coolah Tops National Park, New South Wales (April 2010). Image (C) by M. Rix.
Austrarchaea smithae sp. n., distribution and habitat: A, topographic map showing the known distribution of Archaeidae in south-eastern Queensland and eastern New South Wales, with collection localities for A. smithae highlighted in yellow (orange localities denote genotyped juvenile specimens of tentative identification); B, satellite image showing detail of inset (A); C, wet eucalypt forest at the type locality – Mount Wilson, Blue Mountains National Park, New South Wales (April 2010). Image (C) by M. Rix.
Austrarchaea helenae sp. n., distribution and habitat: A, topographic map showing the known distribution of Archaeidae in south-eastern Queensland and eastern New South Wales, with collection localities for A. helenae highlighted in yellow (red highlighted localities denote juvenile specimens of tentative identification); B, satellite image showing detail of inset (A); C, subtropical rainforest at the type locality – Macquarie Pass, Macquarie Pass National Park, New South Wales (April 2010). Image (C) by M. Rix.
Austrarchaea mcguiganae sp. n., distribution and habitat: A, topographic map showing the known distribution of Archaeidae in south-eastern Queensland and eastern New South Wales, with collection localities for A. mcguiganae highlighted in yellow (red highlighted localities denote specimens of tentative identification; orange highlighted localities denote genotyped juvenile specimens of tentative identification); B, satellite image showing detail of inset (A); C, wet eucalypt forest at the type locality – Link Road, Monga National Park, New South Wales (April 2010). Image (C) by M. Rix.
Supplementary nexus file. (doi: 10.3897/zookeys.123.1448.app) File format: NXS
Explanation note: Nexus file of aligned COI and COII nucleotide sequences (see Table 2) with Bayesian command block, used for the molecular phylogenetic analysis (see Fig. 3).
Copyright notice: This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Citation: Rix MG, Harvey MS (2011) Australian Assassins, Part I: A review of the Assassin Spiders (Araneae, Archaeidae) of mid-eastern Australia. ZooKeys 123: 1–100. doi: 10.3897/zookeys.123.1448.app