(C) 2012 Favízia Freitas de Oliveira. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A third species of the rare augochlorine bee genus Chlerogelloides Engel et al. (Halictinae, Augochlorini) is described and figured. Chlerogelloides nexosa sp. n. is most similar to the type species, Chlerogelloides femoralis Engel et al., in that both have modified midlegs in the males. The former, however, can be distinguished on the basis of its terminalia, which in some respects more closely resembles those of Chlerogelloides simplex Engel and Brooks. Brief comments on the secondary features of males and relationships of the genus are provided. A key to the species of the genus is provided and floral records of Cordia nodosa Lam. (Boraginaceae) and Gonzalagunia dicocca Cham. & Schltdl. (Rubiaceae) are noted.
Apoidea, Anthophila, Halictidae, Halictinae, Augochlorini, Chlerogelloides, taxonomy, Brazil
Numerous lineages of bees within the New World tribe Augochlorini (Halictinae) are known to have characteristically elongate heads, typically involving an elongation of the malar space and clypeus, and often the supraclypeal area, and presumably adaptations for obtaining nectar from flowers with deep corollas, although few floral records are available (e.g.,
Herein we report the discovery of a third species in the genus, specimens of which were captured while visiting flowers of Cordia nodosa Lam. (Boraginaceae: sometimes placed in its own order, Boraginales) and Gonzalagunia dicocca Cham. & Schltdl. (Gentianales: Rubiaceae), and representing the first occurrence of the genus in Pará State, Brazil.
Material and methodsMorphological terminology follows that of
urn:lsid:zoobank.org:act:C5A48B27-0DC1-4AC6-9C17-9AD8E9D3C617
http://species-id.net/wiki/Chlerogelloides_nexosa
Figs 1–3, 5, 7–10♂, Brazil, Pará (Melgaço, Reserva Caxiuanã, Estação Ecológica Ferreira Penna, 01°43'S, 51°29'W, 18–21.ix.2011 [18–21 September 2011], Rech, Coelho, Correa & Carmo Leg. // coletada na flor: Cordia nodosa Lam. (Boraginales - Cordiaceae) – durante o curso de Polinização 2011 // Holotype male Chlerogelloides nexosa Oliveira, Engel & Mahlmann. The specimen is deposited in the Entomological Collection of the Museu Paraense Emílio Goeldi (MPEG), in Belém, Pará, Brazil.
1♂, with same label data as holotype and deposited in the Entomological Collection of the Zoological Museum of the Federal University of Bahia (MZUFBA), in Salvador, Bahia, Brazil. 5♂♂, Guyane Française [French Guiana], Saül, Carbet ONF de Galbao, 27.viii.2003 [27 August 2003], on Gonzalagunia dicocca, leg. J. Munzinger; deposited in the Department of Entomology, Royal Belgian Institute of Natural Sciences, Brussels, Belgium and one in the Division of Entomology, University of Kansas Natural History Museum, Lawrence, Kansas, USA. 1♂, Guyane Fr. [French Guiana], Patawa, viii.1999 [August 1999], PM; deposited in the Department of Entomology, Royal Belgian Institute of Natural Sciences, Brussels, Belgium.
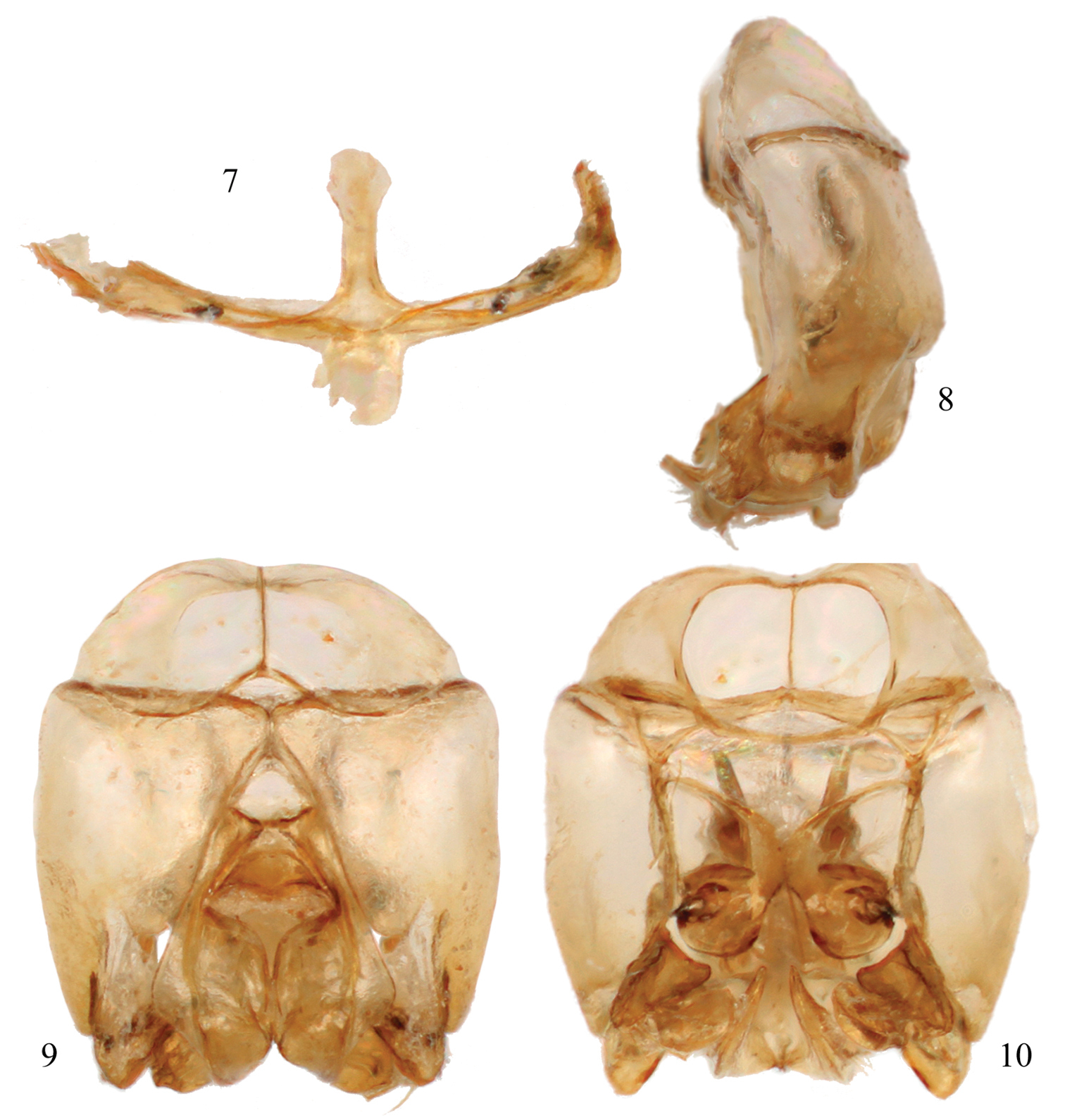
Integument predominantly honey yellow with metallic olive green highlights only on the head, mesoscutum, mesoscutellum, and metanotum (Figs 2, 3); postgena uniformly covered by plumose setae except; mesotrochanter without prominent ventral tubercle and not strongly bent (Fig. 5); mesofemur greatly expanded and flattened along inner surface, with a prominent tubercle on ventral surface in apical third (Fig. 5); mesotibia with inner surface twisted and flanked by non-contiguous, ill-defined carinas converging medioapically (Fig. 5); mesobasitarsus about twice as long as wide and weakly concave on inner surface; male terminalia as in figures 7–10.
♂: Structure: Total body length 6.60 mm; forewing length 4.50 mm. Head elongate, length 1.80 mm, width 1.47 mm (Figs 2, 3); clypeus longer than maximum width, length 0.60 mm, width 0.47 mm, almost entirety of clypeus (85%) set below lower tangent of compound eyes; frontal line weakly carinate between antennae, becoming a faintly impressed line from there to median ocellus; antennal scape relatively short (excluding basal bulb), length 0.50 mm; pedicel about as long as F1; F1 wider than long, slightly longer than F2; F2–F5 slightly wider than long; F6–11 progressively becoming longer than previous flagellomeres; distance from median ocellus to lateral ocellus 0.05 mm; between lateral ocelli 0.20 mm; ocellocular distance 0.22 mm (1.46× ocellar diameter); compound eye about 3.25x wider than gena in profile (width of compound eye 0.65 mm, width of gena 0.20 mm), beginning a little below middle of compound eyes. Intertegular distance 0.95 mm; mesoscutellum less than twice as long as metanotum (mesoscutellum length 0.35 mm, metanotum length 0.20 mm); dorsal surface of propodeum faintly concave, elongate (as for the genus: vide
Sculpturing: Integument smooth and polished, faintly imbricate in some places, with sparse small to minute punctures, except face above level of antennal toruli with punctures more closely spaced as well as on mesoscutum, mesoscutellum, and metanotum; punctures separated by 2–5× a puncture diameter on mesepisternum; dorsal surface of propodeum smooth, polished, and glabrous.
Coloration: Integument predominantly honey yellow with brownish areas laterally pronotum near pronotal lobe, dorsal surface of propodeum, apex of metafemur, external surface of metatibia, apical third of T1, apical two-thirds of T2–T3, and T4–T7 with yellowish areas slightly darker than elsewhere (darker areas wider and longer in paratype). Head metallic olive green except honey yellow on clypeus (sometimes with some brown areas extending from base in paratypes from French Guiana), labrum, mandible, malar area, and antennal scape; pedicel flagellum dark brown. Mesoscutum, mesoscutellum, and metanotum metallic olive green, less shiny than on head; axilla dark brown with metallic reflections; tegula translucent brown; axillary sclerites brown, base of C+Sc honey yellow otherwise wing venation brown to dark brown; pleura honey yellow except mostly brown on hypoepimeral area, with weak metallic green highlights (almost imperceptible in most places); wing membranes hyaline, slightly and faintly infumate apically, with some iridescence depending on lighting.
Pubescence:Pubescence largely consisting of golden simple setae. Head with scattered, largely simple setae, those on supraclypeal area, vertex, gena, and postgena longer; setae dorso-apically on scape longer, between one-third and one-half DS, remainder much shorter; a few short, branched setae in lower paraocular area and surrounding upper border of supraclypeal area; gena with uniformly distributed branched setae, setae with branches arising from one side of rachis in apical two-thirds; setae along borders with compound eyes very short. Mesosomal setae generally simple except more plumose around pronotal lobe and surrounding propodeal spiracle; disc of mesoscutum with relatively short and sparse setae, shorter than those of mesepisternum, although denser than on the latter; disc of mesoscutellum with setae little longer than those of mesoscutum, distinctly longer along posterior margin; metanotum with yellowish, short, plumose setae intermixed with longer, branched, golden setae, laterally longer, about 1.5DS; lateral and posterior surfaces of propodeum with long (about 2DS), largely-simple, scattered setae, although less numerous than on mesoscutum. Leg pubescence typical for male Augochlorini except distal half of anterior surface of procoxa with dense, long, branched setae, such setae about 1.2DS; anterior area formed by carinae along ventral surface of mesobasitarsus glabrous and separate from posterior area covered by minute setae. Wing membranes uniformly pilose. Metasoma generally with sparsely-scattered, simple, long setae, on T1–T3 simple setae mostly distributed across discs, with apical margins glabrous, such setae varying from 1–1.5DS in length, setae becoming progressively longer on more apical segments, on T4–T7 with short, dense, plumose setae intermixed with long setae.
♀:Unknown.
Male of Chlerogelloides nexosasp. n.collecting resources at flowers ofCordia nodosa Lam. (Boraginaceae) at the Estação Ecológica Ferreira Penna (Caxiuanã Reserve, Melgaço, Pará, Brazil). Photograph by
Photomicrographs of male paratype of Chlerogelloides nexosa sp. n. 2 Lateral habitus 3 Facial view.
Male midlegs of species of Chlerogelloides. 4 Chlerogelloides femoralis Engel et al. 5 Chlerogelloides nexosa sp. n. 6 Chlerogelloides simplex Engel and Brooks.
Male terminalia of Chlerogelloides nexosa sp. n. 7 Hidden and fused sterna VII+VIII 8 Genital capsule, lateral view 9 Genital capsule, dorsal view 10 Genital capsule, ventral view.
The specific epithet is taken from the Latin word nexosus, meaning “complicated”, and is a reference to the complex morphology of this and other species in the genus.
A single female, collected in French Guiana with one of the males, is indistinguishable from that of Chlerogelloides simplex (specimen from Patawa, deposited in Brussels). It is possible that it is a female of Chlerogelloides nexosa, but given that definitive Chlerogelloides simplex is known from the same area we cannot be certain that it is a definitive female of the new species. Only further collecting in the region will be able to determine the correct association of females for this complex genus of bees.
The present key is modified from that provided by
| 1 | Males | 2 |
| – | Females | 4 |
| 2 | Mesofemur greatly swollen and with one or two inner apical teeth (Figs 4, 5); metallic reflections of head and mesosoma tending toward green (Figs 2, 3, 13, 14); male terminalia as in figures 7–10 or 19–22 | 3 |
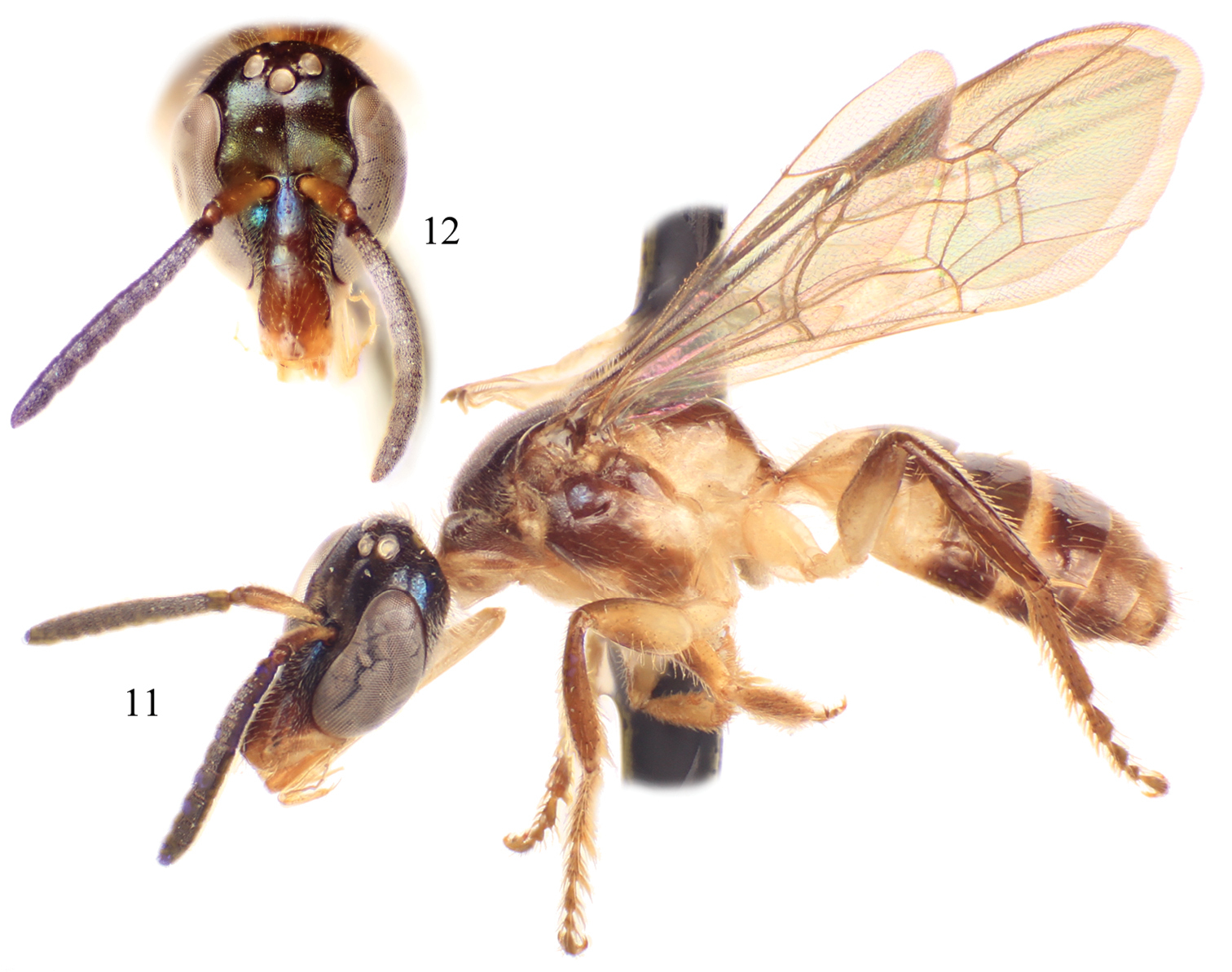
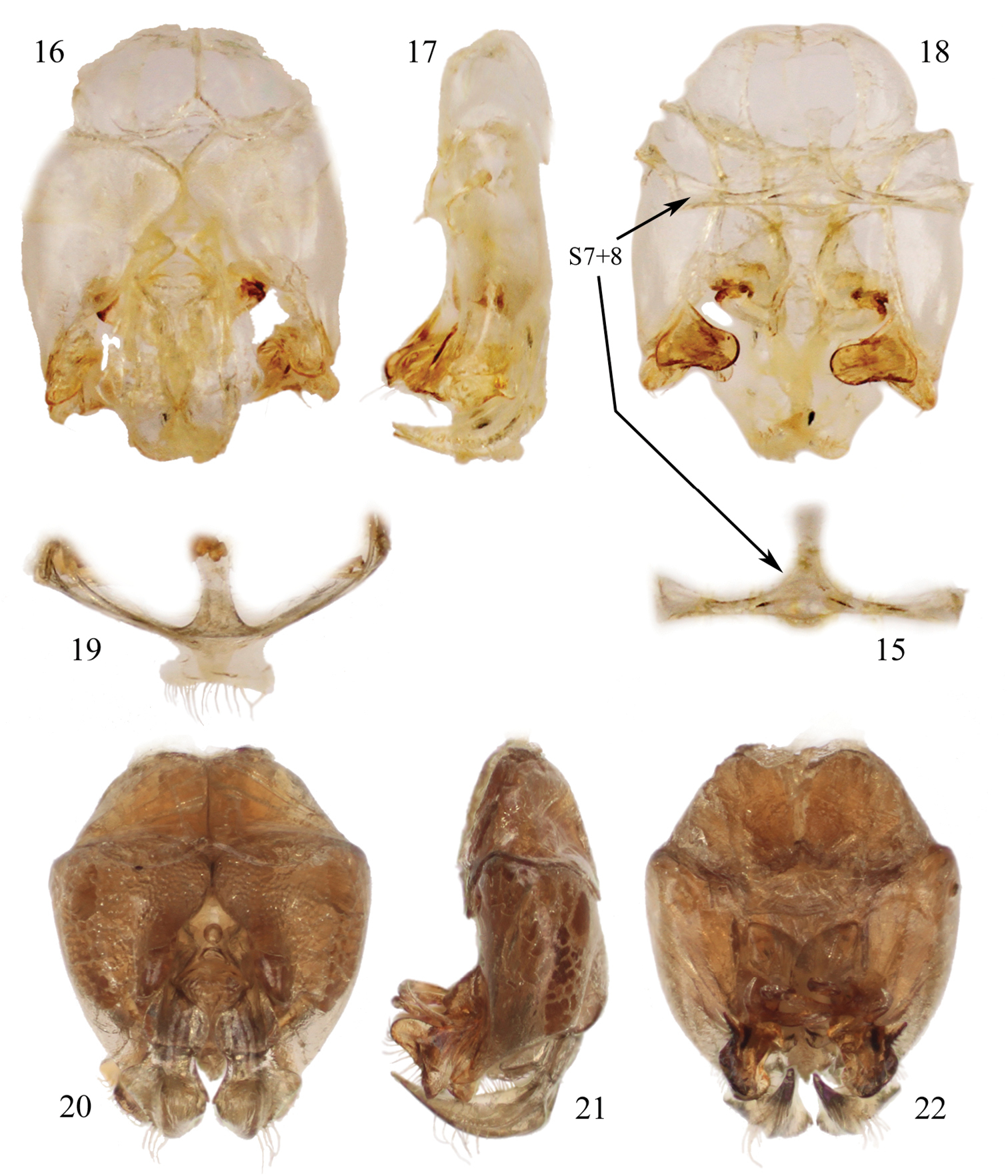
| – | Mesofemur simple, unmodified (Fig. 6); metallic reflections of head and mesosoma tending toward blue (Figs 11, 12); gena lacking distinctive tuft of setae; face above level of antennae with minute punctures closely packed; apical quarter to one-third of clypeus yellow; axilla dark brown; male terminalia as in figures 15–18 | Chlerogelloides simplex Engel & Brooks |
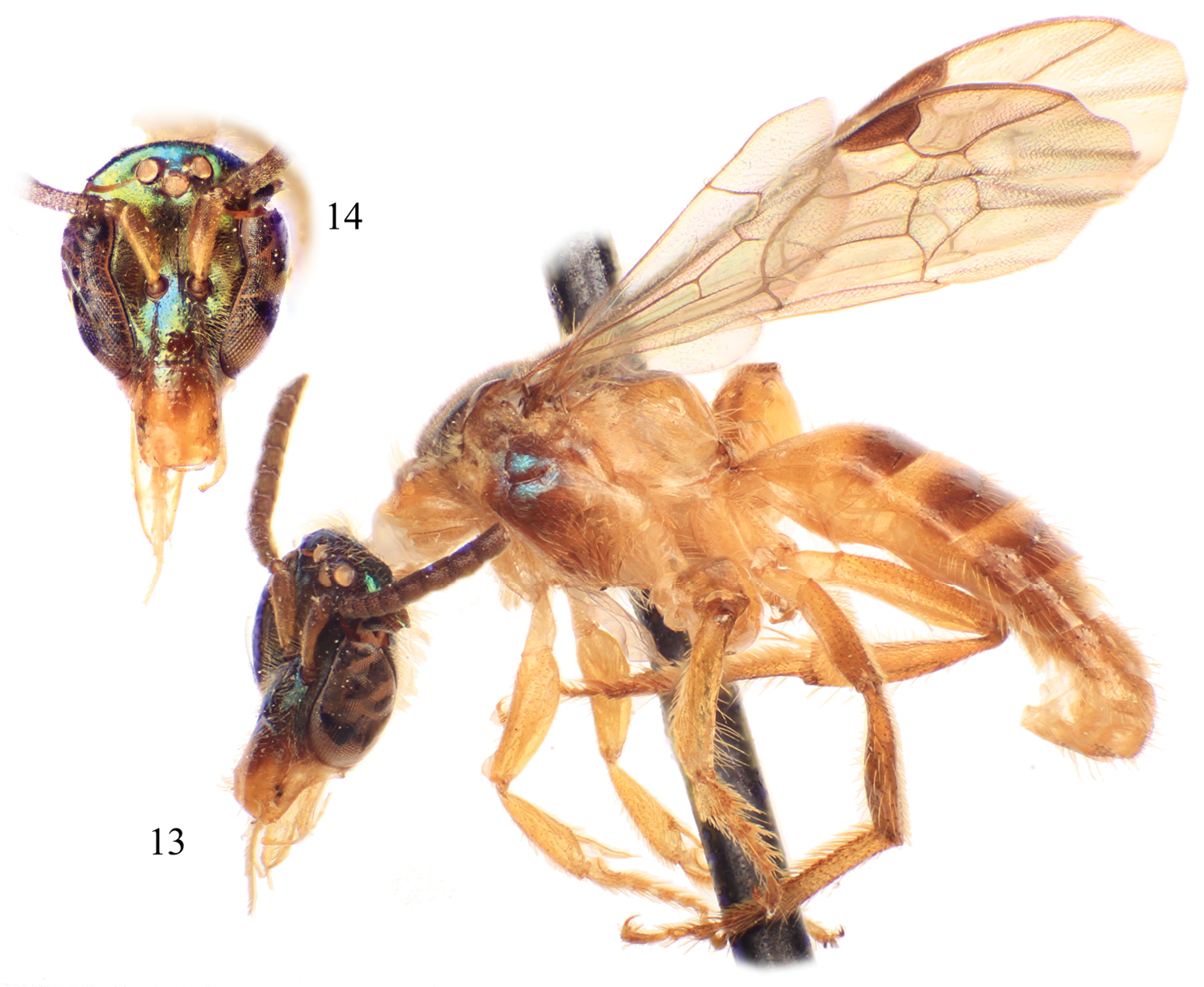
| 3 | Mesotrochanter with strong inner projection (Fig. 4); gena with distinctive tuft of long, plumose setae; face above level of antennae with minute punctures widely scattered over glabrous integument; apical two-thirds of clypeus yellow (Fig. 14); axilla yellow | Chlerogelloides femoralis Engel et al. |
| – | Mesotrochanter without strong inner projection (Fig. 5); gena without tuft of long, plumose setae; face above level of antennae with minute punctures closely packed; clypeus entirely but sometimes with extensive areas of brown extending from base (Fig. 3); axilla brownish | Chlerogelloides nexosa sp. n. |
| 4 | Clypeus and basal area of propodeum yellow | Chlerogelloides femoralis Engel et al. |
| – | Clypeus and basal area of propodeum brown | Chlerogelloides simplex Engel & Brooks |
Male of Chlerogelloides simplex Engel and Brooks. 11 Lateral habitus 12 Facial view.
Male of Chlerogelloides femoralis Engel et al. 13 Lateral habitus 14 Facial view.
Male terminalia of species of Chlerogelloides (15–18 Chlerogelloides simplex Engel and Brooks; 19–22 Chlerogelloides femoralis Engel et al.). 15 Hidden and fused metasomal sterna VII+VIII (in situ with capsule in figure 18) 16 Genital capsule, dorsal view 17 Genital capsule, lateral view 18 Genital capsule, ventral view (with hidden sterna VII+VIII in situ). 19 Hidden and fused sterna VII+VIII 20 Genital capsule, lateral view 21 Genital capsule, lateral view 22 Genital capsule, ventral view.
The three known species of Chlerogelloides are all quite similar, immediately noticeable for their modified, elongate clypeus which is basally intruded upon by a deeply acute and projecting epistomal sulcus, such that the narrowed epistomal lobe nearly reaches the clypeal apex. In addition, the species share a short malar space, a pronotum in which the dorsal surface is expanded, and a serrate inner metatibial spur in males and females (
At present the genus is known only by a meager diversity and relative paucity of material, despite the seemingly wide range of the lineage across South America. Accordingly, it is premature to attempt too contemplative of an investigation into the evolution of this genus, particularly given that the resulting three-taxon statement would have little explanatory power in the absence of more complete life-history or biological data for the constituent species. Nonetheless, if we presume that the modified midlegs of Chlerogelloides femoralis and Chlerogelloides nexosa sp. n. represent a derived feature indicating a close relationship between these two species, then Chlerogelloides simplex would appear to fall basal within the genus. This assumption seems safe for the moment given that such modifications of the midleg podites are not known in related or other genera of Augochlorini (
The authors are grateful to Dra. Márcia Motta Maués (EMBRAPA Amazônia Oriental, Belém do Pará, Brazil) and Dra. Marlúcia Bonifácio Martins (Museu Paraense Emílio Goeldi, Belém do Pará, Brazil), Dra. Blandina Felipe Viana (UFBA, Salvador, Bahia, Brazil), and Dr. Peter G. Kevan (University of Guelph, Canada) for organizing the Pollination Course 2011, during which the insects described herein where collected. The authors are also grateful to the students of the Pollination Course 2011, André Rodrigo Rech (UNICAMP, Campinas, São Paulo, Brazil), Christiano Peres Coelho (UFG, Goiânia, Goiás, Brazil), Fabrício da Silva Correa (EMBRAPA, Belém, Pará, Brazil), and Valdevino Santana do Carmo (CEPLAC, Bahia, Brazil), for their efforts in collecting during the course, including the capture of the two specimens recorded here and capturing the photograph reproduced as figure 1. We are indebted to all the institutions and companies supporting and making possible the Pollination Course 2011 [Empresa Brasileira de Pesquisa Agropecuária (EMBRAPA), Museu Paraense Emílio Goeldi (MPEG), Estação Ecológica Ferreira Penna, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Food and Agriculture Organization of the United Nations (FAO), Global Environment Facility (GEF), Fundo Brasileiro para a Biodiversidade (FUNBIO), Secretaria do Estado de Ciência, Tecnologia e Informação (SEDECTI), Ministério do Meio Ambiente (MMA), Prefeitura do Município de Portel, Canadian Pollinator Initiative (CANPOLIN), International Union of Biological Sciences (IUBS), Cooperativa Agrícola Mista de Tomé-Açu (CAMTA), Frigorífico FRIGOL and Fazenda Marupiara]. The senior and third authors are further grateful to the Brazilian Pollinator Initiative and the BIOSIS-UFBA Laboratory [CNPq, Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB), FAO, GEF, FUNBIO, MMA]. Lastly, we are grateful to A. Pauly, V.H. Gonzalez, and M. Ohl for editorial comments that improved the paper. Photomicrography was supported by the Engel Illustration Fund of the University of Kansas College of Liberal Arts and Sciences. This is a contribution of the Division of Entomology, University of Kansas Natural History Museum and was partially supported by US National Science Foundation grant DBI-1057366 (to MSE).