(C) 2011 Graham Olive. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The chemosymbiotic bivalves collected from the mud volcanoes of the Gulf of Cadiz are reviewed. Of the thirteen species closely associated with chemosynthetic settings two Solemyidae, Solemya (Petrasma) elarraichensis sp. n. and Acharax gadirae sp. n., one Lucinidae, Lucinoma asapheus sp. n., and one Vesicomyidae, Isorropodon megadesmus sp. n. are described and compared to close relatives of their respective families. The biodiversity and distribution of the chemosymbiotic bivalves in the Gulf of Cadiz are discussed and compared to the available information from other cold seeps in the Eastern Atlantic and Mediterranean. Although there is considerable similarity at the genus level between seep/mud volcano fields in the Eastern Atlantic and Mediterranean, there is little overlap at the species level. This indicates a high degree of endemism within chemosymbiotic bivalve assemblages.
Bivalvia, chemosymbiotic, taxonomy, Gulf of Cadiz
Chemosynthetic bivalves are prominent constituents of the
fauna of cold seeps and are represented in that setting by five
families: Solemyidae, Lucinidae, Vesicomyidae, Thyasiridae and Mytilidae (
The occurrence of chemosymbiotic bivalves in the extensive mud volcano fields of the Gulf of Cadiz was first reported by
Chemosynthetic bivalve faunas have been discovered elsewhere in the Eastern Atlantic, notably off tropical West Africa (
This paper intends to provide the taxonomic basis for the chemosynthetic bivalves in the Gulf of Cadiz and includes the description of two new species of Solemyidae, one new species of Lucinidae and one new species of Vesicomyidae. Notes on the biogeography of these taxa in the Atlantic are given with special emphasis on the relationships between the Eastern Mediterranean, Gulf of Cadiz and West Africa.
Materials and methods Study areaThe Gulf of Cadiz is located in the NE Atlantic Ocean
between 34°N and 37°15'N and 6°W to 9°45'W. It is enclosed by the
southern Iberian and northern Moroccan margins, west of Gibraltar
Strait. The geological history of the Gulf of Cadiz is intimately
related to plate tectonic interaction between Southern Eurasia and North
Africa and is driven by two major mechanisms: a) subduction associated
with the westward emplacement of the Gibraltar Arc and formation of the
Gulf of Cadiz accretionary wedge, probably not active at present and b)
oblique lithosphere collision between Iberia and Nubia, active at
present and causing active thrusting (
In the shallow Moroccan margin the El Arraiche field
encompasses Renard Ridge (including Pen Duick Escarpment), Vernadsky
Ridge and several mud volcanoes (e.g. Al Idrisi, Mercator, Fíuza,
Gemini, Kidd MVs) located at depths from 200 to approximately 600m
depth: The proximity to the euphotic zone and to the African coast adds
to the great productivity observed in the area. Dead cold-water
scleractinean coral reefs, carbonate crusts and exposed carbonate
chimneys characterize the Renard and Vernadsky Ridges. Carbonate crusts,
rock blocks and clasts are often found in the craters of the shallow
mud volcanoes where mild seepage activity has been recorded (
The western Moroccan field comprises several mud volcanoes (e.g. Meknès, Student, Yuma, Ginsburg, Jesus Baraza, Darwin MVs) at intermediate depths (700–1200m) located along an extensive province of carbonate and mostly dead cold-water coral mounds. The widespread presence of authigenic carbonates and also extensive Neptunea and Bathymodiolus graveyards (usually within the crater of the mud volcanoes) suggest that this was a very active seepage area in the past. Darwin MV differs from the others in this area because its crater is completely covered by large carbonate slabs and crusts. The fissures among slabs and depressions with scattered crust are filled with abundant shell ash and occasionally small clumps of living Bathymodiolus mauritanicus Cosel, 2002. Meknès MV is the southernmost Moroccan mud volcano rising isolated among an extensive field of small coral mounds. The crater is formed by stiff, sometimes heavily disturbed, green mud breccia with scattered clasts and a striking large number of empty shells of the gastropod Neptunea. Except for a few Paromola individuals, living megafauna is rarely sighted in the crater
The deep-water field (1300–4000m), mostly within the
Portuguese margin includes several mud volcanoes (e.g. Captain
Arutyunov, Carlos Ribeiro, Bonjardim and Porto MVs) that are aligned
along major crustal strike–slip faults associated with the
African-Eurasian plate boundary (
Samples were collected between 2002 and 2006 during TTR (Training Through Research) 12, TTR 14, TTR15 and TTR16 cruises onboard RV Prof. Logachev and MSM.01-03 cruise onboard RV Maria S. Merian (IFM–GEOMAR). The material was collected using TV-assisted grabs or USNEL box-corers. Occasionally faunal specimens were also recovered from Reineck box-corer, multiple corer or lander samples that were carried out for different purposes. Whenever possible the specimens were sorted onboard and preserved in 70 or 96% ethanol (the latter preserved for molecular analysis).
Deposition of samplesThe majority of specimens are deposited in the Biological Research Collection of the Department of Biology, University of Aveiro but the holotype; some paratypes and selected specimens are deposited in the National Museum of Wales.
Institutional abbreviations.DBUA, Department of Biology, University of Aveiro (Biological Research Collection); IFM–GEOMAR, Institut für Meereskunde - Forschungszentrum für marine Geowissenschaften; IOC–UNESCO, Intergovernmental Oceanographic Commission – United Nations Educational, Scientific and Cultural Organization; NMW.Z, National Museum of Wales, Cardiff, Great Britain.
MeasurementsAll measurements were made using Sylvac™ vernier calipers accurate to 0.01mm but are given to the nearest tenth.
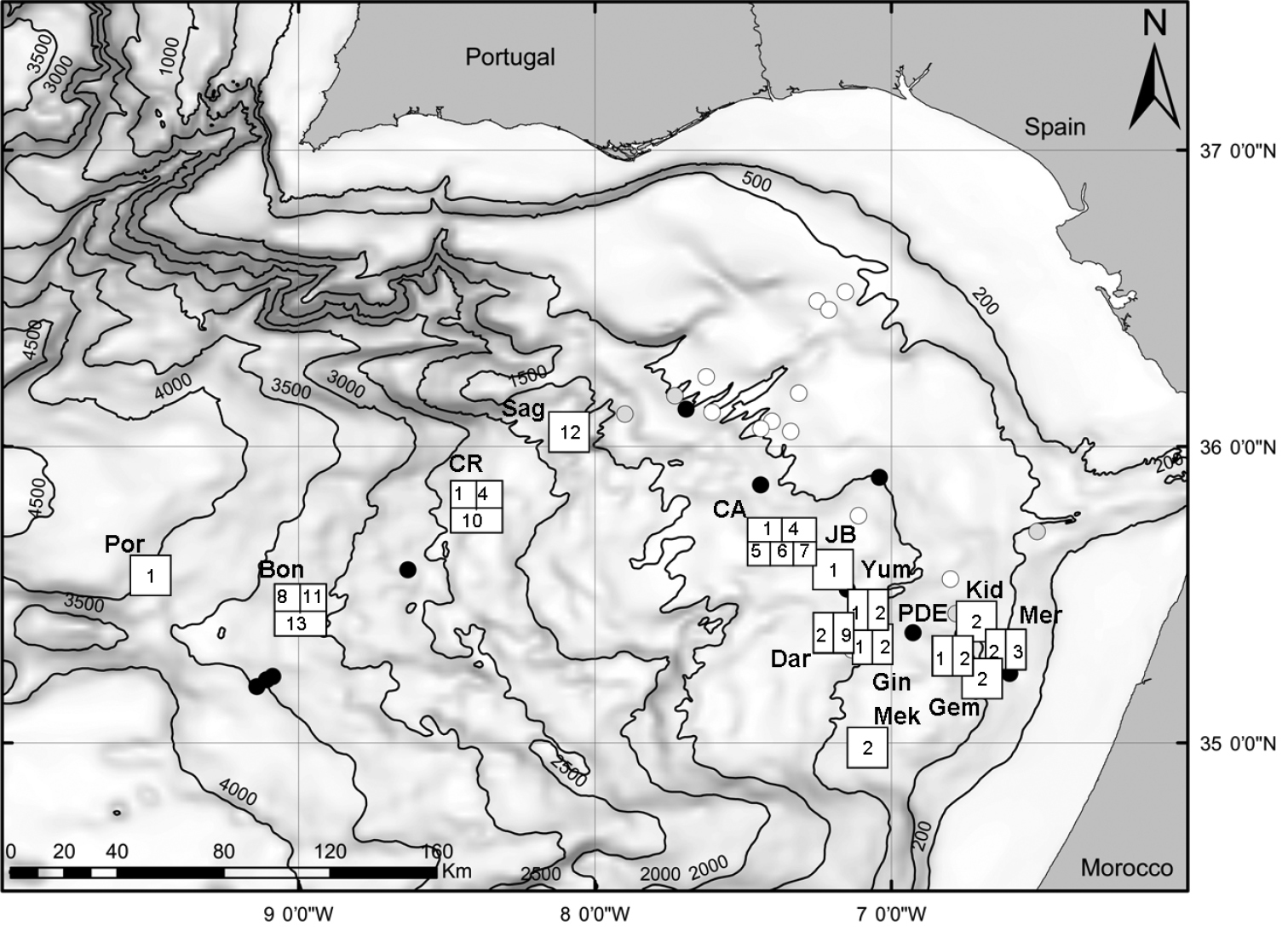
Map of the study area (Gulf of Cadiz) and location of sampling sites. squares with numbers, mud volcanoes with chemosymbiotic bivalves: full black circles, mud volcanoes visited during the study but bivalves not found: grey circles: mud volcanoes and other structures not visited during the study. Bon, Bonjardim MV; CA, Captain Arutyunov MV; CR, Carlos Ribeiro MV; Dar, Darwin MV; Gem, Gemini MV; Gin, Ginsburg; JB, Jesus Baraza MV; Kid, Kidd MV; Mek, Mèknes MV; Mer, Mercator MV; PDE, Pen Duick Escarpment; Por, Porto MV; Sag, Sagres MV; Yum, Yuma MV. The numbers inside the squares indicate the presence of the following species. 1 Acharax gadirae 2 Petrasma elarraichensis 3 Lucinoma asapheus 4 Thyasira vulcolutre 5 Spinaxinus sentosus 6 Isorropodon megadesmus 7 Isorropodon sp. indet. 8 Christineconcha cf. regab 9 Bathymodiolus mauritanicus 10 Idas sp. 11 Laubiericoncha chuni (empty shells only) 12 Callogonia cyrili (empty shells only) 13 Pliocardia sp. (empty shells only).
Superfamily Solemyoidea Gray, 1840
Family Solemyidae Gray, 1840
Solemyids are among the most ancient bivalves dating from the Paleozoic (
Solemyidae taxonomy is complex.
Superficially, all solemyids appear so similar that
specimens discovered at various deep-sea sites might have been
misclassified as Solemya (see review by
Genus Solemya Lamarck, 1818
Solemya borealis Totten, 1834
As given by
urn:lsid:zoobank.org:act:32A6013D-4378-462A-BABA-4AFC4D26FB1E
http://species-id.net/wiki/Solemya_(Petrasma)_elarraichensis
Figs 2, 3A–D, 4Holotype: one specimen, TTR14, stn AT528GR, El Arraiche field, Kidd MV, 35°25.304'N, 06°43.972'W, 489m, 03 August 2004, NMWZ.2010.4.1
Paratypes: ten specimens, same data as holotype, NMWZ.2010.4.2; seven specimens, TTR15, stn AT569GR, El Arraiche field, Mercator MV, 35°17.917'N, 06°38.717'W, 358m, 25 July 2007, DBUA.
Other material examined: eight juveniles specimens, same data as holotype; two specimens, TTR12, stn AT407GR, El Arraiche field, Pen Duick Escarpment, 35°17.695'N, 06°47.082'W, 560m, 15 July 2002; three specimens, TTR14, stn AT560B, El Arraiche field, Kidd MV, 35°25.306'N, 06°43.976'W, 498m, 8 August 2004; one specimen, TTR15, stn AT586GR, Western Moroccan field, Meknès MV, 34°59.146'N, 07°04.380'W, 701m, 28 July 2005; four specimens, TTR16, stn AT604GR, Western Moroccan field, Yuma MV, 35°25.820'N, 07°06.330'W, 1030m, 29 May 2006; two specimens, TTR16, stn AT607GR, Western Moroccan field, Ginsburg MV, 35°22.677'N, 07°04.979'W, 983m, 29 May 2006.
| Station | Length | Height | Posterior Length | |
|---|---|---|---|---|
| Holotype | AT528GR | 33.8 | 14.1 | 9.8 |
| Paratype | AT528GR | 29.2 | 10.5 | 8.0 |
| Paratype | AT528GR | 25.6 | 10.0 | 6.2 |
| Paratype | AT528GR | 23.1 | 8.4 | 6.0 |
| Paratype | AT528GR | 14.7 | 5.3 | 3.6 |
| Paratype | AT528GR | 22.0 | 7.9 | 6.0 |
| Paratype | AT528GR | 11.6 | 4.7 | 2.5 |
| Paratype | AT528GR | 17.6 | 6.8 | 5.2 |
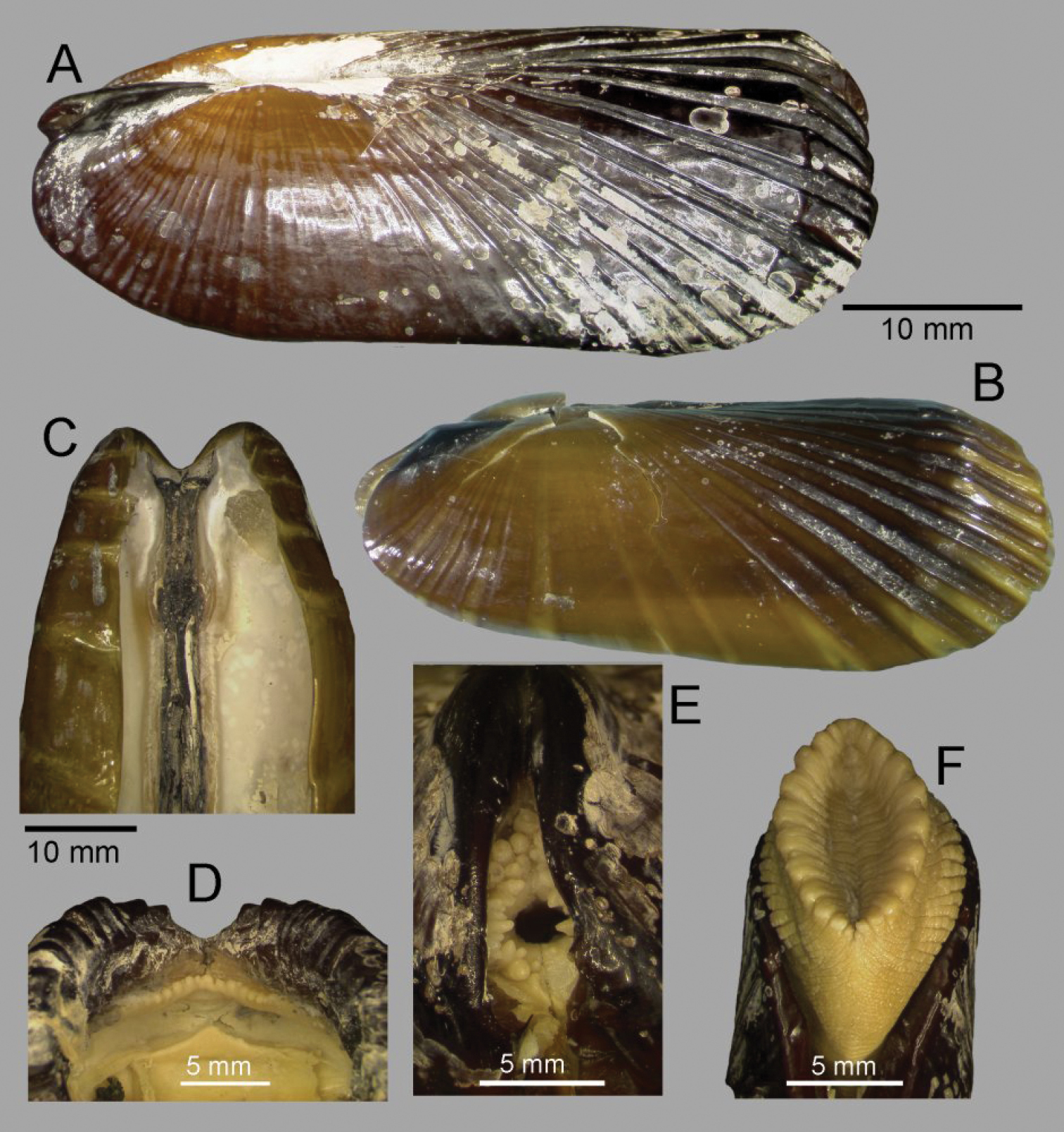
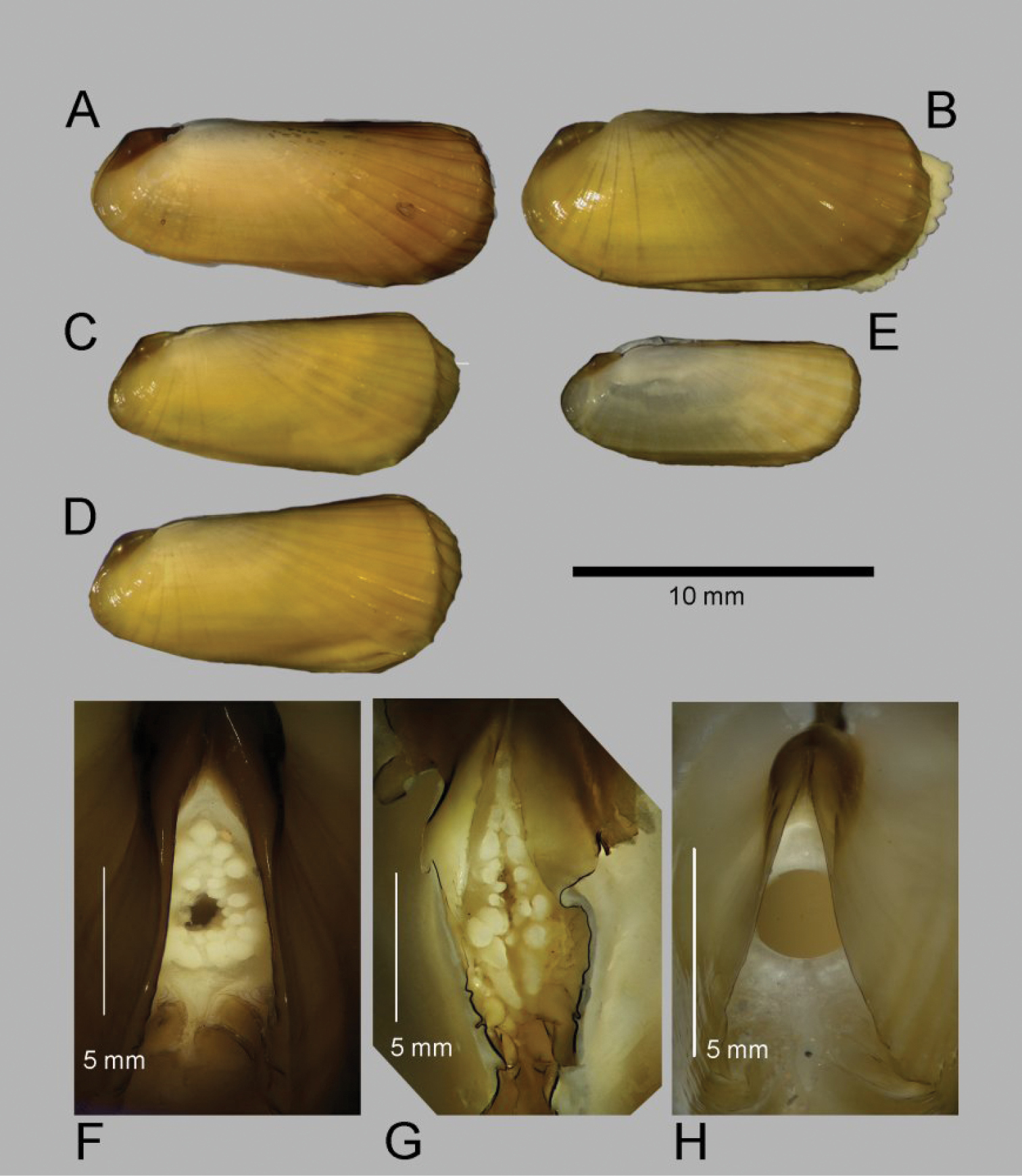
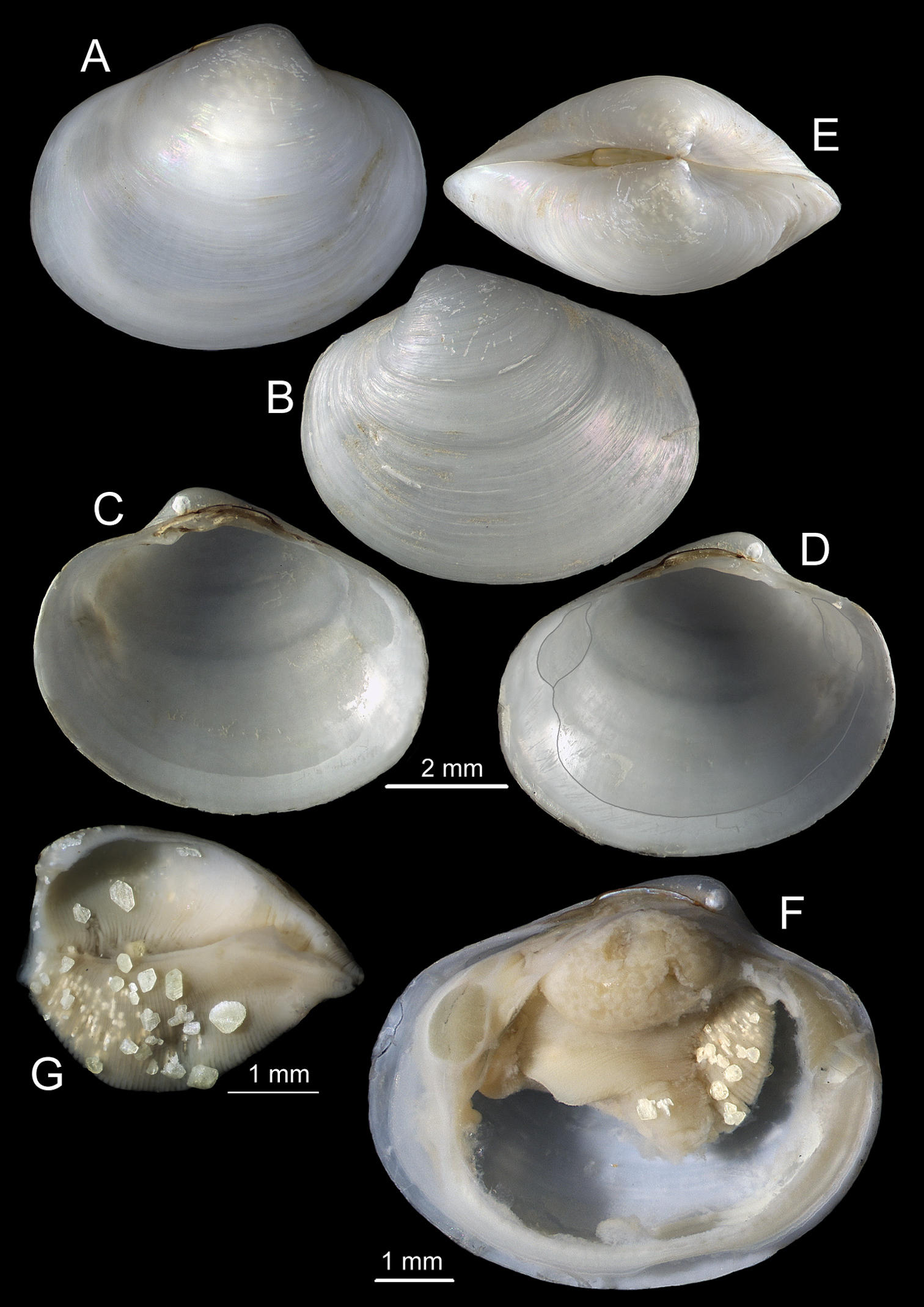
Shell (Figs 2, 3): to 35mm in length. Fragile. Equivalve. Inequilateral, beaks situated at 1/4 length of shell from posterior margin. Outline subcylindrical, compressed, length about 2.6 times height, slightly deeper towards the anterior, dorsal and ventral margins subparallel, anterior margin more broadly rounded than anterior, posterior dorsal margin projecting a little. Beaks indistinct, umbos sunken. Hinge teeth absent. Ligament primarily internal, supported by a prominent chondrophore that extends only slightly as a chondrophore ridge around the posterior adductor, lacking posterior and anterior extensions but a small roughly heart shaped area is present in front of the chondrophore and this is also visible externally just behind the beaks. Periostracum persistent and extending well beyond the shell margin, initially yellowish brown in colour but darkening with growth to a dark chestnut brown. Sculpture of weak radial ridges, 5–6 over the posterior and 10–12 over median and anterior. Adductor scars impressed, dorsal part of posterior scar angulate where bounded by chondrophore ridge, anterior adductor scar larger, spatulate in outline.
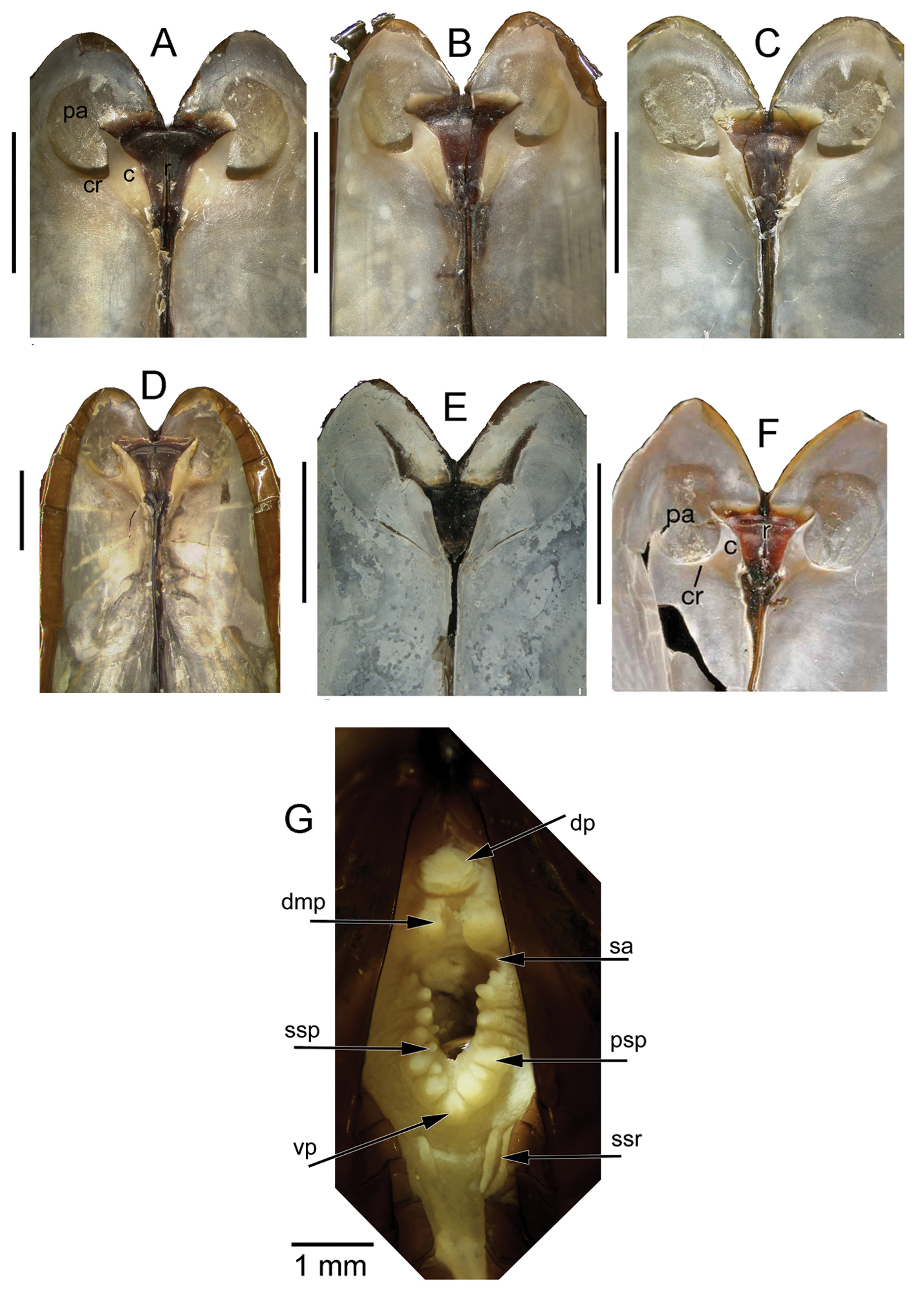
Anatomy (Figs 3G, 4): The posterior siphonal opening is surrounded by a series of papillae: A single large dorsal papilla (dp) lies above two smaller but still large papillae (dmp) on the dorsal margin of the opening, below these is a short smooth section (sa) followed by a series of papillae increasing in size towards the ventral margin, there are 6 primary papillae (psp) on either side and a single ventral median papillae, between these on the inner side are smaller papillae (ssp); a pair of subsiphonal ridges (ssr) are present below the siphonal crown.
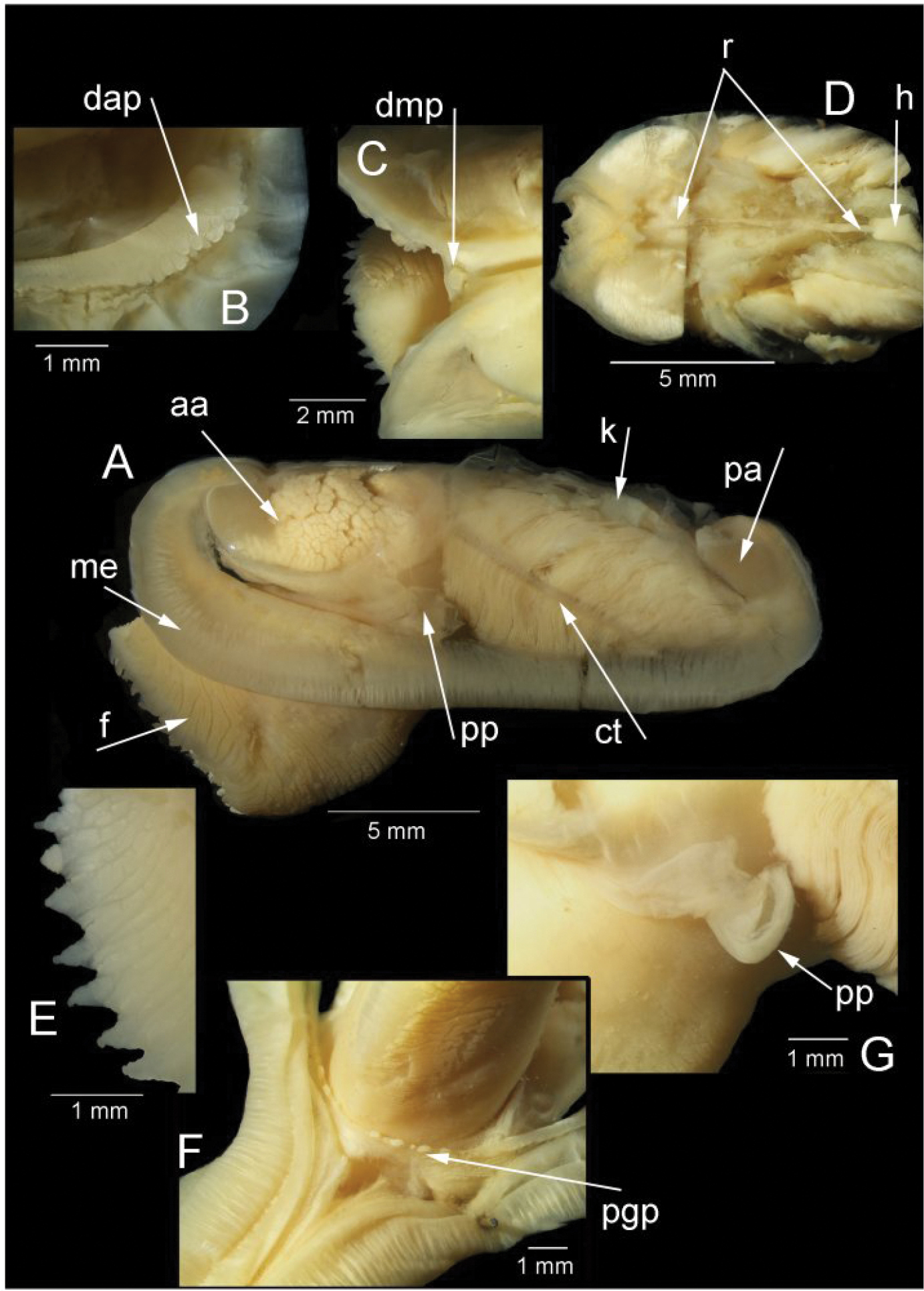
The mantle edge is fused from the posterior siphon for half the length of the ventral margin where there is a large anterior pedal gape. The mantle edge surrounding the rear of the foot bears a few tabulate papillae (pgp). The anterior dorsal mantle edge is prominently papillate (dap) and there is a single papilla on the junction of the mantle edge anterior of the anterior adductor muscle (admp). The foot is very large with a broad oval sole, this fringed by large papillae, all equal in size. The ctenidium is large with numerous laminar filaments attached to a prominent gill axis. The palps are short, twisted and flattened with cup shaped terminations. The gut is present but difficult to examine due to its small dimensions but the hind gut and rectum were easily visible.
Solemya (Petrasma) elarraichensis is presently only known from the mud volcano fields in the Gulf of Cadiz, Eastern Atlantic. The majority of specimens have been taken from the El Arraiche field off the coast of Morocco in Kidd, Fíuza and Mercator MVs and the Pen Duick Escarpment at depths between 358–560m. A few specimens have been taken from the Western Moroccan field at the Meknès, Yuma, Ginsburg and Darwin MVs at the slightly deeper range of 700–1115m.
elarraichensis, denoting the geographic origin of the type locality; the El Arraiche field.
The form of the ligament, which is primarily
internal, supported by a chondrophore and lacks any lateral or
anterior extensions, confirms the placement of Solemya (Petrasma) elarraichensis in the subgenus Petrasma Dall, 1908 (
The subgenus Petrasma
is not known from the North-East Atlantic but is represented in the
Western Atlantic by three species. Two species are known from near shore
waters off the northeast coast of the USA: Solemya (Petrasma) velum (Say, 1822) and Solemya (Petrasma) borealis (Totten, 1834). The third, Solemya (Petrasma) occidentalis (
We note that the curvature of the chondrophore and chondrophore ridge is circular in Solemya (Petrasma) velum (Fig. 3F) but angular in Solemya (Petrasma) elarraichensis (Figs 3A–D). Furthermore, the siphonal papillae of Solemya (Petrasma) velum are by comparison less in number and reduced in development (Morse 1913,
The character of the ligament and chondrophore are rather similar in Solemya (Petrasma) elarraichensis and Solemya (Petrasma) borealis.
Ecologically Solemya (Petrasma) velum and Solemya (Petrasma) borealis are very different from Solemya (Petrasma) elarraichensis
in that they are not associated with deep-water methane seeps. In
contrast they are found in sublittoral or shallow shelf settings with
high organic enrichment (Morse 1913 in
Given the above differences in habitat and form we conclude that none of the Atlantic species is amphi-Atlantic, unlike Solemya (Petrasma) pervernicosa Kuroda, 1948, which is considered to be amphi-Pacific by
Other North Atlantic species referred to as Solemya, Solemya grandis Verrill and Bush, 1898 and Solemya caribbaea Vokes, 1970 are excluded here because both belong to the genus Acharax (
A solemyid living at a pockmark, at a depth of 1607m, has been reported from the Eastern Mediterranean (
In conclusion, there are sufficient morphological and ecological grounds for considering the Gulf of Cadiz species of Petrasma to be new to science.
Solemya (Petrasma) elarraichensis sp. n. A–E from Kidd MV; A–B lateral and dorsal views of holotype C–D lateral and dorsal views of medium sized paratype E lateral view of small paratype. F paired valves from Pen Duick Escarpment G paired valves from Mercator MV H lateral view of specimen from Meknès MV I lateral view of shell from Yuma MV.
Internal views of ligament, scale bars = 5mm. A–D Solemya (Petrasma) elarraichensis sp. n. from A Kidd MV B Pen Duick Escarpment C Mercator MV D Yuma MV. E Solemya togata, Mediterranean F Solemya (Petrasma) velum, Rhode Island (from
Solemya (Petrasma) elarraichensis sp. n., Pen Duick, stn. AT407GR, 560m. Anatomy. A whole animal viewed from left side B papillae on dorsal anterior mantle edge C single, large papilla in dorsal median position D posterior dorsal dissection showing rectum passing through heart E marginal papillae on foot F papillae on mantle edge surrounding pedal gape G palp. aa, anterior adductor muscle. ct, ctenidium. dap, dorsal anterior papillae. dmp, dorsal median papilla. f, foot. h, heart. k, kidney. me, mantle edge. pa, posterior adductor muscle. pgp, papillae surrounding pedal gape. pp, palp. r, rectum.
Solemya johnsoni Dall, 1891
As given by
urn:lsid:zoobank.org:act:A2467D10-D03D-4271-9993-0C2A6CD22944
http://species-id.net/wiki/Acharax_gadirae
Figs 5-6Holotype: one specimen, TTR12, stn AT391GR, Western Moroccan field, Jesus Baraza MV, 35°35.439'N, 07°12.264'W, 1105m, 09 July 2002, NMWZ.2010.4.3.
Paratypes: one specimen, same data as holotype, DBUA; one shell, TTR 12, stn AT392G, deep-water field, Captain Arutyunov MV, 35°39.658'N, 07°20.018'W, 1320m, 9 July 2002, DBUA; one shell, TTR 16, stn AT607GR, Western Moroccan field, Ginsburg MV, 35°22.677'N, 07°04.979'W, 983m. 29 May 2006, NMWZ.2010.4.4.
Other material examined: one specimen, TTR16, stn AT602GR, El Arraiche field, Pen Duick Escarpment, 35°17.693'N, 06°47.089'W, 556m, 28 May 2006; one specimen, TTR16, stn AT604GR, Western Moroccan field, Yuma MV, 35°25.820'N, 07°06.330'W, 1030m, 29 May 2006; one specimen, TTR16, stn AT605GR, same locality, 35°25.046'N, 07°05.450'W, 975m, 29 May 2006; one specimen, TTR16, stn AT615GR, deep-water field, Carlos Ribeiro MV, 35°47.238'N, 08°25.272'W, 2200m, 31 May 2006; one specimen, TTR16, stn AT617K, same locality, 35°47.246'N, 08°25.303'W, 2230m, 31 May 2006; two specimens, MSM01.03, stn 145, deep-water field, Porto MV, 35°33.773'N, 09°30.416'W, 3902m, 3 June 2006.
| Station | Calcified Shell Length | Calcified Shell Height | Calcified ShellPosterior Length | Actual length | Anterior Ribs/Posterior Ribs | |
|---|---|---|---|---|---|---|
| Holotype | AT391GR | 56.3 | 21.1 | 14.5 | 59.5 | 9/4 |
| Paratype | AT391GR | 60.0 | 22.0 | 14.2 | 65.4 | 8/4 |
| Paratype | AT392GR | 67.0 | 25.0 | 19.1 | 85.0 | 8/4 |
| Paratype | AT607 GR | 42.8 | 15.9 | 10.6 | 48.9 | 9//4 |
(Fig. 5) Calcified shell to 67mm in length, to 85mm including periostracal fringe. Robust. Equivalve. Inequilateral, beaks situated at 1/4 length of shell from posterior margin. Outline subcylindrical, compressed, calcified shell length about 3 times height, slightly deeper towards the anterior, dorsal and ventral margins subparallel, anterior margin more broadly rounded than anterior, posterior dorsal margin projecting a little. Including periostracal fringe, anterior appears greatly expanded compared with posterior. Beaks indistinct, umbos sunken. Hinge teeth absent. Ligament external, as a high arched band posterior of the beaks and supported by a thickened shell margin; an oval area of ligament is present immediately behind the beaks and visible internally, anterior of the beaks shell margins fused by periostracal material along entire dorsal margins. Periostracum persistent and extending well beyond the shell margin, initially yellowish brown in colour but darkening with growth to dark brown and black; periostracal frill thickened over ribs but entire. Sculpture of radial ridges, 4 closely spaced over the posterior; median area almost smooth with 2–3 low ribs; anterior with 8–9 deeply cut ribs. Adductor scars impressed, posterior scar subcircular, anterior adductor scar larger, spatulate in outline. Anterior inner shell margin scalloped corresponding to radial ribs.
Posterior siphonal opening surrounded by a series of papillae (Fig. 5E): A single large dorsal papilla (dp) lies above 2–3 pairs of slightly smaller papillae (dmp) on the dorsal margin of the opening, below these surrounding the opening is a series of approximately alternating large and small papillae with those most ventral the largest.
The mantle edge is fused from the posterior siphon for half the length of the ventral margin where there is a large anterior pedal gape. The mantle edge surrounding the rear of the foot is papillate. The anterior dorsal mantle edge is prominently papillate (Fig. 5D). The foot is very large with a broad oval sole, the margin interdigitates between large and small blunt papillae. The ctenidium is large with numerous laminar filaments attached to a prominent gill axis. The palps are short, twisted and flattened with cup shaped terminations. The presence or absence of a gut could not be confirmed.
Acharax gadirae is presently only known from the mud volcano fields in the Gulf of Cadiz, Eastern Atlantic. The specimens have been taken from the Western Moroccan field at Yuma, Ginsburg and Jesus Baraza MVs, and from the deep-water field at Captain Arutyunov, Carlos Ribeiro and Porto MVs at depths between 975 to 3902m. A single specimen was recovered from the shallower El Arraiche field in Pen Duick Escarpment at 556m.
gadirae, from the Phoenician “Gadir” the original name for Cadiz and meaning “walled fortification” and also the root of many Moroccan names such as Agadir. Named to indicate the widespread range across the Moroccan and Iberian margins.
The genus Acharax is recognizable from the large external ligament and the generic placement of Acharax gadirae is confirmed.
The genus is rare in the Atlantic Ocean unlike the situation in the Pacific where species of Acharax are frequently recorded from chemosynthetic settings (
Acharax grandis differs from both Acharax gadirae and Acharax caribbaea in being less inequilateral with the beaks distinctly more towards the mid-line. Acharax caribbaea differs from both Acharax gadirae and Acharax grandis in having very few (4) anterior ribs compared with the 6–8 on Acharax grandis and 8–9 on Acharax gadirae of similar size. Unfortunately, there are no anatomical data for either Acharax grandis or Acharax caribbaea, making a thorough comparison impractical.
There are no given ecological data for either Acharax grandis or Acharax caribbaea. The type locality for Acharax grandis,
which is the region around the Hudson Shelf and Canyon, has no
recorded seep or vent activity. In contrast the region around the type
locality of Acharax caribbaea is known for a variety of chemosynthetic settings (
The bathymetric range of Acharax in the Gulf of Cadiz is large, 556–3902m and specimens have been taken at many mud volcanoes raising the possibility that more than one species is involved. Unfortunately the specimens from the abyssal sites are all small about 10mm or less making comparison with the large specimens from the bathyal sites inconclusive. The specimens from Carlos Ribeiro MV (2200m) (Fig. 6C–D) are prominently wedge shaped in outline compared with the specimen from Porto MV (3902m) (Fig. 6B). The latter is not dissimilar to those from Capt. Arutyunov MV (1325m) (Fig. 6E) with the specimen from Pen Duick Escarpment (556m) (Fig. 6A) somewhat more elongate but not as wedge shaped as those from Carlos Ribeiro MV.
The specimens from the Capt Arutyunov MV are most problematic in that the siphonal opening appears to be devoid of any surrounding tentacles or papillae (Fig. 6H). This does not appear to be a function of size as similar specimens from other sites have siphonal papillae. Should this observation be confirmed in further material it would be appropriate to describe this as a separate species. Comparing the siphonal papillae of specimens from Pen Duick Escarpment (Fig. 6F) and Carlos Ribeiro MV (Fig. 6G) indicates a more complex arrangement in the latter but, with so few specimens, this is inconclusive.
Family Lucinidae Fleming, 1828
The Lucinidae is, by far, the most disparate and species-rich family of chemosymbiotic bivalves and are thoroughly reviewed by
Acharax gadirae sp. n. A Holotype, stn. AT391GR, Jesus Baraza MV B Paratype, stn. AT607GR, Ginsburg MV C interior view of posterior ligament, stn. AT392GR, Jesus Baraza MV D anterior dorsal mantle edge, st. AT391GR E posterior siphon, st AT391GR F foot, stn. AT391GR
Acharax gadirae sp. n. A stn. AT602GR, Pen Duick Escarpment B stn. 145, Porto mud volcano C–D stns AT617GR & AT61GR, Carlos Ribeiro mud volcano E stn. 199, Capt Arutyunov mud volcano. F–E posterior siphon F specimen A G specimen D H specimen E.
Lucina filosa
As given by
urn:lsid:zoobank.org:act:E684B2EE-7C97-4FE9-9445-9B00CFE0259B
http://species-id.net/wiki/Lucinoma_asapheus
Fig. 7Holotype; one complete specimen, live collected, TTR 15, stn AT569GR, El Arraiche field, Mercator MV. 35°17.917'N, 06°38.717'W, 358m, 25 July 2005, NMWZ.2010.4.5.
Paratypes; five specimens, as holotype, NMWZ.2010.4.6.
| Length | Height | Width | Anterior scar length | Anterior scar angle | Lunule width | |
|---|---|---|---|---|---|---|
| Holotype | 33.3 | 30.1 | 15.9 | 15.0 | 15° | 2.2 |
| Paratype | 25.0 | 23.0 | 10.8 | 11.3 | 17° | 1.6 |
| Paratype | 30.7 | 27.6 | 18.0 | 14.6 | 16° | 2.6 |
| Paratype | 28.8 | 27.1 | 13.5 | 13.0 | 15° | 1.7 |
| Paratype | 32.1 | 29.2 | 16.4 | 16.4 | 18° | 2.6 |
| Paratype | 31.7 | 29.4 | 15.4 | 16.4 | 15° | 2.2 |
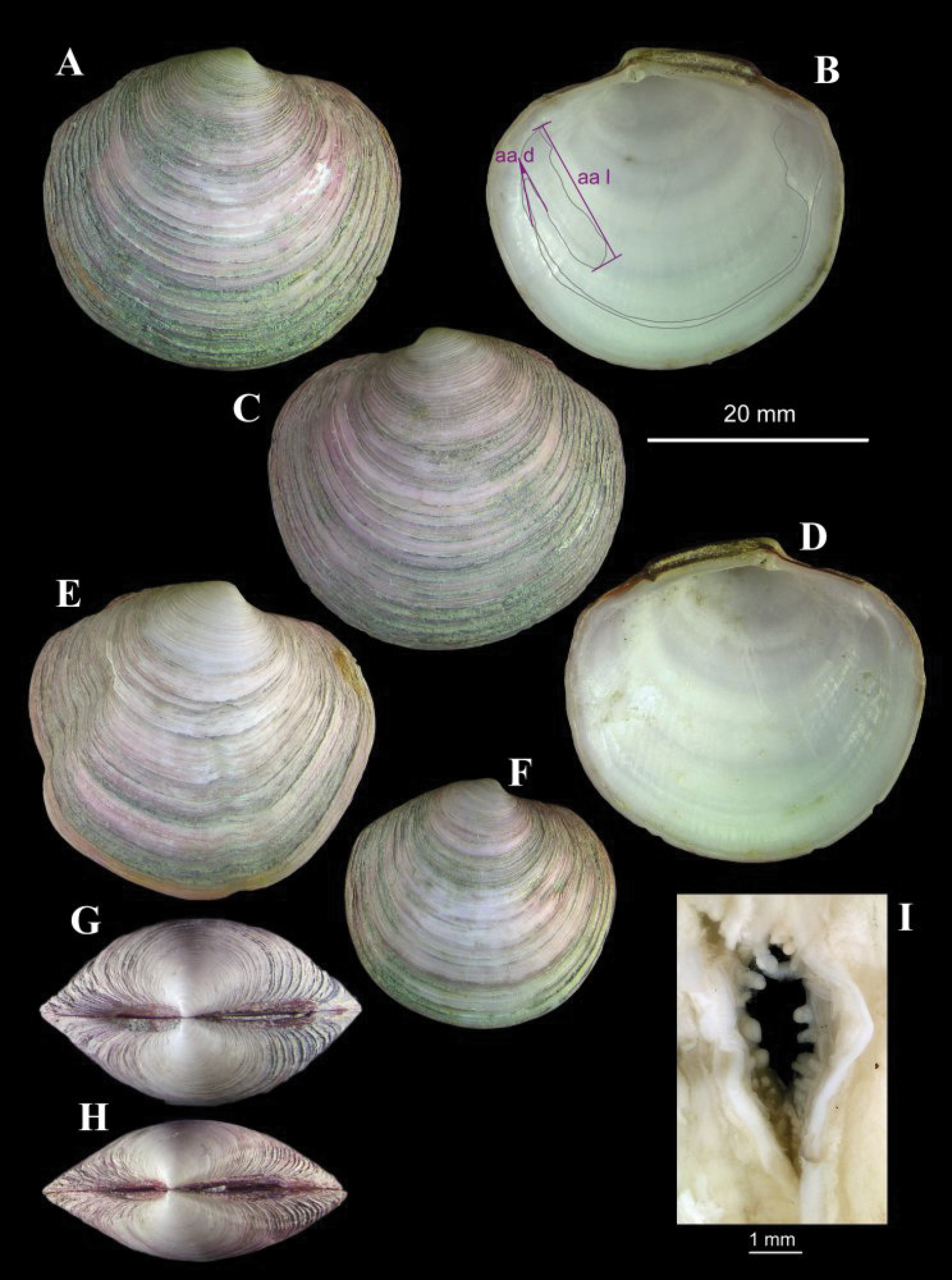
(Fig. 7). Shell to 34 mm in length. Solid. Equivalve. Equilateral. Tumidity variable (Fig. 7G, H) but mostly rather compressed. Umbos low, beaks pointing forward. Outline lenticular; posterior dorsal margin almost straight, sloping gently; posterior margin curved but less so than anterior; anterior dorsal margin short, a little concave. Escutcheon narrow, edges slightly raised, extending the length of the posterior dorsal margin; three-quarters filled by ligament, remainder smooth. Lunule distinct, width dependant on tumidity of shell; edges raised, sharp. Sculpture of numerous, low but erect, thin, concentric lamellae; between lamellae are weak concentric lines. Ligament external as a prominent, raised, arched band. Set on a narrow nymph. Hinge weak; two small cardinal teeth in each valve, RV anterior and LV posterior weakly bifid; anterior lateral protuberance distinct to obscure. Pallial line entire. Anterior adductor scar greatly elongate, approximately 3/4 free from pallial line. Shell white, periostracum thin but persistent, straw coloured (all material collected has been stained in Rose Bengal, thus the pink tinge).
The anatomy is essentially that described for Lucinoma borealis by
The shell can be rather compressed (Fig. 7H) or tumid (Fig. 7G) and this may be related to age rather than size as suggested by
Tissues were sent to Dr. John Taylor (NHM, London) for inclusion in his survey of Lucinidae and the 16S and CO1 genes were compared with those of Lucinoma borealis. The results although not entirely conclusive indicate that the two populations are not conspecific. More recently, John Taylor’s group has demonstrated that Lucinoma kazani and Lucinoma borealis are distinct (J. Taylor pers. comm).
Only found live at Mercator MV in the Gulf of Cadiz (358m).
asapheus from asaphes Greek: meaning “indistinct” and “baffling”, referring to the lack of distinctive morphological characters and the consequent unsettling taxonomic issues.
A morphometric analysis was done comparing the Gulf of Cadiz shells with those of Lucinoma borealis
from numerous localities from around the British Isles. This analysis
could not demonstrate any statistically valid differences in the
outline, the relative size of the anterior adductor scar (aa l on Fig. 7B) or the angle of divergence of this scar from the pallial line (aa d on Fig. 7B).
It should be noted that the Cadiz sample size was small and that
conclusive probability results were unlikely. However, the variation in
tumidity and irregularity of some of the Cadiz shells is not found in
samples of Lucinoma borealis. Anatomically Lucinoma asapheus and Lucinoma borealis
are alike including the papillae that surround the inhalant aperture.
Further evidence for the species level distinction between Lucinoma asapheus and Lucinoma borealis
comes from the molecular data but here again the few specimens
available curtails the analysis. Ecologically one might expect mud
volcanoes and near shore sulphide enriched sediments to support
different species. This argument was used by
Other Eastern Atlantic species are Lucinoma vestita (
Lucinoma asapheus sp. n. stn. AT569GR, Mercator MV. A–D Holotype, aa d, angle of divergence of anterior adductor scar. aa l, length of anterior adductor scar E an aberrant specimen F a small specimen G–H two specimens showing variation in tumidity I the inhalant siphon.
The Thyasiridae of the Gulf of Cadiz were reported on by
Spinaxinus sentosus Oliver & Holmes, 2006b
As given by
http://species-id.net/wiki/Spinaxinus_sentosus
Fig. 8A–DOne live collected specimen, MSM01.03, Stn 190, deep-water field, Captain Arutyunov MV, 35°39.665'N, 07°19.970'W, 1322m, 28 April 2006, NMWZ.2010.4.7
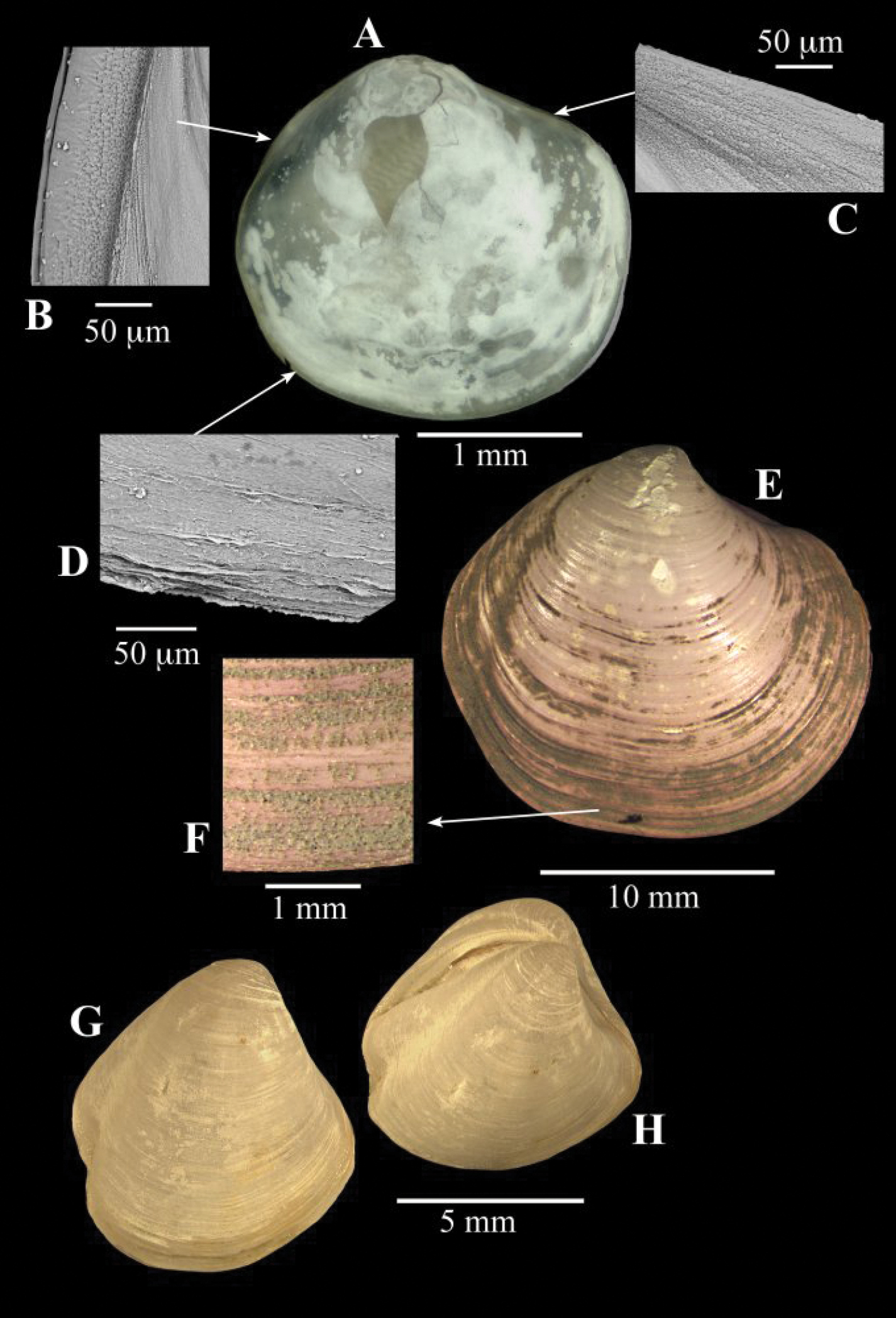
(Fig. 8A–D). This specimen measures only 2.3 mm in length and is damaged. The outline agrees with that of Spinaxinus sentosus
in being extended anteriorly with a long lunule depression and in the
presence of a long but shallow posterior sulcus. These features are in
contrast to the juveniles of Thyasira vulcolutre (
The periostracal spines are typical of Spinaxinus but are not seen in this specimen. The periostracum is coarse and the vestiges of lamellae and projections can be seen on the extreme edges of the shell especially on the ventral margin.
Although the identification is not conclusive the likelihood that this shell is a juvenile Spinaxinus is high and as such represents the first finding of this species in a non-anthropogenic setting. The proximity of the type locality (off northern Portugal) to the Cadiz mud volcanoes makes this supposition more reasonable.
Thyasira vulcolutre
belongs to a group of thyasirids with relatively large shells with
weakly defined posterior sulci. It was concluded that it was most
similar to Thyasira southwardae (
Thyasira striata
Sturany, 1896 has long been known from deep water in the eastern
Mediterranean but was recently re-discovered at cold seep sites (
These new data suggest that those thyasirids closely associated with active cold seeps have restricted ranges within the eastern Atlantic/Mediterranean region.
Family Vesicomyidae Dall & Simpson, 1901
The family Vesicomyidae has become familiar as a group of large chemosymbiotic clams associated with hot vents (
A–D Spinaxinus cf. sentosus Oliver & Holmes, 2006b. stn. 190, Captain Arutyunov MV. A digital image of right valve B SEM, periostracum on posterior margin C SEM, periostracum on anterior dorsal margin D SEM, periostracum on ventral margin. E–F Thyasira sp., Regab pock mark E external of right valve F periostracum. G–H Thyasira striata, Sturany, MEDINAUT, Eastern Mediterranean G external of right valve H oblique view showing posterior sulci.
Isorropodon perplexum Sturany, 1896
As given by
urn:lsid:zoobank.org:act:317BA11E-0B29-4396-8696-DEDCAF00B29F
http://species-id.net/wiki/Isorropodon_megadesmus
Figs 9, 10C–DHolotype: one complete specimen, live collected, MSM01.03, stn 218, deep-water field, Captain Arutyunov MV. 35°39.642'N, 07°20.049'W, 1321m, 30 April 2006, NMWZ.2010.4.8.
Paratypes: ten specimens, four shells and one valve, same data as holotype, NMWZ.2010.4.9.
Other material examined: over thirty decalcified juvenile specimens, MSM01.03, stn 218, deep-water field, Captain Arutyunov MV. 35°39.642'N, 07°20.049'W, 1321m, 30 April 2006; one specimen, MSM01.03, stn 225, same locality, 35°39.707'N, 07°20.020'W, 1322m, 4 May 2006.
| Length | Height | TumidityOne valve (paired) | Ratio (L/T) | |
|---|---|---|---|---|
| Holotype | 11.2 | 8.2 | 2.3 (4.6) | 2.4 |
| Paratype | 14.8 | 11.5 | 3.25 (6.5) | 2.3 |
| Paratype | 10.6 | 7.7 | 2.3 (4.6) | 2.3 |
| Paratype | 6.2 | 4.4 | 1.3 (2.6) | 2.4 |
| Paratype | 6.6 | 4.5 | 1.3 (2.6) | 2.5 |
| Paratype | 9.1 | 6.4 | 1.85 (3.7) | 2.5 |
| Paratype | 9.6 | 7.2 | incomplete |
(Figs 9, 10C–D). To 15mm in length. Thin. Equivalve. Inequilateral, beaks in front of the midline. Compressed, length to tumidity ratio 2.3 to 2.5. Outline subovate, anterior rounded, posterior a little obliquely truncated; ventral curvature at its maximum well to the posterior of the mid line. Lunule indistinct, not depressed. Escutcheon narrow, deeply excavated but entirely occupied by ligament. Sculpture of dense concentric lines and irregular growth stops or wrinkles. Hinge plate prominent dominated by a long nymph supporting a very large external ligament; ligament rises well above the dorsal margin of the shell and extends posteriorly beyond the nymph to fill the escutcheon. Hinge teeth complex; RV with a single prominent anterior lateral tooth situated in front of the beak in the form of a narrow projecting peg with a flat or slightly excavated dorsal surface; below the beak is an arched laminar tooth its anterior end overlapping the lateral tooth, its posterior slopes steeply and ventrally and merges with a second ridge only noticeable by a weak notch mid way on this combined ridge. LV with a thin laminar posterior cardinal angled obliquely plus two combined cardinals in a horizontal orientation the posterior part larger than the anterior with a distinct notch between the two parts. Pallial line entire with a very small straightened section below the posterior adductor scar; adductor scars of about equal size; anterior pedal retractor scar deeply impressed, situated immediately in front of the hinge plate. Periostracum thin, persistent, glossy. Shell white.
Mantle thin, mantle edge unfused except for short inhalant and exhalant siphonal apertures; inhalant aperture with many papillae increasing in size dorsally, exhalant with papillae of equal size. Foot with a distinct finger-like toe and poorly developed heel, pedal retractors prominent, the anterior attached in a deep impression close to the hinge. Anterior adductor muscle oval in cross-section, posterior adductor muscle subcircular, smaller than the anterior one. Ctenidia of a large, single (inner) demibranch, ascending part approximately one half the height of the outer, filaments fine tightly connected.
Isorropodon megadesmus is restricted to Captain Arutyunov MV (1321–1322m).
megadesmus from the Greek mega meaning large and desma meaning bond; referring to the external ligament.
The taxonomy of Isorropodon in the Atlantic and Mediterranean is complex and potentially confused (
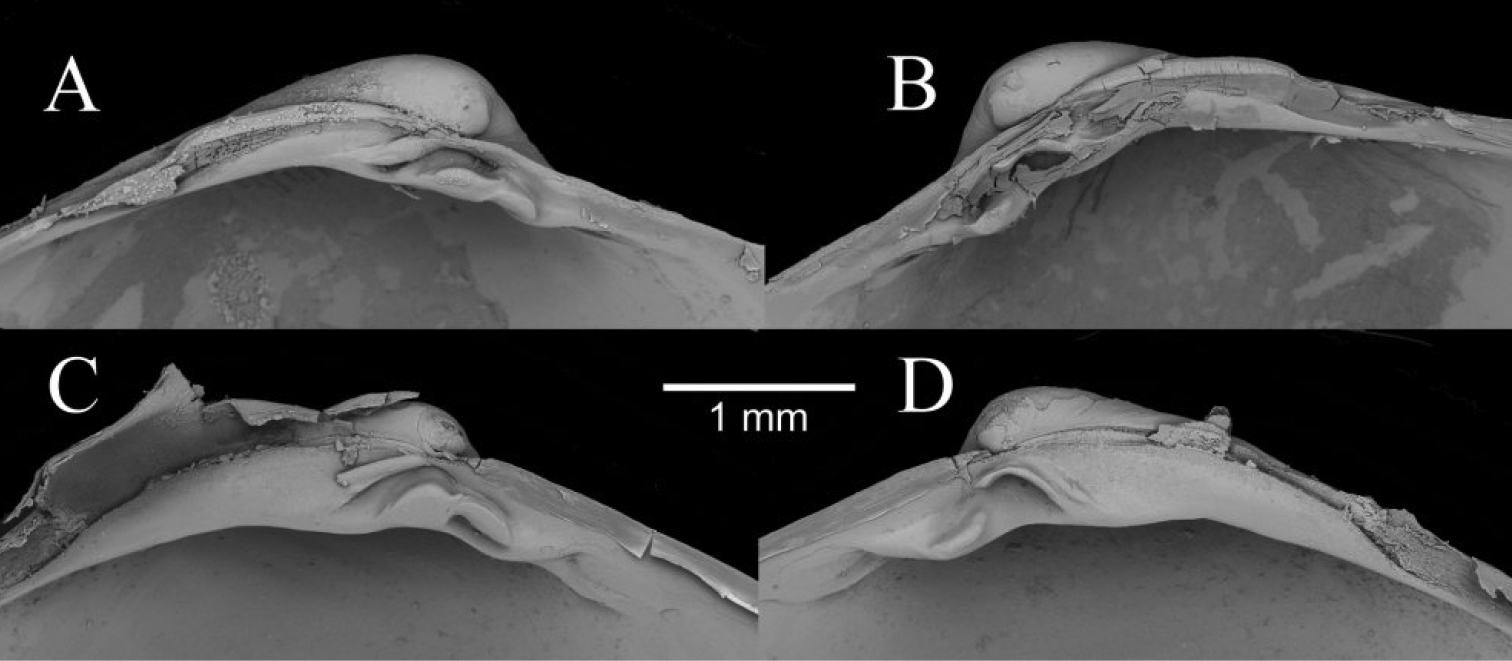
In contrast to the variability given by
The outline of Isorropodon bigoti differs from all of the above in the narrower anterior and distinct angulation of the ventral curve, but is has a short nymph similar to Isorropodon perplexum and the shell from station 180.
Isorropodon atalantae has a more sunken lunule and more angular posterior profile than either of the Gulf of Cadiz taxa. Isorropodon curtum Cosel and Salas, 2001, from off Mauritania, is more circular in outline and I striatum Thiele and Jaeckel, 1931 from off Angola, is a much larger and more elongate form.
Isorropodon megadesmus sp. n. stn. 218, Captain Arutyunov MV. A large right valve, paratype B–D holotype, right valve external, left valve internal, right valve internal E small right valve, paratype F dorsal view, paratype G gross anatomy viewed after removal of right valve and mantle.
Comparison between the hinge teeth of Isorropodon sp. indet. (A, B) and Isorropodon megadesmus sp. n. (C, D).
Figs 10A–B, 11
One complete specimen, live collected, MSM01.03, stn 180, deep-water field, Captain Arutyunov MV. 35°39.740'N, 07°19.960'W, 1323m, 27 April 2006, NMWZ.2010.4.10.
6.4mm (L) × 5.3mm (H) × 3.6mm (T)
(Figs 10A–B, 11). 6.4 mm in length. Thin. Equivalve. Inequilateral, beaks in front of the midline. Inflated, length to tumidity ratio = 1.8. Outline subovate, anterior bluntly rounded, posterior a little obliquely truncated; ventral curvature at its maximum more or less at the midline. Lunule indistinct, slightly depressed. Escutcheon narrow, deeply excavated. Sculpture of dense, concentric, fine lines and few irregular growth stops. Hinge plate narrow, nymph supporting an external ligament; ligament scarcely rises above the dorsal margin of the shell and extends posteriorly to half the length of the escutcheon. Hinge teeth complex; RV with a single prominent anterior lateral tooth situated in front of the beak in the form of a narrow projecting peg with a flat or slightly excavated dorsal surface; below the beak is weakly arched laminar tooth its anterior end overlapping the lateral tooth, its posterior slopes steeply and ventrally and merges with a second ridge only noticeable by a weak notch mid way on this combined ridge. LV with a thin laminar posterior cardinal angled obliquely plus two combined cardinals in a horizontal orientation the posterior part only slightly larger than the anterior with a distinct notch between the two parts. Pallial line entire with a very small straightened section below the posterior adductor scar; adductor scars of about equal size; anterior pedal retractor scar deeply impressed, situated immediately in front of the hinge plate. Periostracum thin, persistent, glossy. Shell white
Mantle thin, mantle edge unfused except for short inhalant and exhalant siphonal apertures; inhalant aperture with few papillae increasing in size dorsally, the latter as short tentacles, exhalant with papillae of equal size. Foot with a blunt finger-like toe and poorly developed heel, pedal retractors prominent, the anterior attached in a deep impression close to the hinge. Anterior adductor muscle pyriform in cross-section, posterior adductor muscle subcircular, smaller than the anterior one. Ctenidia of a large, single (inner) demibranch, filling the majority of the mantle cavity, ascending part approximately one half the height of the outer, filaments fine tightly connected.
The numerous crystalline growths seen on and between the filaments are believed to be natural and not an artifact of preservation.
Isorropodon sp indetis restricted to Captain Arutyunov MV (1323m).
As discussed above for Isorropodon megadesmus.
Isorropodon sp. indet., stn. 180 Captain Arutyunov MV. A–D External and internal views of right and left valves E dorsal view F gross anatomy viewed after removal of right valve and mantle G Excised ctenidium with crystalline artifacts.
Twelve bivalve species have been found in close association with chemosynthetic settings in the Gulf of Cadiz (this paper;
The Gulf of Cadiz mud volcano field is comprised of over thirty seeps of various activity and spread over a bathymetric range of 200–4000m. Of the 25 mud volcanoes sampled, 13 have chemosymbiotic species, which indicates their importance in the structure of the seep assemblages (Fig. 1). Most of the thirteen, chemosymbiotic, species found in the Gulf of Cadiz are restricted to one or two mud volcanoes. This patchy distribution can result from physical or physiological constraints such as depth, distance and fluid flow rates. They were more frequent in the shallower mud volcanoes (200–1500m) but were especially diverse in Captain Arutyunov MV where five different species co-occur (Fig. 1). Some taxa are confined to single mud volcanoes whereas others are more widespread.
The family most frequently encountered in the
chemosynthesis-based assemblages of the mud volcanoes from the Gulf of
Cadiz, is the Solemyidae. The family is represented by two genera, Solemya (Petrasma) with a shallower distribution (358–1030m) and Acharax
with a deeper distribution (556–3902m) but co-occurring at intermediate
depths in the Western Moroccan field. Co-occurrence of these genera has
not been reported elsewhere and may be explained by the apparent
absence of the subgenus Petrasma from seep settings preferring reducing sediments and low oxygen conditions (
This is the first record of this family in cold seeps from the North-east Atlantic, although Solemya (Solemya) togata
is well known from shallow settings such as sea-grass beds in the
Mediterranean. Why this species has not or been unable to colonize the
shallow mud volcanoes is unknown. In contrast, the Pacific Solemya (S.) tagiri
Unlike Solemya (Petrasma), Acharax species are consistently associated with seep or vent settings and some species such as the Pacific Acharax johnsoni have extensive bathymetric ranges from 100 to over 5000m (
In contrast to the solemyids, the lucinid Lucinoma asapheus
has only been collected at Mercator MV, although video observations
revealed presence of lucinids in other mud volcanoes from the Spanish
field (MR Cunha, pers. comm.). Lucinoma asapheus is very similar morphologically to Lucinoma kazani from the Eastern Mediterranean (
The thyasirid Thyasira vulcolutre was only found in the deep-water mud volcano field (
Small vesicomyids including Isorropodon megadesmus and Isorropodon sp. were very abundant in Captain Arutyunov MV.The species Isorropodon perplexum is known only from the Eastern Mediterranean (Table 1), and was shown to harbour sulphur-oxidizing bacteria (
Bathymodioline mussels of the amphi-Atlantic species Bathymodiolus mauritanicus
were only found living in Darwin MV although extensive graveyards of
mussel shell ash are also found in other mud volcanoes of the western
Moroccan field (
When discussing Isorropodon,
The Gulfs of Cadiz and Guinea are most diverse and
almost equally so with 13 and 14 species respectively. The less sampled
Mauritania basin has only 7 recorded species while the well studied
Eastern Mediterranean has only 6 species. The number of species common
to more than two fields is zero and the maximum number of shared species
is two, that for the Gulfs of Cadiz and Guinea. These data suggest
high levels of endemism within fields but where there is overlap,
especially with the larger vesicomyids, that this occurs at deeper
sites. The vesicomyids are the most diverse family but only one species
has colonized the Eastern Mediterranean and they appear to be rare in
the Gulf of Cadiz compared with the Gulf of Guinea. From a geological
history perspective one can explain the poor diversity in the Eastern
Mediterranean from the shorter period of time for colonization since the
re-invasion of Atlantic waters post the hyper-saline event. At this
time there is no evidence to indicate the origins of the Eastern
Atlantic faunas, either by dispersal or local speciation. Molecular
data from the species rich genus Isorropodon may illuminate the relationships and sequence of speciation and we await the study in progress on the Vesicomyidae mentioned by
Distribution of chemosymbiotic taxa known from seep/mud volcano fields in the Eastern Atlantic and Mediterranean.
| Taxon | East.Mediterranean | Gulfof Cadiz | MauritaniaBasin | Gulf ofGuinea |
|---|---|---|---|---|
| Solemyidae | ||||
| Acharax indet. | X | |||
| Acharax gadirae | X | |||
| Solemya (Petrasma) elarraichensis | X | |||
| Solemyidae Eastern Med | X | |||
| Mytilidae | ||||
| Bathymodiolus mauritanicus | X | X | ||
| Bathymodiolus aff. boomerang | X | |||
| Idas modiolaeformis | X | |||
| Idas sp. | X | X | ||
| Lucinidae | ||||
| Lucinoma asapheus | X | |||
| Lucinoma kazani | X | |||
| Lucinoma atalantae | X | |||
| Lucinoma myriamae | X | |||
| Myrtea amorpha | X | |||
| Graecina karinae | X | |||
| Joellina dosiniformis | X | |||
| Thyasiridae | ||||
| Thyasira vulcolutre | X | |||
| Thyasira striata | X | |||
| Thyasira sp. n. | X | |||
| Spinaxinus sentosus | X | |||
| Vesicomyidae | ||||
| Isorropodon perplexum | X | X? | ||
| Isorropodon megadesmus | X | |||
| Isorropodon bigoti | X | X | ||
| Isorropodon curtum | X | |||
| Isorropodon striatum | X | |||
| Isorropodon atalantae | X | |||
| Callogonia cyrili | X | |||
| Callogonia mauritanica | X | |||
| Callogonia valdiviae | X | X | ||
| Christineconcha regab | X? | X | ||
| Wareniconcha guineensis | X | |||
| Elenaconcha guiness | X | |||
| Laubiericoncha chuni | X? | X | ||
| Pliocardia sp. | X | |||
| Abyssogena southwardae | X | |||
| Total( ) shared species | 6 (1?) (0) (0) | (1?) 13 (1) (2) | (0) (1) 7 (2) | (0) (1) (2) 14 |
Thanks are due to the co-chief-scientists Luís Pinheiro (Departamento de Geociências, Universidade de Aveiro) and Michael Ivanov (Moscow State University) for the invitation to participate in the TTR cruises (Training Through Research Programme, IOC–UNESCO), Henk de Haas (chief-scientist of the Microsystems 2007) and to Olaf Pfannkuche (chief-scientist of the MSM01-03. IFMGEOMAR).
This research was partially supported by the HERMES project (European Commission’s Sixth Framework Programme under the priority “Sustainable Development, Global Change and Ecosystems”, EC contract GOCE–CT-2005-511234) and is a contribution to the project HERMIONE (European Commission’s Framework Seven Programme, contract number 226354).
The second author was supported by a PhD grant (SFRH/BD/17085/2004) from Fundação para a Ciência e Tecnologia.
We also wish to thank John Taylor (Natural History Museum, London) for his helpful discussion on many aspects of this paper and to Elena Krylova (P.P. Shirshov Institute of Oceanology) for information on the Vesicomyidae.