(C) 2011 Michael S. Engel. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new species of the cleptoparasitic bee genus Microsphecodes Eickwort and Stage (Halictinae: Halictini) is described and figured from a male and female collected in Jamaica. Microsphecodes xaymacensis Engel, sp. n., is distinguished from its congeners on the basis of integumental coloration and sculpturing, and form of the male pygidial plate and genitalia.
Hymenoptera, Apoidea, Anthophila, Halictinae, Halictini, Sphecodina, new species, taxonomy
The West Indian sphecodine fauna is, much like the
general halictine fauna of the region, poorly documented. Relatively
few species have been described and recorded from the West Indies (Table 1) and for nearly all, the biology remains to be characterized [e.g.,
Herein I provide a brief contribution to this fauna by describing a new species of Microsphecodes from Jamaica, the first representative of this genus from the island.
Checklist of world species of Microsphecodes, and other described Caribbean sphecodines1 (from
| Genus Microsphecodes Eickwort & Stage | ||
| –Continental | ||
| Microsphecodes kathleenae (Eickwort) | Costa Rica, Colombia | |
| Microsphecodes russeiclypeatus (Sakagami & Moure) | Brazil | |
| Microsphecodes trichommus Michener | Colombia | |
| Microsphecodes truncaticaudus Michener | Colombia | |
| –Caribbean | ||
| Microsphecodes dominicanus (Stage) | Dominica | |
| Microsphecodes kittensis Engel | St. Kitts | |
| Microsphecodes solitarius (Ashmead) | St. Vincent2 | |
| Microsphecodes thoracicus (Ashmead) | St. Vincent | |
| Microsphecodes xaymacensis sp. n. | Jamaica | |
| Genus Nesosphecodes Engel | ||
| Nesosphecodes anthracinus Engel | Puerto Rico | |
| Nesosphecodes cubicola Engel | Cuba | |
| Nesosphecodes halictophagus Engel | Dominican Republic | |
| Genus Sphecodes Latreille | ||
| Sphecodes genaroi Engel | Cuba | |
| Sphecodes nigritus Ashmead | St. Vincent | |
| Sphecodes tainoi Engel | Cuba | |
1 Sphecodines as a whole are quite diverse and the monophyly of Sphecodes is questionable. Once phylogenetic studies are completed then the resurrection of former entities such as Drepanium, Proteraner, and Sphecodium should be considered over a retrograde classification lumping all into Sphecodes as has been advocated.
2 The apparent bias in diversity towards St. Vincent
(with three sphecodines recorded) is artificial and simply reflects that
this is one of the few islands, along with Grenada, for which there
is a significant historical monograph (
Material is deposited in the Snow Entomological
Collection, Division of Entomology, University of Kansas Natural
History Museum, Lawrence (SEMC), and the Florida State Collection of
Arthropods, Gainesville (FSCA). Morphological terminology follows that
of
urn:lsid:zoobank.org:act:3048683A-BF8D-4474-A2F3-BD47C2AB3126
http://species-id.net/wiki/Microsphecodes_xaymacensis
Figs 1–12♂, Jamaica: Saint Andrew Parrish, Hard war Gap, 2–3-viii-1985 [2–3 August 1985], C.B. & H.V. Weems, G.B. Edwards (FSCA).
♀, Jamaica: Saint Andrew Parrish, Hard war Gap, 2–3-viii-1985 [2–3 August 1985], C.B. & H.V. Weems, G.B. Edwards (SEMC).
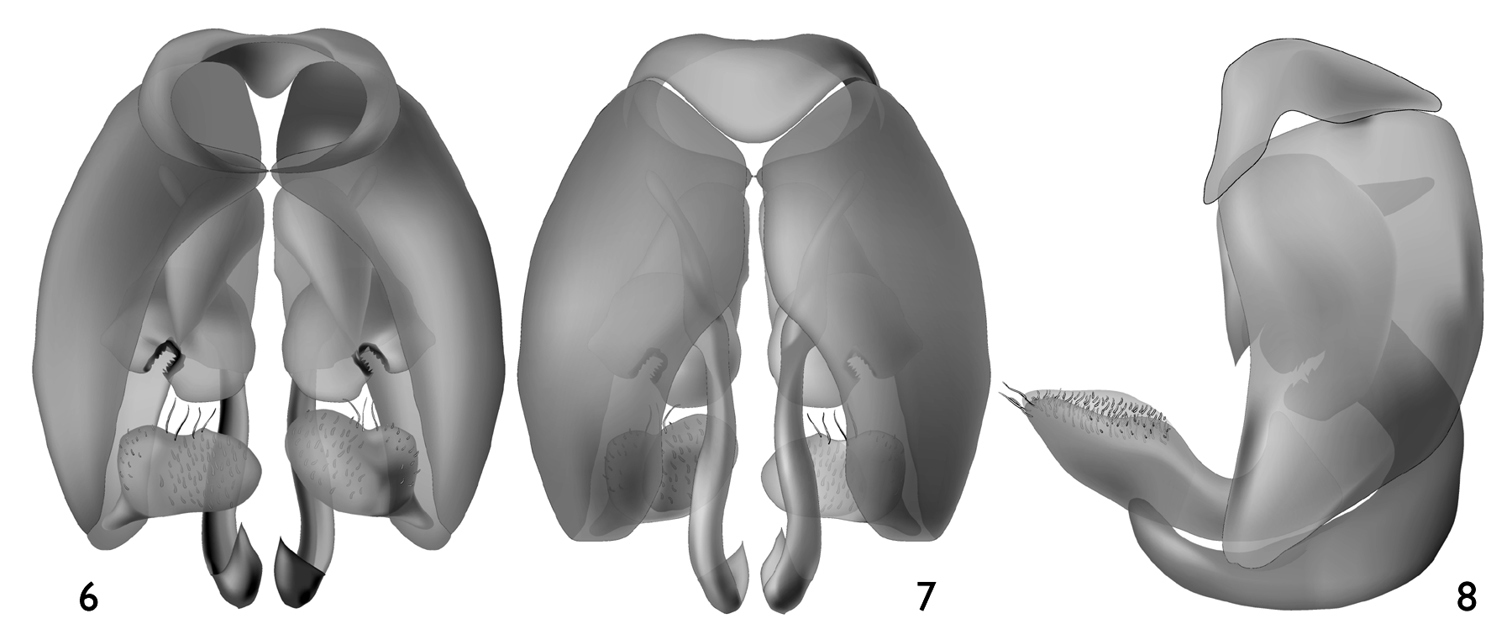
The new species can be readily distinguished from its congeners by the structure of the male genitalia (Figs 6–8) and the combination of the broad male pygidial plate bordered by elongate simple or apically-branched setae (Fig. 4), hyaline wings (Fig. 5), and the rugoso-striate basal area of the propodeum not enclosed by carinae (Figs 3, 12) (the latter generally typical for West Indian species: vide
Male: Total body length 4.85 mm; forewing length 4.1 mm. Head broader than long (width 1.33 mm, length 1.04 mm as measured from clypeal apex to vertex in frontal aspect) (Fig. 2). Frontal line carinate just between antennal toruli to point above upper tangent of toruli equivalent to about torulus diameter, becoming an impressed line from that point onward. Mandibular base meeting lower border of compound eye. Inner margin of compound eye slightly concave just above level of antennal toruli. Gena narrower than compound eye in profile. Scape length 0.42 mm; first flagellomere about as long as second flagellomere. Intertegular distance 0.89 mm. Forewing venation as in figure 5; hind wing with six distal hamuli arranged in a single series. Pygidial plate well delimited, wide, broadly rounded at apex, with slightly depressed shining surface and carinate rim (Fig. 4). Male genitalia as in figures 6–8.
Integument generally shining. Clypeus imbricate with shallow, contiguous punctures; remainder of head distinctly punctate, punctures on lower part of face nearly contiguous, becoming more widely spaced toward upper frons and vertex, separated by 0.25–1.5 times a puncture width, integument between punctures smooth and shining except on lower face finely imbricate, punctures weaker on vertex and sparser around ocelli; postgena faintly imbricate and impunctate. Pronotum with sparsely-scattered, minute punctures, integument between punctures imbricate. Mesoscutum imbricate with punctures separated by 1–2.5 times a puncture width, punctures shallower, fainter, and sparser around median line and along anterior and lateral sections; tegula impunctate and exceedingly faintly imbricate; mesoscutellum sculptured as on mesoscutum except punctures fainter and separated by 2–3 times a puncture width. Metanotum imbricate. Pleura smooth to faintly imbricate, with sparse minute punctures. Basal area of propodeum with strong, rugulose striae radiating from basal margin (Fig. 3), integument between striae finely imbricate; lateral and posterior surfaces of propodeum imbricate with scattered, faint, coarse punctures. Metasomal terga and sterna faintly imbricate except first metasomal tergum smooth.
Mandible, labrum, and labiomaxillary complex ferruginous; remainder of head nearly black or dark brown; antenna dark brown (Figs 1–2). Mesosoma largely ferruginous (Fig. 1) except darker on median and lateral portions of mesoscutum and entirety of mesoscutellum, metanotum, and dorsal surface of propodeum (Fig. 3). Wing veins brown; wing membrane largely hyaline. Legs ferruginous except meso- and metatibiae and meso- and metatarsi brown. Metasoma largely ferruginous except dark brown on more apical terga and sterna; pygidial plate ferruginous (Fig. 4).
Pubescence relatively sparse, white except somewhat yellow on pleura, legs, and metasoma. Setae generally simple and erect, some with minute branches; face with moderately-dense, appressed, short, plumose setae on lower face and clypeus (Fig. 2).
Female: As described for the male except in usual gender differences as well as the following: Total body length 4.80 mm; forewing length 4.2 mm. Head broader than long (width 1.41 mm, length 1.04 mm). Mandible elongate, without dentition, about as long as compound eye (Figs 9–10). Frontal line carinate just between antennal toruli to point above upper tangent of toruli equivalent to twice torulus diameter, becoming a faintly impressed line from that point onward (Fig. 10). Gena only slightly narrower than compound eye in profile (Fig. 9). Scape length 0.52 mm; first flagellomere slightly shorter than second flagellomere. Intertegular distance 0.89 mm. Inner metatibial spur simple.
Mandible and labiomaxillary complex orange testaceous; labrum, clypeus and face dark reddish brown blending to nearly black on vertex (Figs 10–12); gena dark reddish brown; scape and pedicel orange testaceous; flagellum dark brown; mesosoma orange testaceous except more yellowish on pronotal dorsal surface and propodeal dorsal surface (Figs 11–12); legs orange testaceous except dark reddish brown to ferruginous on meso- and metatibiae and meso- and metatarsi; metasoma orange testaceous blending to ferruginous and to dark brown by third tergum, remaining terga largely ferruginous, with dark brown apical portions.
Mesoscutal punctures more well defined posteriorly and separated by 1–1.5 times a puncture width, otherwise as in male with punctures shallower and fainter anteriorly and more widely spaced.
Setae on legs white and on apical portions of metasoma fuscous.
The specific epithet is based on the indigenous Arawakan-speaking Taíno islanders’ name for Jamaica, Xaymaca, and meaning “Land of Springs”.
Photomicrographs of male holotype of Microsphecodes xaymacensis Engel, sp. n. 1 Lateral habitus 2 Facial aspect 3 Dorsal view of posterior mesosoma, highlighting propodeum, metanotum, mesoscutellum and posterior third of mesoscutum 4 Pygidial plate 5 Detail of forewing venation.
Male genitalia of Microsphecodes xaymacensis Engel, sp. n. 6 Ventral aspect 7 Dorsal aspect 8 Lateral aspect.
Photomicrographs of female paratype of Microsphecodes xaymacensis Engel, sp. n. 9 Lateral habitus 10 Facial aspect 11 Dorsal view of head and mesosoma highlighting head and mesoscutum 12 Dorsal view of head and mesosoma highlighting mesoscutellum, metanotum, and propodeum.
I am grateful to James R. Wiley, Florida State Collection of Arthropods, for bringing this material to my attention; to Ismael A. Hinojosa-Díaz, University of Kansas Natural History Museum, for executing the genitalic figures; and to Michael Ohl and an anonymous reviewer for their helpful comments. This is a contribution of the Division of Entomology, University of Kansas Natural History Museum, partially supported by US National Science Foundation grant DBI-1057366.