(C) 2010 Sam W. Heads. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The fossil orthopteran Brauckmannia groeningae Martins-Neto (Orthoptera, Ensifera) from the Early Cretaceous Crato Formation of Brazil, currently misplaced at both the genus and family level, is transferred to the family Schizodactylidae and assigned to the extant genus Schizodactylus Brullé; ergo, Brauckmannia enters synonymy under Schizodactylus and Brauckmanniidae enters synonymy under Schizodactylidae. Schizodactylus groeningae (Martins-Neto), comb. n. agrees in size and general habitus with extant members of the genus, but can be readily separated by the robust, subovoid form of the metatibiae and the distinctive morphology of the lateral metabasitarsal processes. This species represents the first fossil occurrence of Schizodactylidae and the only New World record of this ancient lineage. Phylogenetic relationships of the schizodactylids are reviewed and a sister-group relationship with Grylloidea advocated based on a reappraisal of morphological and molecular evidence.
Orthoptera, Ensifera, Gryllidea, Schizodactylidae, Brauckmanniidae, Brauckmannia, Schizodactylus, phylogeny, Crato Formation, Early Cretaceous, Brazil
The Lower Cretaceous Crato Formation of northeastern
Brazil is famous for the truly exquisite preservation of its remarkable
fossil assemblage (e.g.
Orthoptera (crickets, katydids, grasshoppers and their kin) are well-represented in the Crato Formation (
The unusual orthopteran Brauckmannia groeningae was described by
The holotype of Brauckmannia groeningae
is in the private collection of Rafael Gioia Martins-Neto, referred to
in his publications as the ‘Coleção de Sociedad Brasileira
de Paleoartropodologia’ with the number RGMN 500. This collection
was apparently stored at his home in Ribeirão Preto, São Paulo, though
since his death in August 2010 its whereabouts are unknown. As a
result, any discussion of the holotype presented herein is based
entirely on the illustrations in the original description (
The MfNB specimen was studied using a Zeiss Stemi SV11
zoom stereomicroscope and drawings produced with the aid of a camera
lucida. Macro photographs were taken using a Nikon D700 digital SLR with
a 60 mm macro objective. In addition, the following extant material
was also examined: Schizodactylus brevinotus Ingrisch, 2002; Schizodactylus inexpectatus (Werner, 1901); Schizodactylus burmanus Uvarov, 1935; Schizodactylus monstrosus (Drury, 1773); Schizodactylus hesperus Bei-Bienko, 1967; Comicus arenarius Ramme, 1931; Comicus calcaris Irish, 1986; Comicus campestris Irish, 1986; and Comicus capensis Brunner von Wattenwyl, 1888. Terminology used follows that normally employed for Orthoptera,
with the following distinctions concerning tibial armature: ‘spine’
refers to elongate, distally pointed, unsocketed processes; and ‘spur’
refers to all socketed processes of variable form (e.g. spine-like,
blade-like, lobate, etc.). The geology, stratigraphy, environment
and palaeobiota of the Crato Formation were most recently reviewed by
The Schizodactylidae,
or splay-footed crickets, are a relict group of primitive Ensifera
notable for their uniquely modified tarsi, which bear distinctive
lobe-like lateral processes that serve to support the insects as they
walk around their sandy habitats. The family is traditionally subdivided
into two monotypic subfamilies: Schizodactylinae Blanchard, 1845 containing the type genus Schizodactylus Brullé, 1835 found primarily in India, Pakistan, Afghanistan and parts of Southeast Asia; and Comicinae Ander, 1939 containing the apparently paedomorphic genus Comicus
Brunner von Wattenwyl, 1888 found only in southern parts of Africa.
Schizodactylids are primarily nocturnal and are thought to be active
predators (
The genus Schizodactylus as presently defined comprises all large and robust schizodactylids with wings developed and, with the exception of Schizodactylus inexpectatus in which the wings are reduced, extending beyond the apex of the abdomen where they terminate in a conspicuous coil. Schizodactylus species are further characterised by greatly enlarged mouthparts, a broad diamond-shaped labrum, and strong laterally compressed pro- and mesothoracic legs. The genus is readily separated from Comicus, the only other genus in the family, which is characterised by a markedly smaller and more gracile body (usually less than 25 mm in length), long, slender legs, and complete reduction of the wings.
Given the presence of distinctively coiled wings and well-developed lateral processes on the tarsi, there can be no doubt as to the placement of Brauckmannia groeningae in Schizodactylidae. In addition, there are no characters preserved in either the holotype (so far as can be seen from Martins-Neto’s illustrations) or the new material described below to exclude the species from the genus Schizodactylus. Both fossil specimens match closely the general habitus, tarsal morphology and metatibial armature of extant Schizodactylus species, and there are no convincing apomorphies to support separate generic placement.
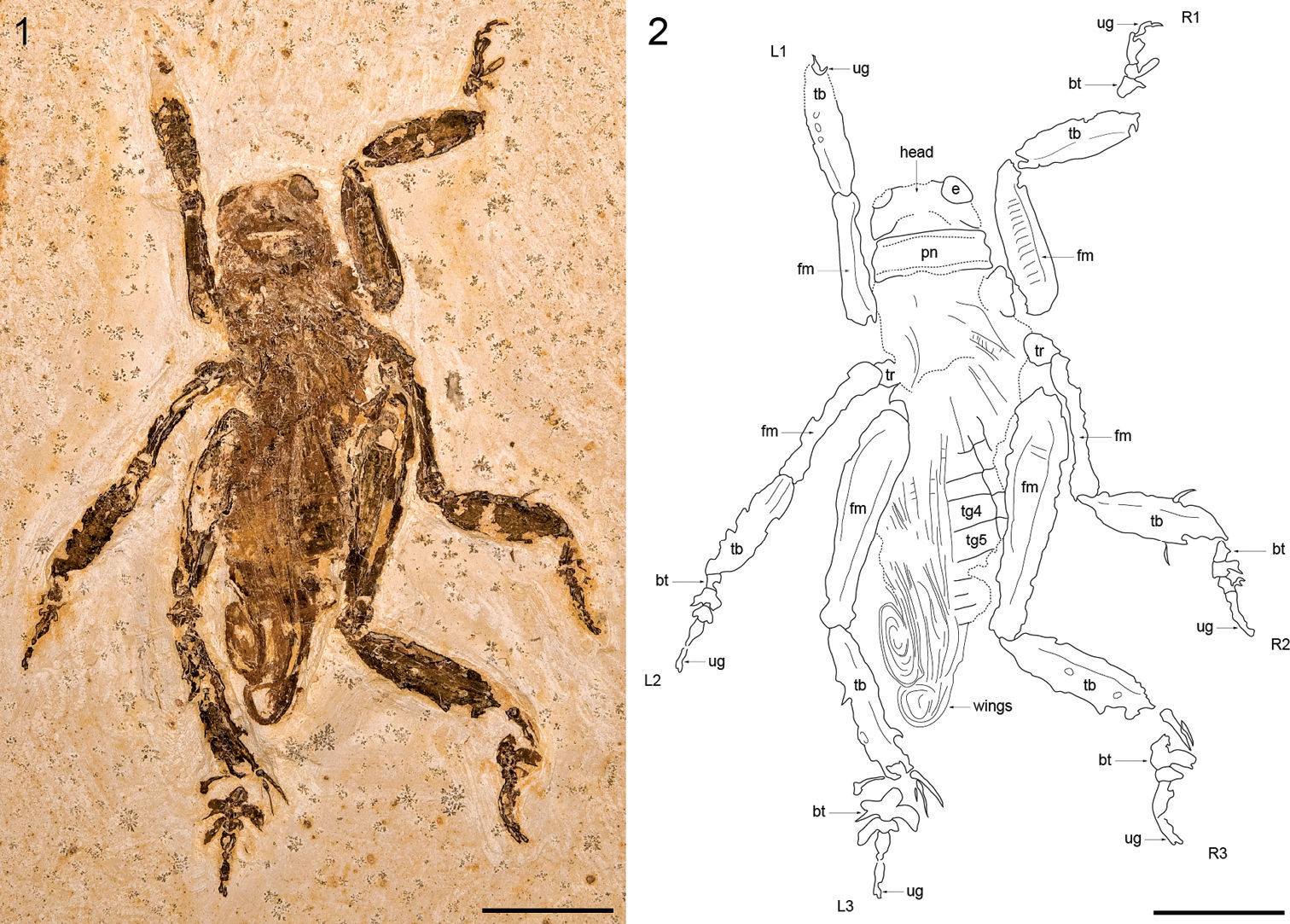
Figs 1–2
Near-complete adult (sex indet.), MfNB-I.2079. Brazil, Ceará, Chapada do Araripe; Crato Formation, Nova Olinda Member, Lower Cretaceous.
Schizodactylus groeningae is distinguished from all congeners by the following characters: [1] robust and acutely subovoid metatibiae (in all extant species the metatibiae are of equal width along their entire length); and [2] distinctive blade- or paddle-like lateral processes arising in the distal half of the metabasitarsus (in all extant species these processes are triangular with a broad base and acute, posteriorly directed apex, and arise within the proximal half of the metabasitarsus).
Large, near-complete specimen preserved in slightly oblique, dorsal aspect (Figs 1, 2). Head capsule robust, 8.46 mm wide at genae; vertex short, c. 2.43 mm from occipital margin as preserved; occipital foramen large, broad; interocular distance 3.98 mm; compound eyes large, c. 2.61 mm wide dorsally. Pronotum markedly wider than long with distinctive marginal sulci; medial length 3.41 mm; width 9.36 mm. Pterothorax poorly preserved, c. 9.20 mm long. Wings incompletely preserved basally, extending posteriorly beyond abdominal apex, tightly folded in a distinctive apical coil. Abdomen somewhat crushed dorsolaterally, c. 16.12 mm long as preserved (apical part missing); first tergite (tg1) largely indistinct, at least 1.45 mm long; tg2 1.96 mm long; tg3 1.83 mm long; tg4 2.17 mm long; tg5 2.35 mm long; remaining tergites incompletely preserved but shorter than previous tergites; right lateral parts of abdominal sternites 1 through 5 visible next to corresponding tergal sclerites; pleural margin distinct. Total body length measured from fastigium verticis to abdominal apex 34.91 mm. Profemora robust and laterally compressed, 12.33 mm long; left profemur preserved in dorsal aspect, with distinct longitudinal dorsal carina; right profemur preserved in lateral aspect, with prominent transverse dorsolateral striae and distinct longitudinal inferior carina. Protibiae robust and lateral compressed, markedly inflated and acutely subovoid in form, 10.24 mm long; left protibia incompletely preserved in oblique dorsal aspect, bearing at least three spur sockets on outer lateral margin, spurs themselves not preserved; right protibia preserved in lateral aspect and somewhat crushed. Left prothoracic leg (L1 in Fig. 2) with distal part missing, only apical part of ungues preserved. Right prothoracic leg (R1 in Fig. 2) with tarsus incompletely preserved, at least 6.52 mm in total length; basitarsus subcylindrical in form, inflated apically, at least 1.75 mm long; second tarsomere short, c. 1.21 mm long, with prominent blade-like lateral process, 2.35 mm long; third tarsomere 2.10 mm long, with stout lateral process, 0.95 mm long; fourth tarsomere indistinctly preserved; ungues incompletely preserved, strongly curved, 1.44 mm long. Mesotrochantora small, c. 2.40 mm long and c. 2.20 mm wide. Mesofemora very slender, somewhat curved, inflated slightly at both the base and geniculae, 11.26 mm long and 1.46 mm wide at midlength. Mesotibiae similar in form to protibiae but somewhat larger and more acutely subovoid in lateral aspect, 10.75 mm long; left mesotibia incompletely preserved in dorsal aspect; right mesotibia preserved in lateral aspect, 3.36 mm wide at midlength, with two short subapical spurs preserved. Left mesothoracic leg (L2 in Fig. 2) with tarsus preserved in dorsal aspect, 8.17 mm long; basitarsus small, 1.15 mm long; second tarsomere 1.25 mm long with broad, incomplete lateral processes at least 1.49 mm long; third tarsomere 1.28 mm long with stout lateral processes at least 0.62 mm long; fourth tarsomere basally inflated, apically slender, 2.18 mm long; ungues at least 2.08 mm long. Right mesothoracic leg (R2 in Fig. 2) with tarsus preserved in lateral aspect, c. 8.10 mm long; basitarsus small, 1.55 mm long; second tarsomere 1.15 mm long with broad, apically incomplete lateral process at least 1.58 mm long; third tarsomere 1.23 mm long with incompletely preserved lateral process at least 0.95 mm long; margin between fourth tarsomere and ungues indistinct, combined length c. 3.91 mm. Metafemora large, robust, 19.78 mm long. Metatibiae markedly shorter than metafemora, 12.58 mm long; acutely subovoid in form, though more elongate than pro- and mesotibiae. Left metatibia preserved in dorsal aspect, somewhat crushed, with poorly preserved spines along dorsolateral margins; first apical spur 3.62 mm long; second apical spur 3.07 mm long; third apical spur incompletely preserved and visible only as a thin, 0.86 mm long fragment immediately adjacent and inferior to the second apical spur; sixth apical spur partially preserved in lateral aspect, 2.88 mm long and 0.96 mm wide apically. Right metatibia preserved in lateral aspect, 3.29 mm wide at midlength, with bases of poorly preserved spines visible along the dorsal margin; first apical spur blade-like, 3.80 mm long; second apical spur incompletely preserved immediately adjacent and inferior to the first apical spur, 2.06 mm long. Left metathoracic leg (L3 in Fig. 2) with tarsus preserved in dorsal aspect, at least 8.76 mm long; basitarsus well-developed, at least 2.80 mm long, with large, broadly paddle-like lateral processes at least 2.41 mm long and 1.78 mm wide; second tarsomere short, 0.98 mm long, with large, blade or paddle-like lateral processes at least 2.28 mm long and 0.94 mm wide; third tarsomere incomplete, at least 1.98 mm long; combined length of fourth tarsomere and ungues c. 2.98 mm. Right metathoracic leg (R3 in Fig. 2) with tarsus preserved in lateral aspect, at least 9.50 mm long; basitarsus well-developed, at least 2.66 mm long, with large, paddle-like lateral process at least 2.37 mm long; second tarsomere short, 1.04 mm long, incompletely preserved; third tarsomere indistinct, with basal part of small lateral process visible; fourth tarsomere and ungues poorly preserved, combined length 3.97 mm.
Schizodactylus groeningae (Martins-Neto, 2007), comb. n. from the Lower Cretaceous Crato Formation of Brazil. 1 Photograph of MfNB-I.2079 2 Camera lucida drawing of MfNB-I.2079. Abbreviations: R1 – right prothoracic leg; L1 – left prothoracic leg; R2 – right mesothoracic leg; L2 – left mesothoracic leg; R3 – right metathoracic leg; L3 – left metathoracic leg; bt – basitarsus; fm – femur; pn – pronotum; tb – tibia; tr – trochanter; ug – ungues. Scale bars represent 10 mm.
The photograph of the holotype provided by Martins-Neto (2007: fig. 1A) is of rather poor quality, though it is obvious that the specimen is not as well preserved as MfNB-I.2079. Moreover, the accompanying drawing (fig. 1B) is not only incomplete (for reasons that are unclear, the drawing only depicts part of the specimen) but does not correspond entirely with features clearly visible in the photograph. Nevertheless, the photograph shows sufficient details for the identification of the holotype as a schizodactylid; namely the presence of distinctive paddle-like lateral processes on the tarsi (clearly visible on both metatarsi though misidentified as ‘well-developed pulvilli’ by Martins-Neto), and apically coiled wings (not mentioned in the original description). Moreover, the holotype is clearly conspecific with MfNB-I.2079, agreeing with it not only in the relative proportions of the legs and overall size and habitus, but also in the distal origin of the metabasitarsal processes.
Schizodactylus groeningae represents the only fossil record of Schizodactylidae
and confirms the antiquity of an extant lineage hitherto unknown from
the fossil record. Especially significant is that the species belongs to
an extant, albeit relict genus, suggesting that the initial radiation
of the Schizodactylidae occurred at least during the Jurassic if not earlier. Moreover, the presence of Schizodactylus groeningae represents the only record of Schizodactylidae
from the New World, confirming presence of the family in the Atlantic
rift zone of South America prior to its complete separation from Africa.
An arid or semi-arid local environment for the Crato hinterland was
first suggested by
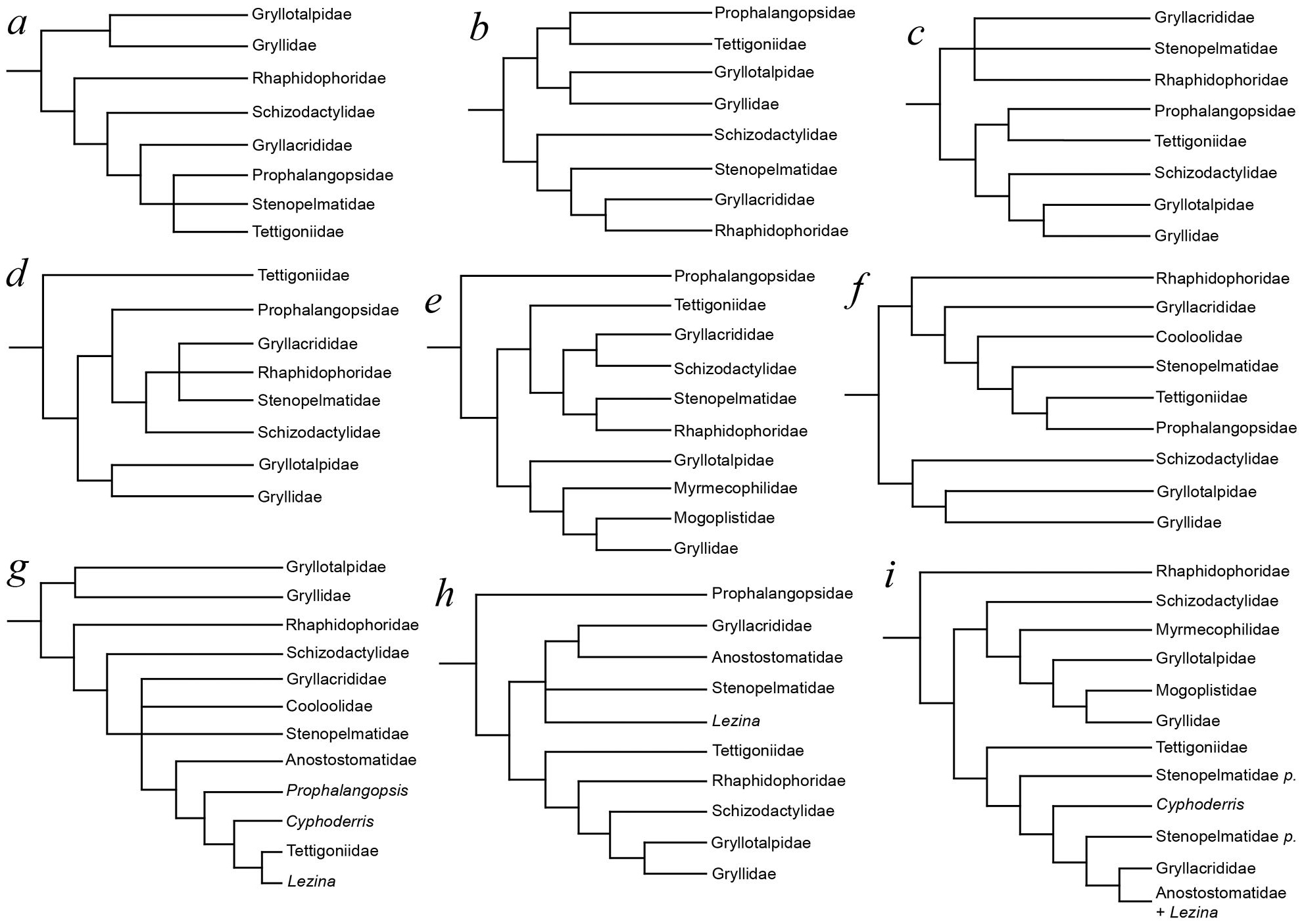
The relationships of the splay-footed crickets have proven somewhat controversial (see Fig. 3), with the group generally regarded as a subfamily within Gryllacrididae (
Competing hypotheses of ensiferan relationships, after: a –
Morphological support for a schizodactylid–grylloid
relationship comes primarily from the morphology and venation of the
wings, namely: [1] marked reduction or loss of costal veins; [2]
development in the tegmina of a longitudinal fold; [3] concurrent
development of a distal medial fan in the tegmina; [4] development of
fan-like folding in the cubital and medial systems of the hind wings;
and [5] hind wing CuA two-branched. In addition,
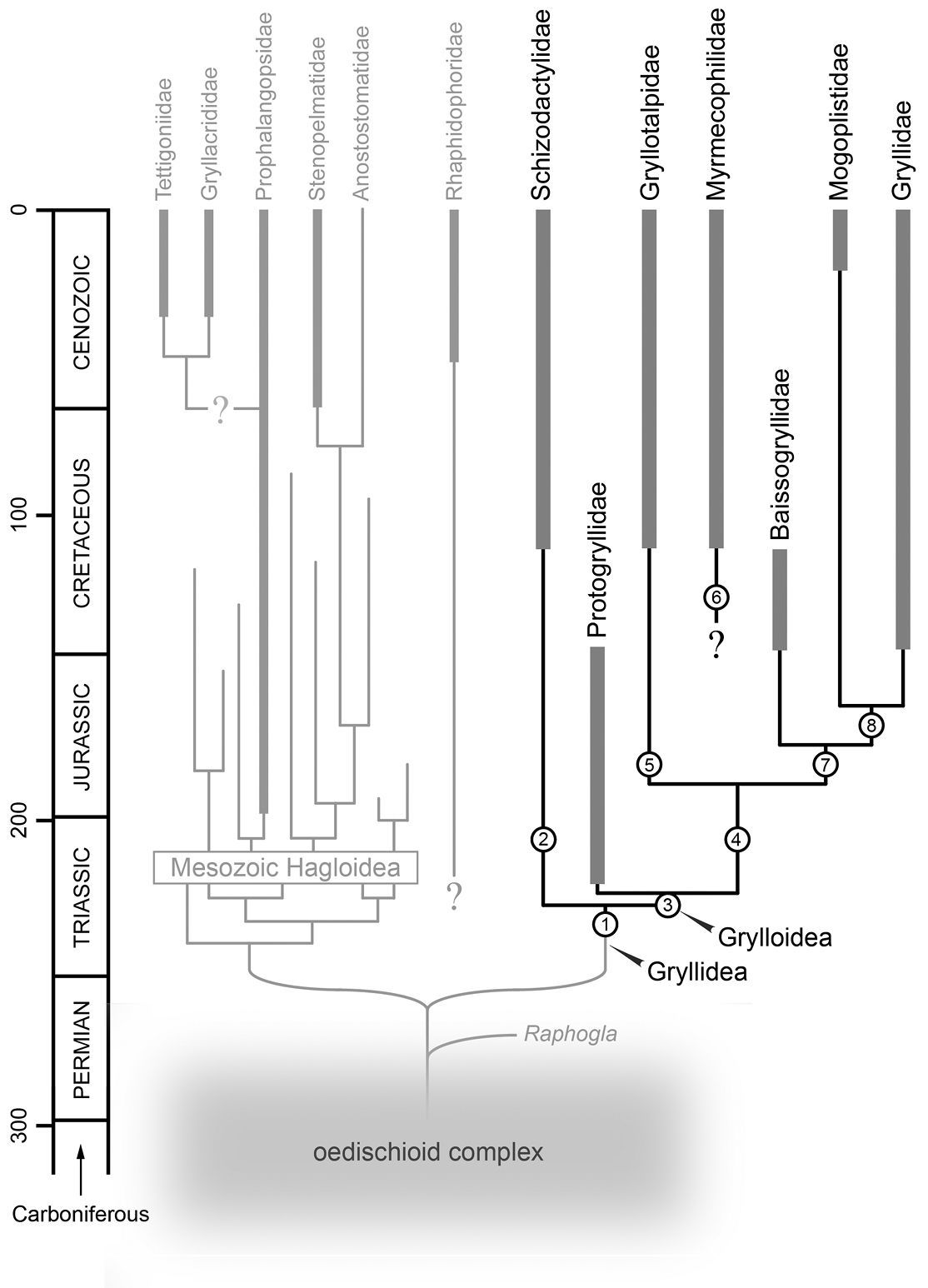
Figure 4
shows a tentative reconstruction of relationships among major ensiferan
groups based on a review of hypotheses presented in the various studies
summarized in Fig. 3. The phylogenetic relationships of the gryllidean taxa are based primarily on the molecular phylogeny of
Possible relationships among major ensiferan groups (both fossil and extant), with an emphasis on the infraorder Gryllidea. Thick lines indicate known geological ranges whilst thinner lines project likely ranges based on sister-group relationships. Arabic numerals at nodes indicate autapomorphic character transformations as follows: 1 (i) reduction or loss of cubitus; (ii) development of longitudinal radio-medial fold in tegmina; (iii) development of a distal medial fan in tegmina; (iv) development of fan-like folding in cubital and medial systems of hind wings; (v) hind wing CuA two-branched; (vi) fusion of abdominal ganglion 7 with posterior ganglionic mass 2 (i) hind wings, when developed, tightly folded at rest and apically coiled in a distinctive ring; (ii) well-developed, blade or paddle-like lateral processes present on the 2nd and 3rd tarsomeres of the pro- and mesotarsi and also on the metabasitarsus; (iii) predatory 3 (i) tarsi reduced to three tarsomeres; (ii) loss of the fastigium verticis; (iii) development of stridulatory file on ventral surface of tegminal CuP; (iv) presence of a dividing vein and harp between CuA2 and CuP 4 (i) tegminal medial fan expanded, forming a subapical medial lobe 5 (i) prothoracic legs fossorial; (ii) ovipositor vestigial 6 (i) compound eyes markedly reduced; (ii) all coxae large and closely approximated; (iii) pseudosegmented cerci; (iv) reduced ovipositor; (v) obligate inquilines of ants 7 (i) development of a tegminal mirror 8 (i) migration of dividing veins in the mirror to a position perpendicular with respect to long axis of tegmen.
Whilst it is clear that major group relationships within Ensifera remain largely unresolved (see
We are grateful to Christian Neumann and Jason Dunlop for providing access to material and facilities; Hwaja Goetz for kindly taking photographs of the specimen; Michael Engel, David Martill, Torsten Wappler, Barbara Mohr and Rolf Kohring for valuable discussion; and two anonymous referees for insightful commentary on an early version of the manuscript. Support was provided by an Orthoptera Research Foundation Fellowship at the Illinois Natural History Survey (to SWH).