(C) 2010 Robert Hershler. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Marstonia comalensis, a poorly known nymphophiline gastropod (originally described from Comal Creek, Texas) that has often been confused with Cincinnatia integra, is re-described and the generic placement of this species, which was recently allocated to Marstonia based on unpublished evidence, is confirmed by anatomical study. Marstonia comalensis is a large congener having an ovate-conic, openly umbilicate shell and penis having a short filament and oblique, squarish lobe bearing a narrow gland along its distal edge. It is well differentiated morphologically from congeners having similar shells and penes and is also genetically divergent relative to those congeners that have been sequenced (mtCOI divergence 3.0–8.5%). A Bayesian analysis of a small COI dataset resolved Marstonia comalensis in a poorly supported sub-clade together with Marstonia hershleri, Marstonia lustrica and Marstonia pachyta. The predominantly new records presented herein indicate that Marstonia comalensis was historically distributed in the upper portions of the Brazos, Colorado, Guadalupe and Nueces River basins, south-central Texas. The species has been live collected at only 12 localities and only two of these have been re-visited since 1993. These data suggest that the conservation status of this snail, which has a critically imperiled (G1) NatureServe ranking and was recently proposed for federal listing, needs to be re-assessed.
Marstonia, Hydrobiidae, Gastropoda, United States, Texas, freshwater, taxonomy, conservation
The freshwater gastropod genus Marstonia (Hydrobiidae: Nymphophilinae)
is composed of 15 small (shell height < 5.0 mm), ovate- to
elongate-shelled species that are distributed in springs, streams and
lakes in eastern North America (
We redescribe Marstonia comalensis
herein based on study of a large series of dry shell and
alcohol-preserved material, most of which was collected by
malacologists J.J. Landye and D.W. Taylor from 1971–1993, and provide
anatomical evidence supporting its current generic allocation. The new
records detailed in this paper considerably expand the geographic range
of Marstonia comalensis,
which lives in springs and fluvial habitats spread among four river
basins in south-central Texas. We also further analyze previously
published molecular data (
Anatomical study was based on specimens that were relaxed with menthol crystals and fixed in dilute formalin. Types and other material of Marstonia comalensis in the collections of the Academy of Natural Sciences of Philadelphia (ANSP); Florida Museum of Natural History (FMNH); National Museum of Natural History, Smithsonian Institution (USNM); and University of Minnesota Bell Museum of Natural History (UMBMNH) were examined during the course of this study.
Variation in the number of cusps on the radular teeth was assessed using the method of
The molecular phylogenetic analysis included single mtCOI sequences from Marstonia comalensis, six other species of Marstonia and representatives of six other North American nymphophiline genera. Hydrobia acuta
(Draparnaud) was used as the root. Sample information, GenBank
accession numbers and publication references for the sequences are in Table 1. Sequence divergences (uncorrected p distance) were calculated using MEGA4 (
Species (specimen codes), locality details, GenBank accession numbers and publication references for mtCOI sequences.
| Species (code) | Locality | GenBank accession number | Reference |
|---|---|---|---|
| Marstonia agarhecta Thompson | Bluff Creek, Pulaski Co., GA | AF520934 |
|
| Marstonia castor Thompson | Mercer Mill Creek, Worth Co., GA | AF520938 |
|
| Marstonia comalensis (Pilsbry & Ferriss) | Old Faithful Spring, Real Co., TX | AF520933 |
|
| Marstonia halcyon Thompson | Ogeechee River, Screven Co., GA | AF520935 |
|
| Marstonia hershleri (Thompson) | Coosa River, Elmore Co., AL | AF520946 |
|
| Marstonia lustrica (Pilsbry) | Stockbridge Bowl, Berkshire Co., MA | AF520945 |
|
| Marstonia pachyta Thompson | Limestone Creek, Limestone Co., AL | AF520939 |
|
| Cincinnatia integra (Say) | Stream north of Fredericksburg, Gillespie Co., TX | AF520948 |
|
| Notogillia wetherby (Dall) (WW) | Weeki Wachee River, Hernando Co., FL | AF367630 |
|
| Notogillia wetherbyi (Dall) (RS) | Rainbow Springs, Marion Co., FL | AF520918 |
|
| Pyrgulopsis bruneauensis Hershler | Bruneau Hot Springs, Owyhee Co., ID | AF520941 |
|
| Rhapinema dacryon Thompson | Chipola River, Jackson Co., FL | AF520932 |
|
| Spilochlamys gravis Thompson | Alexander Springs, Lake Co., FL | AF520919 |
|
| Stiobia nana Thompson | Coldwater Spring, Calhoun Co., AL | AF520921 |
|
| Hydrobia acuta (Draparnaud) | Lagoon 6, Suffolk, East Anglia, United Kingdom | AF354773 |
|
Family Hydrobiidae
Subfamily Nymphophilinae
Genus Marstonia Baker, 1926
Figs 1–3
Figured lectotype, ANSP 91323 (Fig. 1A); paralectotypes (from same lot), ANSP 420575.
TEXAS. USNM 123757, USNM 134007, Guadalupe River. ANSP 134247, Nueces River. Bell County: UMBMNH uncat., Salado Creek, Salado, old US 81 (30.944°N, 97.539°W), 14.IV.1972. Comal County: ANSP 90562, drift of Guadalupe River, 3.2 km above New Braunfels (29.756°N, 98.138°W). UMBMNH uncat., Spring Branch, west of Spring Branch (29.891°N, 98.435°W), 28.III.1963. USNM 473488, New Braunfels (29.702°N, 98.124°W). Kerr County: USNM 874910, South Fork Guadalupe River, ca. 56.4 km northwest of Leakey (29.957°N, 99.456°W), 30.XII.1979. USNM 874932, spring run adjacent to South Fork of Guadalupe River, ca. 11.2 km southwest of Hunt (30.005°N, 99.409°W), 30.XII.1979. USNM 883412, North Fork Guadalupe River (30.054°N, 99.486°W), 26.IV.1993. USNM 874923, North Fork of Guadalupe River at Riverbend Ranch crossing at FM 1340 (30.065°N, 99.373°W), 29.XII.1979. USNM 251887, Japonica (30.064°N, 99.344°W). Kimble County: UMBMNH uncat., Llano River at FM 385, 25.6 km northeast of Junction (30.589°N, 99.598°W), 12–13.IV.1972. Kinney County: UMBMNH uncat., Nueces River, Tularosa Road, A.G. “Tony” Rose Ranch (29.518°N, 100.271°W), 9.VI.1971. USNM 883413, West Nueces River above crossing on Tularosa Road near Spring Ranch, just below Silver Lake (29.523°N, 100.251°W), 25.IV.1993. Real County: UMBMNH uncat., USNM 874926, Old Faithful Spring outflow, Hwy 55, 0.8 km north of Camp Wood (29.680°N, 100.015°W), 8.VI.1971, 31.XII.1979. FMNH 283564, FMNH 283565, FMNH 283573, FMNH 287574, Old Faithful Spring, 1.0 km north of Camp Wood (29.680°N, 100.015°W), 27.I.2001. USNM 883414, Leakey Springs run at Hwy 337 crossing, ca. 0.48 km east of Leakey (29.723°N, 99.757°W), 25.IV.1993. FMNH 283561, Leakey Springs creek, 0.64 km east of Leakey (29.723°N, 99.757°W), 26.I.2001. Uvalde County: UMBMNH uncat., Nueces River, Chalk Bluff, 24 km northwest of Uvalde (29.359°N, 99.984°W), 27.V.1971. USNM 883477, spring at Camp Chalk Bluff, tributary to East Nueces River (29.362°N, 99.984°W), 23.IV.1993. USNM 883666, USNM 883421, East Nueces River at 19 Mile Crossing, 1.6–3.2 km south of Hwy 55 and FM 334 (29.398°N, 100.00°W), 31.XII.1979, 29.IV.1993. UMBMNH uncat., USNM 883420, East Nueces River, Hwy 55, Lake Nueces County Park (29.619°N, 100.01°W), 1-VI-1971, 29.IV.1993.
Shell large for genus (maximum height, 4.6 mm), ovate-conic, openly umbilicate; penis with short filament and oblique, squarish lobe bearing a single terminal gland along its distal edge. Distinguished from congeners having closely similar shells and penes as follows: from Marstonia gaddisorum Thompson by its less convex shell whorls, distinctive pallial roof pigmentation, larger number of cusps on the inner side of the lateral teeth and on the outer marginal teeth, larger penial lobe, narrower terminal gland, and smaller overlap of the bursa copulatrix by the albumen gland; from Marstonia lustrica by its smaller prostate gland, smaller penial lobe, narrower penial filament, straight anterior vas deferens, partly imbedded (in albumen gland) bursal duct, and larger seminal receptacle; and from Marstonia ogmorhaphe Thompson by its smaller size, broader shell, smaller prostate gland, straight anterior vas deferens, and smaller bursa copulatrix.
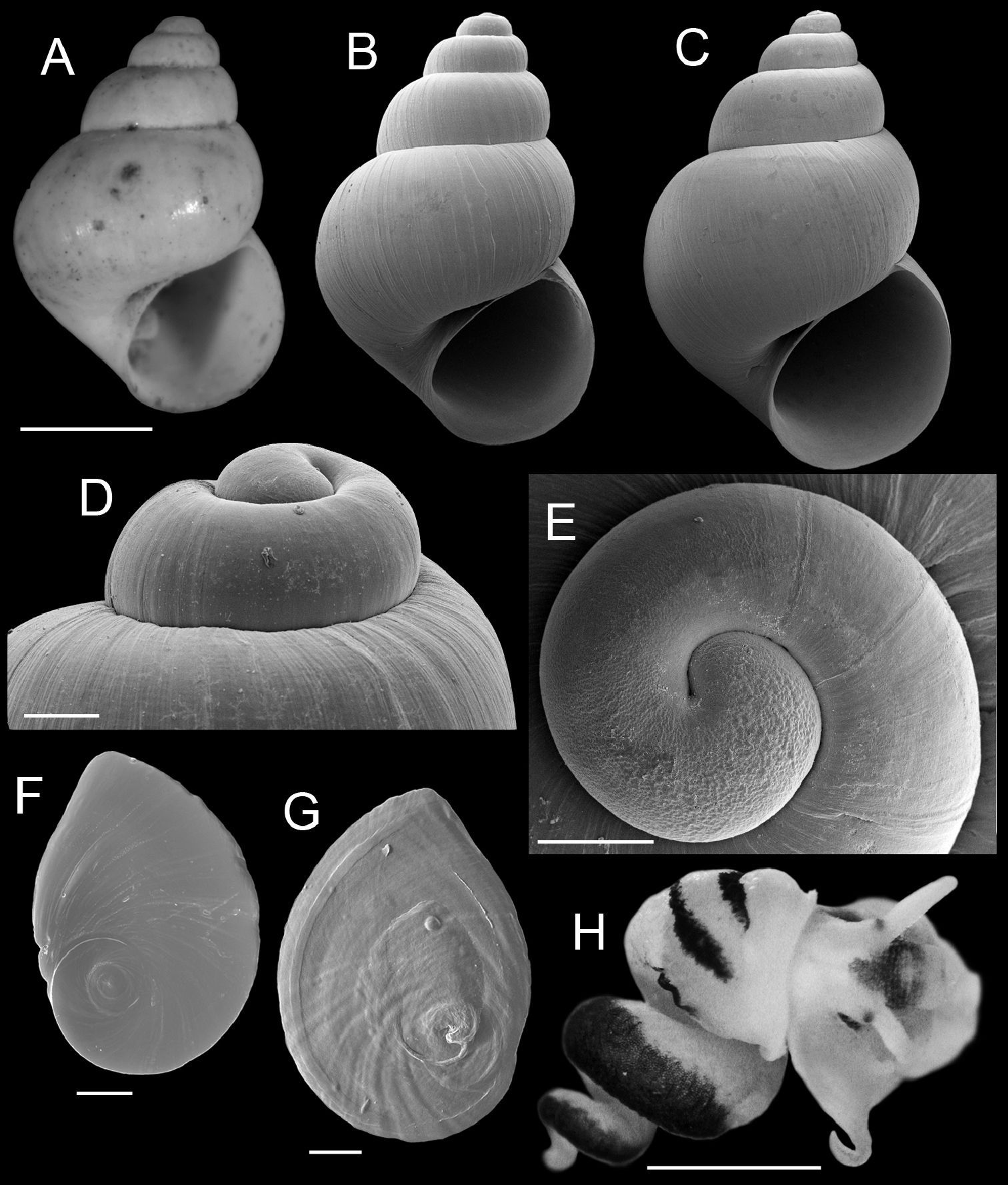
Shell ovate-conic, (Fig. 1A–C); height about 2.6–4.6 mm; whorls 4.5–5.5. Protoconch near planispiral, slightly tilted (Fig. 1D), initial 0.75–1.0 whorl strongly wrinkled (Fig. 1E). Teleoconch whorls weakly convex, often narrowly shouldered, rarely having subsutural angulation; sculpture of strong collabral growth lines, later whorls having numerous weak spiral striae. Aperture pyriform or ovate. Inner lip complete across parietal wall in larger specimens, usually narrowly adnate, rarely slightly disjunct; usually thin, sometimes slightly thickened apically; columellar shelf absent or very narrow; outer lip thin or slightly thickened, orthocline or prosocline. Umbilicus open but small. Measurements of the lectotype and a live-collected series of shells from the Nueces River basin are in Table 2.
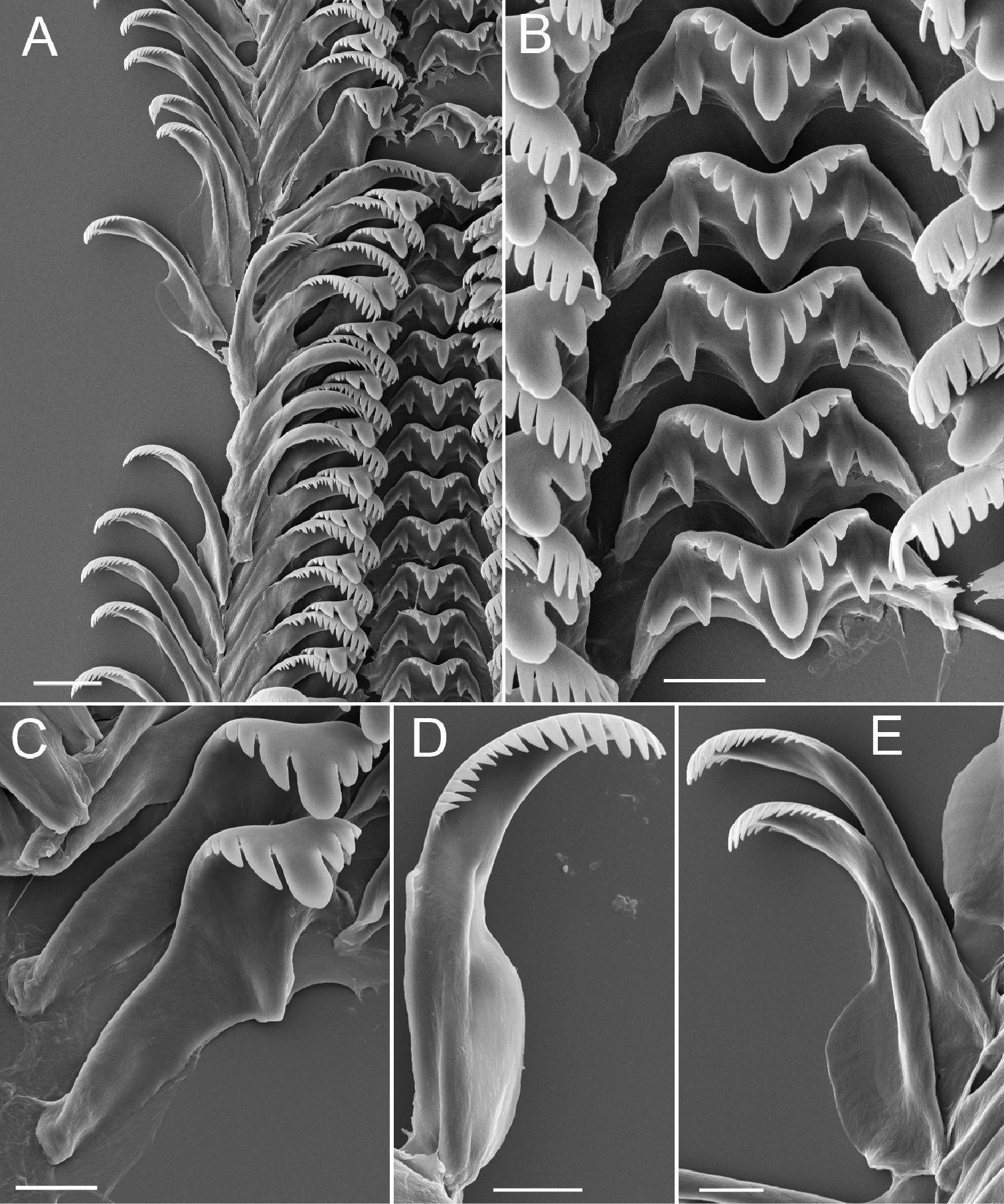
Operculum thin, amber, narrowly ovate, multispiral with eccentric nucleus (Fig. 1F); last 0.25 whorl sometimes frilled on outer side; inner side having well developed rim near outer edge (Fig. 1G); attachment scar border sometimes weakly thickened near nucleus. Radula (Fig. 2A), having about 36 well-formed rows of teeth. Central teeth about 38 µm wide, cutting edge convex (Fig. 2B); lateral cusps 3–8; central cusp pointed or hoe-shaped, parallel-sided proximally or tapering throughout; basal cusps 1–3, small; basal tongue U- or V-shaped, about as long as lateral margins. Lateral tooth face rectangular; central cusp pointed or hoe-shaped (Fig. 2C); lateral cusps 2–5 (inner), 3–7 (outer); outer wing broad, flexed, about 140% length of cutting edge; basal tongue weakly developed. Inner (Fig. 2D) and outer (Fig 2E) marginal teeth both having 14–21 cusps and basally positioned rectangular wing. Radular count data were from USNM 874926.
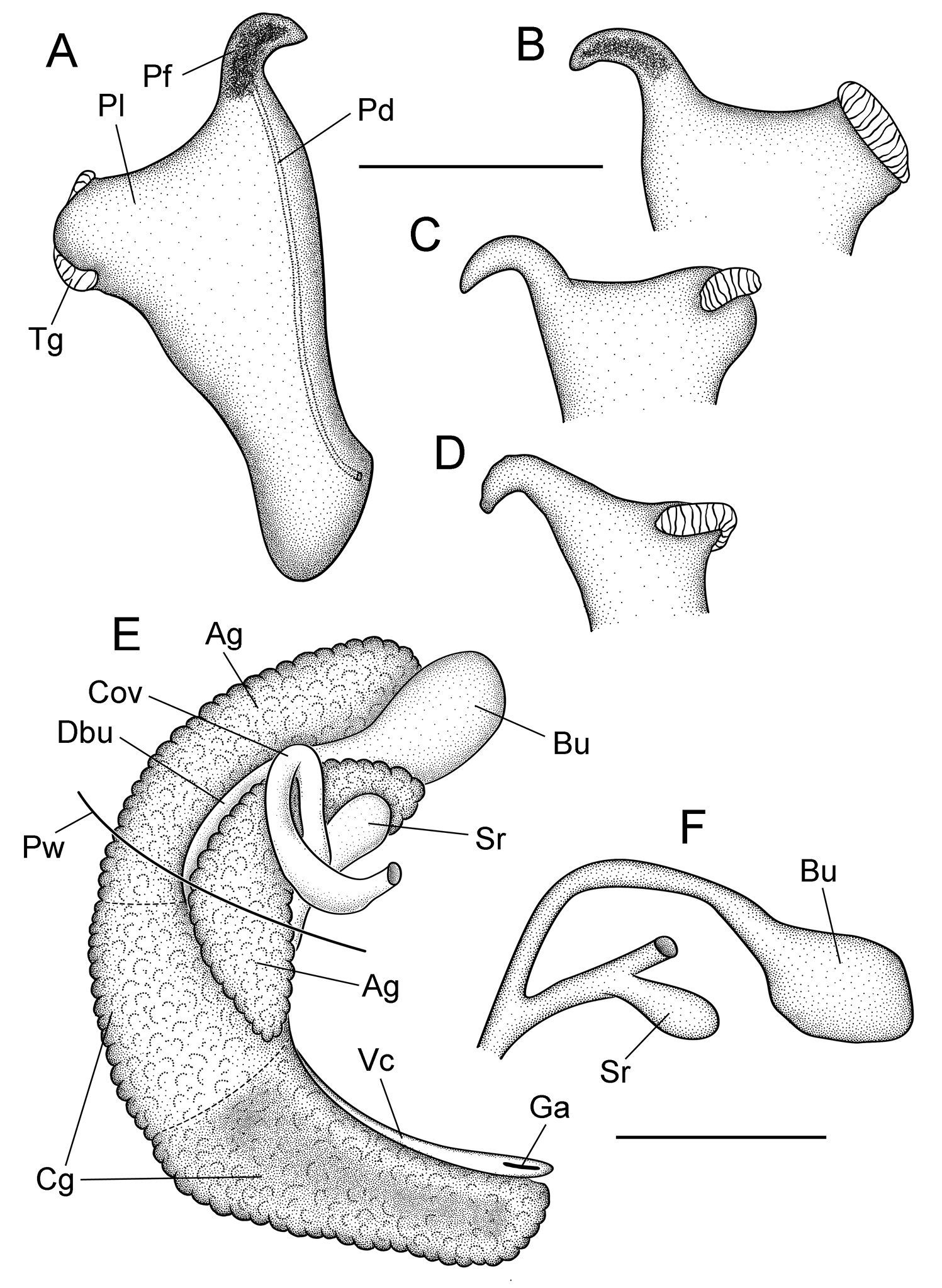
Cephalic tentacles pale except for black eyespots. Snout brown; distal lips pale; foot pale. Pallial roof having black pigment bands along edges of ctenidium and dorsal edge of genital duct (Fig. 1H); visceral coil pale except for black pigment on testis. Ctenidium positioned a little in front of pericardium; ctenidial filaments 24–25 (n=5), broadly triangular, lateral surfaces ridged. Osphradium narrow, positioned slightly posterior to middle of ctenidium. Hypobranchial gland large, overlapping rectum and part of genital duct, thickened alongside kidney. Style sac longer than remaining portion of stomach, posterior stomach having small caecal appendix. Testis large (1.75 whorls), composed of compound lobes, broadly overlapping stomach anteriorly. Seminal vesicle opening near anterior edge of testis, composed of a few thickened coils, positioned along ventral side of anterior 33% of testis. Prostate gland small, pea-shaped, with about 50% of length in pallial roof. Anterior vas deferens opening from antero-ventral edge of prostate gland, section of duct on columellar muscle straight. Penis large, base rectangular, inner edge without folds; filament short, narrow, tapering, oblique; lobe rather medium-sized, squarish, oblique (Fig. 3A). Terminal gland (Fig. 3A–D) narrow, usually transversely positioned along outer edge of lobe (58/86 specimens examined from three samples), less frequently horizontal (28/86), sometimes borne on short stalk. Penial duct narrow, near outer edge, almost straight. Penial filament having black internal pigment core along most of length. Ovary small (0.75 whorl), composed of simple, stalked lobes; slightly overlapping stomach anteriorly. Female glandular oviduct and associated structures shown in Figure 3E–F. Coiled oviduct narrow, vertical. Bursa copulatrix small, ovate, horizontal, about 50% overlapped by albumen gland. Bursal duct longer than bursa, narrow, opening from distal edge, partly embedded in albumen gland proximally, entirely embedded distally, junction with common duct well in front of posterior wall of pallial cavity. Seminal receptacle small, pouch-like, positioned near ventral edge of albumen gland slightly anterior to bursa copulatrix. Albumen gland largely visceral. Capsule gland composed of two distinct tissue sections. Genital aperture a terminal slit.
Shells, opercula and animal, Marstonia comalensis. A Lectotype, ANSP 91323 B, C Shells, USNM 874932, USNM 874926 D Lateral view of shell apex, USNM 874932 E Protoconch, USNM 874926 F, G Opercula (outer, inner sides), USNM 874926 H Relaxed, alcohol-preserved preserved male (dorsal view), showing distinctive pigment bands on the pallial roof, USNM 874926. Scale bars A–C, H, 1.0 mm; D, E, 100 µm; F, G, 200 µm.
Radula, Marstonia comalensis, USNM 874926. A Portion of radular ribbon B Central teeth C Lateral teeth D Inner marginal tooth E Outer marginal teeth. Scale bars A, 20 µm; B-E, 10 µm.
Reproductive anatomy, Marstonia comalensis, USNM 874926. A Penis, dorsal surface B–D Distal portion of penis showing terminal gland variation E Female glandular oviduct and associated structures (viewed from left side) F Bursa copulatrix and seminal receptacle and their ducts. Scale bars = 500 µm. Ag albumen gland Bu bursa copulatrix Cg capsule gland Cov coiled oviduct Dby bursal duct Ga genital aperture Pf penial filament Pl penial lobe Pw posterior wall of pallial cavity Sr seminal receptacle Vc ventral channel of capsule gland.
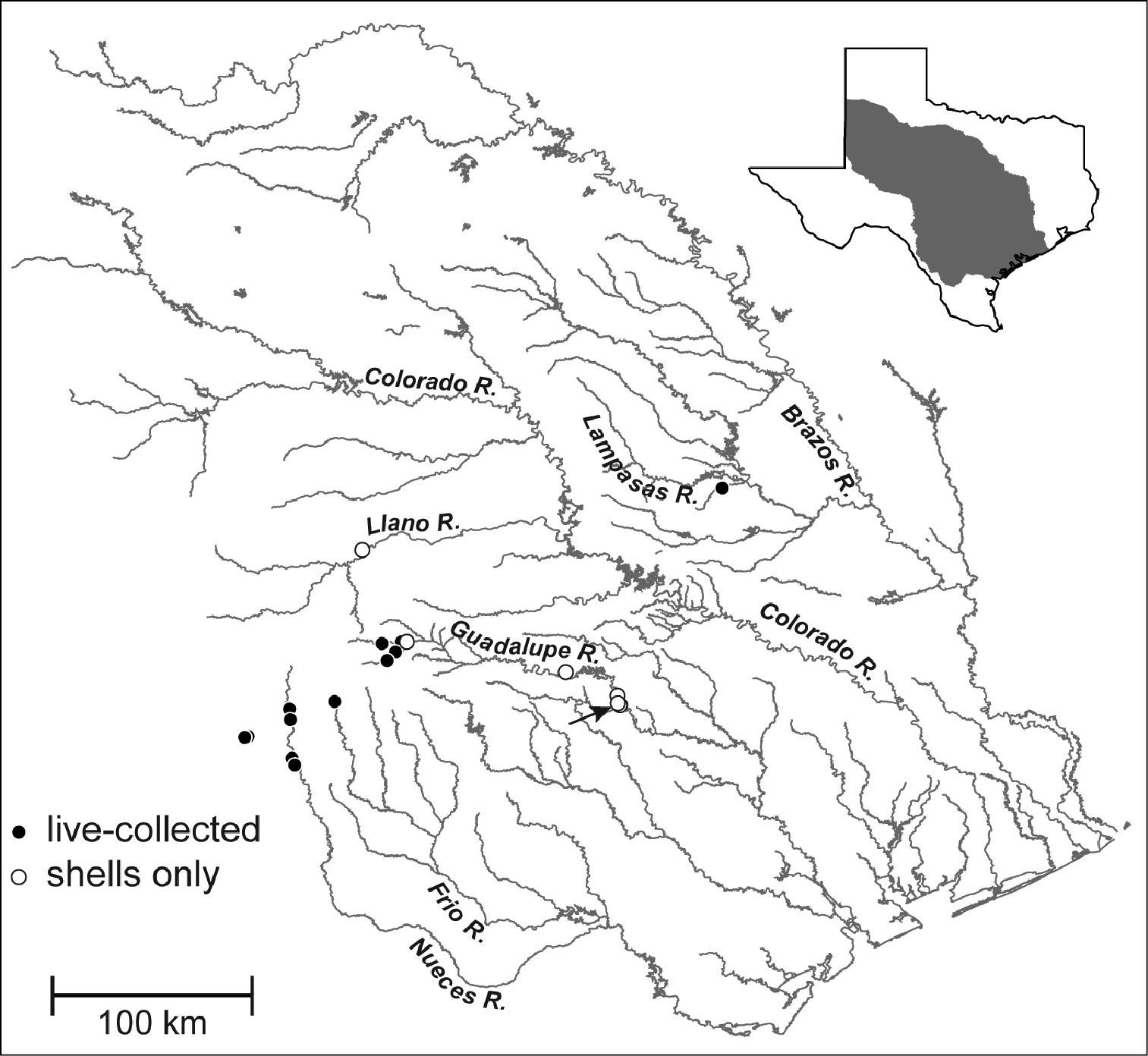
Map showing the distribution of Marstonia comalensis in the Brazos, Colorado, Guadalupe and Nueces River basins, south-central Texas. The arrow indicates the type locality (Comal Creek).
Marstonia comalensis is distributed in the upper portions of the Brazos, Colorado, Guadalupe and Nueces River basins, south-central Texas (Fig. 4);
almost all of these localities are on the Edwards Plateau. We were
unable to confirm a previous report of this species from a drainage
canal near Galveston Bay (
Shell parameters for Marstonia comalensis.
| WH | SH | SW | HBW | WBW | AH | AW | SW/SH | HBW/SH | AH/SH | |
|---|---|---|---|---|---|---|---|---|---|---|
| Lectotype, ANSP 91323 | ||||||||||
| 4.5 | 3.00 | 2.08 | 2.12 | 1.81 | 1.36 | 1.14 | 0.69 | 0.71 | 0.45 | |
| USNM 874926 (n=30) | ||||||||||
| Mean | 4.79 | 3.49 | 2.39 | 2.58 | 2.08 | 1.66 | 1.35 | 0.69 | 0.74 | 0.48 |
| S.D. | 0.18 | 0.17 | 0.09 | 0.11 | 0.08 | 0.09 | 0.05 | 0.03 | 0.02 | 0.02 |
| Range | 4.5–5.0 | 3.20–3.87 | 2.22–2.55 | 2.38–2.84 | 1.93–2.25 | 1.50–1.84 | 1.26–1.45 | 0.63–0.74 | 0.70–0.77 | 0.43–0.51 |
The material referred to Marstonia comalensis
herein, which includes specimens from the Guadalupe River above the
Comal Creek confluence, closely conforms to the types of this species
both in size and shell shape (Fig. 1A–C). This snail clearly belongs to Marstonia
based on its strongly wrinkled shell protoconch, distally bifurcate
penis ornamented with a gland along the edge of the lobe (terminal
gland), and connection between the oviduct and bursal duct well in
front of the posterior pallial wall (
The original collections of Marstonia comalensis are worn shells having the appearance of drift material (Fig. 1A).
We have not seen any live-collected specimens of this species in the
numerous samples that we have examined from the type locality (Comal
Springs) and other waters near New Braunfels. The various reports of
living Marstonia comalensis from this portion of the Guadalupe River basin (e.g.,
Taylor’s (1975) allocation of Amnicola comalensis to Cincinnatia
appears to have been the result of a misidentification as all of the
material in his collection that he referred to this species (per the
original labels), including several lots from the type locality, is Cincinnatia integra
(RH unpublished). Some of these records were detailed in an unpublished
manuscript, “Freshwater molluscs from the Nueces River drainage,
Texas” that Taylor circulated in the mid-1970’s. Cincinnatia integra, which is widely distributed in Texas (
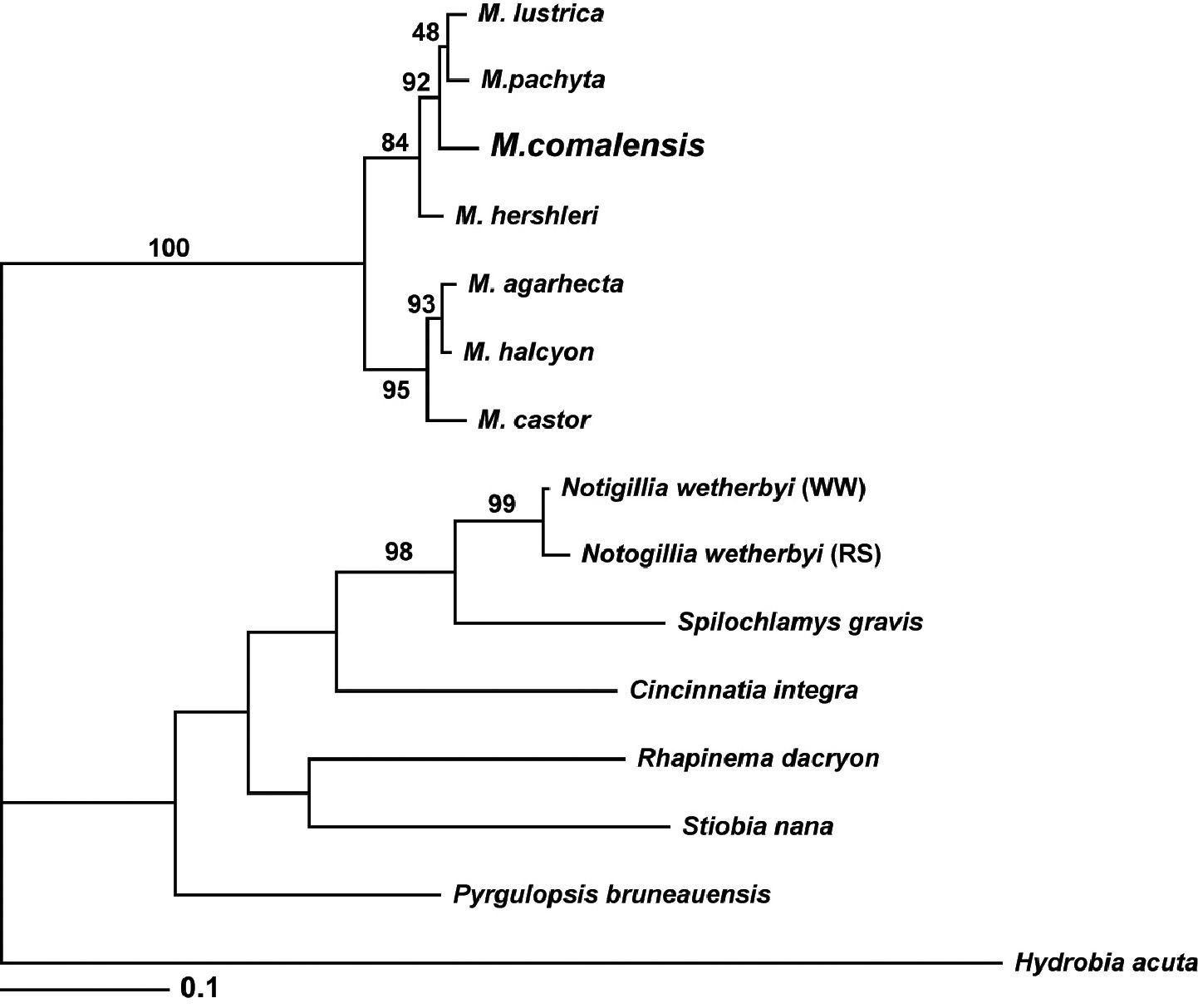
Bayesian tree based on the COI dataset. Posterior probabilities for each node within the Marstonia clade are shown for the reader’s interest; only those >95% are considered significant.
Marstonia comalensis is ranked as critically imperiled (G1) by
We thank Paul Callomon and Amanda Lawless (ANSP), Andrew Simons and Jonathan Slaght (UMBMNH), and Fred Thompson and John Slapcinsky (FLMNH) for loans of specimens under their care. We also thank Peter Diaz and Randy Gibson for providing collections of snails from the type locality area of Marstonia comalensis, and Tierra Curry for sharing information regarding this conservation status of this species. Yolanda Villacampa measured shells and prepared scanning electron micrographs, and Karolyn Darrow inked the anatomical drawings. This paper was improved by the comments provided by two anonymous reviewers.