(C) 2011 Gilles Luquet. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
“Crustaceans are the champions of mineral mobilization and deposition in the animal kingdom”
Lowenstam and Weiner 1989
For growing, crustaceans have to molt cyclically because of the presence of a rigid exoskeleton. Most of the crustaceans harden their cuticle not only by sclerotization, like all the arthropods, but also by calcification. All the physiology of crustaceans, including the calcification process, is then linked to molting cycles. This means for these animals to find regularly a source of calcium ions quickly available just after ecdysis. The sources of calcium used are diverse, ranging from the environment where the animals live to endogenous calcium deposits cyclically elaborated by some of them. As a result, crustaceans are submitted to an important and energetically demanding calcium turnover throughout their life. The mineralization process occurs by precipitation of calcium carbonate within an organic matrix network of chitin-proteins fibers. Both crystalline and stabilized amorphous polymorphs of calcium carbonate are found in crustacean biominerals. Furthermore, Crustacea is the only phylum of animals able to elaborate and resorb periodically calcified structures. Notably for these two previous reasons, crustaceans are more and more extensively studied and considered as models of choice in the biomineralization research area.
ACC, amorphous calcium carbonate, biomineralization, calcification, calcium storage, cuticle, organic matrix
Biomineralization corresponds to the process of mineralized structures formation by living organisms. This word designates also the elaborated mineralized structure itself. This phenomenon, which appeared firstly in Eubacteria and Archea as a biologically-induced process (
The biomineralization process is well developed in several taxa, including Crustacea as one of the most outstanding group in this respect. As a consequence of the presence of a rigid exoskeleton, the growth and the whole physiology of these animals are linked to molting cycles characterized by the complete renewal of the exoskeleton during each molting. All of the arthropods harden their new cuticle by a process called sclerotization (protein-polysaccharide and protein-protein cross-linking by the way of quinonoid-sclerotizing agents) and, in addition, most of the crustaceans proceed furthermore by calcification.
The major source of calcium used for exoskeleton calcification is exogenous: the water in which most crustaceans live. In seawater, calcium concentration is very high but many species also live in freshwater or on land, where the availability of calcium at ecdysis may be low or even absent. Therefore crustaceans have developed different strategies to solve the problem of calcification, in particular by storing calcium during the premolt period (
Next, crustaceans are particularly interesting because of their active calcium metabolism and their ability to form and resorb cyclically (more or less partially) not only an external calcified structure but also, in many species, calcium storage biomineralizations.
Another characteristic of calcium metabolism in crustaceans are the calcium-transporting epithelia very similar to some vertebrate ones, and in this way they represent also good models to understand how they function. Calcium pumps and enzymatic systems, similar to those associated with vertebrate calcium-transporting epithelia, have been evidenced in the cuticular epidermis and the calcium storage epithelium. Furthermore crustaceans are convenient models for studying the hormonal regulation of the calcium turnover with the possible involvement of vertebrate-type hormones such as calcitonin/CGRP and vitamin D presumably evolving from invertebrate counterparts (
In crustaceans, a cellular hypodermis, which underlies the calcified cuticle also called carapace, is responsible for the complete synthesis of the exoskeleton. This cuticle comprises four main layers, from the external to the innermost layer: the epicuticle, the exocuticle, the endocuticle and the membranous layer. Calcification of this cuticle has been particularly well studied in Decapoda (
Until recently, the structure and calcification of the cuticle in isopods have been poorly investigated (
Finally, the cuticle of Ligia italica was used as a model of matrix-mediated calcification in a comparative study using also calcite deposits generated by Synechoccocus cyanobacteria as a biologically-induced calcification model. As previously shown for growth zones of bones (
While the presence of ACC as the main polymorph in calcium storage deposits can be easily understood (see below), the presence of metastable ACC at the level of the cuticle is more surprising. Nevertheless, some advantages of the presence of stable amorphous minerals in cuticles are shape flexibility, plasticity, as well as optimization of strenght and toughness (
From the structural-mineral point of view, the presence of different polymorphs of calcium carbonate within the crustacean cuticles is tightly linked to the presence of specific matrix molecules.
Some molecules, identified as cuticular proteins, have been characterized, the precise function of which is not well elucidated. Some of them, possessing a Rebers-Riddiford domain in their sequence, a hallmark of their chitin-binding ability (
Other proteins, with in vitro calcium-binding ability or which interact with CaCO3 formation, are also thought to play an active role in the in vivo CaCO3 precipitation process. Among them are DD4/crustocalcin from Penaeus monodon (
Except for the Copepoda and Cirripedia (Maxillopoda), calcium storage is a process commonly found in the other groups of crustaceans. The sites of storage as well as the morphologies of the storage structures are very diversified (
The most extensively studied model is the semiterrestrial talitrid amphipod Orchestia cavimana. This animal cyclically stores calcium in two diverticula of the midgut, called posterior ceca (PC), in the form of calcareous concretions (Figure 2;
Adult specimen of the freshwater red claw crayfish, Cherax quadricarinatus, just after molting (at left the exuviae).
The storage organs are composed of a one-layered epithelium forming tubules, which are proximally connected to the midgut and distally blind-ended. During the premolt period, calcium originating from the present cuticle is transported in an ionized form and is precipitated in the PC lumen within an organic matrix synthesized by the PC cells (Figure 2C). Concretions are formed by addition of successive concentric layers of organic matrix, within which calcium carbonate is precipitated as the amorphous polymorph (
Calcium storage in the semiterrestrial amphipod, Orchestia cavimana. A Adult male specimen (2-cm long) B Radiography of an adult specimen just after ecdysis (electron-dense stored calcium is well visible in 2 diverticula of the midgut; arrows) C Calcium is stored as calcareous concretions in paired posterior ceca. c: concretion, hg: hindgut, mg: midgut.
To understand the formation of biomineralized structures, the characterization of the molecular components of the organic matrix is of first interest. Biochemical and molecular biology techniques were used to characterize proteinaceous components of the matrix. One peculiar protein, named Orchestin, has been well characterized and completely sequenced. This phosphorylated calcium-binding protein is probably responsible for the precipitation of calcium carbonate within the storage organs in premolt as well as for the formation of the calcium resorption spherules in postmolt (
Other interesting amphipod models are troglobites of the genus Niphargus. They are able to store calcium in posterior ceca as amorphous calcium carbonate concretions, similarly to Orchestia amphipods, but also as rhomboedric crystalline structures (probably calcitic) within the gut (
Isopods possess a particular biphasic mode of molting: they shed first the posterior half of their cuticle, then the anterior part (
The calcified storage structures are composed of amorphous hydrated calcium carbonate precipitated within an organic matrix of spherules (
The formation and resorption of these sternal plates seem closely related to the biphasic molting cycle of these crustaceans. The calcium deposits, fully developed before ecdysis of the posterior part, are completely resorbed before ecdysis of the anterior part. It has been suggested that the calcium, stored as calcospherules in the edysial space, is used to calcify the posterior cuticle (
The order Decapoda represents the largest group of crustaceans living on land as well as in water, and storage strategies are well developed and very diversified in decapods.
For calcifying the cuticle, calcium ions are translocated from an endogenous or exogenous source through the haemolymph. In some species this medium is used as a storage site. In the freshwater/land crab, Holthuisana transversa, the haemolymph has been observed to contain small-size calcified granules representing a way of transporting a great amount of calcium in a short period while avoiding toxification (
Hepatopancreas is also used by some crabs like Cancer pagurus, Carcinus maenas, Callinectes sapidus and Paratelphusa hydrodomous as a storage organ where calcified granules of calcium phosphate have been found, also considered by some authors as playing a role in metal detoxication processes (
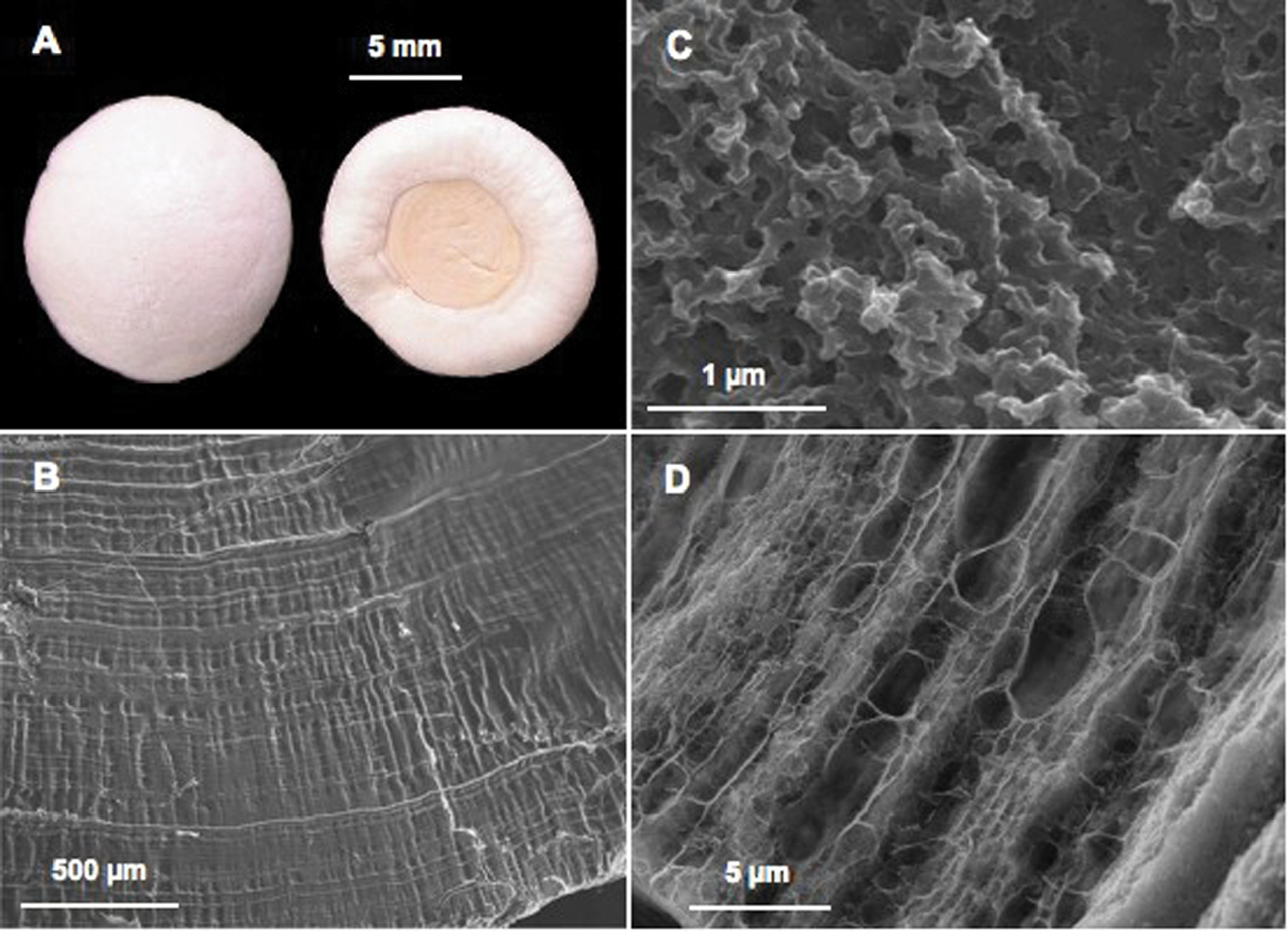
Finally some decapods store calcium in their cardiac stomach wall between the one-layered epithelium and a cuticle as so-called gastroliths (
Calcium storage as gastroliths in decapods. A Pair of gastroliths from the crayfish Cherax quadricarinatus (Light Microscopy) B Internal striated structure visible on natural fracture after slight acetic acid decalcification (SEM) C Ultrastructure well visible after natural fracture and high magnification (SEM): the mineral is precipitated as nanospheres D Organic matrix network revealed after acetic acid decalcification (SEM).
Four organic matrix proteins from gastroliths have been well characterized and sequenced so far. The first one, named GAMP, was obtained from the crayfish Procambarus clarkii (
How the biogenic ACC polymorph can be stabilized in time is still an open question that received recent attention. It was previously suggested that specialized macromolecules (acidic proteins, phosphoproteins, sulfated glycoproteins) or ions such as magnesium or phosphate could contribute to this stabilization (
The formation of the majority of biominerals is under biological control. The knowledge of the physical and chemical features of the matrix components (proteins, polysaccharides, proteoglycans, lipids, low-molecular weight components...) is a prerequisite to understand, at the molecular level, how a biomineralization is elaborated, how the matrix molecules are involved in the nucleation and precipitation processes, how they influence the determinism of the polymorph obtained, and finally how a demineralizing process may occur.
In consideration of their ability to cyclically elaborate and resorb biomineral composites, crustaceans appear as convenient models for such prospects. They are not only able to synthesize a calcified exoskeleton but also calcium storage structures, which differ in their morphology and in their mineral composition. The calcium storage deposits are transient reservoirs of calcium ions that must be quickly mobilizable after ecdysis, and, for this reason, the storage structures are composed mainly of ACC. The cuticle reveals also a remarkable biocomposite because of the simultaneous presence of different polymorphs of CaCO3 and ACP as well. Recent investigations suggest that if some general structural features may be common to all cuticles, it seems likely that the cuticle mineral composition, linked to the molecular content, not only could vary from one order of crustaceans to another but could also depend on the ecophysiology of each species.
To understand why the amorphous calcium carbonate state is stabilized in time whereas the same purely inorganic mineral is completely unstable, as well as how the switch of the transformation from the amorphous to crystalline phases occurs, is also of great interest in the biomaterial and nanotechnology fields (
Finally, by means of comparative studies performed in other calcifying phyla, crustaceans are useful to determine why and how calcification could have emerged on Earth. The sequence analysis of the matrix proteins and the genes encoding these proteins could lead to the understanding of the strategy used by evolution to built and select different mineralizing systems: convergence of different biological systems for a similar mineralizing function by exaptation of initially non-mineralizing molecules or evolution and adaptative divergence from an ancestral biomineral system still undeciphered?
The author is greatly indebted to Prof. François Graf for the photos of Orchestia cavimana and for many fruitful discussions on crustaceans and biomineralizations. The author also thanks Andreas Ziegler, Nada Žnidaršič and the editorial committee for valuable comments.