(C) 2010 Adam J. Brunke. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Staphylinidae (Rove Beetles) from northeastern North America deposited in the University of Guelph Insect Collection (Ontario, Canada) were curated from 2008–2010 by the first author. The identification of this material has resulted in the recognition of thirty-five new provincial or state records, six new Canadian records, one new record for the United States and two new records for eastern Canada. All records are for subfamilies other than Aleocharinae and Pselaphinae, which will be treated in future publications as collaborative projects. Range expansions of ten exotic species to additional provinces and states are reported. The known distributions of each species in northeastern North America are summarized and presented as maps, and those species with a distinctive habitus are illustrated with color photographs. Genitalia and/or secondary sexual characters are illustrated for those species currently only identifiable on the basis of dissected males. The majority of the new records are in groups that have been recently revised, demonstrating the importance of curation and local insect surveys to the understanding of biodiversity, even for taxa and areas considered ‘relatively well-known’.
rove beetles, exotic species, Sepedophilus, Erichsonius, Gabrius, Philonthus, Quedius
The rove beetles of northeastern North America, defined
here as a region from Ontario eastward and south to Virginia, are well
known faunistically compared to other regions of the world, with the
exception of the western Palaearctic. For the large subfamilies Staphylininae, Tachyporinae, Steninae, Pselaphinae and to some degree Omaliinae, modern monographs by (
Although the University of Guelph Insect Collection (DEBU) is Canada’s third or fourth largest collection of invertebrates, relatively few of its staphylinid specimens were considered in the course of the above-mentioned revisions. We assume this oversight was due to the collection’s reputation for its coverage of Nearctic and Neotropical Diptera, and the corresponding incorrect assumption that other orders are not well-represented. Although it is true that over half of the 2.5 million or so specimens in the collection are flies, the University of Guelph collection includes several historically important beetle collections and continues to accumulate Coleoptera in the course of ongoing surveys of Ontario’s parks and protected areas as well as routine collecting associated with course work and general collection development. The acquisition of the Alan and Anne Morgan Collection (AAMC) in early 2010 further augmented this material. Previously deposited at the University of Waterloo, this collection of mostly Coleoptera has strengths in the subarctic, boreal and eastern deciduous fauna of Canada. Most Staphylinidae at DEBU were inadequately curated and mostly unidentified prior to recent curatorial work by the senior author, but now all rove beetles in the collection are identified at least to the genus level (except the Aleocharinae and Pselaphinae) and a large proportion of identified specimens are now entered into the central database. We here report on the faunistic discoveries made in the process of curating this material and discuss their importance in the context of previous knowledge. Known distributions are summarized for each species and those species with a distinctive habitus are illustrated to aid in their identification. Where necessary, the aedeagi and/or secondary sexual characters of species are illustrated. Identification of the Pselaphinae and Aleocharinae at DEBU is in progress and future publications on these subfamilies are planned.
Materials and methodsSpecimens were examined with a WILD Heerbrugg M5A
stereomicroscope and dissections of male genitalia and genital segments
of both sexes were performed in distilled water after preparation
following
CANADA: ON: Chatam-Kent Co., Tilbury, pitfall trap, 9-VI-1994 (2), T. Sawinksi; Essex Co., Leamington, pitfall trap, 15 to 22-V-1992 (1), 22-V-1992 (1), 9-VI-1993 (1), Palichuck; Point Pelee Natl. Pk., Visitor Centre, malaise and pans, 22 to 29-V-2000, (2), 26-IX to 10-sX-2000 (1) O. Lonsdale. Huron Co., Auburn, Londesboro Rd. nr. Hwy 8, 43.728, -81.529, hedgerow, pitfall, 26-X-2009, A. Brunke (1); Benmiller, Sharpes Creek Line, 43.691, -81.608, hedgerow nr. creek, pitfall, 2-XI-2009, A. Brunke (1); Brucefield, London Rd. nr. Centennial Rd., 43.509, -81.516, hedgrerow nr. creek, pitfall, 27-IX-2009 (1), 12-X-2009 (4) A. Brunke; Waterloo Reg., Blair, Dickie Settlement Rd. nr. WhistleBear golf course, 43.373, -80.400, hedgerow, pitfall, 28-IX-2009 (6), 13-X-2009 (2) A. Brunke; Blair, Dickie Settlement Rd. nr. WhistleBear golf course, 43.373, -80.400, hedgerow, canopy trap in buckthorn, 10-XI-2009, A. Brunke (1); Blair, Fountain St. S. nr Speed River, 43.391, -80.373, hedgerow, pitfall, 24-XI-2009, A. Brunke (1); Blair, Whistlebare Rd. and Township Rd.1, 43.372, -80.362, hedgerow, canopy trap in buckthorn, 18-V-2010, A. Brunke (2); Blair, Whistlebare Rd. and Township Rd.1, 43.372, -80.362, hedgerow, pitfall, 1-VI-2010, A. Brunke (1); Blair, Whistlebare Rd. and Township Rd. 1, 43.367, -80.358, hedgerow, canopy trap in buckthorn, 18-V-2010 (1), 21-IX-2010 (1), A. Brunke; Blair, Whistlebare Rd. and Township Rd. 1, 43.367, -80.358, hedgerow, pitfall, 5-X-2010, A. Brunke (1); St. Jacobs, ‘Stuart pitfall’, 3-VI-1993 (1), 14-VI-1994 (1), T. Sawinksi.
As the genus Omalium currently lacks a rigorous definition and consists of a heterogeneous assemblage of species (
Dorsal habitus. 1 Omalium repandum Erichson 2 Porrhodites fenestralis (Zetterstedt) 3 Ischnosoma flavicolle (LeConte) 4 Tachyporus browni Campbell 5 Eustilicus tristis (Melsheimer) 6 Bisnius cephalicus (Casey).
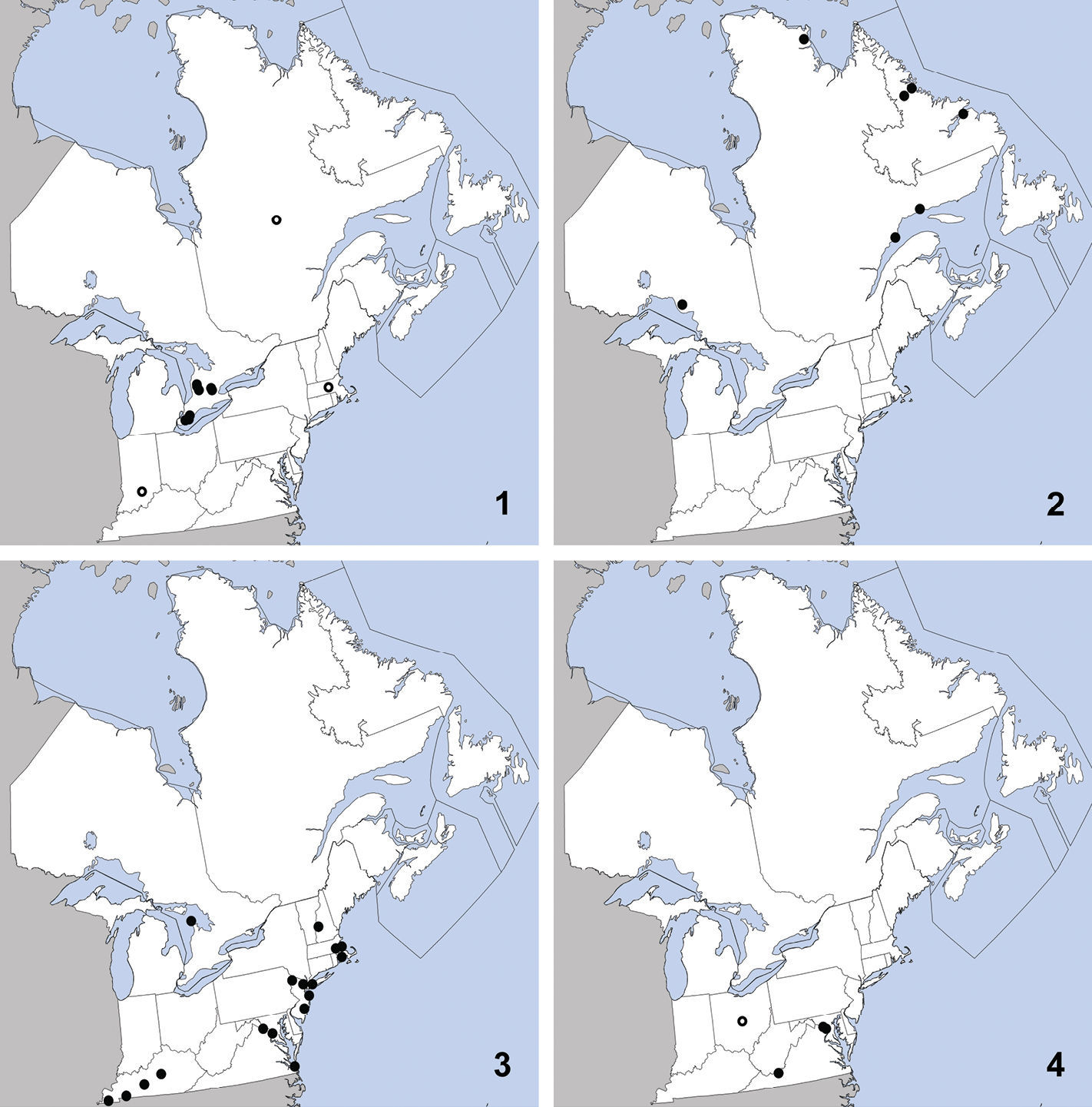
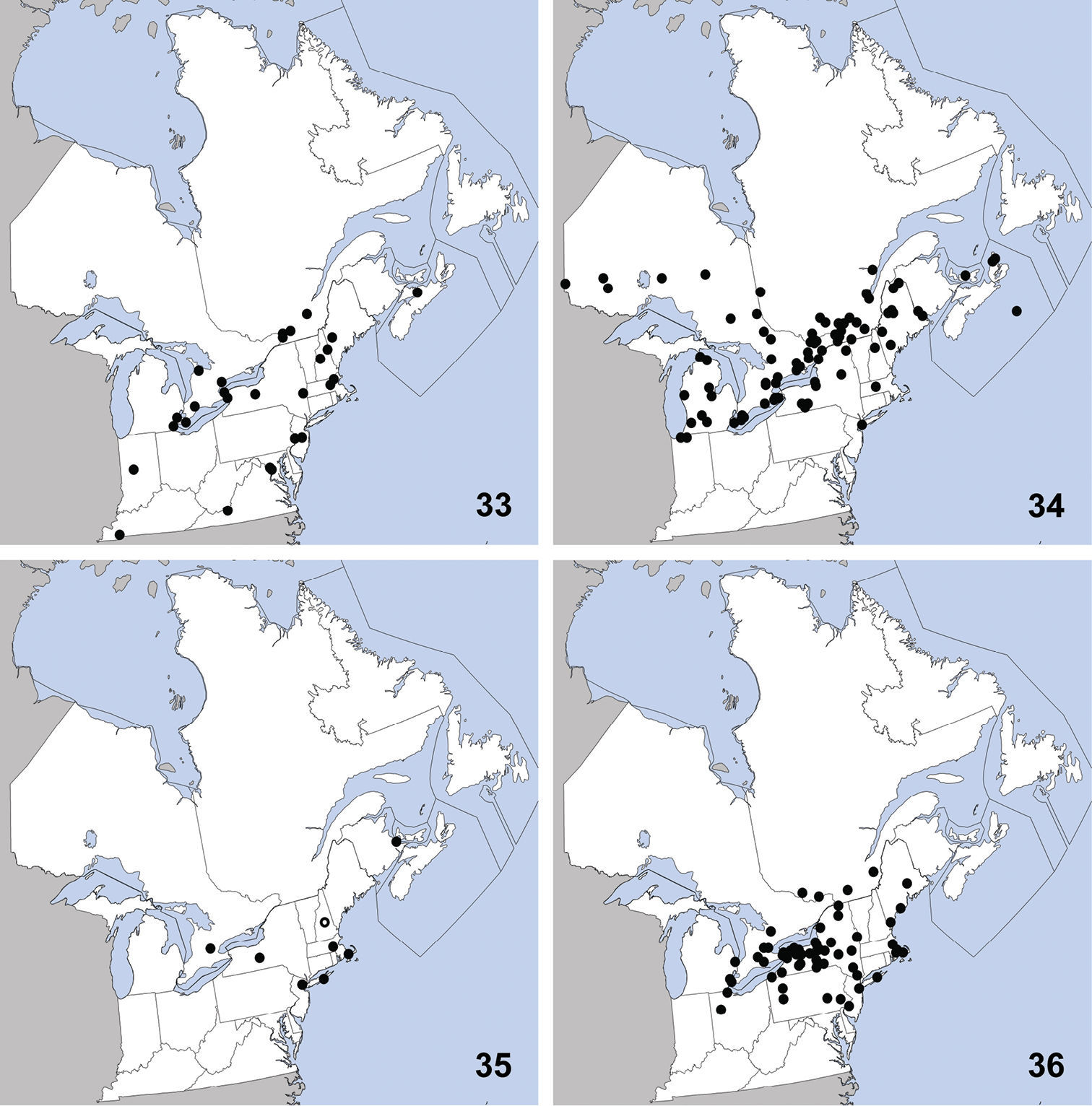
Omalium repandum was previously known from Missouri, South Carolina, Texas (
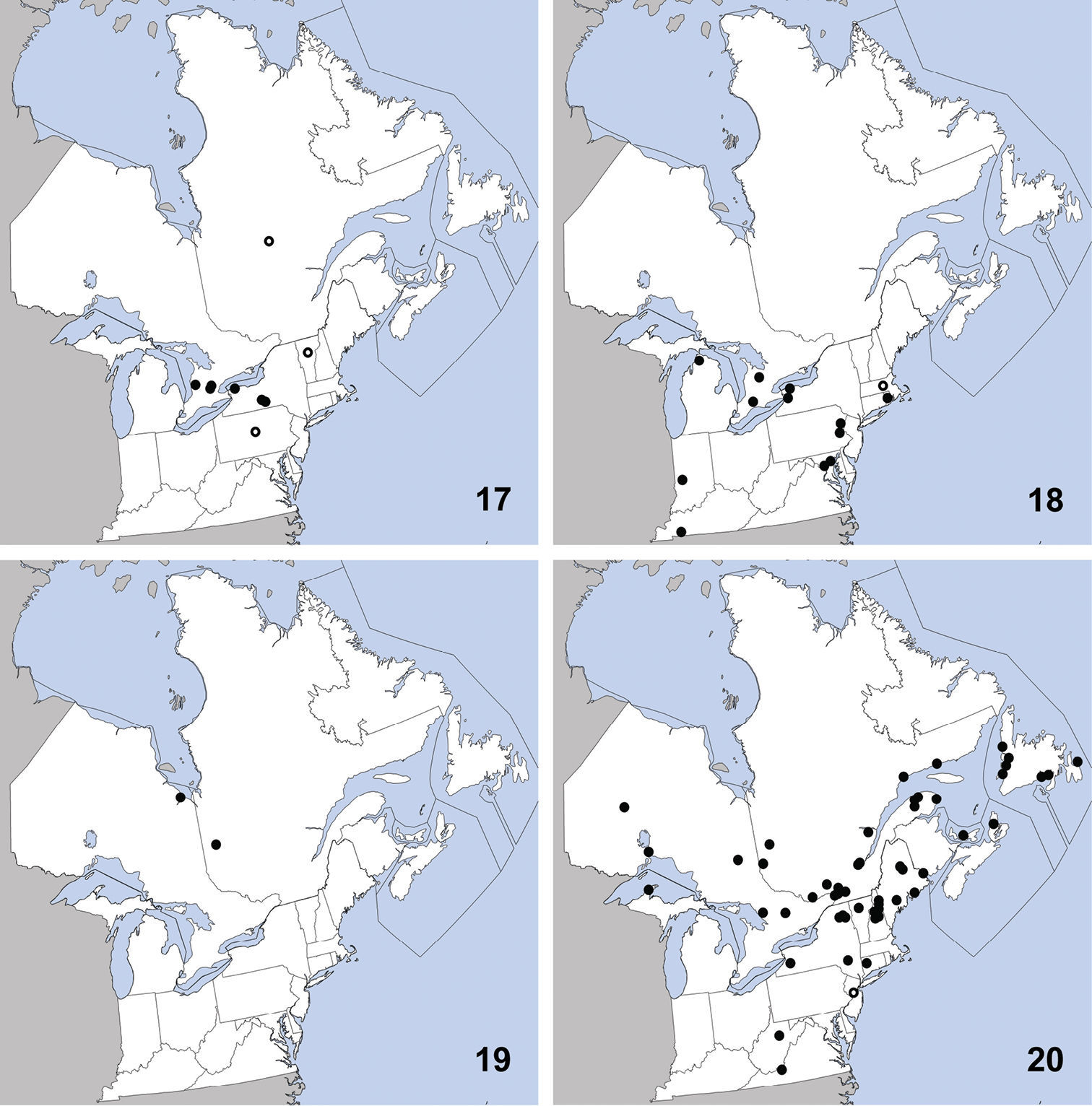
Distribution in northeastern North America, sources of data other than DEBU are quoted in parentheses. 1 Omalium repandum Erichson (
CANADA: ON: Thunder Bay Distr. Pukaskwa Natl. Pk. beach trail, dunes, 30-VII-2003, S.M. Paiero (1).
This species can be readily distinguished from Porrhodites inflatus (Hatch), the only other member of the genus in North America, by the combination of: pronotal margins evenly arcuate; metasternum without microsculpture; antennomere two distinctly longer than segment three (Campbell 1984) (Fig. 2).
Porrhodites fenestralis
is a holarctic, boreal to subarctic species known in the Nearctic
region from Alberta, British Columbia, Manitoba, Newfoundland,
Northwest Territories, Quebec, Yukon Territory, and Alaska, with a
relict population in the Rocky Mountains of Montana and Wyoming
(Campbell 1984).In the Palaearctic it is known from Austria, Czech
Republic, Finland, Germany, Italy, Norway, Russia (North European
Territory, Far East, East Siberia, and West Siberia), Sweden, and
Switzerland (
CANADA: ON: Bruce Co., Dorcas Bay, dunes, pans under malaise, 5 to 13-VI-1999, S.A. Marshall (1).
Ischnosoma flavicolle is easily distinguished from others of the genus by the distinctly bicolored elytra that each lack a humeral spot (
This species is primarily southeastern in
distribution, with a northward extension along the Atlantic coast, and
was known previously from Alabama, Arkansas, District of Columbia,
Florida, Georgia, Illinois, Kansas, Kentucky, Louisiana, Maryland,
Massachusetts, Mississippi, Missouri, New Hampshire, New Jersey,
New York, North Carolina, Oklahoma, South Carolina, Tennessee,
Texas, and Virginia (
UNITED STATES: VA: Giles Co., Cascades Recreation Area, sifted from leaf litter in hardwood forest, 11 to 25-V-2008, A. Brunke (1).
Sepedophilus campbelli is distinguished from other species of the genus in northeastern North America by the combination of: pronotum and elytra without microsculpture and without pale or reddish markings; small size (<1.7mm from the clypeus to the elytral apex); middle-tibia with two apical spines; basal abdominal segments with long lateral bristles.
When
UNITED STATES: NH: Coos Co., Jefferson, under bark, 20-IV-2010, T. Murray (1).
CANADA: ON: Essex Co., Kingsville, 14-V-1973, R. Roughly (1); Waterloo Reg., Blair, RARE, Cruickston Creek, yellow pan traps, 15 to 20-VI-2006, S.A. Marshall and M. Bergeron (1); Blair, Dickie Settlement Rd. nr. WhistleBear golf course, 43.373, -80.400, hedgerow, pitfall, 13-X-2009 (1), 10-XI-2009 (1), A. Brunke;Blair, Fountain St. S. nr Speed River, 43.391, -80.373, hedgerow, pitfall, 13-X-2009 (1), 10-XI-2009 (1);Blair, Whistlebare Rd. and Township Rd.1, 43.372, -80.362, hedgerow near soybean field, pitfall trap, 2-XI-2010 (2); Wellington Co., Arkell, 27-IX-1986, L. Work (1); Belwood Lake, lake margin, fallen log overhang, 3-VI-2008, S.A. Marshall (1); Eramosa, Wellington County Rds. 124 and 29, 43.615, -80.215, hedgerow, pitfall, 4-V-2010, A. Brunke (1); Guelph, 19-V-1981, G.M. Grant (1); Guelph, in rotten wood, 20-IV-2007, S.P.L. Luk (1); Guelph, Preservation Park, under bark, 21-IX-2010, S.P.L. Luk (1).
Sepedophilus marshami may be distinguished from all other members of the genus in eastern North America except Sepedophilus testaceus
by the following combination of characters: body size large (>2.3mm
from clypeus to elytral apex); tergite seven with a white, apical,
palisade fringe and at least one pair of bristles; elytra reddish but
without distinct, reddish basal markings; middle-tibia with two apical
spines (
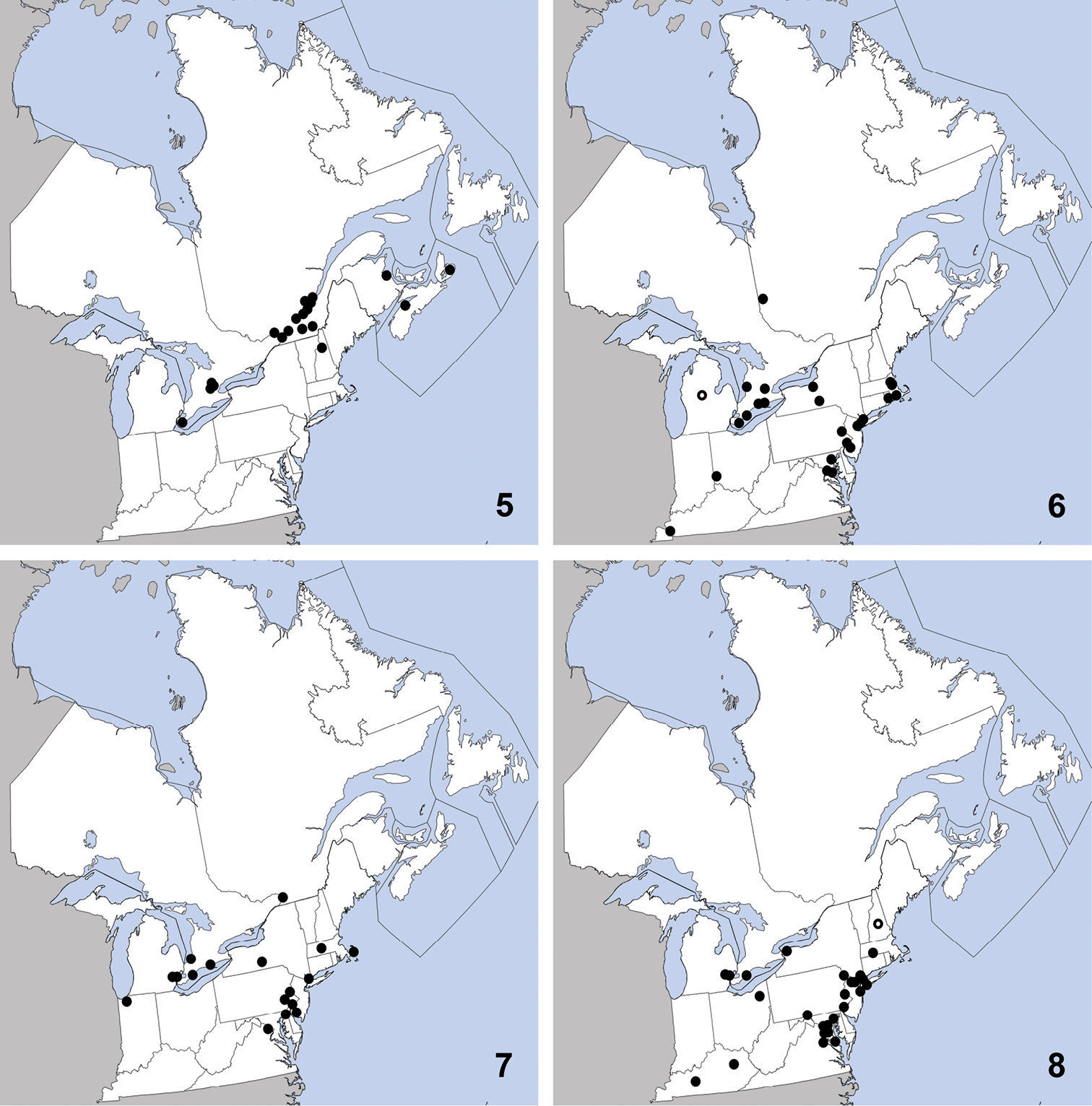
This exotic, Palaearctic species was first collected in North America in Quebec in 1959 (
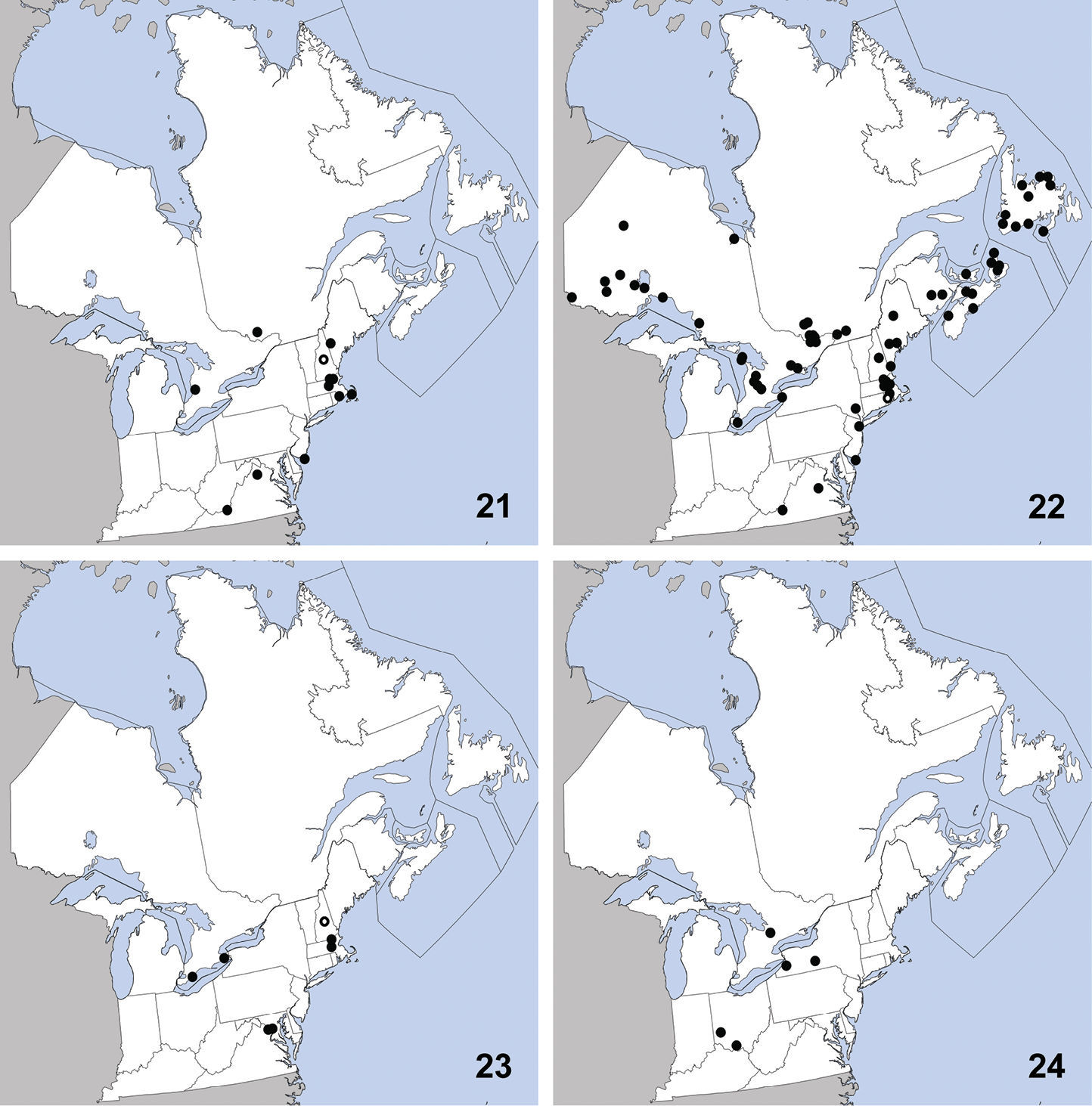
Distribution in northeastern North America, sources of data other than DEBU are quoted in parentheses. 5 Sepedophilus marshami (Stephens) (
CANADA: ON: Huron Co., Benmiller, Sharpes Creek Line, 43.691, -81.608, hedgerow nr. creek, canopy trap in buckthorn, 22-VI-2009, A. Brunke (1); Elgin Co., Orwell, 15-VI-1978, D. Morris (1); Essex Co., Point Pelee Natl. Pk., wood area by W beach, malaise/pan traps, 10 to 23-IX-1999, O. Lonsdale (1); Hald.-Norfolk Reg., Turkey Point Provincial Park, malaise trap, 3 to 28-VIII-2009, S. Paiero (1); Kent Co., Rondeau Prov. Park, spicebush trail, 42°18'9N, 81°51'6W, Carolinian forest, WPT, 16to17-VI-2003, Paiero and Carscadden (1); Wellington Co., Guelph, University arboretum nature reserve, ex. Beech litter, 3-V-2009, Brunke and Cheung (1); Guelph, Victoria Rd. and Conservation Line, 43.580, -80.275, hedgerow, pitfall, 2-VI-2009, A. Brunke (1).
Sepedophilus occultus can be distinguished from other northeastern Sepedophilus
with a reddish area at the base of each elytron by the combination of:
basal abdominal segments with short bristles only; elytral epipleuron
with few or no setae on its basal half; pronotum uniformly colored;
elytra without coarse bristles laterally; apical ctenidium of mesotibia
restricted to the apex (
This species is widely distributed in eastern
North America and was previously known from Connecticut, District of
Columbia, Georgia, Illinois, Iowa, Kentucky, Maryland,
Massachusetts, Michigan, Mississippi, New Jersey, New York, Ohio,
Pennsylvania (
CANADA: ON: Hald.-Norfolk Reg., Backus Tract Woods, sifting leaf litter under mushrooms, 7-VI-2009, A. Brunke and L. DesMarteaux (5); Backus Tract Woods, sifted litter in sugar maple-dominated, mesic forest, 2-IV-2010, A. Brunke (1); Kent Co., Rondeau Prov. Pk., spicebush trail, carolinian forest, malaise, 16 to 29-VII-2003, S. Marshall et al. (1); Rondeau Prov. Pk., south point trail, slough forest, sifting leaf litter, 27-IX-2009, A. Brunke and D.K.B. Cheung (1). Lambton Co., Pinery Prov. Pk., Carolinian Trail, hardwood forest, litter around white pines, 17-IV-2010, A. Brunke (3).
Sepedophilus opicus
can be distinguished from other members of genus in northeastern North
America by a combination of: base of elytra with reddish markings
extending laterally to the margin; basal abdominal segments with long
bristles; elytral epipleuron uniformly setose; pronotal microsculpture
distinct (
This widely distributed species is known from
Alabama, Florida, Illinois, Indiana, Iowa, Maryland,
Massachusetts, Michigan, Missouri, New Jersey, New York, North
Carolina, Pennsylvania, Québec, Texas, and Virginia (
CANADA: ON: Kent Co., Rondeau Prov. Pk., spicebush trail, Carolinian forest, malaise, 3 to 16-VII-2003 (1), 15-VIII to 7-IX-2003 (1), Marshall et al., 16 to 29-VII-2003, S.A. Marshall (1).
Sepedophilus versicolor can be easily separated from others of the genus except Sepedophilus crassus and Sepedophilus ctenidialis by the apical ctenidium of the mesotibia, which extends upwards along the lateral edge (
This species is broadly distributed in
eastern North America and was previously known from District of
Columbia, Florida, Georgia, Illinois, Iowa, Kansas, Kentucky,
Maryland, Massachusetts, Michigan, Minnesota, Missouri, New
Hampshire, New Jersey, New York, North Carolina, Ohio,
Pennsylvania, South Carolina, and Virginia (
UNITED STATES: MA: Middlesex Co., Groton, 22-IV-2010, T. Murray (1).
Tachinus corticinus is easily distinguished from congeners in northeastern North America by the combination of: pronotum and elytra lacking microsculpture; pronotum with at least borders paler than head; female tergite eight with all lobes of similar size; male sternite seven without apical lobes; small size (3.00–3.75 mm from clypeus to apex of elytra).
This exotic species was first collected in
North America in St. Cyrville, Québec in 1967 and was first recognized
in North America by
Distribution in northeastern North America, sources of data other than DEBU are quoted in parentheses. 9 Tachinus corticinus (Gravenhorst) (
CANADA: ON: Huron Co., Benmiller, Sharpes Creek Line, 43.691, -81.608, soybean field, pitfall, 18-IX-2009, A. Brunke (1).
UNITED STATES: NH: Coos Co., Dixville, leaf litter, 6-IV-2010, T. Murray (1); Jefferson, leaf litter, grassy area near stream, 20-IV-2010, T. Murray (1); Dixville, 4-V-2010, T. Murray (4). MA: Middlesex Co., Groton, sifting hay, flood debris in farm field nr. drainage ditch, 30-IV-2010, T. Murray (1). VT: Orange Co., Topsham, sweeping low vegetation, 22-VI-2010, T. Murray (1).
Tachyporus browni can be easily recognized amongst other northeastern Tachyporus by the combination of a bicolored abdomen and elytra without black discal markings (Fig. 4). Rarely, specimens occur with a small black marking on the scutellum but it does not extend half the length of the elytra as in Tachinus elegans Horn. Additionally, Tachinus elegans lacks dark markings on the pronotum.
This distinctive species was known from 12
specimens at the time of its description, all collected from September
to November in southern Québec and Connecticut (
CANADA: ON: Wellington Co., Belwood Lake, lake margin, fallen log overhang, 3-VI-2008, S.A. Marshall (1).
Tachyporus ornatus can be distinguished from all other large northeastern members of the genus except Tachinus lecontei, by the combination of a non-bicolored abdomen and the crisp, dark markings on the elytra. From Tachinus lecontei it is most easily identified by the fine microsculpture of the elytra which produces a strong metallic sheen (the former species completely lacks microsculpture).
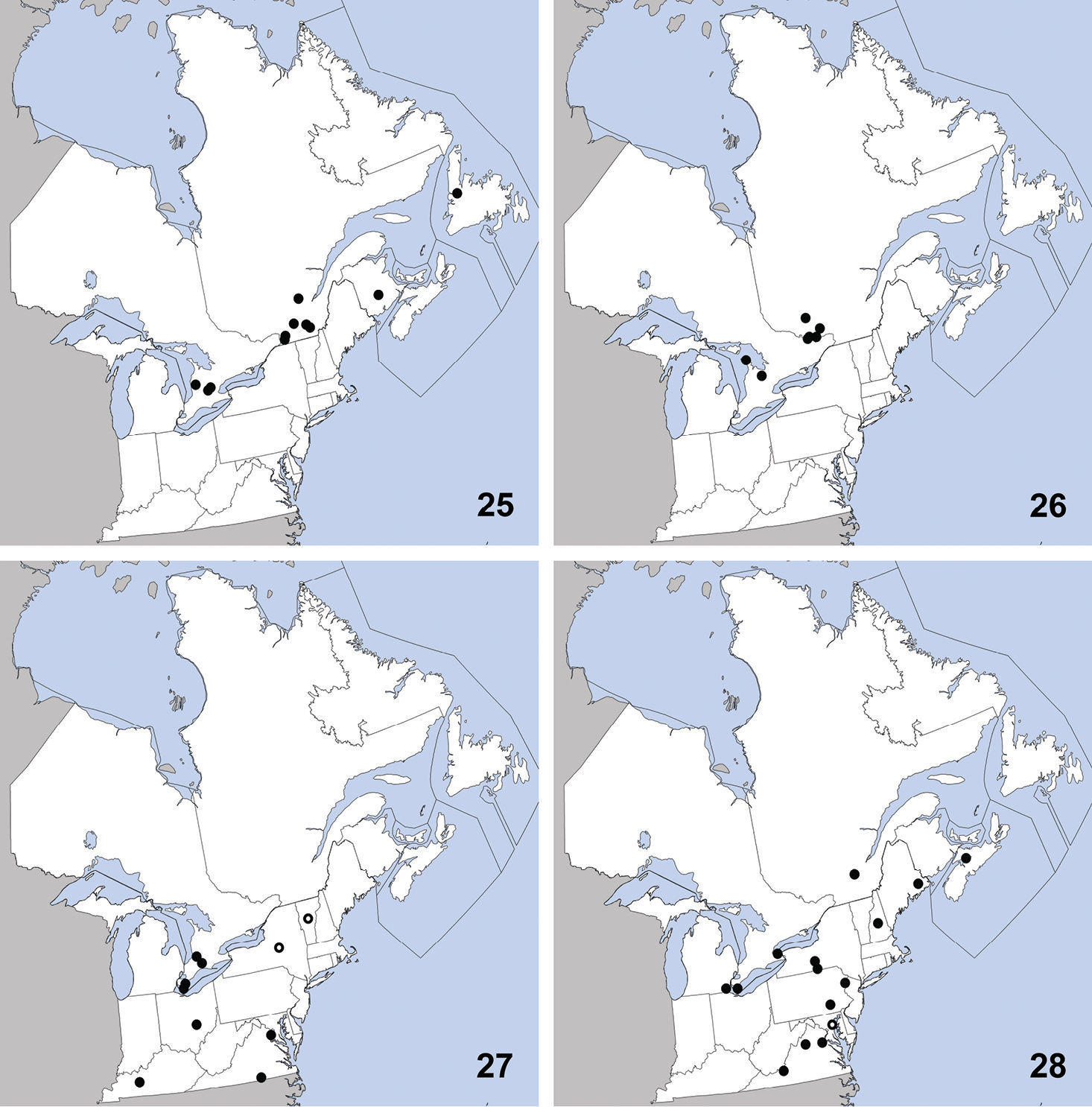
This species is transcontinental in North
America with a disjunct population in the Rocky Mountains of Colorado.
It was previously known from the following states and provinces:
Alberta, Colorado, Manitoba, Massachusetts, New Hampshire, New
Jersey, New York, North Dakota, Pennsylvania, Québec, Saskatchewan,
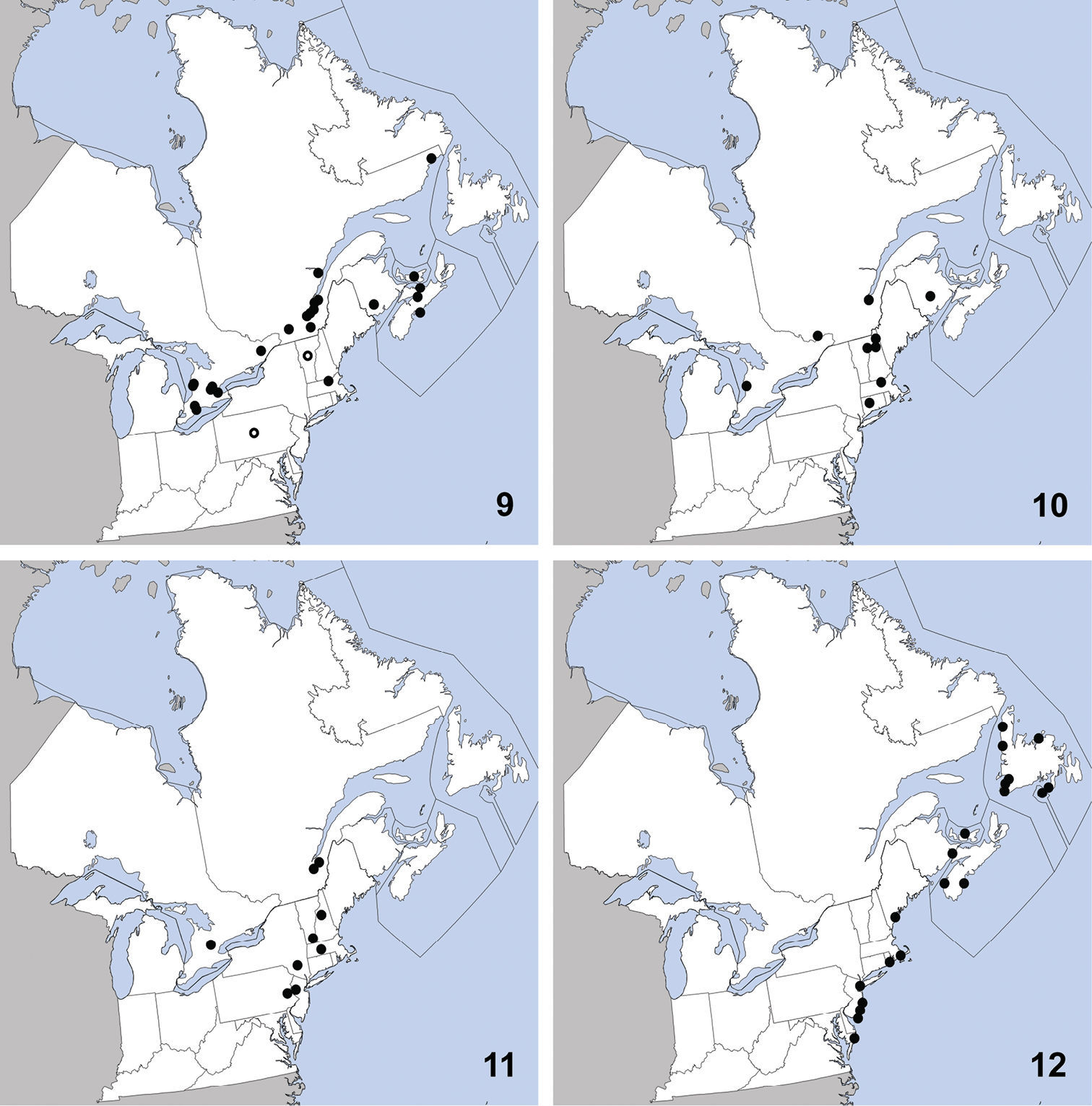
and Vermont. Herein we newly record it from Ontario (Map 11). The only habitat data in
CANADA: PEI: Stanhope Beach, National Park, débris vég. sur plage (=beach debris), 23-VII-1979 (2) R. Sexton.
Bledius neglectus can be identified to the Bledius basalis
group of species by the combination of the complete elytral epipleuron,
the undivided labrum and the lack of a suture on the epipleuron (
This species is widely distributed along the
coast of eastern North America and was previously known from Georgia,
Maine, Maryland, Massachusetts, Newfoundland, New Jersey, New York,
North Carolina, Nova Scotia, Rhode Island (
UNITED STATES: MA: Middlesex Co., Groton, 30-IV-2010, T. Murray, (1).
CANADA: ON: Halton Reg., Milton, Derry Rd. and 4th line, grass field, yellow pans, 23 to 24-VI-2001, S. Paiero, (1); Huron Co., Auburn, Hullett-McKillop Rd. nr. Limekiln Line, 43.744, -81.507, soybean field, pitfall, 4-VIII-2010 (1), A. Brunke; Auburn, Limekiln Line, 43.736, -81.506, hedgerow, pitfall, 26-V-2010 (1), A. Brunke; Benmiller, Sharpes Creek Line, 43.691, -81.608, hedgerow nr. creek, canopy trap in buckthorn, 11-V-2009 (2), A. Brunke;Brucefield, London Rd. nr. Centennial Rd., 43.509, -81.516, hedgrerow nr. creek, pitfall, 11-V-2009 (1), A. Brunke; Ottawa, Carleton Place, 12-IX-1992, W. Bennett, (1); Peel Reg., Cooksville, pond margin, 30-V-1993, C. Krupke, (1); Waterloo Reg., Blair, Whistlebare Rd. and Township Rd.1, 43.372, -80.362, hedgerow, pitfall, 18-V-2010 (1), A. Brunke; Wellington Co., Guelph, 10-IX-1980, Y. Deedat; Guelph, 17-IX-1980, Y. Deedat; Guelph, University Arboretum, 1-X-2005, M. Bergeron, (1); Guelph, Victoria Rd. and Conservation Line, 43.580, -80.275, soybean field, canopy trap, 23-VI-2009 (1), A. Brunke; York Reg., Stouffville, V-1982, Brian Brown, (1);
Stenus clavicornis is, at present, only reliably distinguished from congeners in North America by its characteristic aedeagus (Fig. 14).
Distribution in northeastern North America, sources of data other than DEBU are quoted in parentheses. 13 Stenus clavicornis (Scopoli) (
This exotic, Palaearctic species was first recognized in North America by
United States: VA: Giles Co., Ripplemead, Rte. 460 at bridge, flood debris, 11 to 25-V-2008 (6) A. Brunke.
Eustilicus tristis is the only northeastern member of the genus and its distinct habitus will readily identify it as a Eustilicus (Fig. 5).
This species is rarely collected and at the
time of the most recent revision, it was only known from scattered
localities in District of Columbia, Kentucky, New Jersey, Missouri,
Oklahoma, Texas (
CANADA: ON: Huron Co., Auburn, Hullett-McKillop Rd. nr. Limekiln Line, 43.742, -81.514, hedgerow, pitfall, 26-V-2010 (1) A. Brunke; Auburn, Limekiln Line, 43.736, -81.506, hedgerow, canopy trap in buckthorn, 26-V-2010 (2) A. Brunke; Benmiller, Sharpes Creek Line, 43.691, -81.608, hedgerow nr. creek, pitfall, 11-V-2009 (1) A. Brunke; Muskoka Reg., S. Waseosa Rd., 8-VII-1996 (1)W. J. Crins; Wellington Co., Guelph, 26-V-1978 (1) Ron O. Kreazer; Guelph, under rock, 16-III-1983 (1) Brian Brown; Guelph, University Arboretum nature reserve, sifting beech litter, 3-V-2009 (4) A. Brunke and D.K.B. Cheung, sifting litter, 6-VI-2009 (1) A. Brunke; York Co., Toronto, 2-V-1959 (2) R. J. Pilfrey.
The genus Medon is in need of revision in North America, and Medon fusculus is currently recognizable in North America only from the characteristic modifications of the male seventh sternite and aedeagus (Fig. 15–16).
This exotic, Palaearctic species was first recognized in North America by
CANADA: ON: Huron Co., Auburn, Hullett-McKillop Rd. nr. Limekiln Line, 43.742, -81.514, soybean field, pitfall, 23-VI-2010 (6), 7-VII-2010 (6), 21-VII-2010 (4), 1-IX-2010 (1), A. Brunke; Benmiller, Sharpes Creek Line, 43.691, -81.608, hedgerow nr. creek, canopy trap in buckthorn, 22-VI-2009 (1) A. Brunke; Brucefield, London Rd. nr. Centennial Rd., 43.509, -81.516, soybean field, canopy trap, 22-VI-2009 (1) A. Brunke; Waterloo Reg., Blair, Dickie Settlement Rd. nr. WhistleBear golf course, 43.373, -80.400, soybean field, pitfall, 23-VI-2009 (1), 7-VII-2009 (2), 21-VII-2009 (1), A. Brunke; Blair, Fountain St. S. nr Speed River, 43.391, -80.373, soybean field, pitfall, 23-VI-2009 (3), 7-VII-2009 (1) A. Brunke; Blair, Whistlebare Rd. and Township Rd.1, 43.372, -80.362, soybean field, pitfall, 13-VII-2010 (4), A. Brunke; Blair, Whistlebare Rd. and Township Rd. 1, 43.367, -80.358, soybean field, pitfall trap, 15-VI-2010 (3), 27-VII-2010 (1), A. Brunke. Wellington Co., Eramosa, Wellington County Rds. 124 and 29, 43.615, -80.215, soybean field, pitfall, 13-VII-2010 (2); Guelph, Victoria Rd. and Conservation Line, 43.580, -80.275, soybean field, pitfall trap, 23-VI-2009 (23), 7-VII-2009 (4), 21-VII-2009 (11), 4-VIII-2009 (1), 18-VIII-2009 (2), 1-IX-2009 (1), 15-IX-2009 (4); Guelph, Victoria Rd. and Conservation Line, 43.580, -80.275, soybean field, vacuum sampled from soybean foliage at 8pm, 16-VII-2009.
The diverse genus Scopaeus is greatly in need of revision in North America and thus, Scopaeus minutus can only be recognized currently by the form of the aedeagus (Fig. 17).
This exotic Palaearctic species was first reported from North America by
CANADA: ON: Huron Co., Auburn, Hullett-McKillop Rd. nr. Limekiln Line, 43.742, -81.514, hedgerow, canopy trap in buckthorn, 26-V-2010 (1); Waterloo Reg., Blair, Whistlebare Rd. and Township Rd. 1, 43.367, -80.358, hedgerow, pitfall, 4-V-2010 (1); Wellington Co., Eramosa, Wellington County Rds. 124 and 29, 43.615, -80.215, hedgerow, pitfall, 4-V-2010 (1).
Sunius melanocephalus may be easily recognized among other northeastern members of the genus by the combination of the non-serrate lateral margins of the pronotum and the bicolored body.
This species was accidentally introduced from
the Palaearctic region to North America and was first recognized on the
continent by
Distribution in northeastern North America, sources of data other than DEBU are quoted in parentheses. 17 Sunius melanocephalus Fabricius (
CANADA: ON: Middlesex Co. London, Southern Crop Protection Research Centre, pitfall trap/Masner trap, 21-VI-1995, T. Sawinski (1); Simcoe Co., Noisy River, Prov. Nature Res., beaver lodge, 28-IX-2008, S.A. Marshall (1).
At present, Acylophorus agilis is reliably separated from others in the diverse Acylophorus pronus-group only by the characteristically shaped paramere of the aedeagus (Fig. 169 in
This species is widely distributed in eastern
North America, and was previously known from Indiana, Maryland,
Massachusetts, Michigan, New York, North Carolina, Pennsylvania,
Rhode Island, Missouri, (
CANADA: ON: Cochrane Dist., N. Moosonee, sandy beach, ridge along coastal marsh, Picea, Populus, Alnus and herbs, pitfall trap, 23-VI-1990, J. Pilny, (1).
Diagnosis. Bisnius cephalicus is readily distinguished from others of the genus in the northeast by the combination of: body bicolored with orange elytra; pronotum with five punctures in each dorsal row; eyes small, with the space behind them about three times longer (Fig. 6).
At the time of the most recent revision of
the genus, this species was known from only two specimens, from
Alberta and Manitoba (
UNITED STATES: VA: Giles Co., Mountain Lake, on dead fox squirrel, 11 to 25-V-2008, A. Brunke (3).
CANADA: PEI: Long Pond, National Park, milieu marécageux (=marshy environment), 30-VII-1979, R. Sexton (2); West Covehead, débris sur la plage (=beach debris), 25-VII-1979, R. Sexton, (3).
Bisnius siegwaldii
is easily recognized among other species of the genus in the northeast
by the combination of: body not bicolored; elytra dark; pronotum with at
least five punctures in each dorsal row; head with punctures arranged
to form a ‘V’ (
Dorsal habitus. 7 Bisnius siegwaldii (Mannerheim)8 Erichsonius brachycephalus Frank9 Erichsonius parcus (Horn) 10 Neobisnius terminalis (LeConte)11 Philonthus vulgatus Casey12 Quedius cinctus (Paykull).
This species is transcontinental across
northern North America with several collections made further south in
both the east and west. It is currently known from Alaska, Alberta,
British Columbia, California, Connecticut, Maine, Manitoba,
Michigan, Montana, Newfoundland, New Brunswick, New Hampshire, New
York, North Carolina, Nova Scotia, Northwest Territories, Ontario,
Oregon, Québec, Saskatchewan, Tennessee, Vermont, Washington, West
Virginia, Wisconsin, and Yukon Territory (
CANADA: ON: Huron Co., Brucefield, London Rd. nr. Centennial Rd., 43.509, -81.516, hedgerow nr. creek, canopy trap in buckthorn, 11-V-2009, A. Brunke (1); QC: La Valleé-de-la-Gatinaeu, Martindale, hutte à castor (=beaver lodge), 19-IX-1976, R. Sexton (26).
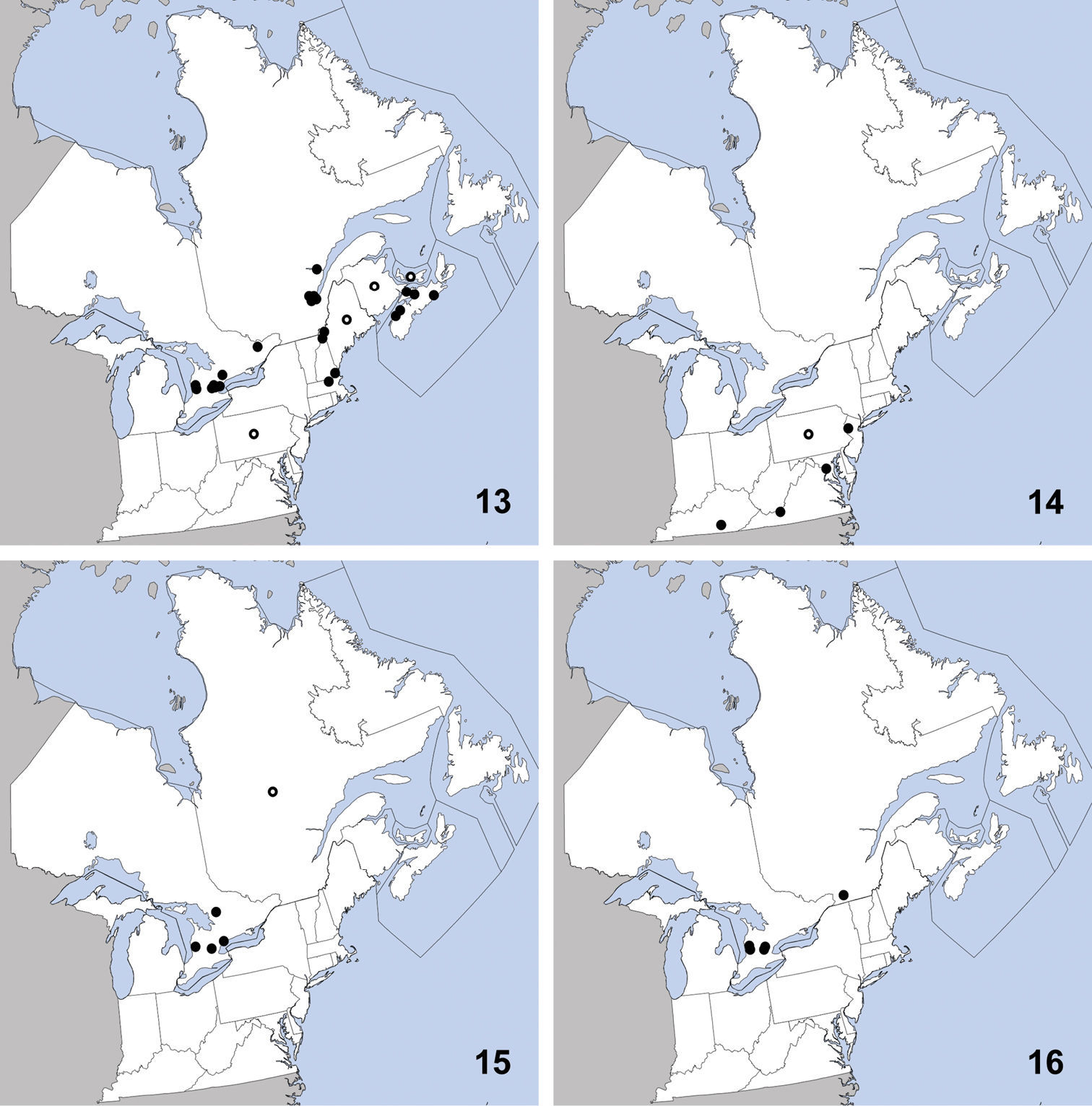
This species is easily recognized among others of the genus with a sparsely punctate forebody by its large size (>4.7mm from clypeus to abdominal apex) and transverse head with slightly converging temples (Fig. 8).
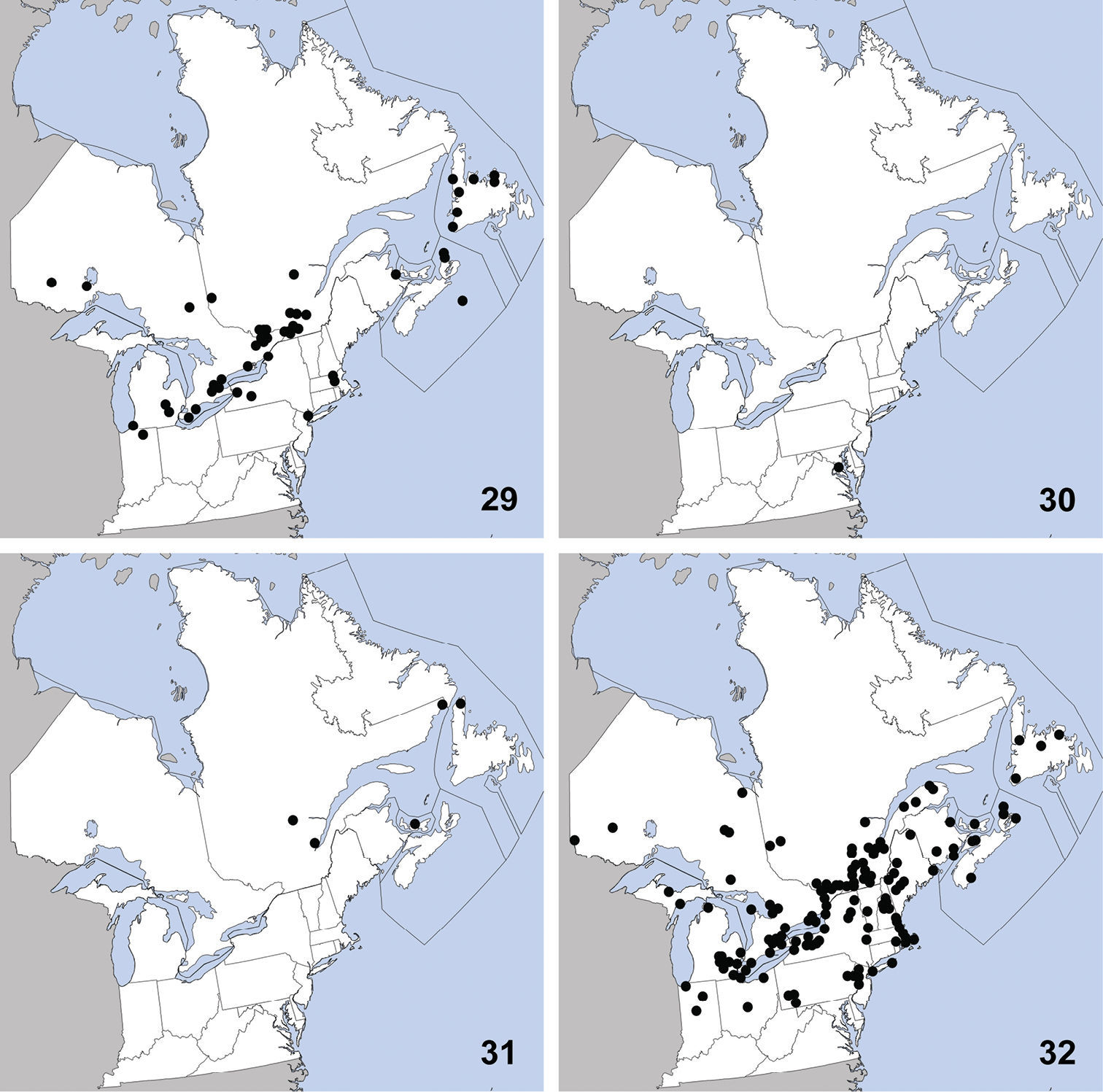
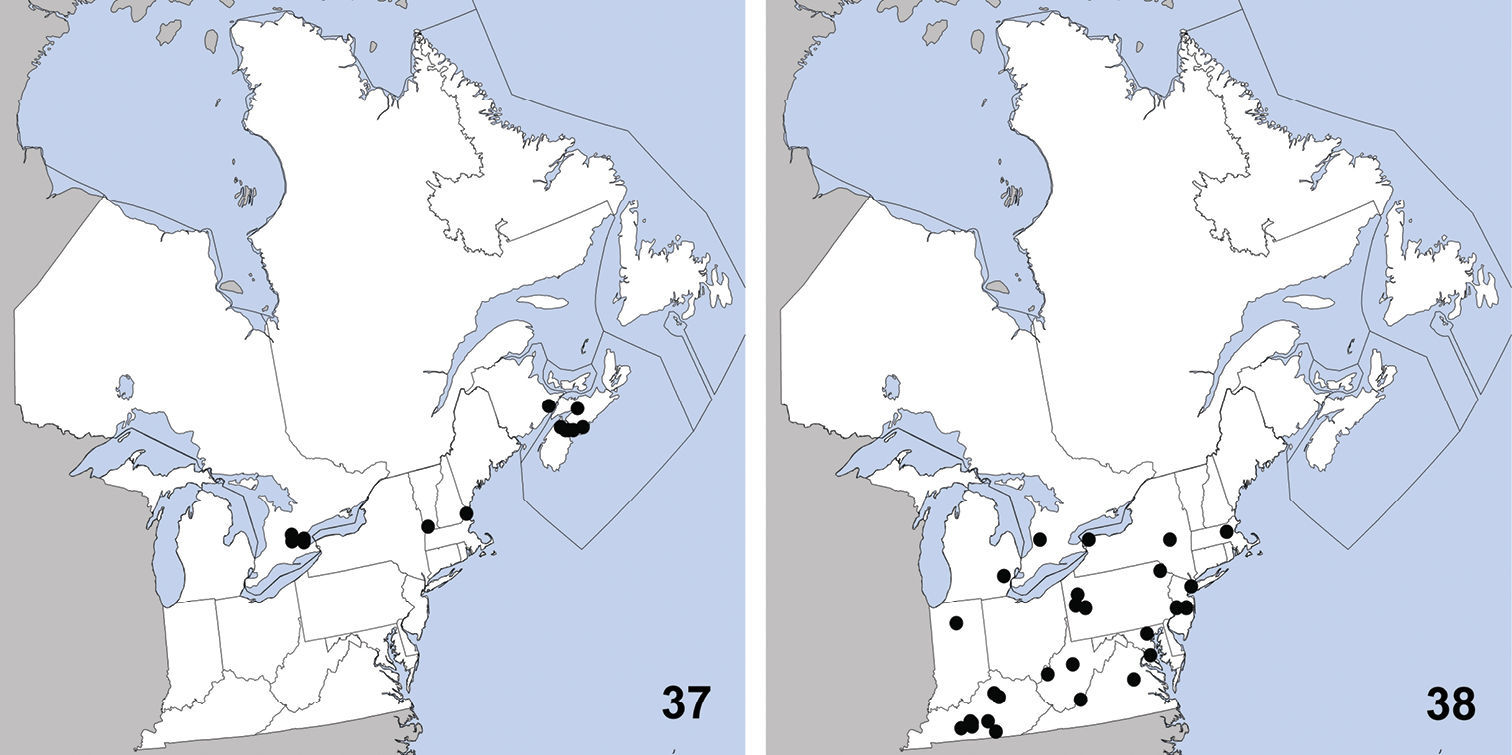
Erichsonius brachycephalus was previously known from Illinois, Maine, Massachusetts, New Jersey, Texas (
Distribution in northeastern North America, sources of data other than DEBU are quoted in parentheses. 21 Erichsonius brachycephalus Frank (
CANADA: PEI: Long Pond National Park, milieu marécageux (=marshy environment), 30-VII-1979, R. Sexton (2).
Erichsonius nanus can be recognized among the other densely punctate species in northeastern North America by its larger size (>4.4mm, from clypeus to abdominal apex), and the apical portion of the median lobe of the aedeagus, which is distinctly thin and sinuate in lateral view (Fig. 18).
This species is widely distributed and was
previously known from Alaska, British Columbia, Illinois, Maine,
Massachusetts, New Brunswick, New Hampshire, Newfoundland, New
Jersey, New York, Northwest Territories, Nova Scotia, Ontario,
Québec, Washington, Wisconsin (
CANADA: ON: Kent Co., Rondeau Prov. Pk., spicebush trail, Carolinian forest, yellow pans, 25-V-2003, M. Buck and S. Paiero (1); Rondeau Prov. Pk., spicebush trail, Carolinian forest, yellow pans, 16 to 17-VI-2003, Buck and Carscadden (1); Wainfleet bog, 8km S of Welland, DIE pt. 1 –north ditch (control), 10 to 24-V-1988, A. Stirling (1); 7 to 13-VI-1988, all collected by A. Stirling: D2H pt. 2 – ‘1962 zone’(1), D4H pt. 4 – ‘1980 zone’ (1), D5H pt. 5 – ‘1985 zone’(1), D5E pt. 5 – ‘1985 zone’ (1).
Erichsonius parcus
can be easily distinguished from other northeastern species of the
genus with a sparsely punctate forebody, with the exception of Erichsonius pusio, by its small size (<3.6mm from clypeus to abdominal apex).
This species was previously known from Florida (Frank 1981), District of Columbia, Massachusetts, Louisiana, Virginia (
CANADA: ON: Simcoe Co. Midhurst, forest nr. Neretva St., under bark of large beech trunk, 4-IX-2009, A. Brunke and K. Brunke (1).
Gabrius amulius may be recognized by the combination of: large size (at least 5.0mm long from clypeus to abdominal apex); eyes large, with temple that is distinctly less than twice as long as the eye; forebody without a greenish metallic lustre; elytra with sparsely distributed punctures that are separated by two to three times their diameter; area between basal lines on tergites two and three punctate
This apparently rare species was known from only five specimens at the time of its description (
CANADA: ON: Huron Co., Auburn, Hullett-McKillop Rd. nr. Limekiln Line, 43.744, -81.507, soybean field, pitfall, 23-VI-2010 (1), 7-VII-2010 (2), A. Brunke; Auburn, Limekiln Line, 43.736, -81.506, soybean field, pitfall, 23-VI-2010 (1), 7-VII-2010 (1), 4-VIII-2010 (1), 18-VIII-2010 (1), A. Brunke; Waterloo Reg., Blair, Dickie Settlement Rd. nr. WhistleBear golf course, 43.373, -80.400, soybean field, pitfall, 23-VI-2009 (1), 7-VII-2009 (1), 21-VII-2009 (6), 4-VIII-2009 (1), A. Brunke; Blair, Dickie Settlement Rd. nr. WhistleBear golf course, 43.373, -80.400, hedgerow, canopy trap in buckthorn, 27-X-2009, A. Brunke (1); Blair, Fountain St. S. nr Speed River, 43.391 -80.373, soybean field, canopy trap, 15-IX-2009, A. Brunke (1);Blair, Whistlebare Rd. and Township Rd.1, 43.372, -80.362, hedgerow, pitfall, 4-V-2010, A. Brunke (1); Blair, Whistlebare Rd. and Township Rd.1, 43.372, -80.362, soybean field, pitfall, 29-VI-2010, A. Brunke (1); Blair, Whistlebare Rd. and Township Rd. 1, 43.367, -80.358, soybean field, pitfall trap, 13-VII-2010 (1), 25-VIII-2010 (1), A. Brunke; Wellington Co., Eramosa, Wellington County Rds. 124 and 29, 43.615, -80.215, hedgerow, pitfall, 4-V-2010, A. Brunke (2); Eramosa, Wellington County Rds. 124 and 29, 43.615, -80.215, soybean, pitfall, 15-VI-2010 (3), 29-VI-2010 (3), A. Brunke; Guelph, Victoria Rd. and Conservation Line, 43.580, -80.275, soybean field, pitfall, 21-VII-2009 (1), 4-VIII-2009 (6), 1-IX-2009 (4), A. Brunke; Guelph, Victoria Rd. and Conservation Line, 43.580, -80.275, soybean field, canopy trap, 4-VIII-2009 (1), 18-VIII-2009 (1), A. Brunke
Gabrius appendiculatus can be distinguished from congenersin the northeast by the following combination of characters: area between basal lines on tergites two and three impunctate; basal antennomeres not distinctly paler than rest of antenna; males with sternite eight broadly notched; females with tergite 10 pointed (as opposed to truncate) apically.
This Palaearctic species was first recognized in North America by
Distribution in northeastern North America, sources of data other than DEBU are quoted in parentheses. 25 Gabrius appendiculatus (Sharp) (
CANADA: ON: Bruce Co., Bruce Pen. Natl. Pk., Upper Andrew Lake, beaver lodge, 26-VII-2008, S. A. Marshall (1); Simcoe Co., Noisy River Prov. Pk., Res., beaver lodge, 28-IX-2008, S.A. Marshall (1).
Gabrius vindex is easily distinguished from all other species of the genus in northeastern North America except Gabrius astutoides by the combination of: punctures of the elytra dense, separated by their widths or less; size large (at least 6.0mm from clypeus to abdominal apex); the area between the basal lines of tergites two and three punctate. From Gabrius astutoides it is most easily separated by the shape of the pronotum which is narrowed anteriorly in Gabrius vindex and parallel in Gabrius astutoides.
This species is transcontinental in northern
North America and was previously known from Alaska, Manitoba,
Minnesota, and Québec (
CANADA: ON: Essex Co., East Sister Is. Prov. Nature Res., dry pond bed, yellow pans, 30-VII-2003, S.A. Marshall (1); Leamington, pitfall trap, 17-VIII-1993 (1); Huron Co., Centralia, Dev 1A, pitfall, 16-VIII-1992 (1); Middlesex Co., London, southern crop protection research centre, corn pitfalls 3, 19-VII-1993 (1); London, southern crop protection research centre, pitfall/Masner trap, 2-VIII-1995, T. Sawinski (1).
This species can be distinguished from other orange and black Neobisnius in northeastern North America by the combination of head completely lacking microsculpture, elytra with apically paler area limited to a narrow strip, and the maxillary palpi with at least one segment darkened.
Neobisnius occidentoides
is a widespread species and was previously known from Alberta,
Alabama, Arkansas, Arizona, California, Colorado, Idaho, Illinois,
Kansas, Kentucky, Louisiana, Manitoba, Minnesota, Mississippi,
Missouri, Montana, Nebraska, Nevada, New Mexico, New York, North
Carolina, North Dakota, Ohio, Oklahoma, South Dakota, Tennessee,
Texas, Utah, Vermont, Virginia, Washington, and Wyoming (
CANADA: ON: Essex Co., Middle Is., shore, yellow pans, 11-VI-2003, S.A. Marshall (1); Niagara Reg., Grimsby, J. Pettit (1).
Neobisnius terminalis is easily recognized among other orange and black species of the genus in northeastern North America by the elytra with a broad, pale apical area (Fig. 10). In other northeastern species, this pale area is restricted to a narrow strip.
This species was previously known from
Arizona, California, Colorado, Iowa, Maine, Maryland, Michigan,
New Hampshire, New Mexico, New York, Nova Scotia, Pennsylvania,
Québec, Texas, and Virginia (
CANADA: PEI: Brackley Beach, National Park, milieu marécageux (=marshy environment), 2-VIII-1979, R. Sexton (1); Long Pond, National Park, milieu marécageux (=marshy environment), 30-VII-1979, R. Sexton (2).
This species is, at present, best
identified by the shape of the median lobe of the aedeagus and branches
of the paramere (Fig. 499 in
Philonthus couleensis
was previously known from Alberta, British Columbia, Idaho,
Illinois, Indiana, Manitoba, Massachusetts, Michigan, New
Brunswick, Newfoundland, New Jersey, Northwest Territories, New
York, Nova Scotia, Ontario, Saskatchewan, Washington, and Wisconsin
(
Distribution in northeastern North America, sources of data other than DEBU are quoted in parentheses. 29 Philonthus couleensis Hatch (
UNITED STATES: MD: Prince George Co., Cedarville St. Pk., at lights, 24 to 29-VII-2008, S. Paiero (1).
Philonthus gavius is recognized among other northeastern Philonthus by the combination of: tergite eight distinctly emarginate in both sexes; elytra with most punctures separated by their widths; eyes longer than the temples.
This species was previously known from Arkansas, Illinois, Louisiana, Oklahoma, Tennessee, and Texas (
CANADA: PEI: Brackley Beach, National Park, milieu marécageux (=marshy environment), 2-VIII-1979, R. Sexton, (3).
Philonthus leechensis may be recognized among other northeastern Philonthus except for Philonthus umbrinoides
This species was previously known from
Alaska, Alberta, Arizona, British Columbia, California, Colorado,
Idaho, Manitoba, Minnesota, Montana, Newfoundland, Northwest
Territories, Oregon, Québec, Saskatchewan, Washington, Wisconsin,
and Yukon Territory (
CANADA: PEI: Brackley Beach, National Park, milieu marécageux (=marshy environment), 2-VIII-1979, R. Sexton (6).
Philonthus lindrothi can be distinguished fromnortheastern congeners other than Philonthus pseudolodes
Smetana 1996 by the combination of: pronotum with five punctures in the
dorsal row on at least one side; head with hind angles present but
rounded; tergite eight not emarginate in either sex; elytra without
distinct markings on the disc; hind tarsus with first segment shorter
than last segment; antennae with basal segments not distinctly paler
than others and with segments seven and eight elongate. Males can be
readily separated from Philonthus pseudolodes by the notch in sternite eight not continuing as a grove towards its base (
This widespread species was previously known
from Alaska, Alberta, Arizona, British Columbia, California,
Colorado, Idaho, Illinois, Indiana, Iowa, Kansas, Maine,
Manitoba, Massachusetts, Michigan, Minnesota, Missouri, Montana,
Nebraska, Nevada, New Brunswick, Newfoundland, New Hampshire, New
Jersey, New York, North Carolina, North Dakota, Northwest
Territories, Nova Scotia, Ohio, Ontario, Oregon, Pennsylvania,
Québec, Rhode Island, Saskatchewan, South Dakota, Vermont,
Washington, and Wisconsin (
UNITED STATES: VA: Giles Co., Ripplemead, rte 460 at bridge, flood debris, berlese, 11 to 25-V-2008, A. Brunke (4).
Philonthus neonatus is separated from other northeastern Philonthus by the combination of: pronotum with six punctures in both dorsal rows; pronotum no more than vaguely narrowed anteriorly; elytra distinctly red and without dark markings; elytra with micropunctures between the regular punctures; abdominal segments paler apically.
This species was previously known from
Arkansas, District of Columbia, Indiana, Iowa, Kansas, Kentucky,
Maine, Maryland, Massachusetts, Michigan, Mississippi, Missouri,
New Hampshire, New Jersey, New York, Ontario, Pennsylvania, and
Québec (
Distribution in northeastern North America, sources of data other than DEBU are quoted in parentheses. 33 Philonthus neonatus Smetana (
CANADA: PEI: Brackley Beach, National Park, milieu marécageux (=marshy environment), 2-VIII-1979, R. Sexton (13).
This species can be easily distinguished from other northeastern Philonthus by the combination of: pronotum with punctures widely distributed and not in rows; legs and antennae completely dark; head with hind angles indistinct (Fig. 11).
Philonthus vulgatus
was previously known from Alaska, Alberta, British Columbia, Idaho,
Illinois, Indiana, Iowa, Kansas, Maine, Manitoba, Massachusetts,
Michigan, Minnesota, Montana, Nebraska, New Hampshire, New York,
North Dakota, Nova Scotia, Ontario, Québec, Saskatchewan, South
Dakota, Utah, Washington, and Wisconsin (
CANADA: ON: Wellington Co., Guelph, University Arboretum, rotting Polyporus squamosus, 29-VI-2008, A. Brunke (2); Guelph, University campus nr. horse pen, grass sweep, 13-VII-2008, C. Ho (1).
This species may be separated from other northeastern Quedius by the combination of: elytra with three rows of coarse punctures on the disc; head without a pair of punctures between the ocular punctures; pronotum with three punctures in each dorsal row (Fig. 12).
This Palaearctic species was first detected in North America by
CANADA: ON: Essex Co., Windsor, ~1.5km S Ojibway Prairie, forest-prairie edge, malaise trap, 15-V to 1-VI-2001, S. Paiero (1); Windsor, ~1.5 km S Ojibway Prairie, private prairie, malaise, 5 to 12-VI-2001, S. Paiero (3); Windsor, ~1.5km S Ojibway Prairie, private prairie, malaise, 19 to 30-VI-2001, P. Pratt (2); Hald. –Norfolk Reg., Charlotte 2 Rd., ~480m E of Charlotteville, West Quarterline Rd., ‘C.C.S.N. -5’, purple prism trap, 13 to 19-VI-2009, S.M. Paiero (1); Halton Reg., Milton, Derry Rd. and 4th Line, under composter, 16-X-2008, S. M. Paiero (1); Oxford Co., Woodstock, trails nr. river, 14-VI-2008, S.A. Marshall (1). Simcoe Co., Midhurst, forest nr. Neretva St., 28-IX-2008, A. Brunke and K. Brunke (1); Wellington Co., Guelph, University Campus, dairy bush, dry Polyporus squamosus, 22-IX-2008, A. Brunke (1).
Quedius cruentus may be distinguished from other northeastern Quedius by the combination of: elytra evenly punctate; labrum distinctly bilobed; eyes distinctly shorter than temples; antennomeres one to three distinctly paler than others; distal antennomeres strongly transverse; pronotum with sublateral row of punctures longer than dorsal row.
This Palaearctic species was first detected by
UNITED STATES: VT: Bennington Co., Woodford, sifting leaf litter near stream, 1-IV-2010, T. Murray (1).
CANADA: ON: ‘Ont.’, 30-IX-1982, G. Abayo (1); Halton Reg., Oakville, nr hwy 25 and Burhamthorpe Rd., meadow, yellow pans, 12 to 14-IX-2003, S.M. Paiero (1); Hamilton Reg., Hamilton, 3-VIII-1984, M.T. Kasserra (1); Waterloo Reg., Blair, Fountain St. S. nr Speed River, 43.391, -80.373, hedgerow, pitfall, 28-IX-2009, A. Brunke (1); Wellington Co., Eramosa, Wellington County Rds. 124 and 29, 43.615, -80.215, hedgerow, pitfall, 4-V-2010 (1), 18-V-2010 (1), 2-XI-2010 (1), A. Brunke; Guelph, 17-VIII-1976, David Levin (1); Guelph, 7-VI-1983, C.F. Langlois (1); Guelph, University Arboretum, hand collected, 16-III-1983, L.B. Carlson (1); Guelph, 30-IX-1983, A. Harris (1); Guelph, 5-VII-1984, ‘maple’, T. Young (1); Guelph, 3-IV-1991, M. Kovacevick (1); Guelph, 14-X-1998, T. Phillips (1); Guelph, Victoria Rd. and Conservation Line, 43.580, -80.275, hedgerow, pitfall, 19-V-2009 (1), 17-XI-2009 (1), A. Brunke.
Diagnosis. Quedius curtipennis can be distinguished from other northeastern Quedius by the combination of: elytra with even punctation; labrum not bilobed; scutellum impunctate; basal antennomeres not distinctly darker than the other segments (Fig. 13).
Dorsal habitus of Quedius curtipennis Bernhauer.
14 Stenus clavicornis (Scopoli), aedeagus in parameral view 15 Medon fusculus (Mannerheim), 7th sternite 16 Medon fusculus, aedeagus in lateral view 17 Scopaeus minutus Erichson, aedeagus in lateral view 18 Erichsonius nanus (Horn), aedeagus in lateral view 19 Erichsonius parcus (Horn), aedeagus in parameral view.
This exotic Palaearctic species was first correctly reported in North America by
Distribution in northeastern North America, sources of data other than DEBU are quoted in parentheses. 37 Quedius curtipennis Bernhauer (
CANADA: ON: Huron Co., Seaforth, 7-VII-1955, D. Keys (1).
Quedius fulgidus may be distinguished from other northeastern Quedius by the combination of: elytra evenly punctate; labrum distinctly bilobed; eyes distinctly shorter than temples; antennomeres one to three not distinctly paler than others; distal antennomeres only slightly transverse; pronotum with sublateral row of punctures longer than dorsal row.
This Palaearctic species was first correctly recognized in North America by
Curation of over 32, 000 staphylinids deposited in the
University of Guelph Insect Collection resulted in the discovery of
thirty-five new provincial or state records, six new Canadian records,
one new record for the United States and two new records for eastern
Canada. Many of these specimens were aleocharines and a future
publication is planned to report on the discoveries made while curating
this subfamily. The majority of the records presented herein involved
species of which were included in recent revisions (after 1970),
suggesting that even of ‘well-known’ groups, our knowledge of
staphylinid distributions remains incomplete. Two boreal beetles, Porrhodites fenestralis and Bisnius cephalicus
were newly recorded in Ontario, joining previous records from adjacent
provinces to suggest a transcontinental distribution. Six staphylinid
species were newly discovered in Canada at the northern extreme of their
range, two of which are apparently entirely restricted in Canada to
the Carolinian ecoregion (Sepedophilus versicolor and Erichsonius parcus). This relatively small area of southernmost Ontario is the most biodiverse region in Canada (
The results of this paper demonstrate the key role of curated insect collections in understanding biodiversity in the boreal region, the imperilled ‘Carolinian’ region in Canada, and northeastern North America in general. An improved understanding of rare or or potentially rare insect species, and the effective detection of exotic species, depends on the routine identification of specimens in collections and the regular implentation of regional insect surveys. We recommend increased support for these activities to develop and maintain a clear picture of biodiversity, and biodiversity change, in northeastern North America.
We would like to thank Alan and Anne Morgan (Waterloo, Ontario, Canada) for their donation of the AAMC, and the Southern Crop Protection Research Group (London, Ontario, Canada) for the donation of their collection. We thank Tom Murray (Groton, Massachusetts) for his donation of specimens to DEBU. Ales Smetana (Canadian National Collection of Insects) kindly verified the identity of Gabrius amulius and Margaret Thayer (Field Museum of Natural History) provided the initial identification of Omalium repandum. We thank the Nature Conservancy of Canada and rare Charitable Research Reserve for their assistance with and permission to conduct surveys on their properties. We would also like to acknowledge the Ontario parks and protected areas, especially Bruce Peninsula National Park, Rondeau Provincial Park, Ojibway Provincial Nature Reserve, and Point Pelee National Park that have encouraged our survey work and collecting. In particular, we thank Bill Crins for expediting our paperwork to continue sampling in provincial reserves, and Scott Parker for facilitating our work on the Bruce Peninsula. This work was partially supported by an NSERC PGS-M awarded to A. Brunke.