(C) 2012 Akito Y. Kawahara. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The endemic Hawaiian moth genus Hyposmocoma includes 348 described species and perhaps twice as many that remain undescribed. The genus is unusual within Lepidoptera in that its larvae create distinctive silk cases in which they perambulate while protected and camouflaged. An extraordinary diversity of case types exists, and to date more than ten different types have been identified, each corresponding roughly to a separate evolutionary lineage. In this study, we describe three new species of Hyposmocoma: Hyposmocoma ipohapuu sp. n. from Big Island, Hyposmocoma makawao sp. n. from Makawao Forest Reserve in Mauiand Hyposmocoma tantala sp. n. from Mt. Tantalus, Oahu, all of which produce tubular purse cases during their larval stage. We also describe the female of Hyposmocoma inversella Walsingham, which was previously undescribed, and re-describe two closely related species, Hyposmocoma auropurpurea Walsingham and Hyposmocoma nebulifera Walsingham, neither which have been formally described in recent years. We present for the first time, primer sequences for a 705 bp fragment of CAD, designed for Hyposmocoma and relatives. The molecular phylogeny based on mitochondrial and nuclear loci demonstrates that all are distinct species. The discovery of a new, endemic species from Mt. Tantalus, an area with many invasive species, suggests that even relatively degraded areas in Hawaii would be worthy of active conservation efforts.
Case-bearing, endemism, Hawaii, conservation, Microlepidoptera, moth, new species
Hyposmocoma Butler, 1881 includes 348 described species endemic to the Hawaiian Islands. The archipelago is one of the most threatened species “diversity hotspots” in the world, and large endemic radiations, including Hyposmocoma, are thought to have already lost many of their species (e.g.
Unlike most cosmopterigids which are internal feeders of leaves, seeds and stems (
The total diversity of tubular-purse case Hyposmocoma remains largely unknown because historic sampling has focused primarily on the adult and there are very few records of larvae. From our examination of specimens in the Bishop Museum, Honolulu (BPBM), University of Hawaii Insect Museum (UHIM) and Smithsonian National Museum, Washington D.C. (USNM), there are seven species of tubular purse case Hyposmocoma that have been described prior to this study. These include, but may not be limited to the following species on the following islands: Hyposmocoma auropurpurea Walsingham, 1907 (Oahu), Hyposmocoma ekemamao Schmitz & Rubinoff, 2009 (Laysan), Hyposmocoma fuscopurpurea Walsingham, 1907 (Maui), Hyposmocoma inversella Walsingham, 1907 (Oahu), Hyposmocoma mokumana Schmitz & Rubinoff, 2009 (Necker), Hyposmocoma nebulifera Walsingham, 1907 (Oahu), and Hyposmocoma rubescens Walsingham, 1907 (Kauai).
Here we describe three new species of tubular purse case Hyposmocoma, Hyposmocoma ipohapuu Kawahara & Rubinoff, sp. n., Hyposmocoma makawao Kawahara & Rubinoff, sp. n.and Hyposmocoma tantala Kawahara & Rubinoff, sp. n. from the islands of Oahu, Maui and Hawai’i. While it is best to incorporate new species descriptions as part of a thorough revision, we have chosen to describe new species separately because of the sheer diversity of Hyposmocoma and the urgent conservation need of the genus. We present the first molecular phylogeny of purse-cased Hyposmocoma to shed light on the relationships between purse-cased species and assess its phylogenetic placement. We chose an integrative approach of combining traditional morphological data with molecular characters (
Genitalia preparation techniques and morphological terminology follow previous work on Hyposmocoma (e.g.
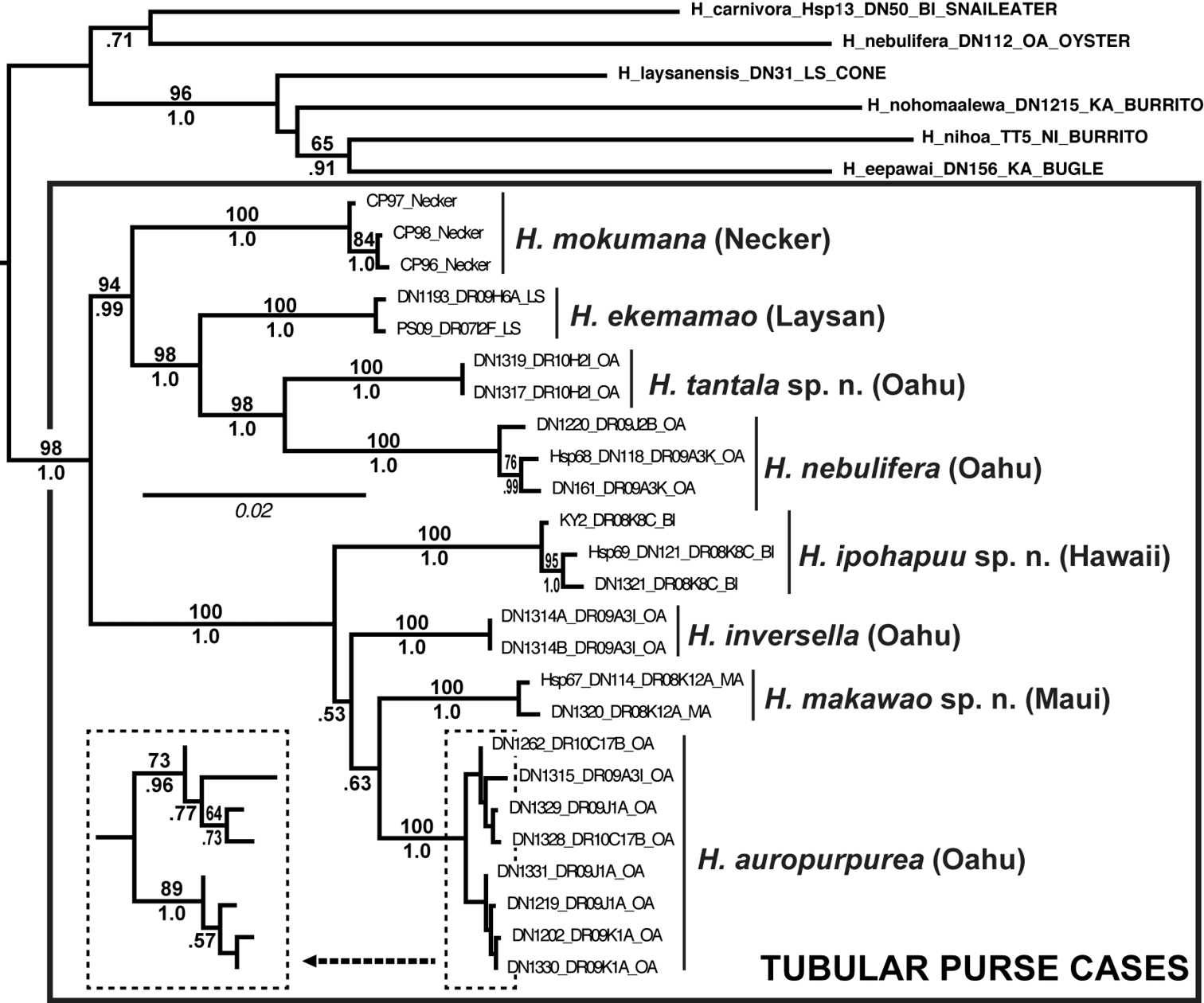
We included sequences of eight species of tubular purse-cased Hyposmocoma: Hyposmocoma auropurpurea; Hyposmocoma ekemamao; Hyposmocoma inversella; Hyposmocoma mokumana; Hyposmocoma nebulifera; and three new species: Hyposmocoma ipohapuu, Hyposmocoma makawao and Hyposmocoma tantala. We also included sequences for six outgroups: Hyposmocoma alliterata Walsingham, 1907; Hyposmocoma carnivora Schmitz & Rubinoff, 2011a; Hyposmocoma eepawai Schmitz & Rubinoff, 2011b; Hyposmocoma laysanensis Schmitz & Rubinoff, 2009; Hyposmocoma nihoa Schmitz & Rubinoff, 2009; and Hyposmocoma nohomaalewa Schmitz & Rubinoff, 2011a; all known to be distantly related to purse-cased Hyposmocoma (
Taxa sequenced for the present study along with UH log, extract, and GenBank accession numbers. An asterisk indicates a species that constructs a tubular case.
| Species | UH Log No. | Extract No. | CAD | EF-1α | COI |
|---|---|---|---|---|---|
| Hyposmocoma alliterata Walsingham, 1907 | DR08J7H | DN112 | GU560396 | GU560714 | GU560555 |
| Hyposmocoma auropurpurea Walsingham, 1907* | DR09A3I | DN1315 | JQ182760 | - | JQ231052 |
| DR09J1A | DN1219 | JQ182756 | JQ231030 | JQ231047 | |
| DR09J1A | DN1329 | JQ182763 | JQ231038 | JQ231058 | |
| DR09J1A | DN1331 | - | JQ231040 | JQ231060 | |
| DR09K1A | DN1202 | JQ182754 | JQ231028 | JQ231045 | |
| DR09K1A | DN1330 | - | JQ231039 | JQ231059 | |
| DR10C17B | DN1262 | JQ182758 | JQ231032 | JQ231049 | |
| DR10C17B | DN1328 | JQ182762 | JQ231037 | JQ231057 | |
| Hyposmocoma carnivora Schmitz & Rubinoff, 2011a | DR08F5A | DN50 | GU560342 | GU560660 | GU560501 |
| Hyposmocoma eepawai Schmitz & Rubinoff, 2011b | DR09B7B | DN156 | JQ182764 | JQ231041 | JQ231061 |
| Hyposmocoma ekemamao Schmitz & Rubinoff, 2009* | DR07I2F | PS09 | GU560311 | GU560631 | GU560472 |
| DR09H6A | DN1193 | JQ182753 | JQ231027 | JQ231044 | |
| Hyposmocoma inversella Walsingham, 1907* | DR09A3I | DN1314A | JQ182759 | - | JQ231050 |
| DR09A3I | DN1314B | - | - | JQ231051 | |
| Hyposmocoma ipohapuu Kawahara & Rubinoff, sp. n.* | DR08K8C | DN121 | GU560405 | GU560723 | GU560564 |
| DR08K8C | DN1321 | - | JQ231036 | JQ231056 | |
| DR08K8C | KY2 | - | - | JQ231063 | |
| Hyposmocoma laysanensis Schmitz & Rubinoff, 2009 | DR07I2D | DN31 | GU560320 | GU560640 | GU560481 |
| Hyposmocoma makawao Kawahara & Rubinoff, sp. n.* | DR08K12A | DN114 | JQ182752 | JQ231026 | JQ231043 |
| DR08K12A | DN1320 | - | JQ231035 | JQ231055 | |
| Hyposmocoma mokumana Schmitz & Rubinoff, 2009* | DR04I1 | CP96 | GU560267 | GU560601 | GU560442 |
| DR04I1 | CP97 | GU560268 | GU560602 | GU560443 | |
| DR04I1 | CP98 | GU560269 | GU560603 | GU560444 | |
| Hyposmocoma nebulifera Walsingham, 1907* | DR09J2B | DN1220 | JQ182757 | JQ231031 | JQ231048 |
| DR09A3K | DN118 | GU560402 | GU560720 | GU560561 | |
| DR09A3K | DN161 | JQ182765 | JQ231042 | JQ231062 | |
| Hyposmocoma nihoa Schmitz & Rubinoff, 2009 | DR07G10 | TT05 | GU560312 | GU560632 | GU560473 |
| Hyposmocoma nohomaalewa Schmitz & Rubinoff, 2011a | DR09B12D | DN1215 | JQ182755 | JQ231029 | JQ231046 |
| Hyposmocoma tantala Kawahara & Rubinoff, sp. n.* | DR10H2I | DN1317 | JQ182761 | JQ231033 | JQ231053 |
| DR10H2I | DN1319 | - | JQ231034 | JQ231054 |
Genomic DNA was extracted from all specimens using the DNeasyTM animal blood and tissue extraction kit following recommended protocols (Qiagen, Inc., Valencia, CA). The tissue was digested at 56° C for 24 hours, 200 ml of EB buffer was used to elute the DNA and extracts were stored at -20° C. COI was amplified in a single fragment: the forward primer Jerry (CAA CAT TTA TTT TGA TTT TTT GG) and reverse primer Pat-2 (TCC AAT GCA CTA ATC TGC CAT ATT A;
Phylogenetic analyses were conducted with maximum likelihood (ML) as implemented in GARLI 1.0 (
http://species-id.net/wiki/Hyposmocoma_auropurpurea
Figs 1, 11, 18This species is unique among species of Hyposmocoma because it has metallic purple wings with a narrow, diagonal orange band near the wing apex.
Male. (n = 2; Fig 1). Forewing length 4.8–5.0 mm.Head with a mixture of copper and metallic purple colored scales arranged radially from compound eye. Haustellum with light brown scales. Maxillary palpus reduced. Labial palpus curved with copper and metallic purple colored scales, scale color similar on all surfaces of palpus. Antenna brown with a mosaic of metallic purple scales. Thorax mostly copper; dark brown/purple scales present along anterior margin. Foreleg coxa with brown and metallic purple scales; femur, tibia, and tarsomeres mostly dark brown. Midleg as foreleg, but spurs covered in a mixture of dark and light-brown scales. Hindleg as midleg, but longer with long hairs on dorsal margin of tibia. Forewing metallic with a narrow diagonal orange band near wing apex. Diffuse orange patch in proximal region of FW along anal margin. Fringe orange and brown/metallic purple, longer scales tending to be brown/metallic purple, shorter ones orange. Hindwing brown with brown fringe. Abdomen dorsally dark brown; ventrally metallic brown/purple, with tuft of long dark brown scales covering lateral surface of genitalia.
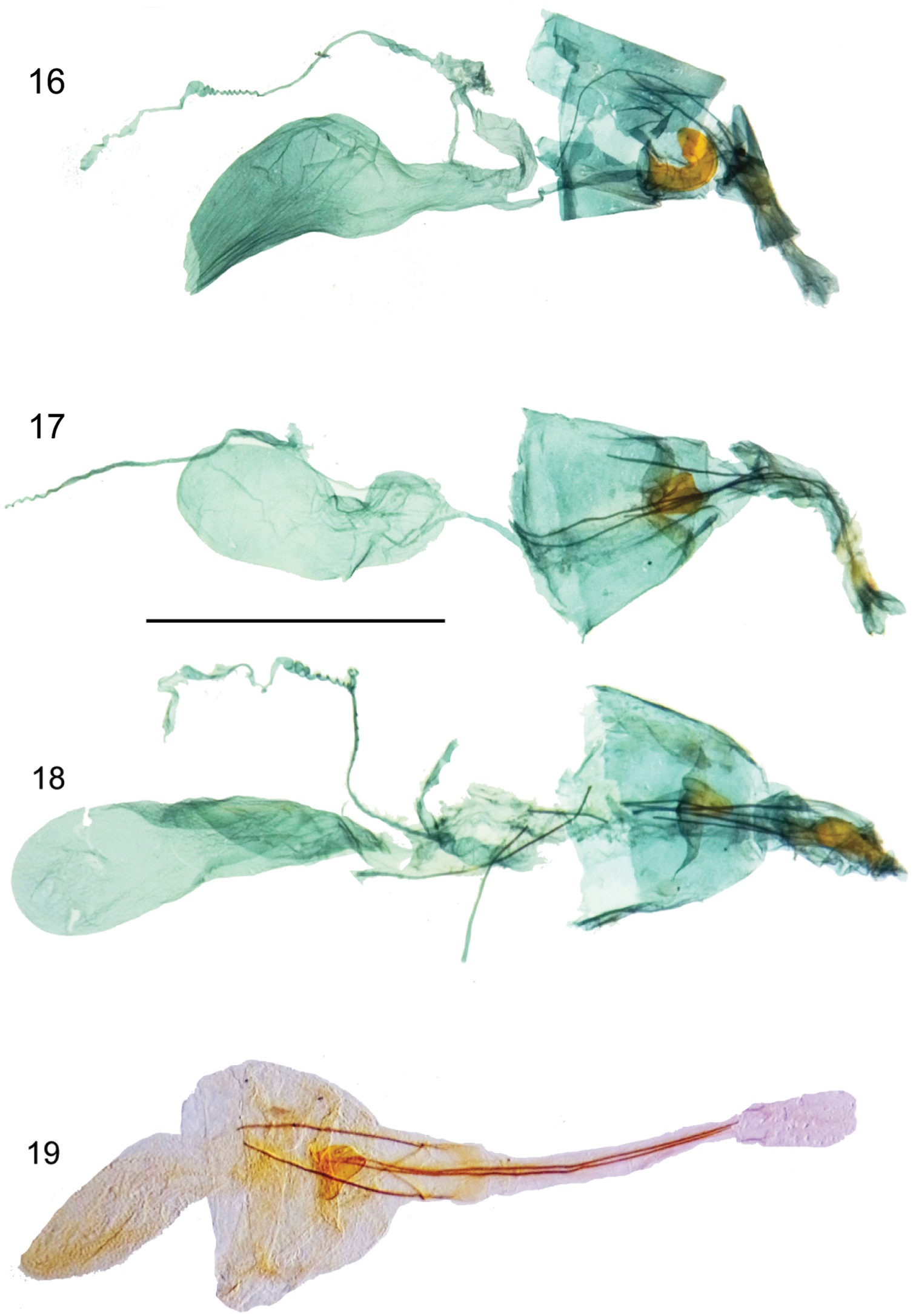
Purse-cased Hyposmocoma. 1 Hyposmocoma auropurpurea, male2 Hyposmocoma inversella, male. 3 Hyposmocoma ipohapuu sp. n.holotype male 4 Hyposmocoma makawao sp. n.holotype male 5 Hyposmocoma nebulifera, male 6 Hyposmocoma tantala sp. n.holotypefemale. In order to examine genitalia, the abdomen of each was removed after photographs were taken. Scale bar = 5 mm.
Male genitalia (Fig. 11).Right brachium of uncus sickle shaped and curved at 1/2, tapering gradually, heavily sclerotized. Left brachium small, not sclerotized. Tegumen wide and sclerotized. Valvae roughly symmetrical, two long thin setae arising from approximately 2/3 length along dorsal margin of both valvae. Dense row of fine, hair-like setae disposed comblike along inner ventral margin of valva. Phallus stout, heavily sclerotized, open ventrally, tapered, and bent ventrad at about 1/2 of length; vesica without spines or cornuti. Anellus with two symmetrical rounded lobes with short setae.
Female. (n = 4). Externally as male, forewing length 5.2–5.5 mm.
Female genitalia. (Fig 18). Papillae anales very short and setose. Anterior and posterior apophyses slender, long, posterior apophyses about same length as anterior apophyses. Ostium bursae small, heavily sclerotized, externally protruding, roughly triangular, not snail-shaped. Corpus bursae oval and elongate, with very light scobination; signum absent. Ductus bursae long and narrow, slightly twisted, about equal in length of corpus bursae. Apical margin of tergum VIII with median emargination.
Larval case (n = 7).Dark brown, smooth, 7–9 mm in length and 1.7–2 mm wide.
(2♂, 4♀). 1♂, 1♀: HI: Oahu, Waianae Range, Palikea trail, “purse” case 15-I-09, em. 23-III-2009, #DR09A3I, coll. P. Schmitz, D. Rubinoff, W. Haines, J. Eiben. 1♂, 1♀: HI: Oahu, Waianae Mountains, Palikea area, ~ elev. 850 on Pittosporum sp. leaves, 23-X-2009, em. 22-I-2010, #DR09J1A, coll. P. Krushelnycky. 1♀: HI: Oahu, Waianae Mountains, Palikea area, ~elev. 850 m, 5-XI-2009, em. 28-I-2010, #DR09K1A, coll. P. Krushelnycky. 1♀: HI: Oahu, South Waianae Mountains, Palikea area, 800–925 m, 30-III to 8-IV-2010, em 15-V-2010, #DR10C7B, coll. P. Krushelnychy and OANRP staff. All specimens from the UHIM.
Eight specimens from Oahu, Hawaii, USA (UH log numbers DR09A3I (1), DR09J1A (3), DR09K1A (2), DR10C17B (2)), extraction codes DN1202, DN1219, DN1262, DN1315, DN1328, DN1329, DN1330, and DN1331.
Case-making larvae were collected from October to April. Larvae were typically found in leaf litter, but in some cases were found on leaves such as Pittosporum sp. (Pittosporaceae).
Known only from the Waianae Mountain Range, Oahu.
http://species-id.net/wiki/Hyposmocoma_inversella
Figs 2, 17This species has a large, orange, “V”-shaped marking on the forewing found in no other described species of Hyposmocoma.
Male. (n = 1; Fig 2). Forewing length 4.9 mm.Head with copper-colored scales arranged radially from compound eye. Haustellum with light brown scales. Maxillary palpus reduced. Labial palpus curved with copper colored scales, scale color similar on all surfaces of palpus. Antenna brown with a mosaic of copper scales. Thorax mostly copper; dark brown scales present along anterior margin. Foreleg coxa with brown and copper scales; femur, tibia, and tarsomeres mostly dark brown with light brown ring at distal margin of femur, tibia, and tarsomeres I-V. Midleg as foreleg, but spurs covered in a mixture of dark and light-brown scales. Hindleg as midleg, but longer with long hairs on dorsal margin of tibia. Forewing brown, two transverse orange fascia form a “V”. Proximal orange band widens along anal margin, distal band uniform in width. Fringe orange and brown, longer scales tending to be brown, shorter ones orange. Hindwing brown with brown fringe. Abdomen dorsally dark brown; ventrally copper, with tuft of long dark brown scales covering lateral surface of genitalia.
Male genitalia. Right brachium of uncus sickle shaped, slender, heavily sclerotized, widening at 1/2 length, slightly twisted to left. Left brachium small, not sclerotized. Tegumen wide and sclerotized. Four long thin setae along dorsal margin of left valva, three shorter setae at same location of right valva. Left valva slightly wider than right. Dense row of fine, hair-like setae disposed along inner ventral margin of valva. Phallus stout, blunt tipped, heavily sclerotized, open ventrally, and bent ventrad at about 1/2 of length; vesica without spines or cornuti. Anellus with two symmetrical rounded lobes with short setae.
Female. (n = 1). Externally as male, forewing length 4.7 mm.
Female genitalia. (Fig 17). Papillae anales short and setose. Anterior and posterior apophyses slender, long, anterior apophyses slightly longer than posterior apophyses. Ostium bursae small, heavily sclerotized, externally protruding, roughly triangular, not snail-shaped. Corpus bursae oval and elongate, with very light scobination; signum absent. Ductus bursae long and narrow, slightly twisted, about equal in length of corpus bursae. Apical margin of tergum VIII with median emargination.
Larval case. (n = 2).Dark brown, smooth, 7.8–8.5 mm in length and 1.8–2 mm wide. The larval case is very similar to that of Hyposmocoma auropurpurea. Though both species are found in the Waianae Mountains, adult morphology and DNA sequence confirms these are distinct species.
(1♂, 2♀). 1♂: HI: Oahu, Waianae Range, Palikea Trail, “purse” case, 15-I-2009, emergence. 16-III-2009, #DR09A3I, coll. P. Schmitz, D. Rubinoff, W. Haines, J. Eiben. Specimen in perfect condition. Male genitalia slide #AK100. 1♀: same data as male. Female genitalia slide #AK101. 1♀: HI: Oahu, Pahole NAR. Northern Waianae Mts. Elev. 500 m, on Pipturus albidus (Urticaceae). 14-XII-2010, #DR10L1C, em. 24-III-2011, coll. P. Krushelnycky. Additional voucher collection #AR0803, spec/lot #PKSP11751. Molecular ID: AK-259-11. All specimens from the UHIM.
Two specimens from Oahu, Hawaii, USA (UH log number DR09A3I), extraction codes DN1314A, DN1314B.
Case-making larvae were collected in the Waianae Mountains of Oahu on the Palikea Trail during December and January. Larvae were found in leaf litter and on rotting logs. Adult emergence occurred between March and April. Because we have collected fairly extensively in the Waianae Mountains, we predict that this species univoltine, with larvae active during the winter months and adults emerging in the spring.
Known only from the Waianae Mountain Range, Oahu.
urn:lsid:zoobank.org:act:DBFC2894-9314-41EE-81BB-F19198CA3F6F
http://species-id.net/wiki/Hyposmocoma_ipohapuu
Figs 3, 7, 14, 19Hyposmocoma ipohapuu has a forewing pattern that differs from any other described species in the genus. A wide pale yellow band is present along the anal margin of the forewing and curves proximally at the wing margin towards the costa. A small, round, dark brown mark is present approximately two thirds of the way from the wing base to the apex.
Male (n = 2; Fig. 3). Forewing length 5.0 mm.Head with pale brown scales radiating from compound eye. Haustellum with a mixture of light and dark brown scales. Maxillary palpus reduced. Labial palpus recurved and covered in pale brown scales, which are dark laterally. Antennal flagellum pale brown, with a ring of dark brown scales extending from the proximal margin of each flagellomere. Thorax mainly pale brown, dark brown at cervical margin and near posterior margin of notum. Foreleg dark brown with pale brown ring at distal margin of femur. Midleg as foreleg, but with pale brown ring at distal margin of tibia and tarsomeres I-V, spurs pale brown. Hindleg as midleg but longer with long pale brown hairs. Forewing pale brown, with dark brown band extending from costal margin of wing base to apex, mark forming a convex arch near apex. A separate brown band extends along anal margin of forewing. Fringe pale brown. Hindwing and hindwing fringe grayish brown. Abdomen dorsally dark brown; ventrally pale brown with a tuft of long pale brown scales on either side of genitalia.
Larval cases of some purse-cased Hyposmocoma. 7 Hyposmocoma ipohapuu sp. n. 8 Hyposmocoma makawao sp. n. 9 Hyposmocoma nebulifera 10 Hyposmocoma tantala sp. n. Scale bar = 1 mm.
Male genitalia. (Fig. 14). Right brachium of uncus sickle shaped, rather thick, heavily sclerotized, slender at apical 1/3, slightly twisted to left. Left brachium small, not sclerotized. Tegumen wide and sclerotized. Valvae asymmetrical, left valva slightly wider at middle than right, with three long sclerotized club-shaped setae along dorsal margin curving posteriorly. Dorsal margin of right valva smooth, lacking setal sockets. Phallus stout, blunt tipped, heavily sclerotized, and bent ventrad at about 1/2 of length; vesica without spines or cornuti. Anellus with two symmetrical rounded lobes, thin until apex, both adorned with few small setae.
Male genitalia. 11 Hyposmocoma auropurpurea 12 Hyposmocoma nebulifera 13 Hyposmocoma tantala sp. n. 14 Hyposmocoma ipohapuu sp. n. 15 Hyposmocoma makawao sp. n. Scale bar = 1 mm. Figs 11–14 stained with Chlorazol Black, Fig. 15 stained with Orange G.
Female. (n = 1). Externally as male, but with longer forewing length (5.8 mm) and small dark brown mark medially below dark band on forewing.
Female genitalia. (Fig. 19). Papillae anales long and setose. Anterior and posterior apophyses thin and straight, slightly enlarged at posterior end, posterior apophyses about 2x length as anterior apophyses. Ostium bursae small, heavily sclerotized, externally protruding into a triangle, not snail-shaped. Corpus bursae oval with light scobination; signum absent. Ductus bursae thick, approximately 1/2 length of corpus bursae. Apical margin of tergum VIII with median emargination.
Female genitalia.16 Hyposmocoma tantala sp. n., lateral view 17 Hyposmocoma inversella, ventral view 18 Hyposmocoma auropurpurea, ventral view 19 Hyposmocoma ipohapuu sp. n., ventral view. Scale bar = 1 mm. Figs 16–18 stained with Chlorazol Black, Fig. 19 stained with Orange G.
Larval case. (n = 5; Fig 7). The mature case is 5.9–6.6 mm in length and 1.2–1.5 mm wide, smooth with banding that follows the length of the case. The case widens slightly at both ends.
Holotype: ♂, [1] Printed white label: ‘H[AWAI]I: Hawaii, Volcano village | Pearl ave[nue], on tree fern | “purse” case, X-30-08 | em[ergence]. III-13-[20]08, #DR08K8C | coll[ector]. J[esse]. Eiben, M[elissa]. Dean’; [2] Printed red label: ‘HOLOTYPE | Hyposmocoma ipohapuu Kawahara & Rubinoff’. Male genitalia slide #AK110. Specimen in perfect condition. Paratypes: (1♂, 1♀). 1♂: same data as holotype but emergence 2-March-2009. 1♀: Data same as male paratype, but emergence 13-March-2009. Female genitalia slide #AK114. Cases (5): same data as holotype. All pinned specimens and slides deposited in the UHIM.
Three specimens from Big Island, Hawaii, USA (UH log number DR08K8C), extraction codes DN121, DN1321 and KY2. This species was tentatively called “Hsp69” before given this formal name.
This species is named “ipohapuu”, which means “tree-fern lover” in Hawaiian.
Case-making larvae were collected on the abaxial surface of old fronds of a Hawaiian tree fern (Hāpu‘u, Cibotium glaucum (Sm.) Hook. & Arn., Cibotiaceae) in the rain forest at Volcano Village near Hawaii Volcano National Park, Hawaii (Big) Island. The habit of residing in old tree fern fronds, still attached to the stump, is typical of several purse case species including Hyposmocoma filicivora Meyrick, 1935.
Known only from Volcano Village, Hawaii Island. Probably restricted to the rainforest areas around Hawaii Volcanoes National Park, where it appears to be uncommon. The elevation for the type locality is approximately 1200 m.
urn:lsid:zoobank.org:act:98EE02FA-5A90-4675-A4E2-A2236F8787FC
http://species-id.net/wiki/Hyposmocoma_makawao
Figs 4, 8, 15Hyposmocoma makawao differs from any other species in the genus. No other species has a single, thick, transverse orange band near the base of the forewing.
Male. (n = 1; Fig. 4). Forewing length 4.8 mm. Head brown with iron-red scales near outer margin of eye; scales large near vertex. Haustellum pale brown. Maxillary palpus reduced. Labial palpus curved with pale brown scales, scales dark brown along lateral margin of labial palpus. Antennal flagellum dark brown along dorsal surface, lighter brown ventrally. Thorax reddish brown, laterally brown with patches of lighter brown scales below forewing. Foreleg and midleg with brown scales, scales dark brown laterally. Hindleg same as midleg, but with long scales along dorsal margin. Spines on legs light brown. Forewing dark brown with a single, wide fiery red-brown transverse fascia one fourth from the base of the wing to apex and narrowing towards costal margin. Abdomen covered in dark brown scales dorsally, light brown scales covering ventral surface.
Male genitalia. (Fig. 15). Right brachium of uncus sickle shaped, slender, heavily sclerotized, widening slightly at 1/2 length, slightly twisted to left. Left brachium small, not sclerotized. Tegumen wide and sclerotized. Valvae asymmetrical, left valva slightly wider than right, valvae without large sclerotized setae or sockets along dorsal margin. Dense row of fine, hair-like setae along inner ventral margin of both valvae. Phallus stout, blunt tipped, heavily sclerotized, open ventrally, and bent ventrad at about 1/2 of length; vesica without spines or cornuti. Anellus with two symmetrical rounded lobes with fine setae.
Female. (n = 1). Same as male, but with forewing length 4.9 mm.
Larval case. (n = 7; Fig. 8). The case is 4.1–5.0 mm in length and 1.2–1.6 mm wide, smooth with banding that follows the length of the case. Two wide, dark bands form a “V” that crosses over the central region of the case.
Holotype: ♂, [1] Printed white label: ‘H[AWAI]I: Maui, Makawao Forest Reserve | elev[ation]: 3500 ft, on Koa branches | “purse” case, I-30-[20]09, #DR08K12A | coll[ector]. W[illiam]. Haines’; [2] Printed red label: ‘HOLOTYPE | Hyposmocoma makawao Kawahara & Rubinoff’. Male genitalia slide AYK103. Specimen in perfect condition. Paratype: 1♀: same data as holotype, but emergence date 13-Feb-2009, abdomen missing. Cases (7): same data as holotype. All specimens stored in the UHIM.
Two specimens from Maui with UH log number DR08K12A, extraction codes DN114, DN1320. The specimen from which extract DN114 was taken was tentatively called “Hsp67” before being given this formal name.
This species is named “makawao” after its type locality, Makawao Forest Reserve, Maui.
Case-making larvae were collected at Makawao Forest Reserve (MFR), Maui. Samples were collected using a beat-sheet, placed under branches of the koa tree (Acacia koa Gray).
Known only from the MFR, which harbors an extraordinary diverse natural fauna and flora, and is the only known locality for several species of Hyposmocoma, including Hyposmocoma domicolens (Butler, 1881), Hyposmocoma molluscivora Rubinoff & Haines, 2005, Hyposmocoma opuulaau Schmitz & Rubinoff, 2011, Hyposmocoma pukoa Schmitz & Rubinoff, 2011, and Hyposmocoma pupumoehewa Schmitz & Rubinoff, 2011. Unfortunately, the native habitat has been in sharp decline, even during the short course of this project, due to ongoing damage from invasive ungulates. If conservation action is not soon taken, it is likely that many of the rare plants and animals that remain in this mixed mesic forest will disappear.
http://species-id.net/wiki/Hyposmocoma_nebulifera
Figs 5, 9, 12Hyposmocoma nebulifera is similar to Hyposmocoma rubescens from Kauai, but differs in having a larger dark brown C-shaped forewing mark, and much smaller spurlike specialized setae on the valva. It is also similar to Hyposmocoma ekemamao but is larger, and has two central round spots on the forewing, while Hyposmocoma ekemamao only has one.
Male. (n = 3; Fig. 5). Forewing length 5.8 – 6.0 mm.Head light brown with scales near outer margin of eye, scales large near vertex. Haustellum pale brown. Maxillary palpus reduced. Labial palpus curved with pale brown scales, scales dark brown at terminus. Antennal flagellum light with dark brown bands. Thorax light brown, with lighter brown scales on tegula. Foreleg and midleg with brown scales and bands of light brown. Hindleg same as midleg, but with long scales along dorsal margin formed into a brush-like patch. Spines on legs light brown. Forewing light brown with a C-shaped dark brown mark 1/3 of distance to forewing apex, dark brown spot at base of forewing. One dark brown mark with light border at center of wing, another mark about 1/3 distance between first mark and apex. Abdomen covered in brown scales.
Male genitalia. (n = 1; Fig. 12). Right brachium of uncus sickle shaped, thin, long, and slightly twisted to left. Tegumen wide and sclerotized. Valvae asymmetrical, left valva medially slightly wider than right. Valvae without large sclerotized setae, but adorned with fine hair-like setae disposed comblike on inner surface of ventral margin. Phallus stout, blunt tipped, heavily sclerotized, and gradually curved ventrad at approximately 2/3 of length; vesica without spines or cornuti. Anellus with two symmetrical rounded lobes, thin until apex, both adorned with few small setae.
Female. (n = 2). Externally as male, but with larger wing span (6.5–7.2 mm).
Female genitalia. Papillae anales short and setose. Anterior and posterior apophyses thin, slightly curved, posterior apophyses slightly longer than anterior apophyses. Ostium bursae heavily sclerotized, externally protruding, C-shaped curled left, not triangular. Corpus bursae oval with light scobination; signum absent. Ductus bursae long, narrow, and approximately 2/3 length of corpus bursae. Apical margin of tergum VIII with median emargination.
Larval case. (n = 1; Fig. 9). Dark brown, smooth, 8.1 mm in length and 2.5 mm wide.
Paratype: ♀, [1] Printed white labels: ‘[HAWAII, Oahu, ] Waianae Mts. | 3000 ft. OAHU | Hawaiian Is. | IV. 1892. | Perkins. 225133’; [2] ‘Fauna Hawaiiensis | Collection’; [3] Hand written and printed white label: ‘Hyposmocoma | nebulifera | Wlsm. | PARA-TYPE 1/3’; [4] BPBM Paratype pink label: ‘No 32412 | Hawaiian Coll. | BISHOP Museum’ (BPBM). Non-type material: 1♂, HI: Oahu, Waianae Mountains, Palikea trail, 15 January 2009, emergence 13 February 2009, #DR09A3K, coll. P. Schmitz, D. Rubinoff, W. Haines, J. Eiben, male genitalia slide #AK113 (UHIM). 1♂, 1♀: HI: Oahu, Waianae Mountains, Palikea area, elev: 850 m, in leaf litter, extracted in Berlese funnel, “purse case”, emergence 29 October 2009, #DR09J2B, coll. P. Krushelnycky, female genitalia slide #AK109 (UHIM).
Two specimens from Palikea, Oahu, UH log number DR09A3K, extraction codes DN118, DN161. One specimen from Oahu, UH log number DR09J2B, extraction code DN1220. The specimen from which extract DN118 was obtained was tentatively called “Hsp68”.
Known only from the Waianae Mountain Range, Oahu. With nearly thirty described species of Hyposmocoma (
urn:lsid:zoobank.org:act:5B0155F3-0795-4706-895B-B728E4C575FC
http://species-id.net/wiki/Hyposmocoma_tantala
Figs 6, 10, 13, 16Hyposmocoma tantala is similar to Hyposmocoma nebulifera, but differs in having a dark forewing background color and much thicker dark forewing markings. The male genitalia has large sclerotized spines on the left valva that are absent in Hyposmocoma nebulifera.
Male. (n = 1). Forewing length 5.5 mm.Head light brown with light brown scales near outer margin of eye; scales large near vertex. Haustellum pale brown. Maxillary palpus reduced. Labial palpus curved with pale brown scales, scales dark brown at terminus. Antennal flagellum light with dark brown bands. Thorax light brown, with lighter brown scales on tegula. Foreleg and midleg with brown scales and bands of light brown. Hindleg same as midleg, but with long scales along dorsal margin formed into a brush-like patch. Spines on legs light brown. Forewing light brown with a large dark brown mark extending from costal margin 1/3 of distance to forewing apex and a smaller dark brown mark at 2/3 of distance to apex. Abdomen covered in brown scales.
Male genitalia (Fig. 13).Right brachium of uncus thick and curved ventrad, smooth, gradually tapering, slightly twisted to left. Left brachium small, not sclerotized. Tegumen wide and sclerotized. Shape of valva largely symmetrical, but left valva with three long tapered narrow spurlike setae along dorsal margin near apex; right valva without large sclerotized setae. Dense row of fine, hair-like setae disposed along inner ventral margin of both valvae. Phallus stout, blunt tipped, heavily sclerotized, open ventrally, and bent ventrad at about 1/2 of length; vesica without spines or cornuti. Anellus with two symmetrical rounded lobes with fine setae.
Female. (n = 2; Fig 6). Externally as male, but with larger wing span (6.2 mm).
Female genitalia. (Fig 16). Papillae anales short and setose. Anterior and posterior apophyses thin and straight, posterior apophyses slightly longer than anterior apophyses. Ostium bursae heavily sclerotized, externally protruding, and C-shaped curled left. Ductus bursae long and of small girth. Corpus bursae roughly kidney-shaped, with light scobination; signum absent. Apical margin of tergum VIII with median emargination.
Larval case. (n = 1; Fig 10). Dark brown, smooth, 9 mm in length and 2 mm wide.
Holotype: ♀, [1] Printed white label: ‘H[AWAI]I: Oahu, Tantalus, Manoa | Cliffs Trail, n[ea]r. Round Top Dr[ive]. | Purse Case DR10H2I [in bold type] | 22-Aug-2010, em[ergence]. 25-Oct[ober]-2010 | A. Y. Kawahara, W. Haines, | C. Yee, C. Atta collectors; [2] Printed red label: ‘HOLOTYPE | Hyposmocoma tantala Kawahara & Rubinoff’. Specimen in perfect condition. Female genitalia slide # slide AK108 (UHIM); Non-type material: 1♂, HI: Oahu: Mt. Tantalus, 4 km N of Manoa, 600 m., 8-VI-1991, coll. W. E. Steiner et al. (USNM).
Two specimens from Oahu (UH log number DR10H2I), extraction codes DN1317, DN1319.
This species is named “tantala” after Mt. Tantalus, from where the type specimen was collected. While Mt. Tantalus has experienced a tremendous amount of destruction from invasive species in the past century, it has historically been a locality with very high endemism, and the type locality of several other endemic insects, including flies (
Adults were reared from case-making larvae collected on bark of a damp dead tree covered partially with lichen.
Known only from Mt. Tantalus, Oahu.
Our morphological investigation coupled with molecular sequence data supports the separation of all three species described as new in this study. Interspecific genetic divergence in COI for sister-species for other Lepidoptera range from slightly less than 1% to nearly 4% (e.g.
While we have sampled Hyposmocoma broadly on all Hawaiian islands, all species in this study and those previous appear to be largely restricted to one volcano on one island. Unfortunately, restricted ranges pose challenges for conservation since many species are vulnerable to extinction because of widespread habitat destruction. Mount Tantalus on Oahu, the only known locality for Hyposmocoma tantala, is the type locality for endemic flies, beetles and at least two other species of Hyposmocoma. The area is heavily infested with invasive species, but does retain some endemism, though the native habitat is still declining. The discovery of a new, endemic species from Mt. Tantalus suggests that even relatively degraded areas would be worthy of active conservation efforts to control invasive species. The same is true for the Makawao Forest Reserve on Maui that straddles an area between rainforest and dry forest, and thus supports a particularly diverse, highly unique endemic flora and fauna. This forest reserve is also the type locality for six species of Hyposmocoma. As such, native species in these refugia continue to decline, and this must include endemic species of Hyposmocoma, all of which are found nowhere else. We hope this information will encourage more active conservation, not only to protect these moths, but also the immense diversity of geographically restricted species displaying amazingly specialized life histories and the remnant native habitats that sustain them.
ML phylogeny of tubular purse-cased Hyposmocoma and relatives. Numbers above branches are ML bootstrap values, numbers below are Bayesian posterior probabilities. Scale bar = 0.02 substitutions/site.
This project benefited from numerous discussions with Patrick Schmitz and William Haines. We thank Walter Domingo, Kathy Fan, Beth Iseri, Jennifer Kameoka, Kellie Kanegawa, Daniel Nitta, Miki Sadamori, and Keri Yatogo for their help rearing, sequencing, and preparing voucher specimens for this project. Calder Atta, Jesse Eiben, Paul Krushelnycky, Steven Montgomery, Natalia Tangalin and Celeste Yee provided invaluable assistance with the collection of samples. Samuel ‘Ohukani‘ōhi‘a Gon III (The Nature Conservancy, USA) helped with Hawaiian names. We thank John Cumming, Fern Duvall, Betsy Gagné, Galen Kawakami, Cynthia King, Ryan Peralta, Glenn Shishido, David Smith (Hawaii Division of Forestry and Wildlife, Department of Land and Natural Resources), Rhonda Loh (Hawaii Volcanoes National Park), Pat Bily and Stephanie Loo Jefts (The Nature Conservancy, USA) for permits and access to parks. This project was supported by the National Science Foundation (NSF) award #DEB-0918341 and in part by grants from the National Geographic Society’s Committee for Research and Exploration, the State of Hawaii’s U.S. Fish and Wildlife Service State Wildlife Grant (T-3-P), USDA-NIFA Agreement No. 58-5320-9-430, and Hatch projects HAW00942-H and HAW00956-H, administered by the College of Tropical Agriculture and Human Resources, University of Hawaii at Manoa.