(C) 2012 Alejandro Zaldívar-Riverón. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Five new species belonging to the poorly known Neotropical doryctine parasitoid wasps genera Heerz Marsh (Heerz ecmahla sp. n. and Heerz macrophthalma sp. n.), Lissopsius Marsh (Lissopsius pacificus sp. n. and Lissopsius jalisciensis sp. n.) and Ondigus Braet, Barbalho & van Achterberg (Ondigus cuixmalensis sp. n.) are described from the Chamela-Cuixmala Biosphere reserve in Jalisco, Mexico. Keys to the described species of the above three genera are provided. The phylogenetic placement of the examined taxa is investigated based on mitochondrial (COI) and nuclear (28S, 2nd and 3rd domain regions) DNA sequence data.
new species, Heerz, Lissopsius, Ondigus, 28S, COI
The Doryctinae represents one of the largest subfamilies of braconid parasitoid wasps, probably only behind Braconinae, Microgastrinae, Alysiinae and Opiinae (
A recent barcoding study carried out in the Chamela-Cuixmala Biosphere Reserve (CCBR) in Jalisco, Mexico, has revealed the existence of an extraordinary, largely neglected doryctine fauna (
Three new species representing novel records for two doryctine genera in the Mexican territory have so far been described from the CCBR (Neoheterospilus Belokobylskij and Iare Barbalho & Penteado-Días:
All the specimens included in this work were collected in several field trips carried out during 2009–2011 to the Chamela Biological station (within the CCBR) owned by the Instituto de Biología, Universidad Nacional Autónoma de México. All the collected specimens were preserved in 100% ethanol, kept at -20°C until they were processed for DNA sequencing, and subsequently dried, labelled and pinned. The material examined in this study is deposited in the Colección Nacional de Insectos, Instituto de Biología, Universidad Nacional Autónoma de México (IB-UNAM CNIN), Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Buenos Aires, Argentina (MACN), and the University of Wyoming Insect Museum (UWIM).
The terminology employed follows
The phylogenetic placement of the new taxa described in this study was reconstructed based on two widely used gene markers, around 658 bp of the barcoding locus [cytochrome oxidase I (COI) mitochondrial (mt) DNA gene], and a ~650 bp fragment corresponding to the second and third domain regions of the nuclear 28S rDNA gene. For the specimens belonging to Ondigus, a single leg was removed, placed in a 96-well lysis plate and posted to the University of Guelph for DNA extraction, amplification and sequencing (see laboratory protocols in
All the sequences generated for this study are deposited in GenBank (see accession numbers below). These sequences will be also available in the project file ‘Parasitoid Wasps (Braconidae: Doryctinae) of Chamela-Cuixmala Biosphere Reserve’ (ASDOR project) in the projects section of the Barcode of Life Data System (www.barcodinglife.org).
Genetic distances of the COI marker within and among the newly described taxa examined were calculated using the K2Pdistance model (
We ran a Bayesian MCMC partitioned analysis with Mrbayes version 3.1.2 (
Genetic distances of the COI marker among the three examined species of Heerz using the K2P distance model ranged from 11.1 to 14.6%. Within Heerz ecmahla sp. n., the COI sequence fragment varied from 0.18 to 0.37%, whereas a unique haplotype was found among the three sequenced specimens of Heerz macrophthalma sp. n. Lissopsius pacificus sp. n. and Lissopsius jaliscoensis sp. n. also each had unique haplotypes, with a sequence divergence of 8% between them. The COI distance between the sequenced specimens of Ondigus cuixmalensis sp. n. and Ondigus bicolor Braet, Barbalho & van Achterberg was of 11.4%.
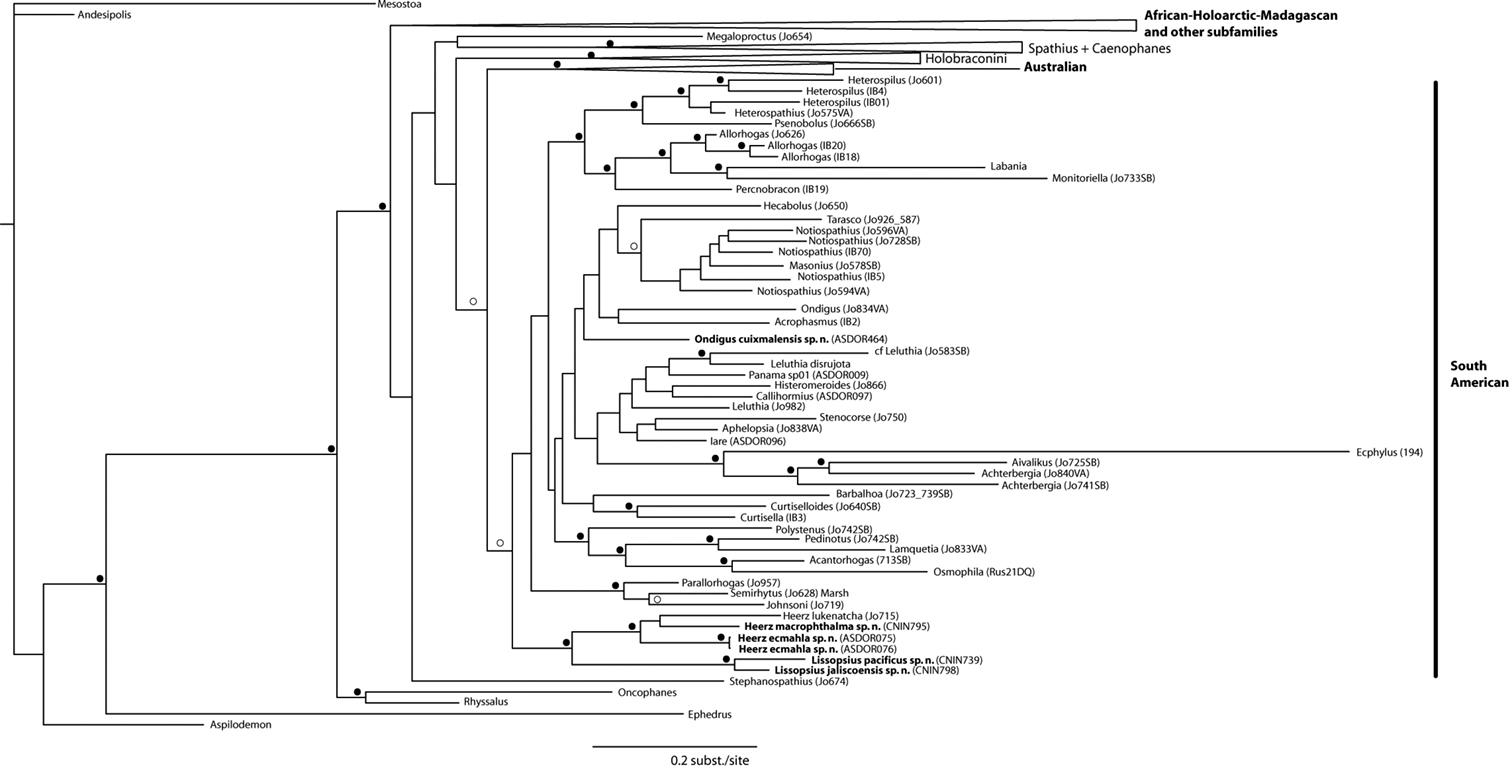
Our reconstructed Bayesian phylogram based on the concatenated 28S+COI data sets (Fig. 1) recovered the species of the three genera examined in this study within a major ‘South American’ clade (PP = 0.93) also recovered in previous phylogenetic studies of the Doryctinae (
Bayesian phylogram showing the phylogenetic placement of the taxa described in this study within the Doryctinae. Black circles near branches represent posterior probabilities ≥ 0.95; blank circles represent posterior probabilities between 0.90 and 0.94. Names of the major clades are according to
Heerz lukenatcha Marsh
Heerz distinguishes from other doryctine genera by the following combination of features: (1) frons excavated (Fig. 2D), (2) propodeum with a longitudinal median carina followed by a pentagonal areola (Figs 2C, 3D), (3) second metasomal tergite entirely or partially sculptured, contrasting with third one, which is smooth, polished and with a transverse furrow (Figs 2C, 3D), (4) vein r-m of fore wing present (Fig. 2F), (5) vein M+CU of hind about as long as vein 1M, (6) vein cu-a of hind wing straight or slightly curved apically towards wing apex, (7) male hind wing without pterostigma, and (8) hind coxa with a distinct basoventral tooth. Species of Heerz are very similar in habitus to those of Lissopsius, all having a body mostly smooth and shiny, propodeum with a longitudinal median carina followed by a pentagonal areola, and vein M+CU of hind wing slightly shorter to larger than vein 1M. However, Heerz differs from Lissopsius by having the vein r-m of fore wing present (Fig. 2F) (absent in Lissopsius), hind coxa with a basoventral tooth (absent in Lissopsius), and ovipositor distinctly sclerotised apically (uniformly slcerotised in Lissopsius).
Small to moderate size, 2.5-7.0 mm; eyes large, moderately to distinctly emarginated opposite antennal sockets; frons concave; occipital carina present, meeting hypostomal carina before mandible; labrum distinctly concave; hypoclypeal depression small and round; clypeus short; malar suture absent; maxillary palpi 5-segmented, labial palpi 4-segmented; head and mesosoma smooth or weakly sculptured; mesoscutum declivous anteriorly; prepectal carinae present; precoxal sulcus smooth; surface of propodeum smooth on anterior half, slightly rugose on posterior half, with a median longitudinal carina followed by a pentagonal areola; metapleural flange present; fore tibia with a row of spines along anterior edge; hind coxa with a distinct basoventral tooth; vein m-cu of fore wing antefurcal to vein 2RS, thus (RS+M)b present; vein 1cu-a postfurcal to vein 1M; vein r-m of fore wing present; second submarginal cell distinctly short; first subdiscal cell of fore wing open at apex; vein M+CU of hind wing slightly shorter to larger than vein 1M; males without pterostigma on hind wing; basal sternal plate (acrosternite) of first metasomal tergite short, 0.2-0.3 times the length of tergum; first and second metasomal tergites scupltured; third metasomal tergite smooth with a transverse furrow; remaining metasomal tergites smooth; ovipositor strongly sclerotised apically; nodes reduced, only one or absent.
Brazil, Costa Rica and Mexico.
The two new species of Heerz described below considerably modify the previous concept of the genus. The two previously described species, Heerz lukenatcha and Heerz tooya Marsh, are characterised by their smooth mesosoma, dusky wings and relatively large body size. The Mexican species, on the other hand, have a coriaceous mesoscutum, uniformly hyaline wings and are considerably smaller, especially Heerz ecmahla. Moreover, frons excavation is more conspicuous in the two new species compared to Heerz lukenatcha and Heerz tooya. Despite these morphological differences, our comparisons with type material and our DNA sequence data (see below) led us to include the new species within Heerz.
| 1 | Wings partially or totally infuscate (Fig. 2B), mesoscutum mostly smooth | 2 |
| – | Wings hyaline (Fig. 2A, 3A), mesoscutum coriaceous (Fig. 3C) | 3 |
| 2 | Wings yellow on basal ¾, dusky on apical ¼, all femora and tibiae black | Heerz lukentacha Marsh |
| – | Wings evenly dusky; all femora and tibiae yellow | Heerz tooya Marsh |
| 3 | Eyes considerably large, their height 5.0 times longer than malar space, inner orbit clearly emarginated (Fig. 3B); second metasomal tergite mostly striate (Fig. 3D); ovipositor 0.5 times as long as metasoma (Fig. 3A) | Heerz macrophthalma sp. n. |
| – | Eyes small, their height about 3.0 times length of malar space (Fig. 2D), weakly emarginated; second metasomal tergite mostly coriaceous (Fig. 2C); ovipositor slightly longer (about 1.1 times) than metasoma (Fig. 2A) | Heerz ecmahla sp. n. |
urn:lsid:zoobank.org:act:79D0180F-1C9F-4825-97A6-6E4C3C706CCB
http://species-id.net/wiki/Heerz_ecmahla
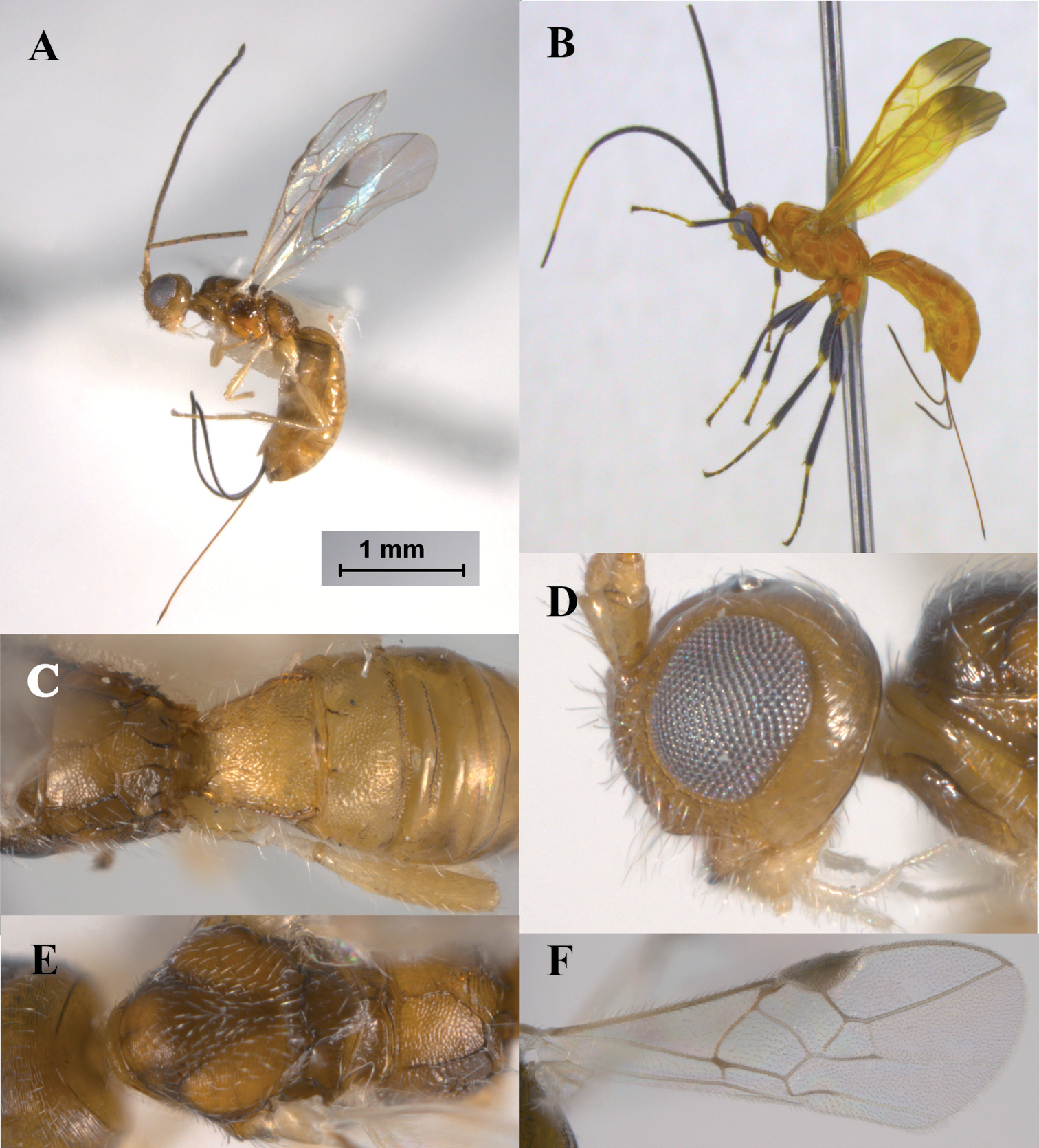
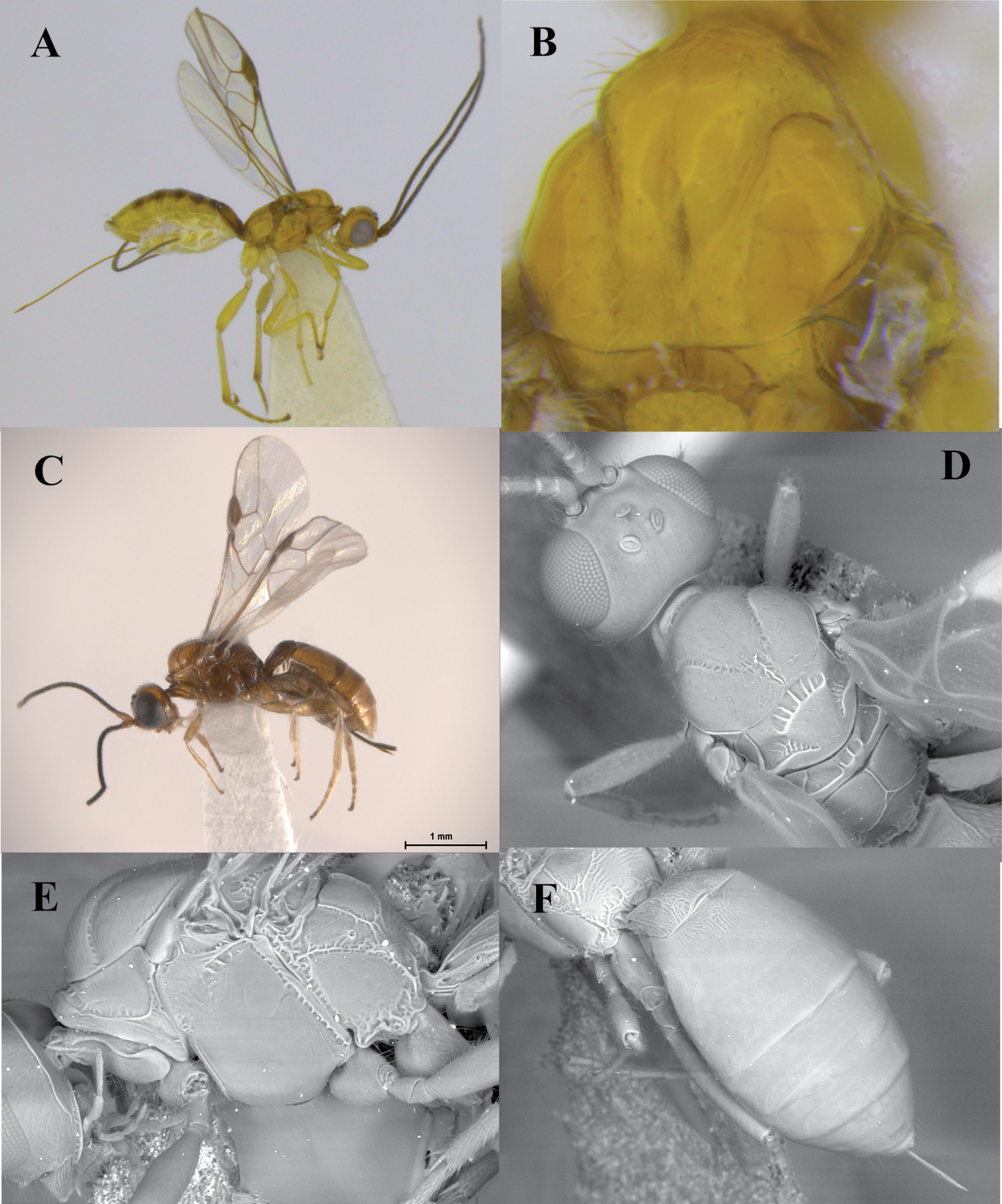
Figs 2A–FHeerz ecmahla distinguishes from the remaining species of the genus by the uniformly coriaceous sculpture on the first and second metasomal tergites (Fig. 2C). It also distinguishes from the Central and South American species, Heerz tooya and Heerz lukenatcha, by its entirely hyaline wings (Fig. 2A) [wings partially or totally infuscate in the latter two species (Fig. 2B)], and from Heerz macrophtalma by its relatively smaller eyes (Fig. 2D) [considerably large in Heerz macrophtalma (Fig. 3B)] and distinctly longer ovipositor (Fig. 2A) [about 0.5 times as long as metasoma in Heerz macrophtalma (Fig. 3A)].
Female. Colour: Body honey yellow, antennae honey yellow, turning darker apically; legs yellow, median lobe of mesoscutum and upper half of mesopleuron light brown; metasoma slightly lighter; wings hyaline, veins, pterostigma and tegula light brown. Body length: 2.5 mm. Head: vertex, frons and temple striate, gena smooth, face acinose; eyes large, its height 1.3 times its maximum width (lateral view); malar space about 0.3 times eye height (lateral view); ocello-ocular distance two times the diameter of lateral ocellus and 1.5 times longer than posterior ocellar line; antennae with 21 flagellomeres. Mesosoma: length of mesosoma 1.9 times its maximum height; pronotum essentially smooth, pronotal groove largely smooth, with a few rugae medially; mesoscutal lobes coriaceous; notauli scrobiculate, obscured in a posterior rugose median area; scutellum smooth, scutellar sulcus deep and scrobiculate, with four longitudinal carinae; mesopleuron largely smooth, posterior mesopleural sulcus distinct and scrobiculate; subalar groove scrobiculate; precoxal sulcus smooth; metapleuron rugulose; dorsolateral areas of propodeum coriaceous; propodeal areola distinctly delimited by carinae and essentially coriaceous. Legs: fore tibia with a row of 12 spines; hind coxa mostly smooth, with a distinct basoventral tooth. Wings: fore wing 2.9 times longer than wide; length of pterostigma 2.7 times its maximum width, 0.6 times length of vein R; vein r 1.5 times longer than vein 3RSa; vein 3RSa 0.8 times length of r-m; vein m-cu distinctly antefurcal; (RS+M)b present, 0.3 times length of vein 2RS; hind wing with vein M+CU 0.8 times as long as 1M and 1.5 times length of vein r-m; vein m-cu slightly curved towards wing apex. Metasoma: first metasomal tergite short, 1.1 times as long as its apical width, entirely coriaceous, with two dorsolateral carinae running through anterior half of median tergite, without a median dorsal area fully delimited by carinae; second metasomal tergite coriaceous, with two slightly convergent furrows basally; third metasomal tergite smooth, turning coriaceous laterally and with a transverse scrobiculate furrow; remaining metasomal tergites smooth and polished; ovipositor 1.1 times length of metasoma, with a single, reduced node.
Male. Essentially as female, body length 2.7 mm.
Variation. Females: Body length 2.5–2.7 mm; eyes 1.1–1.2 times higher than wide (lateral view); fore wing length 2.5–2.6 times its maximum width; length of pterostigma 2.6–2.7 times its maximum width; hind wing vein M+CU 0.8–1.0 times as long as vein 1M.
Holotype. IB-UNAM CNIN. Female. Mexico, Jalisco, Estación Biológica Chamela, cerca del Laboratorio, 19.49N, -105.04E, 23–24.vi.2009, 95 msnm, light trap, selva baja caducifolia, H. Clebsch, A. Zaldívar, A. Polaszek col., DNA voucher no. ASDOR076 (CHAM-076), GenBank accession nos JF912210, HQ200616 (IB-UNAM CNIN).
Paratypes. IB-UNAM CNIN, MACN. Two specimens. One female, Mexico, Jalisco, Estación Biológica Chamela, cerca del laboratorio, 19.49N, -105.04E, 23–24.vi.2009, 95msnm, light trap, selva baja caducifolia, H. Clebsch, A. Zaldívar, A. Polaszek col., DNA voucher no. CHAM-075, GenBank accession nos JF912209, HQ200615; one male, Mexico, Jalisco, Estación Biológica Chamela, cerca del laboratorio, 19.49N, -105.04E, 05.v.2011, 99–122 msnm, light trap, selva baja caducifolia, A. Zaldívar, S. Zaragoza, A. Ibarra col., DNA voucher nos CNIN796, ASDOR076 (CHAM-076), GenBank accession nos JQ268746, JF912210, HQ200616.
The specific epithet is an anagram of Chamela, the type locality of this species.
Heerz ecmhala sp. n. (holotype) (A, C–F) and Heerz lukenatcha Marsh (B): A, B habitus, lateral view C propodeum and basal half of metasoma, dorsal view D head, lateral view E mesosoma, dorsal view F fore wing.
urn:lsid:zoobank.org:act:2F240FAD-E0C2-43FD-ACBC-B66E432EA6B2
http://species-id.net/wiki/Heerz_macrophthalma
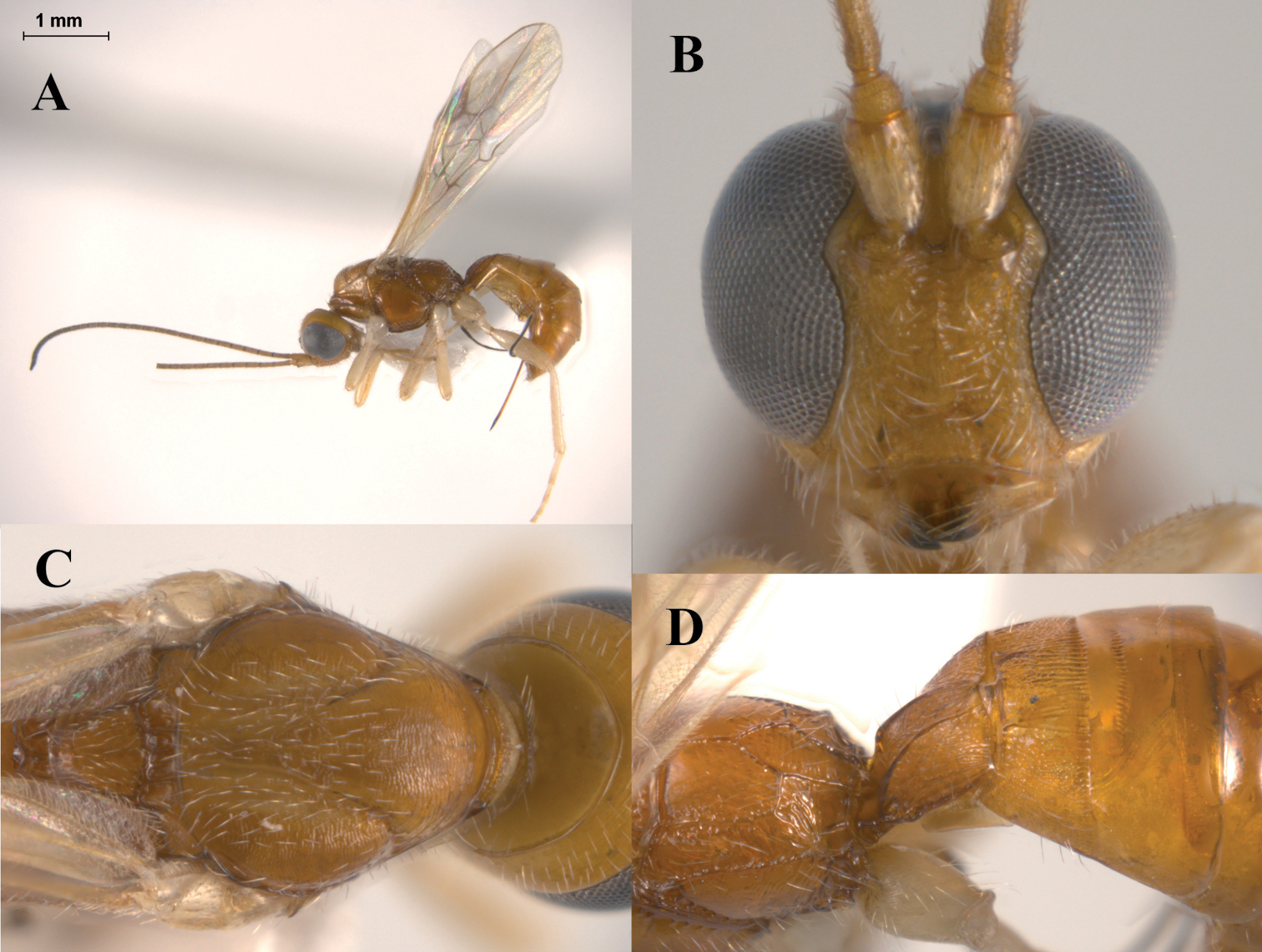
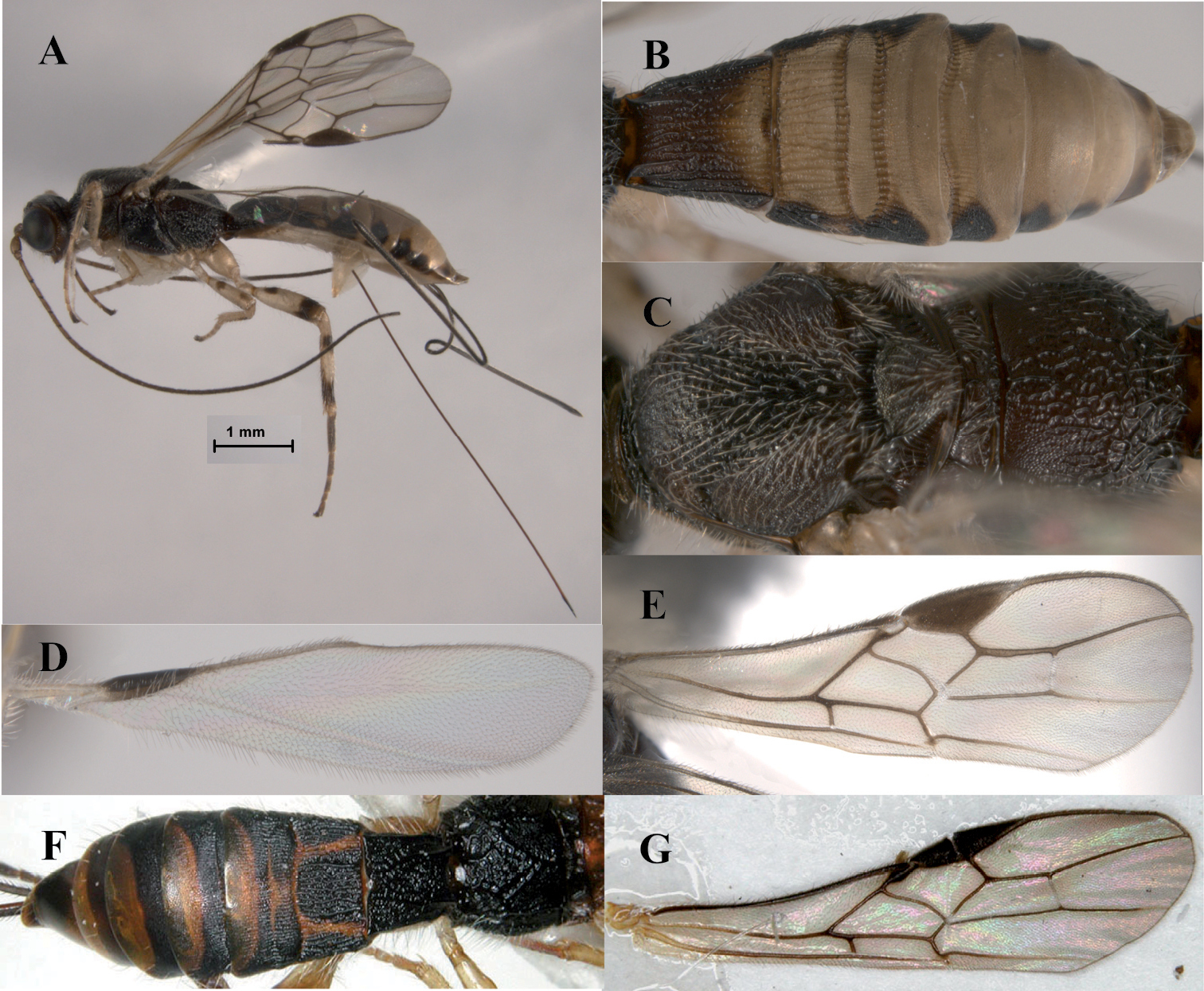
Figs 3A–DHeerz macrophthalma distinguishes from the remaining species of the genus by its considerably larger eyes (Fig. 3B). It can also be distinguished from the Central and South American species, Heerz tooya and Heerz lukenatcha, by its entirely hyaline wings (Fig. 3A) [wings partially or totally infuscate in the latter two species (Fig. 2B)] and from Heerz ecmahla by the striate sculpture on the second metasomal tergite (Fig. 3D) [coriaceous in Heerz ecmahla (Fig. 2C)].
Female. Colour: Body mostly honey yellow, antennae honey yellow, turning darker apically; legs creamish white, with apex of tarsomeres darker; wings hyaline, veins, pterostigma and tegula light brown. Body length: 4.7 mm. Head: vertex, frons and temple striate, face acinose; eyes large, 1.3 times higher than wide (lateral view); malar space about 0.2 times eye height; ocello-ocular distance less than 0.8 times diameter of lateral ocellus and as long as posterior ocellar line; antennae with 31 flagellomeres. Mesosoma: length of mesosoma 1.8 times its maximum height; pronotum smooth, pronotal groove scrobiculate; mesoscutal lobes coriaceous; notauli deep and scrobiculate, obscured in a posterior striate-rugose median area; scutellum smooth, scutellar sulcus deep and scrobiculate, with six longitudinal carinae; mesopleuron largely smooth, striate near subalar furrow; posterior mesopleural sulcus distinct and scrobiculate; subalar groove scrobiculate; precoxal sulcus smooth; metapleuron smooth; propodeal areola distinctly delimited by carinae and with crossing transversal rugae. Legs: fore tibia with a row of 15 spines; hind coxa striate dorsally, smooth ventrally, with a distinct basoventral tooth. Wings: fore wing 2.8 times longer than wide; pterostigma 0.6 times the length of vein R; vein r 1.5 times longer than vein 3RSa; vein 3RSa 0.8 times as long as vein r-m; vein m-cu distinctly antefurcal, vein (Rs+M)b present, 0.2 times length of vein 2RS; hind wing with vein M+CU as long as vein 1M and twice length of vein r-m; vein m-cu slightly curved towards wing apex. Metasoma: first metasomal tergite short, about as long as its apical width, with median dorsal area delimited by two dorsolateral carinae, median area rugulose-coriaceous, lateral areas striate; second metasomal tergite striate medially, with coriaceous sculpture in between striations, with two more conspicuous slightly convergent furrows; third metasomal terigte smooth, turning coriaceous laterally, with a transverse scrobiculate furrow; remaining metasomal tergites smooth and polished; ovipositor 0.5 times length of metasoma, without distinct nodes.
Male. Smaller than female, body length 2.7–2.8 mm.
Variation. Female: Body length 4.1–4.6 mm; eyes 1.3–1.4 times higher than wide (lateral view); malar space 0.1–0.2 times eye height (lateral view); ocello-ocular distance 0.7-0.8 times diameter of lateral ocellus; antennae with 28–29 flagellomeres; fore wing length 2.8–2.9 times its maximum width; length of pterostigma 2.6–2.8 times its maximum width; hind wing vein M+CU 1.0–1.1 times longer than vein 1M.
Holotype. IB-UNAM CNIN. Female. Mexico, Jalisco, Estación Biológica Chamela, cerca laboratorio, 19.49, -105.04, 5.v.2011, 99-122 msnm, light trap, selva baja caducifolia, Cham-084, Zaldívar, Zaragoza, Ibarra col. DNA voucher no. CNIN795, GenBank accession nos JQ268745, JQ268749 (IB-UNAM CNIN).
Paratypes. IB-UNAM CNIN, MACN, UWIM. Three specimens. One female, Mexico, Jalisco, Estación Biológica Chamela, camino Búho, 19.49/19.49 N, -105.04/-105.04 E, 25.ii.2010, 106 msnm, sweeping net, selva baja caducifolia, A. Zaldívar col., DNA voucher no. ASDOR761, GenBank accession no. HQ200977; two males, Mexico, Jalisco, Estación Biológica Chamela, cerca laboratorio, 19.49 N, -105.04 E, 20.ii.2011, 99-122 msnm, light trap, selva baja caducifolia, A. Zaldívar, col., DNA voucher nos ASDOR551 (Cham-500), ASDOR555 (Cham-504), GenBank accession nos HQ200979, HQ200967 (IB-UNAM CNIN).
The specific epithet derives from the greek words makros and ophthalmos, in reference to the very large compound eyes of this species.
Heerz macrophthalma sp. n. (holotype): A habitus, lateral view B head, frontal view C mesosoma, dorsal view D propodeum and basal half of mesosoma, dorsolateral view.
Lissopsius flavus Marsh, 2002.
This genus distinguishes from other recognised doryctine genera by the following combination of features: (1) body mostly smooth and polished (Figs 4A-E, 5B-F), (2) propodeum with a median longitudinal carina followed by a pentagonal areola (Figs 4B, 5D), (3) vein r-m of fore wing absent (Fig. 4F), (4) vein M+CU of hind wing larger than vein 1M (Fig. 4F), (5) vein cu-a of hind wing curved at apex towards wing tip (Fig. 4F), (6) hind coxa angled at base, without distinct tubercle or tooth, and (7) ovipositor uniformly sclerotised and with a single nodus (Fig. 4G). Lissopsius is closely related to Heerz (see above) and both are morphologically similar, with a body mostly smooth and polished, propodeal areola present and vein M+CU of hind wing slightly shorter to longer than vein 1M. However, Lissopsius differs from Heerz by having the vein r-m of fore wing absent (always present in Heerz), hind coxa without basoventral tooth (present in Heerz), and ovipositor uniformly sclerotised (strongly sclerotised at apex in Heerz).
Small size, 2.3-4.5 mm; eyes large, emarginated opposite antennal sockets; occipital carina present, ending before reaching hypostomal carina; labrum distinctly concave; hypoclypeal depression small and round; clypeus short; malar suture absent; maxillary palpi 5-segmented, labial palpi 4-segmented; head, mesosoma and metasoma mostly smooth and polished; mesoscutum declivous anteriorly; prepectal carinae present; precoxal sulcus shallow and almost indistinct; surface of propodeum smooth on anterior half, slightly rugose on posterior half, with median longitudinal carina anteriorly and pentagonal areola posteriorly; metapleural flange present; fore tibia with a row of at least 10 spines along anterior edge (Fig. 4H); hind coxa angled at base, without basoventral tooth; vein m-cu of fore wing considerably antefurcal to vein 2RS, vein (RS+M)b present; vein 1cu-a considerably postfurcal to vein 1M; vein r-m of fore wing absent; first subdiscal cell of fore wing open at apex; vein M+CU of hind wing equal to or slightly longer than vein 1M; males without stigma-like enlargement on hind wing; basal sternal plate (acrosternite) of first metasomal tergite about 0.25 length of tergite.
Costa Rica and Mexico.
In their study describing new ovipositor diagnostic features for the subfamily Doryctinae,
| 1 | Ovipositor considerably short, no more than 0.25 times as long as metasoma (Figs 4A, G, 5C, F); mesoscutum with triangular, longitudinally rugose area in posteromedian area (Figs 4B, 5D) | 2 |
| – | Ovipositor long, slightly shorter than metasoma; mesocutum entirely smooth (Fig. 5B) | Lissopsius flavus Marsh |
| 2 | Second metasomal tergite mostly smooth, only slightly costate basally (Fig. 4E); ventral part of mesopleuron, precoxal sulcus and venter of mesosoma darker that the rest of the body (Fig. 4A); notauli ending before first half of mesoscutum (Fig. 4B) | Lissopsius pacificus n. sp. |
| – | Second metasomal tergite distinctly costate basomedially, remaining area smooth (Fig. 5F); body uniformly yellow (Fig. 5C); notauli ending after first half of mesoscutum (Fig. 5D) | Lissopsius jaliscoensis n. sp. |
urn:lsid:zoobank.org:act:0DDA9D93-E265-40B9-A525-7E185502A6EB
http://species-id.net/wiki/Lissopsius_pacificus
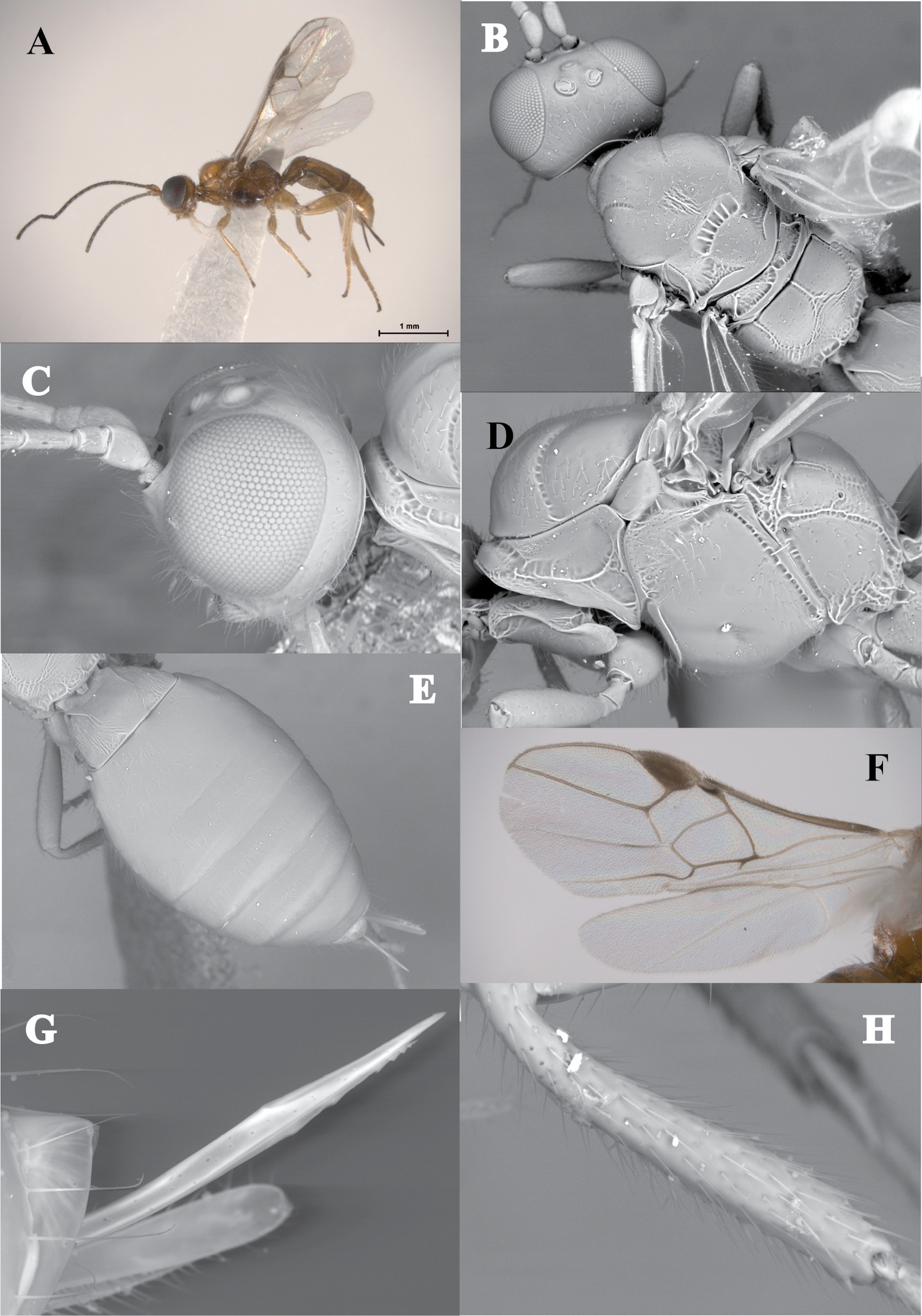
Figs 4 A–HThis new species distinguishes from Lissopsius jaliscoensissp. n.and Lissopsius flavus in having a second metasomal tergite mostly smooth, only slightly costate basally (Fig. 4E) [distinctly costate basally, remaining area smooth in Lissopsius flavus; distinctly costate basomedially, remaining area smooth in Lissopsius jaliscoensissp. n.(Fig. 5F)]; ventral part of mesopleuron, precoxal sulcus and venter of mesosoma darker that the rest of the body (Fig. 4A) [mesosoma completely yellow in Lissopsius flavus and Lissopsius jaliscoensis (Figs 5A, B)]; and notauli ending before first half of mesoscutum (Fig. 4B) [ending after first half of mesoscutum in Lissopsius flavus and Lissopsius jaliscoensissp. n. (Figs 5B, D)]. Lissospius pacificus also distinguishes from Lissopsius flavus in having a very short ovipositor, about 0.25 times as long as metasoma (
Female. Colour: head yellow, ventral part of mesopleuron, precoxal sulcus and venter of mesosoma light brown, remainder part of mesosoma yellow; metasoma yellow with some areas light brown; pedicel and flagellum yellow to light brown; legs yellow, hind tarsi light brown; wings hyaline, veins and pterostigma brown, tegula yellow. Body length: 3.4 mm. Head: entirely smooth, vertex and temple pilose, face strongly pilose; eyes large, malar space about 0.2 times eye height; ocello-ocular distance about 1.5 times diameter of lateral ocellus; eye 1.2 times higher than wide (lateral view); antennae broken, with at least 20 flagellomeres; scape with the same length as first flagellomere; first flagellomere longer than second. Mesosoma: length of mesosoma 1.7 times its maximum height; pronotum smooth dorsally, slightly rugose ventrally, pronotal groove slightly scrobiculate; mesoscutal lobes smooth, sparsely pilose medially, with a triangular longitudinal rugose area in the posteromedian area of mesoscutum; notauli deep and scrobiculate, not joining, ending before anterior half of mesoscutum; posterolateral sides of scutellum rugose, remaining areas smooth; scutellar sulcus deep and scrobiculate, with five longitudinal carinae; mesopleuron smooth, posterior mesopleural sulcus distinct and scrobiculate, subalar groove puctate; precoxal sulcus shallow and smooth, ending on anterior half of mesopleuron; metapleuron smooth, propodeum smooth on basal half, slightly rugose on apical half, with a median longitudinal carina followed by a distinct pentagonal areola. Legs: hind coxa smooth, protruding forward in ventro-anterior corner, about 1.4 times longer than its maximum width. Wings: Fore wing length 3.2 times its maximum width, length of pterostigma 2.9 times its maximum width, vein m-cu clearly antefurcal to vein 2RS, vein 1cu-a clearly postfurcal to vein 1M; hind wing vein M+CU 1.4 times longer than vein 1M; vein cu-a curved at apex toward wing tip. Metasoma: first metasomal tergite short, about 0.8 times as long as its apical width, medially smooth, laterally slightly costate-punctate; second metasomal tergite slightly costate basally, remaining area smooth; suture between second and third metasomal tergites poorly defined; remaining metasomal tergites smooth and polished; ovipositor very short, about 0.3 times length of metasoma.
Male. Similar to female; body length 2.3–2.8 mm.
Variation. Females: body length 3.0–4.5 mm; eyes 1.1–1.3 times higher than wide (lateral view); malar space 0.1–0.2 times eye height (lateral view); ocello-ocular distance 1.4–1.5 times diameter of lateral ocellus; antennae with 22–26 flagellomeres; fore wing length 2.9–3.2 times its maximum width; length of pterostigma 2.9–3.5 times its maximum width; hind wing vein M+CU 1.4–1.8 times longer than vein 1M.
Holotype. IB-UNAM CNIN. Female. Mexico, Jalisco, Estación de Biología de Chamela, UNAM, camino Búho, 19.49 N, -105.04 E, 65 msnm, 26–27 June 2009, light trap, tropical dry forest, H. Clebsch, A. Zaldívar-Riverón, A. Polaszek col., DNA voucher no. CNIN740, GenBank accession no. JQ268738 (IB-UNAM CNIN).
Paratypes. IB-UNAM CNIN, MACN, UWIM. One hundred and thirteen specimens. Twenty three females, same data as holotype; 37 females, two males, Mexico, Jalisco, Estación de Biológica de Chamela UNAM, camino Búho, 19.49N, -105.04E, 95 msnm, 24–26 June 2009, light trap, tropical dry forest, H. Clebsch, A. Zaldívar, A. Polaszek col.; 49 females, Mexico, Jalisco, Estación Biológica de Chamela, UNAM, cerca del laboratorio, 19.49N, -105.04E, 95 msnm, 23–25 June 2009, light trap, tropical dry forest, H. Clebsch, A. Zaldívar, A.Polaszek col.; four females, Mexico, Jalisco, Estación de Biológica de Chamela, UNAM, camino Chachalaca, 19.49N, -105.03E, 56 msnm, 25 June 2009, light trap, tropical dry forest, H. Clebsch, A. Zaldívar, A. Polaszek col.; one female, Mexico, Jalisco, Estación de Biológica de Chamela, UNAM, near lab, 19.49N, -105.04E, 99 msnm, 5 May 2011, light trap, tropical dry forest, H. Clebsch, A. Zaldívar, A. Polaszek col. DNA voucher nos CNIN739, 740, 742, 743, GenBank accession nos JQ268737, JQ268739-40, JQ268747 (IB-UNAM CNIN). Additional material: About three hundred of specimens preserved in 100% ethanol and kept at -20°C.
The two new species of Lissopsius described in this study apparently have nocturnal habits, since all their specimens were only collected with light traps. These species appear to be generalist parasitoids of various species of lepidopterans according to an ongoing study (Zaldívar-Riverón et al. in prep.) that is being carried out based on molecular analyses of parasitoid linkages (MAPL;
The specific name refers to the area where the species was collected, which is situated near the Mexican Pacific coast.
Lissopsius pacificus sp. n. (holotype): A habitus, lateral view B head and mesosoma, dorsal view C head, lateral view D mesosoma, lateral view E metasoma, dorsal view F fore and hind wing G ovipositor H fore tibia.
urn:lsid:zoobank.org:act:169B8D3A-CE52-4AE6-BCA0-F1D752F2B28E
http://species-id.net/wiki/Lissopsius_jaliscoensis
Figs 5AThis species distinguishes from Lissopsius pacificus and Lissopsius flavus by having the second metasomal tergite distinctly costate basomedially (Fig. 5F) [mostly smooth, only slightly costate basally in Lissopsius pacificus (Fig. 4D); distinctly costate basally, remaining area smooth in Lissopsius flavus]. Lissopsius jaliscoensis is morphologically very similar to Lissopsius pacificus, but differs from this species by having the mesosoma entirely yellow (Fig. 5C) [ventral part of mesopleuron, precoxal sulcus and venter of mesosoma darker that the rest of the body in Lissopsius pacificus (Fig. 4A)], and notauli ending after first half of mesoscutum (Fig. 5D) [ending before first half of mesoscutum in Lissopsius pacificus (Fig. 4B)].
Female. Colour: head and mesosoma yellow, metasoma yellow with some areas light brown; pedicel and flagellum yellow to light brown; legs yellow; wings hyaline, veins and pterostigma light brown, tegula yellow. Body length: 3.3 mm. Head: entirely smooth, vertex and temple pilose, face strongly pilose; eyes large, malar space about 0.2 times eye height; ocello-ocular distance about 2.0 times diameter of lateral ocellus; eye 1.1 times higher than wide (lateral view); antennae broken, with at least 24 flagellomeres; scape with the same length as first flagellomere; first flagellomere longer than second. Mesosoma: length of mesosoma about 1.7 times its maximum height; pronotum smooth to slightly rugose dorsally and ventrally, pronotal groove slightly scrobiculate; mesoscutal lobes smooth, sparsely pilose; notauli deep and scrobiculate, joining in a triangularly rugose area at the end of mesoscutum; posterolateral sides of scutellum rugose, remaining areas smooth; scutellar sulcus deep and scrobiculate, with five longitudinal carinae; mesopleuron smooth, posterior mesopleural sulcus narrow and scrobiculate, subalar groove slightly punctate; precoxal sulcus almost indistinct and smooth, ending on anterior half of mesopleuron; metapleuron smooth, propodeum smooth on basal half, slightly rugose on apical half, with a median longitudinal carina followed by a distinct pentagonal areola. Legs: hind coxa smooth, protruding forward in ventro-anterior corner, about 1.3 times longer than its maximum width. Wings: Fore wing length 3.1 its maximum width, length of pterostigma 3.0 times its maximum width, vein m-cu clearly antefurcal to vein 2RS, vein 1cu-a clearly postfurcal to vein 1M; hind wing vein M+CU 1.8 times longer than vein 1M; vein cu-a curved at apex toward wing tip. Metasoma: first metasomal tergite short, 0.8 times as long as its apical width, basomedially smooth, remaining area costate with punctate microsculpture; second metasomal tergite distinctly costate with punctate microsculpture basomedially, remaining area smooth; suture between second and third metasomal tergites almost indistinct; remaining metasomal tergites smooth and polished; ovipositor very short, about 0.3 times length of metasoma.
Male. Similar to female. Body length 3.1 mm. Hind wing without stigma-like enlargement.
Variation. Females: body length 3.1-3.7 mm; eye 1.1–1.3 times higher than wide (lateral view); malar space 0.1–0.2 times eye height (lateral view); ocello-ocular distance 1.5–2.0 times diameter of lateral ocellus; all with antennae broken and less than 20 flagellomeres remaining. Wings: fore wing length 2.8–2.9 times its maximum width; length of pterostigma 3.8–4.0 times its maximum width; hind wing vein M+CU 1.6–1.8 times longer than vein 1M.
Holotype. IB-UNAM CNIN. Female. Mexico, Jalisco, Estación Biológica de Chamela, UNAM, camino Búho, 19.49N, -105.04E, 65 msnm, 26–27 June 2009, light trap, tropical dry forest. H. Clebsch, A. Zaldívar, A. Polaszek col, . DNA voucher no. CNIN798, GenBank accession nos JQ268742, JQ268748 (IB-UNAM CNIN).
Paratypes. IB-UNAM CNIN, MACN, UWIM. Four specimens. Three females Same data as holotype; one male, Mexico, Jalisco, Estación Biológica de Chamela, UNAM, camino Búho, 19.49N, -105.04E, 95 msnm, 24–25 June 2009, light trap, tropical dry forest, H. Clebsch, A. Zaldívar, A. Polaszek col. DNA voucher nos CNIN741, CNIN798-800, GenBank accession nos JQ268741-44 (IB-UNAM CNIN).
The specific name refers to Jalisco, the Mexican state where the species was found.
Only five specimens of this species were collected during all our field trips, contrasting with the approximately 300 specimens collected for Lissopsius pacificus.
Lissopsius flavusMarsh: A habitus, lateral view B mesoscutum, dorsal view. Lissopsius pacificus sp. n. (holotype): C habitus, lateral view D head and mesosoma, dorsal view E mesosoma, lateral view F metasoma, dorsal view.
http://species-id.net/wiki/Ondigus
Ondigus bicolor Braet, Barbalho & van Achterberg
This genus distinguishes from the remaining doryctine genera by having the following combination of features: (1) first six metasomal tergites sculptured, fourth to sixth granulate (Figs 6B, F); (2) suture between the second and third metasomal tergites wide, deep, scrobiculate and slightly sinuate (Figs 6B, F); (3) third metasomal tergite with a deep, scrobiculate transverse groove (Figs 6B, F); (4) first subdiscal cell of fore wing open at apex (Figs 6E, G); (5) hind coxa with a distinct basoventral tooth; hind wing vein M+CU approximately the same length of vein 1M (Figs 6E, G); (6) all femora with subbasal tubercles (Fig. 6A).
Moderate size, female 5.6–6.1 mm, male 4.2 mm; eyes large, moderately emarginated opposite antennal sockets; maxillary palpi 5-segmented, labial palpi 4-segmented; face densely setose; occipital carina present and complete; hypoclypeal depression elliptic; clypeus short and wide; malar suture absent; mesosoma setose and mostly coriaceous; mesoscutum declivous anteriorly; notauli distinct anteriorly, scrobiculate, obscured at half of mesoscutum in a longitudinally rugose area; prepectal carinae present; metapleural flange present; all femora with a subbasal protuberance; fore tibia with a row six to eight spines along anterior edge; hind coxa granulate, pilose, with a basoventral tooth; only known male with pterostigma on hind wing; vein m-cu of fore wing interstitial or slightly antefurcal to vein 2RS; vein 1cu-a considerably postfurcal to vein 1M; vein r-m of fore wing present; second submarginal cell of fore wing long; first subdiscal cell of fore wing open at apex; hind wing vein M+CU equal to or slightly longer than vein 1M; first metasomal tergite about 1.1–1.2 times longer than its apical width; suture between second and third metasomal tergite wide, deep, scrobiculate and slightly sinuate; third metasomal tergite with a deep, scrobiculate transverse groove; basal sternal plate (acrosternite) of first metasomal tergite about 0.2 length of tergum.
French Guyana, Mexico.
The new species of Ondigus described here differs in some of the morphological features originally proposed by
| 1 | Vertex striate, propodeum without a median longitudinal carina and areola, dorsolateral carinae of first metasomal tergite ending before its anterior half; second metasomal tergite without a pair of sublateral depressions | Ondigus cuixmalensis sp. n. |
| – | Vertex smooth; propodeum with a median longitudinal carina and a pentagonal areola; dorsolateral carinae of first metasomal median tergite complete; second metasomal tergite with a pair of sublateral depressions | Ondigus bicolor Braet, Barbalho & van Achterberg |
urn:lsid:zoobank.org:act:5A802A1E-8FE2-4880-8BBF-BA3516F403D3
http://species-id.net/wiki/Ondigus_cuixmalensis
Figs 6 A–GThis species differs from the other described species of the genus, Ondigus bicolor by having a vertex striate (smooth in Ondigus bicolor), propodeum without a median longitudinal carina and areola (Fig. 6C) [both present in Ondigus bicolor (Fig. 6F)], dorsolateral carinae of first metasomal tergite ending before its anterior half (Fig. 6B) [running through the apical end of first metasomal tergite in Ondigus bicolor (Fig. 6F)], and second metasomal tergite without a pair of sublateral depressions (Fig. 6B) [present in Ondigus bicolor (Fig. 6F)].
Female. Colour: head and mesosoma dark brown, eye orbits honey yellow; scape and pedicel brown, flagellomeres brown, turning black to apex; palpi pale yellow; first metasomal tergite dark brown, with a semicircular area yellow apically; remaining metasomal tergites yellow, with brown irregular areas laterally; fore, middle and hind coxae, trochanter and trochantellus pale yellow, femora and tibiae pale yellow with irregular specks medially and apically, tarsi light brown to brown; wings hyaline, veins and pterostigma light brown, tegula pale yellow. Body length: 5.6 mm; ovipositor 2.5 mm. Head: face, frons and vertex striate, temple and gena smooth; malar space about 0.2 times eye height; occipital carina ending just before reaching hypostomal carina; ocello-ocular distance about the same length than diameter of lateral ocellus; eye 1.2 times higher than wide (lateral view); antennae broken, with at least 31 flagellomeres; scape longer than first flagellomere; first flagellomere longer than second. Mesosoma: length of mesosoma about 2.0 times its maximum height; pronotum rugose dorsally and ventrally, pronotal groove scrobiculate; mesoscutal lobes coriaceous, slightly transversally rugose at the edges of notauli; notauli deep and scrobiculate, joining before mesoscutum in a rugose area; scutellar disc coriaceous; scutellar sulcus large, deep and scrobiculate, interrupted by scutellar disc, with at least nine longitudinal carinae on each side; mesopleuron porcate-coriaceous dorsally, coriaceous medially and ventrally; precoxal sulcus complete, wide, deep and scrobiculate; venter of mesopleuron coriaceous; posterior mesopleural sulcus narrow and scrobiculate; metapleuron rugose-areolate with coriaceous microsculpture, propodeum longitudinally rugose with coriaceous microsculpture, with a median longitudinal carina on apical half. Legs: hind coxa, femur and tibia coriaceous, about 1.4 times longer than its maximum width. Wings: Fore wing length 3.7 times its maximum width, length of pterostigma 2.7 times its maximum width, vein m-cu interstitial to vein 2RS, vein 1cu-a clearly postfurcal to vein 1M; hind wing vein M+CU about the same length as vein 1M. Metasoma: first metasomal tergite about the same length as its apical width, costate with rugose microsculpture, with dorsolateral carinae ending before its anterior half; second metasomal tergite costate with rugose microsculpture; third metasomal tergite longitudinally striate with granulose microsculpture, with a deep, wide and scrobiculate transversal groove basally; suture between third and fourth metasomal tergites wide, deep, scrobiculate and sinuate; fourth metasomal tergite granulate with basal longitudinal striae; fifth to seventh metasomal tergites granulate, remaining ones smooth; ovipositor long, about 1.7 times length of metasoma.
Male. Smaller than female, body length 4.2 mm; vertex dark brown, rest of head honey yellow; mesopleuron and dorsal and lateral areas of pronotum dark brown to black, remaining part of mesosoma and basal two thirds of first metasomal tergite brown; 30 flagellomeres (complete); vein m-cu antefurcal to vein 2RS; hind wing with stigma; suture between second and third metasomal tergites straight.
Holotype. IB-UNAM CNIN. Female. Mexico, Jalisco, Estación de Biología de Chamela, UNAM, camino Calandria, 19.50N, -105.03W, 45 m, 3 September 2009, sweeping net, tropical dry forest, Hans Clebsch, Alejandro Zaldívar-Riverón, collectors. DNA voucher no. ASDOR464 (CHAM-368), GenBank accession nos HQ201295, HQ200886 (IB-UNAM CNIN).
Paratype. IB-UNAM CNIN, MACN. One specimen. Male. Mexico, Jalisco, Estación de Biología de Chamela, UNAM, camino Calandria, 19.50N, -105.03W, 45 m, 20 February 2010, light trap, tropical dry forest, Alejandro Zaldívar-Riverón, collector. DNA voucher no. ASDOR514 (CHAM-463), GenBank accession no. HQ201294 (IB-UNAM CNIN).
Mexico.
The COI sequences generated in this work allowed us to associate the only two collected specimens of Ondigus cuixmalensis, one male and one female, as conspecific. This is the first known male for the genus, and is characterised by having a stigma-like enlargement on the hind wing.
The specific name refers to the CCBR, where this species was collected.
Ondigus cuixmalensis sp. n. (holotype): A habitus, lateral view B metasoma, dorsal view C mesosoma, dorsal view D hind wing, lateral view (male, paratype) E fore wing, lateral view. Ondigus bicolor Braet, Barbalho & van Achterberg: F propodeum and metasoma, dorsal view G fore wing.
We thank Hans Clebsh, Vladimir S. De Jesús-Bonilla, Andrew Polaszek and Andrés Reséndiz-Flores for their help during our first two collecting trips in Chamela; Yves Braet for sending pictures of Ondigus bicolor; Alberto Jorge García for his help taking the SEM photographs, and María cristina Mayorga Martínez for mounting part of the specimens. This work was supported by grants given by the Universidad Nacional Autónoma de México (IACOD-DGAPA 2011), Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO; grant no. HB-033), CONACyT (Proyecto Ciencia Básica no. 511; Red Temática del Código de Barras de la Vida) and by the Ministerio de Ciencia e Innovación (CGL2010-15786; Spain) to AZR; by the National Fundation grant DEB-10-20751 to SRS (Caterpillars and parasitoids in the Eastern Andes of Ecuador, CAPEA); and by two postdocoral fellowship given by the Universidad Nacional Autónoma de México (UNAM; DGAPA fellowships) to JJM and FSC. Any opinions, findings, and conclusions expressed are those of the authors and do not necessarily reflect the views of the National Science Foundation.