(C) 2010 André R. S. Garraffoni. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Current knowledge of freshwater gastrotrich fauna from Brazil is underestimated as only two studies are available. The present communication is a taxonomic account of the first-ever survey of freshwater Gastrotricha in Minas Gerais State. Samplings were carried out yielding six species of three Chaetonotidae genera: Aspidiophorus cf. pleustonicus, Ichthydium cf. chaetiferum, Chaetonotus acanthocephalus, Chaetonotus heideri, Chaetonotus cf. succinctus, Chaetonotus sp., and also an undescribed species belonging to the genus Redudasys (incertae sedis): this is the first finding of specimens of Redudasys outside of original type locality. These preliminary observations suggest that the knowledge of the biodiversity of Gastrotricha in the Minas Gerais State, as well as in the whole Brazil, will certainly increase as further investigations are undertaken, and that freshwater Macrodasyida may be more common than previously thought.
freshwater Gastrotricha, Macrodasyida, Chaetonotida, biodiversity, meiofauna
Gastrotricha are aquatic free-living microinvertebrates
(< 1 mm), with a worldwide distribution in freshwater, estuarine,
and marine benthic habitats where they are an important component of the
benthos and periphyton (
The taxon consists of nearly 750 named species grouped into two orders, Macrodasyida and Chaetonotida (but see
The biodiversity of the Gastrotricha fauna in Brazil is still underestimated (
Regarding the freshwater habitat,
Thus, the aim of the present study is to provide the
first records of the Gastrotricha fauna from the State of Minas Gerais.
This is the first of a series of surveys that will be realized as an
effort to increase the taxonomic and biogeographic knowledge of the
Brazilian Gastrotricha, with special emphasis on the State of Minas
Gerais. Furthermore, with the aim to stimulate new research on this
group in Brazil,
Samples of the upper sediment were taken from 7 distinct stations located along two small watercourses and one river near Diamantina city at an altitude of 1300 m: Soberbo (18º11'38.11"S – 43º34'13.03"W), Água Limpa (18º12'51.95"S – 43º37'01.96"W), and Preto River (18º7.50'23"S – 43º20.15'53"W). Other sampling locations were: an unnamed stream in the Itambé Peak (18°23'50"S – 43°19'44"W), at an altitude of 1680 m, and unnamed stream in Cabral Mountains (17°46'03.7"S – 44°17'09.6"W), at an altitude of 1209 m, and an unnamed stream, near Gouveia City, (18°31'48.5"S – 43°53'55.8"W; 18°32'19.2"S – 43°53'52.8"W), at an altitude of 1174 m. Gastrotrichs were extracted after repeated washing of small amounts of sediment with 2% MgCl2 aqueous solution. Living individuals were located by examining the supernatant under an Olympus SZ40 stereomicroscope at 40x magnification, and were removed by micropipette to a glass slide. Further observations and photographies were done under a Zeiss Photomicroscope equipped with differential interference contrast optics (DIC) and an Olympus CH30 microscope without DIC.
The morphological study and the identification of
gastrotrichs were performed using the terminologies and identification
keys presented in
Descriptions of putative new taxa are beyond the scope of the present study, and their definitive affiliation will be made at the end of the ongoing taxonomical surveys in forthcoming papers. However, we provide a photograph of each taxon and the measurement of the main structures, for the benefit of researchers working in the same area who might find them in the meantime.
All adult formalin–glycerin whole-mounts specimens are kept in the meiofauna collection of the senior author at the Universidade Federal dos Vales do Jequitinhonha e Mucuri.
TaxonomyOrder Chaetonotida Remane, 1925
Family Chaetonotidae Gosse, 1864
Genus Aspidiophorus Voigt, 1904
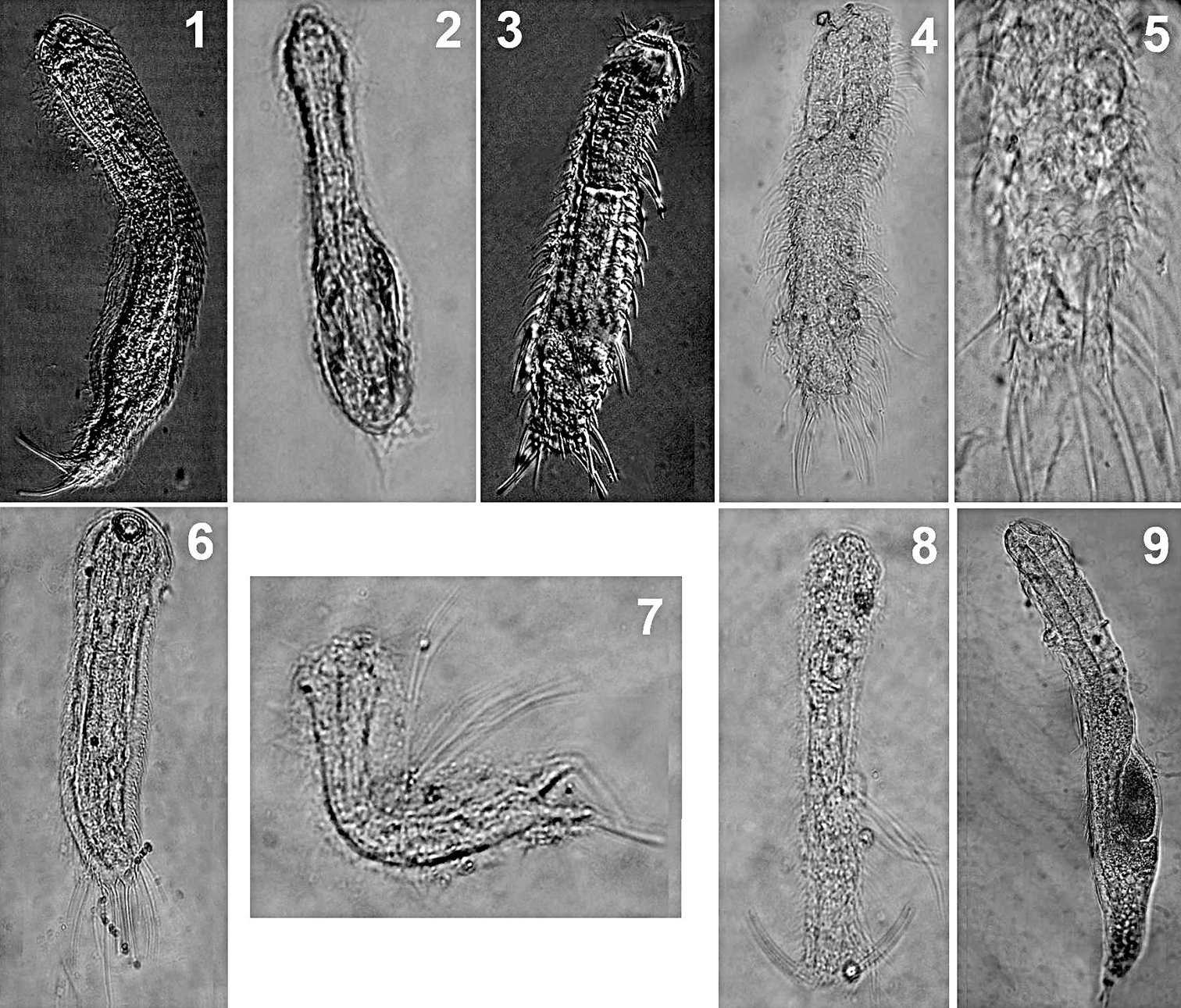
Fig. 1, Table 1
Soberbo: 2 specimens, Água limpa: 2 specimens, Preto River: 2 specimens, Gouveia: 2 specimens.
The description is based on a single adult specimen, 212.5 μm in total length. Head with oval edge and body long and wide. Body medium-sized, with head and neck weakly defined, but trunk and caudal base clearly distinct. Head with slightly five lobes and two pairs of ciliary tufts. Hypostomion weakly developed as a fine transverse furrow appearing as a thin line. Pharynx 56.25 μm in length from the posterior edge of the mouth to the pharyngo-intestinal junction, that lies at U26. Alternating columns of pedunculated, unkeeled, elongate scales along the body.
Freshwater psammic Gastrotricha from Brazil. 1 Aspidiophorus cf. pleustonicus: ventral view2 Ichthydium cf. chaetiferum 3 Chaetonotus acanthocephalus: dorsal view4 Chaetonotus heideri: dorsal view5 Chaetonotus heideri: close-up of the dorsal scales 6 Chaetonotus sp. ventral view 7 Chaetonotus cf. succinctus: lateral view 8 Chaetonotus cf. succinctus: dorsal view9 Redudasys sp.: dorso-lateral view.
Morphometrical features of Aspidiophorus cf. pleustonicus. N= number of specimens measured.
| Features | Range | N | Literature data from Brazil |
|---|---|---|---|
| Body length | 212.5–275 µm | 6 | 191–208 µm |
| Length of adhesive tube | 31.25–37.5 µm | 6 | 9.5–11.5 µm |
| Pharynx length | 56.25–93.75 µm | 6 | 22.6–27.2 µm |
| Diameter of mouth ring | 11.25–18.75 µm | 6 | 5 µm |
| Cephalion length | 25.97 µm | 6 | - |
| Cephalion width | 19.48 µm | 1 | 16 µm |
The genus Aspidiophorus counts 30 species in the world with 9 marine species and 21 freshwater (
Brazil: Diamantina, Gouveia (Minas Gerais State); São Paulo (São Paulo State).
Genus Ichthydium Ehrenberg, 1830
Fig. 2, Table 2
Água limpa: 1 specimen.
The description is based on a single adult specimen, 108.49 μm in total length. Head with five lobes and two pair of ciliary tufts, with a pair of large “ocellar” granules. Pharynx 28.30 μm in length from the posterior edge of the mouth to the pharyngo-intestinal junction, that is at U26. 8 Spines present on the ventrolateral body side.
Morphometrical features of Ichthydium cf. chaetiferum. N= number of specimens measured.
| Features | Measures | N | Literature data from Brazil |
|---|---|---|---|
| Body length | 108.49 µm | 1 | 107–117 µm |
| Length of adhesive tube | 16.98 µm | 1 | 12.5–14 µm |
| Pharynx length | 28.30 µm | 1 | 25–29 µm |
| Diameter of mouth ring | 4.01 µm | 1 | 3 µm |
| Cephalion length | 4.8 µm | 1 | 8.5–9 µm |
| Cephalion width | 9.6 µm | 1 | 11 µm |
The specimens collected in the present study resemble those described in
Brazil: Diamantina (Minas Gerais State), Juréia Ecological Reserve (São Paulo State).
Genus Chaetonotus Ehrenberg, 1830
Subgenus Primochaetus Kisielewski, 1997
Fig. 3, Table 3
Água Limpa: 2 specimens, Soberbo: 1 specimens, Preto River: 5 specimens.
The description is based on an adult specimen, 236 μm in total length. Head with three lobes and a one pair of ciliary tufts. Five peculiar cephalic scales with long spines present on the head. Two ventral plates at the sides of the hypostomion. Pharynx 65 μm in length from the posterior edge of the mouth to the pharyngo-intestinal junction that is at U27. The general long-spine distribution pattern shows two pairs of conspicuous lateral neck spines. Two pairs of long lateral spines at the furcal base.
Morphometrical features of Chaetonotus acanthocephalus. N= number of specimens measured.
| Features | Range | N | Literature data from Brazil | Literature data from Europe |
|---|---|---|---|---|
| Body length | 169–236 µm | 2 | 123–175 µm | 100–148 µm |
| Length of adhesive tube | 27.5–28 µm | 2 | 11–17 µm | 14–16 µm |
| Pharynx length | 62.5–65 µm | 2 | 34–54 µm | 37–49 µm |
| Diameter of mouth ring | 8–8.75 µm | 2 | 5 µm | 6.5–8 µm |
| Cephalion length | 20 µm | 1 | - | - |
| Length of neck scales | 7 µm | 1 | 6–7 µm | 4–7 µm |
| Length of trunk scales | 10 µm | 1 | 5.5–9.5 µm | 5.5–8 µm |
| Maximum length of the neck spines | 24–27 µm | 2 | 7.5–19.5 µm | 11–15 µm |
| Maximum length of the trunk spines | 30–35 µm | 2 | 12.5–28 µm | 16.5–22 µm |
| Length of terminal spines | 19–31.25 µm | 2 | 8–19.5 µm | - |
| Number of scales in a single longitudinal row | 17 | 2 | 17 | 16–18 |
Brazil: Diamantina (Minas Gerais State); São Carlos (São Paulo State), Juréia Reserve (São Paulo State), Corumbá (Mato Grosso do Sul State); Poland: Lake Piaseczno; Germany; Bulgaria.
Figs 4–5, Table 4
Água Limpa: 1 specimen, Soberbo: 2 specimens, Preto River: 2 specimens.
The description is based on an adult specimen 137.5 μm in total length. Head with three lobes and two pairs of ciliary tufts. Pharynx 41 μm in length from the posterior edge of the mouth to pharyngo-intestinal junction, that is at U29. Anterior scales rounded and posterior ones pentagon-like shaped. Lateral spine denticle located near to the spine end.
Morphometrical features of Chaetonotus heideri. N= number of specimens measured.
| Features | Range | N | Literature data from Brazil | Literature data from Europe |
|---|---|---|---|---|
| Body length | 137.5–137.96 µm | 2 | 188–196 µm | 106–220 µm |
| Length of adhesive tube | 25–38.8 µm | 2 | 22–25 µm | 21–32 µm |
| Pharynx length | 41 µm | 2 | 48.5–50 µm | 45–56 µm |
| Diameter of mouth ring | 7.55–10.62 µm | 2 | 10–11.5 µm | 10.5–13 µm |
| Length of trunk spines | 37.5–37.96 µm | 2 | 22–37 µm | 46–68 µm |
| Length of egg | 11 µm | 1 | - | - |
Brazil: Diamantina (Minas Gerais State), Juréia Ecological Reserve and São Carlos (São Paulo State), Benevides (Pará State); USA: Ohio; Germany; England; Italy; Poland; Romania; Russia; Czech Republic; Switzerland; France: Gironde.
Subgenus Lepidochaetus Kisielewski, 1991 [
Gouveia: 3 specimens.
The description is based on an adult specimen 236.95 μm in total length. Head with three lobes and one pair of ciliary tufts. Pharynx 64.93 μm in length from the posterior edge of the mouth to the pharyngo-intestinal junction (PhIJ), at U27. Hypostomion as a weak transverse furrow. Three pairs of lateral parafurcal spines, the two posteriormost longer than the adhesives tube. Adhesive tubes very long and thin.
Morphometrical features of Chaetonotus sp. N= number of specimens measured.
| Features | Range | N |
|---|---|---|
| Body length | 150.76–236.95 µm | 3 |
| Length of adhesive tube | 26.15–32.46 µm | 3 |
| Pharynx length | 35.38–63 µm | 3 |
| Diameter of mouth ring | 10–14 µm | 3 |
| Cephalion length | 30.96 µm | 3 |
| Length of the egg | 100 µm | 3 |
| Length of rearmost lateral spines | 38.46–71.42 µm | 3 |
The genus Lepidochaetus was originally described by
Brazil: Diamantina, Gouveia (Minas Gerais State).
Subgenus Zonochaeta Remane, 1927
Figs 7–8, Table 6
Cabral Mountains:1 specimen; Gouveia:1 specimen; Preto River: 1 specimen.
The description is based on an adult specimen 201.38 μm in total length. Head with five lobes and two pairs of ciliary tufts. Pharynx 55.48 μm in length from the posterior edge of the mouth to the pharyngo-intestinal junction, lying at U27. On the middle trunk region, a transverse band of five long dorsal spines, all terminally bifurcated, and of equal length (77.6 μm) and thickness. Paired spines at the furca base, not extending beyond the adhesive tube end.
Morphometrical features of Chaetonotus cf. succinctus. N= number of specimens measured.
| Features | Range | N |
|---|---|---|
| Body length | 165.27–201.38 µm | 2 |
| Length of adhesive tube | 41.6–43.05 µm | 2 |
| Pharynx length | 54.1–55.55 µm | 2 |
| Diameter of mouth ring | 4.13–6.89 µm | 2 |
| Length of trunk “band” spines | 71.42–72.6 µm | 2 |
Brazil: Diamantina, Cabral Mountains (Minas Gerais State), Belém (Pará State); Poland; Romania; England; Italy; Germany; South Korea.
Remarks. Within the subgenus Zonochaeta, four species (Chaetonotus bisacer, Chaetonotus cestacanthus, Chaetonotus dracunculus, Chaetonotus succinctus)are characterized by the presence of a series of long dorsal spines with concave apices (
Order Macrodasyida Remane, 1925
Genus Redudasys Kisielewski, 1987
Fig. 9, Table 7
Água limpa: 8 specimens; Cabral Montains: 4 specimens. Video sequence (format .mov) is available at http://www.megaupload.com/?d=1F7NJ1XI
The description is based on an adult specimen 461.54 μm in total length. Cephalic cilia occur in one transverse dorsal row as well as in irregularly distributed tufts located at the anterolateral head margin. The mouth opening has a diameter of 10.1 µm. Pharynx 153.85 μm in length from the posterior edge of the mouth to the junction with the intestine. Two elongated caudal lobes, 25.64 µm long and 4.76 µm wide. Median caudal cone absent. Only anterior and caudal adhesive tubes are typically present. One anterior tube per side located laterally in the anterior part of the body. Seven tacticle bristles per side along the lateral body and one per side on the caudal end. Two pairs of caudal adhesive tubes. The inner tube (7.14 µm long) is usually 2/5 shorter than the external one (11.9 µm long).
Morphometrical features of Redudasys sp. N= number of specimens measured.
| Features | Range | N |
|---|---|---|
| Body length | 280–461.54 µm | 14 |
| Pharynx length | 87.5–153.85 µm | 14 |
| Diameter of mouth ring | 10.1–11.0 µm | 14 |
| Length of external caudal tube | 11.9–17.5 µm | 14 |
| Length of inner caudal tube | 7.14–12.5 µm | 14 |
Brazil: Diamantina, Cabral Mountains (Minas Gerais State).
The specimens found in Minas Gerais State areundoubtedly members of the genus Redudays, an incertae sedis Macrodasyida taxon recorded from a freshwater environment (
The findings presented here allow us to draw some remarks
on the Gastrotricha of the State of Minas Gerais. It is worthwhile
noting that a poor sampling effort has allowed us to obtain very
interesting faunistic data and to identify seven distinct species,
which suggest a high biodiversity of Gastrotricha in this State. Up to
now, 22 species of Gastrotricha Chaetonotida had been described from
Brazilian rivers with slow water current and quiet habitats (
The most striking result of this study was the report of Redudasys specimens from different streams in Minas Gerais State. Thus, the discovery of Redudasys specimens outside of the original record is of great biogeographic interest, as the adaptation of this macrodasyidan taxon to the freshwater habitats could have been followed by considerable radiation, mainly in the neotropical region.
Albeit a high diversity of endemic gastrotrich chaetonotidans has been recorded in the Brazilian fauna (e.g. Undula, Arenotus -
Based on preview studies (
We would like to express our gratitude to Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG) for financial support (CRA-APQ-00292-09). To Cecília Amaral, at Universidade Estadual de Campinas, and Gustavo Henrique Bahia de Oliveria, at Universidade Federal dos Vales do Jequitinhonha e Mucuri, for the use of laboratory space and equipment. To Sílvio Nihei, at Universidade de São Paulo, for helping us with literature, and Fabiane Costa, Rafael Pessoa and Leonardo Lessa, at Universidade Federal dos Vales do Jequitinhonha e Mucuri, for collecting samples in Cabral Moutains, Gouveia city, and Preto River. Two anonymous referees are also acknowledged for offering suggestions that greatly improved the paper.