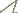(C) 2010 Michael E Soleglad. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Multiple populations of Hadrurus pinteri from Baja California Sur, Mexico have been examined. It is demonstrated that the southern populations of this species have a larger number of accessory trichobothria (neobothriotaxy) than the northern populations, numbers exceeding the maximum currently recorded for the genus. Examination of carapace and chela coloration and its patterns show a close affinity between Hadrurus pinteri and the dark phase of Hadrurus concolorous. A new morphometric ratio of the carapace is defined that distinguishes Hadrurus from Hoffmannihadrurus, further supporting the monophyly of the latter genus.
Caraboctonidae, Hadrurus, Hoffmannihadrurus, neobothriotaxy, Baja California Sur, Mexico
ABDSP, Anza-Borrego Desert State Park, San Diego and Riverside Counties, California, USA.
DepositoriesCAS, California Academy of Sciences, San Francisco, California, USA; MES, Personal collection of Michael E. Soleglad, Borrego Springs, California, USA; VF, Personal collection of Victor Fet, Huntington, West Virginia, USA.
MaterialThe following material was examined for analysis
and/or illustrations provided in this paper. It must also be noted that
many observations and statistics provided in this paper are augmented,
in part, from other data previously collected and discussed in
Hadrurus arizonensis arizonensis Ewing, 1928: Carrizo Badlands, Vallecito Creek, ABDSP, California, USA ♂ (MES), 41.2 mi. E San Luis, Sonora, Mexico, ♂ (MES); Hadrurus arizonensis austrinus Williams, 1970: Oakies Landing, Baja California, Mexico, ♂ ♀ (MES); Hadrurus concolorous Stahnke, 1969: 8 km S Tambobiche, Baja California Sur, Mexico, ♀ (CAS), 5 mi SW San Miguel de Comondú, Baja California Sur, Mexico, ♂ (CAS), Santa Rosalia, Baja California Sur, Mexico, ♂ ♀ (MES), Las Bombas, Baja California Sur, Mexico, ♂ (MES); Hadrurus hirsutus (Wood, 1863): Cabo San Lucas, Baja California Sur, Mexico, 2 ♂ (MES); Hadrurus obscurus Williams, 1970: Indian Gorge, ABDSP, California, USA, 2 ♀ (MES); Hadrurus pinteri Stahnke, 1969: Oakies Landing, Baja California, Mexico, 3 ♀ 4 ♂ 3 J (MES), Arroyo Calamajué, Baja California, Mexico, 2 ♀ (MES), Punta Trinidad, Baja California Sur, Mexico, 2 ♂ (CAS), San Ignacio, Baja California Sur, Mexico, 2 ♀ (CAS), Bahia Concepción, Baja California Sur, Mexico, ♀ (CAS), 5 mi SW San Miguel de Comondú, Baja California Sur, Mexico, ♂ (CAS), 5–10 mi SW San Miguel de Comondú, Baja California Sur, Mexico, 5 ♂ 4 ♀ (CAS), 5.6 mi SW Loreto, Baja California Sur, Mexico, ♀ (CAS), 7.2 mi SW Loreto, Baja California Sur, Mexico, ♂ (CAS), 8.3 mi SW Loreto, Baja California Sur, Mexico, ♀ (CAS), 14.7 mi SW Loreto, Baja California Sur, Mexico, ♀ (CAS), Isla Danzante (NW side), Baja California Sur, Mexico, 5 ♀ (CAS), Agua Verde Bay, Baja California Sur, Mexico, 2 ♀ (CAS); Hadrurus spadix Stahnke, 1940: Hawthorne, Mineral Co., Nevada, USA (VF).
Genus Hoffmannihadrurus Fet & Soleglad, 2004:Hoffmannihadrurus aztecus (Pocock, 1902): Tehuacán, Puebla, Mexico, 3 ♂ 1 ♀ (MES), Tomellín, Oaxaca, Mexico, ♂ (MES); Hoffmannihadrurus gertschi (Soleglad, 1976), Azcala, Guerrero, Mexico, paratype ♀ (CAS), Iguala, Guererro, Mexico, ♀ (MES).
Neobothriotaxy in Hadrurus pinteriNeobothriotaxy in Hadrurus
was first reported by Gertsch and Soleglad (1972: figs 96–107)
when the first trichobothrial pattern for this genus was illustrated for
Hadrurus arizonensis. The unusual and complicated pattern exhibited in this genus was later investigated by
In this study, we have analyzed a collection of southern populations of Hadrurus pinteri
from the California Academy of Sciences. We tabulated the number of
chelal accessory trichobothria of the ventral, internal, and external
surfaces and compared it to the data of the northern populations. Figure 1 shows the geographic localities of Hadrurus pinteri
from which the accessory trichobothria data are derived. The northern
samples, totaling 13 specimens, are from the northern half of their
known range, the majority of specimens from Oakies Landing. The most
southern of these specimens is from the Arroyo Calamajué. The southern
samples, comprised of 28 specimens, span the entire southern range
originally outlined by
Reported distribution of Hadrurus pinteri. The two dotted line areas partition Hadrurus pinteri
distribution into the northern and southern portions of Baja California
Peninsula, Mexico. These areas, in general, denote the original
distribution reported by
Table 1 shows the statistical breakdown of accessory trichobothria for the entire “hirsutus” group, involving more than 260 samples. Of importance to this discussion is the breakdown of Hadrurus pinteri into its northern and southern populations. As predicted by
Statistics showing neobothriotaxy of the pedipalp chela of the Hadrurus “hirsutus” group. In particular, two disjunct populations of Hadrurus pinteri are contrasted showing that for two of the three trichobothrial series, the southern population (i.e., Baja California Sur) has the largest number of accessory trichobothria, roughly a 7.5 % increase. MVD = mean value difference; p-value = Anova output. Statistical data group: minimum–maximum (mean) (±SDEV) [N] {standard error range} (coefficient of variability) (SDEV/mean). * includes orthobothriotaxic trichobothria V1–V4. Many of the statistics are from previous studies, as well as new material examined in this project. See Soleglad (1976), Soleglad and Fet (2004), Fet et al. (2004), and Fet and Soleglad (2008).
| Chela Neobothriotaxy Statistics for Hadrurus “hirsutus” Group | ||
|---|---|---|
|
| ||
| Ventral* | 22–26 (24.292) (±1.429) [024] {22.863–25.721} (0.059) | |
| Internal | 5–6 ( 5.692) (±0.471) [026] { 5.222–6.163} (0.083) | |
| External | 3–4 ( 3.500) (±0.511) [024] { 2.989–4.011} (0.146) | |
|
| ||
| Ventral* | 23–32 (26.245) (±1.870) [053] {24.375–28.115} (0.071) | MVD/p-value: Sur > 8.0%/2.06E-05 |
| Internal | 5–7 ( 5.611) (±0.564) [054] { 5.048–6.175} (0.100) | MVD/p-value: Sur < 1.4%/ 0.527192 |
| External | 3–5 ( 3.759) (±0.612) [054] { 3.147–4.372} (0.163) | MVD/p-value: Sur > 7.4%/ 0.074043 |
|
| ||
| Ventral* | 22–32 (25.636) (±1.960) [077] {23.677–27.596} (0.076) | |
| Internal | 5–7 ( 5.637) (±0.534) [080] { 5.104–6.171} (0.095) | |
| External | 3–5 ( 3.679) (±0.592) [078] { 3.087–4.272} (0.161) | |
|
| ||
| Ventral* | 15–20 (17.804) (±1.170) [158] {16.634–18.974} (0.066) | |
| Internal | 3–5 ( 4.154) (±0.493) [162] { 3.661–4.647} (0.119) | |
| External | 1–2 ( 1.226) (±0.420) [159] { 0.807–1.646} (0.342) | |
|
| ||
| Ventral* | 14–16 (15.682) (±0.568) [022] {15.114–16.250} (0.036) | |
| Internal | 4–5 ( 4.048) (±0.218) [021] { 3.829–4.266} (0.054) | |
| External | 1–2 ( 1.048) (±0.218) [021] { 0.829–1.266} (0.208) | |
Of particular interest, we found that the southern populations of Hadrurus pinteri exhibited the largest number of accessory trichobothria found in the three individual chelal surfaces for the entire genus Hadrurus (as well as for its sister genus Hoffmannihadrurus). Previously, based on data presented in
Hadrurus pinteri, chelal neobothriotaxy showing examples of patterns with the largest number of accessory trichobothria (closed circles). 2 External view of left chela (reversed), juvenile male, Punta Trinidad, Baja California Sur, Mexico, showing five external accessory (Ea) trichobothria, including the diagnostic Ea on the base of the fixed finger (db, dst, and dt are not shown) 3 Internal view of left chela (reversed), adult female, Loreto, Baja California Sur, Mexico, showing nine internal trichobothria, including ib–it and seven accessory trichobothria (ia). Note that the accessory trichobothria reduce in size somewhat as they occur basally 4 Ventral view of right chela, adult female, Bahia Concepción, Baja California Sur, Mexico, showing 32 ventral trichobothria, including V1–V4, and Et1 (note that V1–V4 are not distinguished from accessory trichobothria).
While examining the southern populations of Hadrurus pinteri, we discovered that two of the specimens were in fact not Hadrurus pinteri, but Hadrurus concolorous. Based on coloration and patterns these two specimens certainly looked like Hadrurus pinteri, only after detailed trichobothrial analysis could we isolate the two specimens from Hadrurus pinteri. One specimen, from Tambobiche, had a somewhat small number of accessory trichobothria, only 14–15 ventral and three internal. The other specimen, from San Miguel de Comondú, exhibited 18–19 ventral, five internal, and one external accessory trichobothria. In both specimens, the diagnostic accessory trichobothrium on the fixed finger was absent. Of special interest, the specimen from San Miguel de Comondú was contained in a vial with a large Hadrurus pinteri male, thus they were collected together.
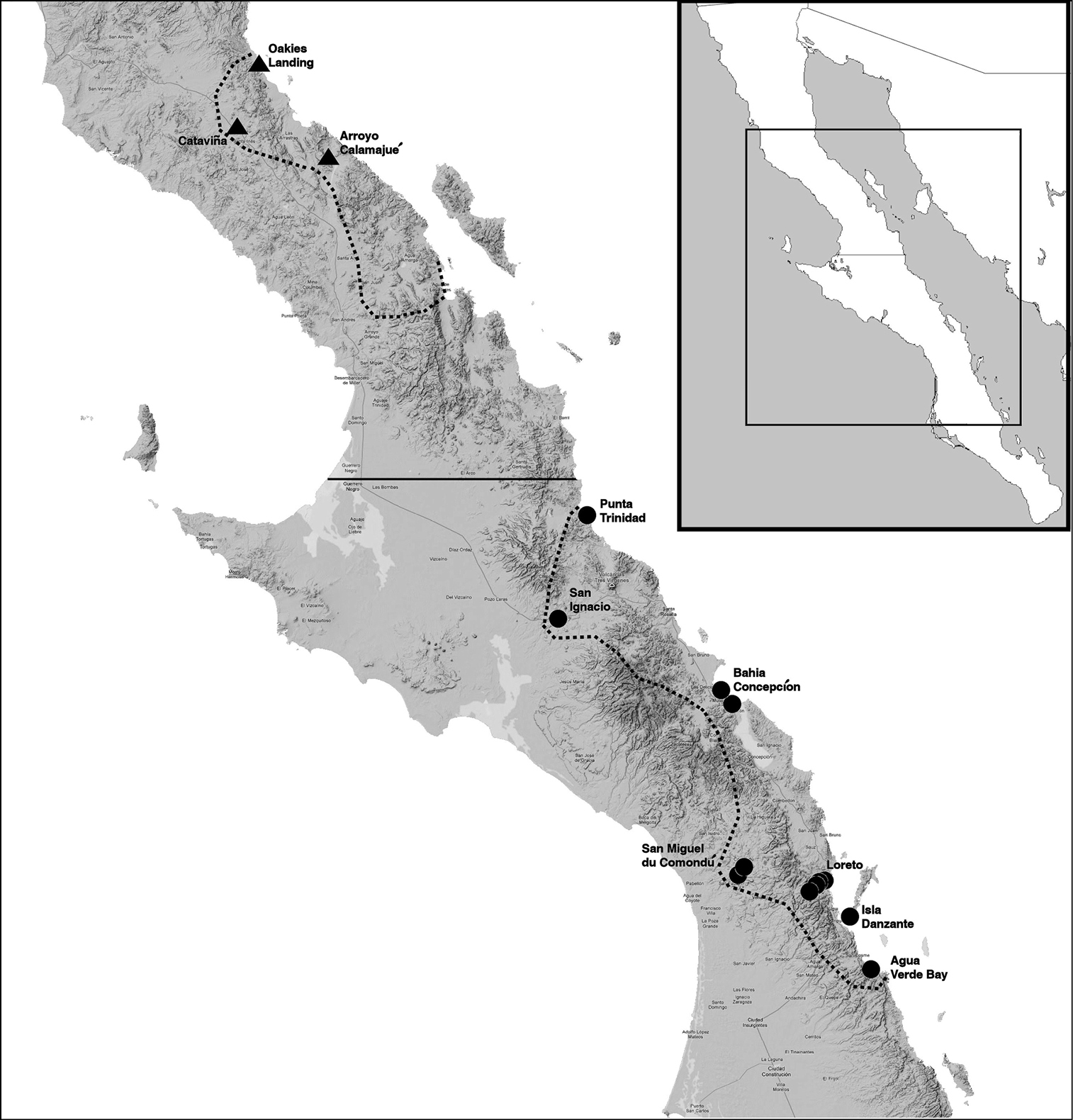
Figures 5–9 show the carapaces of Hadrurus pinteri, the two Hadrurus concolorous misidentified for Hadrurus pinteri, and two additional color phases of Hadrurus concolorous. The carapaces of Hadrurus pinteri and the Hadrurus concolorous from Tambobiche are indistinguishable, both uniformly dark in color. The carapace of the specimen from San Miguel de Comondú is lighter in color, more close to the reddish specimen from Santa Rosalia (Fig. 8). The specimen from the sand dune area in Las Bombas (Fig. 9) is typical of Hadrurus concolorous, as indicated by its name “concolorous”.
The chela of Hadrurus pinteri (Fig. 13) and the Hadrurus concolorous from San Miguel de Comondú (Fig. 14) are indistinguishable. Again, the chela from the Las Bombas specimen (Fig. 15) is typical of the “concolorous” phase of Hadrurus concolorous.
Figures 11–12 show the carapacial coloration and patterns of Hoffmannihadrurus gertschi and Hoffmannihadrurus aztecus. Although the carapace of Hoffmannihadrurus gertschi
is considerably darker, its interocular area is lighter in color,
exhibiting similar light/dark patterns as seen in its sister species Hoffmannihadrurus aztecus. It is clear that the carapace of Hoffmannihadrurus gertschi is not patterned as in Hadrurus pinteri (Fig. 5). Similarly the chela of Hoffmannihadrurus gertschi (Fig. 16) is darker than that seen in Hadrurus pinteri (Fig. 13).
Carapaces and chelae of Hadrurus and Hoffmannihadrurus showing the variability in coloration and its patterns. Of particular interest is the range of variability in Hadrurus concolorous (Figs 6–9, 14–15) exhibiting dark coloration patterns essentially identical to Hadrurus pinteri (Figs 5, 13) to pale yellow with little or no patterns. Also of interest is the darken posterior half of the carapace in Hoffmannihadrurus gertschi, matching the same area that is also darkened in Hoffmannihadrurus aztecus 5, 13 Hadrurus pinteri, male, San Miguel de Comondú, Baja California Sur, Mexico 6 Hadrurus concolorous, female, Tambobiche, Baja California Sur, Mexico 7, 14 Hadrurus concolorous, male, San Miguel de Comondú, Baja California Sur, Mexico (same locality as Hadrurus pinteri in Fig. 5) 8 Hadrurus concolorous, male, Santa Rosalia, Baja California Sur, Mexico 9, 15 Hadrurus concolorous, male, Las Bombas, Baja California Sur, Mexico 10 Hadrurus hirsutus, male, Cabo San Lucas, Baja California Sur, Mexico 11, 16 Hoffmannihadrurus gertschi, female paratype, Azcala, Guerrero, Mexico 12 Hoffmannihadrurus aztecus, female, Tehuacán, Puebla, Mexico.
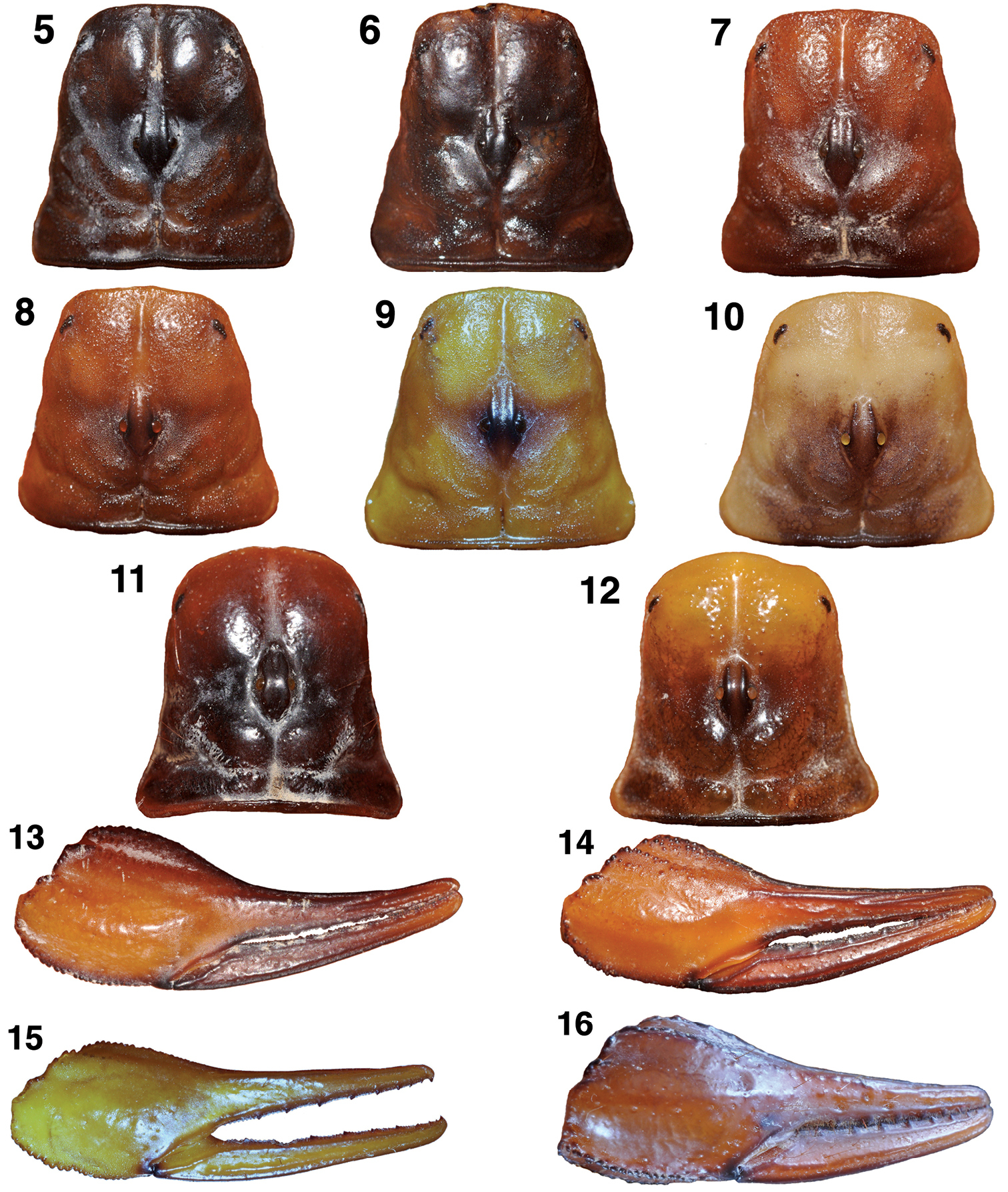
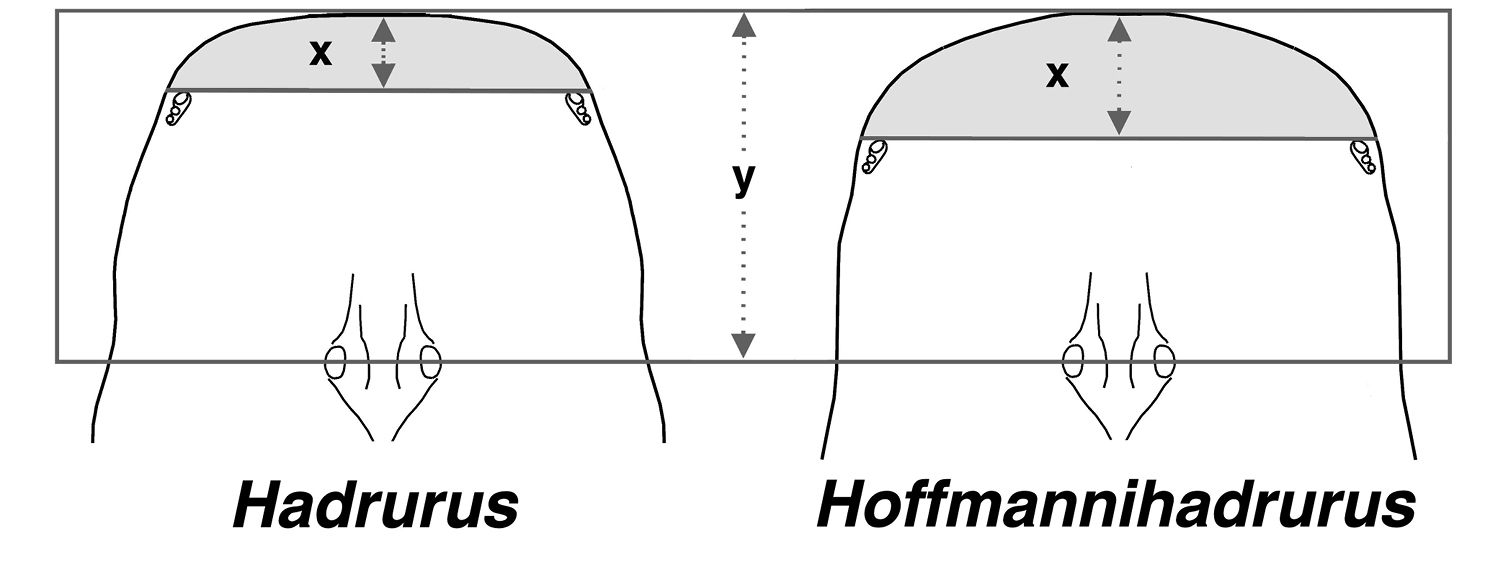
While studying the carapacial coloration and its patterns in Hadrurus and Hoffmannihadrurus we observed that the convexed anterior edge exhibited in both genera was considerably more exaggerated in Hoffmannihadrurus. This is quite visible in the photographs presented in Figs 5–12 as well as in
Method of measurement of carapace for genera Hadrurus and Hoffmannihadrurus for determining morphometric ratio anterior_edge_ length (x) / median_tubercle_position (y). Shaded area indicates anterior_edge_length. Diagrammatic drawings based on Hadrurus pinteri and Hoffmannihadrurus gertschi.
Figure 17 illustrates exactly how these two measurements are taken and Table 2 presents the results involving 30 samples spanning all species of Hadrurus (20 samples), and Hoffmannihadrurus (ten samples). The sampling included four Hoffmannihadrurus gertschi, six Hoffmannihadrurus aztecus, and ten samples each from the Hadrurus “arizonensis” group (i.e., both Hadrurus arizonensis subspecies, Hadrurus obscurus, and Hadrurus spadix), and the “hirsutus” group (i.e., Hadrurus pinteri, Hadrurus concolorous, and Hadrurus hirsutus). The mean value differences of this morphometric ratio between these genera is 47.7%, implying that in Hoffmannihadrurus, the anterior edge of the carapace is roughly 50 % longer than in Hadrurus. [note that the carapace of Hadrurus concolorous from Tambobiche (Fig. 6) was not included in the morphometric sampling due to its obvious damaged anterior edge.] Other relevant statistical indicators are no overlap of the absolute range, over 200 % separation of the standard error range, and a very small anova p-value of 6.12E-16.
Statistics showing differences in the length of the carapace anterior edge in subfamily Hadrurinae based on the following morphometric ratio: anterior_edge_length / median_tubercle_position. See Fig. 17 for methods of measurement. Data shows that the anterior edge of Hoffmannihadrurus is approximately 48 % longer than in Hadrurus. Large standard error range separation and a very small p-value from variance analysis further support the significant statistical difference between the two genera. Statistical data group: minimum–maximum (mean) (±SDEV) [N] {standard error range} (coefficent of variability). * Mean value difference, standard error range separation, and analysis of variance. Statistical data derived from specimens examined and the following references: Williams (1970), Stahnke (1971), Soleglad (1976), and Fet et al. (2004).
| Carapace Anterior Edge Ratio for Subfamily Hadrurinae | |
|---|---|
| Hoffmannihadrurus gertschi | 0.351–0.383 (0.3663) [4] |
| Hoffmannihadrurus aztecus | 0.327–0.368 (0.3461) [6] |
| 0.327–0.383 (0.3542) (±0.0166) [10] {0.338–0.371} (0.047) | |
| Hadrurus pinteri | 0.219–0.257 (0.2320) [4] |
| Hadrurus concolorous | 0.219–0.246 (0.2331) [4] |
| Hadrurus hirsutus | 0.220–0.250 (0.2349) [2] |
| Hadrurus arizonensis | 0.233–0.267 (0.2553) [4] |
| Hadrurus obscurus | 0.206–0.253 (0.2322) [4] |
| Hadrurus spadix | 0.256–0.260 (0.2584) [2] |
| 0.206–0.267 (0.2399) (±0.0185) [20] {0.218–0.262} (0.092) | |
Statistical comparisons between genera:
MVD * = 47.7
SERS * = 238.8
ANOVA* p–value = 6.12E-16
The new character described above showing differences in the anterior edge length between Hadrurus and Hoffmannihadrurus represents the fifth new character supporting monophyly of Hoffmannihadrurus (i.e., four were previously identified by
We thank Anthea Carmichael, Willis J. Gertsch, Matthew R. Graham, Charles Griswold, František Kovařík, Gary A. Polis, and Darrell Ubick for the loans and/or gifts of specimens. Finally, we extend our gratitude to two anonymous reviewers of this paper.