(C) 2011 Lena Menzel. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Mesocletodes Sars, 1909a encompasses 37 species to date. Initial evidence on intraspecific variability and sexual dimorphism has been verified for 77 specimens of Mesocletodes elmari sp. n. from various deep-sea regions, and ontogenetic development has been traced for the first time. Apomorphies are a strong spinule-like pinna on the mx seta that is fused to the basis, P2–P4 exp3 proximal outer seta lost, P1–P4 enp2 extremely elongated, furcal rami elongated, female body of prickly appearance, female P2–P4 enp2 proximal inner seta lost. Intraspecific variability involves spinulation, ornamentation and size of the body and setation and spinulation of pereiopods. Sexually dimorphic modifications of adult females include prickly appearance of the body, P1 enp exceeds exp in length, P1 coxa externally broadened, seta of basis arising from prominent protrusion, hyaline frills of body somites ornate. Sexual dimorphism in adult males is expressed in smaller body size, haplocer A1, 2 inner setae on P2–P4 enp2 and on P5 exp, P5 basendopodal lobe with 2 setae. Some modifications allow sexing of copepodid stages. The female A1 is fully developed in CV, the male A1 undergoes extensive modifications at the last molt. P1–P4 are fully developed in CV. Mesocletodes faroerensis and Mesocletodes thielei lack apomorphies of Mesocletodes and are excluded.
ANDEEP, CROZEX, DIVA, Great Meteor Bank, meiofauna, Mesocletodes elmari sp. n., NODINAUT, ontogeny, Porcupine , Abyssal Plain
Expeditions to the Southeast Atlantic (DIVA-1 [
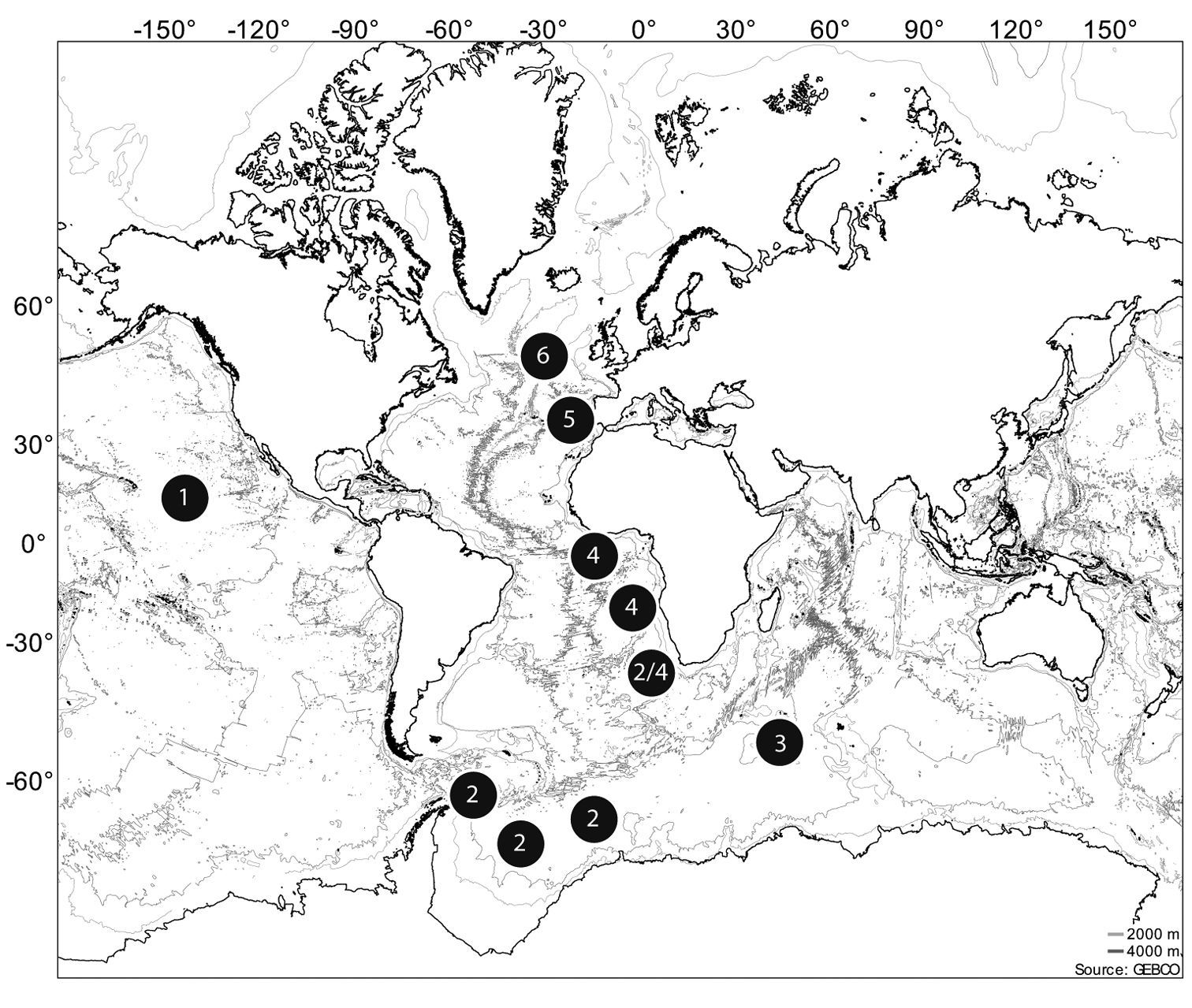
Positions of the sampled stations containing the species studied. 1 NODINAUT 2 ANDEEP 3 CROZEX 4 DIVA 5 GMB 6 PAP.
Mesocletodes nowadays comprises 36 species (
The sex ratio of harpacticoid copepods in the deep sea is strongly biased towards females (e.g.
Most of the species descriptions of Mesocletodes
are based on few adult specimens (29 descriptions contain one to five
type specimens, three descriptions are based on six to ten specimens,
four descriptions are based on 11 to 16 specimens). Thus, neither
intraspecific variability nor the process of ontogenetic development is
reported for any species of Mesocletodes. Expeditions during the DIVA and ANDEEP campaigns yielded 54 out of 66 adults of Mesocletodes elmari
sp. n. (more than 80%). The comparatively high frequency of specimens
is probably explicable by the greater sampling effort in contrast to the
CROZEX, NODINAUT, OASIS expeditions and sampling at the PAP as well
as during previous campaigns. Repeated multicorer sampling of the same
station (
The aim of this publication is to convey an initial impression of the extent of sexually dimorphic modifications, ontogeny and intraspecific variability for the genus Mesocletodes, using Mesocletodes elmari sp. n. as an example.
Material and methodsSediment samples were taken with a multicorer (
Specimens of Mesocletodes elmari sp. n. discovered, containing information on gender, number of eggs, ontogenetic stage, body length and remarks on intraspecific variability. f = female; m = male.
| Project | Expedition | Station | gender | number of eggs | ontogenetic stage | body length [mm] | remarks on intraspecific variability | |
|---|---|---|---|---|---|---|---|---|
| ANDEEP I | PS61 | 43/4-6 | f | adult | 0.70 | |||
| ANDEEP I | PS61 | 46/4-1 | f | ca. 15 | adult | 0.82 | ||
| ANDEEP I | PS61 | 46/4-5 | f | adult | 0.63 | P2 enp2 with outer seta | ||
| ANDEEP I | PS61 | 46/4-8 | f | adult | 0.70 | hyaline frill not ornate | ||
| ANDEEP I | PS61 | 46/6-5 | m | CV | 0.50 | |||
| ANDEEP I | PS61 | 46/6-3 | f | adult | 0.80 | P2-P4 enp2 with outer seta | ||
| ANDEEP I | PS61 | 46/6-3 | f | adult | 0.87 | |||
| ANDEEP I | PS61 | 129/5-4 | f | 4 | adult | 0.78 | ||
| ANDEEP II | PS61 | 131/11-A | f | 4? 20? | adult | 0.62 | ||
| ANDEEP II | PS61 | 138/11-4 | f | adult | 0.71 | |||
| Paratype 6 | ANDEEP II | PS61 | 138/11-4 | CIII | 0.47 | |||
| CROZEX | D300 | 15773/31 | f | 11 | adult | 0.80 | ||
| CROZEX | D300 | 15775/32 | f | adult | 0.92 | FR: setular tuft near seta VII | ||
| CROZEX | D300 | 15775/36 | m | adult | 0.43 | |||
| DIVA-1 | M48/1 | 325/6-2 | f | adult | ? | FR lost | ||
| DIVA-1 | M48/1 | 342/2-1 | f | adult | 0.71 | FR: setular tuft near the base | ||
| DIVA-1 | M48/1 | 346/1-3 | m | adult | 0.68 | |||
| DIVA-1 | M48/1 | 346/1-7 | m | adult | 0.54 | |||
| DIVA-1 | M48/1 | 346/2-3 | f | adult | 0.83 | |||
| DIVA-1 | M48/1 | 346/2-3 | f | 12 | adult | 0.87 | ||
| DIVA-1 | M48/1 | 346/2-8 | f | 17 | adult | 0.86 | ||
| DIVA-1 | M48/1 | 346/2-9 | f | 3? | adult | 0.74 | ||
| DIVA-1 | M48/1 | 346/2-11 | f | adult | 0.82 | FR: setular tuft near seta VII, P1-P4 enp shorter | ||
| DIVA-1 | M48/1 | 346/3-9 | f | adult | 0.91 | |||
| DIVA-1 | M48/1 | 346/4-10 | f | adult | 0.85 | FR: setular tuft near seta VII, P1-P4 enp shorter | ||
| DIVA-1 | M48/1 | 346/5-3 | f | adult | 0.80 | hyaline frill not ornate | ||
| DIVA-1 | M48/1 | 346/5-9 | f | adult | 0.82 | |||
| DIVA-1 | M48/1 | 346/5-10 | f | 10 | adult | 0.66 | ||
| DIVA-1 | M48/1 | 346/6-2 | f | adult | 0.82 | |||
| DIVA-1 | M48/1 | 346/7-7 | f | adult | 0.85 | |||
| DIVA-1 | M48/1 | 346/7-8 | f | adult | 0.79 | |||
| DIVA-1 | M48/1 | 346/7-10 | f | adult | 0.78 | |||
| DIVA-1 | M48/1 | 346/7-10 | f | CV | 0.75 | |||
| Paratype 4 | DIVA-1 | M48/1 | 346/7-10 | m | CV | 0.59 | ||
| DIVA-1 | M48/1 | 346/8-3 | f | adult | 0.86 | |||
| Paratype 2 | DIVA-2 | M63/2 | CAPE/35-7 | f | adult | 0.90 | FR: setular tuft near seta VII | |
| DIVA-2 | M63/2 | CAPE/36-10 | f | 3 | adult | 0.57 | ||
| DIVA-2 | M63/2 | CAPE/36-11 | f | adult | 0.66 | |||
| DIVA-2 | M63/2 | CAPE/36-11 | m | adult | 0.40 | |||
| DIVA-2 | M63/2 | CAPE/36-11 | m | CV | 0.40 | FR: setular tuft near the base, P2-P4 enp2 with only 1 inner seta | ||
| DIVA-2 | M63/2 | GUINEA E/56-5 | m | adult | 0.53 | |||
| DIVA-2 | M63/2 | GUINEA E/57-1 | f | 2? | adult | 0.70 | FR: setular tuft near the base | |
| DIVA-2 | M63/2 | GUINEA E/57-8 | f | adult | 0.67 | |||
| DIVA-2 | M63/2 | GUINEA E/58-10 | f | 7 | adult | 0.75 | ||
| DIVA-2 | M63/2 | GUINEA E/58-12 | f | 5 | adult | 0.63 | ||
| DIVA-2 | M63/2 | GUINEA E/59-10 | f | adult | 0.64 | |||
| DIVA-2 | M63/2 | GUINEA E/59-12 | m | adult | 0.51 | |||
| DIVA-2 | M63/2 | GUINEA E/61-4 | m | adult | 0.50 | |||
| DIVA-2 | M63/2 | GUINEA E/62-6 | f | adult | 0.81 | |||
| DIVA-2 | M63/2 | GUINEA E/62-6 | m | adult | 0.53 | |||
| DIVA-2 | M63/2 | GUINEA W A/74-4 | f | adult | 0.78 | hyaline frill not ornate, FR longer | ||
| Holotype | DIVA-2 | M63/2 | GUINEA W A/75-7 | f | adult | 0.78 | ||
| DIVA-2 | M63/2 | GUINEA W A/76-6 | f | 7 | adult | 0.80 | ||
| DIVA-2 | M63/2 | GUINEA W A/78-6 | f | adult | ? | FR lost | ||
| Paratype 1 | DIVA-2 | M63/2 | GUINEA W A/78-7 | m | adult | 0.42 | ||
| DIVA-2 | M63/2 | GUINEA W A/79-4 | f | adult | ? | Abdomen lost | ||
| Paratype 3 | DIVA-2 | M63/2 | GUINEA W A/79-4 | f | CV | 0.55 | P1 0-0 0, 1, 1, P2 0-0 0, 2, 0 | |
| DIVA-2 | M63/2 | GUINEA W B/95-10 | m | adult | 0.43 | |||
| DIVA-2 | M63/2 | GUINEA W B/96-8 | f | adult | 0.80 | FR: setular tuft near the base, hyaline frill not ornate, FR longer | ||
| DIVA-2 | M63/2 | GUINEA W B/97-6 | f | adult | 0.87 | |||
| DIVA-2 | M63/2 | GUINEA W B/97-7 | m | CIV | 0.52 | FR: setular tuft near the base, P1–P4 enp2 with outer seta | ||
| DIVA-2 | M63/2 | GUINEA W B/97-7 | m | CIV | 0.49 | FR: setular tuft near the base, P1–P4 enp2 with outer seta | ||
| DIVA-2 | M63/2 | GUINEA W B/97-7 | m | CIV | 0.64 | FR: setular tuft near the base, P1–P4 enp2 with outer seta | ||
| DIVA-2 | M63/2 | GUINEA W B/97-7 | m | CIV | 0.47 | FR: setular tuft near the base, P1–P4 enp2 with outer seta | ||
| Paratype 5 | DIVA-2 | M63/2 | GUINEA W B/97-7 | m | CIV | 0.43 | FR: setular tuft near the base, P1–P4 enp2 with outer seta | |
| DIVA-2 | M63/2 | GUINEA W B/99-10 | f | 10 | adult | 0.71 | FR: setular tuft near the base, P2-P4 enp2 with outer seta, denticulation of hyaline frill more dense | |
| DIVA-2 | M63/2 | GUINEA W B/100-6 | f | adult | 0.87 | |||
| DIVA-2 | M63/2 | GUINEA W B/100-7 | f | 5 | adult | 0.90 | ||
| GMB | M42/3 | 505 | f | adult | 0.61 | |||
| GMB | M42/3 | 505 | f | adult | 0.61 | |||
| GMB | M42/3 | 566 | f | adult | 0.76 | FR: setular tuft near the base | ||
| NODINAUT | 1599/7-2 | f | 4 | adult | 0.65 | |||
| NODINAUT | 1599/7-3 | f | adult | 0.66 | ||||
| NODINAUT | 1602/10-7 | f | 5 | adult | 0.69 | |||
| NODINAUT | 1602/10-8 | f | 5 | adult | 0.67 | |||
| NODINAUT | 1603/11-1 | f | adult | 1.06 | ||||
| PAP | Mar 1997/13077-12 | f | 1? | adult | 0.63 | FR: setular tuft near the base |
Altogether 77 specimens (56 adult females, 10 adult males, 2 CV females, 3 CV males, 5 CIV males and 1 CIII) were found. The type material of Mesocletodes elmari sp. n. consists of 7 specimens (2 females plus 1 each of the other discovered stages). The type material was deposited in the collection of the Senckenberg Forschungsinstitut und Naturmuseum Frankfurt (Germany). The remaining 70 specimens are mounted on slides and kept in the collection of the DZMB in Wilhelmshaven (Germany).
The material was mounted on separate slides using glycerol as the embedding medium. Identification at the species level and drawings were carried out using a Leica microscope DM2500 equipped with a camera lucida and interference contrast with a maximum magnification of 1600x.
The CLSM photograph of a Congo-red stained female was
taken with a Leica TCS SP5 mounted in a Leica DM5000. Preparations and
settings were made according to
Abbreviations used in the present paper are: A1
(antennula), A2 (antenna), aes (aesthetasc), benp (baseoendopod),
CI–CV (copepodid stages 1–5), cphth (cephalothorax), enp (endopod),
exp (exopod), FR (furcal rami), GF (genital field), md (mandibula),
mx (maxilla), mxl (maxillula), mxp (maxilliped), P1–P6 (pereiopods
1–6), STE (Subterminal Tubular Extension, according to
I could examine other material for comparison: Type material of Mesocletodes parabodini Schriever, 1983, (1 dissected female, ZMK Cop. No. 1319). Mesocletodes farauni Por, 1967 (1 female, dissected, HUJ Cop no. 69 plus one additional specimen), Mesocletodes glaber Por, 1964a (1 female, dissected, HUJ Cop no. 33) and Mesocletodes monensis (Thompson, 1893) (3 females, dissected, on one slide each, HUJ Cop no. 63, 93, 138).
Taxonomy
Argestidae Por, 1986a
Mesocletodes irrasus (T. and A. Scott, 1894), (described as Cletodes irrasa)
Mesocletodes contains 37 species (
(amended from
urn:lsid:zoobank.org:act:8056C1B4-FC83-4410-BE24-7F495F056509
http://species-id.net/wiki/Mesocletodes_elmari
Figs 2–14The name is dedicated to the author’s father, Elmar Menzel.
Guinea Basin, RV “Meteor“, Cruise M63/2 (DIVA-2), station 75/7 (0°50.0'N, 5°35.0'W, 5139m), March 19, 2005.
7 individuals Holotype: 1 female, dissected, mounted on 17 slides, coll. no. SMF 37012/1–17, RV “Meteor“, Cruise M63/2 (DIVA-2) at station 75/7 (0°50.0'N, 05°35.0'W, 5139m), March 19, 2005.
Paratypes: Paratype 1 (Allotype): 1 male, dissected, mounted on 9 slides, coll. no. SMF 37013/1–9, RV “Meteor“, Cruise M63/2 (DIVA-2) at station 78/7 (0°50.1'N, 05°35.1'W, 5136m), March 19, 2005.
Paratype 2: 1 female, mounted on 1 slide, coll. no. SMF 37014, RV “Meteor“, Cruise M63/2 (DIVA-2) at station 35/7 (28°6.8'S, 7°20.7'E, 5033m), March 03, 2005.
Paratype 3: 1 CV female dissected, mounted on 6 slides, coll. no. SMF 37015/1–6, RV “Meteor“, Cruise M63/2 (DIVA-2) at station 79/4 (0°50.0'N, 05°35.1'W, 5140m), March 19, 2005.
Paratype 4: 1 CV male dissected, mounted on 2 slides, coll. no. SMF 37016/1–2, RV “Meteor“, Cruise M48/1 (DIVA-1) at station 346-7/10 (16°17.0'S, 05°27.0'E, 5389m), July 27, 2000.
Paratype 5: 1 CIV male dissected, mounted on 8 slides, coll. no. SMF 37017/1–8, RV “Meteor“, Cruise M63/2 (DIVA-2) at station 97/7 (0°37.2'N, 06°28.1'W, 5168m), March 23, 2005.
Paratype 6: 1 CIII dissected, mounted on 7 slides, coll. no. SMF 37018/1–7, RV “Polarstern“, Cruise PS61 (ANT-XIX/4 (ANDEEP II)) at station 138-11/4 (62°58.03'S, 27°54.08'W, 4541m) March 18, 2002.
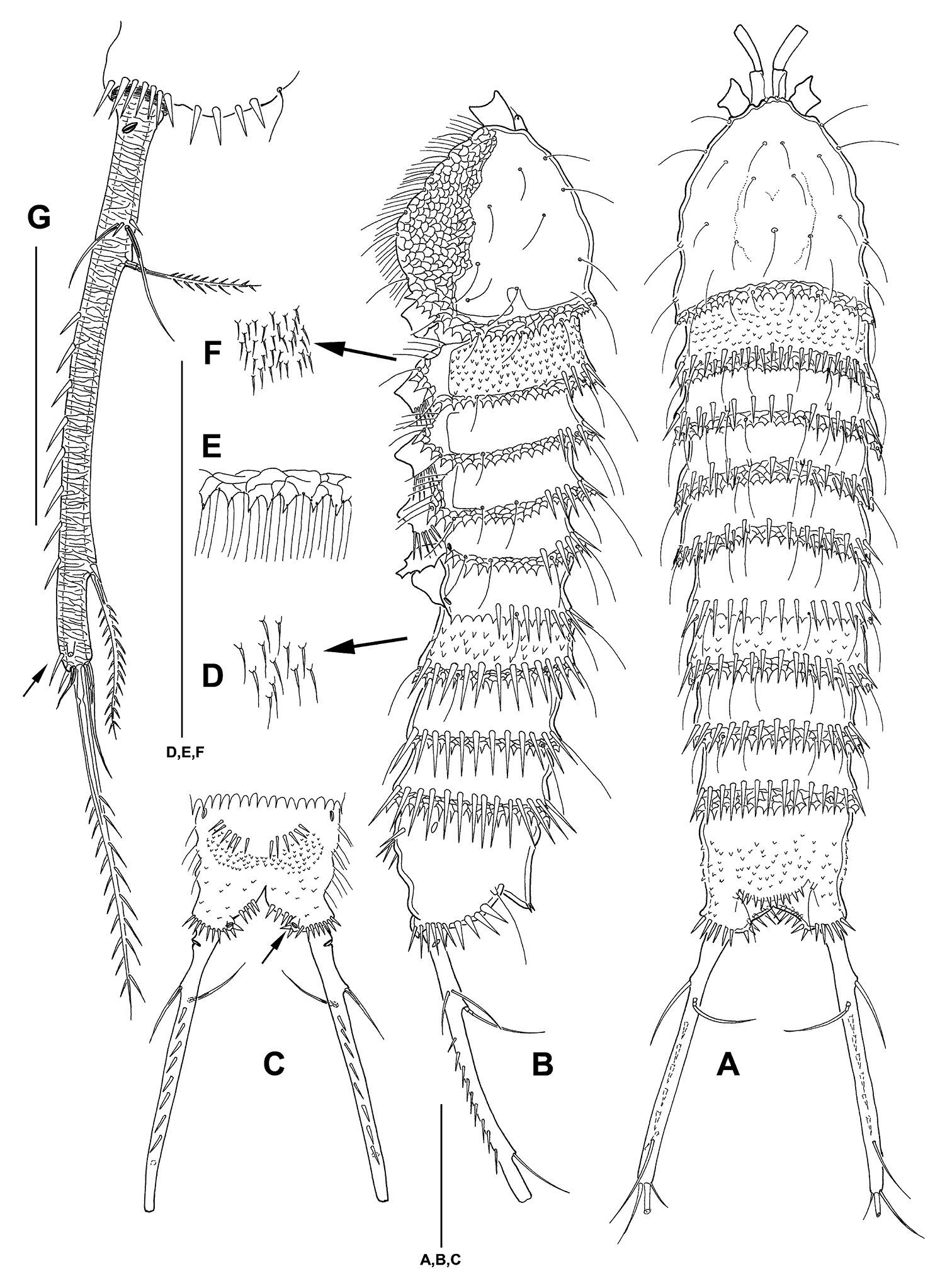
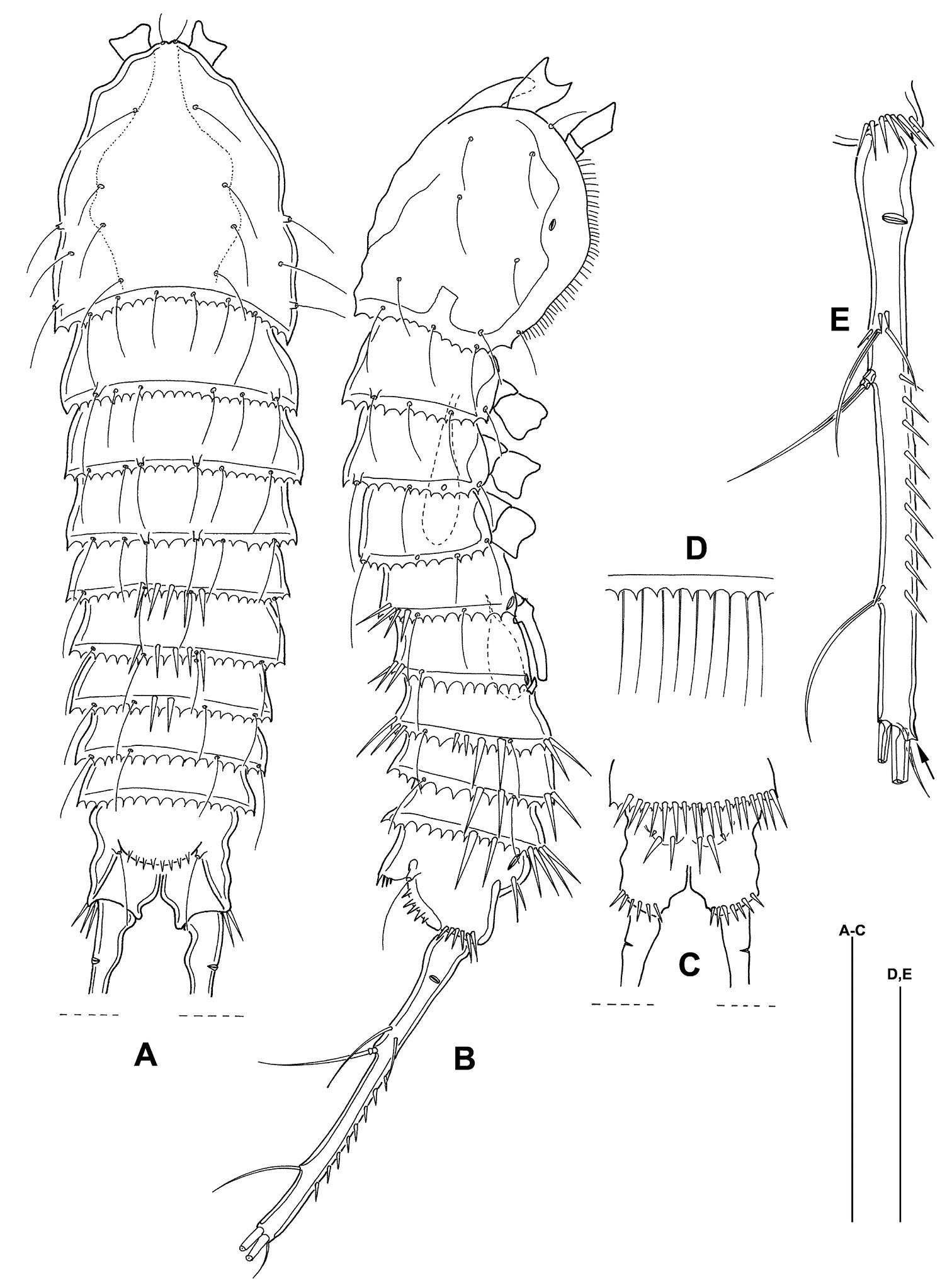
(Figs 2–8) Habitus (Figs 2 [paratype], 3 A – B) of cylindrical shape, no clear distinction between prosome and urosome. Body length including FR 0.78 mm. Distal margins of cphth, prosomites and urosomites with conspicuous coarsely ornate and denticulated hyaline frill with many setules (Fig. 3 E). Body with several remarkably long sensilla. Distal margins of prosomites with long spinules: only dorsally in prosomites and first urosomite, in urosomites also laterally and ventrally. Distal margin of last urosomite without sensilla. Rostrum not protruding, with 2 sensilla. Body of prickly appearance, caused by small protrusions bearing one setule each, protrusions in urosomites and telson coarser than in prosomites (Fig. 3 D, F). Notch-like pores ventrolaterally on P4 – P5 bearing somites. Genital double somite fused ventrally. Telson (Fig. 3 A–C) as long as 2 preceding urosomites together, almost square from lateral and dorsal view. Ventrally with 2 rows of 6 long spinules each and on the outer edges, close to hyaline frill of last urosomite. 1 ventral notch-like pore on each side at inner edge near insertion of FR. Operculum with several denticles (Fig. 3 A).
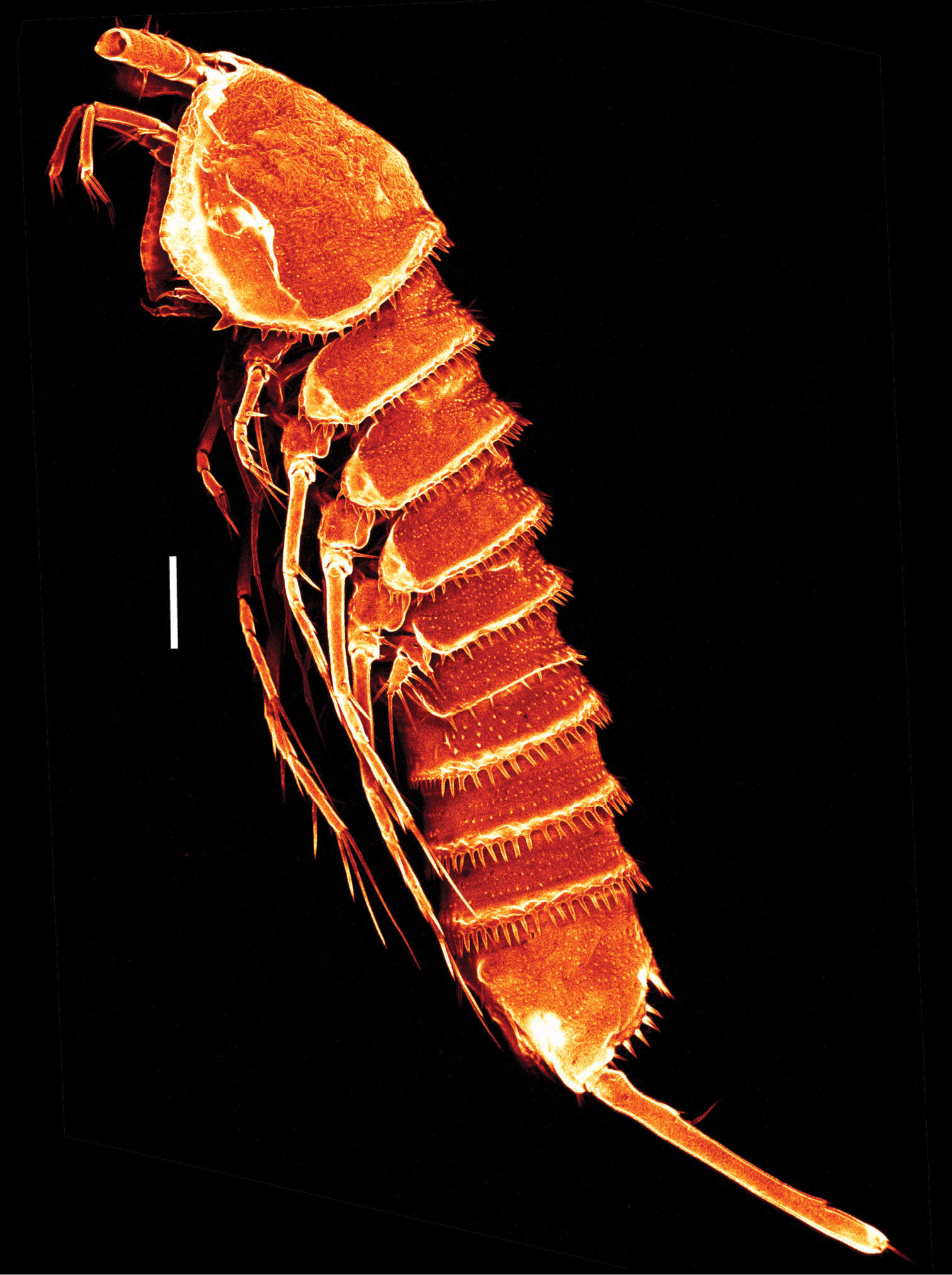
Mesocletodes elmari sp. n., adult female, paratype 2. CLSM photograph of a Congo-red stained specimen, lateral view. Scale bar: 100 µm
Mesocletodes elmari sp. n., adult female, holotype. A habitus dorsal view B habitus lateral view C telson ventral view, internal notch-like pores indicated by arrow D detail of urosomal setules E detail of hyaline frill F detail of prosomal setules G FR lateral view, tube pores indicated by arrow. Scale bars: A–C: 100 µm; D–G: 50 µm
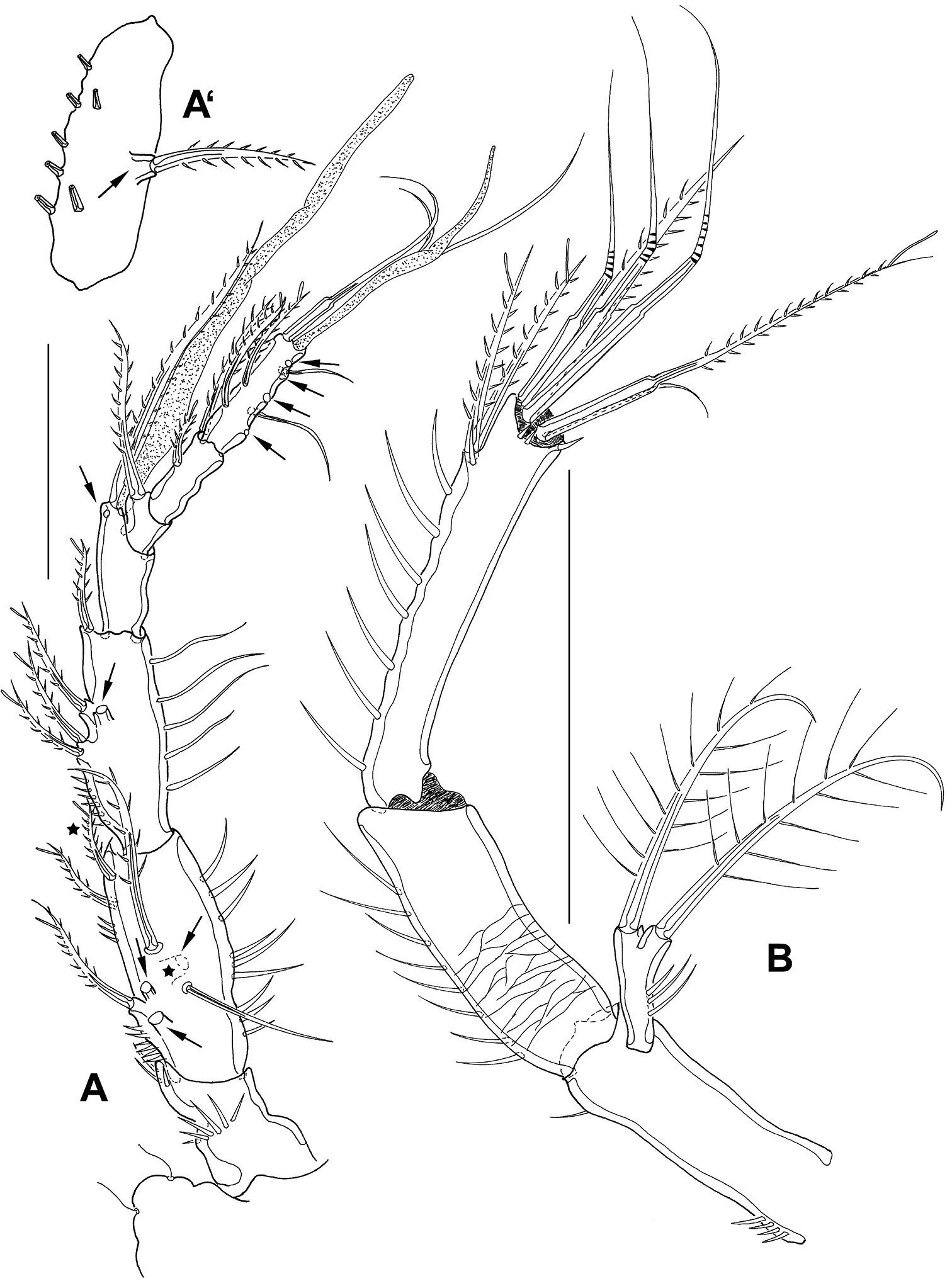
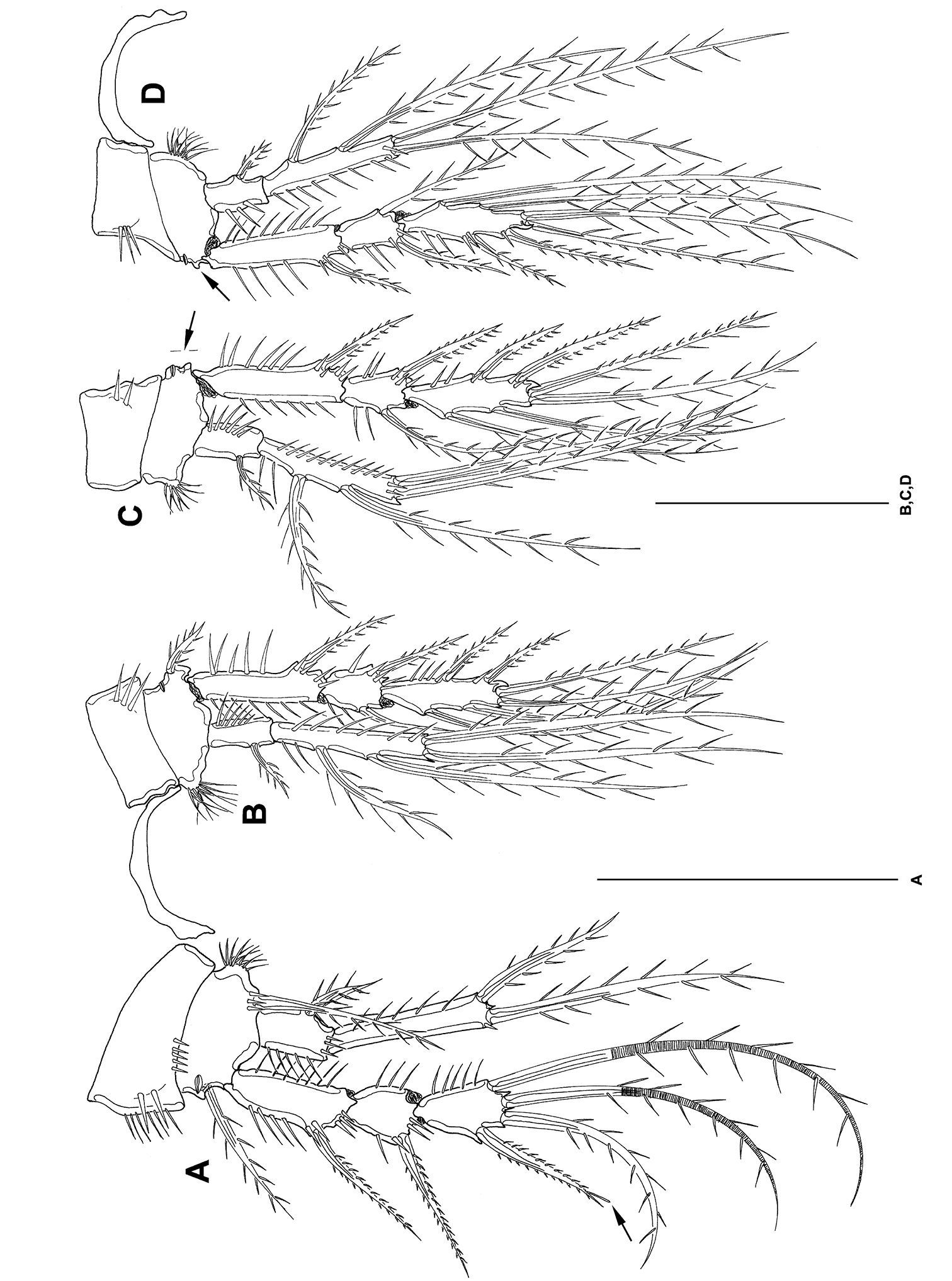
A1 (Fig. 4 A, A’) 7-segmented, reticulated as shown for proximal part of A2 enp1 (Fig. 4 B). Segments 4 and 7 with aes. Second segment of paratype 2 (A’) large, with 1 protrusion bearing 1 bipinnate seta (seta lost during preparation of holotype). Spines with STEs. First and second segment bear inner and outer spinules, third segment with outer spinules. Setal formula: 1: 0; 2: 8; 3: 5; 4: 2+aes; 5: 1; 6: 2; 7: 9+acrothek (=11+aes).
Mesocletodes elmari sp. n., adult female. A A1, holotype, dorsal view. Missing setae indicated by arrows. Asterisks mark the 2 setae presumably occurring in CV. A’ second A1 segment, paratype 2, ventral view, arrow indicates characteristic protrusion with seta B A2 holotype. Scale bars: 50 μm
A2 (Fig. 4 B) with basis, reticulate ornamentation as shown for part of enp1. Exp 1-segmented, with 1 terminal and 1 subterminal seta. Enp 2-segmented, both segments with strong outer spinules. Enp2 with 2 bipinnate spines subterminally. 3 geniculate and 2 pinnate spines, and 1 naked seta terminally. Naked terminal seta fused basally to 1 outer pinnate spine. The innermost element is a reduced seta. Spines with STEs.
Labrum (Fig. 5 A) with 1 medial and 2 lateral rows of spinules, setules at oral surface.
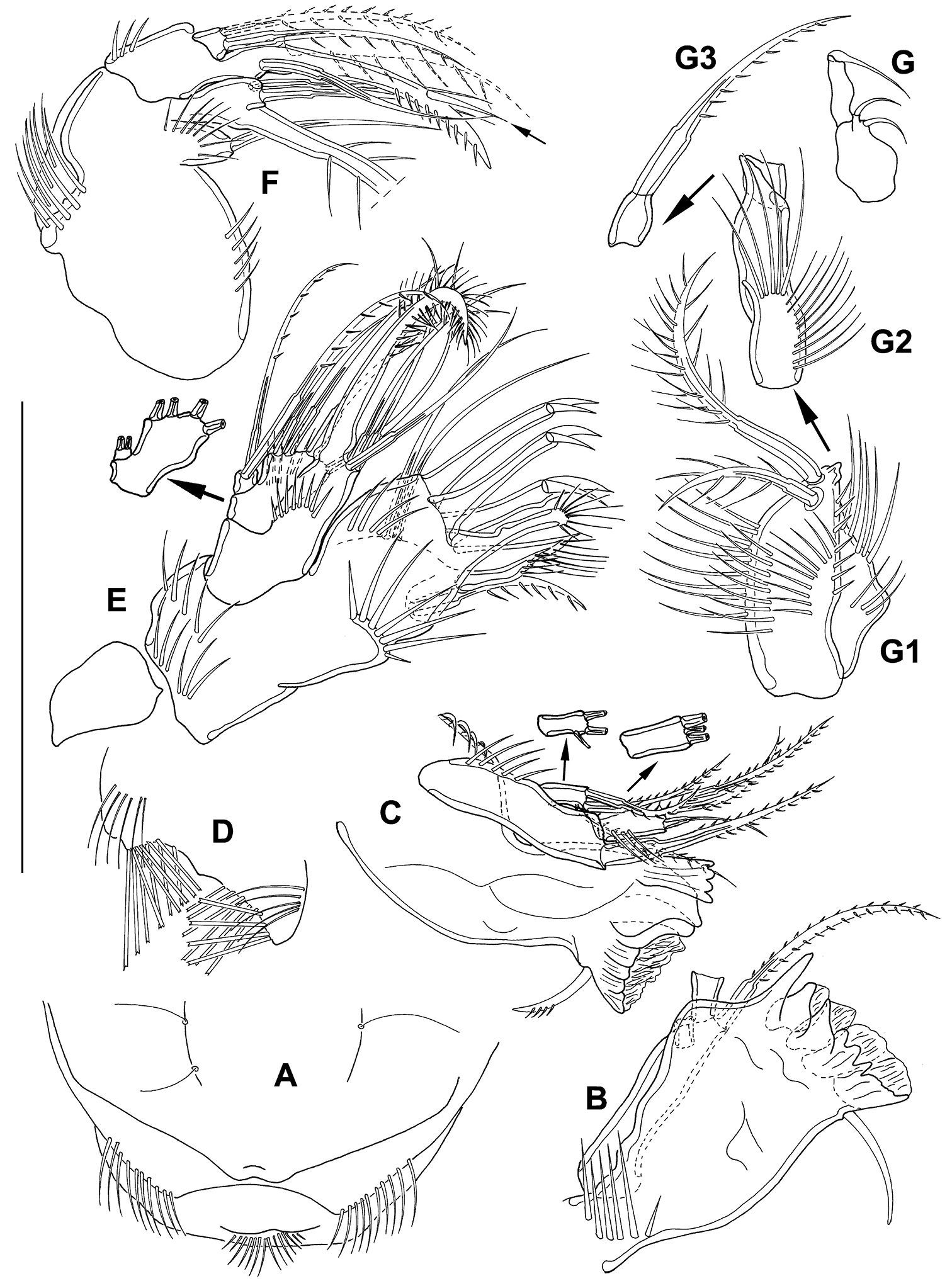
Mesocletodes elmari sp. n., adult female. A labrum, holotype B+C md, holotype, D paragnaths, holotype E mxl, holotype F mx, holotype, basal seta supplemented after counterpart, dash-depicted endopodal seta supplemented after paratype 2. Arrow dicates the peculiar spinulelike pinna G mxp, paratype 2, unfragmented, G1–G3 mxp details, holotype. Scale bar: 50 µm
Md (Fig. 5 B, C) gnathobase formed by 5 tooth-like projections: 1 dentate, 1 broad tooth, 3 strong teeth partly fused to broad grinding face. Strong seta close to grinding face. Md palpus 3-segmented, exp and enp articulated. 1 strong basal seta terminally, exp with 2 terminal and 1 subterminal setae, enp with 3 terminal setae.
Paragnaths (Fig. 5 D) on each side with 2 rows of traverse arranged brush-like setae orally and 1 row of long spinules at the surface.
Mxl (Fig. 5 E) praecoxal arthrite terminally with 6 strong elements: 3 hooks with 1 strong spinule each, 1 brushlike seta fused to arthrite and 2 unipinnate setae. Subterminally with another pinnate spine and 2 bare setae aborally. Coxa with 4 elements terminally: 1 strong seta fused to coxa and 3 bare setae. Basis with 2 bare setae. Enp incorporated into basis, with 2 bare setae, exp 1-segmented with 2 pinnate setae.
Mx (Fig. 5 F) syncoxa with 2 endites, the proximal one bearing 1 seta. Distal endite with 3 setae, the biggest one fused to segment. 2 strong setae fused to basis, distal one shows a suture, proximal one with 1 conspicuous strong spinule-like pinna (indicated by arrow in Fig. 5 F). Basis additionally with 1 bare seta. Enp 1-segmented, with 2 bipinnate setae of equal length (dash-depicted seta supplemented from paratype 2).
Mxp (Fig. 5 G, G1–G3) prehensile, syncoxa (Fig. 5 G1) slightly shorter than basis (proximal part of Fig. 5 G2), with 2 setae and several spinules. Basis slender, with spinules of different sizes. Enp 2-segmented. Enp1 (distal part of Fig. 5 G2) small, bare of setae. Enp2 (Fig. 5 G3) terminally fused to strongly pinnate claw, suture visible.
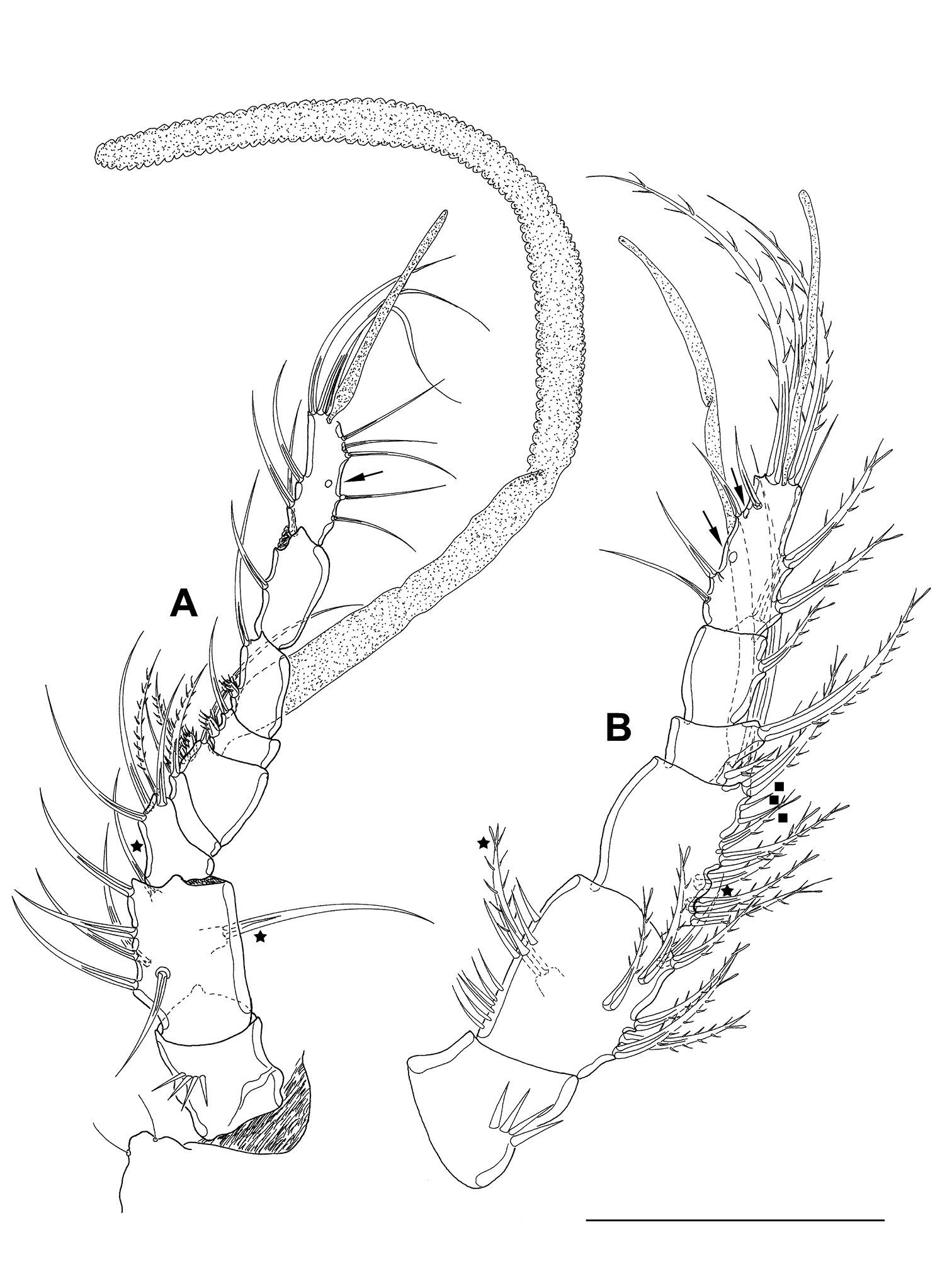
P1 (Fig. 6 A) with 3-segmented exp and 2-segmented enp. Intercoxal sclerite long and bow-like. Coxa 1/3 broader than basis, with several spinules on ventral margin. Basis with outer spine, outer pore, long inner spine ventrally oriented and several rows of spinules. Exp1 and exp2 without inner seta. Exp3 with 4 elements. Enp1 short, with strong inner spine inserted medially. Enp2 extremely long, surpassing exp in length, with 1 outer, 1 terminal and 1 inner seta. Enp2 with 1 peculiar spinule subterminally. For setal formula see Table 2.
Mesocletodes elmari sp. n., adult female, holotype. A P1, tube pores indicated by arrows B P2. Scale bars: 50 µm
Mesocletodes elmari sp. n., setal formula of P1–P4 of adults and copepodid stages. Pereiopodal setation of CV female and CV male is analogous to adults. – = segment is missing
| exp1 | exp2 | exp3 | enp1 | enp2 | ||
|---|---|---|---|---|---|---|
| P1 | adult female | I-0 | I-0 | I, I1, 1 | 0-1 | 1, 1, 1 |
| adult male | I-0 | I-0 | I, I1, 1 | 0-1 | 0, 1, 1 | |
| CIV male | I-0 | 2, I1, 1 | – | 0-1 | 1, 1, 1 | |
| CIII | I-0 | 2, I1, 1 | 0-1 | 0, 1, 1 | ||
| P2 | adult female | I-0 | I-1 | II, I1, 2 | 0-1 | 0, 2, 1 |
| adult male | I-0 | I-1 | II, I1, 2 | 0-1 | 0, 2, 2 | |
| CIV male | I-0 | III, I1, 3 | – | 0-1 | 1, 2, 1 | |
| CIII | I-0 | III, I1, 2 | – | 0-1 | 0, 2, 1 | |
| P3 | adult female | I-0 | I-1 | II, I1, 2 | 0-1 | 0, 2, 1 |
| adult male | I-0 | I-1 | II, I1, 2 | 0-1 | 0, 2, 2 | |
| CIV male | I-0 | III, I1, 3 | – | 0-1 | 1, 2, 1 | |
| CIII | I-0 | II, I1, 2 | – | 0-1 | 0, 2, 0 | |
| P4 | adult female | I-0 | I-1 | II, I1, 2 | 0-1 | 0, 2, 1 |
| adult male | I-0 | I-1 | II, I1, 2 | 0-1 | 0, 2, 2 | |
| CIV male | I-0 | III, I1, 3 | – | 0-1 | 1, 2, 1 | |
| CIII | III, I1, 0 | – | – | 0, 2, 0 | – | |
P2–P4 (Figs 6 B, 7 A, B) with 3-segmented exps and 2-segmented enps. Intercoxal sclerites long and bow-like. Coxae little larger than bases. Bases twice as broad as long. Bases with outer spines, at inner margin with setular tufts. Outer margins of coxa with strong spinules, inner margins of coxa and basis with setules. Exp1 as long as exp2 and exp3 together. Exp1 without inner seta. Exp3 terminally with cuticular hooks. Enp1 short. Enp2 extremely long, decreasing in length from P2-P4, measured in relation to exp1. Enp2 with 1 strong, short spinule subterminally. Outer terminal seta of enp2 decreasing in length from P2–P4. Inner terminal seta in P2 enp2 lost during preparation (indicated by arrow in Fig. 6 B). Setation of exp and enp as in Table 2.
Mesocletodes elmari sp. n., adult female, holotype. A P3 B P4. Scale bar: 50 µm
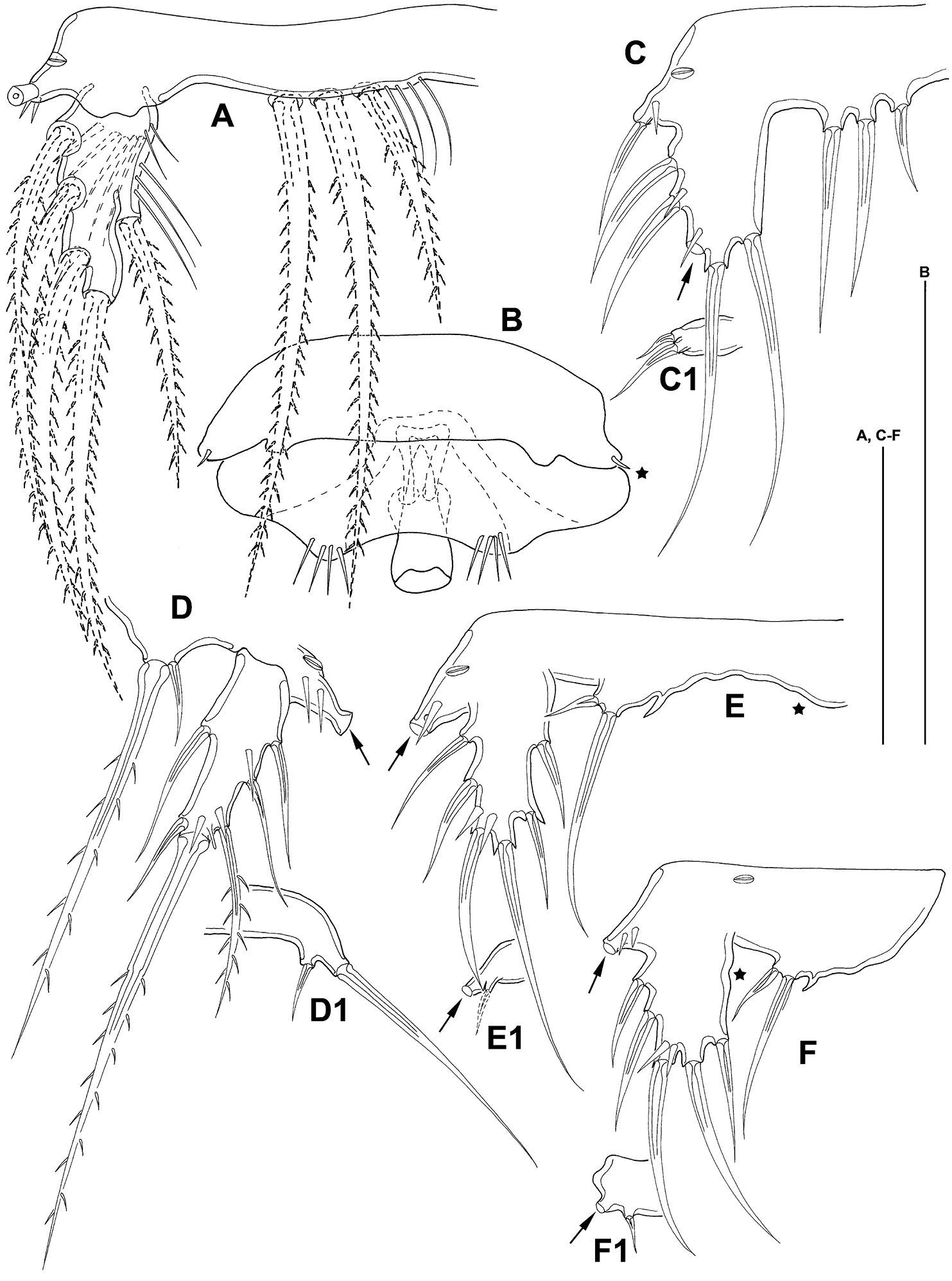
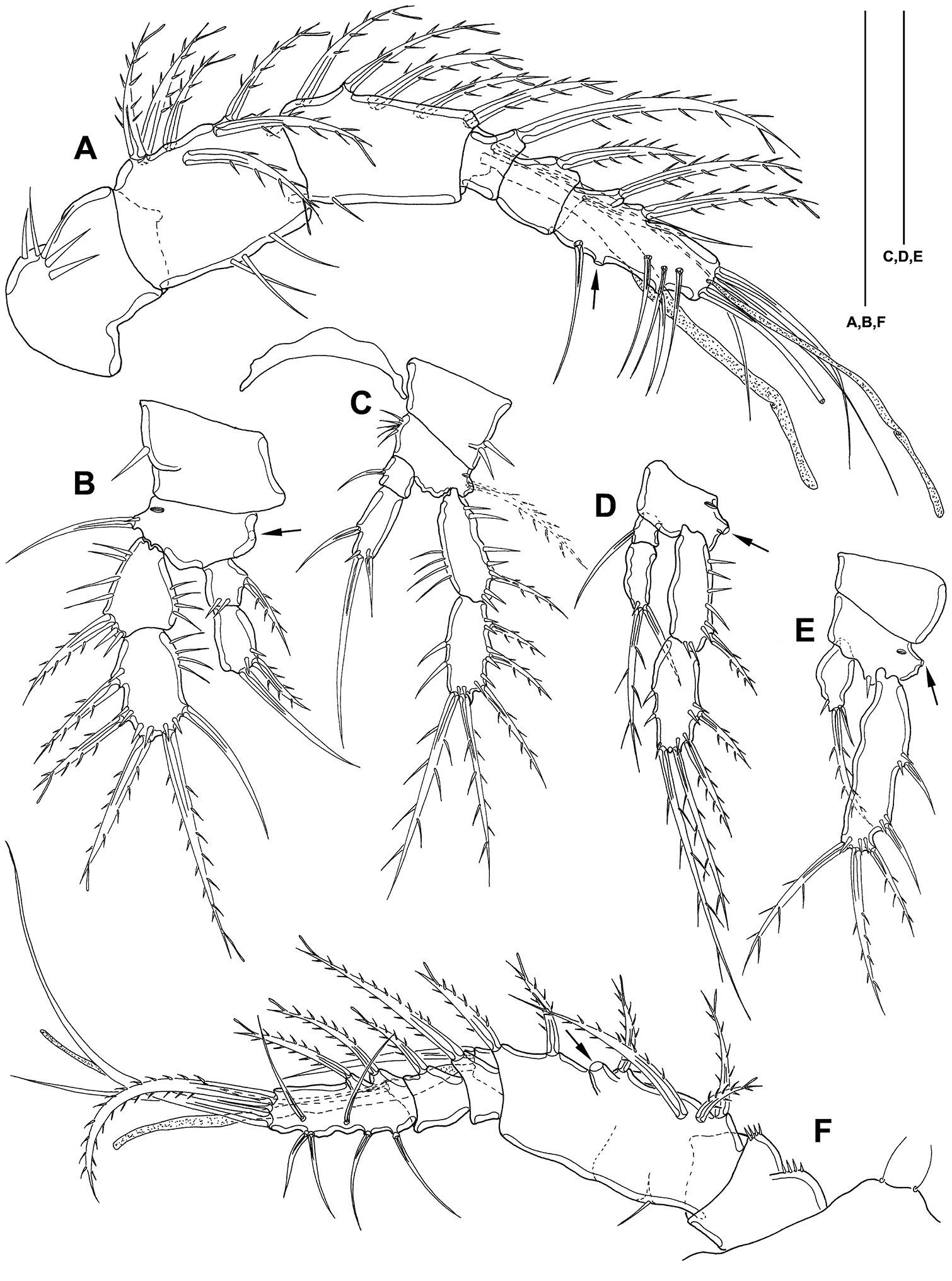
P5 (Fig. 8 A) benp with setophore with 2 spinules and 1 long bipinnate seta. Endopodal lobe not protruding, with 3 setae. Exp about 2 times as long as broad at base, bearing 3 outer, 1 terminal and 1 inner seta (dash-depicted setae supplemented from paratype 2).
Mesocletodes elmari sp. n. A adult female holotype, P5, dorsal view B adult female holotype, GF, P6 indicated by asterisk C CV female paratype 3, P5 ventral view C1 CV female paratype 3, P6 ventral view D adult male paratype 1, P5 ventral view D1 adult male paratype 1, P6 ventral view E CV male paratype 4, P5 ventral view, asterisk on the right side of the endopodal lobe indicates where a cuticular protrusion analogous to the one on the left side can be expected E1 CV male paratype 4, P6 ventral view F CIV male paratype 5, P5 ventral view, asterisk marks the inner depression on P5 exp F1 CIV male paratype 5, P6 ventral view. Missing setae indicated by arrows. Scale bars: 50 µm.
P6 integrated into GF (Fig. 8 B), reduced to a fused opercular plate, armed with 1 short spine on each side (see asterisk in Fig. 8 B). GF with single aperture, accompanied by 1 row of spinules on each side.
FR (Fig. 3 G) long and slender, ornate, ventral spinules between seta VII and III. Approximately 13 times as long as broad (measured at base). Close to base ventrolaterally with 1 notch-like pore at external side (Fig. 3 G, C). Extremely elongated between setae VII and III. Seta I close to seta II. Seta VII triarticulate. Seta III located on dorsal side subterminally. Setae IV–VI located terminally. FR laterally with subterminal tube pore (see arrow in Fig. 3 G).
(Allotype) (Figs 8–11) The adult male corresponds to the adult female in all morphological characters unless deviations are mentioned below.
Habitus (Fig. 9 A, B) much smaller than adult female, body length including FR 0.40 mm. Body not of prickly appearance (Fig. 9 A–C), hyaline frill (Fig. 9 D) not ornate. Distal margins of first and second urosomites with long spinules dorsally, of third urosomite dorsally, laterally and ventrally, of last 2 urosomites only laterally and ventrally. With 2 spermatophores: first one inside first urosomite, second one inside second and third prosomite. Gut empty. FR (Fig. 9 E) as described for female.
Mesocletodes elmari sp. n., adult male paratype 1. A habitus dorsal view B habitus lateral view C telson ventral view D detail of hyaline frill E FR, lateral view, arrow indicates terminal tube pore. Scale bars: A–C 100 µm, D+E 50 µm.
A1 (Fig. 10 A) 9-segmented, haplocer. Segments 5 and 9 with aes. Second segment large, with 1 protrusion bearing 1 bare seta. Segments 5, 6 and 7 with modified setae. Setae of most segments bare. Setal formula: 1: 0; 2: 8; 3: 4; 4: 2; 5: 4+aes; 6: 2; 7: 2; 8: 2; 9: 9+acrothek (=11+aes).
Mesocletodes elmari sp. n. A adult male paratype 1, A1 dorsal view B CV male paratype 4, A1 dorsal view, minute setae on third segment highlighted by solid squares. Asterisks mark the 2 setae occurring in CV. Missing setae indicated by arrows. Scale bar: 50 µm.
A2, Md, Mxl, Mx and Mxp as described for adult female.
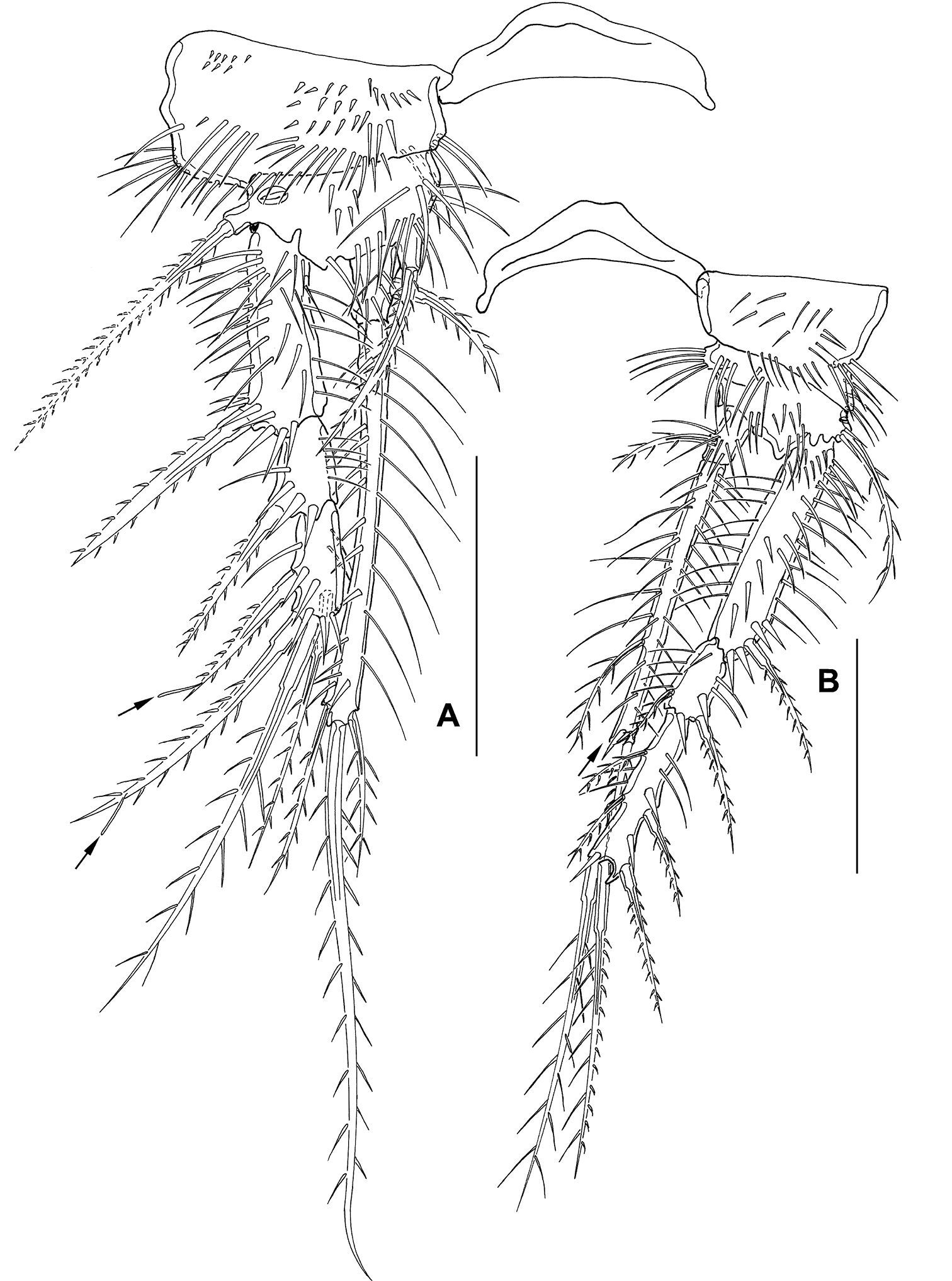
P1–P4 (Fig. 11 A–D) intercoxal sclerites, coxae, bases and segmentation of enp and exp as described for adult female, but with fewer spinules. P1 exp3 with 1 spine and 3 setae, the 2 innermost of wreathed appearance. P2–P4 inner exopodal setae long. P2–P4 enp2 with 2 long inner setae. Basal seta of P3 and P4 broken (indicated by arrow in Fig. 11 C, D). For setal formula see Table 2.
Mesocletodes elmari sp. n., adult male paratype 1. A P1 B P2 C P3 D P4. Missing setae indicated by arrows. Scale bars: 50 µm.
P5 (Fig. 8 D) with setophore (seta lost during dissection, see arrow in Fig. 8 D) with few spinules and 1 notch-like pore laterally. Endopodal lobe barely protruding, with 2 setae, outermost very short. Exp about twice as long as broad (measured at base), bearing 3 outer, 1 terminal and 2 inner setae.
P6 (Fig. 8 D1) with 2 setae.
(paratypes 3–6) (Figs 8, 10, 12–14) CV female (Fig. 12 C, C1): body length including FR 0.58 mm. Body not of prickly appearance. Penultimate urosomite is not formed. Distal margins of body somites with smooth hyaline frill and, except penultimate one, with sensilla. Extremities A1–P4 (not depicted) as described for adult female but smaller. P5 (Fig. 8 C) exp not separated from benp, setation of exp and basendopodal lobe as in adult female but smaller. P6 (Fig. 8 C1) with 2 setae. GF not expressed.
Mesocletodes elmari sp. n. A CIII paratype 6, habitus lateral view, terminal TP on FR indicated by arrow A1 CIII paratype 6, telson ventral view, internal notch-like pores indicated by arrow B CIV male paratype 5, habitus lateral view, terminal TP on FR indicated by arrow B1 CIV male paratype 5, telson ventral view, internal notch-like pores and setular tuft on FR indicated by arrows C CV female paratype 3, habitus lateral view, terminal TP on FR indicated by arrow C1 CV female paratype 3, telson ventral view, internal notch-like pores indicated by arrow. Scale bar: 100 µm.
CV male: body as in CV female. A1 (Fig. 10 B) 6-segmented. Segments 3 and 6 with aes. Second segment large, with a protrusion bearing 1 seta. Setal formula: 1: 0; 2: 8; 3: 9+aes; 4: 2; 5: 2; 6: 9+acrothek (=11+aes). A2–mxp as described for adult female. P1–P4 (not depicted) and P6 (Fig. 8 E1) as described for adult male but smaller. P5 (Fig. 8 E) exp not separated from benp, setation of exp as in adult male but smaller. Right basendopodal lobe with 2 setae and 1 cuticular protrusion, which is missing on the counterpart (see asterisk in Fig. 8 E).
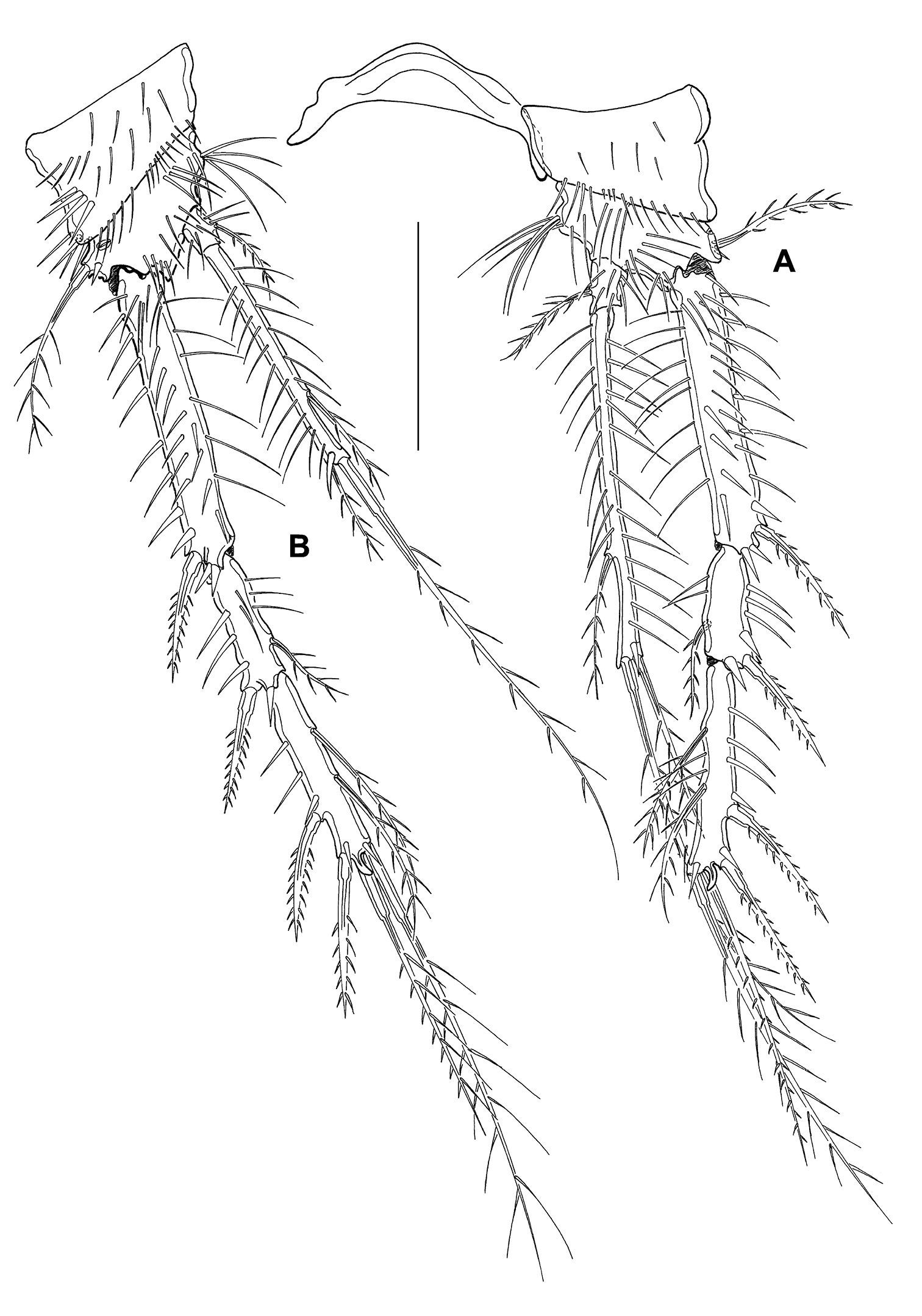
CIV male (Fig. 12 B, B1): body length including FR 0.50 mm. Body not of prickly appearance. 2 penultimate urosomites not formed. Distal margins of body somites with smooth hyaline frill and, except the penultimate one, with sensilla. A1 (Fig. 14 A) 6-segmented. Segments 3 and 6 with aes. Setal formula: 1: 0; 2: 6; 3: 6+aes; 4: 1; 5: 2; 6: 9+acrothek (=11+aes). A2–mxp (not depicted) as described for adult female but smaller P1–P4 (Fig. 13 A–D) with 2-segmented enp and 2-segmented exp. P1–P4 enp2 with 1 inner seta and 1 subterminal, outer seta. For setal formula see Table 2. Setal elements developed as in adult male, P5 (Fig. 8 F) exp not separated from benp. Basendopodal lobe with 2 setae and 1 cuticular protrusion, P5 not fused in the middle. P6 (Fig. 8 F1) with 2 setae. GF not expressed. FR with setular tuft (Fig. 12 B1) close to insertion in telson.
Mesocletodes elmari sp. n., CIV male paratype 5. A P1 B P2 C P3 D P4. Missing setae indicated by arrows. Scale bars: 50 µm.
Mesocletodes elmari sp. n. A CIV male paratype 5, A1 dorsal view B CIII paratype 6, P1 C CIII paratype 6, P2, outer basal seta supplemented according to counterpart D CIII paratype 6, P3 E CIII paratype 6, P4 F CIII paratype 6, A1. Missing setae indicated by arrows. Scale bars: 50 µm.
CIII (Fig. 12 A, A1): body length including FR 0.42mm. Body not of prickly appearance. 3 penultimate urosomites not formed. Distal margins of body somites with smooth hyaline frill. A1 (Fig. 14 F) 5-segmented. Setal formula: 1: 0; 2: 8+aes; 3: 1; 4: 2; 5: 9+acrothek (=11+aes). A2–mxp (not depicted) as described for adult female but smaller.
P1–P3 (Fig. 14 B–D) with 2-segmented enp and 2-segmented exp. Exp1 longer than exp2. P4 (Fig. 14 E) exp and enp 1-segmented. For setal formula see Table 2. P5 lost during preparation, P6 not expressed.
(cf. Table 1). The body length including FR is variable: for adult females between 0.57 and 1.06 mm (the majority measured 0.7 to 0.9 mm), for adult males between 0.4 and 0.7 mm, for CV females between 0.5 and 0.75 mm, for CV males between 0.5 and 0.59 mm, for CIV males between 0.4 and 0.64 mm.
The spinulation also seems to be highly variable: the row of spinules ventrally at the telson ranges from numerous, long and slender to few, short and stout. In total, 16 specimens show setular tufts in the FR: six adult females, one CV male and the five CIV males bear setular tufts close to the telson, four adult females close to seta VII. The amount of spinules in A1 segment 3 varies. Four out of 56 adult females, all adult males and copepodid stages possess a non-ornate hyaline frill. A very rare feature (in two adult females, all CIV males) is also the presence of outer setae in P2–P4 enp2 or just in P2 enp2 (one adult female). The number of eggs (2–20) is variable, too.
Allocation of Mesocletodes elmari sp. n. to the taxon Mesocletodes is indisputable since all specimens show the apomorphies recognized by
The phylogenetic relationships within Mesocletodes are still under discussion. However, a first approach is possible: Mesocletodes elmari sp. n. is considered to belong to the “Mesocletodes inermis
group” as it lacks the characteristic cuticular processes on
cephalothorax and telson that are regarded to be autapomorphic to the Mesocletodes abyssicola-group (
Mesocletodes elmari
sp. n. shows a distinct mxl exopodal segment, and the enp is
incorporated into the basis. By contrast, a distinct endopodal segment
is described for the mxl of Mesocletodes bodini (
From a morphological point of view Mesocletodes elmari sp. n. is similar to Mesocletodes bodini and Mesocletodes parabodini as these three are the only species of Mesocletodes with elongated P1–P4 enp2. Mesocletodes elmari sp. n., however, shows clear autapomorphies [plesiomorphic states in brackets] that justify it as a new species:
1) mx seta that is fused to the basis, bears a conspicuously strong spinule-like pinna [seta without spinule-like pinna]
2) P2–P4 exp3 proximal outer seta lost [seta present]
3) P1–P4 enp2 extremely elongated [not elongated]
4) FR strongly elongated between setae III and VII [not elongated]
5) female body of a prickly appearance created by setules that are widened at their bases [no prickly appearance]
6) female P2–P4 enp2 proximal inner seta lost [seta present]
Character 1): The mx seta that is fused to the basiscarries a conspicuously strong spinule-like pinna in Mesocletodes elmari sp. n. The corresponding seta in other species of Mesocletodes is usually bipinnate with the pinnae of equal size. The loss of all pinnae except one at the anterior side plus the modification of this pinna towards a spinule-like appearance is not recorded for any other species of Mesocletodes or Argestidae and is therefore regarded here as derived. This modification thus is considered to be autapomorphic to Mesocletodes elmari sp. n.
Character 2): Mesocletodes elmari
sp. n. lacks the proximal outer seta on P2–P4 exp3. The reduction of
outer pereiopodal ornamentation is considered to be derived according to
the rule of oligomerization (
Character 3): Endopodal segments of species of Mesocletodes are very short and there are never more than two of them in this genus, many species even have only one single segment. The extreme elongations in P1–P4 enp2 are unique for Mesocletodes elmari sp. n. and are considered to be the result of lengthening of the distal endopodal segment. Ontogenetic stages of males do not show a suture that might indicate a fusion of the distal segment with the preceding. Extreme elongations of P1–P4 enp2 are therefore considered here to be autapomorphic to Mesocletodes elmari sp. n. A less extreme elongation of these segments, however, occurs also in Mesocletodes bodini and Mesocletodes parabodini.
Character 4): The FR of Mesocletodes
are longer than wide, with setae IV, V and VI located terminally,
whereas setae I, II, III and VII are located closer to or in the
proximal part of the ramus. An extreme elongation between setae III and
VII has been discussed as an apomorphy for the Mesocletodes abyssicola-group (
Character 5): Females of Mesocletodes elmari sp. n. are characterized by the prickly appearance of the body somites dorsally and laterally. Such coverage is absent in other species of Mesocletodes and is therefore regarded here as derived, i.e. an autapomorphic character for Mesocletodes elmari sp. n.
Character 6): Endopodal segments do not seem to be fused in Mesocletodes elmari sp. n. (see character 3). The proximal inner seta on P2–P4 enp2 in males is considered to be reduced in females. The lack of the proximal inner seta on P2–P4 enp2 is therefore considered here to be autapomorphic to females of Mesocletodes elmari sp. n.
Intraspecific variability in Mesocletodes elmari sp. n.Intraspecific variability in deep-sea harpacticoids has recently been revealed to be extremely high. For instance,
For Mesocletodes
intraspecific variability has not yet explicitly been recorded.
However, five species were redescribed at least once, indicating that
detected specimens deviate minimally from the type specimen: Mesocletodes abyssicola (T. and A. Scott 1901;
Although clear apomorphies were recognized for Mesocletodes elmari sp. n., careful morphological examination of the 77 specimens revealed high intraspecific variability (cf. Table 1).
The total length of FR, the number and the shape of spinules in
various parts of the body, the ornamentation of the hyaline frill and
the setation of P2–P4 enp2 is variable. Moreover, few specimens bear
setular tufts in various positions on the FR. Setular tufts on the FR
near seta VII have only been recorded for Mesocletodes bodini (
Many morphological characters of species belonging to Mesocletodes are entirely different in both genders. Nevertheless, the identification keys for Mesocletodes are exclusively based on the morphology of females (e.g.
Sexual dimorphism in adults. The descriptions of Mesocletodes contain only females, with the exception of four species: exclusively the male is described for Mesocletodes angolaensis and Mesocletodes fladensis (the latter description is poorly detailed). Both genders are described for Mesocletodes faroerensis and Mesocletodes thielei. However, these two species bear a proximal outer spine in P1 exp3 and 3 inner setae on P3 exp3. Moreover, Mesocletodes faroerensis bears an inner seta on P1 exp2 and 3 inner setae on P3 exp3, and the md gnathobase of Mesocletodes thielei does not form a strong grinding face. Consequently, both species lack autapomorphies of Mesocletodes (cf.
Sexually dimorphic modifications in males of basal Argestidae, such as Argestes (
However, not only the empty gut or the reduction of
mouthparts indicates the abandonment of feeding in adult males, but
also the A1: most setae on the A1 of the adult male of Mesocletodes elmari sp. n. are smooth, merely some in the grasping region of the A1 (segments 3–6) are bipinnate (Fig. 10 A).
However, all setae that are smooth in the adult male are strongly
pinnate in the two preceding copepodid stages (Figs. 10 B, 14 A). Thus,
the loss of pinnae is regarded as another sexually dimorphic
modification in adult males since the regression or poorer development
of setal elements is typical of non-feeding male copepods (
Females are generally considered to show the whole
character set of a species while the modifications in males are
considered to be due to sexual dimorphism (but see
Sexual dimorphisms in juveniles. Sexually dimorphic modifications expressed in copepodid stages of Mesocletodes elmari
sp. n. allow sexing during ontogenetic stages, at least from CV
onwards; it is only partially resolved for this species if sexing of CIV
is possible because all discovered CIV seem to be of the same gender. A
similar constraint applies to the single individual of CIII. This
copepodid stage, however, is assumed not to show sexual dimorphism
(e.g.
Sexing of CV. The male CV and the female CV of Mesocletodes elmari sp. n. are distinguishable from the adults by virtue of the overall smaller body size, the lack of the penultimate urosomite and the non-articulated P5 exp. Moreover, the female CV lacks the GF, the male CV lacks the spermatophores and shows strong differences from the adult male in the A1 (Fig. 15 B, C): only six out of nine A1 segments are articulated and several setae are lacking. The position and number of developed setae in these segments, however, resemble the adult male A1 more than the adult female A1 (compare Figs 4 A, 10 A, B, 15 A–C).
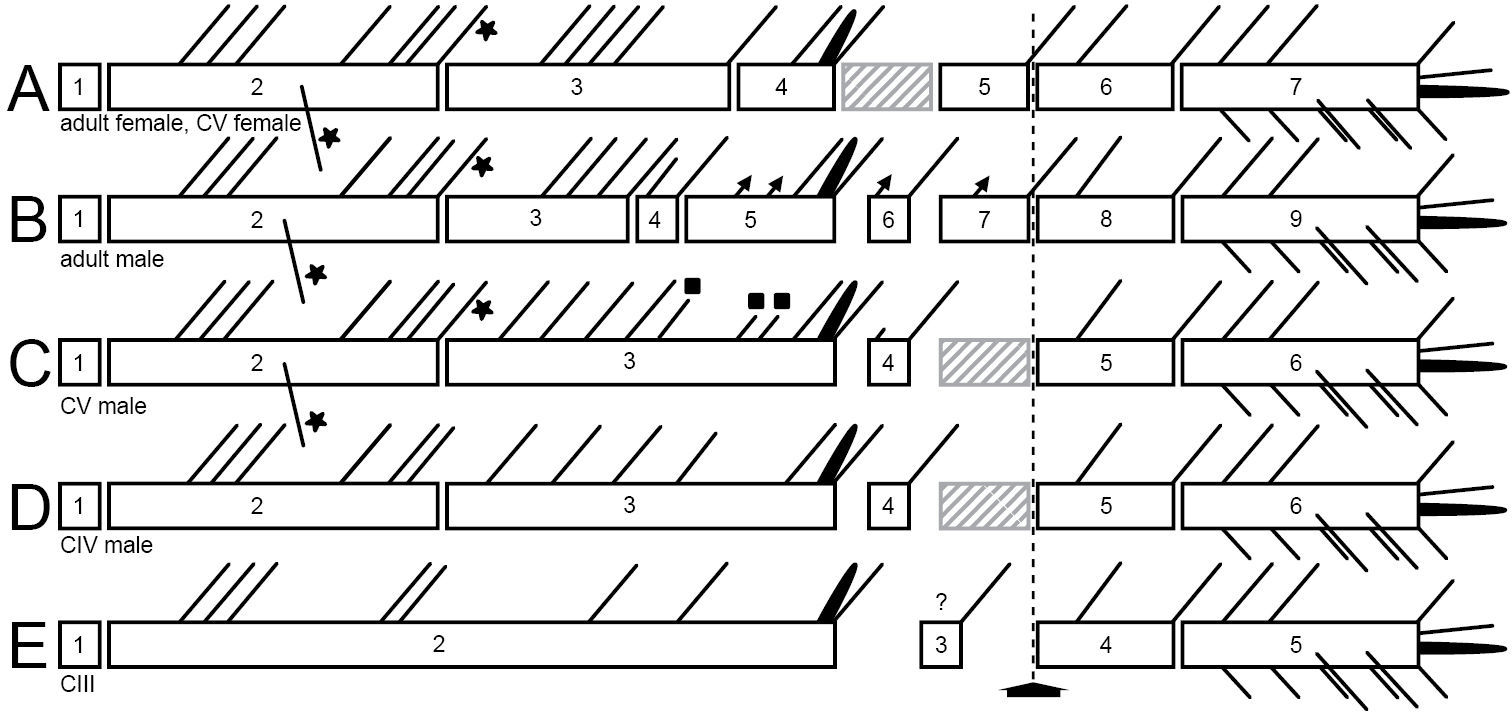
Schematic showing the A1 segmentation and setation of both genders and different copepodid stages of Mesocletodes elmari sp. n. A adult female and CV female B adult male C CV male D CIV male E CIII. Crosshatched segments are considered to be missing or not formed. Solid triangles: sexually dimorphically modified setae, solid squares=setae added at the molt to CV male, solid asterisks=characteristic Mesocletodes seta and the subterminal seta in segment 2 in CV and adults. Arrow marks geniculation.
Sexing of CIV. Careful examination of the A1 and the P5 suggests sexing of the discovered CIV as males.
The five inner setae on the third segment of the CIV A1 (Figs 14 A, 15 D) are almost evenly distributed as is the case in the CV male (Figs 10 B, 15 C). The CV female A1 (cf. Fig. 4 A) has the aes on the fourth segment, while it is on the third in the CV male (Figs 10 B, 15 C). As follows, if the CIV were females, a separation of the aes-bearing segment from the third segment should happen at the next molt. This does not seem plausible, however, because four setae on female segment 3 (Figs 4 A, 15 A) are close to each other in the middle of the segment, the fifth seta inserts distally. An elongation proximally and distally of the evenly distributed four setae in CIV segment 3, plus shortening of the distances between these setae, is not likely. However, an addition of three inner setae at the molt from CIV (Figs 14 A, 15 D) to CV (Figs 10 B, 15 C) in the distal part of this segment (see solid squares in Figs 10 B, 15 C) and maintenance of the distances between the five setae addressed above appear likely. The A1 of the CIV is therefore considered herein to show male characteristics.
The P5 endopodal lobe of the four CIV (Fig. 8 F)
has one short, outer seta, one long medial seta and one inner
cuticular protrusion, and is therefore in accordance with the CV male (Fig. 8 E).
The setation of P5 exp, however, resembles the CV female.
Nevertheless, the small depression on the proximal inner edge of the
exp (see asterisk in Fig. 8 F)
might indicate the emergence of a seta at the next molt, which is only
present in males. It is unclear, however, whether harpacticoid CIV
show sexually dimorphic modifications in P5 exp. It seems that the CIV
of Mesocletodes elmari sp. n. do, whereas the opposite is reported for the CIV of an undescribed species of Orthopsyllus Brady and Robertson, 1873 (
P2–P4 enp2 of the discovered CIV bear one inner seta,
which is in accordance with female adults and CV. The male adult and
CV bear two inner setae in these segments, with the distal seta being
homologous to the single seta in the adult female. However, previous
studies suggest that endopodal setation is not complete in harpacticoid
CIV (
Although copepodid stages amount to between 30% and
more than 50% of the total deep-sea harpacticoid assemblage, they are
excluded from faunistic analyses because confident specific allocation
is not possible for many families. For investigations on phylogeny,
however, juveniles may be the key to plausible theories (e.g.
Many species descriptions contain short remarks on Mesocletodes
relationships with other genera and species within the genus.
Phylogenetic investigations have been subject to one study to date (
A2, mouthparts and FR of Harpacticoida are complete with respect to segmentation and setation from CI onwards (cf.
A1. The female A1 of Mesocletodes elmari sp. n. is complete at least at CV, whereas the male A1, which is available from CIV onwards, undergoes extensive modifications at each molt. Segments 3 to 5 of the adult male are part of the third compound segment in CIV males, three setae (marked by solid squares in Figs 10 B, 15 C) are added to this compound segment at the molt to CV. The strongest modifications appear at the molt to adult: the third compound segment is simultaneously separated into segments 3, 4 and 5. Segment 6 of the adult male is distinct at least from CIV onwards, but the proximal seta is added at the molt to CV. Segment 7, directly preceding the geniculation, is not present prior to the molt to adult male.
The characteristic Mesocletodes seta (strong, bipinnate, arising from a conspicuous protrusion, see
Although sexing of the single discovered CIII was impossible, its A1 provides valuable ontogenetic information for Mesocletodes elmari sp. n. with respect to the first and the last two A1 segments. These segments, moreover, are not sexually dimorphically modified in CIV or later stages.
Segment 1 lacks a seta at least from CIII onwards (Figs 4 A, 10 A, B, 14 A, F).
The presence of a seta on this segment in CI and CII, but the loss at
the molt to CIII is discussed to be the case for some harpacticoid
species (cf.
P1–P5. Copepodid development of CI to CV
implies extensive changes in P2–P5 with respect to segmentation and
setation at each molt. P1 exopodal setation, however, is complete from
CI, endopodal setation from CII (
Outer elements on the pereiopods of Mesocletodes elmari sp. n. occur earlier during ontogeny than inner setae, exps and enps are affected likewise (see P2–P4 of CIII and CIV, Figs 13 B–D, 14 C–E) (cf.
In CIV males the P5 endopodal lobe corresponds to the one in CV and adult, whereas the P5 exp lacks the proximal inner seta (Fig. 8 D, E, F) (see section Sexual dimorphisms in juveniles).
On the basis of adult specimens,
The characteristic Mesocletodes
seta on the second A1 segment is developed from CV onwards of both
genders. This segment does not show sexually dimorphic modification,
except that the setae of females are bipinnate, whereas males bear bare
setae. All investigated stages of Mesocletodes elmari sp. n. lack the proximal outer spine on P1 exp3. According to
Various taxa of benthic harpacticoid copepods show
distribution ranges at the species level that extend over thousands of
kilometers across Atlantic, Southern Ocean and Pacific abyssal plains: Ancorabolidae Sars, 1909a (
In the case of Mesocletodes,
as well, the sampling localities known up to now suggest an extremely
wide distribution of this genus: the North Atlantic (Scandinavian coast
[
The record of Mesocletodes elmari sp. n. in the North Atlantic Ocean and South Atlantic Ocean, the Southern Ocean, the Pacific Ocean and the South Indian Ocean extends the knowledge on the distribution of Mesocletodes and points a worldwide distribution at the species level. Future studies will have to deal with the means of dispersal as well as ecological and biological needs of species belonging to Mesocletodes to help explain the distributional patterns.
I greatly appreciate the contributions of colleagues who provided material from different expeditions: ANDEEP I–III and DIVA-2 was sorted by Marco Bruhn, Jutta Heitfeld, Annika Hellmann and Eric Gutzmann. The sorting of material of DIVA-1 was coordinated by Sybille Seifried. CROZEX samples were handled by Hanna Lösekann, NODINAUT samples by Radith Mahatma, OASIS samples by Marco Büntzow, Marco Bruhn, Jutta Heitfeld and Annika Hellmann, Porcupine Abyssal Plain samples by Vassiliki Kalogeropoulou. The type material of Mesocletodes farauni, Mesocletodes glaber and Mesocletodes monensis was kindly provided by Francis Dov Por and Ariel Chipman from the Hebrew University of Jerusalem (Israel). Thanks go to Nechama Ben-Eliahu for her hospitality during my stay in Jerusalem. The type material of Mesocletodes parabodini was kindly provided by Dirk Brandis from the Zoologisches Museum Kiel (Germany). Thanks are also due to Kai Horst George, who helped to improve this manuscript. I am very grateful for the valuable and constructive criticism of two reviewers. Thanks go to Brigitte Ebbe for proofreading the English. This study was carried out within the CeDAMar project. Financial support was obtained from the DFG (GE 1086/6-1 and GE 1086/11-1).