(C) 2010 Neil Adam Smith. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Although flightless alcids from the Miocene and Pliocene of the eastern Pacific Ocean have been known for over 100 years, there is no detailed evaluation of diversity and systematic placement of these taxa. This is the first combined analysis of morphological and molecular data to include all extant alcids, the recently extinct Great Auk Pinguinus impennis, the mancalline auks, and a large outgroup sampling of 29 additional non-alcid charadriiforms. Based on the systematic placement of Mancallinae outside of crown clade Alcidae, the clade name Pan-Alcidae is proposed to include all known alcids. An extensive review of the Mancallinae fossil record resulted in taxonomic revision of the clade, and identification of three new species. In addition to positing the first hypothesis of inter-relationships between Mancallinae species, phylogenetic results support placement of Mancallinae as the sister taxon to all other Alcidae, indicating that flightlessness evolved at least twice in the alcid lineage. Convergent osteological characteristics of Mancallinae, the flightless Great Auk, and Spheniscidae are summarized, and implications of Mancallinae diversity, radiation, and extinction in the context of paleoclimatic changes are discussed.
Cenozoic, convergence, diversity, evolution, fossil, Miomancalla
Alcidae
Primarily owing to the penguin-like characteristics of the flightless Great Auk Pinguinus impennis (
Although all extant alcids are volant, two lineages of
extinct flightless auks are known. These flightless auks superficially
resemble penguins, and share many morphological features convergent
with those southern hemisphere wing-propelled divers such as an
elongated first metacarpal and humeri with anteriorly rotated humeral
heads (
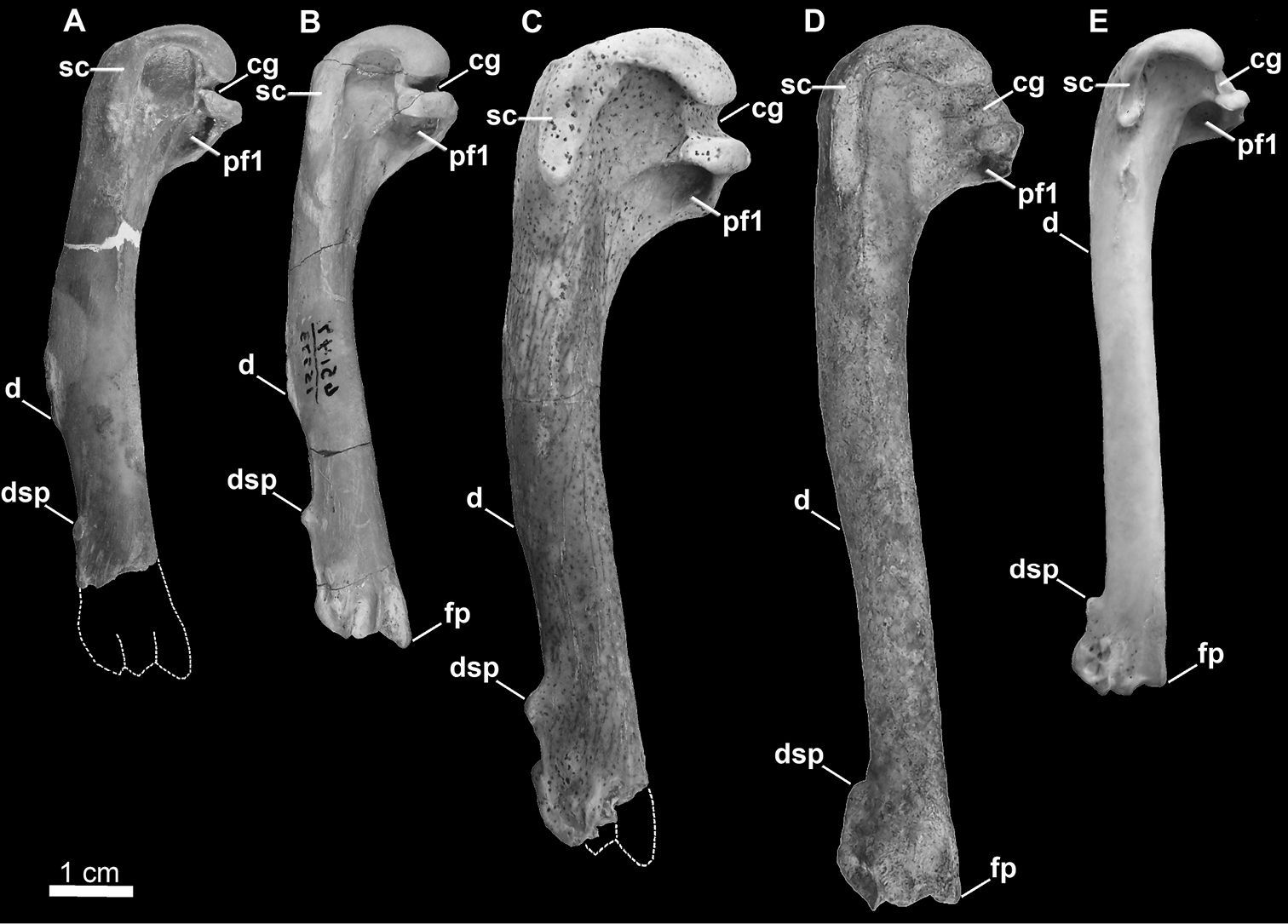
Comparison of alcid humeri in posterior view. Previously recognized Mancallinae holotype humeri along with examples of Pinguinus impennis and volant Alca torda humeri for comparison (dotted lines represent reconstructed parts of humeri). A Holotype specimen of Mancalla californiensis (USNM 4976) B Holotype humerus of Mancalla cedrosensis (LACM 15373) C Holotype specimen of Miomancalla wetmorei (LACM 42653) D Pinguinus impennis (USNM 623465) E Alca torda (NCSM 20058). Anatomical abbreviations: cg capital groove d deltopectoral crest dsp dorsal supracondylar process fp flexor process pf1 primary pneumotricipital fossa sc supracoracoidal crest.
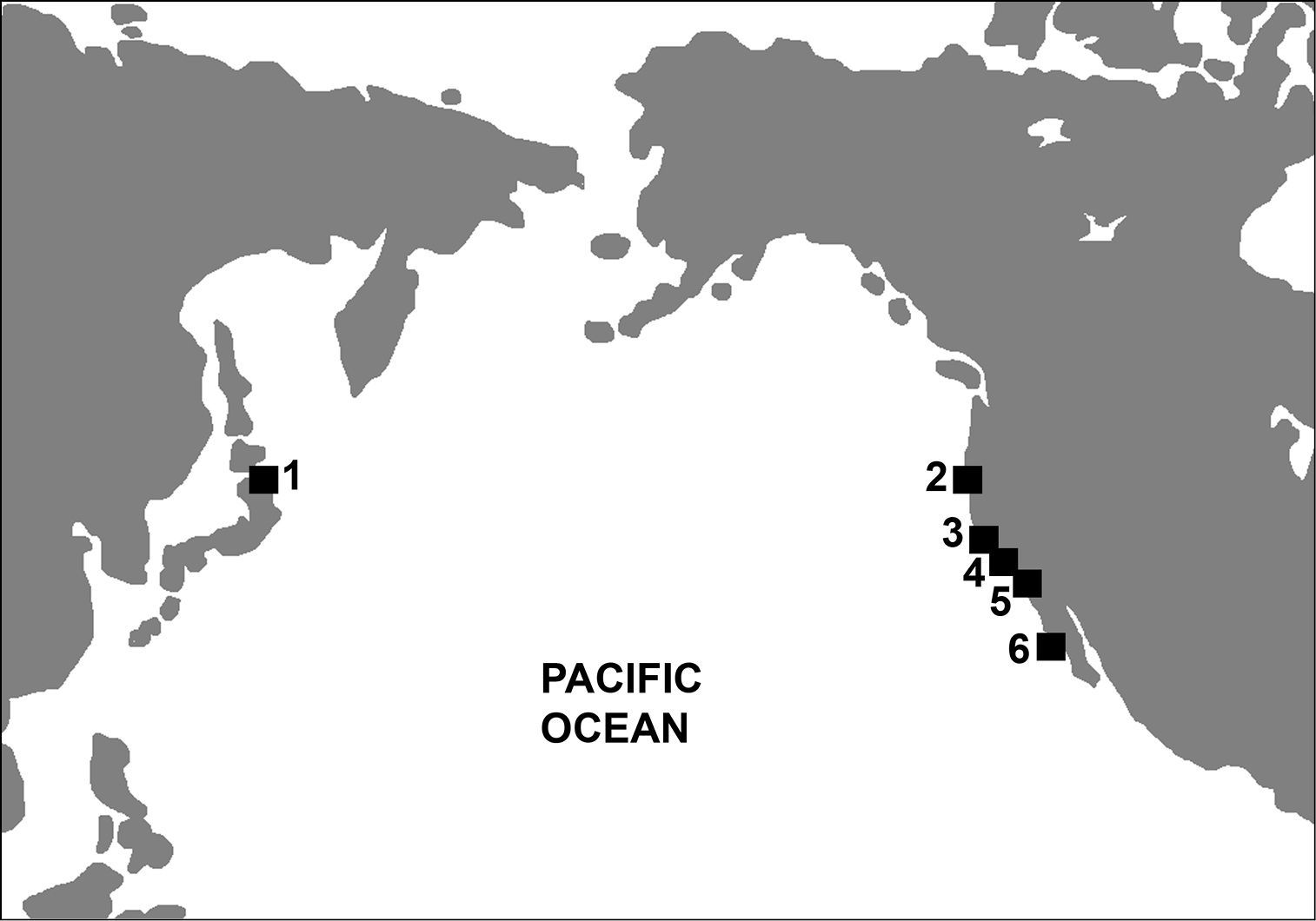
Fossil records of Mancallinae are restricted to the northern Pacific Ocean basin. Miocene and Pleistocene aged fossils have been reported from Japan (
Map depicting Mancallinae fossil localities. 1 Shiriya, Honshu, Japan 2 Humboldt County, CA, USA 3 Los Angeles, CA, USA 4 Laguna Hills, and Laguna Niguel, CA, USA 5 San Diego, CA, USA 6 Cedros Island, Baja California, Mexico.
Mancallinaeholotype material. See Appendix 1 for details of the taxonomic revision.
| Taxon | Holotypematerial | Provenience | Age | Reference | Taxonomic Status |
|---|---|---|---|---|---|
| Mancalla californiensis | Humerus | Los Angeles, CA | EarlyPliocene |
|
Mancalla californiensis |
| Mancalla diegensis | Femur | San Diego, CA | EarlyPliocene |
|
Pan-Alcidae incertae sedis |
| Praemancalla lagunensis | Distal Humerus | Laguna Hills, CA | LateMiocene |
|
Mancallinae incertae sedis |
| Alcodes ulnulus | Ulna | Laguna Hills, CA | MiddleMiocene |
|
Pan-Alcidae incertae sedis |
| Mancalla milleri | Femur | San Diego, CA | EarlyPliocene |
|
Pan-Alcidae incertae sedis |
| Mancalla cedrosensis | PartialSkeleton | Baja Calif., Mexico | LateMiocene |
|
Mancalla cedrosensis |
| Praemancalla wetmorei | Humerus | Laguna Niguel, CA | LateMiocene |
|
Praemancalla wetmorei |
| Mancalla emlongi | Ulna | San Diego, CA | EarlyPliocene |
|
Mancallinae incertae sedis |
| Miomancalla howardi | PartialSkeleton | San Diego, CA | LateMiocene | Smith 2011 | Miomancalla howardi |
| Mancall alucasi | PartialSkeleton | San Diego, CA | EarlyPliocene | Smith 2011 | Mancall alucasi |
| Mancalla vegrandis | PartialSkeleton | San Diego, CA | EarlyPliocene | Smith 2011 | Mancalla vegrandis |
Discovery of an articulated partial skeleton referable to Mancallinae (SDSNH 68312) from the Early Pliocene Capistrano Formation of Orange County California prompted a re-examination of diversity and morphological variation within this clade. Previously reported Mancallinae remains are reviewed (Appendix 1), and the results of an extensive survey of Mancallinae remains are reported. Three new species of Mancallinae are described, and the systematic placement of Mancallinae within Alcidae, as well as the inter-relationships of Mancallinae species is evaluated in a combined phylogenetic analyses of morphological and molecular sequence data. This study represents the first time that relationships among all 23 extant alcids and 29 other charadriiform outgroup taxa have been assessed in the context of a combined phylogenetic analysis.
Materials and methods Anatomical terminology and taxonomic conventionsDescription of anatomical features primarily follows
the English equivalents of the Latin osteological nomenclature
summarized by
With the exception of species names (e.g., Fratercula arctica), which follow the 7th edition of the Checklist of North American Birds (
All extinct taxa were evaluated by direct observation
of holotype and referred specimens. Whenever available, a total of
five or more specimens of each extant species (Appendix 2) including
both sexes were evaluated to account for intraspecific character
variation and sexual dimorphism respectively. Only adult specimens,
assessed based upon degree of ossification (
The cladistic matrix (Appendix 4) includes 72
terminals, scored for a maximum of 344 morphological characters (284
binary; 60 multistate;15 ordered). All 23 extant alcids, the recently
extinct Great Auk Pinguinus impennis Linnaeus, 1758, 18 Mancallinae specimens, and a Mancallinae
supraspecific terminal are included in the matrix. Twenty-nine other
extant charadriiforms comprise the remainder of the taxa analyzed, and
provide a dense outgroup taxonomic sample to test the monophyly of
extant and extinct alcids. with respect to other
charadriiforms.Morphological characters include osteological (n = 223), integumentary (n = 32), ethological (n = 16), myological (n = 24) and micro-feather (n
= 52). One hundred and fifty-five characters were newly identified for
this analysis. The other 189 characters were drawn from the work of
The cladistic matrix also includes a molecular
sequence alignment of 11, 601 base pairs from eight DNA sequence types
(including gaps). See Appendix 5 for details of sequence availability,
inclusion for each species, and sequence authorship. Molecular sequence
data (mitochondrial: ND2, ND5, ND6, CO1, CYTB; ribosomal RNA: 12S,
16S; and nuclear: RAG1) were downloaded from GenBank. Preliminary
sequence alignments for each gene were obtained using the program
ClustalX v2.0.6 (
A combined approach of phylogeny estimation was used to evaluate the systematic position of Mancallinae
species. Simulations show that the combination of molecular and
morphological data often provides a more accurate estimate of phylogeny
with respect to both extant and extinct organisms (
AMNH—American Museum of Natural History, New York, NY, USA; GCVP—Georgia College and State University Vertebrate Paleontology Collection, Milledgeville, GA, USA; IVPP—Institute of Vertebrate Paleontology and Paleoanthropology, Beijing, China; LACM—Natural History Museum of Los Angeles County, Los Angeles, CA., USA; LM—Loye Miller Collection, location presently unknown; NSM PO—National Museum of Nature and Science Paleontology Osteological Collection, Tokyo, Japan; NCSM—North Carolina Museum of Natural Sciences, Raleigh, NC, USA; SDSNH—San Diego Natural History Museum, San Diego, CA, USA; TMM—Texas Natural Science Center Vertebrate Paleontology Laboratory, Austin, TX, USA; UCMP—University of California Museum of Paleontology, Berkeley, CA, USA; USNM—National Museum of Natural History, Smithsonian Institution, Washington, D.C., USA.
Systematic PaleontologyAVES Linnaeus, 1758
CHARADRIIFORMES Huxley, 1867
PAN-ALCIDAE new taxon.
Pan-Alcidae (contents = Alcidae Leach, 1820 (i.e., the alcid crown clade) + Mancallinae) is differentiated from all other Charadriiformes by the following characteristics: quadrate apneumatic (38:1); reduced pneumatic foramen of anterior sternum (59:0); omal ext
Unambiguously optimized morphological characters with a CI of 1.0 supporting alcid clades in the resultant phylogenetic tree (Fig. 15). Character numbers from Appendix 3 are followed by character state symbols (e.g., 23:0 = character number 23, state 0). ‘*’ indicates selected locally optimized apomorphies with a CI of < 1.0.
| Clade | Character numbers and states that support monophyly |
|---|---|
| Pan-Alcidae + Stercorariidae | *63:0; *124:1; *190:1; 315:1; 343:1 |
| Pan-Alcidae | 35:0; 38:1; 75:1; 77:1; 162:1 |
| Alcidae (crown clade) | 68:1; 153:1; 172:1 |
| Alcinae | 49:1; 270:1; 281:1 |
| Alcini | 185:1; 237:1; 239:1; 274:1 |
| Fraterculinae | *10:1; *13:0; *52:1; *67:1; *72:1 |
| Fraterculini | 29:1; 35:1; 40:1; 63:1; 112:0; 275:1; 286:1; 287:1 |
| Aethiini | 11:1; 86:0; 94:1; 201:1 |
| Mancallinae | 104:2; 120:1; 139:1; 140:1; 148:1; 150:1; 183:0; 184:1 |
| Mancalla | *130:1; 137:1 |
Mancallinae (contents = Mancalla + Miomancalla gen. n.) is referable to Pan-Alcidae based upon dorsoventral compression of the humeral shaft (141:2). The humeral shafts of Pan-Alcidae are more dorsoventrally compressed than in all other Charadriiformes. Mancallinae is differentiated from all other alcids on the basis of the following unambiguously optimized humeral apomorphies: deltopectoral crest extends past the midway point of the humeral shaft rather than restricted to the proximal half of the humeral shaft (104:2); presence of a ‘mancalline muscle scar’ extending distally from the primary pneumotricipital fossa (discussed below; 120:1); capital groove communicates with transverse ligament sulcus resulting a notched rather than rounded appearance of ventral margin of the humeral head in anterior view (136:2); humeral head rotated anterodorsally rather than in-line with humeral shaft (139:1); humeral shaft arced rather than sigmoidal (140:1); presence of fossae in tricipital sulci (150:1); anterior surface of the ventral condyle rounded rather than flattened (153:0). Additional proposed apomorphies of Mancallinae include distal elongation (184:1) and anterior flattening of the first metacarpal (185:1). These characteristics are present in Mancalla cedrosensis Howard, 1971, Miomancalla howardi sp. n., and two additional associated specimens referable to Mancallinae (SDSNH 77966 and LACM 107028). Although these two characters are also diagnostic for Alcini Storer, 1960, the clade composed of Alca, Pinguinus, Alle Link, 1806, and Uria Brisson, 1760, the degree of distal elongation and anterior flattening in Mancalla exceeds that observed in Alcini.
(sensu
. Mancalla is differentiated from Miomancalla on the basis of the following humeral characteristics: supracoracoidial crest does not broaden proximally (113:2); distal margin of the primary pneumotricipital fossa convex rather than concave (126:0); ventral margin of the ventral tubercle narrow and ventrally expanded (i.e., convex) rather than wide and deeply grooved (134:0); capital groove constricted rather than wide (137:1). Additional proposed apomorphies which are present in Mancalla cedrosensis and two additional associated specimens (SDSNH 77966 and LACM 128870) referable to Mancalla but not to species include: ulna shorter than carpometacarpus (180:1); ulna and radius more dorsoventrally compressed than other alcids; extension of the dorsal ulnar condyle farther distally to the ventral ulnar condyle than in other alcids (182:0); pisiform process of carpometacarpus reduced or absent (188:1).
urn:lsid:zoobank.org:act:31389B4B-0A03-48E5-8A0B-C71CDBCE7164
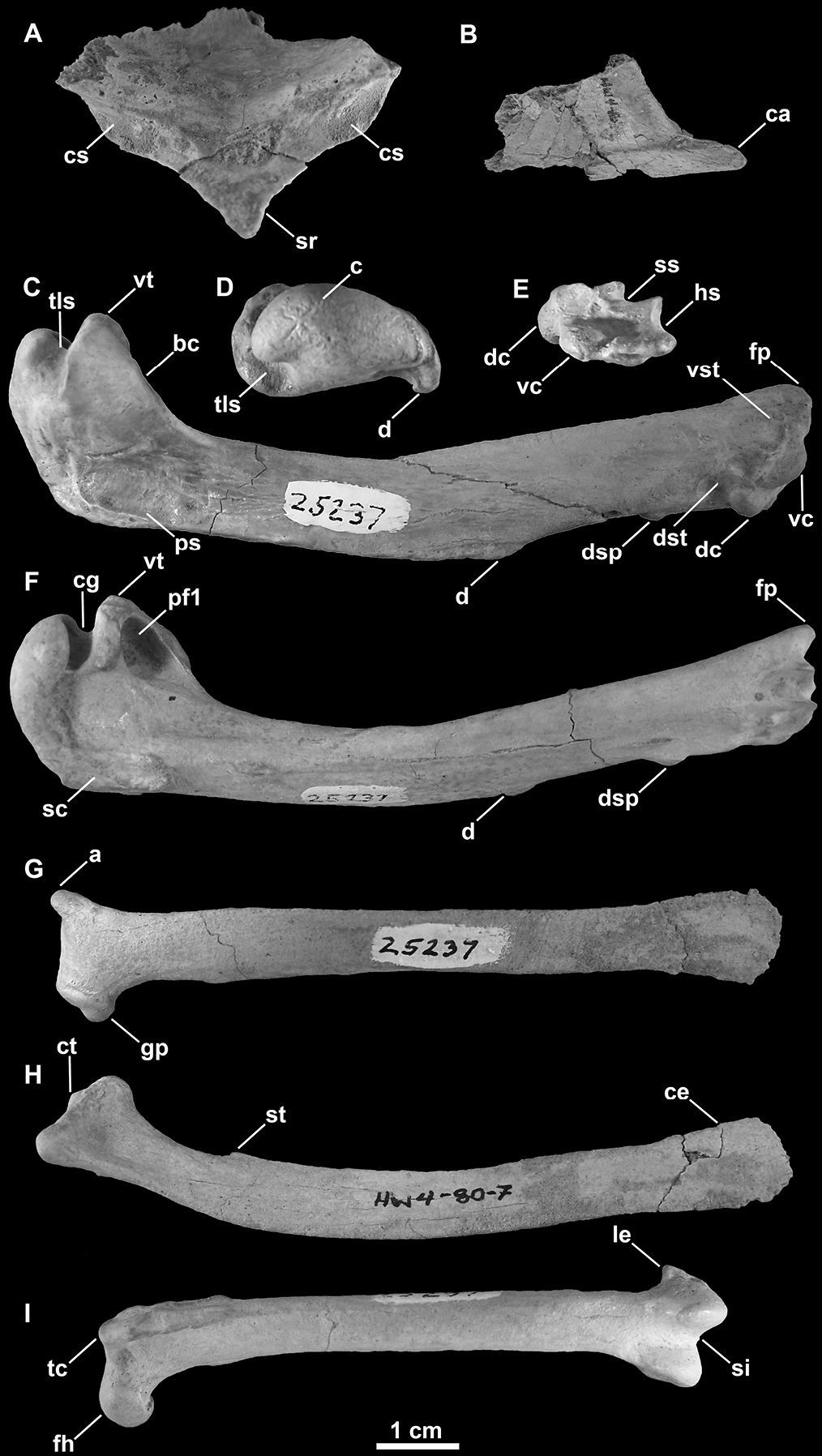
SDSNH 25237: a partial postcranial skeleton comprising the following elements: right and left scapulae, partial sternum, right and left humeri, left femur (Fig. 3; Tables 1, 2 and 3). The holotype specimen was collected by H. M. Wagner in April, 1980.
Holotype specimen of Mancalla lucasi (SDSNH 25237). A Fragment of anterior sternum in anterior view B Carinal apex of sternum in right lateral view C Right humerus in anterior view D Left humerus in proximal view E Left humerus in distal view F Left humerus in posterior view G Left scapula in lateral view H Right scapula in medial view I Left femur in anterior view. Anatomical abbreviations: a acromion process bc bicipital crest c caput ca carinal apex ce caudal extremity of scapula cg capital groove cs coracoidal sulcus ct coracoidal tubercle d deltopectoral crest dc dorsal condyle dsp dorsal supracondylar dst dorsal supracondylar tubercle fh femoral head fp flexor process gp glenoid process hs humerotricipital sulcus le lateral epicondyle pf1 primary pneumotricipital fossa ps pectoralis scar sc supracoracoidal crest si sulcus intercondylaris sr sternal rostrum ss scapulotricipital sulcus st scapulotricipital tubercle tc trochanteric crest tls transverse ligament sulcus vc ventral condyle vst ventral supracondylar tubercle vt ventral tubercle.
Measurements of Mancallinae
holotype humeri (mm). Abbreviations: (Glh) greatest length of humerus;
(Bph) breadth of proximal humerus; (Diph) diagonal of proximal humerus;
(Whs) width of humeral shaft; (Bdh) breadth of distal humerus; (Ddh)
depth of distal humerus. Measurements according to
| Species | Specimen # | Glh | Bph | Diph | Whs | Bdh | Ddh |
|---|---|---|---|---|---|---|---|
| Mancalla californiensis | USNM4976 | ~75.0 | 19.0 | 18.4 | 8.9 | ___ | ___ |
| Mancalla cedrosensis | LACM15373 | 73.3 | 17.8 | 17.1 | 9.1 | 13.0 | 7.1 |
| Mancalla lucasi | SDSNH25237 | 90.2 | 21.7 | 21.2 | 11.1 | 13.4 | 8.0 |
| Mancalla vegrandis | SDSNH77399 | 61.8 | 15.1 | 14.3 | 7.4 | 9.5 | 5.8 |
| Miomancalla wetmorei | LACM42653 | ~86.0 | 21.5 | 21.1 | 12.7 | 8.7 | 9.5 |
| Miomancalla howardi | SDSNH24584 | 103.2 | 22.9 | 22.2 | 11.1 | 12.2 | 8.7 |
| Miomancalla howardi | SDSNH68312 | ___ | ~25.0 | ~24.0 | ___ | ___ | ___ |
Measurements of new associated Mancallinae holotype specimens (in mm). ‘-’ = missing data due to damage or lack of comparable element.
| Miomancalla howardi | Mancalla lucasi | Mancalla vegrandis | |
| SDSNH 68312 | SDSNH 25237 | SDSNH77399 | |
| SKULL & MANDIBLE | |||
| Greatest length of skull | 122.9 | - | - |
| Greatest breadth of frontal | 11.4 | - | - |
| Greatest length of rostrum | 84.2 | - | - |
| Greatest height of rostrum | 21.1 | - | - |
| Greatest length of mandible | 127.8 | - | - |
| STERNUM | |||
| Smallest width between costal processes | - | - | 5.9 |
| FURCULA | |||
| Dorsoventral height of apophysis | - | - | 2.8 |
| CORACOID | |||
| Greatest length | - | - | 45.8 |
| SCAPULA | |||
| Greatest proximal height | - | 15.1 | 10.9 |
| CARPOMETACARPUS | |||
| Greatest length | 46.8 | - | - |
| Length of metacarpal one | 23.2 | - | - |
| Proximal breadth | 11.9 | - | - |
| PELVIS | |||
| Greatest length | 127.8 | - | 74.8 |
| FEMUR | |||
| Greatest length | 79.9 | 67.8 | - |
| Medial length | 78.0 | 64.9 | - |
| Proximal breadth | 17.8 | 12.9 | - |
| Proximal depth | 10.9 | 9.2 | - |
| Breadth of shaft | 8.3 | 7.5 | - |
| Distal breadth | 18.0 | 12.5 | - |
| TIBIOTARSUS | |||
| Greatest length (preserved) | 113.7 | - | - |
| Breadth of shaft | 7.8 | - | - |
This new species is named in honor of Frederic A. Lucas who described the first known remains of Mancalla.
Late Pliocene or Early Pleistocene (Zanclean or Calabrian) Niguel Formation of Orange County, California. Latitude, longitude, and elevation data are on file at SDSNH (locality 3202). Details of the geologic setting are provided in Appendix 6.
SDSNH 59049: a complete left humerus from the Middle Pliocene to Early Pleistocene San Diego Formation (SDSNH locality 3506; Fig. 4E).
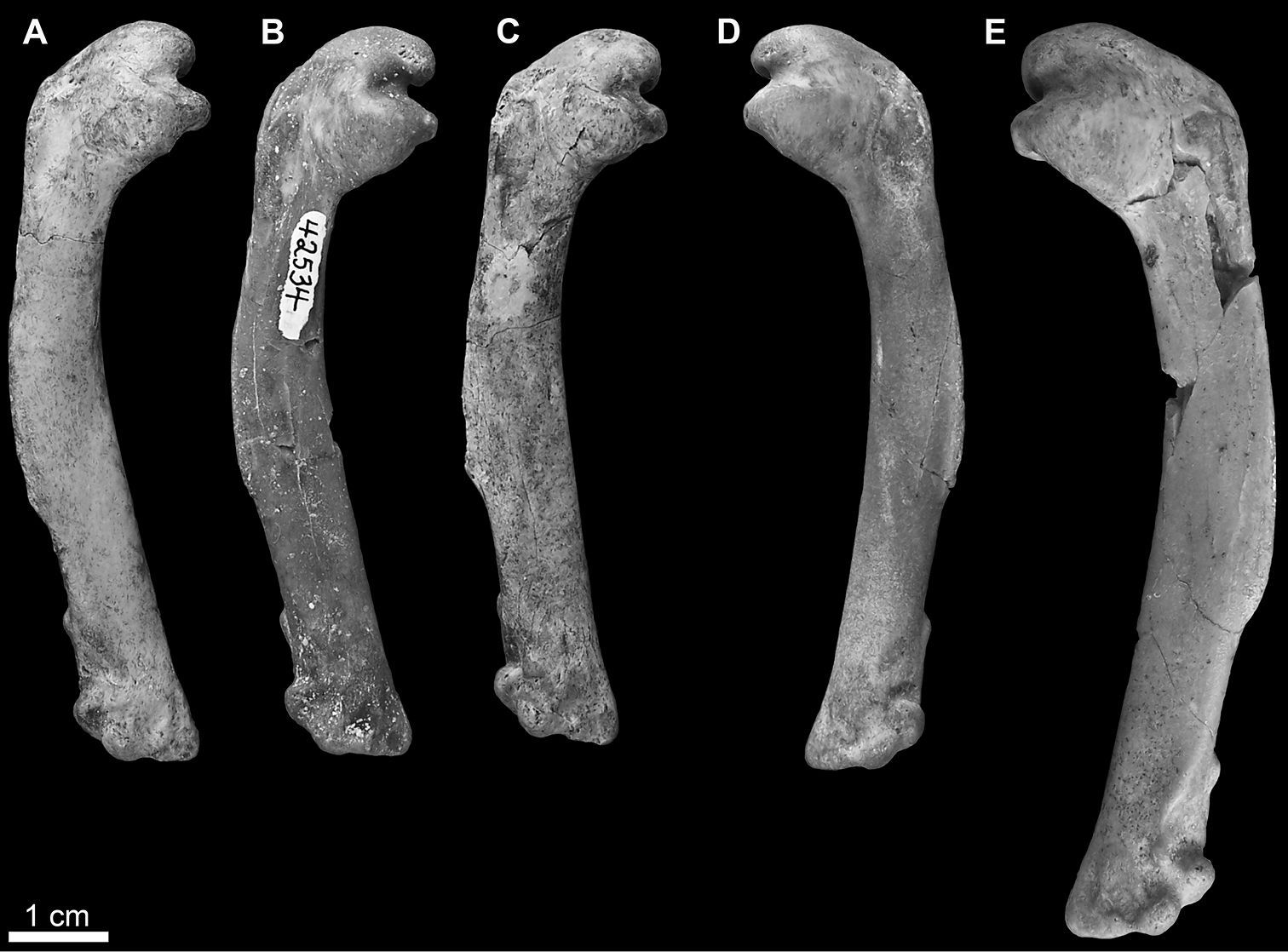
Mancalla referred humeri in anterior view. A Mancalla vegrandis SDSNH 28152 B Mancalla vegrandis SDSNH 42534 C Mancalla vegrandis SDSNH 75051 D Mancalla vegrandis SDSNH 42532 E Mancalla lucasi SDSNH 59049.
Scar extending into primary pneumotricipital
fossa is raised in relief to the floor of the primary pneumotricipital
fossa and the humeral shaft as in Mancalla cedrosensis, rather than an excavated pit as in Mancalla vegrandis sp. n. and Mancalla californiensis
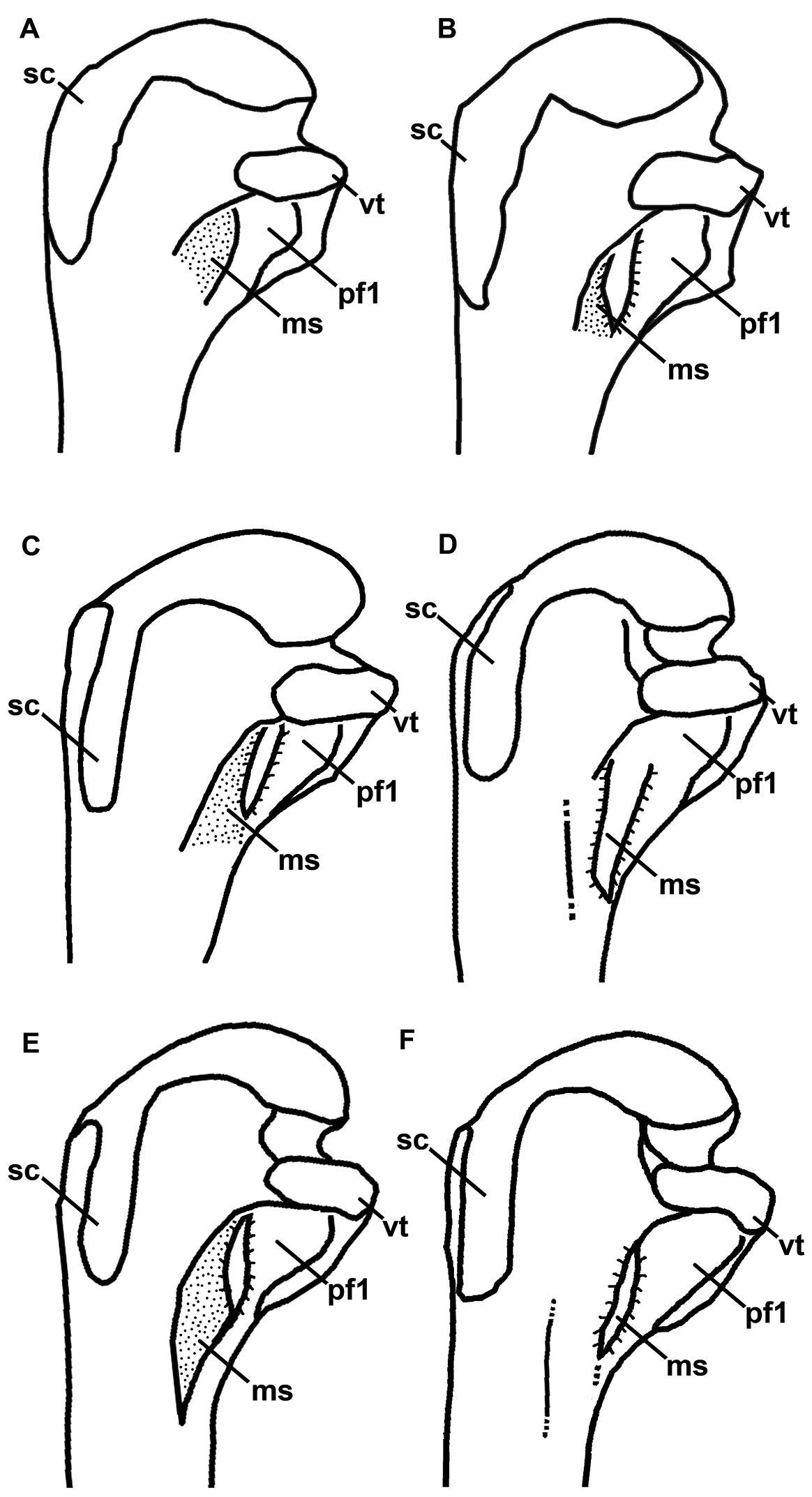
Line drawings for comparison of Mancallinae proximal humeri in posterior view (not to scale). A Miomancalla wetmorei B Miomancalla howardi C Mancalla californiensis D Mancalla cedrosensis E Mancalla vegrandis F Mancalla lucasi. Anatomical abbreviations: ms mancalline scar pf1 primary pneumotricipital fossa sc supracoracoidal crest vt ventral tubercle.
Both scapulae are preserved (Fig. 3G, H). As in all Alcidae, the scapular shaft is mediolaterally compressed throughout its entire length. The proximal end of the scapular shaft is more rounded in other Charadriiformes. As in Mancalla vegrandis, the acromion projects farther anteriorly than that of Mancalla cedrosensis and other alcids (e.g., Uria, Aethia). As in Mancalla cedrosensis, the coracoidal tubercle is less pronounced than in Mancalla vegrandis. As in Mancalla vegrandis and Mancalla cedrosensis, a scapulotricipital tubercle is present just distal to the glenoid process on the ventral margin of the scapular shaft. This feature is also present in other flightless wing-propelled divers such as Spheniscidae and Pinguinus, but is not known in any volant alcid. As in Mancalla vegrandis, the scapular shaft, including the caudal extremity, is slightly more robust than in other alcids (e.g., Alca, Aethia). The caudal extremity is less dorsoventrally expanded than in Mancalla vegrandis. The caudal extremity is not known for Mancalla cedrosensis.
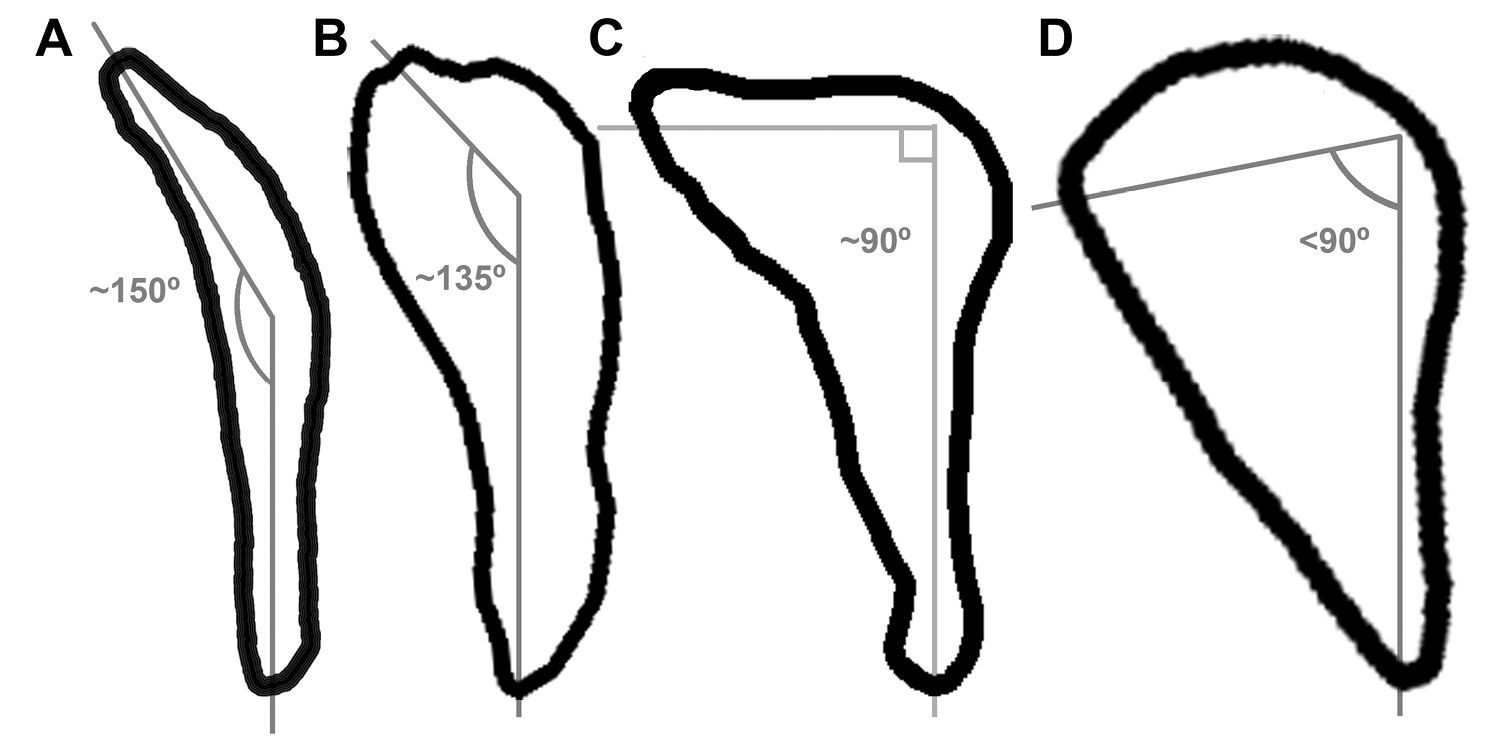
Fragments of the sternum preserve the sternal rostrum, coracoidal sulci, and the carinal apex (Fig. 3A, B). These features are not preserved in Miomancalla howardi and comparisons are therefore limited to extant alcids and specimens of Mancallinae that are not presently referable to species. The morphology of the sternal rostrum is consistent with that of all other Alcidae. Although no coracoid is preserved in the holotype specimen of Mancalla lucasi, the shape of the coracoidal sulci of the sternum is consistent with the ~150° angle of the sternal articulation of the coracoid in Mancalla cedrosensis and Mancalla vegrandis. The sternal articulation of the coracoid, and the coracoidal sulci of the sternum in other alcids curves more acutely (e.g., ~90° in Alca torda; Fig. 6).
Comparison of sternal facet curvature in charadriiform left coracoids (sternal view; not to scale). A Stercorarius B Mancalla C Alca D Aethia.
Complete right and left humeri are preserved (Fig. 3C, D, E and F). Based upon humeral proportions, Mancalla lucasi represents the largest known species of Mancalla (Table 2). As in other Mancalla species, the ventral margin of the ventral tubercle is convex, and the capital groove is relatively narrower than other Alcidae. The ventral tubercle does not project as far ventrally as in Mancalla californiensis (Fig. 5). The distal end of the deltopectoral crest transitions to the shaft more abruptly than in Mancalla vegrandis. As in other Mancalla, the humeral head is rotated anteriorly, and the supracoracoideus muscle scar does not broaden proximally. Mancallinae
is characterized by a scar of unknown function that is positioned
adjacent to the primary pneumotricipital fossa (hereafter referred to as
the ‘mancalline scar’; Fig. 5). The position of the ‘mancalline scar’ suggests an accessory insertion of m. humerotriceps (
The left femur is preserved (Fig. 3I) and is smaller (~15%; Table 2)than in Miomancalla howardi sp. n. (Table 3), and larger (~19%) than in Mancalla cedrosensis
(Howard, 1971). Extant alcids do not display statistically significant
degrees of sexual dimorphism in their size, plumage, or osteological
morphology (
Mancalla lucasi corresponds in size and some humeral characteristics with material previously referred to Mancalla diegensis. However, Mancalla diegensis is considered Alcidae incertae sedis (see Appendix 1 for details of the taxonomic revision).
urn:lsid:zoobank.org:act:8F6D55BF-C827-47C3-AAB6-777632C92DB6
SDSNH 77399: a partial postcranial skeleton comprising the following elements: two cervical vertebrae, one costal and one vertebral rib, partial furcula, scapulae, left coracoid, partial right coracoid, partial sternum, left humerus, and pelvis (Figs 7 and 8; Tables1, 2 and 3). The holotype specimen was collected by W. T. Stein in October, 1961.
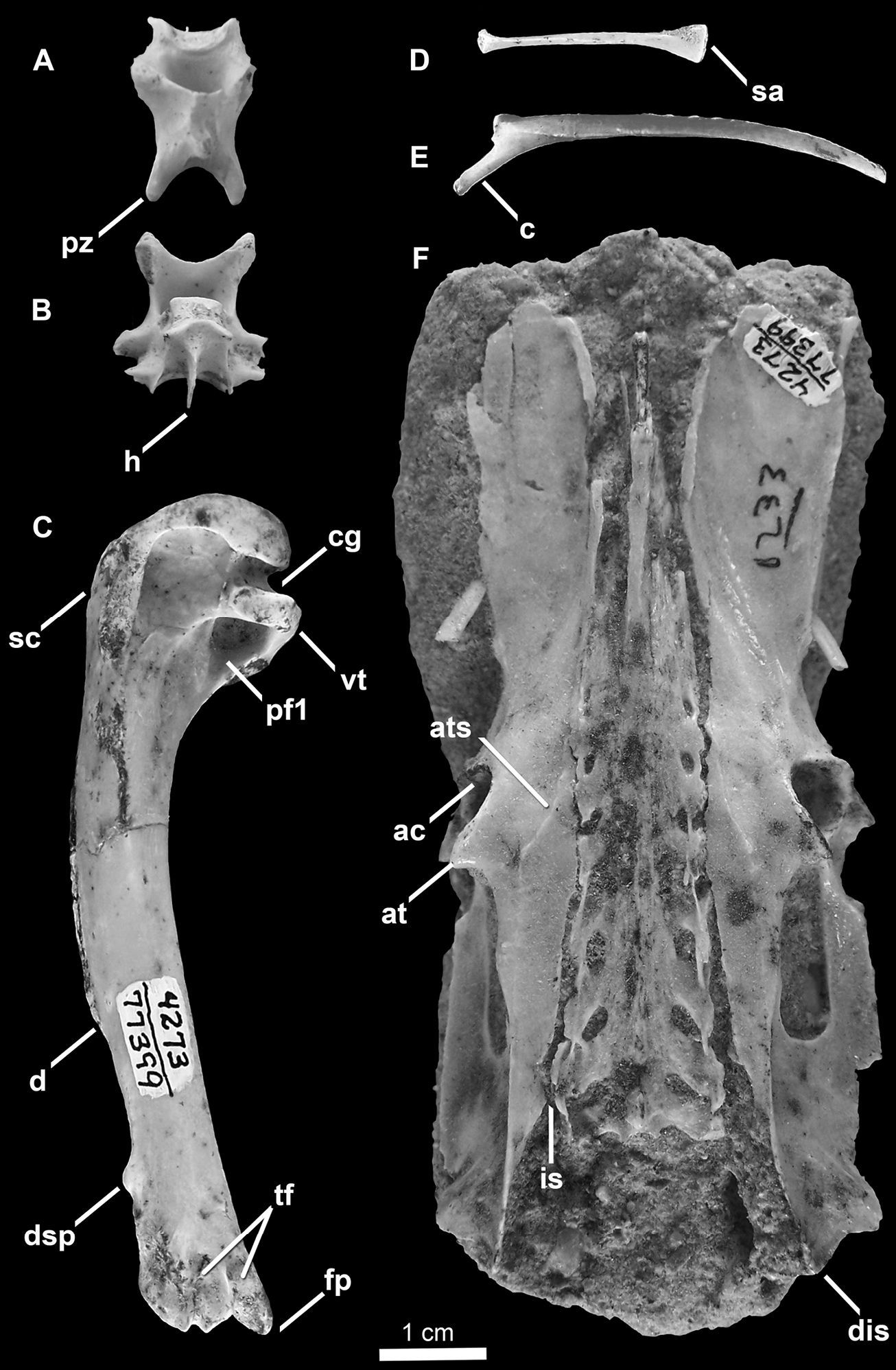
Holotype specimen of Mancalla vegrandis (SDSNH 77399) A Cervical vertebra (C3?) in dorsal view B Cervical vertebra (C4?) in ventral view C Left humerus in posterior view D Costal rib E Vertebral rib F Pelvis in dorsal view. Anatomical abbreviations: ac acetabulum at antitrochanter ats antitrochanteral sulcus c capitulum of vertebral rib cg capital groove d deltopectoral crest dis dorsal illiac spine dsp dorsal supracondylar process fp flexor process h hypapophysis is iliosynsacral suture pf1 primary pneumotricipital fossa pz postzygapophysis sa sternal articulation of costal rib sc supracoracoidal crest tf tricipital fossae vt ventral tubercle.
The species name vegrandis reflects the diminutive size of this taxon compared to other known Mancalla species (vegrandis, from the Latin for small, diminutive or tiny).
Middle Pliocene to Early Pleistocene (Zanclean-Calabrian) San Diego Formation of San Diego County, California. Latitude, longitude, and elevation data are on file at SDSNH (locality 4273). Details of the geologic setting are provided in Appendix 6.
SDSNH 42532: a complete left humerus from the Middle Pliocene to Early Pleistocene San Diego Formation of San Diego County, California (SDSNH locality 3468); SDSNH 42534: a complete right humerus from the Middle Pliocene to Early Pleistocene San Diego Formation of San Diego County, California (SDSNH locality 3468); SDSNH 28152: a complete right humerus from the Early Pliocene upper member of the San Mateo Formation of San Diego County, California (SDSNH locality 3161); SDSNH 75051: a complete right humerus from the Early Pliocene upper member of the San Mateo Formation of San Diego County, California (SDSNH locality 2643; Fig. 4A–D).
Dorsal and ventral edges of the mancalline scar extending into primary pneumotricipital fossa taper to a point as in Mancalla lucasi, rather than remaining parallel as in Mancalla californiensis and Mancalla cedrosensis (123:1; Fig. 5); mancalline scar extending into primary pneumotricipital fossa is an excavated pit as in Mancalla californiensis rather than raised in relief to the floor of the primary pneumotricipital fossa and the humeral shaft as in Mancalla cedrosensis and Mancalla lucasi (121:0); humerus shorter than other known Mancalla (Tables 2 and 3).
Two cervical vertebrae are preserved (Fig. 7A and B). Comparisons with Miomancalla howardi are limited to generalities regarding shape in dorsal view, for which the morphology of Mancalla vegrandis is consistent with that of Miomancalla howardi. Only thoracic vertebrae are known for Mancalla cedrosensis. One of the vertebrae (Fig. 7A) is mediolaterally narrower than the other (Fig. 7B). Although the width of cervical vertebrae other than the axis and atlas do not vary considerably in extant Alcidae, the 3rd and 4th cervical vertebrae of some charadriiforms (e.g., Larosterna inca Lesson, 1827) are mediolaterally narrower than cervical vertebra posterior to the 4th (i.e., C5, C6, C7). The dorsal surface of the broader vertebra (Fig. 7B) is perforated by a small foramen (i.e., perforation of laminae arcocostales). In extant alcids, only the third and fourth cervical vertebrae are perforated. Typically in extant Alcidae, the third cervical vertebra is punctured by a small foramina, whereas the foramina in the fourth cervical vertebra is much larger, leaving only a thin strut of bone bordering it laterally. The morphology of the preserved vertebrae is suggestive of C3 and C4; however, definitive assignment cannot be made at this time.
One complete cervical rib and one complete costal rib (Fig. 7D and E) are preserved along with several other rib fragments (not figured). No morphological differences were evident between the ribs of Mancalla vegrandis, Mancallinae specimen SDSNH 25236, and other alcids for which the ribs are known.
All but the omal extremities of the furcula are preserved (Fig. 8D). The furcular rami are mediolaterally compressed as in all other Alcidae. The anterior surface of the furcular rami dorsal to the apophysis is rounded or convex as in Uria, rather than grooved as in Cepphus. The furcular apophysis does not bear the ventrally expanded, bladelike interclavicular process characteristic of extant Alcidae. However, the possibility that this feature was lost to damage cannot be ruled out. No additional morphological differences were evident between the preserved portions of the furcula of Mancalla vegrandis and other alcids for which the furcula is known.
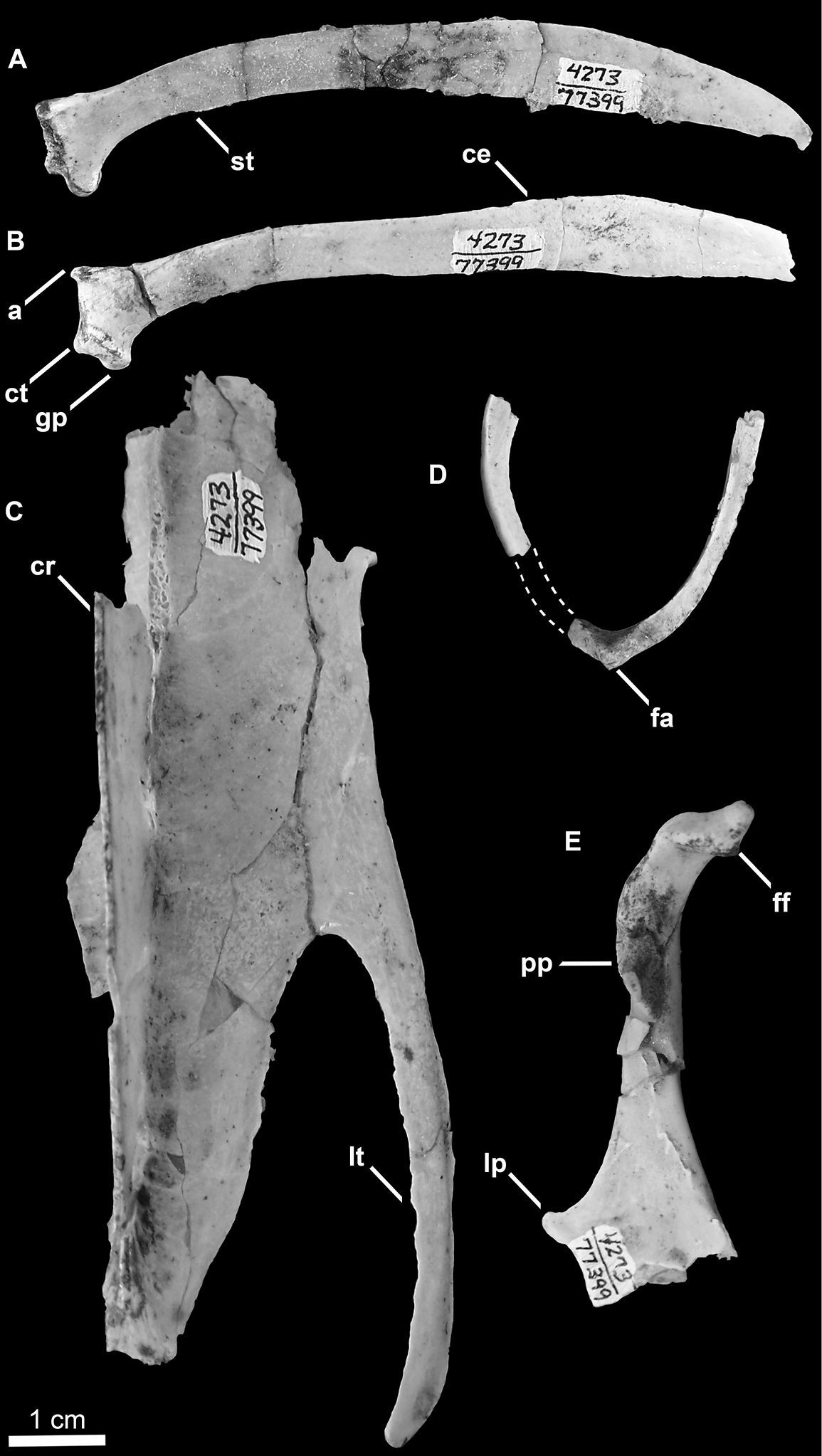
Holotype specimen of Mancalla vegrandis (SDSNH 77399). A Right scapula in medial view B Left scapula in lateral view C Partial sternum in ventral view D Partial furcula in posterior view (dashed lines represent missing portion of left ramus) E Left coracoid in posterior view. Anatomical abbreviations: a acromion process ce caudal extremity of scapula cr sternal carina ct coracoidal tubercle fa furcular apophysis ff furcular facet of coracoid gp glenoid process lp latral process of coracoid lt lateral trabeculae of sternum pp procoracoid process st scapulotricipital tubercle.
The left coracoid is complete except for a small portion of the medial margin of the sternal facet (Fig. 8E). A fragment of the right coracoid preserves the medial margin of the sternal facet and the sternal portion of the coracoidal shaft (not figured). As in Mancalla cedrosensis the furcular facet is rounded, rather than oval as in Aethia and Fratercula. The head of the coracoid is apneumatic as in all Alcidae, but the brachial tuberosity is deeply undercut as in Alca and Pinguinus. The humeral articulation is more rounded than in extant Alcidae. As in Cepphus, the scar marking the position of m. supracoracoideus is less distinct than in other Alcidae. As in Mancalla cedrosensis, Aethia, and Alle, the procoracoidal process is not punctured by a foramen for passage of the tendon of m. supracoracoideus. The procoracoid process points dorsomedially as in all Alcidae except Aethia, in which the procoracoid points more ventromedially. As in Mancalla cedrosensis, Brachyramphus, Uria, Aethia, and Ptychoramphus Brandt, 1837, the sternal margin of the procoracoid process is concave, rather than convex as in Cerorhinca, Fratercula, and Pinguinus. As in many alcids (e.g., Alca, Brachyramphus) a single, distinct, straight ridge, which extends from the lateral angle of the sternal facet towards the humeral facet is present. This ridge does not extend sternally in Synthliboramphus, Cepphus, Fratercula, Aethia, Ptychoramphus, and Cerorhinca. This ridge is less pronounced and positioned farther laterally in Mancalla cedrosensis. A well-developed lateral process is present. This feature is absent in Mancalla cedrosensis. The dorsal margin of the medial sternal process is notched as in most alcids (e.g., Alca torda). As in Mancalla cedrosensis, the posterior surface of the sternal end of the coracoid is more excavated than in extant Alcidae and the sternal facet is curved ~150°.
Right and left scapulae are preserved (Fig. 8A and B). As in all Alcidae, the scapular shaft is mediolaterally compressed throughout its entire length. As in Mancalla lucasi, the acromion projects farther anteriorly than that of other alcids (e.g., Uria, Aethia). The acromion of Mancalla cedrosensis does not project as far anteriorly as that of Mancalla vegrandis. The coracoidal tubercle is more pronounced than in Mancalla lucasi and Mancalla cedrosensis. As in Mancalla lucasi and Mancalla cedrosensis, a scapulotricipital tubercle is present just distal to the glenoid process on the ventral margin of the scapular shaft. As in Mancalla lucasi, the scapular shaft, including the caudal extremity, is slightly more robust than in other alcids (e.g., Alca, Aethia). The caudal extremity is more dorsoventrally expanded than in Mancalla lucasi. The caudal extremity is not known for Mancalla cedrosensis.
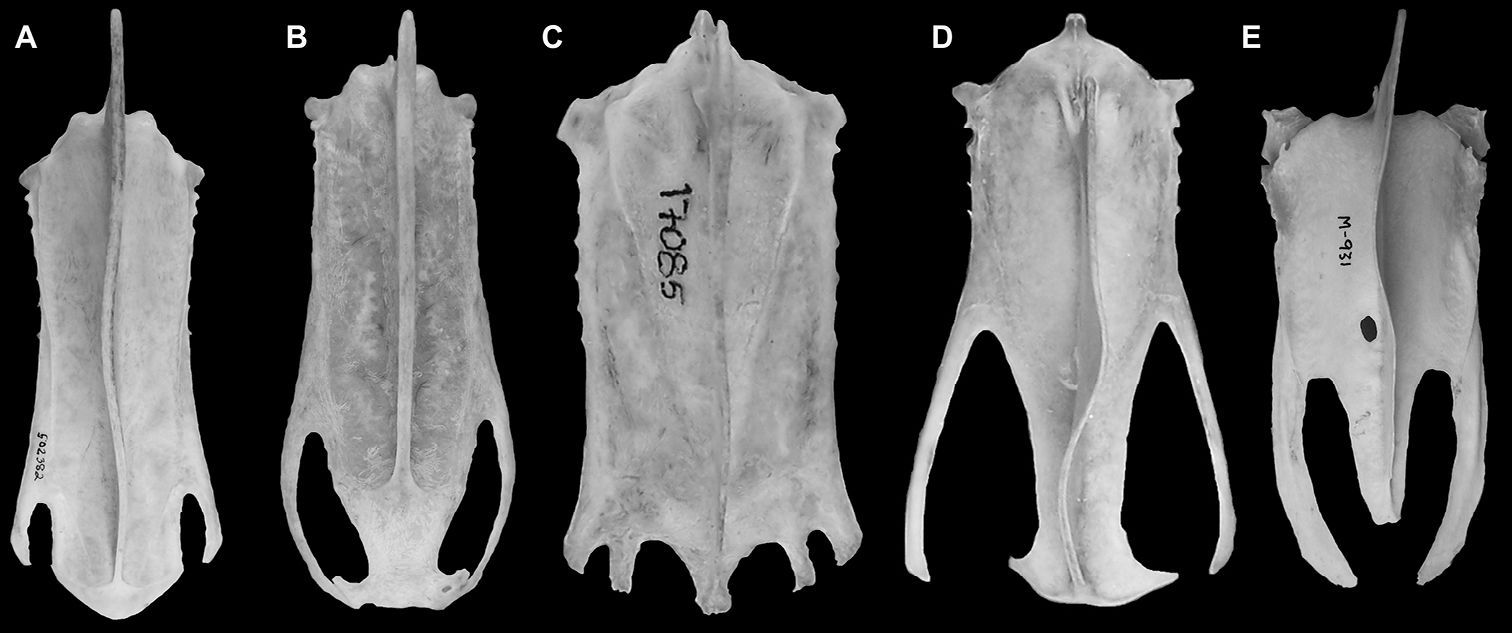
Parts of the left distal end of the sternum including the distal end of the carina, and the left lateral process are preserved (Fig. 8C). Mancalla lucasi and Miomancalla howardi do not preserve the same portions of the sternum so comparisons cannot presently be made between the sterni of Mancallinae. As a result of the deep incisure of the lateral notches the lateral processes of Mancalla vegrandis are more elongate that any other alcids for which the sternum is known. In other Charadriiformes this condition is present only in the Glareolidae and Scolpacidae, and resembles the sternum in Spheniscidae (Fig. 9).
Comparison of charadriiform and sphenisciform sterni. A Alca torda (USNM 502382) B Aethia psittacula (NCSM 18514) C Sterna anaethetus (NCSM 17085) D Hydrophasianus chirurgus (USNM 490566) E Eudyptula minor (TMM M-391).
The left humerus is preserved (Fig. 7C). Based upon humeral proportions, Mancalla vegrandis represents the smallest known species of Mancalla (Table 2). As in other species of Mancalla, the ventral margin of the ventral tubercle is convex, and the capital groove is relatively narrower than other Alcidae. The ventral tubercle does not project as far ventrally as in Mancalla californiensis. The distal end of the deltopectoral crest transitions to the shaft less abruptly than in Mancalla lucasi. As in other Mancallinae, the humeral head is rotated anteriorly and the supracoracoideus muscle scar does not broaden proximally. The ‘mancalline scar’ is excavated as in Mancalla californiensis, rather than raised in relief like that of Mancalla cedrosensis and Mancalla lucasi (Fig. 5). As in Mancalla lucasi, the ‘mancalline scar’ extends from a point just proximal to the junction of the bicipital crest with the humeral shaft and tapers to a point, and extends into the primary pneumotricipital fossa. The margins of this scar remain parallel in Mancalla californiensis and Mancalla cedrosensis. As in all Mancallinae, the humeral shaft is arced rather than sigmoidal or straight. As in other Mancalla, the dorsal supracondylar tubercle is separated from the dorsal epicondyle by a small notch. A tubercle or papilla is present on the posterior side of the distal end of the humerus adjacent to the dorsal condyle (Howard, 1966). As with all Mancallinae, the anterior surface of the ventral condyle is rounded, rather than flattened as in all other Alcidae. Rounded fossae are present at the proximal ends of the humerotricipital and scapulotricipital grooves. The flexor process extends distal to the ventral condyle as in all Mancallinae and Pinguinus.
The pelvis is preserved in dorsal view (Fig. 7F). Comparisons of pelves within Mancallinae are limited to Miomancalla howardi. As in all alcids the anteroposterior length of the pelvis is greater than two times the mediolateral width across the antitrochanters. The relative length of the pelves of other charadriiforms is anteroposteriorly shorter. The proximal end of the preacetabular ilium is wide as in Miomancalla howardi and most alcids (e.g., Brachyramphus). The distal end of the preacetabular ilium is relatively broader than in Miomancalla howardi. As in Miomancalla howardi the antitrochanteral sulcus does not extend proximally to contact the antitrochanter. As in most Alcidae (e.g., Brachyramphus), the post-acetabular dorsal ilium narrows, rather than broadens as in Uria, Cepphus, and some Fraterculinae. The iliosynsacral suture is perforated as in Uria, Alca, Pinguinus, and Synthliboramphus, rather than fused along its entire length as in Cepphus, Brachyramphus, and Fraterculinae. The dorsal iliac spine has a pointed tip as in all alcids other than Aethia and Ptychoramphus, in which the end of the spine is blunt.
Mancalla vegrandis corresponds in size and humeral characteristics with some material previously referred to Mancalla milleri Howard, 1970. However, Mancalla milleri is considered Alcidae incertae sedis (see Appendix 1 for details of the taxonomic revision).
urn:lsid:zoobank.org:act:6280FCDF-06BA-46F8-A795-3AFF52A5A001
Miomancalla howardi sp. n.
Mio to reflect Miocene occurrences of known species within the taxon, and mancalla to reflect the sister group relationship with Mancalla Lucas, 1901.
Miomancalla is differentiated from Mancalla by the following humeral characteristics: capital groove wider (137:0); supracoracoidial crest (sensu
Based upon phylogenetic results (see below) and apomorphies shared with Miomancalla howardi (see diagnosis above), Praemancalla wetmorei
urn:lsid:zoobank.org:act:BF31D07E-0CFF-4202-BE8C-C5E97F49F625
SDSNH 68312: a partial skeleton collected by B. O. Riney on May 31, 1990 and comprising the following elements: partial skull, mandible, two cervical vertebrae, partial sternum, partial right humerus, left carpometacarpus, pelvis, femora, tibiotarsi, left tarsometatarsus (Figs 10, 11; Tables 1, 2 and 3).
Holotype specimen of Miomancalla howardi (SDSNH 68312). A Photograph with contrast digitally adjusted to better display bone against similarly colored matrix B Line drawing of holotype specimen showing position of preserved elements with bones in light grey and matrix in dark grey.
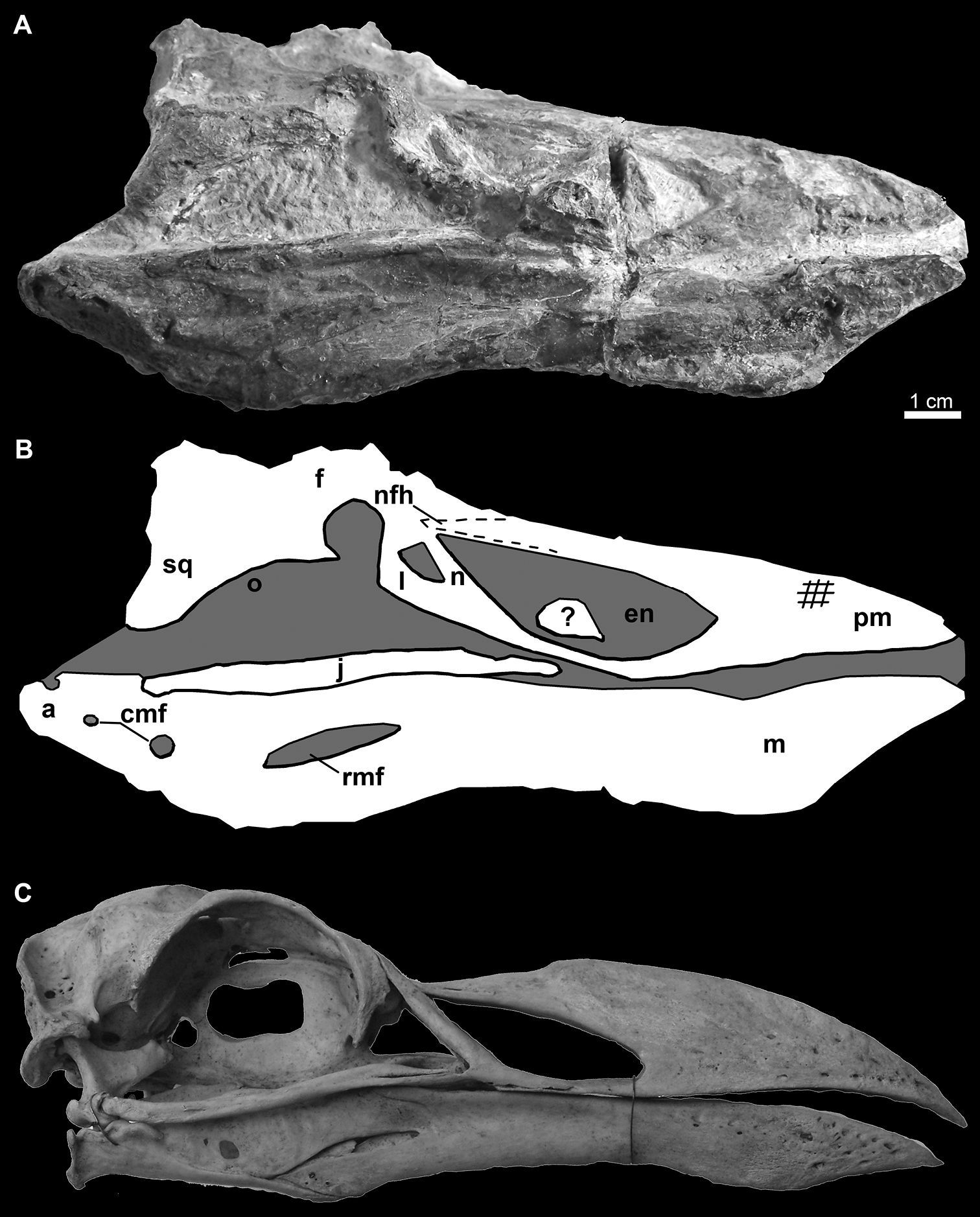
Photograph A and line drawing B of the skull of Miomancalla howardi compared with the skull of Pinguinus impennis (C; not to scale; USNM 346387). Cross-hatched lines on the premaxilla represent abrasion and dotted lines represent approximate reconstruction of incomplete elements. Anatomical abbreviations: a articular cmf caudal mandibular fenestrae en external nares f frontal j jugal l lacrimal m mandible n nasal pm premaxilla nfh nasofrontal hinge o orbit rmf rostral mandibular fenestra sq squamosal; ? unidentified bone fragment.
This new species is named in honor of Hildegarde Howard in recognition of her many contributions to the systematics of extinct Alcidae.
Early Pliocene (Zanclean;
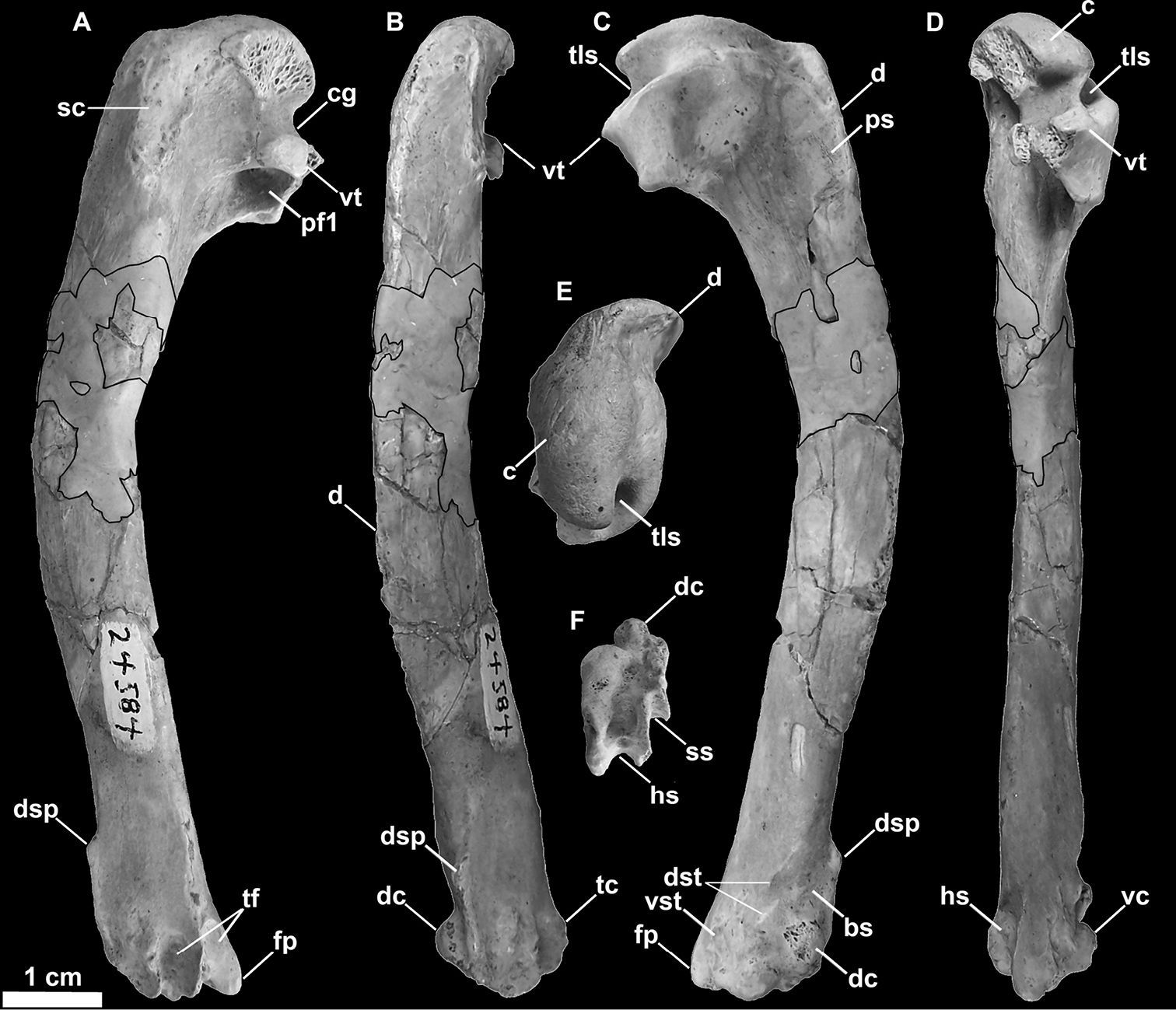
SDSNH 24584, a left humerus (Fig. 12)
from the Late Miocenelower member(Messinian) of the San Mateo Formation
of San Diego County, California (SDSNH locality 3177). This specimen
was noted but not named or described by
Referred left humerus of Miomancalla howardi (SDSNH 24584; dark outlined areas represent reconstructed areas obscured by repair). A posterior view B dorsal view C anterior view D ventral view E proximal view F distal view. Anatomical abbreviations: bs brachialis scar c caput cg capital groove d deltopectoral crest dc dorsal condyle dsp dorsal supracondylar process dst dorsal supracondylar tubercles fp flexor process hs humerotricipital sulcus pf1 primary pneumotricipital fossa ps pectoralis scar sc supracoracoidal crest ss scapulotricipital sulcus tc tricipital crest tf tricipital fossae tls transverse ligament sulcus vc ventral condyle vst ventral supracondylar tubercle vc ventral condyle vst ventral supracondylar tubercle vt ventral tubercle.
Differs from Miomancalla wetmorei
in the following characteristics: ventral margin of ventral tubercle
more deeply grooved; transverse ligament furrow deeper, with lateral
lip extended farther medially; capital groove wider, and flatter;
dorsal supracondylar process less dorsally projected; groove between
dorsal supracondylar process and dorsal condyle wider; ventral
supracondylar tubercle more prominent; tubercle present proximal to
dorsal condyle as in Mancalla cedrosensis (155:1); humerus ~20% longer (Table 2;
The holotype specimen is preserved in a matrix of dark grey, highly indurated, siltstone (Fig. 10). Some elements areslightly crushed and many cortical bone surfaces are considerably abraded, obscuring fine morphological details in many portions of the specimen.
Elements of the skull are exposed in oblique right lateral view (Figs 10, 11). The premaxilla, maxilla, nasal, lacrimal, jugal, frontal, and squamosal are present. Additional fragments of bone adjacent to the posterior frontal may represent a portion of the parietal. An unidentified fragment of bone protrudes from the external narial opening. The premaxilla is relatively shorter and mediolaterally compressed in comparison with the only other known premaxillae referable to Mancallinae (LACM 103940; SDSNH 25236; Fig. 13), which resemble the more terete bills of some other Alcidae (e.g., Uria). The maxilla, which broadens anteriorly before fusion with the premaxilla, is complete but broken at approximately its midpoint. As in many alcids (e.g., Cepphus, Alca) the nasal contacts the maxilla at ~45° angle. This angle is ~60° in the puffins and auklets (i.e., Fratercula, Cerorhinca, Aethia, and Ptychoramphus). As in Pinguinus, and in contrast to other alcids, the lacrimal appears to be directed ventrally rather than posteroventrally. However, crushing of the skull may have changed the relative orientation of elements and it is possible that distortion is responsible for this condition. The jugal is preserved in contact with the mandible. Fusion between the jugal and the jugal process of the premaxilla is visible. The frontal is distorted by crushing and most morphological details obscured in this element. The outline of the right orbit is visible, but is deformed by ventrolateral displacement of the lateral margin of the frontal. The frontal bears a robust orbital rim as in Uria, Miocepphus, Alle, Alca, and Pinguinus.
Skull of Mancallinae (SDSNH 25236). A Dorsal view of skull B Dorsal view of mandible C Left lateral view of skull D Left lateral view of mandible E Ventral view of skull F Ventral view of mandible (sketches by Michael Emerson).
The mandible is preserved in right lateral view (Figs 10, 11). The mandibular symphysis is elongate as in Uria and Fratercula. The mandibular rami are fused along a relatively shorter distance in some alcids (e.g., Alle). The proximal and distal ends of the mandible are dorsoventrally expanded, similar to the condition in Alca and Pinguinus. A pair of small posterior mandibular fenestrae is present as in other known Mancallinae mandibles (LACM 103940; SDSNH 25236; Fig. 13), Fraterculini Storer, 1960, and some charadriiforms (e.g., Stercorarius longicaudus Vieillot, 1819).
At least two cervical vertebrae are partially exposed on the surface of the slab (Fig. 10). Fine morphological details are obscured by matrix and the poor preservation of the vertebrae. One vertebra resembles the axis, but positive identification is hindered by matrix and damage to the element. The other is a cervical vertebra exposed in dorsal view. Mancallinae vertebrae are known only from the holotype specimens of Mancalla cedrosensis and Mancalla vegrandis. Comparisons with Mancalla cedrosensis are not possible because only a single thoracic vertebra is preserved in the holotype specimen. The shape of the dorsal surface of the cervical vertebrae of Miomancalla howardi is consistent with that of Mancalla vegrandis. Further preparation of the holotype specimen of Miomancalla howardi, or discovery of additional material referable to this species is necessary before more details of vertebral anatomy can be described for this species.
Fragments of the sternum are preserved adjacent to the humerus in what appears to be ventral view (Fig. 10). The craniolateral process appears to point dorsally, rather than anteriorly as in Mancalla lucasi, although the possibility that crushing of this element altered the relative orientation of that feature cannot be ruled out. Other morphological details are obscured by matrix and the poor preservation of the sternum.
The holotype specimen preserves the proximal end of the right humerus in posterior view (Fig. 10).
In addition to the head of the humerus, which is slightly crushed,
the outline of the proximal half of the humeral shaft is visible as an
impression in matrix. A complete left humerus (SDSNH 24584; Fig. 12) is referable to Miomancalla howardi based upon its similar proportions (i.e., larger than any other known Mancallinae; Table 2),
and the fact that the ventral surface of ventral tubercle is more
deeply grooved than in any other alcid. The ventral surface of the
ventral tubercle is also grooved in Pinguinus and Miomancalla wetmorei, but the degree of excavation of this groove is more pronounced in Miomancalla howardi. The ventral margin of the ventral tubercle of Mancalla is convex. The capital groove is relatively wider than that of other species of Mancallinae, and it is incised more deeply into the transverse ligament sulcus in anterior view than in Miomancalla wetmorei. The proximal end of the deltopectoral crest is less pronounced than in Miomancalla wetmorei. The distal end of the deltopectoral crest transitions to the shaft less abruptly than in Mancalla. The humeral head is rotated more anteriorly than in Miomancalla wetmorei, and is more similar to the condition in Mancalla. As in Miomancalla wetmorei and Fratercula, and in contrast to the condition in Mancalla species, the supracoracoideus muscle scar broadens proximally. In Miomancalla howardi and Miomancalla wetmorei
the ‘mancalline scar’ extends from a point just proximal to the
junction of the bicipital crest with the humeral shaft and tapers to a
point that meets the dorsal border of the primary pneumotricipital fossa
(i.e., crus dorsale fossae of
The left carpometacarpus is preserved in dorsal view (Fig. 10). Although hundreds of Mancallinae carpometacarpi are known from Pliocene marine deposits in California, the holotype specimens of Miomancalla howardi and Mancalla cedrosensis are the only associated specimens that allow for species-level referral of carpometacarpi. The carpometacarpus of Miomancalla howardi is larger than that of Mancalla cedrosensis (~23%; Table 3;
The pelvis is exposed in dorsal view (Fig. 10). Comparisons within Mancallinae are limited to Mancalla vegrandis. As in all alcids the anteroposterior length of the pelvis is greater than two times the mediolateral width across the antitrochanters. The relative length of the pelves of other charadriiforms is anteroposteriorly shorter. The proximal end of the preacetabular ilium is wide as in Mancalla vegrandis and most alcids (e.g., Brachyramphus). The distal end of the preacetabular ilium narrows more so than in Mancalla vegrandis. As with Mancalla vegrandis the antitrochanteral sulcus does not extend proximally to contact the antitrochanter. The dorsal iliac spine has a pointed tip as in all alcids other than Aethia and Ptychoramphus, in which the end of the spine is blunt.
The distal ends of both tibiotarsi are missing or embedded in matrix (Fig. 10). The poor preservation of these elements limits comparisons with the smaller holotype tibiotarsi of Mancalla cedrosensis to size (~26% larger; Table 3;
The right femur is exposed in posterolateral
view along the edge of the block but is severely abraded: however, the
left femur is well-preserved and exposed in anterior view (Fig. 10). The femur is robust and less sigmoidal in shape in comparison with the femora of extant alcids such as Alle or Uria, resembling the condition in Mancalla lucasi and Mancalla cedrosensis, the only other Mancallinae from which the femur is known. The intercondylar sulcus is relatively broader and more well-defined proximally than that of Mancalla lucasi and Mancalla cedrosensis. As in Cepphus, Brachyramphus, and Synthliboramphus,
the distally extending and anteriorly projected crest of the femoral
trochanter is convex in shape. This feature is flattened (e.g., Alca and Uria) or concave (e.g., Fratercula and Cerorhinca) in other alcids. The femoral head appears relatively smaller in comparison with this element in Mancalla cedrosensis and Mancalla lucasi. The length of the femur is greater than in Mancalla cedrosensis and Mancalla lucasi (Table 3;
The left tarsometatarsus is preserved in anterior view (Fig. 10). The anterior surface of the shaft is deeply grooved as in Mancalla cedrosensis and Fratercula. Associated specimens with tarsometatarsi that would allow for referral of isolated tarsometatarsi to species are not currently known from other Mancallinae. The outlines of trochlea are visible but the distal end of the element is too badly abraded to discern fine morphological details.
Owing to the incomplete and fragmentary preservation of most Mancallinae specimens referable to species, preliminary analysis of the systematic relationships of Mancalla resulted in an unresolved polytomy among Alcidae sub-clades (i.e., relationships between Mancallinae, Cepphus, Brachyramphus, Synthliboramphus, Alcini, and Fraterculinae (contents = Fraterculini Storer, 1960 + Aethiini Storer, 1960) unresolved at the base of a monophyletic alcid clade (results not shown). Two additional phylogenetic analyses were performed to investigate the position of Mancallinae within Charadriiformes, and the interrelationships of Mancallinae species. The primary phylogenetic analysis included a Mancallinae supraspecific terminal (SST) constructed by combining scorings from 19 Mancallinae specimens (including all holotype material; Appendix 4). The referral of all Mancallinae specimens used to construct the SST was evaluated based upon the unambiguously optimized apomorphies listed in the diagnosis section for Mancallinae above. Note that due to damage or missing elements in Mancallinae holotype specimens, five of the specimens used to construct the Mancallinae supraspecific terminal preserve morphological data not preserved by the holotype specimens, thus providing a more compete picture of morphological variation in Mancallinae than if only the holotype specimens were analyzed. The results of the first analysis were used to constrain the topology of trees accepted during a secondary tree search in which the species-level relationships of Mancallinae were evaluated.
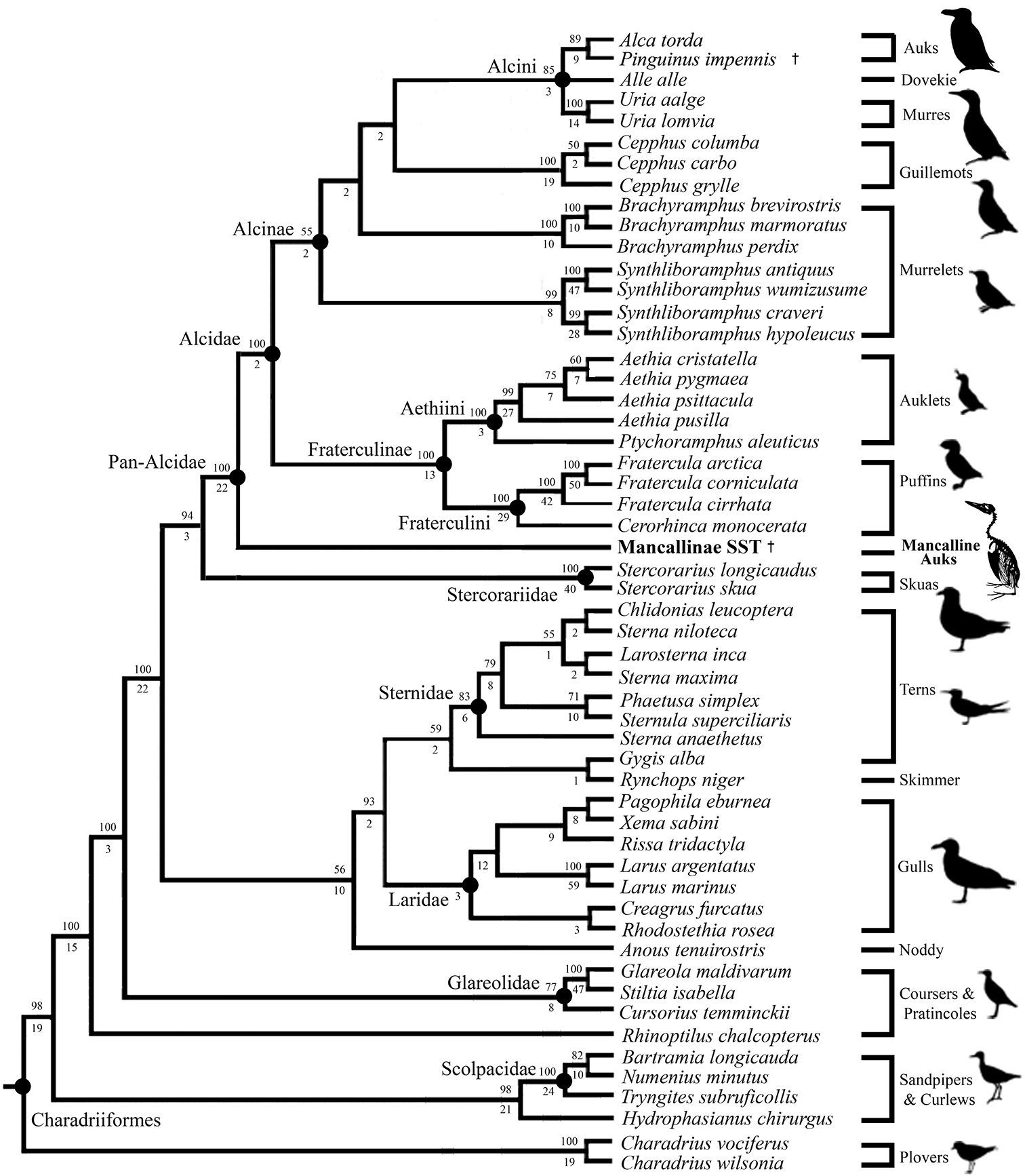
The primary combined phylogenetic analysis of the cladistic matrix including a Mancallinae SST resulted in two most parsimonious trees (MPT’s) of 15, 974 steps (Fig. 14;
CI: 0.38; RI: O.50; RCI: 0.19). Additional analyses performed with all
characters unordered did not result in topological differences, or an
increase in the number of MPT’s recovered. Pan-Alcidae is recovered as the sister to Stercorariidae, a result that is congruent with the results ofprevious molecular based analyses(
Results of primary phylogenetic analysis including the Mancallinae SST (2 MPT’s; TL: 15, 974; CI O.38; RI O.50; RCI 0.19). Bootstrap values (>50%) are displayed above nodes, and Bremer support values are displayed below nodes.
Only the systematic position of Alle alle Link, 1806 remains unresolved within Alcini (Fig. 14). The systematic position of Alle alle is potentially the most contentious issue within alcid systematics, as it has been recovered as the sister to Alca + Pinguinus (
Mancallinae is placed as the sister taxon to all other Alcidae (i.e., placed outside of crown clade Alcidae; Fig. 14). This result is consistent with the only previous analysis that included Mancallinae (
The combined analysis recovered relationships among the
29 charadriiform outgroup taxa that are largely congruent with prior
molecular-based analyses of the clade, but do not support previous
morphology-based results. Larus and Hydrophasianus (i.e., gulls and jacanas) are recovered as more closely related to one another than either are to Charadriius (i.e., plovers), as in the results obtained by
Also of interest is the placement of Rynchops (i.e., skimmers). Recent molecular analyses recovered Rynchops as the sister to Laridae (
Anous (i.e., noddies) was recovered as the sister to Sternidae + Laridae + Rynchopidae in the combined analysis, a placement consistent with the molecular-based results reported by
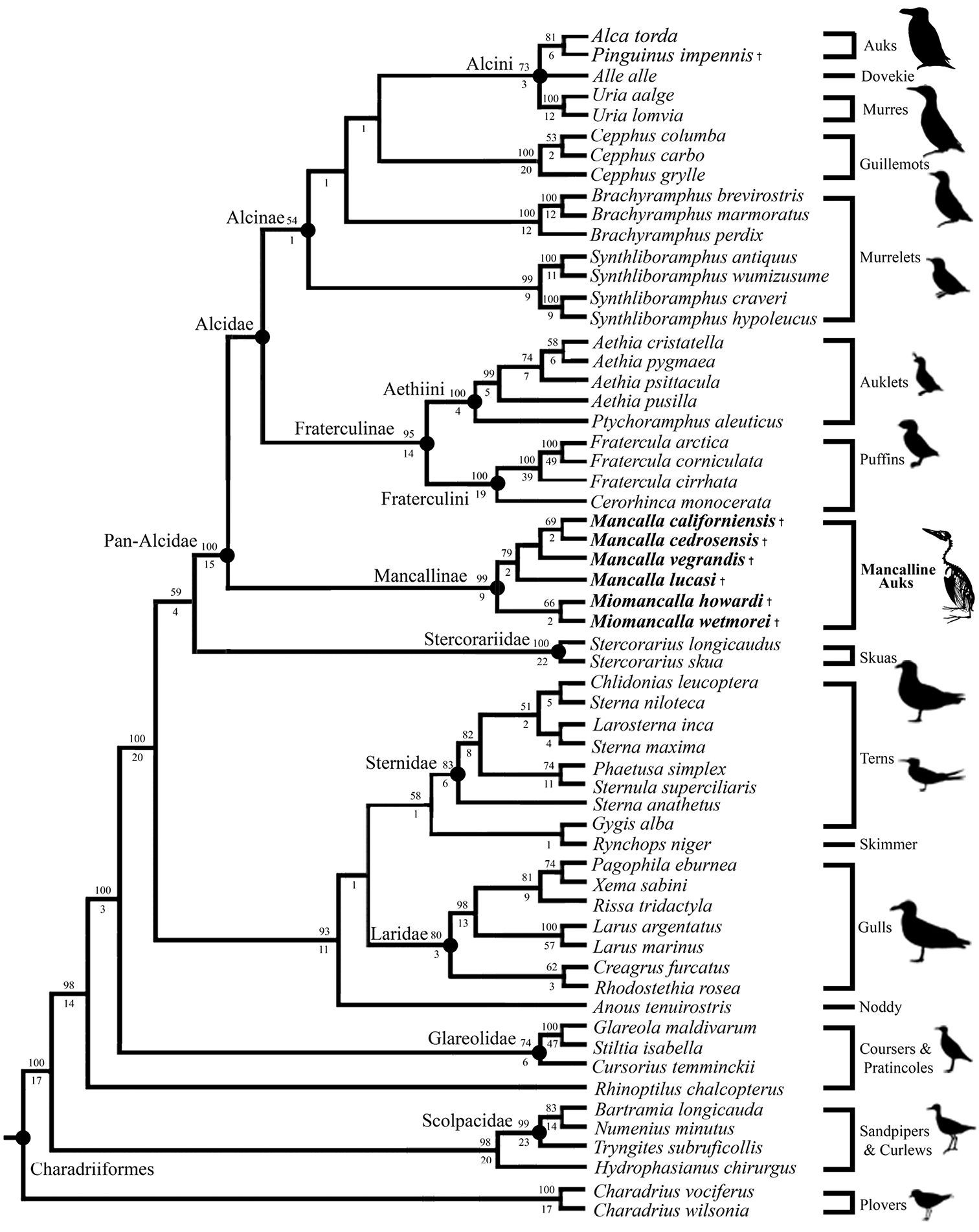
The secondary phylogenetic analysis, which evaluated the interrelationships among Mancallinae resulted in two MPT’s of 15, 971 steps (Fig. 15; CI: 0.37; RI: O.51; RCI: 0.19). Binary characters are interpreted as ambiguity (i.e., treated the same as ‘?’ scorings) when they are scored as polymorphic (e.g., 0&1 scorings), explaining the shorter tree length of the secondary analysis as compared to the primary analysis including the Mancallinae SST. The monophyly of Mancallinae is supported by eight UOMC’s (Table 4). Miomancalla wetmorei and Miomancalla howardi are placed as sister taxa, and Miomancalla monophyly is supported by three locally optimized morphological characters (LOMC; 105:0; 113:1; 134:1). Miomancalla is placed as the sister taxon to Mancalla. Mancalla monophyly is supported by one UOMC (137:1) and an additional LOMC (130:1). The placement of Mancalla californiensis as the sister taxon of Mancalla cedrosensis is supported by one UOMC (123:0), and an additional LOMC (109:1). Mancalla vegrandis and Mancalla lucasi are placed as successive outgroups to the clade composed of Mancalla californiensis and Mancalla cedrosensis (Fig. 15).
Results of secondary phylogenetic analysis of Mancallinae inter-relationships (2 MPT’s; TL: 15, 971; CI O.38; RI O.51; RCI 0.19). Bootstrap values (>50%) are displayed above nodes, and Bremer support values are displayed below nodes.
The taxonomic revision and description of new Mancallinae species herein confirms previous estimates of high diversity in Mancallinae (
Although impressive with regard to the quantity of taxa sampled (n = 242) and the number of morphological characters scored for those taxa (n = 1107), comparisons with the results of a recent morphology based analysis of Charadriiformes (
Referral of specimens to named species, or
recognition of new species, based solely upon size, or provenience,
or age, or any combination of those three criteria, run the risk of
incorrectly assigning specimens to species, or incorrectly assessing
species diversity (
The etymology of Mancalla (mancus-from the Latin for crippled or lame, and ala from the Latin for wing;
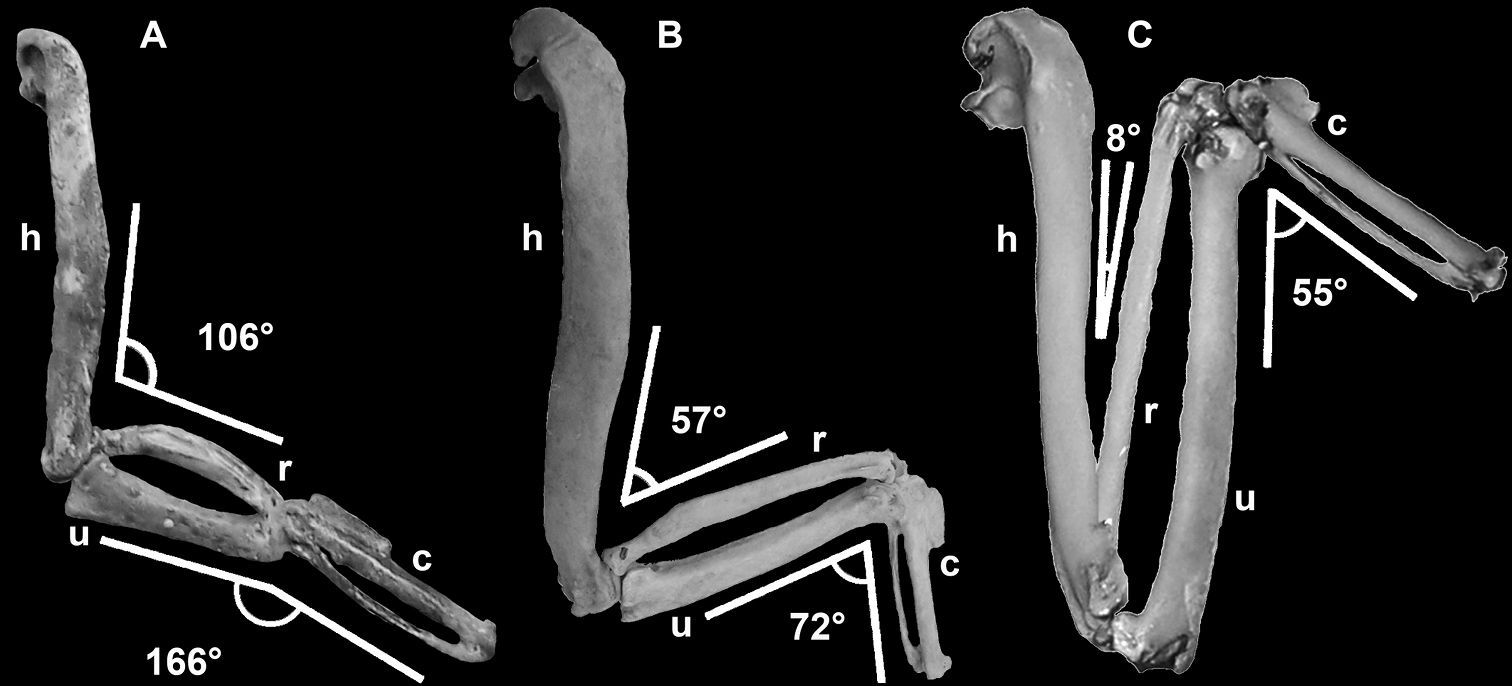
Wing elements of flightless and volant auks depicting decreased range of motion and shortening of distal wing elements. Elements not to scale and degree of flexion estimated based on manual articulation of specimens: A Mancalla (composite LACM 154560) B Pinguinus impennis (composite USNM 346387) C Alca torda (NCSM 20502). Anatomical abbreviations: c carpometacarpus h humerus r radius u ulna.
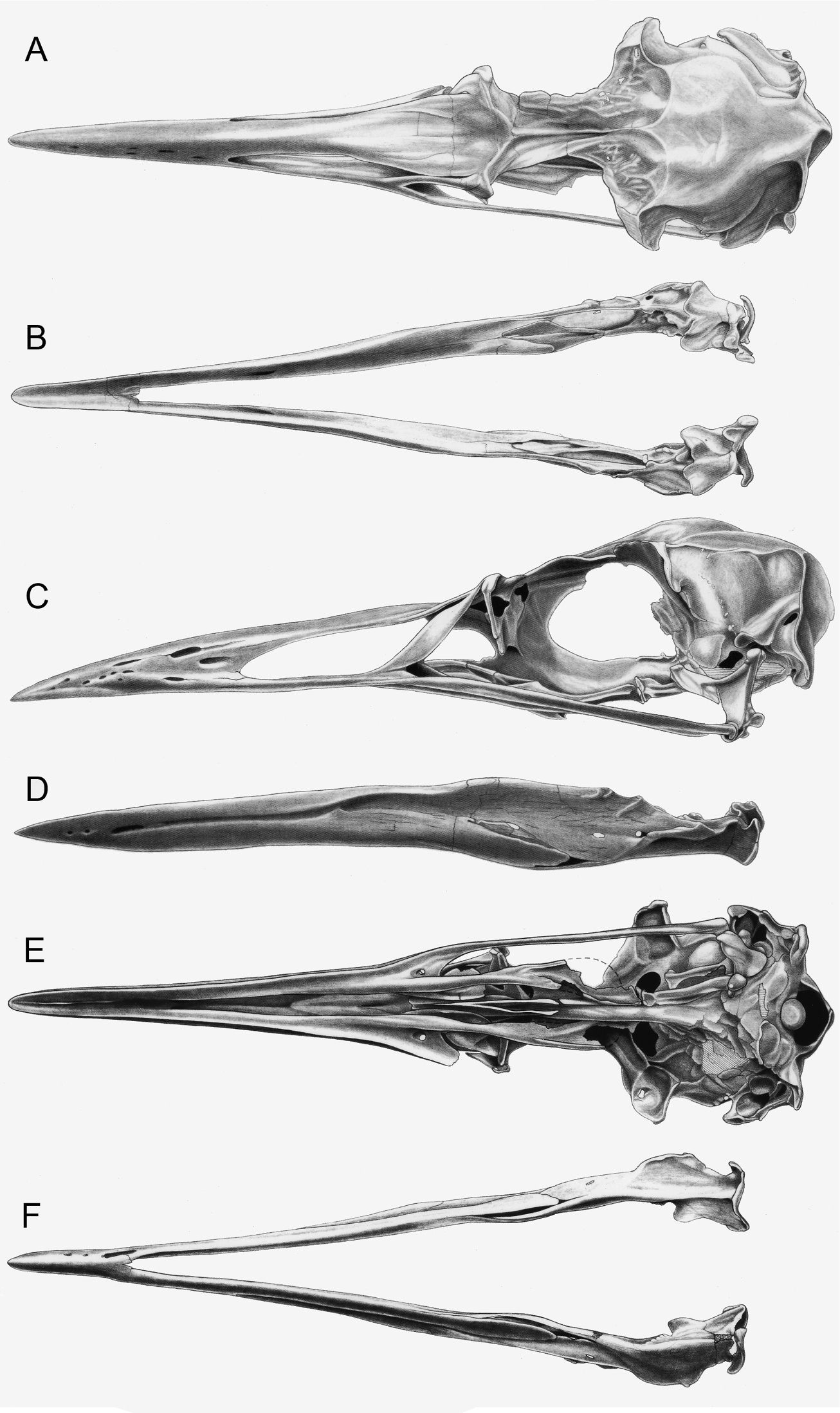
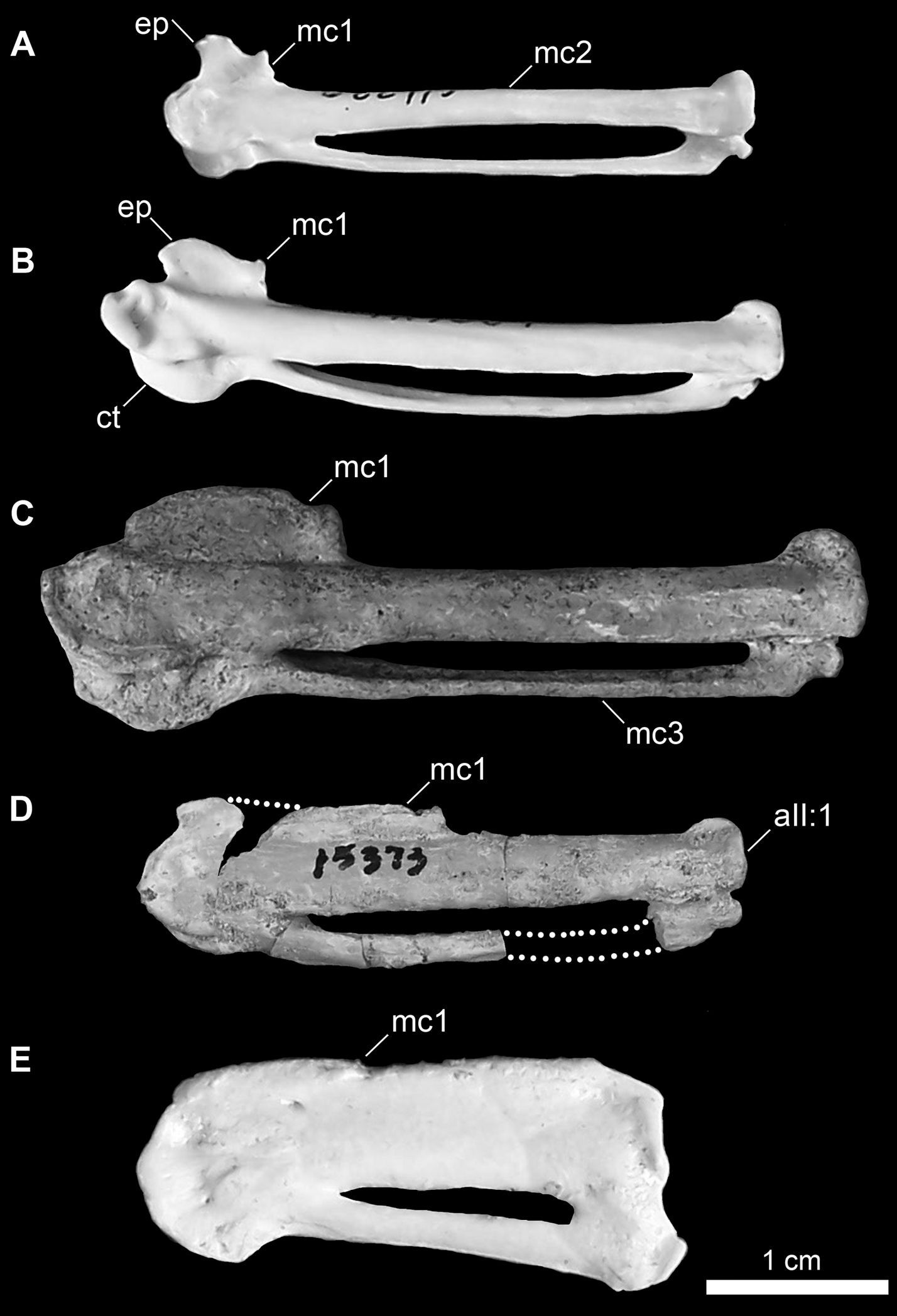
Comparison of charadriiform and sphenisciform carpometacarpi. A Anous minutus (USNM 622415) B Cerorhinca monocerata (USNM 620641) C Pinguinus impennis (USNM 623465) D Mancalla cedrosensis (LACM 15373) E Eudyptula minor (TMM M-931). Anatomical abbreviations: aII:1 articulation of digit II phalanx 1 ct carpal trochlea ep extensor process mc1 first metacarpal mc2 second metacarpal mc3 third metacarpal.
One characteristic that is unique to Mancallinae
among all known flightless birds, is the shorter length of the ulna
compared with that of the carpometacarpus (180:1). In most birds these
proportions are opposite of that observed in Mancallinae, with the ulna being longer than the carpometacarpus. Three associated Mancallinae specimens (LACM 107028; SDSNH 77966), including the holotype specimen of Mancalla cedrosensis
(LACM 15373) display this characteristic. Statistical analysis of
osteological proportions of flightless alcids quantified the
dorsoventral compression of wing elements and shortening of distal wing
elements, but surprisingly,
The relatively large size of Pinguinus and some Mancallinae as compared to other alcids (
Body size in extant alcids has been correlated with dive depth and feeding ecology (
The oldest unequivocal fossil alcid (GCVP 5690) is from Late Eocene deposits of the Hardie Mine, Gordon, Georgia, USA (
The taxonomic status of all but one earlier (i.e.,
Mesozoic, Paleocene, and Early-mid Eocene) fossil referred to
Charadriiformes (
The fossil record of Mancallinae ranges in age from Middle Miocene through Late Pleistocene (i.e., Turtonian-Calabrian;
Just as coldwater upwelling is linked to biological productivity in modern seabird communities (
Known diversity of extinct Atlantic alcids now
approaches that of extinct Pacificalcids (~16–19 species ranging from
Miocene-Pleistocene age; Smith and Clarke in review). The
differential extinction of Atlantic alcids, compared with that of
Pacific lineages, may be linked to climatic changes that effected the
Atlantic and Pacific Oceans in different ways. The alcid Pacific Ocean
origin hypothesis is based primarily on higher extant diversity in the
Pacific Ocean; however, higher extant diversity in the Pacific is not
evidence of origination area, and the two oldest known alcid fossils
are both from Atlantic deposits (
Regardless of the ancestral area of the clade (i.e.,
Atlantic or Pacific), hypotheses regarding the spread of alcids from
one ocean basin to another include dispersal by ice-free northern
passage through the Bering Strait and Arctic Ocean, and southern
dispersal across the submerged Isthmus of Panama (
As suggested by
Rigorous taxonomic evaluation of alcid fossil material resulted in a more refined picture of diversity within Mancallinae,
and facilitated phylogenetic analysis of species-level relationships
within the clade. The combined analysis and total evidence approaches
adopted herein resulted in a well-resolved and strongly supported
hypothesis of the position of Mancallinae with respect to other Charadriiformes, and the inter-relationships of Mancallinae species. The phylogenetic position of Mancallinae as the sister taxon to all other Alcidae (i.e., crown clade Alcidae) suggests extensive ghost lineages in Pan-Alcidae,
provides further evidence that the charadriiform fossil record is
quite incomplete, and demonstrates that flight was lost independently
in at least two lineages of alcids. The stem-lineage position of Mancallinae recovered in this analysis is consistent with previous phylogenetic placement of this clade (
Miomancalla howardi is placed as the sister taxon of Miomancalla wetmorei, and is the largest known species of Mancallinae. The large size and resemblance of the bill of Miomancalla howardi to that of the Great Auk Pinguinus impennis provides an example of within-lineage convergence between two species separated by time and geography. The independent acquisition of morphological characteristics in both lineages of flightless alcids (i.e., Mancallinae and Pinguinus), and the similarity of these modifications to those of penguins and plotopterids, strongly suggests correlation between these morphologies and mode of locomotion. The study of convergence within Alcidae may provide insights about the evolution of flightlessness in penguins, in which there are no known volant species.
Similarly diverse lineages of alcids inhabited the eastern and western coasts of North America during the Miocene and Pliocene. Approximately coeval Early Pliocene deposits in California and North Carolina record the replacement of Miocepphus by Alca in the Pliocene of the Atlantic, and the replacement of Miomancalla by Mancalla in the Pliocene of the Pacific. Global-scale environmental perturbations such as increased cooling following the MMCO, may have contributed to similar scenarios involving species turnover in Pan-Alcidae in both ocean basins.
I thank J. Clarke, C. Bell, D. Cannatella, T. Rowe, J. Sprinkle, H. Matsuoka, B. Boessenecker, C. Boyd, D. Eddy, A. DeBee, D. Ksepka, S. Nesbitt, J. Stewart, the editors, and reviewers for comments that improved this manuscript. I thank B. Chandler at GCVP, M. Carrino, T. Deméré, and K. Randall at SDSNH, S. McLeod and H. Thomas at LACM, M. Goodwin and P. Holroyd at UCMP, J. Dean, M. Florence, and S. Olson at USNM, P. Brinkman, B. Desjardins, J. Gerwin, and V. Schneider at NCSM for fossil preparation and access to fossil and extant comparative specimens. Special thanks to T. Deméré and Michael Emerson for sketches of SDSNH 25236. Financial support from the Frank M. Chapman Memorial Fund, Section of Ornithology, American Museum of Natural History, the Smithsonian Institution Office of Fellowships, and the Geological Society of America is gratefully acknowledged. This project was also supported as part of NSF DEB 0949897 "Collaborative Research: Wings to Flippers - Phylogenetics, character acquisition, and feather biomechanics in the evolution of wing-propelled diving".
Owing to the recognition of several Mancallinae species based upon non-diagnostic material, the systematics of Mancallinae required extensive revision. The following review of the Mancallinae fossil record is presented to clarify the systematic position of previously named species and referred fossil material, and to justify the exclusion of some previously named species from the phylogenetic analysis.
Although more than 100, 000 avian fossils are now known from sediments in California (
The second report of Mancalla remains (humerus; catalog # uncertain) came from the Early Pliocene San Diego Formation exposed in San Diego, California (
Mounting evidence that more than one species of Mancalla was present during the Early Pliocene came from Howard in 1949. At that time approximately 118 specimens representing Mancalla
were known, including two size classes of carpometacarpi from
localities in Los Angeles, San Diego, and Corona del Mar, California.
Although no associated remains were known, carpometacarpi were
referred to Mancalla based upon characters such as an elongated first metacarpal, a morphology considered convergent with that of penguins (i.e., Spheniscidae) by
The growing number of specimens from the San Diego Fm. prompted a review known remains of Mancalla (
In 1966 Howard described a new Mancallinae
taxon from the Late Miocene based upon isolated elements including a
distal humerus, carpometacarpi, a partial coracoid, the proximal end
of a scapula, and the articular portion of a mandible. Praemancalla lagunenesis
Howard, 1966 was considered by that author to be less specialized with
respect to features associated with loss of aerial flight, and the
possibility that Praemancalla might represent a less derived ancestor of Mancalla was proposed. All elements referred to Praemancalla lagunenesis
were isolated, so only the holotype distal humerus (LACM 15288) can be
compared with previously recognized taxa to evaluate the taxonomic
validity of this species. The holotype specimen of Praemancalla lagunensis is weathered smooth, obscuring many fine morphological details. Although LACM 15288 is referable to Mancallinae based upon the rounded anterior surface of the ventral condyle (153:0), all of the characteristics that
Another species of alcid with characteristics interpreted as “progressing towards flightlessness” (
Although the reviewby
Mancalla cedrosensis Howard, 1971 was the first species of Mancalla described from associated remains, and also the first that was directly comparable to Mancalla californiensis (Howard, 1971). The holotype specimen (LACM 15373; Fig. 2)
and additional referred specimens were recovered from Early Pliocene
deposits on Cedros Island off the coast of Baja California, Mexico (
Praemancalla wetmorei Howard, 1976 was described based upon a nearly complete humerus (LACM 42653; Fig. 2) from Late Miocene sediments in Laguna Niguel, California. Several features distinguish this species from other Mancalla (see diagnoses below). An associated specimen (LACM 107028) was tentatively referred to Praemancalla wetmorei by
Mancalla emlongi was described based upon a complete ulna from Early Pliocene San Diego Fm. sediments in San Diego, California (
Additional material from the San Diego Fm. including a well-preserved skull and mandible (SDSNH 25236; Fig. 3) was tentatively referred to Mancalla emlongi by
Although Mancalla remains were reported from Pleistocenesediments inShiriya, Japan (
Aethia cristatella Crested Auklet:
Skins: NCSM 6564, 6565, 6567, 16419, 17749.
Skeletons: NCSM 17749; USNM 223707, 488675, 498282, 561934, 61094.
Eggs: USNM 32126, 32128, 32131, 33167.
Aethia psittacula Parakeet Auklet:
Skins: NCSM 16423, 16424, 18387; USNM 89143, 493708.
Skeletons: NCSM 14147, 14804, 18387, 18514, 20177; NSM PO 355; USNM 12640, 226451, 610513, 610514, 610937.
Eggs: USNM 42123, 42124, 42125, 42126.
Dissection: NCSM 20881.
Aethia pusilla Least Auklet:
Skins: NCSM 17735, 17736, 17751, 17797.
Skeletons: NCSM 17734, 17736, 17737; USNM 224009, 224010, 498285; NSM PO 356, 357.
Eggs: USNM 16725, 18052, 25103, 33886.
Aethia pygmaea Whiskered Auklet:
Skins: NCSM 13159; USNM 4163, 67399, 85617, 92971, 110194.
Skeletons: USNM 344544; UMMZ 204592, 224279, 224882, 224883.
Eggs: Scored from
Alca torda Razorbill:
Skins: NCSM 298, 299, 2236, 4455, 18760, 20015.
Skeletons: NCSM 20058, 20502; USNM 18062, 347946, 501644, 502378, 502382, 502387, 502388, 502389, 502549, 555666, 555668.
Eggs: NCSM 13447, 13448; USNM 18476, 21571, 23259.
Alle alle Dovekie:
Skins: NCSM 301, 302, 303, 304, 20111, 20630, 40060, .
Skeletons: NCSM 18374; USNM 344740, 344748, 499471, 560929.
Eggs: USNM 2634, 18490, 18491, 19053.
Dissection: NCSM 21042.
Anous tenuirostris Lesser Noddy:
Skins: USNM 486718, 486723, 486725, 486728.
Skeletons: USNM 488400, 622578.
Bartramia longicauda Upland Sandpiper:
Skins: NCSM 825, 826, 827, 828, 3093.
Skeletons: USNM 227823, 347894, 610844, 610845, 501160, .
Brachyramphus brevirostris Kittlitz’s Murrelet:
Skins: NCSM 35213; USNM 286494, 333257, 589672.
Skeletons: USNM 288086, 288087.
Eggs: USNM 47733.
Brachyramphus marmoratus Marbled Murrelet:
Skins: NCSM 5669, 5670, 18144, 18145, 18146, 18148.
Skeletons: NCSM 18143, 18144, 18145, 18146, 18147, 18148, 18149; NSM PO 354, 358, 551.
Eggs: USNM 21545, 28473, 40125, 417778.
Brachyramphus perdix Long-billed Murrelet:
Skins: USNM 108952, 109985, 120704, 200411, 200412.
Skeletons: USNM 582506, 599498.
Charadrius vociferus Killdeer:
Skins: NCSM 791, 792, 17610, 18671, 19284.
Skeletons: NCSM 18305, 21905; USNM 61432, 492870, 553817, 622526.
Eggs: NCSM 13382, 13383, 13384, 13385, 13386, 13387.
Charadrius wilsonia Wilson’s Plover:
Skins: USNM 220535, 338822, 338823, 524172.
Skeletons: NCSM 5818; USNM 1250, 556652, 610801.
Eggs: NCSM 13388, 13389; USNM 43430, 43431, 43432.
Cepphus carbo Spectacled Guillemot:
Skins: USNM 40637, 102199, 406348, 424970.
Skeletons: USNM 347755, 347756, 347757.
Cepphus columba Pigeon Guillemot:
Skins: NCSM 16153, 16155, 16414, 16438, 16439.
Skeletons: NCSM 18094, 18095, 18096, 18097.
Eggs: NCSM 13449; USNM 19063, 21546, 27059.
Dissection: NCSM 21075.
Cepphus grylle Black Guillemot:
Skins: NCSM 6830; USNM 331585, 393556, 394525.
Skeletons: USNM 344759, 344760, 347265, 612213, 612214.
Eggs: NCSM 7435, 13450, 13451; USNM 2578, 18494.
Cerorhinca monocerata Rhinoceros Puffin:
Skins: NCSM 8064, 10628, 16420, 16421, 16430.
Skeletons: NSM PO 189; USNM 557613, 557614, 561468, 620641, 620643.
Eggs: USNM 12866, 24634, 27632, 27633.
Chlidonias leucopterus White-winged Black Tern:
Skins: NCSM 11351, 11352, 11358, 11470, 11471.
Skeletons: USNM 43173, 290154, 430844, 431172, 488879.
Creagrus furcatus Swallow-tailed Gull:
Skins: NCSM 183825. USNM 115967, 115968, 131674, 543878, 543879.
Skeletons: USNM 18492, 19029, 498301.
Cursorius temminckii Temminck’s Courser:
Skins: USNM 448378, 520019, 545851, 545853, 545854.
Skeletons: 429182, 431709.
Fratercula arctica Atlantic Puffin:
Skins: NCSM 17824, 17825; USNM 589716, 627638.
Skeletons: USNM 18055, 18057, 18058, 224189, 621331.
Eggs: NCSM 13452; USNM 2637, 14977, 31034.
Fratercula cirrhata Tufted Puffin:
Skins: NCSM 16147, 16148, 16150, 16433, 18098.
Skeletons: NCSM 17823, 18099, 18100; USNM 19449, 488748.
Eggs: NCSM 13453, 13454; USNM 16335, 12861.
Fratercula corniculata Horned Puffin:
Skins: NCSM 7761, 10629, 18102; USNM 610504, 612200, 499957.
Skeletons: NCSM 17835, 18083, 18388; USNM 499961, 499964.
Eggs: USNM 16329, 19706, 22052, 25095, 29216.
Dissection: NCSM 21095.
Glareola maldivarum Oriental Pratincole:
Skins: NCSM 9756, 11059, 11060, 11061, 11062.
Skeletons: USNM 19580.
Gygis alba White Tern:
Skins: NCSM 7859, 7860, 8021, 18890, 18932.
Skeletons: NCSM 16895; USNM 498081, 498415, 559583, 621328.
Hydrophasianus chiurgis Pheasant-tailed Jacana:
Skins: NCSM 10609, 11018, 11019, 11473.
Skeletons: USNM 226034, 431604, 431609, 490560, 490566.
Larosterna inca Inca Tern:
Skins: USNM 15503, 15516, 212050, 212051, 371303.
Skeletons: USNM 292869, 430271, 430375, 430580, 430625, 631761.
Larus argentatus Herring Gull:
Skins: NCSM 17738, 21188, 21444, 21462, 21791.
Skeletons: NCSM 8624, 10116, 10211, 10251, 22218.
Eggs: NCSM 5934, 13395.
Larus marinus Great Black-backed Gull:
Skins: NCSM 7376, 7861, 7863, 7941, 7992.
Skeletons: NCSM 6590, 16190, 102451; USNM 491592, 502396.
Eggs: NCSM 5968; USNM 42295, 42296, 42297.
Numenius minutus Little Curlew:
Skins: NCSM 1907, 22227, 22228, 22229, 22230.
Skeletons: USNM 347648.
Pagophila eburnea Ivory Gull:
Skins: USNM 17766, 22217, 22221.
Skeletons: NCSM 17766; USNM 344734, 491595, 491596, 491597.
Phaetusa simplex Large-billed Tern:
Skins: NCSM 22224. USNM 316370, 326609, 349836, 512940.
Skeletons: USNM 345827, 345828.
Pinguinus impennis Great Auk:
Skins: USNM 57388 (eye and mouth color scored based on Smith, 1879).
Skeletons: USNM 346387 (composite), 557975 (composite), 623465 (composite) additional series of disarticulated USNM material from the expedition to Funk Island (Lucas, 1890).
Eggs: USNM 15141, 15144.
Ptychoramphus aleuticus Cassin’s Auklet:
Skins: NCSM 5666, 7222, 10624, 19137, 19140.
Skeletons: NCSM 18088; USNM 491305, 491845, 491846, 557607, 557609, 557611.
Eggs: NCSM 7901; USNM 2353, 16635, 16636.
Rhinoptilus chalcopterus Bronze-winged Courser:
Skins: USNM 117798, 216168, 437251, 448203, 460101.
Skeletons: USNM 321515.
Rhodostethia rosea Ross’s Gull:
Skins: NCSM 22222, 22223. USNM 93346, 93356, 93357, 332306, 495943.
Skeletons: USNM 491606, 491607, 491608, 491609, 491611.
Rissa tridactyla Black-legged Kittiwake:
Skins: NCSM 18072, 18073, 18074, 18075, 18076.
Skeletons: NCSM 18123, 18124, 18125, 18126.
Eggs: NCSM 13403.
Rynchops niger Black Skimmer:
Skins: NCSM 281, 282, 287, 289, 20262.
Skeletons: NCSM 4228, 6280, 6281, 7790, 7791, 9725, 19048, 19063
Eggs: NCSM 13441, 13442, 13443, 13444, 13445.
Stercorarius longicaudus Long-tailed Skua:
Skins: NCSM 8385, 10269, 11725, 17144, 17801.
Skeletons: NCSM 10269, 17801; USNM 491643, 491951, 501243.
Eggs: USNM 7789, 11692, 11694, 11681, 11699.
Stercorarius skua Great skua:
Skins: NCSM 13193, 14891, 22191, 22192.
Skeletons: NCSM 11747; USNM 488294, 488295, 560938, 576076, 623300.
Eggs: USNM 14918, 24541, 34243, 42219, 42221, 46504.
Sterna anaethetus Bridled Tern:
Skins: NCSM 4066, 6037, 6039, 6042, 6086.
Skeletons: NCSM 10268, 17085, 19073; USNM 488397, 554970, 554972, 558277.
Sterna maxima Royal Tern:
Skins: NCSM 7213, 7294, 7614, 20050, 20668.
Skeletons: NCSM 1640, 10248, 16010, 17514.
Eggs: NCSM 2603, 2604, 5317, 13245, 13424, 13426.
Sterna niloteca Gull-billed Tern:
Skins: NCSM 242, 10461, 11469, 15044, 15046.
Skeletons: 10228, 15046, 17188, 289676, 501253, 610912.
Eggs: NCSM 8397, 8398, 8399, 9943, 9944.
Sternula superciliaris Yellow-billed Tern:
Skins: USNM 283, 682, 401268, 512943, 512944.
Skeletons: USNM 227482, 345825, 345826.
Stiltia isabella Australian Pratincole:
Skins: USNM 279023, 405699, 405698, 405700, 405701.
Skeletons: AMNH 9599.
Synthliboramphus antiquus Ancient Murrelet:
Skins: NCSM 16146, 17742, 18089, 19143.
Skeletons: NCSM 17742, 18089, 18090; NSM PO 351, 352, 427, 428, 564; USNM 488688, 561926.
Eggs: USNM 16618, 27130, 27131, 28369.
Dissection: NCSM 21074.
Synthliboramphus craveri Craveri’s Murrelet:
Skins: USNM 544024, 544034, 597160, 597163.
Skeletons: SDSNH 36390, 36391, 37767.
Eggs: USNM 42144, 46625, 46627, 46628.
Synthliboramphus hypoleucus Xantus’ Murrelet:
Skins: USNM 544886, 544887, 544889, 544893.
Skeletons: USNM 19387, 291879, 345427, 345428, 500652.
Eggs: USNM 28131, 31480, 46623, 46624.
Synthliboramphus wumizusume Japanese Murrelet:
Skins: USNM 15803, 85796, 111653, 114529, 466256.
Skeletons: NSM PO 10, 353, 359; UMMZ 152355, 152356, 152357, 152358, 152359, 152360.
Tryngites subruficollis Buff-breasted Sandpiper:
Skins: NCSM 7621, 21581, 22225, 22226.
Skeletons: USNM 7995, 227481, 227771, 492110.
Uria aalge Common Murre:
Skins: NCSM 8074, 11188, 18115, 18992, 20551.
Skeletons: NCSM 17822, 18116, 18117, 18118, 18234.
Eggs: NCSM 5935, 5936, 13455, 13456, 13457, 13773.
Dissection: NCSM 21070.
Uria lomvia Thick-billed Murre:
Skins: NCSM 6347, 16144, 16145, 17754, 17779.
Skeletons: NCSM 18114, 19414; USNM 344435, 561265.
Eggs: USNM 18502, 18504, 18505, 19049, 24420.
Xema sabini Sabines Gull:
Skins: NCSM 3678, 16393, 16394, 17777, 17778.
Skeletons: NCSM 17778; USNM 499111, 533882, 533905, 557605, 557606.
Osteology: Characters 1–223
Integument: Characters 224–255
Reproduction: Characters 256–266
Diet: Characters 267–268
Myology: Characters 269–292
Feather Microstructure: Characters 293–344
Skull
1. Premaxilla, anterior tip: (0) decurved; (1) hooked. The anterior tip of the premaxilla is hooked ventrally in a raptorial fashion in some alcids (e.g., Alca torda). The anterior tip of the premaxilla in other alcids (e.g., Brachyramphus marmoratus) is decurved slightly ventrally but does not possess a hooked tip.
2. Premaxilla, dorsal margin (modified from
3. Maxilla, fenestra adjacent to junction of maxilla and palatine: (0) absent; (1) present. The ventral surface of the distal end of the maxilla is fenestrated in some alcids (e.g., Cerorhinca monocerata). This characteristic is absent in many other alcids (e.g., Cepphus grylle). In life the fenestra is covered by a thin membrane. Because the fenestra does not serve as a passageway for muscle, tendon, or nerves, its purpose may be related to flexion or weight reduction.
4. Nasal, anterior projection along the ventral surface of the premaxilla (Chandler, 1990b, character 9): (0) contacting; (1) separated. The nasals converge beneath the premaxilla in some species (e.g., Uria aalge), while in other species (e.g., Fratercula cirrhata) the lateral nasal bars merge with the ventral premaxilla but remain separated.
5. Nasal, maxillary spine on nasal bar (
6. Nasal bar, angle with respect to jugal: (0) ~45 degrees; (1) ~60 degrees. The angle between the nasal and the jugal of most alcids (e.g., Uria aalge) is ~45 degrees, while in the auklets and puffins (e.g., Fratercula cirrhata) this angle is around ~60 degrees.
7. Maxillopalatine strut (
8. Maxillopalatine process shape (
9. Maxillopalatine process orientation (
10. Maxillopalatine process, anterior end, medial margin in ventral view (
11. Palatine, ventral extent of the medial margin of the ventral crest relative to the palatine shelf (crista ventralis medialis,
12. Palatine, anterior margin of the medial palatal crest (crista ventralis medialis;
13. Palatine, posterior margin of the medial palatal crest (crista ventralis medialis;
14. Palatine, lateral margin anterior to contact with the pterygoids (angulus caudolateralis;
15. Palatine, posterior extension (
16. Vomer, anterior curvature (
17. Vomer, anterior tip shape: (0) pointed; (1) bifurcated. The anterior tip of the vomer is pointed in most alcids (e.g., Alca torda), while in some species (i.e., Cepphus grylle) the anterior tip of the vomer is bifurcated.
18. Lamina dorsalis, segmentation:
(0) not segmented; (1) segmented. The lamina dorsalis is an extension
of the mesethmoid that lies against the ventral side of the frontal (
19. Lamina dorsalis, size: (0) large; (1) small. The lamina dorsalisof most alcids (e.g., Alca torda) is a large (mesethmoid margin interrupted only by suture between it and the lamina dorsalis), triangular, anteriorly pointing structure with a medial crest , while in some species (e.g., Alle alle) it is reduced to a small (lamina dorsalis not continuous with margin of mesethmoid, appears to be a separate accessory structure), elongate point.
20. Frontal, nasal fossa, salt glands, depth (modified from
21. Frontal, supraorbital rims (lateral to fossa glandulae nasalis;
22. Mesethmoid, fenestra in nasal capsule anterior to nasofrontal hinge: (0) small fenestra; (1) large fenestra. In contrast with many closely related charadriiforms (e.g., Larus marinus) that have only a small (i.e., fenestra height <=1/3 height of septum) interorbital fenstra, alcids possess a large (i.e., fenestra height >1/3 height of septum) interorbital fenstra.
23. Mesethmoid, fenestra in nasal capsule anterior to nasofrontal hinge (0) fenestrated; (1) not fenestrated. Between the lamina dorsalis and the ectethmoid, the mesethmoid of some alcids (e.g., Fratercula arctica) is fenestrated. The mesethmoid of other alcids (e.g., Alca torda) is not fenestrated.
24. Foramen opthalmicum internum (
25. Fonticulus orbitocranialis (
26. Lacrimal, articulation with ectethmoid (
27. Lacrimal, position in lateral view: (0)posteroventrally directed; (1) ventrally directed. With the exception of Pinguinus impennis and Rynchops niger, the lacrimal of all taxa examined in this study are directed posteroventrally. In contrast, the lacrimal of Pinguinus impennis extends ventrally. The condition shared by Pinguinus impennis and Rynchops niger is not considered homologous here, as the cranium of Rynchops niger is extremely derived(with respect to other charadriiforms).
28. Lacrimal, supraorbital process (
29. Sclerotic ring, shape (from
30. Squamosal, zygomatic process, shape (
31. Squamosal, temporal fossa depth (
32. Squamosal, temporal fossa, medial extent: (0) not medially extended; (1) separated by a thin flat space; (2) separated only by a thin crest. In many alcids (e.g., Aethia psittacula) the temporal fossa is not expressed on the dorsal surface of the skull, although, in some species (e.g., Alca torda) the temporal fossa nearly converge on the dorsal surface of the skull. In Pinguinus impennis the temporal fossa are very deep and separated only by a thin crest. Ordered
33. Squamosal, temporal fossa, shape of medial margin: (0) narrow; (1) broad. In species that possess medially expanded temporal fossa (see character 32) the medial–most extent of the temporal fossa varies in alcids from a broad, relatively ‘U-shaped’ curve (e.g., Alca torda) to a more pointed, medially narrowing groove (e.g., Uria aalge).
34. Supraoccipital foramina (foramen venae occipitalis externae;
35. Cerebellar prominence (
36. Foramen magnum, dorsal margin shape (modified from
37. Secondary articulation of mandible (ala parasphenoidalis;
38. Quadrate (
Mandible
39. Mandible, length of symphysis (modified from
40. Mandible, contact distal to symphysis: (0) non-contacting; (1) contacting. In most alcids (e.g., Alca torda) the mandibular rami are in contact only where fused at the symphysis. In Fraterculini (e.g., Fratercula arctica) the mandibular rami, although not fused, remain in contact posterior to the mandibular symphysis.
41. Mandible, ventral expansion: (0) absent; (1) present. The mandibles of most alcids (e.g., Cepphus columba) are not ventrally expanded. The mandibles of some species (e.g., Fratercula arctica) have a pronounced ventral expansion at the anterior end of the mandible (i.e., beak tip).
42. Mandible, thickening of junction between pars dorsalis and dorsal splenial (Chu, 1998 character 56): (0) flat to moderate; (1) gross, forming massive longitudinal crista. The dorsomedial surface of the mandible is noticeably thickened in terns (e.g., Sterna maxima). In the Alcidae (e.g., Cepphus columba) and most other charadriiforms, the medial surface of the mandible is flat (i.e., lateromedially compressed).
43. Mandible, mediolateral curvature: (0) laterally concave; (1) laterally convex. The mandibular rami of many alcids (e.g., Fratercula arctica) are laterally concave distal to the tip of the bill, while in other alcids (e.g., Alle alle) the rami are curved outward or laterally convex.
44. Prearticular, anterior end (modified from
45. Surangular, fenestration: (0) absent; (1) present. The posterior mandible in many charadriiforms (e.g., Larus marinus) is perforated (fenestra caudalis mandibulae;
46. Surangular, fenestration, quantity: (0) one; (1) two. Most alcids (e.g., Cepphus grylle) have a single caudal mandibular fenestra, while the Fraterculini (puffins; e.g., Fratercula arctica) and Mancallinae (e.g., Miomancalla howardi) are characterized by the presence of two small caudal mandibular fenestrae perforating the dorsal surangular.
47. Articular, medial articular process foramen (foramen pneumaticum articulare;
48. Articular, medial articular process, shape:
(0) anteroposteriorly compressed; (1) dorsoventrally compressed; (2)
rounded point. The medial articular process of the mandible, which
articulates with the parasphenoid process (
49. Articular, medial articular process, orientation: (0) projects medially; (1) projects posteromedially. The medial articular process of the mandible points medially in some alcids (e.g., Fratercula cirrhata), while in other alcids (e.g., Cepphus grille) this same process points more posteriorly.
50. Articular facet in ventral view, shape: (0) rounded knob; (1) anteromedial projection. In ventral view, the articular facet of the mandible is visible as a small, often rounded knob in some alcids (e.g., Alca torda). In some alcids (e.g., Cepphus grylle) this facet is more pointed and projects anteromedially.
51. Articular, retroarticular process (modified from
52. Articular, retroarticular process length (modified from
Vertebrae
53. Atlas, flange on the lateral margins of the arcus atlanticus in dorsal view: (0) straight; (1) laterally angled. The posteriorly-projecting processes for articulation with the axis project posteriorly in most species of alcids (e.g., Uria aalge). In some species of alcids (e.g., Fratercula arctica) the zygapophyses angle laterally.
54. Axis, dorsal extension of neural spine: (0) short; (1) long. In posterior view, the neural spine of the axis in most alcids (e.g., Uria aalge) is short (i.e., less than half of the length of the neural spine extends above the level of the anapophyses), although in some alcids(e.g., Alca torda) this projection of the axis is lengthened and extends to a point well above the anapophyses (i.e., more than half of the length of the neural spine extends above the level of the anapophyses).
55. Thoracic vertebrae, hypapohyses: (alae cristae ventralis;
56. Thoracic vertebrae, number of hypapohyses (crista [processus] ventralis corporis;
57. Pygostyle, dorsal margin (
Sternum
58. Sternum, coracoidal sulci, separation (modified from
59. Sternum, anterior pneumatic foramen: (0) reduced; (1) pneumatic. The pneumatic foramen located at the anterior end of the sternal basin is reduced to a tiny ‘pin-sized’ hole in alcids (e.g., Alca torda). In most other charadriiforms examined (e.g., Larus marinus) this feature is a deep pneumatic foramen.
60. Sternum, sternocoracoidal process, orientation (
61. Sternum, costal processes, quantity (
62. Sternum, width (modified from
63. Sternum, medial notch (
64. Sternum, medial notch, shape: (0) a notch; (1) a fenestra. Among alcids, only the puffins (Fraterculini) retain the remnant of the medial sternal notch as a medial sternal fenestra.
65. Sternum. lateral notch, shape (
66. Sternum, lateral notch, anterior extent of incisure: (0) shallow; (1) deeply incised. In most charadriiforms, the extent to which the lateral sternal notches incise proximally is limited (e.g., Larus marinus), while in some charadriiforms (e.g., Mancalla vegrandis), these incisures are extensive.
67. Sternum, posterior extension of carina relative to lateral sternal notches/fenestrae (modified from
68. Sternum, supracoracoideus scar, position: (0) angled medially; (1) straight. In contrast to the condition observed many charadriiforms (e.g., Sterna maxima)
in which the scar for the supracoracoideus muscle on the ventral
surface of the sternum angles medially from the coracoidal sulcus
towards the carina, in Alcidae
this scar extends posteriorly for almost the entire length of the
carina. This feature is correlated with the increased resistance during
the upstroke experienced by alcids while flying underwater (
69. Sternum, posterior margin ossification (margo caudalis sterni;
70. Sternum, length of area between distal extent of medial fenestra and posterior margin (modified from
71. Sternum, length: (0) short; (1) long. When compared to their immediate outgroup, the Stercorariidae, alcids have an elongated sternum (i.e., sternum >2× long than wide), a character which has been associated with diving (
Furcula
72. Furcula, symphysis (apophysis), size (modified from
73. Furcula, anterior surface of rami (
74. Furcula, cristae on anterior surface of rami: (0) absent; (1) present. The anterior surface of the furcular rami dorsal to the apophysis is characterized by the presence of small cristae/tubercles in some alcids (e.g., Alca torda).
75. Furcula, curvature of omal extremity (
76. Furcula, dorsoventral expansion of omal extremity: (0) absent; (1) present. Ventral to the coracoidal facet, the clavicles of most alcids (e.g., Pinguinus impennis) are dorsoventrally expanded and lateromedially compressed (i.e., bladelike; scapular tuberosity much thinner than clavicular shaft ventral to the coracoidal facet). The clavicles of many other charadriiforms (e.g., Tryngites subruficollis) are more circular in cross section and much less dorsoventrally expanded (i.e., scapular tuberosity same width or thicker than clavicular shaft ventral to the coracoidal facet).
77. Furcula, coracoidal tuberosity, position relative to coracoidal facet (
Scapula
78. Scapula, acromium, attachment of acrocoracoacromiale ligament in proximal view, : (0) anteriorly oriented pit; (1) laterally oriented scar. In some alcids (e.g., Uria aalge), the attachment of the acrocoracoacromiale ligament is an anteriorly oriented excavation of the ventral surface of the acromium process bordered medially by a crest. This same attachment point in other alcids (e.g., Aethia cristatella) is rotated laterally and is characterized by a relatively smooth attachment surface.
79. Scapula, acromium, shape in lateral view: (0) blunt, rounded; (1) angular, pointed. The acromium process of all extant alcids (e.g., Uria aalge) has a pointed proximal tip, while the tip of the acromium in some larids (e.g., Larus marinus) is rounded/truncated and does not project anteriorly.
80. Scapula, scapulotricipital tubercle: (0)
absent; (1) present. A raised process for attachment of m.
scapulotriceps on the ventral surface of the scapula (tuberculum m.
scapulotricipitis,
81. Scapula, width of distal extremity: (0) tapering; (1) dorsoventrally expanded. The dorsal margin of the scapula (margo dorsalis;
82. Scapula, shape of distal extremity: (0) curved; (1) angled. In contrast to the gently ventrally curving distal extremity of many charadriiforms (e.g., Larus argentatus), the scapulae of all known alcids are characterized by a ventrally directed angular bend proximal to the distal most extremity.
Coracoid
83. Coracoid, furcular facet shape (modified from
84. Coracoid, furcular facet, notch posterior to bicipital tubercle: (0) absent; (1) present. The ventral margin of the furcular facet is curves dorsally just posterior to the process for the attachment of the bicipital muscle in some species of alcids (e.g., Uria aalge). The ventral margin of this feature in other alcids (e.g., Alca torda) is gently curved but not notched.
85. Coracoid, supracoracoidal sulcus: (0) pneumatic; (1) apneumatic, but deeply undercut; (2) not deeply undercut. The medial side of the distal end or head of the coracoid of some charadriiforms (e.g., Anous tenuirostris) are characterized by a pneumatic excavation. The coracoids of all alcids are apneumatic, although the brachial crest is deeply undercut for the passage of the supracoracoideus muscle in some species of alcids (e.g. Cepphus grille), while in some alcids (e.g., Cerorhinca monocerata) the brachial crest is not deeply undercut (i.e., ventrally concave). Ordered
86. Coracoid, brachial tuberosity, shape: (0) a tubercle; (1) a crest. The brachial tuberosity is developed as an anteroposteriorly oriented crest in some alcids (e.g., Cepphus grille), while in other alcids (e.g., Aethia psittacula) the brachial tuberosity is developed simply as a small rounded tubercle positioned roughly at the midpoint on the neck of the coracoid. The term brachial crest is used here to describe the latter condition.
87. Coracoid, brachial tuberosity, shape in medial view: (0) approximately straight; (1) distinctly curved. In species that possess a brachial crest rather than a brachial tubercle (see character 88), the crest varies from an approximately straight crest (e.g., Alle alle) to a distinctly concave curve (e.g., Alca torda).
88. Coracoid, neck in dorsal view (
89. Coracoid, supracoracoideus scar development: (0) a distinct ridge; (1) ridge reduced or absent. Contact with the supracoracoideus creates a distinct, medially oriented ridge/scar in most alcids (e.g., Alca torda) that gives the shaft of the coracoid a distinctly angular cross-section, while in Cepphus this structure is greatly reduced or absent and the cross-section of the coracoid element is more rounded.
90. Coracoid, supracoracoidal nerve foramen (
91. Coracoid, position of supracoracoidal nerve foramen (0) distal; (1) proximal. In alcids that possess a coracoidal foramen, the position of this feature is typically near the midpoint of the of the procoracoid process near the shaft of the coracoid (e.g., Pinguinus impennis), although in Cepphus this foramen is positioned on the extreme anteroproximal edge of the procoracoid process leaving only a very thin strut of bone which forms the dorsal margin of the procoracoid process.
92. Coracoid, procoracoid process, shape: (0) rectangular; (1) triangular; (2) wing-shaped. The procoracoid process of some alcids (e.g., Aethia psittacula) is ‘strap-like’ and has a roughly rectangular shape, resembling the condition in the outgroup of Alcidae. The shape of the procoracoid process in most alcids (e.g., Fratercula cirrhata) is triangular.
93. Coracoid, tip of procoracoid: (0) straight; (1) hooked. In Brachyramphus, the tip of the procoracoid is hooked anteriorly. This feature is absent in all other Alcidae for which the coracoid is known.
94. Coracoid, procoracoid process, orientation: (0) points dorsomedially; (1) points ventromedially; (2) points anteriorly. The tip of the procoracoid process in the auklets (e.g., Aethia pygmaea) is hooked, and points ventromedially, while in all other alcids (e.g., Uria aalge) the tip of the procoracoid process points dorsomedially. In many other charadriiforms (e.g., Larus marinus) the procoracoid process is noticeably hooked to provide passage for the supracoracoideaus tendon, and as a result the tip of the procoracoid process points anteriorly.
95. Coracoid, procoracoid process, shape of proximal edge: (0) concave; (1) convex. In posterior view the proximal edge of the procoracoid process (lower or sternal side) in some alcids (e.g., Cerorhinca monocerata) curves convexly. The procoracoid process of other alcids (e.g., Uria aalge) is concave in curvature.
96. Coracoid, scar on anterior face of lateral edge of coracoid: (0) absent; (1) present. Alcids (e.g., Cepphus grylle) possess a distinct scar along the anterior surface of the lateral process that is lacking in other charadriiforms (e.g., Bartramia longicauda). The exact origin of this scar is unclear, although
97. Coracoid, scar extension along anterior surface of lateral process: (0) extends to sternal articulation; (1) bordered sternally by crest.This scar is less medially and sternally extended and more excavated in the auklets and puffins (e.g., Fratercula cirrhata) than in other alcids (e.g., Alca torda) in which this scar is less excavated and extends to the sternal margin of the coracoid (i.e., not bordered sternally by a crest).
98. Coracoid, crest along sternal edge of lateral process: (0) absent; (1) present. In anterior view the sternal edge of the lateral process of some alcids (e.g., Uria aalge) is characterized by a crest or thickening of the sternal margin. This characteristic is absent in some alcids (e.g., Alca torda).
99. Coracoid, lateral (sternocoracoidal) process length (modified from Strauch, 1985, character 11):
(0) elongate, with anteriorly pointing tip; (1) short, with laterally
pointing tip. The lateral process of the coracoid is a well developed,
elongate projecting process with an anteriorly projecting tip in most
alcids (e.g., Alca torda) the Laridae and most other charadriiforms; it is absent or poorly developed in some of the auklets (e.g., Aethia pusilla). These differences are illustrated by
100. Coracoid, medial sternal process, notch in dorsal margin:
(0) absent; (1) present. The posteromedial margin of the proximal
coracoidal shaft just distal to the medial sternal articulation (angulus
medialis;
101. Coracoid, sternal facet curvature (
Humerus
102. Humerus, head, distal extent of posterior margin (caput): (0) convex curve; (1) triangular point. The distally overturned posterior margin of the humeral head of some alcids (e.g., Cepphus grylle) is characterized by a distinct, distally extending point. This feature is rounded in most other alcids (e.g., Alca torda).
103. Humerus, dorsal caput, posterior side (modified from
104. Humerus, deltopectoral crest, distal extension (modified from
105. Humerus, deltopectoral crest, transition to shaft: (0) smooth; (1) abrupt. As noted by
106. Humerus, deltopectoral crest, dorsal curvature: (0) concave; (1) flat. In dorsal view, the area between the dorsal surface of the deltopectoral crest and the dorsal tubercle (i.e., the dorsal shaft distal to the head) is concave in many charadriiforms (e.g., Creagrus furcatus). In all alcids except Alca stewarti this space is flat or slightly convex in some cases (e.g., Brachyramphus marmoratus).
107. Humerus, impressio coracobrachialis scar, depth (
108. Humerus, distal edge of bicipital crest, angle with respect to long axis of shaft: (0) not perpindicular; (1) nearly perpindicular. The ventral edge of the bicipital crest forms a nearly perpindicular angle to the shaft in some species (e.g., Pinguinus impennis) while in other species (e.g., Alca torda) the bicipital crest is positioned at an obtuse angle with respect to the long axis of the humeral shaft.
109. Humerus, biciptal crest, transition to shaft: (0) smooth; (1) notched. This character, noted by Olson and Winker (2009), varies from a condition where (in anterior view) the bicipital crest transitions smoothly onto the humeral shaft (e.g., Aethia pusilla) to a condition in which there is a distinct notch or separation between these structures (e.g., Alle alle).
110. Humerus, coracobrachial sulcus, conformation: (0) open sulcus; (1) closed duct. As noted by Olson and Winker (2009), the coracobrachial sulcus is an open sulcus in most species of alcids (e.g., Aethia pusilla), although in some species of alcids (e.g., Alca torda) the sulcus is enclosed to form a duct.
111. Humerus, coracobrachial sulcus, curvature: (0) dorsal; (1) ventral. The distal most point of the bicipital surface, as defined by the curvature of the coracobrachial sulcus, which curves or angles dorsal to the bicipital crest on the anterior surface of the humerus in some alcids (e.g., Pinguinus impennis), while in other alcids (e.g., Alle alle) the coracobrachial sulcus and the distal edge of the bicipital surface extend ventrally to terminate where the bicipital crest contacts the ventral surface of the humeral shaft.
112. Humerus, supracoracoideus scar, depth: (0) deep; (1) shallow. The attachment for the supracoracoideus muscle on the posterior humerus is a deep (i.e., excavated) scar in puffins (e.g., Fratercula arctica) and a shallow (i.e., basically flat) impression in others (e.g., Cepphus grylle).
113. Humerus, supracoracoideus scar, shape:
(0) round; (1) long, proximally broadening; (2) long, does not broaden
proximally. The attachment of the supracoracoideus muscle on the
proximal humerus of most charadriiforms (e.g., Larus marinus) is a rounded scar, while in alcids this scar is distally elongated (Crista m. supracoracoidei;
114. Humerus, supracoracoideus scar, transition into the secondary pneumatic fossa (pf2): (0) pf2 borders scar; (1) scar separated from pf2; (2) margo caudalis widely separates pf2 and scar. In most alcids (e.g., Alca torda) the dorsal extent of the excavation of the second pneumatic fossa parallels the ventral margin of the supracoracoideus scar. In some species (e.g., Cerorhinca monocerata)the excavation for pneumatic fossa 2 is separated from the supracoracoideus scar by a thin, flat, latero-medially oriented projection of the humeral shaft (which is most like the very reduced remains of the margo caudalis). The supracoracoideus attachment point in many other charadriiforms (e.g., Larus marinus) is widely separated from the medial portion of the humeral shaft by the margo caudalis and does not extend as far distally as the condition seen in alcids. Ordered
115. Humerus, medial crest between pneumatic fossae, extension relative to the bicipital crest (modified from
116. Humerus, primary pneumatic fossa, excavation for insertion of humerotriceps muscle:
(0) absent; (1) present. The interior (i.e., anterior wall) of the
ventral pneumatic fossa (fossa pneumotricipitalis ventralis;
117. Humerus, primary pneumatic fossa, accessory ridge: (0) absent; (1) present. The interior (i.e., anterior wall) of the ventral pneumatic fossa in most alcids (e.g., Alca torda) is smooth. In some Fraterculini (e.g., Cerorhinca monocerata) this area is characterized by a small accessory ridge.
118. Humerus, primary pneumatic fossa, depth: (0) deeply pneumatic; (1) moderately deep; (2) shallow. In contrast to the deeply pneumatic (i.e., deeper than wide) first fossa of most charadriiforms (e.g., Larus marinus), the first pneumatic fossa of most alcids (e.g., Uria aalge) is moderate in depth (i.e., ~ as deep as wide). In true auks (e.g., Alca torda) the first pneumatic fossa is very shallow and constricted (i.e., less deep than wide). Ordered
119. Humerus, primary pneumatic fossa, shape: (0) round; (1) oval. The first pneumatic fossa varies in shape from rounded (e.g., Pinguinus impennis) to oval (e.g., Cerorhinca monocerata).
120. Humerus, mancalline scar on posterior side of proximal humerus: (0) absent; (1) present. In Mancalla a deep scar extends along the humeral shaft distal to the first pneumatic fossa. This distinct scar, hereafter referred to as the ‘mancalline scar’, is absent in all other charadriiforms. And its its homology is, therefore, uncertain. Although it is possible that this scar may represent an additional insertion point of m. humerotriceps.
121. Humerus, mancalline scar on posterior side of proximal humerus, conformation: (0) excavated; (1) raised. The dorsal and ventral borders of the scar on the posterior side of the proximal humerus of Mancalla extend parallel to one another in some species (e.g., Mancalla californiensis). In other species (e.g., Miomancalla wetmorei) these borders converge proximally, giving this scar a more triangular shape.
122. Humerus, mancalline scar on posterior side of proximal humerus, proximal extension relative to the first pneumatic fossa: (0) extends within the first pneumatic fossa; (1) scar terminates near the distal margin of the first pneumatic fossa. The proximal extent of this scar varies from a condition in which the scar extends well within the first pneumatic fossa (e.g., Mancalla californiensis) to a condition in which this scar terminates near the distal margin of the first pneumatic fossa (e.g., Miomancalla wetmorei).
123. Humerus, mancalline scar on posterior side of proximal humerus, shape: (0) ridges parallel; (1) ridges converge proximally. The dorsal and ventral borders of the scar on the posterior side of the proximal humerus of Mancalla extend parallel to one another in some species (e.g., Mancalla californiensis). In other species (e.g., Miomancalla wetmorei) these borders converge proximally, giving this scar a more triangular shape.
124. Humerus, attachment of scapulohumeralis caudalis, position: (0) medial; (1) ventral. The insertion point of scapulohumeralis caudalis (crus ventrale fossae;
125. Humerus, scapulohumeralis caudalis attachment scar, depth: (0) flat or slightly concave; (1) a deep pit. As noted by
126. Humerus, primary pneumatic fossa, shape of distal edge: (0) convex; (1) straight; (2) concave. The distal edge of the pneumatic fossa is concave (e.g., Aethia pusilla) or straight (e.g., Alca torda) in alcids. This feature is convex in most other charadriiforms (e.g., Charadrius wilsonia).
127. Humerus, secondary pneumatic fossa, (fossa pneumotricipitalis dorsalis;
128. Humerus, secondary pneumatic fossa, depth (fossa pneumotricipitalis;
129. Humerus, secondary pneumatic fossa, division: (0) absent; (1) present. The pneumatic fossa II of some alcids (e.g., Aethia pygmaea) is divided by a medial crest, creating two separate points for muscle insertion. This feature is absent in most alcids (e.g., Cerorhinca monocerata).
130. Humerus, ridge between ventral tubercle and secondary pneumatic fossa: (0) absent; (1) present. On the posterior side of the humerus in Brachyramphus a slight ridge extends distally from underneath the distally overturned head of the humerus and contacts dorsal margin of the ventral tubercle, thus dividing the second pneumatic fossa from the capital groove.
131. Humerus, ventral tubercle, shape: (0) long and thin; (1) short and thick. In ventral view the ventral tubercle of some species of alcids (e.g., Fratercula arctica) is fairly thin and extends posteriorly to a point roughly level with the posterior extent of the caput. In other alcids (e.g., Alca torda) this feature does not extend as far posteriorly, and is more robust.
132. Humerus, ventral tubercle, lateral margin curvature: (0) single concavity; (1) double concavity. When viewed ventrally the lateral margin of the ventral tubercle of all alcid species other than Cerorhinca monocerata is a single concave curve. This feature in Cerorhinca monocerata is characterized by two concave curves. This character is the result of the crus ventrale fossae of Cerorhinca monocerata being divided into two sections.
133. Humerus, ventral tubercle, shape of posterior tip: (0) rounded or oval; (1) elongate. In Brachyramphus the posterior-most extension/point of the ventral tubercle is dorsally expanded into an elongate shape. In other alcids (e.g., Fratercula arctica) this feature is rounded or oval in shape.
134. Humerus, ventral tubercle, ventral margin curvature: (0) not deeply grooved; (1) deeply grooved. In anterior or posterior view the point at which the ventral tubercle and the ventral margin of the first pneumatic fossa merge varies in its shape from ventrally convex or flat (e.g., Fratercula corniculata) to ventrally concave (e.g., Pinguinus impennis).
135. Humerus, m. latissimus dorsi scar, curvature: (0) straight; (1) curves dorsally. The latissimus dorsi scar in most alcids (e.g., Fratercula arctica) extends distally straight down the shaft of the humerus. The latissimus dorsi scar of some alcids (e.g., Cepphus grylle) curves anteriorly across the dorsal surface of the humeral shaft.
136. Humerus, capital groove, anterior expression (modified from
137. Humerus, capital groove, width: (0) wide; (1) constricted. In all alcids (e.g., Fratercula arctica) except Mancalla the capital groove is an open ‘U’ shaped groove. Only in Mancalla does the caput overhang the capital groove, giving the proximal wall of the capital groove a convex shape, and constricting this passageway.
138. Humerus, capital groove, shape: (0) ‘U’ shaped; (1) pointed anteriorly. In ventral view the capital groove is ‘U’ shaped in most alcids (e.g., Aethia psittacula). In Mancalla the capital groove is constricted anteriorly.
139. Humerus, orientation of head relative to shaft: (0) in line with shaft; (1) rotated anteriorly. As noted by
140. Humerus, longitudinal shape of shaft, : (0) sigmoidal; (1) arced. As noted by
141. Humerus, cross-sectional shape of shaft, : (0) rounded; (1) semi-rounded; (2) flattened. As noted by Howard(1978, 1982), the humeral shaft of most alcids (e.g., Alca torda) is flattened in cross-section (flattened oval). The shaft of some alcids (e.g., Cepphus grylle) is more rounded (i.e., semi-rounded) in cross-section. The humeral shaft of other charadriiforms (e.g., Larus marinus) is rounded in cross-section. Ordered
142. Humerus, shaft thickness: (0) robust; (1) gracile. The thickness of the humeral shaft varies from robust (i.e., width of shaft in anterior view greater than or equal to half the width of the humeral head; e.g., Brachyramphus marmoratus) to gracile (i.e., width of shaft in anterior view less than or equal to half the width of the humeral head; e.g., Alle alle).
143. Humerus, dorsal supracondylar process, shape: (0) large dorsally pointing projection;(1) small dorsally pointing projection; (2) smoothly transitioning; (3) square; (4) rounded knob. The attachment point for M. extensor carpi along the dorsal margin of the distal humerus projects dorsally away from the shaft in many charadriiforms (e.g., Larus marinus; state 0) while in all alcids this feature is elongated along the shaft of the humerus medially and does not project as far dorsally. States within Alcidae include: (1) a small dorsally projecting point (e.g., Alca torda), (2) square, ~ 90° contact with shaft (e.g., Fratercula arctica) (3) smoothly transitioning to the shaft (e.g., Pinguinus impennis), In Mancalla this process is a rounded knob that is separated from the distal extent of the dorsal supracondylar prominence (crest) by a gap.
144. Humerus, dorsal supracondylar process, length: (0) short; (1) long. The dorsal supracondylar process of most alcids (e.g., Aethia pygmaea) is short (i.e., the proximodistal length measured from the distal end of the humerus to the proximal termination of the crest on the humeral shaft is shorter than the greatest distal width of the humerus measured from the entepicondyle to the dorsal condyle). The dorsal supracondylar process of some alcids (e.g., Mancalla lucasi) extends further proximally onto the humeral shaft.
145. Humerus, dorsal sulcus: (0) continuous; (1) divided. The sulcus for passage of extensor metacarpi radialis, which runs between the dorsal supracondylar process and the dorsal condyle is continuous in all alcids (e.g., Pinguinus impennis) except the Fraterculini (e.g., Fratercula arctica), in which this sulcus is divided by a bony crest, forming a round pit on the posterior edge of the dorsal condyle.
146. Humerus, ventral epicondyle, orientation relative to shaft: (0) flared ventrally; (1) nearly straight. As noted by
147. Humerus, tricipital sulci, width (modified from
148. Humerus, tricipital crest, orientation: (0) straight, projects posteriorly; (1curved dorsally over scapulotricipital sulcus. The crest that divides the tricipital sulci (humerotricipital and scapulotricipital sulci) is a low posteriorly projecting ridge in most alcids (e.g., Uria aalge). In Mancalla this ridge veers dorsally and merges with the dorsal margin of the scapulotricipital sulcus on its lateral side.
149. Humerus, humerotricipital sulcus, shape in distal view: (0) flattened; (1) ‘U’ shaped or curved. In distal view the humerotricipital sulcus of all alcids other than Alle alle and Uria aalge is curved or ‘U’ shaped.
150. Humerus, tricipital fossae: (0) absent; (1) present. The scapulotricipital and humerotricipital sulci of Mancallinae are characterized by fossae positioned at the proximal end of the sulci. The sulci of other alcids transition smoothly onto the posterior face of the humeral shaft.
151. Humerus, relative distal extension of condyles (
152. Humerus, ventral condyle in distal view, posterior trochlear process: (0) absent; (1) present. As noted by
153. Humerus, ventral condyle, shape (Chandler, 1990b, character 59): (0) rounded; (1) flattened. The anterior face of the ventral humeral condyle is flattened in most alcids (e.g., Pinguinus impennis), while the ventral condyle is rounded in all other charadriiforms examined during this study (e.g., Larus argentatus).
154. Humerus, separation of humeral condyles: (0) absent; (1) present. In distal view the humeral condyles of Brachyramphus are separated, whereas the ventral margin of the dorsal condyle and the dorsal margin of the ventral condyle of other alcids (e.g., Synthliboramphus antiquus) contact one another.
155. Humerus, tubercle adjacent to dorsal condyle: (0) absent; (1) present. As noted by
156. Humerus, tubercles dorsal to scapulotricipital groove: (0) absent; (1) present. Many alcids (e.g., Brachyramphus perdix) possess a tubercle along the dorsal border of the scapulotricipital sulcus. In alcids this tubercle is located distal to paired fossae that lye between the raised dorsal margin of the scapulotricpital sulcus and the dorsal sulcus.
157. Humerus, tubercles dorsal to scapulotricipital groove, quantity: (0) a single tubercle; (1) paired tubercles. Rather than a single tubercle, some alcids (e.g., Mancalla cedrosensis)possess two tubercles dorsal to the scapulotricipital sulcus.
158. Humerus, ventral supracondylar tubercle (anterior ligament scar): (0) triangular; (1) rounded. On the anterior surface of the distal shaft of the humerus ventral to the brachialis scar, the ventral supracondylar tubercle, which is the attachment for the ventral collateral ligament, varies in its shape from triangular (e.g., Brachyramphus marmoratus), to an oval/rounded pit (e.g., Alca torda).
159. Humerus, pit associated with anterior ligament scar: (0) absent; (1) present. A small scar on the distal humerus marks the attachment point of the M. pronator sublimis in some species of alcids (e.g., Alca torda), but is absent in many species (e.g., Uria aalge).
160. Humerus, position of pit adjacent to anterior ligament scar: (0) proximal; (1) ventral; (2) detached. The position of the small pit, which marks the origination point of the M. pronator sublimis varies in its position. In some species (e.g., Aethia pygmaea) this feature is located at the proximal tip of the anterior ligament scar, while in other species of alcids (e.g., Aethia psittacula) it is located along the dorsal margin of this scar. In some other charadriiforms (e.g., Phaetusa simplex), this scar is detached from the anterior ligament scar.
Radius
161. Radius, bicipital tubercle: (0)reduced;(1)distinct. The auklets (e.g., Ptychoramphus aleuticus) and murrelets (e.g., Synthliboramphus antiquus) lack the distinct bicipital tubercle found in other alcids (e.g., Alca torda).
162. Radius, bicipital tubercle, shape: (0) a crest; (1) a round tubercle. The shape of the bicipital tubercle in Alcidae (e.g., Alca torda) is an elongated crest-like structure, rather than the rounded tubercle of other charadriiforms (e.g., Larus marinus).
163. Radius, bicipital tubercle, position: (0) contacts papilla; (1) separate. The bicipital tubercle of most alcids (e.g., Uria lomvia) is a swollen area along the distal margin of what
164. Radius, sulcus tendinosus (
165. Radius, notch in distal end: (0) absent; (1) present. In anterior view, the crest associated with the scapho-lunar facet of some alcids (e.g., Aethia pygmaea) extends far enough distally so that a notch is formed between that crest and the ventral-most articulation surface of the distal radius with the radiale. In other alcids (e.g., Alle alle) the crest on the anterior surface of the radius transitions smoothly into the distal end of the radius.
Ulna
166. Ulna, olecranon, length: (0) long; (1) short; (2) truncate. The olecranon of most alcids (e.g., Alca torda), is a long (i.e., projects well past the medial extent of the ventral cotyla) medially projecting point. In some species (e.g., Pinguinus impennis) the olecranon is truncated (i.e., does not extend past the medial extent of the ventral cotyla), while the condition in other alcids (i.e., Aethia pusilla) is intermediate (i.e., olecranon short). Ordered
167. Ulna, olecranon, curvature: (0) flares posteriorly; (1) curves anteriorly. In dorsal view the posterior margin of the ulnar head of most alcids (e.g., Pinguinus impennis) flares posteriorly to form the posterior edge of the olecranon, while in some species (e.g., Alca torda) this margin curves anteriorly.
168. Ulna, ventral collateral ligament tubercle, shape: (0) triangular; (1) rounded. The scar for the attachment of the ventral collateral ligament is triangular in some alcids (e.g., Alca torda) and more rounded in others (e.g., Aethia psittacula).
169. Ulna, crest extending from the ventral cotyla to the anterior margin of the ventral collateral ligament tubercle (modified from
170. Ulna, crest extending from the ventral cotyla to the posterior margin of the ventral collateral ligament tubercle: (0) absent; (1) present.Although most alcids (e.g., Alca torda) lack a crest, which extends from the ventral cotyla to contact the posterior margin of the ventral collateral ligament scar, several alcids (e.g., Brachyramphus brevirostris) possess this character.
171. Ulna, dorsal cotylar process, anterior margin shape: (0) rounded; (1) straight. The dorsal condyle of alcids is bordered on the posterior margin by a posteriorly projecting bladelike process for attachment of the scapulotriceps muscle. The anterior margin of this feature in dorsal view can be either rounded (e.g., Alca torda) or straight (e.g., Fratercula cirrhata).
172. Ulna, dorsal cotylar process, development (
173. Ulna, proximal radial depression, shape:
(0) a round pit; (1) a triangular pit; (2) broad and flat. In contrast
to the distinctly triangular shape of the proximal radial depression of
most charadriiforms (e.g., Larus marinus), the proximal radial depression of all extant alcids (e.g., Uria aalge) is a round pit situated distal to the ulnar cotylae. Insome charadriiforms not closely related to Alcidae (e.g., Bartramia longicauda) the radial depression is broad and flat.In Miomancalla wetmorei
the proximal radial depression is a broad flat space bordered dorsally
and ventrally by distinct crests that occupies the entire anterior
surface of the ulna (
174. Ulna, brachial impression, breadth: (0) thin; (1) broad. As noted by
175. Ulna, intramuscular line: (0) non-distinct; (1) distinct, raised ridge. As noted by
176. Ulna, shaft, shape: (0) rounded; (1) semi-flattened; (2) flattened. As noted by
177. Ulna, dorsal condyle, shape: (0) rounded; (1) angular. The entire posterior margin of the dorsal ulnar condyle of some alcids (e.g., Pinguinus impennis) is rounded, while in other alcids (e.g., Alca torda) the dorsal condyle has an angular bend distal to the contact with the ulnar shaft.
178. Ulna, carpal tubercle, shape: (0) flat or angled distally; (1) concave. The distal margin of the carpal tubercle of some alcids (e.g., Alca torda) is flat or angles slightly distally in some specimens. In Cepphus this surface is concave, giving the distal surface of the carpal tubercle a hooked appearance.
179. Ulna, sulcus intercondylaris: (0) concave; (1) flat. In distal view the groove between the ulnar condyles is a concave ‘U’ shaped depression in some species (e.g., Alca torda). In other species (e.g., Pinguinus impennis) the posterior surface of the ventral condyle angles anteriorly from the sulcus towards the ventral surface of the ulna, forming a flat almost 90° angle between the condyles (i.e., ‘stairstep-like’).
180. Ulna, length: (0) longer than carpometacarpus; (1) shorter than carpometacarpus. The ulnae of most alcids (e.g., Cepphus grylle) are longer than their carpometacarpi, while the ulnae of Mancallinae are shorter (e.g., Mancalla cedrosensis). This condition is not known in other flightless birds, but interestingly, is found in some hummingbirds.
181. Ulna, ventral condyle, orientation (
182. Ulna, dorsal condyle, distal extension (
Carpometacarpus
183. Carpometacarpus, extensor process of metacarpal 1: (0) present; (1) absent. The carpometacarpi of all alcids except Mancalla (e.g., Mancalla cedrosensis) have an extensor process on the anterior margin of metacarpal 1.
184. Carpometacarpus, metacarpal 1, length (modified from
185. Carpometacarpus, extensor process of metacarpal 1, anterior margin shape (modified from
186. Carpometacarpus, proximal intermetacarpal spatium, position relative to the distal extent of metacarpal 1 (modified from
187. Carpometacarpus, posterior extension of ventral trochlear margin relative to metacarpal III (modified from
188. Carpometacarpus, pisiform process, development: (0) distinct; (1)reduced. The Pisiform process of most alcids (e.g., Miomancalla wetmorei) is a distinct ventral projection. The Pisiform process of Mancalla cedrosensis is reduced to a small scar. Similar to the condition observed in penguins, the reduction of this feature in Mancalla may be related to the stiffening of the wing that is associated with the lack of these highly specialized wing propelled divers need to flex the manus.
189. Carpometacarpus, distal end of tendinal groove (i.e., sulcus interosseous;
190. Carpometacarpus, minor digit articulaton: (0) level with facies articularis digitalis major; (1) proximal to facies articularis digitalis major. The articulation surface of the minor digit (III:1) is located proximally to the articulation surface for the major digit (II:1) in some species (e.g., Pinguinus impennis), whereas in other species (e.g., Alca torda) the articulation surfaces are approximately level.
Major Phalanx
191. Manual digit II, phalanx 1, fenestration: (0) absent; (1) present. The major phalanx of many charadriiforms (e.g., Larosterna inca) is penetrated by two fenestrae. These fenestrae are absent in all alcids.
192. Manual digit II, phalanx 1, shape of process on dorsal surface of the distal end: (0) rounded; (1) rectangular. A bladelike process projects posteriorly from the distal end of the first phalange of the second digit. In dorsal view this process varies from rounded (e.g., Aethia pusilla) to rectangular (e.g., Alca torda).
193. Manual digit II, phalanx 1, length: (0) <1/2 length of carpometacarpus; (1) >1/2 length of carpometacarpus. The greatest length of the major phalanx of some charadriiforms (e.g., Sterna maxima) is >1/2 the greatest length of the carpometacarpus. The length of the major phalanx is <1/2 the length of the carpometacarpus in all alcids except Pinguinus impennis, in which the relative length of the wing elements has been reduced in association with flightlessness.
Pelvis
194. Ilium, pre-acetabular ilium, lateral expansion: (0) not expanded, narrow; (1) expanded laterally, spatulate. As noted by
195. Synsacral strut extending to acetabulum (
196. Renal depression (
197. Antitrochanteral sulcus, distal extension: (0) terminates at antitrochanter; (1) extends past antitrochanter. The antitrochanteral sulcus (sulcus antitrochantericus,
198. Iliosynsacral suture: (0) fused; (1)
perforated. The contact between the lateral processes of the sacral
vertebrae and the ilium, termed the iliosynsacral suture (sutura
iliosynsacralis,
199. Ilium, post acetabular dorsal iliac crest: (0) broadens; (1) narrows. The dorsal iliac crest (crista dorsolateralis ilii,
200. Ilium, dorsolateral iliac spine orientation: (0) dorsal; (1) dorsolateral. The dorsolateral iliac spine (spina dorsolateralis ilii,
201. Ilium, dorsolateral iliac spine shape: (0) pointed; (1) square. The dorsolateral iliac spine (spina dorsolateralis ilii,
202. Ischium, relative length of ischial angle and posterior projection (
203. Pelvis, width: (0) broad; (1) narrow. In contrast to the broad (i.e., length of pelvis from anterior-most illium to distal point of the dorsal iliac spine <= 2× width across pelvis at antitrochanter) pelvi of many other charadriiforms (e.g., Sterna maxima), the pelvi of all alcids are narrow (i.e., length of pelvis from anterior-most illium to distal point of the dorsal iliac spine>2× width across pelvis at antitrochanter).
Femur
204. Femur, trochanteric ridge, shape: (0) convex; (1) straight; (2) concave. In most alcids the anterior margin of the femoral trochanter in lateral view is straight (e.g., Alca torda) or convex (e.g., Alle alle). In puffins (e.g., Fratercula arctica) the trochanter is slightly concave.
205. Femur, trochanteric ridge, length: (0) long; (1) short. As noted by
Tibiotarsus
206. Tibiotarsus, cnemial crests, shape in proximal view: (0) ‘T’ shaped; (1) ‘L’ shaped. In some alcids (e.g., Aethia cristatella) the medial cnemial crest extends posteriorly along the medial margin of the femoral articulation surface. This gives this feature a ‘T’ shape in proximal view. Some alcids (e.g., Uria lomvia) lack this posterior extension and as a result the cnemial crests appear ‘L’ shaped in proximal view.
207. Tibiotarsus, cnemial crests, distal extent (
208. Tibiotarsus, lateral cnemial crest orientation: (0) directed anterolaterally; (1) directed laterally. In proximal view the external cnemial crest of some alcids (e.g., Alca torda) is directed anterolaterally, while the lateral cnemial crest of other alcids (e.g., Aethia psittacula) is directed laterally, which results in a more constricted incisura tibialis.
209. Tibiotarsus, notch in lateral margin of medial condyle (
210. Tibiotarsus, lateral projection of crest lateral to the groove for peroneus profundus tendon, posterior view: (0) a distinct projection; (1) not visible in dorsal view. The lateral edge of the groove for the peroneus profundus tendon projects far enough laterally in some species of alcids (e.g., Alca torda) to be visible in posterior view.
211. Tibiotarsus, supratendinal bridge: (0) not fully ossified; (1) fully ossified. The supratendinal bridge of all alcids (e.g., Alle alle) except Pinguinus is ossified.
212. Tibiotarsus, length: (0) <2× greatest length of tarsometatarsus; (1) >2× greatest length of tarsometatarsus. The greatest length of the tibiotarsus is greater than two times the greatest length of the tarsometatarsus in most alcids (e.g., Alca torda), but in some species of alcids (e.g., Synthliboramphus antiquus) the tibiotarsus is less than twice the length of the tarsometatarsus.
Tarsometatarsus
213. Tarsometatarsus, tendinal canal No. 1 of hypotarsus, conformation (modified from
214. Tarsometatarsus, tendinal canal No. 2 of hypotarsus, position (modified from
215. Tarsometatarsus, tendinal canal No. 3 of hypotarsus, conformation (modified from
216. Tarsometatarsus, calcaneal ridges of hypotarsus, distal extension: (0) short; (1) long. The calcaneal ridges of the hypotarsus extend further distally (i.e., proximodistally longer than lateromedially wide) in some species of alcids (e.g., Synthliboramphus wumizusume) while in others (e.g., Ptychoramphus aleuticus), the calcaneal ridges are shorter (i.e., proximodistally shorter than lateromedially wide).
217. Tarsometatarsus, medial crest of hypotarsus: (0) absent; (1) present. The medial crest of the hypotarsus in many charadriiforms (e.g., Sterna maxima) is a well-developed posteriorly projecting and distally extending structure. In all alcids (e.g., Alca torda) the medial hypotarsal crest is reduced (i.e., relatively the same size as other hypotarsal crests).
218. Tarsometatarsus, anterior groove, conformation (
219. Tarsometatarsus, cross-sectional shape: (0) square; (1) rectangular. The tarsometatarsus of many alcids at mid-shaft is much wider than it is deep (e.g., Fratercula cirrhata), while in other alcids (e.g., Cepphus columba) the tarsometatarsus is approximately as wide as it is deep.
220. Tarsometatarsus, proximal vascular foramina, penetration of medial calcaneal ridge: (0) absent; (1) present. There are two proximal vascular foramina in alcids. The medial foramina penetrates the medial calcaneal ridge all species (e.g., Alca torda) except (e.g., Ptychoramphus aleuticus) in which this foramina is positioned distal to the distal extent of the medial calcaneal ridge.
221. Tarsometatarsus, trochlear proportions (
222. Tarsometatarsus, trochlea II in lateral view, overlap of trochlea III (
223. Tarsometatarsus, length: (0) tarsometatarsus longer than femur; (1) tarsometatarsus shorter than femur. The tarsometatarsi of a few alcids (e.g., Synthliboramphus antiquus) are longer than their femurs. Most alcids have femurs that are longer than their tarsometatarsi (e.g., Alca torda).
Integument
224. Maxillary rhamphotheca, color of tip (modified from
225. Maxillary rhamphotheca, color (modified from
(e.g., Synthliboramphus wumizusume), to shades of red, yellow or orange (e.g., Fratercula arctica).
226. Maxillary rhamphotheca, lateral surface ornamentation (modified from
227. Maxillary rhamphotheca, horn at base of maxilla: (0) absent; (1) present. A dorsally projecting horn is present on the base of the posterior maxillary rhamphotheca of Cerorhinca monocerata and Aethia pusilla. This feature is absent in all other alcids (e.g., Alle alle) and the nearest outgroups to Alcidae.
228. Maxillary rhamphotheca, seasonal change (
229. Mouth tissue color (modified from
230. External nares, orientation: (0) laterally or dorsally directed oval or slit; (1) ventrally directed, medially oriented slit. Most alcids (e.g., Cepphus grylle) have oval shaped dorsally oriented nares. A few alcids (e.g., Fratercula arctica) have nares in the form of long, ventrally opening, medially oriented slits.
231. Nostril feathering (
232. Eye color: (0) darkly colored; (1) lightly colored. The eye color of most alcids (e.g., Alca torda) is brown, although a few alcids (e.g., Aethia pygmaea) have yellow or grey colored eyes.
233. Eye scales (
234. Plume in front of eye (
235. Plume behind eye (
236. Plume on forehead (
237. Head plumage (
238. Neck plumage (
239. White tips on secondaries (
240. White wing patch (
241. Number of primaries: (0) eleven; (1) nine. All alcids (e.g., Cepphus grylle) and many other charadriiforms have 10 functional primary flight feathers and an eleventh reduced primary, while some charadriiforms (e.g., Charadriius wilsonia) have 9 primaries.
242. Number of retrices (
243. Shape of retrices (
244. Winter plumage (
245. Juvenile plumage: (0) resembles winter adults; (1) resembles summer adults. The juvenile plumage of all acids (e.g., Uria lomvia) except Alca torda and Alle alle resembles the winter plumage of adults, in which the juvenile plumage resembles the summer plumage of adults (
246. Moult: (0) simultaneous; (1) gradual. Most alcids (e.g., Uria lomvia) moult their flight feathers simultaneously, resulting in a roughly 45-day period of flightlessness. Only the auklets (e.g., Aethia pygmaea) moult their flight feathers gradually, maintaining the ability of flight year-round (
247. Tail shape (modified from
248. Foot color (modified from
249. Scutellation (modified from
250. Ungual (claw) of inner toe, shape (modified from
251. Foot webbing: (0) absent; (1) present. All alcids (e.g., Alca torda) have webbing between the second, third and fourth toes. Some charadriiforms (e.g., Charadriius wilsonia) lack this characteristic.
252. Hallux (
253. White face color in breeding plumage (
254. Belly color during breeding plumage (
255. Barred breeding plumage (
Reproduction
256. Incubation patches (
257. Nest sites (modified from
258. Nesting dispersion (
259. Nesting proximity to shore: (0) near-shore; (1) inland. Although most alcids (e.g., Alca torda) nest on sea-cliffs or rocky beaches near-shore, a few alcids (e.g., Brachyramphus marmoratus) nest further inland.
260. Clutch size (
261. Egg shape (modified from
262. Egg markings, scribbling: (0) absent; (1) present. The eggs of some alcids (e.g., Pinguinus impennis) display complex ‘scribbles’, although the eggs of most alcids (e.g., Aethia cristatella) lack this feature.
263. Egg texture: (0) smooth; (1) granular. The eggs of some alcids (e.g., Alca torda) have a rough, granular texture. The eggs of other alcids (e.g., Cepphus grylle) and most charadriiforms have a smooth texture.
264. Egg luster: (0) non-glossy; (1) glossy. The luster of murrelet (e.g., Synthliboramphus antiquus) eggs varies from all other alcids (e.g., Alca torda) in having a glossy luster.
265. Color of downy chicks: (0) variable; (1) primarily brown; (2) primarily black; (3) primarily grey; (4) primarily buff or white. The down feathering of charadriiform chicks is predictably colored in most species (e.g., black in Cepphus grylle), although the color of the down feathers in some terns (e.g., Sterna maxima) is variable (i.e., sometimes black, sometimes buff).
266. Post-hatching development pattern (
Diet
267. Adult prey preference: (0) primarily
invertebrates; (1) primarily vertebrates; (2) significant amounts of
invertebrates and vertebrates. Many of the smaller alcids (e.g., Alle alle) are specialized feeders on small invertebrates, while some larger alcids (i.e., Fratercula arctica) subsist on a diet of mostly fish (
268. Chick diet: (0) primarily invertebrates;
(1) primarily vertebrates; (2) significant amounts of invertebrates and
vertebrates. The diet that alcids feed to their chicks varies from
primarily invertebrates such as copepods, amphipods, and euphausiids (
Musculature
(see
269. M. pectoralis abdominalis insertion on (
270. Anterior head of M. subcoracoideus (
271. M. propatagialis longus dilation at wrist (
272. M. propatagialis (
273. Patagial fan sesamoid (
274. M. deltoideus minor dorsal head (
275. Swelling in M. triceps tendons (
276. Swelling in humero-ulnar pulley (
277. M. biceps brachii (
278. M. flexor digitorum sublimis dilation at base of phalanx 1 (
279. M. ulnimetacarpalis dorsalis ventral head (
280. M. ambiens (
281. Pars iliofemoralis of M. piriformis (
282. Pars interna of M. gastrocnemius (
283. Pars interna of M. gastrocnemius (
284. Pars medialis of M. gastrocnemius (
285. M. plantaris (
286. Sesamoid of M. scapulotriceps (
287. Sesamoid of M. humerootriceps (
288. Sesamoid of humero-ulnar pulley (
289. Sesamoid of propatagialis longus at wrist (
290. Sesamoid of flexor digitorum profundus in hand (
291. Sesamoid of flexor digitorum sublimis at base of phalanx 1 (
292. Sesamoid of flexor digitorum longus (
Feather microstructure
(see
293. Subpennaceous region (modified from
294. Subpennaceous region pigmentation (modified from
295. Subpennaceous region pigmentation position (modified from
296. Subpennaceous length (modified from
297. Barbule base pigmentation (modified from
298. Barbule base length (modified from
299. Barbule base cells (modified from
300. Barbule base cell composition (modified from
301. Barb length (modified from
302. Barb pigmentataion (modified from
303. Barb pigmentation position (modified from
304. Barbule pigmentation (modified from
305. Barbule pigmentation position (modified from
306. Node expansion (modified from
307. Node expansion location (modified from
308. Density of nodes per barbule (modified from
309. Proximal node shape (modified from
310. Midsection nodes shape (modified from
311. Distal nodes (modified from
312. Distal node shape (modified from
313. Nodal spines (modified from
314. Nodal spine position (modified from
315. Nodal prongs (modified from
316. Nodal points (modified from
317. Nodal point position (modified from
318. Proximal node pigment (modified from
319. Proximal node pigment shape (modified from
320. Mid-node pigment (modified from
321. Mid-node pigment shape (modified from
322. Distal node pigmentation (modified from
323. Distal pigment distribution (modified from
324. Nodal pigment intensity at basal nodes (modified from
325. Nodal pigment intensity at basal nodes (modified from
326. Nodal pigment at distal nodes (modified from
327. Nodal pigment intensity at distal nodes (modified from
328. Pigment color (modified from
329. Morphology of first node (modified from
330. Internode pigmentation (modified from
331. Internode pigmentation (modified from
332. True down pigmentation (modified from
333. True down pigmentation (modified from
334. True down nodes (modified from
335. True down nodes (modified from
336. True down pigment shape (modified from
337. True down pigmented like contour down (modified from
338. True down pigmented like afterfeather down (modified from
339. Afterfeather pigmentation (modified from
340. Afterfeather pigmentation (modified from
341. Afterfeather down pigmented like contour feather down (modified from
342. Villi (modified from
343. Distal prongs (modified from
344. Distal prong morphology (modified from
DNA sequence data
(see Appendix 5for Genbank accession numbers)
345. – 1487. cytb
1488. – 2528. ND2
2529. – 4343. ND5
4344. – 4865. ND6
4866. – 6416. CO1
6417. – 9287. RAG1
9288. – 10331. 12S
10332. – 11945. 16S
Rejected characters
The following characters from the dataset of Chandler (1990) were rejected due to intraspecies variability: 15, 32, 48, 55, 72, 73, 77, 78; or because they were parsimony uninformative (i.e., they did not vary among taxa examined): 27, 31, 34, 45, 54, 56, 57, 66, 68, 76.
The following characters of
All the characters of
Although all characters from
Morphological character scorings for all extant and extinct taxa, 18 Mancallinae specimen terminals (LACM, SDSNH, and USNM catalog numbers), and a Mancallinae supraspecific terminal (Mancallinae SST) are listed below. Polymorphic scorings are represented by capital letters (A:0&1; B:0&2; C:1&2).
| 1 | 0 | 2 | 0 | 3 | 0 | 4 | 0 | 5 | 0 | 6 | 0 | 7 | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aethia cristatella | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | - | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 4 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | - | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
| Aethia pygmaea | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | - | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 5 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | - | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
| Aethia psittacula | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | - | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 5 | 0 | 1 | 0 | 1 | 2 | 1 | 0 | - | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
| Aethia pusilla | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | - | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 4 | 0 | 1 | 0 | 1 | C | 1 | 0 | - | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
| Ptychoramphus aleuticus | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | - | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 4 | 0 | 1 | 0 | 1 | 2 | 1 | 0 | - | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
| Cerorhinca monocerata | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | - | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 2 | 0 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
| Fratercula arctica | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | - | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
| Fratercula corniculata | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | - | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
| Fratercula cirrhata | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | - | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
| Alca torda | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | - | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 |
| Pinguinus impennis | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 2 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | ? | ? | 0 | 1 | 0 | 0 | 2 | 0 | 0 | - | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 |
| Alle alle | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | - | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 3 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | - | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 |
| Uria aalge | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | A | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | - | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 |
| Uria lomvia | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | - | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 |
| Cepphus carbo | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 2 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | - | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 |
| Cepphus columba | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | - | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 |
| Cepphus grylle | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | - | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 |
| Brachyramphus brevirostris | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | - | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | - | 1 | 0 | 0 | 1 | 0 | - | 1 | 0 | 0 | 0 | 1 |
| Brachyramphus marmoratus | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | - | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | - | 1 | 0 | 0 | 1 | 0 | - | 1 | 0 | 0 | 0 | 1 |
| Brachyramphus perdix | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | - | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | - | 1 | 0 | 0 | 1 | 0 | - | 1 | 0 | 0 | 0 | 1 |
| Synthliboramphus antiquus | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | - | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | - | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 |
| Synthliboramphus wumizusume | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ? | ? | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | - | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 2 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | - | 1 | 0 | 0 | 1 | 0 | - | 1 | 0 | 1 | 0 | 1 |
| Synthliboramphus craveri | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | - | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 2 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | - | 1 | 0 | 0 | 1 | 0 | - | 1 | 0 | 1 | 0 | 1 |
| Synthliboramphus hypoleucus | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | - | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | - | 1 | 0 | 0 | 1 | 0 | - | 1 | 0 | 1 | 0 | 1 |
| Stercorarius longicaudus | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | - | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 2 | 0 | - | 0 | - | 0 | 0 | 0 | - | 1 | 0 | 1 | 0 | 1 | 1 | 0 | - | 0 | 0 | 1 | 0 | 0 | - | 0 | 0 | 1 | 0 | 0 |
| Stercorarius skua | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | - | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 2 | 0 | 0 | 0 | - | 1 | 0 | 0 | - | 1 | 0 | 1 | 0 | 1 | 1 | 0 | - | 0 | 0 | 1 | 0 | 0 | - | 0 | 0 | 1 | 0 | 0 |
| Anous tenuirostris | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 2 | 0 | 0 | 0 | - | 0 | 0 | 0 | - | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | - | 0 | 0 | 1 | 0 | 0 |
| Chlidonias leucoptera | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | - | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | - | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 2 | 0 | 0 | 0 | - | 0 | 0 | 0 | - | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 0 | 1 | 0 | 0 |
| Sterna niloteca | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | - | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 2 | 0 | 0 | 0 | - | 0 | 0 | 0 | - | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 0 | 1 | 0 | 0 |
| Larosterna inca | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | - | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 2 | 0 | 0 | 0 | - | 1 | 0 | 0 | - | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 0 | 1 | 0 | 0 |
| Sterna maxima | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | - | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 2 | 2 | 0 | 1 | 0 | - | 0 | 0 | 0 | - | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 0 | 1 | 0 | 0 |
| Phaetusa simplex | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | - | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 2 | 0 | 1 | 0 | - | 0 | 0 | 0 | - | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 0 | 1 | 0 | 0 |
| Sternula superciliaris | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 2 | 2 | 0 | 0 | 0 | - | 0 | 0 | 1 | 3 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | 1 | 1 | 1 | 0 | 0 |
| Sterna anaethetus | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | - | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 2 | 0 | 0 | 0 | - | 0 | 0 | 0 | - | 1 | 0 | 1 | 0 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 1 | 1 | 0 | 0 |
| Gygis alba | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | - | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | - | 0 | 2 | 0 | 0 | 0 | - | 0 | 1 | 0 | - | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 0 | 1 | 0 | 0 |
| Rynchops niger | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 2 | 2 | 0 | 0 | 0 | - | 0 | 1 | 0 | - | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 0 | 1 | 0 | 0 |
| Creagrus furcatus | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 2 | 0 | 0 | 0 | - | 0 | 0 | 0 | - | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Rhodostethia rosea | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | - | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 2 | 0 | 1 | 0 | - | 0 | 0 | 0 | - | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 0 | 1 | 0 | 0 |
| Larus argentatus | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 2 | 0 | 1 | 0 | - | 0 | 0 | 0 | - | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | - | 0 | 0 | 1 | 0 | 0 |
| Larus marinus | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 2 | 0 | 1 | 0 | - | 0 | 0 | 0 | - | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | - | 0 | 0 | 1 | 0 | 0 |
| Pagophila eburnea | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | - | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 2 | 0 | 1 | 0 | - | 0 | 0 | 0 | - | 1 | 0 | 0 | 0 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 0 | 1 | 0 | 0 | 0 |
| Xema sabini | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | - | 0 | 0 | 0 | - | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 0 | 1 | 0 | 0 |
| Rissa tridactyla | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 1 | 0 | - | 0 | 1 | 0 | - | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 0 | 1 | 0 | 0 |
| Cusorius temmneckii | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | - | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 2 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | - | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | ? | 0 | 0 | 0 | - | 1 | 0 | 1 | 0 | 0 |
| Glareola maldivarum | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | - | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | ? | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | - | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | ? | 0 | 0 | 0 | - | 0 | 0 | 0 | 0 | 0 |
| Stiltia isabella | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | - | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 2 | 2 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 3 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | - | 1 | 0 | 1 | 0 | 0 |
| Rhinoptilus chalcopterus | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | - | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 2 | 0 | 0 | 0 | - | 0 | 0 | 1 | 4 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | ? | 0 | 0 | 0 | - | 1 | 1 | 0 | 0 | 0 |
| Bartramia longicauda | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | - | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | - | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | - | 1 | 0 | 1 | 0 | 0 |
| Numenius minutus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | - | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | - | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 4 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | ? | 0 | 1 | 0 | - | 1 | 0 | 0 | 0 | 0 |
| Tryngites subruficollis | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | - | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 2 | 0 | 0 | 0 | - | 0 | 0 | 0 | - | 1 | 1 | 0 | 0 | 1 | 1 | 0 | - | 0 | 0 | 0 | 0 | 0 | - | 1 | 0 | 1 | 0 | 0 |
| Hydrophasianus chirurgus | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | - | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | - | 1 | 0 | 0 | 0 | 0 | 1 | 0 | - | 0 | 1 | 0 | 0 | 0 | - | 1 | 1 | 1 | 0 | 0 |
| Charadrius vociferus | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | 1 | 1 | 0 | - | 1 | 0 | 0 | 0 | 0 | - | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | - | 1 | 1 | 0 | 0 | 0 |
| Charadrius wilsonia | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | 1 | 1 | 0 | - | 1 | 0 | 0 | 0 | 0 | - | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | 1 | 0 | 0 | 0 | 0 |
| LACM 15410 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| LACM 128870 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| LACM 15425 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| LACM 23739 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| USNM 243765 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| LACM 107028 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| SDSNH 24262 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 1 | 0 | 1 | 2 | 1 | ? | ? | 0 | 1 | 0 | 0 | 0 | - | 1 | ? | ? | ? | ? |
| SDSNH 59047 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| SDSNH 28152 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| SDSNH 42532 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| SDSNH 21295 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 0 | 0 | 1 | 2 | 1 | ? | ? | ? | ? | ? | ? | ? | ? | ? | 0 | 0 | 0 | 1 |
| LACM 103940 | ? | 0 | ? | ? | 1 | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 1 | ? | ? | ? | ? | ? | ? | ? | ? | ? | 1 | 1 | 0 | ? | ? | ? | ? | ? | ? | ? | 0 | 0 | ? | 1 | 1 | 0 | 1 | 2 | 0 | 1 | 1 | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| SDSNH 25236 | 0 | 0 | 1 | 0 | 1 | 0 | ? | 1 | ? | 0 | 0 | 1 | ? | ? | ? | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | ? | 0 | 0 | 1 | ? | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Mancalla californiensis | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Mancalla cedrosensis | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Mancalla lucasi | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 0 | 0 | 1 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Mancalla vegrandis | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 1 | 0 | - | 0 | 1 | 0 | 0 | ? | ? | ? | ? | 0 | 0 | 1 |
| Miomancalla howardi | 0 | 1 | ? | ? | ? | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 0 | 1 | ? | ? | ? | ? | ? | ? | ? | ? | ? | 1 | 1 | ? | ? | ? | ? | ? | ? | 1 | 0 | 1 | ? | ? | ? | 1 | 1 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Miomancalla wetmorei | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Mancallinae SST | 0 | A | 1 | 0 | 1 | 0 | ? | 1 | ? | 0 | 0 | 1 | ? | ? | ? | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | ? | 0 | 0 | 1 | ? | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | A | 0 | 0 | 1 | 1 | A | 1 | 2 | 0 | 1 | 1 | 0 | ? | ? | ? | ? | ? | A | 0 | 1 | 2 | 1 | 0 | - | 0 | 1 | 0 | 0 | 0 | - | 1 | 0 | 0 | 0 | 1 |
| 8 | 0 | 9 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 2 | 0 | 1 | 3 | 0 | 1 | 4 | 0 | 1 | 5 | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Aethia cristatella | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 2 | 0 | - | 0 | 0 | 0 | - | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 3 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | - | - | - | 0 | 1 | 2 | 0 | - | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Aethia pygmaea | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 2 | 0 | - | 0 | 0 | 0 | - | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 3 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | - | - | - | 1 | 0 | 1 | 0 | - | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Aethia psittacula | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 2 | 0 | - | 0 | 0 | 0 | - | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 3 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | - | - | - | 0 | 1 | 2 | 0 | - | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Aethia pusilla | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 2 | 0 | - | 0 | 0 | 0 | - | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 3 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 2 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | - | - | - | 0 | 0 | 2 | 0 | - | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Ptychoramphus aleuticus | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 2 | 0 | - | 0 | 0 | 0 | - | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 3 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 2 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | - | - | - | 0 | 0 | 2 | 0 | - | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Cerorhinca monocerata | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 3 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 2 | 1 | 0 | - | - | - | 1 | 1 | 2 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Fratercula arctica | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 3 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | - | - | - | 1 | 1 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Fratercula corniculata | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 3 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | - | - | - | 1 | 1 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Fratercula cirrhata | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 3 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | - | - | - | 1 | 1 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Alca torda | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 2 | 0 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 1 | 1 | 1 | 2 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | - | - | - | 1 | 0 | 1 | 0 | - | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 |
| Pinguinus impennis | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 2 | 0 | 1 | 1 | 0 | 1 | 2 | 1 | 0 | 1 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | - | - | - | 1 | 0 | 1 | 0 | - | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 3 | 0 | 0 | 1 | 1 | 0 | 1 | 0 |
| Alle alle | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 2 | 1 | 0 | 1 | 0 | 0 | - | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 3 | 1 | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 0 | 1 | 1 | 2 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | - | - | - | 0 | 0 | 1 | 0 | - | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| Uria aalge | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 2 | 1 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | - | - | - | 1 | 0 | 1 | 0 | - | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 3 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| Uria lomvia | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 2 | 1 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | - | - | - | 1 | 0 | 1 | 0 | - | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 3 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| Cepphus carbo | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 2 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | - | - | - | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 3 | 0 | 0 | 1 | 1 | 0 | 1 | 0 |
| Cepphus columba | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | - | - | - | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 3 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| Cepphus grylle | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | - | - | - | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 3 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| Brachyramphus brevirostris | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | - | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | - | - | - | 1 | 0 | 1 | 0 | - | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 |
| Brachyramphus marmoratus | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | - | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 1 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | - | - | - | 1 | 0 | 1 | 0 | - | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 |
| Brachyramphus perdix | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | - | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | - | - | - | 1 | 0 | 1 | 0 | - | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 |
| Synthliboramphus antiquus | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | - | - | - | 0 | 0 | 1 | 0 | - | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 3 | 1 | 0 | 1 | 0 | 0 | 1 | 0 |
| Synthliboramphus wumizusume | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | - | - | - | 1 | 0 | 2 | 0 | - | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 3 | 1 | 0 | 1 | 1 | 0 | 1 | 0 |
| Synthliboramphus craveri | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | - | - | - | 1 | 0 | 2 | 0 | - | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 3 | 1 | 0 | 1 | 1 | 0 | 1 | 0 |
| Synthliboramphus hypoleucus | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | - | - | - | 0 | 0 | 2 | 0 | - | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 3 | 1 | 0 | 1 | 0 | 0 | 1 | 0 |
| Stercorarius longicaudus | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 2 | 0 | 0 | - | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | - | - | - | 1 | 1 | 0 | 0 | - | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Stercorarius skua | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | - | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | - | - | - | 1 | 1 | 0 | 0 | - | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Anous tenuirostris | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 0 | 0 | - | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | - | - | - | 0 | 1 | 0 | 0 | - | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chlidonias leucoptera | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 2 | 0 | 0 | - | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | - | - | - | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sterna niloteca | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | - | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | - | - | - | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Larosterna inca | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 2 | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | - | - | - | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sterna maxima | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 2 | 0 | 0 | - | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | - | - | - | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Phaetusa simplex | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 2 | 0 | 1 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | - | - | - | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sternula superciliaris | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | ? | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 2 | 0 | 0 | - | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | ? | ? | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | ? | 0 | - | - | - | 0 | 1 | 1 | 1 | 0 | 0 | ? | 1 | 0 | ? | 0 | 0 | 0 | 0 | ? | 0 | 0 | 0 | ? | 0 | 1 | 0 | 0 | 0 | 0 | 0 | ? |
| Sterna anaethetus | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | - | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | - | - | - | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gygis alba | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | - | - | - | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rynchops niger | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 2 | 0 | 0 | - | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | - | - | - | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Creagrus furcatus | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 2 | 0 | 0 | - | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | - | - | - | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rhodostethia rosea | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 2 | 0 | 0 | - | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | - | - | - | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Larus argentatus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 2 | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | - | - | - | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Larus marinus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 2 | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | - | - | - | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pagophila eburnea | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | - | - | - | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Xema sabini | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | - | 1 | 0 | 1 | 1 | 0 | - | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | - | - | - | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rissa tridactyla | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | - | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | - | - | - | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cusorius temmneckii | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | ? | ? | ? | 0 | 1 | 0 | - | 0 | 0 | 2 | 0 | 0 | - | 0 | 0 | ? | 1 | 0 | 0 | 0 | 1 | ? | 2 | 0 | ? | ? | 0 | 1 | 0 | 2 | 1 | 0 | 0 | 1 | ? | 0 | - | - | - | 0 | 0 | 0 | 0 | - | 0 | ? | 0 | 0 | ? | 0 | 0 | 1 | 0 | ? | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ? |
| Glareola maldivarum | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | ? | ? | ? | 0 | 1 | 0 | - | 0 | 0 | 2 | 0 | 0 | - | 0 | 0 | ? | 1 | 0 | 0 | 0 | 1 | ? | 1 | 0 | ? | ? | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 1 | ? | 0 | - | - | - | 0 | 1 | 0 | 0 | - | 0 | ? | 1 | 0 | ? | 0 | 0 | 0 | 0 | ? | 0 | 0 | 0 | ? | 0 | 1 | 0 | 0 | 0 | 0 | 0 | ? |
| Stiltia isabella | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | - | 0 | 0 | 2 | 0 | 0 | - | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 1 | 1 | 0 | - | - | - | 0 | 1 | 1 | 0 | - | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rhinoptilus chalcopterus | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | ? | 1 | ? | 1 | 0 | 0 | - | 0 | 0 | 2 | 0 | 0 | - | 0 | 0 | ? | 1 | 0 | 0 | 0 | 1 | ? | 2 | 0 | ? | ? | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | ? | 0 | - | - | - | 1 | 0 | 0 | 0 | - | 0 | ? | 1 | 0 | ? | 0 | 1 | 1 | 0 | ? | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ? |
| Bartramia longicauda | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | - | 0 | 0 | 2 | 0 | 0 | - | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | - | - | - | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Numenius minutus | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | ? | 1 | ? | 0 | 1 | 0 | - | 0 | 0 | 2 | 0 | 0 | - | 0 | 0 | ? | 0 | 0 | 0 | 0 | 1 | ? | 1 | 0 | ? | ? | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 1 | ? | 0 | - | - | - | 1 | 0 | 0 | 0 | - | 0 | ? | 1 | 0 | ? | 0 | 0 | 1 | 0 | ? | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ? |
| Tryngites subruficollis | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | - | 0 | 0 | 2 | 0 | 0 | - | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | - | - | - | 0 | 1 | 0 | 0 | - | - | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Hydrophasianus chirurgus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | - | 0 | 0 | 2 | 1 | 0 | - | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | - | - | - | 0 | 1 | 1 | 0 | - | - | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Charadrius vociferus | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 2 | 0 | 0 | - | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | - | - | - | 0 | 1 | 0 | 0 | - | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Charadrius wilsonia | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 2 | 0 | 0 | - | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | - | - | - | 0 | 1 | 0 | 0 | - | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| LACM 15410 | ? | ? | 0 | 0 | 1 | ? | ? | 1 | 0 | 1 | 1 | 0 | 1 | 0 | ? | ? | ? | 0 | ? | ? | 1 | 1 | 0 | ? | ? | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 2 | ? | ? | ? | 0 | 0 | 0 | 1 | 1 | 1 |
| LACM 128870 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 1 | 2 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 0 | - | 0 | ? | ? | ? | ? | ? | ? | 2 | ? | ? | ? | ? | ? | 0 | 4 | 1 | 0 | 0 | 0 | 1 | 1 | 1 |
| LACM 15425 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| LACM 23739 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| USNM 243765 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| LACM 107028 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| SDSNH 24262 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| SDSNH 59047 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 0 | 0 | 2 | 1 | 1 | 2 | 0 | 0 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 2 | 1 | 1 | 1 | 0 | 1 | - | - | 0 | 0 | - | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | 1 | 1 | 2 | 0 | 4 | 1 | 0 | 0 | 0 | 1 | 1 | 1 |
| SDSNH 28152 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 0 | 0 | 2 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 1 | - | - | 0 | 0 | - | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | 1 | 1 | 2 | 0 | 4 | 1 | 0 | 0 | 0 | 1 | 1 | 1 |
| SDSNH 42532 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 0 | 0 | 2 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 1 | - | - | 0 | 0 | - | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | 1 | 1 | 2 | 0 | 4 | 1 | 0 | 0 | 0 | 1 | 1 | 1 |
| SDSNH 21295 | 1 | 1 | ? | ? | ? | ? | ? | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | - | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | ? | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| LACM 103940 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 2 | 1 | 1 | ? | 0 | ? | ? | ? | ? | 0 | - | ? | ? | 1 | 0 | 1 | 0 | ? | 2 | ? | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| SDSNH 25236 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Mancalla californiensis | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 0 | 0 | 2 | 1 | 1 | 2 | 0 | 1 | 0 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 2 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | - | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 1 | 1 | 1 | 1 | 2 | 0 | 4 | ? | ? | ? | ? | ? | ? | ? |
| Mancalla cedrosensis | ? | ? | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | - | 1 | 0 | 0 | 0 | 1 | 1 | 0 | ? | 1 | 0 | 0 | 0 | 2 | 1 | 1 | 2 | 0 | 1 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 2 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | - | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | 1 | 1 | 2 | 0 | 4 | 1 | 0 | 0 | 0 | 1 | 1 | 1 |
| Mancalla lucasi | ? | ? | 0 | 0 | 1 | 1 | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 0 | 0 | 2 | 1 | 1 | 2 | 0 | 0 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 2 | 1 | 1 | 1 | 0 | 1 | ? | - | 0 | 0 | - | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | 1 | 1 | 2 | 0 | 4 | 1 | 0 | 0 | 0 | 1 | 1 | 1 |
| Mancalla vegrandis | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | - | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 1 | ? | - | 0 | 0 | - | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | 1 | 1 | 2 | 0 | 4 | 1 | 0 | 0 | 0 | 1 | 1 | 1 |
| Miomancalla howardi | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 0 | 0 | 2 | 0 | 1 | 2 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 2 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 2 | 0 | - | 0 | 0 | 1 | 0 | 1 | 1 | ? | 2 | 0 | 0 | 1 | 1 | 2 | 0 | 4 | 1 | 0 | 0 | 0 | 1 | 1 | 1 |
| Miomancalla wetmorei | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 0 | 0 | 2 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 2 | 0 | - | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 1 | 1 | 2 | 0 | 4 | 1 | 0 | ? | ? | ? | ? | ? |
| Mancallinae SST | 1 | 1 | 0 | 0 | 1 | 1 | 0 | A | 0 | 1 | 1 | A | 1 | 1 | 0 | - | 1 | 0 | 0 | 0 | 1 | A | 0 | 0 | 1 | 0 | 0 | 0 | 2 | A | 1 | 2 | 0 | A | 0 | A | 1 | C | A | 0 | 0 | 0 | 2 | A | 1 | A | A | A | 1 | 0 | B | 0 | - | A | A | A | 0 | 1 | A | 0 | 2 | A | A | 1 | 1 | 2 | 0 | 4 | 1 | 0 | 0 | 0 | 1 | 1 | 1 |
| 1 | 6 | 0 | 1 | 7 | 0 | 1 | 8 | 0 | 1 | 9 | 0 | 2 | 0 | 0 | 2 | 1 | 0 | 2 | 2 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Aethia cristatella | 1 | 0 | 1 | 0 | 0 | 0 | - | 1 | 0 | - | 0 | - | - | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 |
| Aethia pygmaea | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | - | - | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 2 | 0 |
| Aethia psittacula | 1 | 0 | 1 | 0 | 0 | 0 | - | 1 | 1 | 1 | 0 | - | - | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 |
| Aethia pusilla | 1 | 0 | 1 | 0 | 0 | 0 | - | 1 | 0 | - | 0 | - | - | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 2 | 0 |
| Ptychoramphus aleuticus | 1 | 0 | 1 | 0 | 0 | 0 | - | 1 | 1 | 1 | 0 | - | - | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 |
| Cerorhinca monocerata | 1 | 0 | 1 | 0 | 0 | 0 | - | 1 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 |
| Fratercula arctica | 1 | 0 | 1 | 0 | 0 | 0 | - | 1 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 |
| Fratercula corniculata | 1 | 0 | 1 | 0 | 0 | 0 | - | 1 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 |
| Fratercula cirrhata | 1 | 0 | 1 | 0 | 0 | 0 | - | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 |
| Alca torda | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 |
| Pinguinus impennis | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | - | 0 | - | - | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 |
| Alle alle | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | - | 1 | 1 | - | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 |
| Uria aalge | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | - | 0 | - | - | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 2 | 1 |
| Uria lomvia | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | - | 0 | - | - | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 2 | 1 |
| Cepphus carbo | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | - | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 |
| Cepphus columba | 1 | 0 | 1 | 0 | 0 | 0 | - | 0 | 1 | 0 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 |
| Cepphus grylle | 1 | 0 | 1 | 0 | 0 | 0 | - | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 |
| Brachyramphus brevirostris | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | - | - | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 |
| Brachyramphus marmoratus | 1 | 0 | 1 | 1 | 0 | 0 | - | 0 | 1 | 0 | 0 | - | - | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 |
| Brachyramphus perdix | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | - | - | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 |
| Synthliboramphus antiquus | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | - | 0 | - | - | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 2 |
| Synthliboramphus wumizusume | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | - | - | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 2 |
| Synthliboramphus craveri | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | - | - | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 |
| Synthliboramphus hypoleucus | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | - | 0 | - | - | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Stercorarius longicaudus | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | - | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Stercorarius skua | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | - | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Anous tenuirostris | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | - | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 2 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 |
| Chlidonias leucoptera | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | - | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Sterna niloteca | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | - | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 |
| Larosterna inca | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | - | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 |
| Sterna maxima | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 2 | 1 | 0 | - | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 |
| Phaetusa simplex | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 2 | 1 | 0 | - | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 2 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 |
| Sternula superciliaris | 0 | 0 | 0 | ? | 0 | ? | ? | 1 | 0 | - | 1 | 0 | - | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | ? | 0 | 1 | 1 | 1 | 0 |
| Sterna anaethetus | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | - | 1 | 0 | - | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 |
| Gygis alba | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | - | 1 | 0 | - | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 |
| Rynchops niger | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | - | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 |
| Creagrus furcatus | 0 | 0 | 0 | 0 | 0 | 0 | - | 1 | 1 | 0 | 1 | 0 | - | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 |
| Rhodostethia rosea | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | - | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Larus argentatus | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | - | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 2 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 |
| Larus marinus | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | - | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 2 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| Pagophila eburnea | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | - | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 |
| Xema sabini | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | - | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 2 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 |
| Rissa tridactyla | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 1 | 0 | 1 | 0 | - | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 |
| Cusorius temmneckii | 0 | 0 | 0 | ? | 0 | ? | ? | 1 | 0 | - | 1 | 0 | - | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | ? | 1 | 0 | 0 | 0 | 1 |
| Glareola maldivarum | 0 | 0 | 0 | ? | 0 | ? | ? | 1 | 1 | 2 | ? | ? | ? | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | ? | 1 | 0 | 0 | 0 | 1 |
| Stiltia isabella | 0 | 0 | 0 | 0 | 0 | 0 | - | 1 | 1 | 0 | 1 | 0 | - | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 2 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Rhinoptilus chalcopterus | 0 | 0 | 0 | ? | 0 | ? | ? | 1 | 1 | 0 | 1 | 0 | - | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | ? | 1 | 0 | 0 | 0 | 1 |
| Bartramia longicauda | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | - | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 2 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Numenius minutus | 1 | 0 | 0 | ? | 0 | ? | ? | 1 | 1 | 0 | 1 | 0 | - | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 2 | 0 | 0 | 1 | 1 | 0 | ? | 1 | 0 | 0 | 0 | 1 |
| Tryngites subruficollis | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | - | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 2 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 |
| Hydrophasianus chirurgus | 0 | 0 | 0 | 0 | 0 | 0 | - | 1 | 1 | 0 | 1 | 0 | - | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 2 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Charadrius vociferus | 1 | 0 | 0 | 0 | 0 | 0 | - | 1 | 1 | 0 | 1 | 0 | - | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 2 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Charadrius wilsonia | 1 | 0 | 0 | 0 | 0 | 0 | - | 1 | 0 | - | 1 | 0 | - | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 2 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| LACM 15410 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | - | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| LACM 128870 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | - | ? | ? | ? | ? | ? | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | ? | ? | ? | 1 | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| LACM 15425 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 1 | ? | 0 | 0 | ? | 1 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| LACM 23739 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 0 | 0 | ? | ? | 0 | 0 | 1 | ? | 1 | ? | ? | ? | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | ? | ? |
| USNM 243765 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 2 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | ? | 1 | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| LACM 107028 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 2 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | - | 2 | ? | 1 | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 0 | ? | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | ? | ? | ? |
| SDSNH 24262 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| SDSNH 59047 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | - | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| SDSNH 28152 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | - | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| SDSNH 42532 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | - | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| SDSNH 21295 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| LACM 103940 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| SDSNH 25236 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Mancalla californiensis | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Mancalla cedrosensis | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | - | 1 | 1 | 2 | 1 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | - | 2 | 2 | 1 | 0 | 1 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | ? | ? | ? | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | ? | ? |
| Mancalla lucasi | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | - | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Mancalla vegrandis | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | - | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 1 | ? | ? | 1 | 1 | 1 | 1 | 0 | ? | 1 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Miomancalla howardi | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 0 | 1 | - | 2 | 2 | ? | ? | 1 | ? | ? | ? | 1 | ? | ? | 1 | ? | 1 | ? | 0 | ? | 1 | 0 | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 0 | ? | ? | ? | ? | ? | ? | ? |
| Miomancalla wetmorei | 1 | 0 | 0 | 0 | 0 | ? | ? | 1 | 0 | - | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Mancallinae SST | 1 | 0 | 0 | 0 | A | 1 | A | A | A | 0 | 1 | 1 | 2 | 1 | 0 | 2 | 0 | 1 | 0 | A | A | 0 | 0 | A | 1 | 2 | A | 0 | 0 | 1 | 1 | 0 | 0 | 1 | - | 2 | 2 | 1 | 0 | 1 | ? | ? | ? | 1 | ? | ? | 1 | 1 | 1 | 1 | 0 | ? | 1 | 0 | 0 | 1 | 0 | 0 | A | 1 | A | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | ? | ? |
| 2 | 3 | 0 | 2 | 4 | 0 | 2 | 5 | 0 | 2 | 6 | 0 | 2 | 7 | 0 | 2 | 8 | 0 | 2 | 9 | 0 | 3 | 0 | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Aethia cristatella | 0 | 0 | 0 | 2 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Aethia pygmaea | 0 | 0 | 0 | 2 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Aethia psittacula | 0 | 0 | 0 | 2 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 1 | 1 | 1 | 2 | 1 | 2 | 0 | - |
| Aethia pusilla | 0 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 1 | 1 | 1 | 1 | 1 | 2 | 0 | - |
| Ptychoramphus aleuticus | 0 | 0 | 0 | 2 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 2 | 0 | - |
| Cerorhinca monocerata | 0 | 1 | 1 | ? | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 2 | 0 | - |
| Fratercula arctica | 1 | 0 | 1 | 0 | 1 | 0 | A | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 0 | - |
| Fratercula corniculata | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | ? | ? | ? | ? | ? | ? | ? | ? |
| Fratercula cirrhata | 1 | 0 | 1 | ? | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 2 | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 0 | - |
| Alca torda | 1 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 2 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 2 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 0 | - |
| Pinguinus impennis | 1 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | ? | ? | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | ? | 0 | 1 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Alle alle | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 1 | 1 | 1 | 2 | 1 | 2 | 0 | - |
| Uria aalge | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 2 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 2 | 0 | - |
| Uria lomvia | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 2 | 0 | 2 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | ? | ? | ? | ? | ? | ? | ? | ? |
| Cepphus carbo | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | ? | ? | ? | ? | ? | 2 | ? | 1 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Cepphus columba | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 3 | 0 | 0 | 0 | 2 | 2 | 2 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 2 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 0 | - |
| Cepphus grylle | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 3 | 0 | 0 | 0 | 2 | 2 | 2 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 2 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | ? | ? | ? | ? | ? | ? | ? | ? |
| Brachyramphus brevirostris | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 2 | 0 | 1 | 0 | 3 | 2 | 1 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Brachyramphus marmoratus | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 2 | 1 | 1 | 4 | 1 | 1 | 0 | 2 | 0 | 1 | 0 | 4 | 2 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 0 | - |
| Brachyramphus perdix | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 1 | 1 | 0 | ? | ? | ? | ? | ? | 2 | 0 | 1 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Synthliboramphus antiquus | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 3 | 0 | 0 | 1 | 2 | 1 | 2 | 2 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 2 | 0 | - |
| Synthliboramphus wumizusume | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | ? | ? | ? | ? | ? | 1 | 0 | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Synthliboramphus craveri | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 3 | 0 | 0 | 1 | ? | 1 | 1 | 1 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Synthliboramphus hypoleucus | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 2 | 1 | 0 | 1 | 2 | 1 | 1 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Stercorarius longicaudus | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | ? | 1 | 2 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 3 | 1 | 1 | 1 | 3 | 2 | 2 | 2 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 1 | 0 | - | 1 | 1 | 1 | 1 | 0 |
| Stercorarius skua | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | ? | 1 | 2 | 0 | 0 | 0 | 1 | 1 | 0 | 2 | 1 | 0 | 0 | 0 | 1 | 1 | 3 | 1 | 0 | 1 | ? | 2 | 1 | 1 | 0 | 0 | ? | 0 | 1 | 0 | ? | ? | 2 | ? | 0 | 0 | 0 | 0 | 0 | 0 | ? | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | - | 0 | 0 | 1 | 1 | 0 |
| Anous tenuirostris | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | - | ? | 0 | 2 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | ? | 4 | 0 | 0 | 0 | ? | ? | ? | ? | ? | ? | 2 | 2 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Chlidonias leucoptera | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | - | 0 | ? | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | ? | 3 | 0 | 1 | 1 | ? | ? | ? | ? | 4 | ? | 0 | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Sterna niloteca | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | - | 1 | 2 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | ? | 0 | 0 | 1 | 1 | 3 | 0 | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 | ? | 0 | 0 | 0 | 1 | 1 | ? | ? | 0 | 0 | 1 | 0 | 1 | ? | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | - | 1 | 0 | 1 | 1 | 1 |
| Larosterna inca | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | - | - | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 3 | 0 | ? | 1 | 1 | 0 | ? | ? | ? | ? | ? | 3 | ? | 1 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 1 | 0 | - | 1 | 1 | 1 | 1 | 0 |
| Sterna maxima | 3 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | - | 1 | 2 | 2 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | ? | 0 | 0 | 0 | 1 | 1 | ? | ? | 0 | 0 | 1 | 0 | 1 | ? | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | ? | ? | ? | ? | ? | ? | ? | ? |
| Phaetusa simplex | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | - | 1 | 1 | 3 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | ? | 0 | A | 1 | 1 | ? | ? | ? | ? | ? | ? | 1 | 1 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 1 | 0 | - | 1 | 0 | 1 | 1 | 2 |
| Sternula superciliaris | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | ? | 1 | 3 | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | ? | 0 | 0 | 1 | 1 | ? | ? | ? | ? | ? | ? | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Sterna anaethetus | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | - | 1 | 2 | 2 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 | ? | ? | ? | ? | 3 | ? | 1 | 1 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Gygis alba | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | - | - | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | ? | 4 | 1 | 0 | 0 | ? | ? | ? | ? | 4 | ? | 1 | 1 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 1 | 0 | - | 1 | 0 | 1 | 1 | 2 |
| Rynchops niger | 1 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | ? | 1 | 2 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 2 | 1 | 1 | 0 | 0 | ? | 0 | 0 | 0 | ? | 0 | 1 | ? | 0 | 0 | 0 | 0 | 0 | 0 | ? | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | - | 0 | 0 | 1 | 1 | 0 |
| Creagrus furcatus | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | ? | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 | ? | ? | ? | ? | ? | 0 | 1 | 1 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 1 | 0 | - | 0 | 0 | 1 | 1 | 2 |
| Rhodostethia rosea | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 3 | 0 | 1 | - | 1 | 3 | 0 | 0 | 0 | 1 | 1 | 1 | - | 0 | ? | 0 | 0 | 0 | 1 | ? | ? | ? | ? | 4 | ? | 0 | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 1 | 0 | - | 1 | 0 | 1 | 1 | 0 |
| Larus argentatus | 0 | 0 | 0 | ? | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 4 | 0 | 2 | 2 | 0 | 0 | ? | 0 | 0 | 0 | ? | 1 | 2 | ? | 0 | 0 | 0 | 0 | 0 | 0 | ? | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | - | 0 | 0 | 1 | 1 | 2 |
| Larus marinus | 0 | 0 | 0 | ? | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 3 | 0 | 2 | 2 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Pagophila eburnea | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | - | - | 1 | 2 | 2 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | ? | 0 | 0 | 0 | 1 | ? | ? | ? | ? | 3 | ? | 2 | 2 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 1 | 0 | - | 0 | 0 | 1 | 1 | 2 |
| Xema sabini | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | - | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | ? | 0 | A | 0 | 1 | ? | ? | ? | ? | 4 | 2 | 2 | 1 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 1 | 0 | - | 1 | 0 | 1 | 1 | 0 |
| Rissa tridactyla | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 2 | 2 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | ? | 3 | 0 | 0 | 1 | ? | 0 | 1 | 0 | 4 | ? | 2 | 2 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | - | 0 | 0 | 1 | 1 | 0 |
| Cusorius temmneckii | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | - | ? | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | ? | 0 | 1 | 1 | 2 | ? | ? | ? | ? | 2 | ? | 0 | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Glareola maldivarum | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | - | ? | 1 | 2 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | ? | 0 | 0 | 1 | 1 | ? | ? | ? | ? | ? | ? | 0 | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Stiltia isabella | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | ? | 3 | 1 | 1 | 0 | 0 | 1 | 0 | 2 | 0 | ? | 0 | 0 | 1 | 1 | ? | ? | ? | ? | 4 | 2 | 0 | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 0 | - | - | - | 1 | 1 | 1 | 1 |
| Rhinoptilus chalcopterus | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | - | - | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ? | 0 | 1 | 1 | 1 | ? | ? | ? | ? | 2 | ? | 0 | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 0 | - | - | - | 1 | 1 | 1 | 0 |
| Bartramia longicauda | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | - | ? | 3 | 3 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | ? | 3 | 0 | 1 | 1 | ? | ? | ? | ? | 4 | ? | 0 | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| Numenius minutus | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | - | ? | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 2 | 1 | ? | 3 | 0 | 1 | 1 | ? | ? | ? | ? | ? | ? | 0 | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Tryngites subruficollis | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | ? | ? | ? | 0 | 3 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | ? | 0 | 1 | 1 | 1 | ? | ? | ? | ? | 1 | ? | 0 | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 1 | 0 | - | 0 | 1 | 1 | 1 | 0 |
| Hydrophasianus chirurgus | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | - | ? | 3 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | ? | 3 | 1 | 1 | 1 | ? | ? | ? | ? | 4 | ? | 0 | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Charadrius vociferus | 0 | 0 | 0 | ? | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | ? | ? | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 4 | 2 | 0 | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 |
| Charadrius wilsonia | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | ? | ? | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 4 | 1 | 0 | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| LACM 15410 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| LACM 128870 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| LACM 15425 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| LACM 23739 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| USNM 243765 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| LACM 107028 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| SDSNH 24262 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| SDSNH 59047 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| SDSNH 28152 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| SDSNH 42532 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| SDSNH 21295 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| LACM 103940 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| SDSNH 25236 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Mancalla californiensis | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Mancalla cedrosensis | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Mancalla lucasi | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Mancalla vegrandis | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Miomancalla howardi | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Miomancalla wetmorei | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Mancallinae SST | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| 3 | 1 | 0 | 3 | 2 | 0 | 3 | 3 | 0 | 3 | 4 | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Aethia cristatella | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| Aethia pygmaea | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| Aethia psittacula | 0 | 1 | 0 | 1 | 0 | 0 | - | 0 | 2 | 2 | 1 | 1 | 0 | - | 1 | 0 | - | 1 | 1 | 0 | - | 0 | - | 1 | 0 | 0 | - | 1 | 1 | 1 | 0 | 1 | 1 | 0 | - | 2 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | |||||||||||||||||||||||||||||||
| Aethia pusilla | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 3 | 3 | 1 | 1 | 0 | - | 1 | 0 | - | 1 | 0 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | - | 2 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | |||||||||||||||||||||||||||||||
| Ptychoramphus aleuticus | 0 | 1 | 0 | 1 | 0 | 0 | - | 0 | 3 | 3 | 1 | 1 | 0 | - | 1 | 0 | - | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | - | 2 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | |||||||||||||||||||||||||||||||
| Cerorhinca monocerata | 0 | 1 | 0 | 1 | 1 | 0 | - | 0 | 3 | 3 | 1 | 1 | 0 | - | 1 | 0 | - | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | - | 2 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | |||||||||||||||||||||||||||||||
| Fratercula arctica | 0 | 1 | 0 | 1 | 1 | 0 | - | 0 | 3 | 3 | 1 | 1 | 0 | - | 1 | 0 | - | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | - | 2 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | |||||||||||||||||||||||||||||||
| Fratercula corniculata | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| Fratercula cirrhata | 2 | 1 | 0 | 1 | 1 | 0 | - | 0 | 3 | 3 | 1 | 1 | 0 | - | 1 | 0 | - | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | - | 2 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | |||||||||||||||||||||||||||||||
| Alca torda | 0 | 1 | 0 | 1 | 1 | 0 | - | 0 | 3 | 3 | 1 | 1 | 0 | - | 1 | 0 | - | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | - | 2 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | |||||||||||||||||||||||||||||||
| Pinguinus impennis | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| Alle alle | 0 | 1 | 0 | 1 | 1 | 0 | - | 0 | 3 | 3 | 1 | 1 | 0 | - | 1 | 0 | - | 1 | 0 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | - | 2 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | |||||||||||||||||||||||||||||||
| Uria aalge | 0 | 1 | 1 | 1 | 1 | 0 | - | 0 | 3 | 3 | 1 | 1 | 0 | - | 1 | 0 | - | 1 | 1 | 1 | 1 | 0 | - | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | - | 2 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | |||||||||||||||||||||||||||||||
| Uria lomvia | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| Cepphus carbo | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| Cepphus columba | 2 | 1 | 0 | 1 | 1 | 0 | - | 0 | 3 | 3 | 1 | 1 | 0 | - | 1 | 0 | - | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | - | 2 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | |||||||||||||||||||||||||||||||
| Cepphus grylle | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| Brachyramphus brevirostris | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| Brachyramphus marmoratus | 0 | 1 | 3 | 1 | 1 | 0 | - | 0 | 3 | 3 | 1 | 1 | 0 | - | 1 | 0 | - | 1 | 1 | 0 | - | 0 | - | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | - | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | |||||||||||||||||||||||||||||||
| Brachyramphus perdix | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| Synthliboramphus antiquus | 0 | 1 | 2 | 1 | 1 | 0 | - | 0 | 3 | 3 | 1 | 1 | 0 | - | 1 | 0 | - | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | - | 2 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | |||||||||||||||||||||||||||||||
| Synthliboramphus wumizusume | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| Synthliboramphus craveri | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| Synthliboramphus hypoleucus | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| Stercorarius longicaudus | 2 | 1 | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | - | 1 | 1 | 1 | 1 | 0 | - | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | |||||||||||||||||||||||||||||||
| Stercorarius skua | 1 | 1 | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | - | 0 | - | 0 | - | 0 | - | 0 | - | 0 | - | - | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 1 | |||||||||||||||||||||||||||||||
| Anous tenuirostris | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| Chlidonias leucoptera | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| Sterna niloteca | 0 | 0 | - | - | - | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | - | 0 | - | 0 | - | 0 | - | 0 | - | 0 | - | - | 1 | 0 | - | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | - | 0 | 0 | 0 | - | |||||||||||||||||||||||||||||||
| Larosterna inca | 0 | 1 | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | - | 1 | 1 | 1 | 0 | 0 | - | 1 | 0 | 1 | 0 | 0 | 1 | 0 | - | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | - | |||||||||||||||||||||||||||||||
| Sterna maxima | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| Phaetusa simplex | 0 | 0 | - | - | - | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | - | 0 | - | 0 | - | 0 | - | 0 | - | 0 | - | - | 1 | 0 | - | 0 | - | 1 | 1 | - | 1 | 1 | 0 | - | 1 | 0 | 0 | - | |||||||||||||||||||||||||||||||
| Sternula superciliaris | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| Sterna anaethetus | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| Gygis alba | 0 | 0 | - | - | - | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | - | 0 | - | 0 | - | 0 | - | 0 | - | 0 | - | - | 1 | 0 | - | 0 | - | 1 | 1 | - | 1 | 1 | 0 | - | 1 | 0 | 0 | - | |||||||||||||||||||||||||||||||
| Rynchops niger | 0 | 0 | - | 0 | - | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | - | 0 | - | 0 | - | 0 | - | 0 | - | 0 | - | - | 1 | 0 | - | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | - | 1 | 0 | 0 | - | |||||||||||||||||||||||||||||||
| Creagrus furcatus | 1 | 0 | - | - | - | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 2 | 0 | 1 | 3 | 0 | - | 0 | - | 0 | - | 0 | - | 0 | - | - | 1 | 0 | - | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | - | 1 | 0 | 0 | - | |||||||||||||||||||||||||||||||
| Rhodostethia rosea | 2 | 0 | - | - | - | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | - | 0 | - | 0 | - | 0 | - | 0 | - | - | 1 | 0 | - | 1 | 0 | 1 | 0 | 2 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | - | |||||||||||||||||||||||||||||||
| Larus argentatus | 1 | 0 | - | 0 | - | 1 | 1 | 0 | 0 | 0 | 0 | - | 0 | - | 0 | 1 | 1 | 0 | - | 0 | - | 0 | - | 0 | - | 0 | - | - | 1 | 0 | - | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | ? | |||||||||||||||||||||||||||||||
| Larus marinus | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| Pagophila eburnea | 1 | 0 | - | - | - | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | - | 0 | 1 | 1 | 0 | - | 0 | - | 0 | - | 0 | - | 0 | - | - | 1 | 0 | - | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | - | 1 | 0 | 0 | - | |||||||||||||||||||||||||||||||
| Xema sabini | 0 | 0 | - | - | - | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | - | 0 | - | 0 | - | 0 | - | 0 | - | 0 | - | - | 1 | 0 | - | 1 | 1 | 1 | 0 | 2 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | - | |||||||||||||||||||||||||||||||
| Rissa tridactyla | 2 | 0 | - | - | - | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | - | 0 | 1 | 1 | 0 | - | 0 | - | 0 | - | 0 | - | 0 | - | - | 1 | 0 | - | 1 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | - | 1 | 0 | 0 | - | |||||||||||||||||||||||||||||||
| Cusorius temmneckii | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| Glareola maldivarum | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| Stiltia isabella | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | - | 0 | 1 | 0 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | - | |||||||||||||||||||||||||||||||
| Rhinoptilus chalcopterus | 2 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | - | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | - | |||||||||||||||||||||||||||||||
| Bartramia longicauda | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | - | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | - | |||||||||||||||||||||||||||||||
| Numenius minutus | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| Tryngites subruficollis | 1 | 1 | 3 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | - | |||||||||||||||||||||||||||||||
| Hydrophasianus chirurgus | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| Charadrius vociferus | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | - | |||||||||||||||||||||||||||||||
| Charadrius wilsonia | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| LACM 15410 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| LACM 128870 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| LACM 15425 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| LACM 23739 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| USNM 243765 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| LACM 107028 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| SDSNH 24262 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| SDSNH 59047 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| SDSNH 28152 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| SDSNH 42532 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| SDSNH 21295 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| LACM 103940 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| SDSNH 25236 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| Mancalla californiensis | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| Mancalla cedrosensis | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| Mancalla lucasi | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| Mancalla vegrandis | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| Miomancalla howardi | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| Miomancalla wetmorei | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | |||||||||||||||||||||||||||||||
| Mancallinae_SST | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
Mancallinae material described herein comes from four Miocene and Pliocene aged marine deposits (
San Mateo Formation: The San Mateo Formation is
composed of sandstones, siltstones, and conglomerates that interfinger
with the latest Miocene and earliest Pliocene aged member of the
Capistrano Formation (
The vertebrate assemblages of the San Mateo Fm. were discussed by
Capistrano Formation: The Capistrano Fm. is
composed of sandstones and siltstones that have been correlated with
upper portions of the San Mateo Fm. in northern San Diego County (Elliot
1975;
Niguel Formation: The Niguel Formation is composed of a mixed sequence of marine and non-marine siltstones, sandstones, and conglomerates (
San Diego Formation: The San Diego Formation
predominantly consists of Pliocene and Pleistocene marine sandstones
with minor amounts of conglomerates and claystones, which are,
interpreted as shore-face and shallow depth shelf facies deposits (