(C) 2010 Wendy Moore. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Larvae of the ground beetle genus Eustra Schmidt-Goebel are described and illustrated for the first time and some biological notes are reported. One specimen of an unknown Eustra species was collected while excavating a nest of the ant Pachycondyla javana Mayr, in Taiwan, which is the first report of a paussine associated with a member of the ant subfamily Ponerinae. Several larvae and adults of a second species, Eustra chinensis Bänninger, were collected in Shanghai under bark with no association with ants. First instar larvae of the latter species were also reared in the lab. The occurrence of larvae of the genus Eustra both inside and outside ant nests, together with a report of adults collected inside a nest in Taiwan, suggests that members of this genus may be facultative predators or facultative symbionts of ants, an attribute that has never been reported for this genus. The larvae of Eustra show several unique features, including a peculiar bidentate mandibular apex, an extremely long galea, one of two tarsal claws greatly reduced, abdominal setae (including those of terminal disk) elongate and clavate at apex, urogomphi wide and flattened, and inflated sensilla S-I. Larvae were studied by both optical and scanning electron microscopy, their morphological features are compared with those of other described Paussinae larvae, and their potential phylogenetic and functional significance are discussed.
flanged bombardier beetles, myrmecophily, systematics, larvae, Southeast Asia
Eustra Schmidt-Goebel is an ozaenine genus (Carabidae: Paussinae) containing twenty-two species (reviewed by
Many different animals, especially arthropods, profit
from a facultative or obligate association with ants (myrmecophily),
bypassing the behavioral and chemical defenses of the hosts and adapting
to the peculiar environmental conditions of the nests. Since
myrmecophiles are rare and live in concealed environments, our
knowledge of their behavior is sparse, and most of the information we
do have has been inferred from structural features of adults and larvae (
In general, very little is known about the behaviour of the ozaenines (
Members of the ozaenine genus Physea Brullé are known to live inside the nests of the Neotropical leafcutting ants, Atta, and both larvae and adults have structural adaptations for this lifestyle (
In this paper we: (1) present biological information about the habitats and behaviors of Eustra chinensis and the Eustra species from Taiwan observed in nature and in captivity; (2) describe and illustrate these larvae; (3) discuss the functional significance of several unique characteristics of the genus; and (4) compare them to other described paussine larvae.
Methods Material described(1) Eustra chinensis
Bänninger, 1949. Twenty-five adults and several larvae were collected
in Shanghai on February 9, 2009 and April 6, 2008. They were found
hibernating together in the soft, rotten wood of bristly locust (Robinia hispida Linnaeus, 1767) and weeping willow (Salix babylonica
Linnaeus, 1753). Adults and larvae can be found in Shanghai throughout
the year. They overwinter as both adults and larvae (all larval
instars), from November to April. Presumably, while these larvae are
overwintering they do not feed. In captivity, a third instar larva
overwintered without food for more than six months. During this time
they did not close the opening of their galleries with their terminal
disks, as they do to facilitate feeding during the spring and summer
(as described for other ozaenine larvae, see
(2) Eustra sp. A single third instar larva was collected in northern Taiwan (Shan-shya [sic]) by Gustav Tzh-wei Chen on 9.IX.2003 while excavating a nest of Pachycondyla javana Mayr (Hymenoptera, Formicidae, Ponerinae). The specimen was identified as belonging to the genus Eustra, by a phylogenetic analysis of molecular sequence data obtained from this specimen and from sixty other members of the subfamily Paussinae, including other members of the genus Eustra.
Rearing conditionsLarvae of Eustra chinensis were reared in captivity, where ambient conditions (e.g., temperature, light and humidity) were similar to natural conditions outdoors. Five larvae of each instar were reared in 2 ml centrifuge tubes. Other larvae were reared in a plastic box (18 cm × 11 cm × 12 cm) with the field-collected rotten wood. All larvae were fed springtails once a month.
Morphological analysisPrior to preparing them for microscopy, larvae were
drawn by a stereomicroscope Olympus SZX16 equipped with drawing tube.
One specimen of each instar of Eustra chinensis,
and the single specimen from Taiwan were rehydrated, cleared in 10%
KOH, transferred in hot lactic acid, dehydrated through a series of
EtOH baths of increasing concentration (10-20-50-70-90-95-100%), left
overnight in a clove oil bath, and mounted on slides with Canada
balsam. These specimens were studied and illustrated by using a light
microscope Olympus BX51 equipped with drawing tube. Another first instar
specimen was dehydrated through a series of EtOH baths of increasing
concentration (70-80-90-95-100%), critical point dried (Bal-Tec CPD
030), mounted on a stub (by using self adhesive carbon disks),
sputtered with gold (Emitech k550 sputter coater), and observed with
Philips XL30 scanning electron microscope and FEI Dualbeam FIB/SEM
Helios Nanolab (L.I.M.E. laboratory, University ‘Roma Tre’, Rome). In
this paper, the general terminology of larval structures follows
Generic diagnosis. Body length very small as compared with other Paussinae (1.75mm, first instars); antenna 3-jointed (II+III fused); mandible apically bidentate, with sub-basal retinaculum, ental margin of retinaculum with additional small sub-basal tooth; galea extremely long and apically sharp, distinctly longer than maxillary palp and lacinia; maxillary palpomere 3-jointed (II+III fused); claws of very different size, smaller claw obsolescent; hypopleurite VI with ventrolateral, elongate digitiform protuberance, tipped by strong spine-like seta; most sternal and pleural setae of the abdomen elongate and clavate at apex; lateral plates of terminal disk thin and wing-like, pointed at apex, with dorsal margin straight and ventral margin curved; urogomphi flattened, wider and longer than dorsal plates, composed by 7 short triangular lobes, acute at apex, separated by V-shaped incisures of different depths; lobe X present between C and E2; lobe E1 divided into E1a and E1b; peculiar mushroom-like inflated sensilla S-I of different length present on surface of plates and urogomphi; sensilla S-II of two different types, alternate on dorsal plates and urogomphi: (1) very long and stick-like, pointed at apex; (2) short and clavate at tip; terminal disk covered with peculiar hairy microsculpture.
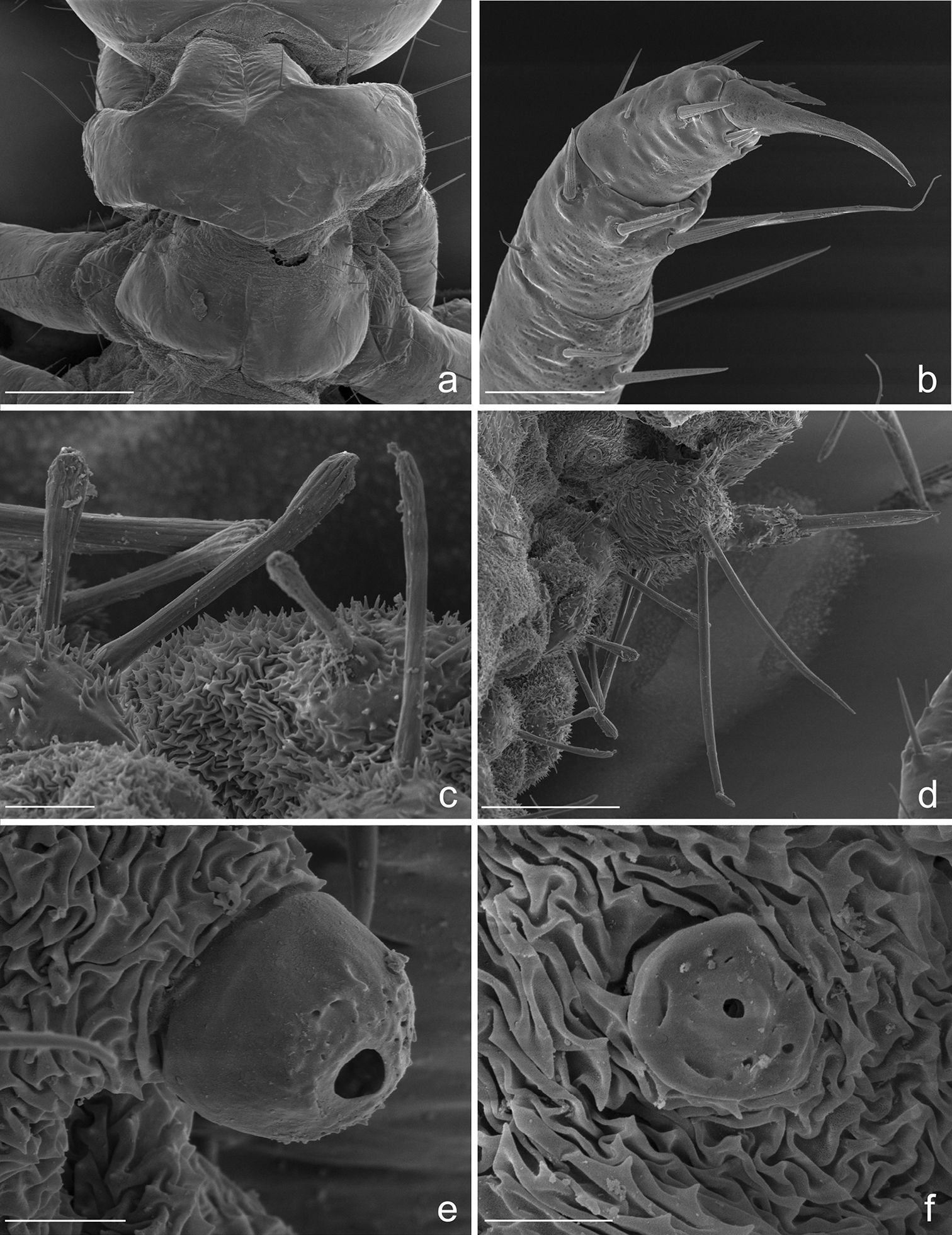
Eustra chinensis first instar larvaHabitus and coloration. Body soft, whitish, weakly sclerotized, not physogastric; abdomen flattened, bellows-like, contracted dorsally elevating the large terminal disk; terminal disk, cephalic capsule and mouthparts well sclerotized, yellowish to light brown; mandibles, laciniae, anterior margin of frontal sclerite, egg-bursters and claws thickly sclerotized and reddish brown.
Microsculpture. Cephalic capsule, mouthparts, thoracic tergites and legs smooth, without or with only sparse, pointed microsculpture (Figs 2, 5a, 5b); anterior margin of frontoclypeolabrale to adnasalia strongly denticulate at sides of median prominence, resulting in a serrate anterior edge (Figs 1a, 2a); anterior frontal keel smooth; basal third of prementum (Figs 1f, 2a, 3f) and stipes (Figs 1e, 2a, 3f) with pointed microsculpture on dorsal surface; membranous areas of the body and sclerites of the abdomen rugulose to rugose (Figs 5a, 5c-f), with pointed or multi-pointed sculpticells, sparse near the setae, longer on epipleurites I-VII; dorsal surface of dorsal plates, basal part of lateral plates and ventral surface of urogomphi covered by transverse rows of 2-6 spines (2-3 µm long), regularly spaced every 3-6 µm (Fig. 7d); surface of terminal disk (Fig. 7) thickly covered by a peculiar hairy microsculpture; pygidium, with pointed to multi-pointed microsculpture.
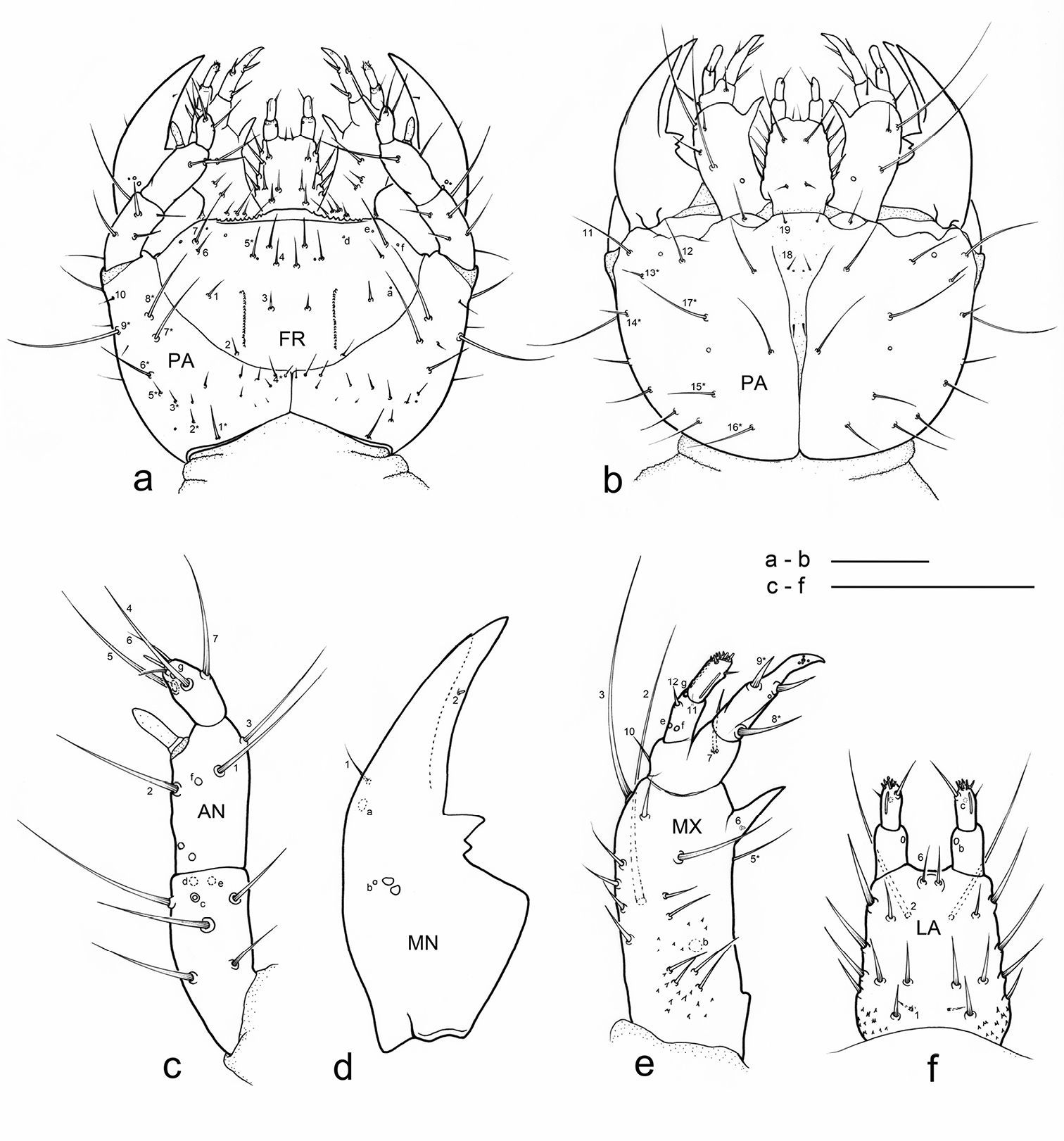
Eustra chinensis first instar larva: line drawings of a head, dorsal view b head, ventral view c left antenna, dorsal view d left mandible, dorsal view e left maxilla, dorsal view f labium, dorsal view. Scale bars: 0.1 mm.
Chaetotaxy. Frontoclypeolabrum (Figs 1a, 2a-c) without additional setae; FR1-4, 6-9 easily distinguished (Fig. 1a); medial prominence of frontoclypeolabrum with 2 minute spine-like setae on dorsal surface (Fig. 2b); FRb absent; several minute filiform sensilla (Fig. 2b) expanded at apex present on anterior part of frontale (Figs 2e-f). Parietale (Figs 1a, 2c) with several small additional setae irregularly positioned mesodorsally, and longer additional setae placed ventrally; some setae of parietale possibly homologous to the ancestral pattern are tentatively assigned in Fig. 1a. Antennomere I (Figs 1c, 2c) with 5 dorsolateral additional setae; ANa, b absent; III with AN1 and ANf displaced apically (Fig. 1c). Mandibles (Fig. 1d) with two large additional pores mesodorsally. Setal group gMX on stipes composed of about 10 setae (Fig. 1e); MX6 very small, dorsal and subbasal on lacinia; galeomere II with one additional seta on ental side and a subapical, dorsal sensorial area (composed of 3 dome-like and 1 longer medial sensilla) (Fig. 3c); maxillary palpomere IV with 1 small additional seta on ental side, 2 longitudinal subapical digitiform sensilla (Fig. 3e) and apical sensorial area composed of several papillae. Prementum (Figs 1f, 3f) with about 10 additional setae on lateral and dorsal surface, LA3, 4, 5 not clearly identifiable; seta LA1 close to the midline; LAa absent, LAc subapical; labial palpomere II with 2 additional setae, 1 dorsal, medially directed and 1 small ventrolateral, 2 longitudinal subapical digitiform sensilla and apical sensorial area composed of several papillae. Pro-, meso- and metanotum (Figs 4a, 5a) with about 25 setae each (identification not possible). Coxa with about 20 setae; trochanter with spiniform setae present mostly on ventral side, including a long TR4; TR8 about as long as TR4 but thinner and more flexible. Meso- and metasternum with MS4 long. Abdominal tergites I-VII (Fig. 4c) with 4 setae on each side. Tergal side of dorsal and lateral plates of terminal disk (Figs 4c, 7b) with stiff pointed setae (sensilla S-VII) of various sizes, with cylindrical bases protruding from the plates: about 14 on each dorsal plate (epipleurite IX + tergite VIII) and about 3 on each lateral plate (epipleurite VIII); distal margin of each dorsal plate with about 12 elongated, straight and deeply corrugated sensilla S-II, of two different sizes and shapes (Fig. 6a) alternately placed: type 1 extremely long (about double than type 2), stick-like, with sharp tip; type 2 thinner than 1 and distinctly clavate at apex; inner edge of each dorsal plate (Fig. 6a) with 2-3 S-II type 2 obliquely directed, increasing in size from base to apex; margin of each lateral plate with 8 sensilla S-II, 5 of type 1; caudal side of the terminal disk with numerous sensilla S-I (Figs 4d, 6a) sparsely distributed: 25-30 S-I on each dorsal plate and about 1-4 on each lateral plate. Epipleurites (Figs. 4c-d, 5c-d) of abdominal segment I without setae, II-V with one elongate sensillum S-II (type 2) each, VI-VII with several setae and S-II type 2. Sternal area of segment I with small simple setae, II-VI with elongate sensilla S-II type 2, VII with simple elongate setae (except for one, see Fig. 4d). Urogomphi (Figs 6a, 7a, c) with many S-I (about 40), mainly on dorsal surface and at margins of branches; branches A, C, X and E1b with S-II type 2 (Fig. 7a), B, E2, E1a with apical long S-II type 1 (Fig. 7a, f). Pygidium without setae (Fig. 7a).
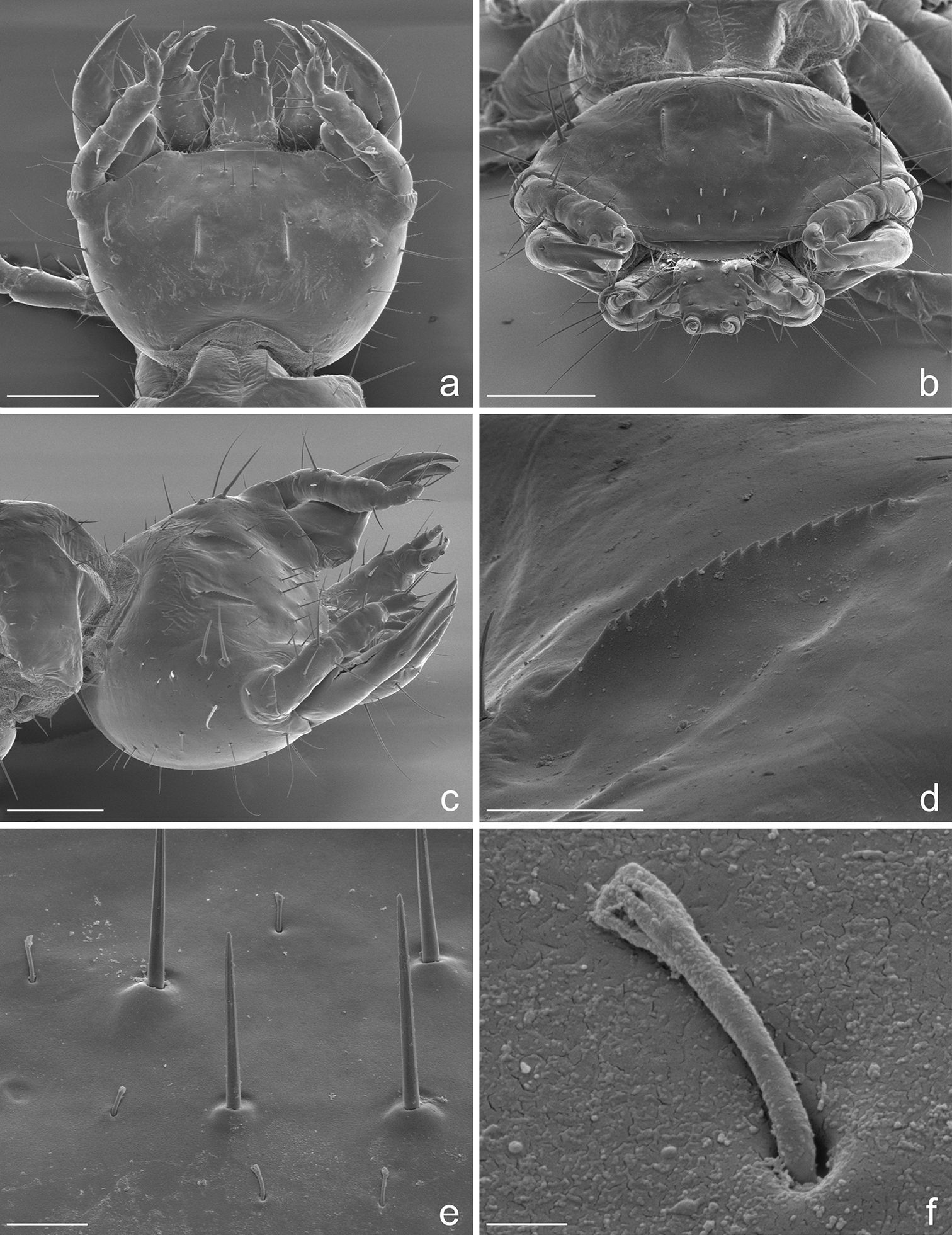
Eustra chinensis first instar larva: SEM micrographs of a head, dorsal view b head, frontal view c head, dorsolateral oblique view d left egg-burster, right dorsolateral oblique view e basal setae of frontoclypeolabrale, with filiform sensilla, dorsal view f close-up of a filiform sensillum of frontoclypeolabrale. Scale bars: a, b, c = 100 µm; d = 20 µm; e = 10 µm; f = 1 µm.
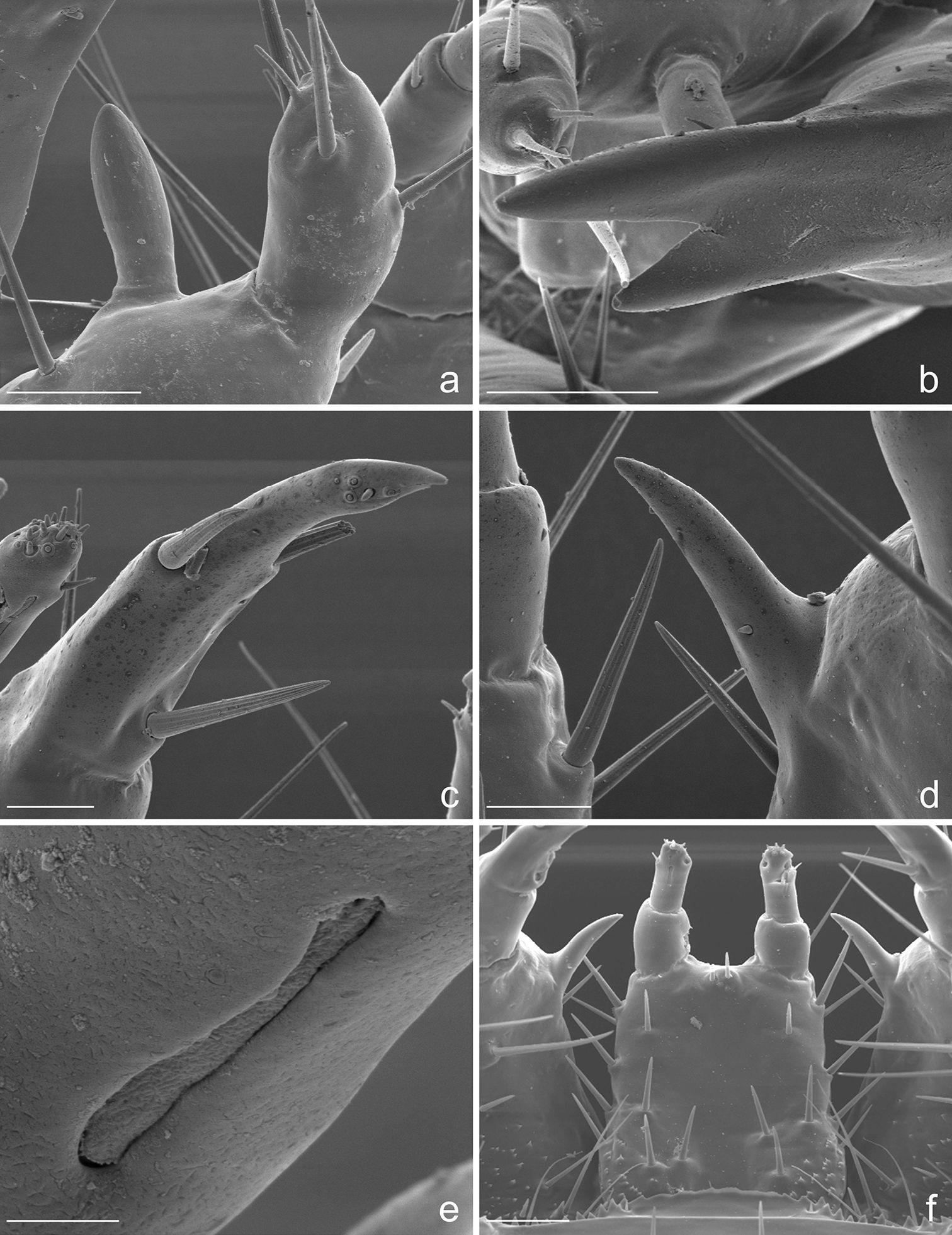
Eustra chinensis first instar larva: SEM micrographs of a apex of left antenna, dorsal view b apex of left mandible, lateral view c apex of left galea, dorsal view d right lacinia, dorsal view e digitiform sensillum of maxillary palpomere IV, lateral view f labium, dorsal view. Scale bars: a = 20 µm; b, f = 25 µm; c, d = 10 µm; e = 2 µm.
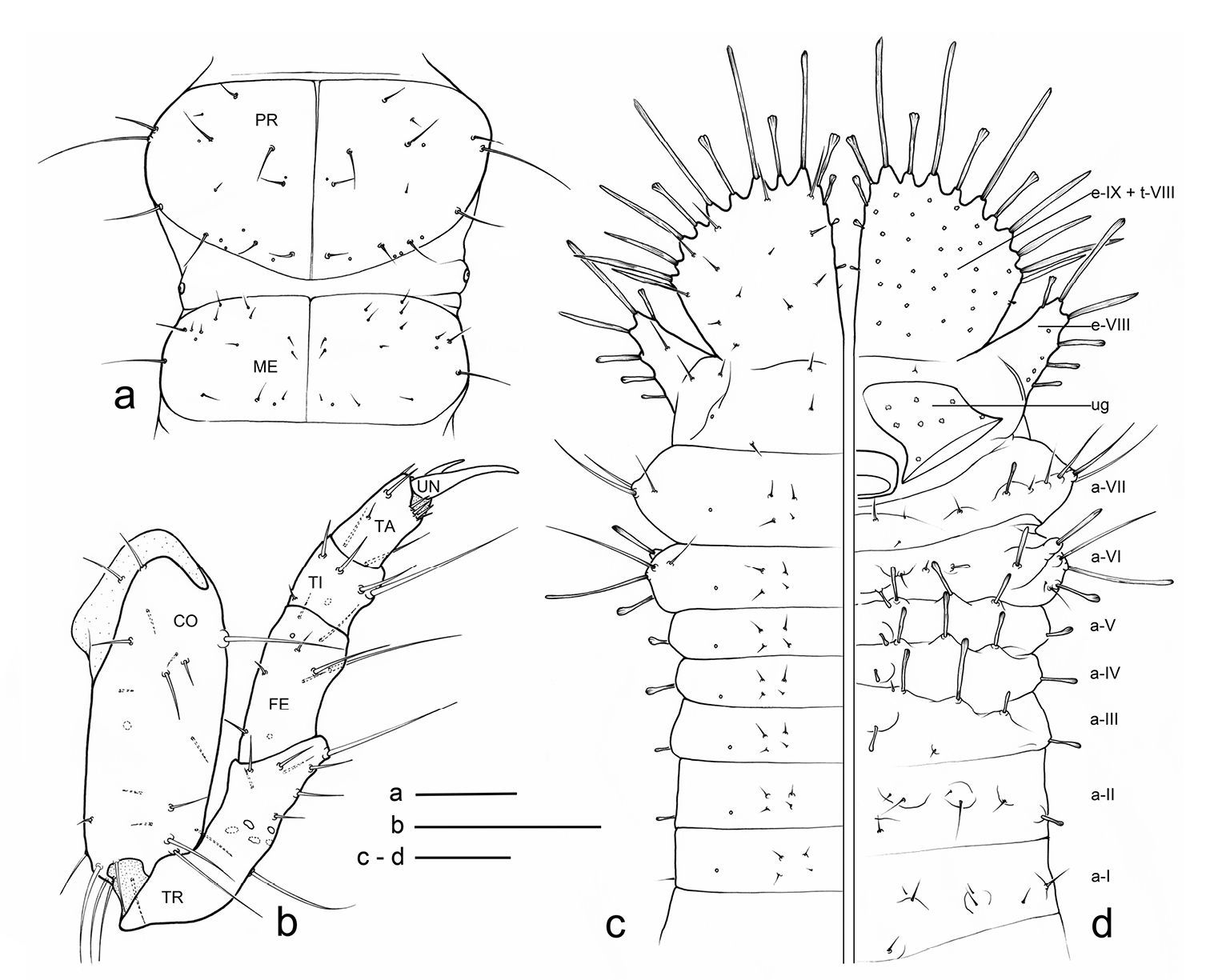
Eustra chinensis first instar larva: line drawings of a pro- and meso-notum, dorsal view b left foreleg, anterior view c right side of abdomen, dorsal view d right side of abdomen, ventral view (right urogomphus not drawn). a- = abdominal segment; e- = epipleurite; t- = tergum; Scale bars: 0.1 mm.
Eustra chinensis first instar larva: SEM micrographs of a pro- and meso-notum, dorsal view b apex of left hind-leg, anterior view c abdominal sensilla on left pleurae, dorsal view d abdominal sensilla on left pleurae, dorsal view e right metathoracic spiracle f abdominal spiracle I. Scale bars: a = 100 µm; b = 30 µm; c = 10 µm; d = 50 µm; e = 5 µm; f = 4 µm.
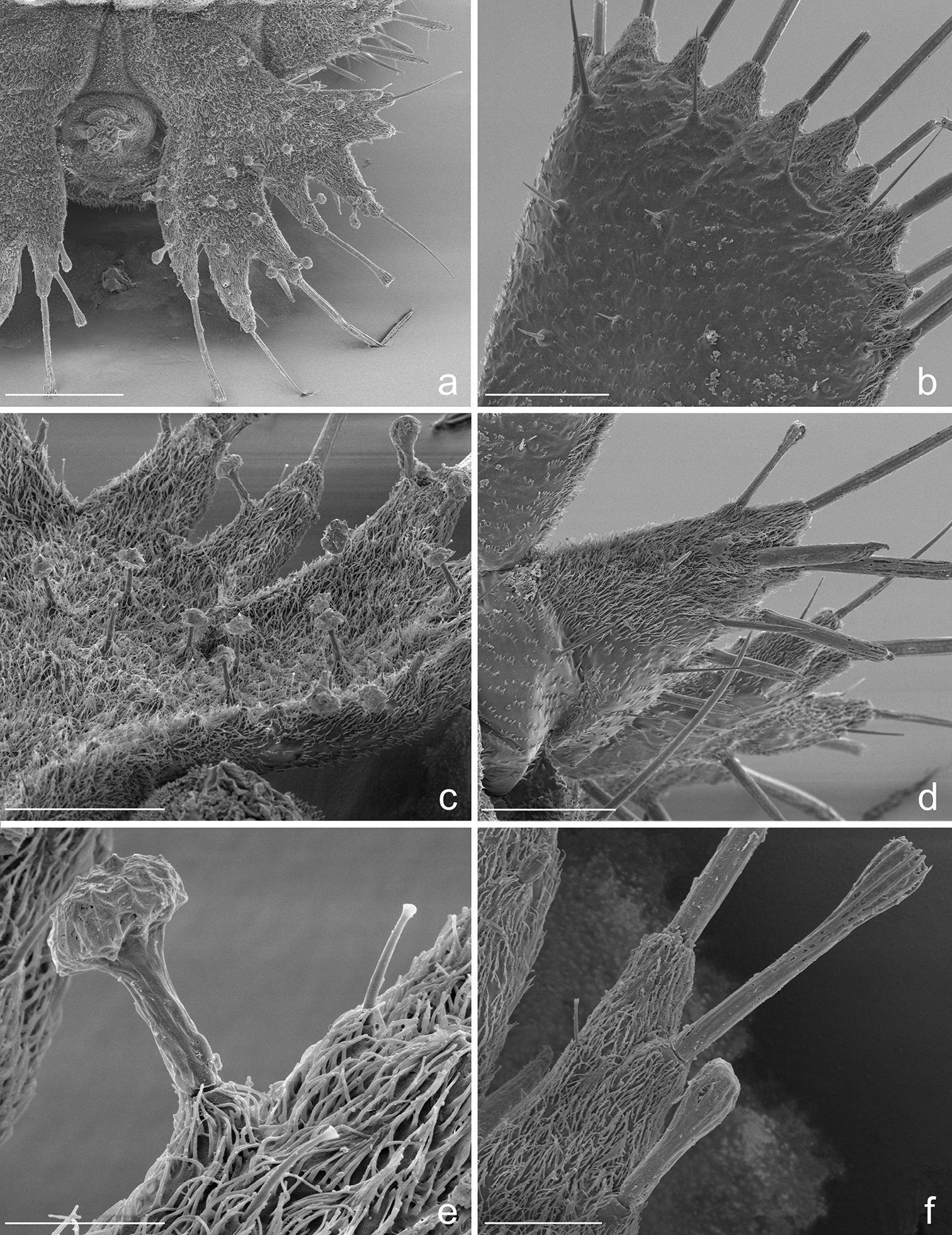
Terminal disks of: a Eustra chinensis first instar b Eustra sp. Taiwanthird instar c Goniotropis kuntzeni first instar d Paussus favieri first instar. DP = dorsal plate; LP = lateral plate; UG = urogomphus; PY = pygidium. Scale bars: a = 0.25 mm; b= 0.5 mm.
Eustra chinensis first instar larva: SEM micrographs of a right urogomphus, dorsal view b left dorsal plate, dorsal view c right urogomphus, left dorso-lateral view d left lateral plate, dorsal view e sensillum S-I on X lobe, lateral view f right urogomphus, lobe A, dorso-lateral view. Scale bars: a = 100 µm; b, c, d = 50 µm; e = 10 µm; f = 20 µm.
Head. Cephalic capsule (Figs 1a-b, 2a-c)
strongly transverse (width/length ratio = 1.86), hyperprognathous,
rounded laterally, regularly tapered at basal half into a distinct
neck; maximum width at antennal insertions about twice as wide as
occipital foramen. Frontoclypeolabrum (Figs 1a, 2a, c)
strongly transverse (width/length ratio = 1.64), with surface medially
convex and anterolaterally concave; basal stem of epicranial suture
short, anterior frontal arms only slightly sinuate; egg-bursters (Fig. 2d)
composed of two longitudinal, multispinulate carinae, each consisting
of about 20 forwardly directed teeth; carinae parallel, about one
third the length of frontoclypeolabrum, widely separated, placed
between FR1 and FR3. Anterior margin of frontoclypeolabrum (Figs 1a, 2a-b) double-edged: dorsal edge smooth, slightly convex, forming a transverse keel (see
Thorax. Tergites (Figs 4a, 5a) scarcely sclerotized, sternum not sclerotized. Pronotum wider than meso- and metanotum, transverse, about two times wider than long. Meso- and metanotum widely transverse, about two and a half times wider than long; longitudinal ecdysial line well marked on pro- and mesonotum, less evident, but present, on metanotum.
Spiracles. Thoracic and abdominal spiracles (Figs 5e, f) annular-uniphorous; mesothoracic spiracles dome-like, anterolateral on mesopleura, more than twice the size of the abdominal spiracle I. Abdominal spiracles rounded, plate-like, not protruding (Fig. 5f), placed dorsolaterally between tergites and epipleurites.
Legs. Legs well developed, 5-jointed (Fig. 4b), forelegs slightly shorter than others, mid and hind legs subequal. Coxa cylindrical, very long, about as long as trochanter and femur combined; trochanter elongate, obliquely truncate and fused apically to femur, about as long as femur and tibia combined; femur about as long as tibia and tarsus combined; tibia very short, cylindrical, slightly shorter than tarsus; tarsus more slender than tibia, conical, tapered from base to apex, with 2 sharp unequal claws (Fig. 5b): anterior claw elongate and strong, slightly longer than tarsus, apically curved; posterior claw very small and somewhat obsolescent.
Abdomen. Abdominal segments I-VII (Figs 4c, d) not sclerotized, bellows-like, usually up-curved, keeping the abdominal apex in an elevated position. Abdominal sclerites barely discernable, recognised by reduction of multipointed microsculpture around setae or sensilla S-II; segments progressively wider from I to VIII. Each segment dorsally flattened, with swollen, setiferous pleural and sternal areas. Hypopleurites setiferous, slightly protruding; hypopleurite VI with ventrolateral, elongate digitiform protuberance, tipped by strong spine-like seta. Epipleurites conical, distinctly protruding, gradually more developed from segment I to VIII; epipleurites of segment VIII (Figs 4c-d, 6a, 7d) flattened and enlarged into two sclerotized lateral plates, smaller than the dorsal plates; lateral plates slender, triangular, about two times longer than wide at base; epipleurites of segment IX greatly enlarged and fused with tergum of segment VIII into two rectangular, sclerotized plates (dorsal plates), slightly enlarged from base to apex and widely separated in the middle by a deep V-shaped notch (Figs 4c-d, 6a); lateral plates widely separated from dorsal plates; lateral plates, dorsal plates and urogomphi forming a terminal disk articulated at base by membranes, dorsal and lateral plates move against urogomphi. Urogomphi (Figs 6a, 7a, c) wide, flattened, each composed of 7 pointed lobes: A, B, C, X, E2, E1b, E1a (respectively from the inner to the outer); A much shorter than B; pygidium (Figs 6a, 7a) protruding, medioventrally positioned between the urogomphal insertions.
Eustra chinensis, second and third instar larvaeGeneral morphology very similar to that described above for the first instar, except for: progressive increasing of relative dimensions (see Table 1); presence of secondary setae on antennomere II (2 setae); sensorial appendage much shorter than antennomere IV; retinaculum progressively longer and more falcate; stipe with wider and sharp sub-basal protuberance; labial palpomere I wider than II; tibia subequal to tarsus; pronotum about as wide as meso- and metanotum; dorsal plates of terminal disk slightly longer; lobes of urogomphi relatively longer and more slender; lobe E1a slightly longer than E1b.
Measurements (mm) of three instars (L1, L2, L3) of Eustra chinensis and the third instar (L3) of Eustra sp.Taiwan. BL = body length (from tip of mandibles to the apex of terminal disk); HW = cephalic capsule maximum width (at the base of the antennae); HL = cephalic capsule medial length (mesodorsally, from occipital foramen to anterior margin of frontoclypeolabrum); PW = prothorax maximum width; PL = prothorax medial length; TDW = terminal disk maximum width (at the level of lateral plates); DPL = dorsal plates length (from base, near articulation, to the medial apex).
| Eustra chinensis L1 | Eustra chinensis L2 | Eustra chinensis L3 | Eustra sp. L3 | |
|---|---|---|---|---|
| BL | 1.75 | 2.7 | 3.02 | - |
| HW | 0.39 | 0.5 | 0.68 | - |
| HL | 0.21 | 0.28 | 0.37 | - |
| PW | 0.32 | 0.45 | 0.65 | 0.8 |
| PL | 0.19 | 0.28 | 0.42 | 0.55 |
| TDW | 0.5 | 0.7 | 0.98 | 1.42 |
| DPL | 0.22 | 0.33 | 0.5 | 0.62 |
Unfortunately, the specimen is damaged and portions of its head and legs are missing. Only basal part of head capsule, basal half of a mandible, thorax, basal part of legs, entire abdomen and terminal disk are intact. However, there is one low-resolution image of the entire specimen, which provides only limited information of some structural details.
General larval structure and most characters of the terminal disk (Fig. 6b) are very similar to those described above, especially as compared with the third instar of Eustra chinensis, except for the following minor differences:
(1) lobe A of urogomphi about as long as B (A much shorter than B in Eustra chinensis); (2) E1a thinner and more elongate than E1b (subequal or only slightly longer to E1b in Eustra chinensis); (3) lobes A very close medially, almost touching (distinctly separated medially in Eustra chinensis).
DiscussionEustra larvae are highly modified compared with the other known larvae of Ozaenini, and have several unique structures that make their identification easy. These include:
1. Antennae 3-jointed (Figs 1c, 2c). Paussinae larvae generally show 4-jointed antennae, a condition typical for adephagans. The reduction to 3 joints in Eustra is clearly due to the fusion of antennomeres II and III.
2. Mandible apically bidentate with sub-basal retinaculum (Figs 1d, 3b). A bidentate mandibular apex is also present in all known myrmecophilous Paussini larvae except Arthropterus, but in this tribe the second tooth is thought to be a subapically displaced retinaculum (
3. Ental margin of retinaculum with additional small sub-basal tooth (Fig. 1d). This margin is straight only in Physea, while it is more or less sinuate (basal half convex, distal half concave) in all other known ozaenine genera. The presence of a sub-basal tooth on the ental margin in Eustra may be an adaptation for piercing and holding their prey.
4. Maxillary palp 3-jointed (Fig. 1e). The reduction of the palpomeres from 4 to 3 is a common feature of known Paussini larvae except for Platyrhopalopsis and Arthropterus. In the genus Paussus the reduction is due to the fusion of basal palpomere with the stipe. In Eustra the basal palpomere is only partially fused with stipe but still recognizable, and the actual reduction is due to the fusion of palpomeres II+III.
5. Galea extremely long and apically sharp (Figs 1e, 3c). The galea of Eustra is two-jointed as is typical of ozaenines but it is highly modified: it is very strong, up-curved, and almost two times longer than the maxillary palp and almost three times longer than the lacinia. The apex is hook-like and unusually sharp, which would provide an effective tool for capturing and holding prey.
6. Strongly asymmetric tarsal claws (Figs 4b, 5b). All Metriini, Mystropomini, and Ozaenini larvae have legs with two tarsal claws of unequal size, the anterior distinctly longer than posterior, while myrmecophilous Paussini larvae have only a single claw (presumably the anterior). In Eustra the posterior tarsal claw is extremely small and almost obsolescent.
7. Hypopleurite VI with ventrolateral, elongate digitiform protuberance, tipped by strong spine-like seta (Figs 4d, 5d). This peculiar sensorial structure is unique to the genus Eustra.
8. Most sternal and pleural setae of the abdomen elongate and clavate at apex (Figs 4c-d, 5c-d). Clavate sensilla have been described in the myrmecophilous genus Arthropterus (sensilla S-VIII possibly homolog to S-II, see
9. Lateral plates of terminal disk transverse, subtriangular and pointed at apex (Figs 6a-b, 7d), with straight margins. The lateral plates of the Metriini, Mystropomini and other Ozaenini are wide and broadly rounded. Lateral plates of Goniotropis (Ozaenini) (Fig. 6c) are transverse and widely separated from dorsal plates similar to those of Eustra.
10. Urogomphi flattened, wider and longer than dorsal plates, composed by 7 short triangular lobes (Figs 6a-b, 7a), acute or bidentate at apex, separated by V-shaped notches of different depths, very shallowas compared to other Ozaenini. The flattening and widening of the urogomphi and the reduction (Physea, Ozaenini) or absence (all Paussini, see for example Fig. 6d) of branches is a typical feature of myrmecophilous larvae (
11. Absence of urogomphal lobe D and presence of the additional urogomphal lobe X (Figs 6a-b, 7a). The lobes of urogomphi were coded first in Metrius by
12. Peculiar inflated sensilla S-I (Figs 7a, c, e) of different lengths present on surface of plates and urogomphi. Inflated sensilla S-I have been described in larvae of Platyrhopalopsis (Paussini) and Physea (Ozaenini) and have been considered as an adaptation to the myrmecophilous lifestyle (
Like in the other ozaenine genera, larvae of Eustra
live in galleries that they dig in humid soil or rotten wood and close
off with their terminal disk, which they use to trap prey. However,
the larvae of Eustra
are so specialized and modified that it is not possible to find clear
synapomorphies with larvae of any of the other known ozaenine genera.
Some of the peculiar adaptations discussed above are similar to, but
not necessary homologous to, characteristics described for the
myrmecophilous larvae of Paussini and Physea (See Table 2). Since the Eustra larva from Taiwan was found inside a nest of Pachycondyla javana,
it is possible that some of these traits are adaptations to a
myrmecophilous lifestyle. However, we think that it is more likely that
these minute larvae feed on very small invertebrates like collembolans
and Drosophila,
which first instar ozaenine larvae consistently consume in the lab
(Moore and Di Giulio, pers. obs.), than it is that they feed on Pachycondlya,
which are relatively large-bodied ants. Instead, many of the unusual
characters observed in these larvae could facilitate feeding on fast
moving prey, including the very long radial mechanoreceptors (sensilla
S-II) of the terminal disk which would sense the approach of fast
collembolans, and modified mouthparts including the bidentate
mandibular apex, second tooth of retinaculum, and hook-like galea
which would help the larva hold onto motile prey. Other characters,
such as the flattening and widening of the urogomphi, could be related
to the miniaturization of the larval body. In the future, we hope to
discover the larvae of the genus Dhanya, and compare its morphological structures with Eustra since they are hypothesized to be sister genera (
Characteristics of Eustra larvae that are similar to those found in mymecophilous larvae.
| Eustra+Physea | |
| galea elongate (but in a completely different way: in Physea galeomere I long, II short and truncate at apex; in Eustra I short and II very long and sharp) | |
| lacinia reduced | |
| prementum elongate and tapered from base to apex | |
| labral spine wide | |
| ligula absent | |
| urogomphal lobes partially fused | |
| head short and transverse | |
| frontoclypeolabrale wide and transverse | |
| coronal suture short | |
| anterior arms of frontal sutures only slightly sinuate | |
| stemmata absent | |
| retinaculum in first instar triangular, inward directed | |
| sensilla S-I inflated | |
| Eustra+Paussini | |
| mandibles apically bidentate | |
| number of maxillary palp articles reduced (but in a completely different way, see Discussion) | |
| second tarsal claw reduced (in Paussini second claw is absent) | |
| sensilla clavate or inflated (only in Arthropterus clavate sensilla S-II, inflated S-I in Platyrhopalopsis) | |
| urogomphal lobes short rather then strongly branched | |
| urogomphi flat and wide | |
| antennae short and strongly directed medially | |
| stemmata absent | |
| sensorial appendage elongate | |
| head shortened and distinctly transverse | |
The authors wish to thank Gustav Tzh-wei Chen for collecting a mysterious paussine larva while excavating a nest of Pachycondyla javana and sending a leg to WM for molecular identification in 2003, and Brian Fisher (California Academy of Sciences) for his help tracking down the voucher specimen many years later. We also thank David Kavanaugh and one anonymous reviewer for helpful comments that improved this paper. This project was partially funded by NSF grant DEB-0908187 to WM.