(C) 2010 Ueangfa Bantaowong. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Four new species of terrestrial earthworms from the zebrus-group in the genus Amynthas Kinberg, 1867, are described from Nan province, north Thailand: Amynthas phatubensis sp. n., from Tham Pha Tub Arboretum, Amynthas tontong sp. n., from Tontong Waterfall, Amynthas borealis sp. n., from Chaloemprakiat district, and Amynthas srinan sp. n., from Srinan National Park.After comparing with the two closely related Laos species Amynthas chandyi Hong, 2008 and Amynthas namphouinensis Hong, 2008, the four new species show clear morphological differences, and also it is confirmed that there are no previous records of the species described here. Amynthas phatubensis sp. n. is the largest (longest) sized of these earthworms and is the only species that lives in limestone habitats. The genital characters are different among them and also from the two Laotian species. Molecular systematics would be a good method for further analysis of the diversity and species boundaries in SE Asian Amynthas.
Amynthas, Earthworm, Taxonomy, New species, Thailand
Previous taxonomic publications on, or including, the Megascolecidae (sensu
Morphological characteristics comparison of Amynthas
species recorded in Thailand. The morphological characters are from the
original description of each nominal species, except for the character
with (*) are from
| Species | Species group | Body length(mm) | Number of segments | Sperma-thecal pores | Genital markings | Genital marking glands | Seminal vesicles | Prostate glands | Intestinal caeca | Distribution** |
|---|---|---|---|---|---|---|---|---|---|---|
| Amynthas hupbonensis (Stephenson, 1931) | aeruginosus | 225 | 142 | 7/8–8/9 | absent | absent | large in XI, XII | XVI–XX | manicate, XXVII– | Chonburi |
| Amynthas alexandri (Beddard, 1900) | corticis | 145 | 133 | 5/6–8/9 | absent | absent | XI, XII | XVII–XX | simple, XXVII–XX | Chiengrai, Chiengmai, Nakornratchasima, Bangkok, Chonburi |
| Amynthas comptus (Gates, 1932) | corticis | 197–260* | 120–134* | 5/6–8/9 | three trios on 18/19–20/21 | sessile | larger in XI, XII | XVIII | simple, XXVII–XXIII | Phrae |
| Amynthas exiguus austrinus (Gates, 1932) | corticis | 33–68 | 73–102 | 5/6–8/9 | two pairs on 17/18, 18/19 | absent | small in XI, XII | XVII–XX | simple, XXVII–XXIV | Chiengmai |
| Amynthas exiguus exiguus (Gates, 1930) | corticis | 43 | 90 | 5/6–8/9 | paired on vii, viii, xix, xx | absent | small in XI, XII | XVII–XIX | simple, XXVII–XXIV | Phrae |
| Amynthas longicauliculatus (Gates, 1931) | corticis | 170 | 138 | 5/6–8/9 | three pairs on 18/19– 20/21 | sessile | XI, XII | XVIII | simple, xxvii-xxiv | Chiengmai, Lumphun, Nakornratchasima |
| Amynthas manicata decorosa (Gates, 1932) | corticis | 40 | 60 | 5/6–8/9 | one pair on xviii | sessile | large in XI, XII | XVII–XIX | manicate, XXVII–XXII | Chiengmai |
| Amynthas mekongianus (Cognetti, 1922) | corticis | 1 meter | 370 | 5/6-8/9 | absent | absent | 10/11-11/12 | XVII-XVIII | simple, XXVII-XXIII | Chiengrai |
| Amynthas defecta (Gates, 1930) | gracilis | >78 | >49 | 5/6–7/8 | absent | absent | small in XI, XII | absent | manicate, XXVII–XXVI | Nakornratchasima |
| Amynthas gracilis (Rosa, 1891) | gracilis | 100 | 88–95 | 5/6–7/8 | clusters on xviii | stalked* | XI, XII* | XVII–XXIII | simple, XXVII–XXIV* | Dor Kiu Koh Ma, north Thailand |
| Amynthas papulosus (Rosa, 1896) | gracilis | 45–50 | 110–115 | 5/6–7/8 | transverse row on XVII–XIX | stalked* | XI, XII | XVI–XXI | simple, XXVII–XXII* | Yala |
| Amynthas morrisi (Beddard, 1892) | morrisi | 52 | 93 | 5/6–6/7 | near spermathecal pore | stalked | XI, XII* | XVII–XXIII* | simple, XXVII–XXIV* | Chiengmai |
| Amynthas fucosus (Gates, 1933) | sieboldi | 120 | 114 | 6/7–8/9 | two pairs on 17/18, 18/19 | sessile | large in XI, XII | XVII–XX | simple, XXVII–XVII | Nakornratchasima |
| Amynthas siam Blakemore, 2011 | sieboldi | >70 | >73 | 6/7-8/9 | one pair postsetal on XVIII | sessile | XI, XII | XVIII- | simple, XXVII- | Sakon Nakhon |
Map of type locality of 1 Amynthas srinan sp. n. from Srinan National Park, Nan province, 2 Amynthas phatubensis sp. n. from Tham Pha Tub Arboretum, Nan province, 3 Amynthas tontong sp. n. from Tontong Waterfall, Pua district, Nan province and 4 Amynthas borealis sp. n. from a small hill near Chaloemprakiat district, Nan province.
Since none of the four species described in this paper seems to fit the descriptions of species described in the past, the purpose of this paper is to formally describe these species as new to science. Their descriptions follow.
Material and methodsEarthworms were collected from deciduous forests in many areas in Nan province, north of Thailand, by carefully digging up the topsoil near casts and by hand sorting the leaf litter. The worms were killed in 30% (v/v) ethanol, photographed, transferred to 5% (w/v) formalin for fixation for approximately 12 hours, and then transferred to 70% (v/v) ethanol for longer term preservation and subsequent morphological studies.
Duplicate specimens and/or tissue samples (in the cases
of morphotypes determined to be unique on field inspection) were
preserved in 95% ethanol for molecular data and DNA barcoding. Tissues
were sent to the Canadian Center for DNA Barcoding (
The descriptions of each species were made during observation under a Stemi DV 4 ZEISS stereoscopic light microscope. Drawings were made of the body segments and the distinct external characters and internal organs, as mentioned above, and are shown in Figures 2–5 for the four new species, respectively. The number of segments and the body width and length were measured in both full adults and juveniles, and are presented as the range (min-max) and mean±one standard deviation.
Type specimens housed at the Department of Biology, Faculty of Science, National University of Laos, Vientiane, Laos (BDNUL), of the two closely related Laos species, Amynthas chandyi Hong, 2008 and Amynthas namphouinensis Hong, 2008, have been critically studied and compared with the new species of this report.
Holotype and paratype specimens have been deposited in the Chulalongkorn University, Museum of Zoology, Bangkok, Thailand (CUMZ). Additional paratypes are housed in the Biozentrum Grindel und Zoologisches Museum, Hamburg, Germany (UHH), and the Natural History Museum, London (NHM).
Anatomical abbreviations: fp, female pore; ic, intestinal caeca; mp, male pores; pg, prostate gland; sc, spermathecae; sp, spermathecal pores; sv, seminal vesicles.
SystematicsAmynthas aeruginosus Kinberg, 1867, by monotypy.
urn:lsid:zoobank.org:act:299379EB-C7CE-4B89-8A40-40E3122DCAB9
http://species-id.net/wiki/Amynthas_phatubensis
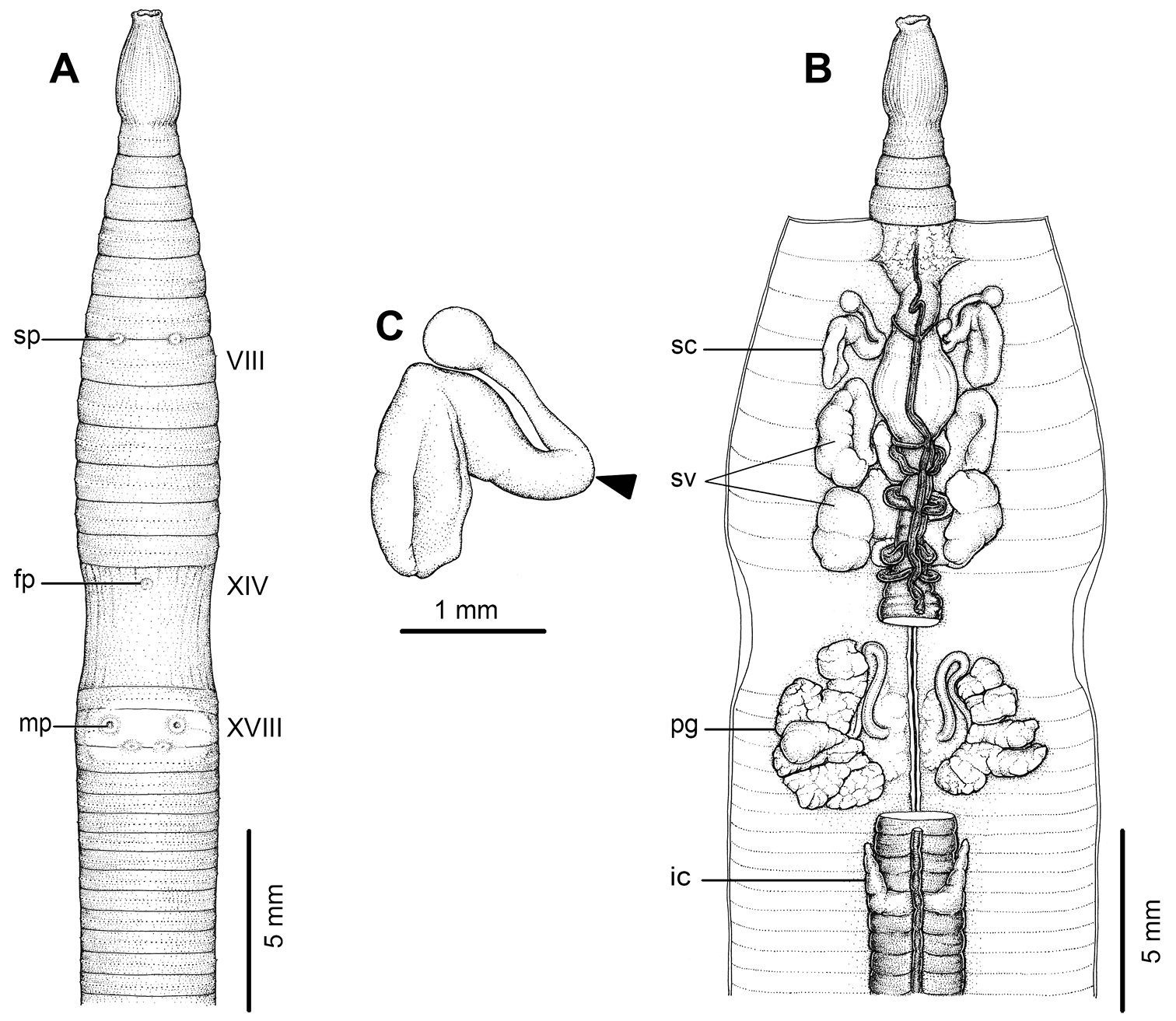
Figs 1, 2Dimensions; 110 mm by 4.3 mm at segment X, 4.3 at segment XX, 4.0 mm at clitellum; body cylindrical with 108 segments. Setae regularly distributed around segmental equators, numbering 51 at VII, 60 at XX, 15 between mp, setae formula AA:AB:ZZ:ZY= 1:1:1:1 at XIII with no ventral gaps. Single fp at XIV. Prostomium epilobic with tongue open. First dorsal pore at 5/6. Clitellum annular XIV–XVI with no setae.
A pair of mp is located ventro-laterally in XVIII, or at 9th seta line, 0.33 circumference apart ventrally, convex structure; distance between mp 4.2 mm. Porophores (protuberances bearing male aperture), papilla-like structures. Each mp surrounded by six flat, circular genital markings almost the same diameter as mp, also one pair is equatorial in XVII in line with the male pores. One pair of sp in intersegmental furrow 7/8, distance between pores 0.32 circumference ventrally apart; distance between sp 3.5 mm. Genital markings, rounded, flat, located close to sp, postsetal paired on VII very near 7/8, presetal paired on VIII.
Septa 5/6 and 6/7 thick, 7/8 thin, 8/9 and 9/10 absent, 10/11–13/14 thin. Gizzard large within VIII–X, intestinal origin in XV, no lymph glands observed. Typhlosole small from XXVII. Intestinal caeca originate from XXVII extending forward to XXIII, simple, long finger-shape. Hearts esophageal in X–XIII. Holandric; testes and funnels in ventrally joined sacs in X–XI. Seminal vesicles paired in XI–XII. Prostates in XVII–XX; prostatic ducts U-shape. Genital marking glands absent.
Ovaries in XIII. Sc one pair in VIII; ampulla large ovate sac, duct stout, short; long stalked diverticulum, convoluted kinks enclosed within membrane, spherical knob terminal. No nephridia on spermathecal ducts. A large sessile genital marking gland corresponding to each external genital marking in VII–VIII.
All the key morphological characters of the holotype and paratype specimens are given in Table 2.
External and internal morphology of holotype (CUMZ 3204) of Amynthas phatubensis sp. n. A External ventral view, B internal dorsal view and C spermatheca, and black arrow indicates the connection of the spermatheca and spermathecal pore.
Holotype and Paratype dimension and other morphological characteristics of Amynthas phatubensis Panha & Bantaowong, sp. n.
| Types \ Characters | Body length (mm) | Number of segments | Location of genital markings | First dorsal pore | Number of setae | Prostate glands | Intestinal caeca | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Preclitellum | Postclitellum | VII | XX | Between male pore | ||||||
| Holotype CUMZ 3204 | 110 | 108 | VII, VIII | XVII, XVIII | 5/6 | 51 | 60 | 15 | XVII–XX | XXVII–XXIII |
| Paratype CUMZ 3205 | ||||||||||
| 1 | 90 | 96 | VII, VIII | XVIII | 5/6 | 60 | 58 | 15 | XVII–XXI | XXVII–XXIV |
| 2 | 105 | 107 | VII, VIII, IX | XVII, XVIII, XIX | 5/6 | 52 | 58 | 12 | XVII–XX | XXVII–XXIV |
| 3 | 100 | 105 | VII, VIII, IX | XVIII | 5/6 | 53 | 60 | 9 | XVII–XX | XXVII–XXIV |
| 4 | 80 | 86 | VII, VIII | XVII, XVIII, XIX | 5/6 | 53 | 65 | 13 | XVII–XX | XXVII–XXIV |
| 5 | 120 | 96 | VII, VIII | XVIII | 5/6 | 58 | 68 | 11 | XVII–XX | XXVII–XXIV |
| 6 | 101 | 85 | VII, VIII | XVIII | 5/6 | 51 | 59 | 9 | XVII–XX | XXVII–XXIV |
| 7 | 131 | 86 | VII, VIII | XVII, XVIII | 5/6 | 64 | 67 | 15 | XVII–XXI | XXVII–XXII |
| 8 | 108 | 98 | VII, VIII | XVII, XVIII | 5/6 | 58 | 62 | 15 | XVII–XXI | XXVII–XXII |
| 9 | 116 | 99 | VII, VIII | XVII, XVIII | 5/6 | 53 | 64 | 11 | XVII–XXI | XXVII–XXIII |
| 10 | 89 | 92 | VII, VIII | XVII, XVIII | 5/6 | 64 | 58 | 12 | XVII–XX | XXVII–XXIV |
| 11 | 99 | 106 | VII, VIII, IX | XVII, XVIII | 5/6 | 60 | 63 | 13 | XVII–XXI | XXVII–XXIV |
| 12 | 112 | 112 | VII, VIII | XVII, XVIII | 5/6 | 52 | 58 | 11 | XVII–XX | XXVII–XXIII |
| 13 | 142 | 110 | VII, VIII | XVII, XVIII | 5/6 | 49 | 58 | 7 | XVII–XX | XXVII–XXIV |
| 14 | 137 | 108 | VII, VIII, IX | XVII, XVIII | 5/6 | 62 | 65 | 11 | XVII–XX | XXVII–XXIII |
| 15 | 80 | 85 | VII, VIII, IX | XVII, XVIII | 5/6 | 54 | 60 | 13 | XVII–XX | XXVII–XXIV |
| 16 | 89 | 111 | VII, VIII, IX | XVII, XVIII | 5/6 | 57 | 59 | 14 | XVII–XXI | XXVII–XXIII |
| 17 | 84 | 105 | VII, VIII | XVII, XVIII | 5/6 | 52 | 59 | 11 | XVII–XX | XXVII–XXIV |
| 18 | 148 | 112 | VII, VIII | XVII, XVIII | 5/6 | 51 | 58 | 12 | XVII–XX | XXVII–XXII |
| 19 | 109 | 114 | VII, VIII | XVII, XVIII | 5/6 | 64 | 59 | 12 | XVII–XX | XXVII–XXII |
| 20 | 144 | 107 | VII, VIII | XVII, XVIII | 5/6 | 53 | 60 | 11 | XVII–XXI | XXVII–XXIV |
| 21 | 84 | 108 | VII, VIII | XVII, XVIII | 5/6 | 64 | 61 | 15 | XVII–XX | XXVII–XXII |
The holotype measures 110 mm body length with 108 segments; the twenty one paratypes range in size from 80–148 mm (108±21.93 mm) body length with 85–114 segments (Table 2).
Tham Pha Tub Arboretum, Nan province, Thailand, 18°51'16.4"N, 100°44'10.1"E, 265 meters elevation (11th October 2009). We also collected another lot of further specimens from Tontong Waterfall, Nan province (location 3 in Figure 1), which is located about a hundred kilometers north of the type locality.
This species was named after the type locality, Tham Pha Tub Arboretum.
The holotype (CUMZ 3204) and 15 paratypes (CUMZ 3205) and 10 paratypes (CUMZ 3212) are deposited in Chulalongkorn University, Museum of Zoology. Another four paratypes will be deposited in the Biozentrum Grindel und Zoologisches Museum, Hamburg, Germany (UHH), and three paratypes in the Natural History Museum, London (NHM).
Found in the top soil at about 10 cm depth, the soil surface was covered with leaf litter in a deciduous limestone forest at Tham Pha Tub Arboretum. The soil was carefully dug close to the casts. Many ariophantid snails, Cryptozona siamensis Pfeiffer, 1856 were on the ground or under leaf litter.
Amynthas phatubensis sp. n. is a medium to large sized terrestrial earthworm with a pair of mp surrounded by six genital papillae on segment XVIII. Within the zebrus-group, this species is diagnosed by the unique combination of dorsal pores in 5/6, simple digitate caeca, ventrally joined testis sacs, genital marking glands in the spermathecal segments, and the spermathecal characters of the large ovate ampulla, stalked diverticulum whose folds are membrane-bound, and spherical knob terminal diverticulum sac.
Amynthas phatubensis sp. n. has very simple characteristics of the genus, but among these, only the superficial male pores are external. In most newly collected specimens it was difficult to observe the pores or marks on the bodies. However, after preservation they can be seen more clearly. The internal organs are much more easily discerned. This new species is quite distinct when compared to the two closely related species from Laos, Amynthas chandyi Hong, 2008 and Amynthas namphouinensis Hong, 2008, which belong in the same zebrus-group. The two Laos species are a little bit smaller than Amynthas phatubensis sp. n., especially Amynthas chandyi. Even though Amynthas namphouinensis is much closer in appearance to Amynthas phatubensis sp. n., there are distinct differences between the type specimens (Figs 6 and 7). For example, the distance between the mp of Amynthas phatubensis sp. n. is 4.2 mm for the holotype and range from 3.0–4.5 mm (4.27±0.57mm), while for Amynthas namphouinensis this was significantly smaller, ranging from 1.4–1.5 mm. The distance between a pair of sp is also different, being 3.5–4.5 mm (4.12±0.4 mm) for Amynthas phatubensis sp. n. and 1.4–2.0 mm in Amynthas namphouinensis. The distance between the male pores as a fraction of the estimated circumference of the 18th segment is 0.30–0.33 in Amynthas phatubensis sp. n., but 0.10–0.14 circumference apart in Amynthas namphouinensis. Moreover, Amynthas phatubensis sp. n. has no genital marking glands on segments XVII–XIX, where Amynthas namphouinensis has sessile genital marking glands, but contains two distinct genital marking glands located close to sc that are absent in Amynthas namphouinensis.
Two populations of Amynthas phatubensis
sp. n. were sampled, one from the type locality and one from Tontong
waterfall. Distinct DNA barcode clusters corresponding to these
populations had intra-cluster Kimura 2 parameter distances of 0.023
(N=9) and 0.016 (N=5) respectively. The inter-cluster divergence between
the two populations is 0.084. Based on the morphological unity and the
fact that the divergence is less than that usually seen between
congeneric species pairs of earthworms (
urn:lsid:zoobank.org:act:3317146B-143D-4EFC-A0C9-60262073BAFF
http://species-id.net/wiki/Amynthas_tontong
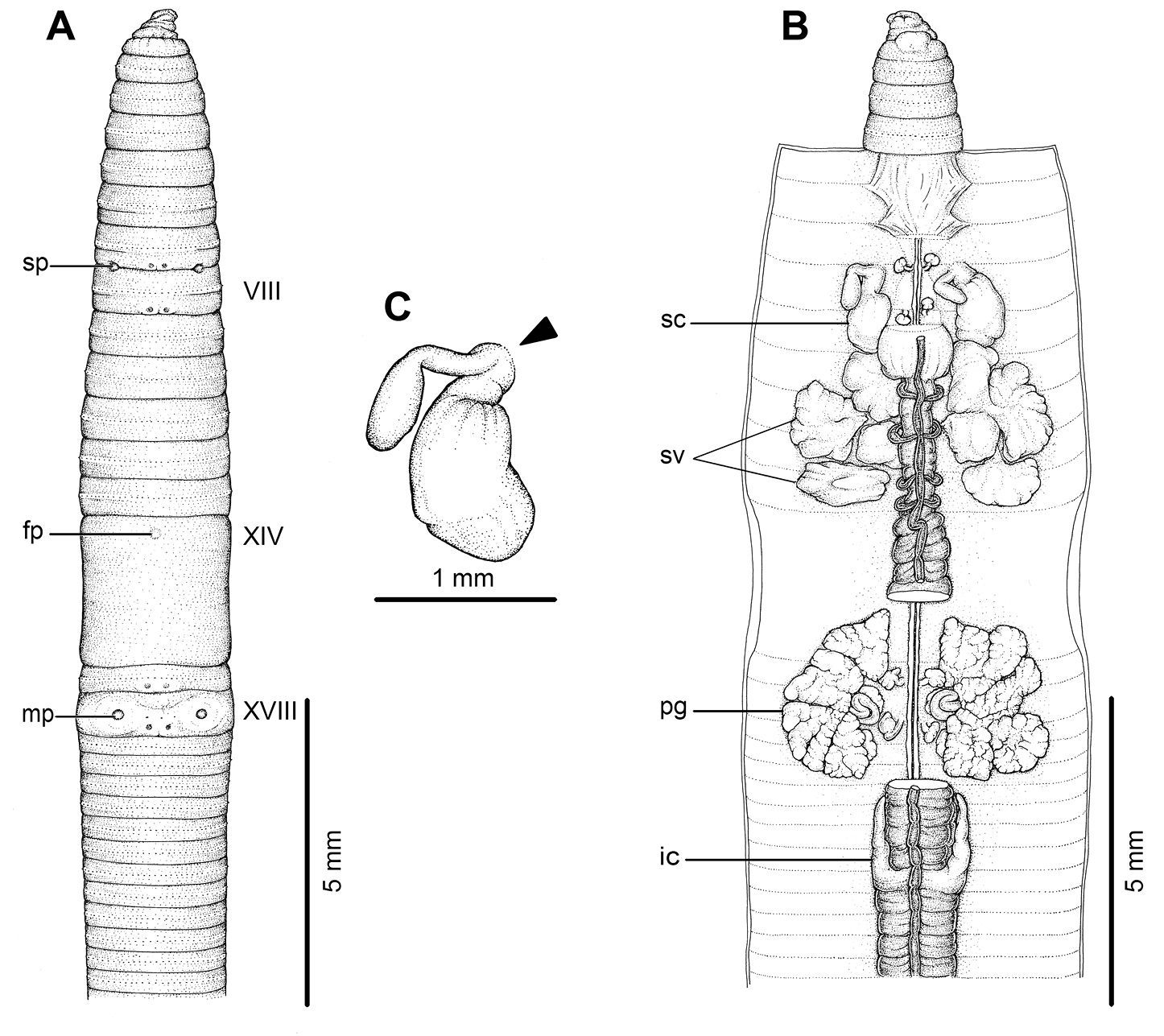
Figs 1, 3Dimensions; 53 mm by 2.7 mm at segment X, 2.6 at segment XX, 2.2 mm at clitellum; body cylindrical with 80 segments. Setae regularly distributed around segmental equators, numbering 42 at VII, 52 at XX, no visible setae between mp, setae formula AA:AB:ZZ:ZY= 1.5:1:1:1 at XIII. Single fp at XIV. Prostomium epilobic. First dorsal pore at 5/6. Clitellum annular XIV–XVI with no setae.
A pair of indistinct rounded mp in XVIII, 0.19 mm circumference apart ventrally; distance between mp 1.0 mm at 5th seta line. Genital markings closely paired located medial to male pore level in intersegment 18/19. Sp paired in 7/8 at 4th seta line, each small, lip-like structure within porophore, 0.10 circumference apart ventrally; distance between sp 1.0 mm. Genital markings near sp absent.
Septa 5/6 and 6/7 thick, 7/8 thin, 8/9 and 9/10 absent 10/11–13/14 thin. Gizzard large within VIII–X, intestinal origin in XV, no lymph glands observed. Typhlosole small from XXVII. Ic originated from XXVII extending forward to XXV, simple finger-shape. Hearts esophageal in X–XIII. Holandric; testes and funnels in ventrally joined sacs in X–XI. Sv paired in XI–XII. Prostates in XVIII; prostatic ducts long slender with U-shape. Genital marking glands absent.
Ovaries in XIII. Sc one pair in VIII; ampulla thumb shape, duct stout, shorter than ampulla. Diverticulum slender stalk with spherical knob terminal, no genital marking glands observed.
All the key morphological characters of the holotype and paratype specimens are given in Table 3.
External and internal morphology of holotype (CUMZ 3206) of Amynthas tontong sp. n. A External ventral view, B internal dorsal view and C spermatheca, and black arrow indicates the connection of the spermatheca and spermathecal pore.
Holotype and Paratype dimension and other morphological characteristics of Amynthas tontong Panha & Bantaowong sp. n.
| Types \ Characters | Body length (mm) | Number of segments | Genital markings | First dorsal pore | Number of setae | Between male pore | Prostate glands | Intestinal caeca | |
|---|---|---|---|---|---|---|---|---|---|
| VII | XX | ||||||||
| HolotypeCUMZ 3206 | 53 | 80 | XVIII | 5/6 | 42 | 52 | 0 | XVII–XX | XXVII–XXIV |
| ParatypeCUMZ 3207 | |||||||||
| 1 | 41 | 71 | XVIII | 5/6 | 41 | 53 | 0 | XVI–XVIII | XXVII–XXV |
| 2 | 39 | 74 | XVIII | 5/6 | 42 | 52 | 0 | XVII–XX | XXVII–XXIV |
| 3 | 41 | 73 | XVIII | 5/6 | 46 | 55 | 0 | XVII–XIX | XXVII–XXIII |
The holotype measures 53 mm body length with 80 segments; the three paratypes range in size from 39–41 mm (40.33±1.15 mm) body length with 71–74 segments (Table 3).
Tontong Waterfall, Nan province, Thailand, 19°12'35.9"N, 101°04'13.7"E, 1, 128 meters elevation (10th October 2009).
This species was named after the type locality, Tontong Waterfall.
The holotype (CUMZ 3206) and two paratypes (CUMZ 3207) are deposited in Chulalongkorn University, Museum of Zoology. Another paratype will be deposited in the Biozentrum Grindel und Zoologisches Museum, Hamburg, Germany (UHH).
Found in the top soil at about 10 cm depth, the soil surface covered with leaf litter of deciduous forest which originated at the Tontong Waterfall area. The soil was carefully dug close to surface casts. Most surrounding areas have been modified to agricultural fields.
Amynthas tontong sp. n. is a small sized terrestrial earthworm with a close indistinct pair of male pores with a pair of genital markings in intersegment 18/19. Spermathecae consists of a thumb shaped ampulla and a spherical terminal knob shaped diverticulum. Genital marking glands absent, first dorsal pore in 5/6, intestinal caeca simple, intestinal origin XV, septa 8/9/10 absent, testis sacs joined ventrally.
Amynthas tontong sp. n., along with Amynthas srinan sp. n. and Amynthas exiguus exiguus, is one of the smallest sized Amynthas ever recorded in Thailand. The basic external characters are easily seen in both newly collected and preserved materials. Compared with the two other closely related species from Laos, Amynthas chandyi Hong, 2008 and Amynthas namphouinensis Hong, 2008, which belong in the same zebrus-group, Amynthas chandyi is similar to Amynthas tontong sp. n. However, it differs in the specific details of the significant characters, such as the distance between the mp in Amynthas tontong sp. n. is 1.0 mm for the holotype and ranged from 1.0–1.2 mm (0.93±0.12 mm), while in Amynthas chandyi it ranged from 1.5–2.4 mm. The distance between the male pores as a fraction of the estimated circumference of the 18th segment is 0.15–0.19 in Amynthas tontong sp. n., but 0.14–0.32 in Amynthas chandyi. The arrangement of the genital markings of both species are totally different, and the distance between a pair of sp is also different, being 0.8–1.0 mm (1.1±0.1 mm) in Amynthas tontong sp. n. and 1.2–1.5 mm for Amynthas chandyi. Moreover, Amynthas tontong sp. n. has no genital markings near to the sp, whilst Amynthas chandyi exhibits circular genital markings in various locations, paired or single mid ventral in VII, VIII; usually 3 or 4 in total.
Alcohol-preserved paratype specimens of Amynthas tontong sp. n. belonged to a single DNA barcode cluster, with an intra-cluster divergence of 0.005 (N=3), and diverging from Amynthas phatubensis sp. n. by 0.294, and by 0.189 for an undescribed species. An undescribed morph at Tham Pha Tub diverged by 0.100, and may represent a subspecies. A consensus sequence is in Appendix 1.
urn:lsid:zoobank.org:act:C2BE17F8-A721-4736-9809-EF9ABDAB0C03
http://species-id.net/wiki/Amynthas_borealis
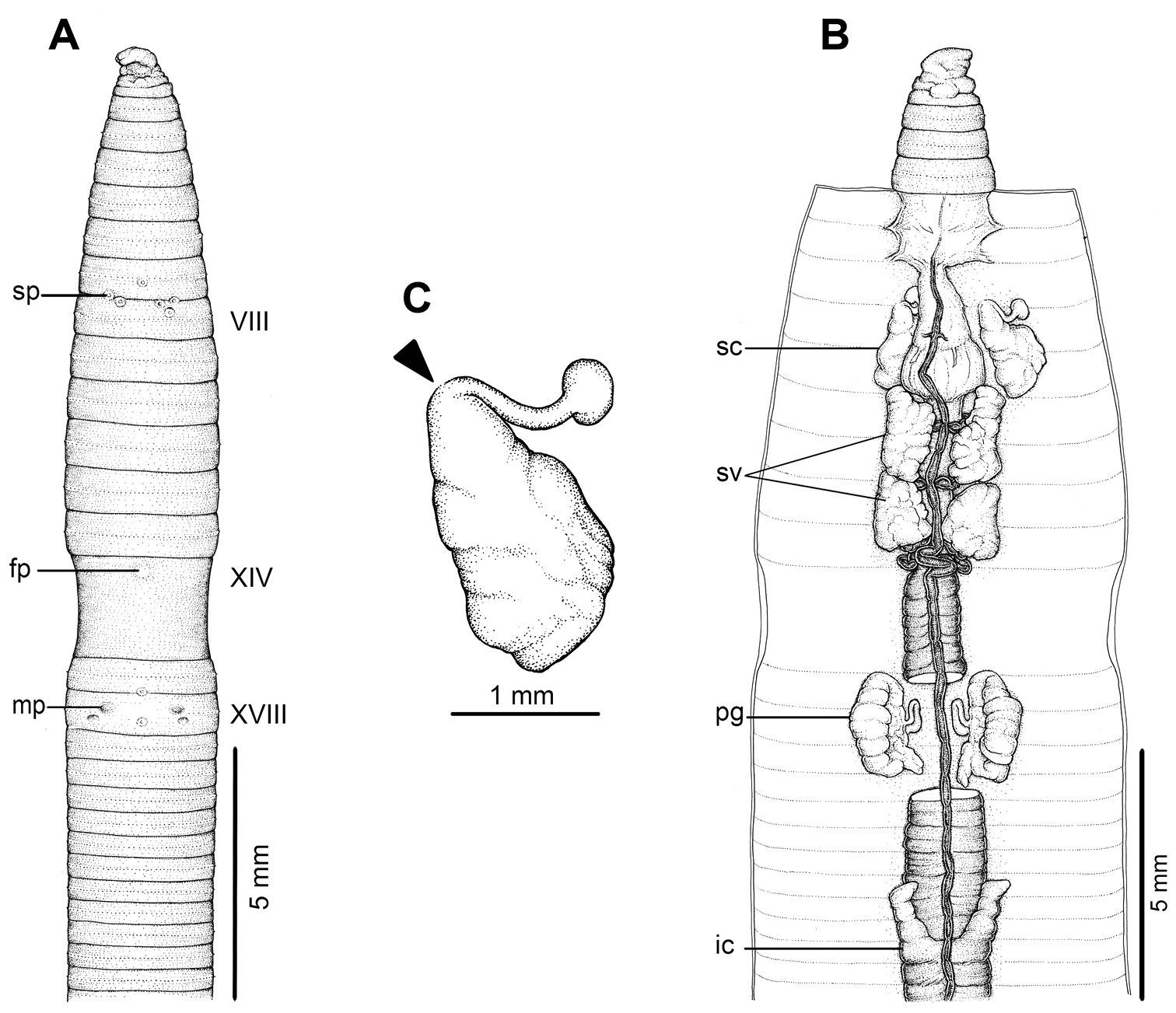
Figs 1, 4Dimensions; 54 mm by 3.5 mm at segment X, 3.8 at segment XX, 3.5 mm at clitellum; body cylindrical with 89 segments. Setae regularly distributed around segmental equators, numbering 39 at VII, 51 at XX, no visible setae between mp, setae formula AA:AB:ZZ:ZY= 2:1:1.5:1 at XIII. Single fp at XIV. Prostomium epilobic. First dorsal pore at 5/6. Clitellum annular XIV–XVI with no setae.
Mp pocket-like structures indistinctly occur in XVIII, 0.10 circumference apart ventrally; distance between mp 1.0 mm; porophores small, lip-like and surrounded by an elevated skin fold at medial pores, and there is a long ridge with a sharp posterior boundary traversing the body in front of the mp. Genital markings absent. Sp paired in 7/8 at 4th seta line, 0.10 circumference apart ventral; distance between sp 1.0 mm. Genital markings absent.
Septa 5/6 and 6/7 thick, 7/8 thin, 8/9 and 9/10 absent, 10/11–13/14 thin. Gizzard large within VIII–X, intestinal origin in XV, no lymph glands observed. Typhlosole small from XXVII. Ic originated from XXVII extending forward to XXV, simple finger-shape. Hearts esophageal in X–XIII. Holandric; testes and funnels in ventrally joined sacs in X–XI. Sv paired in XI–XII. Prostates in XVIII; prostatic ducts long slender bent in U-shape. Genital marking glands absent.
Ovaries at XIII. Sc one pair in VIII; ampulla large sac-shape, flattened by gizzard, narrow duct shorter than ampulla. Diverticulum with elongated tubular shape, stalk attached to duct near body wall, with no genital marking glands.
All the key morphological characters of the holotype and paratype specimens are given in Table 4.
External and internal morphology of holotype (CUMZ 3208) of Amynthas borealis sp. n. A External ventral view, B internal dorsal view and C spermatheca, and black arrow indicates the connection of the spermatheca and spermathecal pore.
Holotype and Paratype dimension and other morphological characteristics of Amynthas borealis Panha & Bantaowong, sp. n.
| Types \ Characters | Body length (mm) | Number of segments | Genital markings | First dorsal pore | Number of setae | Between male pore | Prostate glands | Intestinal caeca | |
|---|---|---|---|---|---|---|---|---|---|
| VII | XX | ||||||||
| HolotypeCUMZ 3208 | 54 | 89 | Absent | 5/6 | 39 | 51 | 0 | XVII–XIX | XXVII–XXV |
| ParatypeCUMZ 3209 | |||||||||
| 1 | 45 | 87 | Absent | 5/6 | 51 | 48 | 0 | XVII–XX | XXVII–XXIV |
| 2 | 42 | 78 | Absent | 5/6 | 49 | 45 | 0 | XVIII–XIX | XXVII–XXIII |
| 3 | 44 | 79 | Absent | 5/6 | 51 | 50 | 0 | XVII–XX | XXVII–XXIII |
| 4 | 42 | 86 | Absent | 5/6 | 54 | 41 | 0 | XVIII–XIX | XXVII–XXIV |
| 5 | 44 | 85 | Absent | 5/6 | 40 | 40 | 0 | XVIII–XIX | XXVII–XXIV |
| 6 | 42 | 85 | Absent | 5/6 | 46 | 48 | 0 | XVII–XIX | XXVII–XXIV |
| 7 | 42 | 77 | Absent | 5/6 | 44 | 50 | 0 | XVII–XX | XXVII–XXV |
| 8 | 42 | 83 | Absent | 5/6 | 48 | 52 | 0 | XVII–XIX | XXVII–XXV |
The holotype measures 54 mm body length with 89 segments; the eight paratypes range in size from 42–45 mm (42.87±1.25 mm) body length with 77–87 segments (Table 4).
Chaloemprakiat district, Nan province, Thailand, 19°34'48.5"N, 101°04'53.1"E, 513 meters elevation (7th August 2010).
The specific epithet “borealis” derived from Latin word “boreal” mean “north”. This name refers to the location of type locality in the north of Thailand.
The holotype (CUMZ 3208) and seven paratypes (CUMZ 3209) are deposited in Chulalongkorn University, Museum of Zoology. Another two paratypes will be deposited in the Biozentrum Grindel und Zoologisches Museum, Hamburg, Germany (UHH), and another two paratypes in the Natural History Museum, London (NHM).
Found in the top soil at about 10 cm depth, the soil surface covered with the leaf litter of a deciduous limestone forest, mostly disturbed. The soil was carefully dug close to the casts.
Amynthas borealis sp. n. is a small sized terrestrial earthworm small male pores, a transverse ridge anterior to the male pores in XVII, and no genital markings. One pair of sc in VIII, each spermathecae consists of a large sac-shaped ampulla and elongated tubular shaped diverticulum. Testis sacs joined ventrally, intestinal origin XV, intestinal caeca simple, first dorsal pore in 5/6.
Amynthas borealis sp. n. is one of the smaller Amynthas. The characteristic male field is difficult to see in newly collected specimens but can be clearly observed after preservation. Compared with the two other closely related species from Laos, Amynthas chandyi and Amynthas namphouinensis, which belong in the same zebrus-group, Amynthas chandyi is similar to Amynthas borealis sp. n. However, distinctive differences include the distance between mp of the new species, being 1.0 mm in the holotype with a range of 0.8–1.0 mm (0.95±0.09 mm) in Amynthas borealis sp. n. compared to 1.5–2.4 mm. The distance between the male pores as a fraction of the estimated circumference of the 18th segment is 0.10–0.14 in Amynthas borealis sp. n., but 0.14–0.32 in Amynthas chandyi. There are no genital markings in the new species; the distance between a pair of sp is also different, being 0.5–1.0 mm (0.9±0.19 mm) in the new species compared to 1.2–1.5 mm for Amynthas chandyi. Moreover, Amynthas borealis sp. n. has no genital marking glands at all, whilst Amynthas chandyi exhibits circular genital markings in various locations, paired or single mid ventral in VII and VIII; usually 3 or 4 in total.
urn:lsid:zoobank.org:act:C3EC91E6-B29A-4C72-908F-1858DE7F21DA
http://species-id.net/wiki/Amynthas_srinan
Figs 1, 5Dimensions; 47 mm by 1.8 mm at segment X, 2.3 at segment XX, 2.3 mm at clitellum; body cylindrical with 77 segments. Setae regularly distributed around segmental equators, numbering 36 at VII, 42 at XX, four between mp, setae formula AA:AB:ZZ:ZY= 1.5:1:2:1 at XIII. Single fp at XIV. Prostomium epilobic with tongue open. First dorsal pore at 4/5 or 5/6. Clitellum annular XIV–XVI with no setae.
Mp on circular porophores in XVIII, 0.30 circumference apart ventrally; distance between mp 1.5 mm. Genital markings small, postsetal, closely paired near mid ventral of XVII and XVIII. Sp paired in 7/8 at 6th setal lines, 0.26 circumference apart ventrally; distance between sp 1.5 mm. Genital markings tiny, closely paired on near mid ventral of VII and VIII.
Septa 5/6 and 6/7 thick, 7/8 thin, 8/9 and 9/10 absent, 10/11–13/14 thin. Gizzard globular within VIII–X, intestinal origin in XV, no lymph glands observed. Typhlosole small from XXVII. Ic originated from XXVII extending forward to XXIII, long finger-shape. Hearts esophageal in X–XIII. Holandric; testes and funnels in ventrally joined sacs in X–XI. Sv paired in XI–XII. Prostates in XVIII, extending between XVII–XX; prostatic ducts tightly folded twice. Genital marking glands paired in XVII and XVIII corresponding to external genital papillae, each consisting of a stalk with terminal multi-lobed glandular part.
Ovaries in XIII. Sc one pair in VIII; ampulla oval to kidney-shaped, with stout duct shorter than ampulla. Diverticulum with oval bulb terminal, stalk attached to duct near body wall. Genital markings stalked, corresponding to external genital papillae; each gland small consisting of a stalk with terminal multi-lobed glandular part.
All the key morphological characters of the holotype and paratype specimens are given in Table 5.
External and internal morphology of holotype (CUMZ 3210) of Amynthas srinan sp. n. A External ventral view, B internal dorsal view and C spermatheca, and black arrow indicates the connection of the spermatheca and spermathecal pore.
Holotype and Paratype dimension and other morphological characteristics of Amynthas srinan Panha & Bantaowong, sp. n.
| Types \ Characters | Body length (mm) | Number of segments | Location of genital markings | First dorsal pore | Number of setae | Between male pore | Prostate glands | Intestinal caeca | ||
|---|---|---|---|---|---|---|---|---|---|---|
| preclitellum | postclitellum | VII | XX | |||||||
| HolotypeCUMZ 3210 | 47 | 77 | VII, VIII | XVII, XVIII | 5/6 | 36 | 42 | 4 | XVII–XX | XXVII–XXIII |
| ParatypeCUMZ 3211 | ||||||||||
| 1 | 35 | 75 | VII, VIII | XVII, XVIII | 5/6 | 40 | 42 | 6 | XVII–XX | XXVII–XXV |
| 2 | 44 | 76 | VII, VIII | XVII, XVIII | 5/6 | 36 | 42 | 5 | XVII–XX | XXVII–XXIV |
| 3 | 39 | 65 | VII, VIII | XVII, XVIII | 5/6 | 37 | 46 | 4 | XVIII–XX | XXVII–XXIV |
| 4 | 44 | 70 | VII, VIII | XVII, XVIII | 5/6 | 36 | 49 | 5 | XVII–XIX | XXVII–XXIV |
| 5 | 47 | 78 | VII, VIII | XVII, XVIII | 5/6 | 38 | 45 | 4 | XVII–XX | XXVII–XXIV |
| 6 | 37 | 68 | VII, VIII | XVII, XVIII | 5/6 | 40 | 44 | 4 | XVII–XX | XXVII–XXV |
| 7 | 38 | 77 | VII, VIII | XVII, XVIII | 4/5 | 43 | 48 | 5 | XVII–XXI | XXVII–XXIV |
| 8 | 37 | 52 | VII, VIII | XVII, XVIII | 4/5 | 38 | 42 | 4 | XVII–XXI | XXVII–XXV |
| 9 | 35 | 57 | VII, VIII | XVII, XVIII | 4/5 | 41 | 44 | 4 | XVII–XX | XXVII–XXIV |
| 10 | 38 | 78 | VII, VIII | XVII, XVIII | 5/6 | 36 | 40 | 4 | XVII–XX | XXVII–XXIV |
| 11 | 42 | 77 | VII, VIII | XVII, XVIII | 4/5 | 42 | 47 | 4 | XVII–XXI | XXVII–XXIII |
| 12 | 45 | 77 | VII, VIII | XVII, XVIII | 5/6 | 39 | 45 | 5 | XVII–XX | XXVII–XXIV |
| 13 | 40 | 77 | VII, VIII | XVII, XVIII | 5/6 | 40 | 48 | 4 | XVII–XIX | XXVII–XXV |
| 14 | 39 | 77 | VII, VIII | XVII, XVIII | 5/6 | 39 | 47 | 4 | XVII–XX | XXVII–XXIV |
| 15 | 43 | 77 | VII, VIII | XVII, XVIII | 5/6 | 40 | 44 | 4 | XVII–XX | XXVII–XXIII |
| 16 | 40 | 75 | VII, VIII | XVII, XVIII | 5/6 | 41 | 49 | 4 | XVII–XX | XXVII–XXIV |
| 17 | 37 | 75 | VII, VIII | XVII, XVIII | 4/5 | 36 | 46 | 4 | XVII–XIX | XXVII–XXIV |
| 18 | 36 | 60 | VII, VIII | XVII, XVIII | 5/6 | 40 | 47 | 5 | XVII–XX | XXVII–XXIV |
| 19 | 39 | 75 | VII, VIII | XVII, XVIII | 5/6 | 37 | 44 | 4 | XVII–XX | XXVII–XXIII |
| 20 | 47 | 78 | VII, VIII | XVII, XVIII | 4/5 | 36 | 42 | 4 | XVII–XX | XXVII–XXV |
| 21 | 42 | 71 | VII, VIII | XVII, XVIII | 5/6 | 40 | 46 | 4 | XVII–XX | XXVII–XXIV |
| 22 | 35 | 56 | VII, VIII | XVII, XVIII | 4/5 | 41 | 43 | 4 | XVII–XIX | XXVII–XXIV |
| 23 | 36 | 69 | VII, VIII | XVII, XVIII | 5/6 | 36 | 45 | 4 | XVII–XX | XXVII–XXV |
| 24 | 42 | 73 | VII, VIII | XVII, XVIII | 5/6 | 36 | 46 | 4 | XVII–XX | XXVII–XXIV |
| 25 | 44 | 76 | VII, VIII | XVII, XVIII | 4/5 | 39 | 47 | 6 | XVI–XX | XXVII–XXV |
| 26 | 35 | 69 | VII, VIII | XVII, XVIII | 5/6 | 36 | 44 | 4 | XVII–XIX | XXVII–XXIII |
| 27 | 38 | 75 | VII, VIII | XVII, XVIII | 5/6 | 37 | 45 | 4 | XVII–XX | XXVII–XXIV |
| 28 | 35 | 78 | VII, VIII | XVII, XVIII | 5/6 | 39 | 44 | 4 | XVII–XIX | XXVII–XXV |
The holotype measures 47 mm body length with 77 segments and the first dorsal pore located at 5/6; the twenty eight paratypes range in size between 35–47 mm (39.75±4.27 mm) body length with 52–78 segments, and first dorsal pore at 4/5 (8 samples) or 5/6 (20 samples) (Table 5).
Srinan National Park, Nan province, Thailand, 18°22'11.1"N, 100°50'23.2"E, 607 meters elevation (30th September 2010).
This species was named after the type locality Srinan National Park.
The holotype (CUMZ 3210) and 25 paratypes (CUMZ 3211) are deposited in Chulalongkorn University, Museum of Zoology. Another five paratypes will be deposited in the Biozentrum Grindel und Zoologisches Museum, Hamburg, Germany (UHH), and four paratypes in the Natural History Museum, London (NHM).
Found in the top soil at about 10 cm depth, the soil surface covered with leaf litters of deciduous forest. The soil was carefully dug close to the castes.
Amynthas srinan sp. n. is the smallest Amynthas ever collected in Thailand. Male pores on distinct round porophores, genital markings paired near mid ventral of VII, VIII, XVII and XVIII; each with genital marking glands. Each spermathecae consists of a kidney-shaped ampulla and an oval shaped diverticulum. Testes sacs ventrally joined, intestinal origin XV, intestinal caeca simple, first dorsal pores at 4/5 or 5/6.
Amynthas srinan sp. n., along with Amynthas exiguus exiguus and Amynthas tontong sp. n., is one of if not the smallest Amynthas recorded so far. It has external characteristics which are easily seen in both newly collected and preserved materials. Compared with the two other closely related species from Laos, Amynthas chandyi and Amynthas namphouinensis, which belong in the same zebrus-group, Amynthas chandyi is very similar in appearance to Amynthas srinan sp. n. However, they clearly differ in certain specific details of their significant characters, such as the distance between the mp which in Amynthas srinan sp. n. is 1.5 mm for holotype and ranged from 1.5–2.0 mm (1.41±4.27 mm), while in Amynthas chandyi this ranged from 1.5–2.4 mm. The distance between the male pores as a fraction of the estimated circumference of the 18th segment is 0.24–0.30 in Amynthas srinan sp. n., and 0.14–0.32 in Amynthas chandyi. This is not convincing as a diagnostic difference, because there is significant overlap with the highly variable Amynthas chandyi. In addition, although genital markings are clearly observed in both Amynthas chandyi and Amynthas srinan sp. n. on the sc and mp areas, Amynthas srinan sp. n. has a much larger number and different arrangement of such markings. The distance between pairs of sp is quite similar, being 1.5–2.0 mm (1.34±2.31mm) in Amynthas srinan sp. n. and 1.2–1.5 mm in Amynthas chandyi.
The genus Amynthas
is widely distributed in the Asian continent, where it is one of the
dominant genera. In Thailand it occurs in various types of lowland
forest habitats, dry evergreen, moist evergreen, deciduous and
limestone forests, encompassing diverse soil pH values, from acidic to
alkali soils (
The four new species range in size, with respect to other Amynthas members, from moderate to very small, of which Amynthas phatubensis sp. n. is the longest. The other three species are almost the same size and close to the two Laotian species, as shown in Table 6.
However, the spermathecae (sc) and genital marking locations of the
four new species are clearly different from the two closely related Laos
species. The four new Amynthas species described here belong to the zebrus-group, as defined by
Morphological characteristics for between these four new species and two know species from Laos
| Characters | Amynthas phatubensis sp. n. | Amynthas tontong sp. n. | Amynthas borealis sp. n. | Amynthas srinan sp. n. | Amynthas namphouinensis | Amynthas chandyi |
|---|---|---|---|---|---|---|
| Body length (mm) | 80–148 | 39–53 | 42–54 | 35–47 | 63–92 | 29–58 |
| Number of segments | 85–112 | 71–80 | 78–89 | 56–77 | 92–94 | 48–52 |
| First dorsal pore | 5/6 | 5/6 | 5/6 | 4/5, 5/6 | 4/5, 5/6, 6/7 | 5/6 |
| Setae number | ||||||
| VII | 51–64 | 41–46 | 39–54 | 36–45 | 52–61 | 44–54 |
| XX | 58–68 | 52–55 | 40–52 | 42–49 | 53–58 | 44–57 |
| between male pores | 9–15 | 0 | 0 | 4–6 | 0–7 | 0–7 |
| Preclitellar genital markings | ||||||
| VII | 2 | 0 | 0 | 2 | 0 | 1–2 |
| VIII | 1–7 | 0 | 0 | 2 | 0 | 1–2 |
| IX | 0–1 | 0 | 0 | 0 | 0 | 0 |
| Postclitellar genital markings | ||||||
| XVII | 0–2 | 0 | 0 | 2 | 2 | 1 |
| XVIII | 6–12 | 2 | 0 | 2 | 0 | 3 |
| XIX | 0–1 | 0 | 0 | 0 | 4 | 1 |
| XX | 0–1 | 0 | 0 | 0 | 0 | 1 |
| Prostate glands | XVII–XX | XVII–XX | XVII–XX | XVII–XX | XVII–XIX | XVI–XXI |
| Genital marking glands | sessile at VII, VIII | Absent | absent | stalked | sessile at XVII–XIX | absent |
| Intestinal caeca | simple, XXVII–XXIII | simple, XXVII–XXV | simple, XXVII–XXV | simple, XXVII–XXII | simple, XXVII–XXIV | simple, XXVII–XXIV |
External and internal morphology of holotype (BDNUL 0001) of Amynthas namphouinensis Hong, 2008 A External ventral view, B internal dorsal view and C spermatheca, and black arrow indicates the connection of the spermatheca and spermathecal pore.
External and internal morphology of holotype (BDNUL 0002) of Amynthas chandyi Hong, 2008 A External ventral view, B internal dorsal view and C spermatheca, and black arrow indicates the connection of the spermatheca and spermathecal pore.
Amynthas phatubensis sp. n. is the only species that lives in limestone habitats in leaf litter and also in shallow mild alkali topsoil. The soil humidity can be quite low and is of a clay loam structure. The other three species are smaller in size and were found in almost harder, muddy sandy clay substrates. Amynthas tontong sp. n. lives in deeper soil of a high humidity around waterfalls. Amynthas borealis sp. n. and Amynthas srinan sp. n. are found in deciduous forests, which have mostly been modified as agricultural fields. The soil is drier and harder. The genital marking glands of Amynthas phatubensis sp. n. and Amynthas srinan sp. n. are distinct from other two species (Table 6 and Figs 2–5), whilst Amynthas tontong sp. n. show two postclitellar genital markings that are absent in Amynthas borealis (Figs 3 and 4) The diagnostic differences are shown in the dichotomous key to the sixteen Thai and two Laotian Amynthas species, below.
The zebrus-group is composed of eleven nominal species: Metaphire hilgendorfi (Michaelsen, 1892), Amynthas palmosus (Chen, 1946), Amynthas magnipapillatus (Qui and Wang, 1992), Amynthas zebrus (Benham, 1896), Amynthas culminus Michaelsen, 1899, Amynthas principalis (Michaelsen, 1932), Amynthas xuongmontis (Thai & Samphon, 1990), Amynthas fasciculus (Qui, Wang & Wang, 1993), Amynthas heaneyi James, 2004, Amynthas namphouinensis Hong, 2008 and Amynthas chandyi Hong, 2008. Within the zebrus-group, the first three species show manicate intestinal caeca, while the current newly described four species have simple finger-shaped intestinal caeca. The three latter nominal species are longer in body length (200–300 mm) compared with the size of these four new species which ranged from 35–148 mm. Amynthas heaneyi can be distinguished by its proandric character (James, 2004), while the four new described species are holandric. Amynthas fasciculus has coiled and kinked spermathecae, whereas Amynthas phatubensis sp. n. has large ovate ampulla, Amynthas tontong sp. n. has thumb shaped ampulla, Amynthas borealis sp. n. has sac-shape ampulla, and Amynthas srinan sp. n. has oval to kidney-shaped ampulla. Amynthas xuongmontis clearly differs from these four new species in the genital marking located on XVIII, whereas located on VII, VIII, XVII, XVIII in Amynthas phatubensis sp. n., located between 18/19 in Amynthas tontong sp. n., absent in Amynthas borealis sp. n. and located on VII, VIII, XVII, XVIII in Amynthas srinan sp. n.
Key to Thai and two Laos species of Amynthas| 1 | First spermathecal pores at 5/6 | 2 |
| – | First spermathecal pores after 5/6 | 12 |
| 2 | Two pairs of spermathecal pores | Amynthas morrisi |
| – | More than two pairs of spermathecal pores | 3 |
| 3 | Three pairs of spermathecal pores | 4 |
| – | More than three pairs of spermathecal pores | 6 |
| 4 | Genital markings absent | Amynthas defecta |
| – | Genital markings present | 5 |
| 5 | Genital markings clustered on XVIII | Amynthas gracilis |
| – | Genital markings transverse rows on XVII, XVIII, XIX | Amynthas papulosus |
| 6 | Genital markings absent | 7 |
| – | Genital markings present | 8 |
| 7 | Body length 1 meter or more | Amynthas mekongianus |
| – | Body length less than 300 mm | Amynthas alexandri |
| 8 | Genital marking glands absent | 9 |
| – | Genital marking glands present | 10 |
| 9 | Genital markings located on 17/18, 18/19 | Amynthas exiguus austrinus |
| – | Genital markings located on VII, VIII, XIX, XX | Amynthas exiguus exiguus |
| 10 | Intestinal caeca, simple | 11 |
| – | Intestinal caeca, manicate | Amynthas manicatus decorosus |
| 11 | Genital markings, paired at 18/19, 19/20, 20/21 | Amynthas longicauliculatus |
| – | Genital markings, three trios at 18/19, 19/20, 20/21 | Amynthas comptus |
| 12 | First spermathecal pores at 6/7 | 13 |
| – | First spermathecal pores after 6/7 | 14 |
| 13 | Genital markings located on 17/18, 18/19 | Amynthas fucosus |
| – | Genital marking located on XVIII | Amynthas siam |
| 14 | Body length more than 200 mm | Amynthas hupbonensis |
| – | Body length less than 200 mm | 15 |
| 15 | Genital markings absent | Amynthas borealis sp. n. |
| – | Genital markings present | 16 |
| 16 | Preclitellar genital markings absent | 17 |
| – | Preclitellar genital markings present | 18 |
| 17 | Genital marking glands absent | Amynthas tontong sp. n. |
| – | Genital marking glands present | Amynthas namphouinensis |
| 18 | Genital marking glands absent | Amynthas chandyi |
| – | Genital marking glands, present | 19 |
| 19 | Genital marking glands, sessile | Amynthas phatubensis sp. n. |
| – | Genital marking glands, stalked | Amynthas srinan sp. n. |
This project was the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission FW646A (2011–2013) to SP, and from Science for Locale Project, Faculty of Science, Chulalongkorn University. We are grateful to Khamla Inkhavilay for kindly permitting us to study the type specimens and relevant reference material of National University of Laos at Vientiane. Thanks also to Thita Krutchuen for excellent drawings, and to all members of the Animal Systematics Research Unit, Chulalongkorn University for assistance in collecting material.
DNA barcode sequences for Amynthas phatubensis sp. n. and Amynthas tontong sp. n. Positions with variable base are indicated by the appropriate ambiguity code: Y= C or T, R= A or G, K= G or T, M= A or C.
The primer sets used, LCO1490 and HCO2198, amplify a 658 bp fragment of the COI gene in a wide range of invertebrate taxa (
LCO1490: 5’-GGTCAACAAATCATAAAGATATTGG-3’
HCO2198: 5’-TAAACTTCAGGGTGACCAAAAAATCA-3’
Consensus of 8 sequences of Amynthas phatubensis sp. n. Paratype CUMZ 3212, GenBank Accession No. HM901031-HM901038.
AACCCTATATTTCATTTTAGGTATTTGAGCCGGTATGATTGGATCTGGAATAAGCCTACTYATCCGAATTGARTT
GAGCCAACCTGGATCCTTCCTAGGCAGAGATCAGCTATACAATACCATTGTTACAGCTCATGCATTCTTAATAAT
YTTCTTTTTAGTTATACCCGTATTTATTGGGGGTTTTGGAAACTGATTAYKACCACTTATACTTGGRGCGCCAGA
TATRGCCTTTCCYCGACTAAACAACATAAGATTCTGATTGCTTCCTCCRTCRCTTATTCTATTAGTAAGCTCTGC
GGCCGTRGAAAAGGGGGCYGGCACTGGATGAACTGTTTACCCMCCCCTAGCTAGAAATGTRGCCCACGCAGGGCC
TTCAGTAGATTTAGCTATTTTCTCACTTCATTTAGCAGGAGCTTCATCTATTTTAGGGGCAATTAATTTCATTAC
AACTGTAATTAATATGCGATGATCCGGACTACGTCTAGAGCGTATCCCACTATTTGTATGGGCCGTRGTTATCAC
AGTAGTACTATTATTACTTTCCCTRCCTGTATTAGCAGGGGCTATCACTATACTACTAACTGAYCGTAATCTAAA
CACATCATTTTTTGATCCTGCTGGGGGCGGCGACCCCATTCTATATCAACACCTA
Consensus of 3 sequences of Amynthas tontong sp. n. Paratype CUMZ 3207, GenBank Accession No. HQ562073-HQ562076.
AACCCTATACTTCATTTTAGGAATTTGAGCTGGAATAATTGGAGCAGGAATAAGACTCCTTATTCGAATTGAGYT
AAGACAGCCCGGATCATTCCTAGGAAGYGATCAACTATACAATACCATTGTTACAGCCCATGCATTCTTAATAAT
TTTCTTCCTRGTAATGCCAGTATTTATTGGGGGCTTTGGAAATTGATTACTTCCACTAATGTTGGGGGCCCCTGA
CATAGCTTTCCCACGACTAAATAATATGAGATTTTGACTACTTCCACCATCATTAATCCTTTTAGTTAGATCCGC
RGCCGTTGAAAAAGGTGCGGGGACAGGATGAACTGTATACCCACCACTAGCAAGGAACATTGCCCATGCTGGCCC
ATCTGTAGATTTAGCAATTTTCTCACTACACTTGGCGGGGGCATCCTCAATCCTGGGGGCTATTAACTTCATTAC
CACAGTAATTAATATGCGATGATCTGGGCTGCGCTTAGAACGAATCCCCCTATTTGTATGAGCTGTAGTAATTAC
AGTAGTACTCCTACTACTATCTTTGCCCGTGCTGGCGGGAGCCATTACAATACTCTTAACAGATCGAAATCTTAA
TACATCATTCTTCGACCCTGCTGGCGGGGGCGACCCTATTCTATACCAGCACCTG