(C) 2010 Pavel Stoev et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The centipede genus Eupolybothrus Verhoeff, 1907 in North Africa is revised. A new cavernicolous species, Eupolybothrus kahfi Stoev & Akkari, sp. n., is described from a cave in Jebel Zaghouan, northeast Tunisia. Morphologically, it is most closely related to Eupolybothrus nudicornis (Gervais, 1837) from North Africa and Southwest Europe but can be readily distinguished by the long antennae and leg-pair 15, a conical dorso-median protuberance emerging from the posterior part of prefemur 15, and the shape of the male first genital sternite. Molecular sequence data from the cytochrome c oxidase I gene (mtDNA–5’ COI-barcoding fragment) exhibit 19.19% divergence between Eupolybothrus kahfi and Eupolybothrus nudicornis, an interspecific value comparable to those observed among four other species of Eupolybothrus which, combined with a low intraspecific divergence (0.3–1.14%), supports the morphological diagnosis of Eupolybothrus kahfi as a separate species. This is the first troglomorphic myriapod to be found in Tunisia, and the second troglomorph lithobiomorph centipede known from North Africa. Eupolybothrus nudicornis is redescribed based on abundant material from Tunisia and its post-embryonic development, distribution and habitat preferences recorded. Eupolybothrus cloudsley-thompsoni Turk, 1955, a nominal species based on Tunisian type material, is placed in synonymy with Eupolybothrus nudicornis. To comply with the latest technological developments in publishing of biological information, the paper implements new approaches in cybertaxonomy, such as fine granularity XML tagging validated against the NLM DTD TaxPub for PubMedCentral and dissemination in XML to various aggregators (GBIF, EOL, Wikipedia), vizualisation of all taxa mentioned in the text via the dynamically created Pensoft Taxon Profile (PTP) page, data publishing, georeferencing of all localities via Google Earth, and ZooBank, GenBank and MorphBank registration of datasets. An interactive key to all valid species of Eupolybothrus is made with DELTA software.
Eupolybothrus kahfi sp. n., Eupolybothrus nudicornis, North Africa, barcoding, cytochrome c oxidase I gene, troglomorphism, habitat preferences, interactive key, cybertaxonomy, semantic tagging, semantic enhancements
The lithobiid subfamily Ethopolyinae
is represented in Europe and Africa by a single genus, Eupolybothrus
Verhoeff, 1907, which currently comprises around 20 valid species as
well as a few poorly known species and subspecies, collectively
arranged in seven subgenera (
The identity of Eupolybothrus nudicornis
has been a subject of controversy for more than a century. The
polymorphic external anatomy shown by the species throughout its broad
geographic range led to the description of several morphologically
similar taxa that were sometimes based only on a single type specimen (
The aim of present paper is to put on record all North African material of Eupolybothrus amassed during recent years and also found in old collections of different European museums. We redescribe Eupolybothrus nudicornis and describe a new species discovered in a cave in Tunisia. The new species is distinguished from the nearest congener morphologically as well as using the cytochrome c oxidase I gene (mtDNA–5’ COI-barcoding fragment). We also discuss the morphological variability and post-embryonic development of Eupolybothrus nudicornis and provide an overview of its habitat preferences and distribution in Africa. We outline some of the existing taxonomic problems in the genus Eupolybothrus and provide a key to all currently valid species of the genus.
Historical account. The earliest record of the genus Eupolybothrus
in North Africa was made by Carl Ludwig Koch (
In 1892 Pocock recorded Lithobius impressus
from Kherrarta, Alger, Constantine and Hammam Ri’irha in Algeria,
and from Tunis in Tunisia (
The first and hitherto only record of the genus in Libya comes from
Collections. Unless stated otherwise, the material treated herein has been
collected from Tunisia in March 2008 and March 2009 by N. Akkari, P.
Stoev and H. Enghoff, and also in the course of individual excursions
of N. Akkari to different regions of the country in the period
2003-2008. The material is preserved in 70% or 96% ethanol and is shared
between the Field Museum of Natural History, Chicago (FMNH), National
Museum of Natural History, Sofia (NMNHS), Natural History Museum of
Denmark, Copenhagen (ZMUC) and Biodiversity Institute of Ontario,
Guelph (BIO). Additional type and non-type specimens of Eupolybothrus
from North Africa housed in the Hungarian Natural History Museum
(HNHM), the Natural History Museum, London (NHM), ZMUC and the
private collection of Marzio Zapparoli (CMZ) were also incorporated in
the present study. Photos were taken mainly with a Leica DFC 420 digital
camera mounted on a Leica MZ16A stereomicroscope, and were processed
using the program Automontage Pro software (Syncroscopy, Cambridge,
UK) for image-stacking 3D focus expansion. Terminology for external
anatomy follows
Molecular methods. Eleven specimens from 5 species were used for genetic examination
of the divergence among species of the genus. Ten specimens that sample 4
species were barcoded in the context of a global campaign on Myriapoda
initiated as a part of the ‘Barcode of Life’ project (iBOL WorkGroup 1.9
‘Terrestrial surveillance’) (Appendix C doi:10.3897/zookeys.50.504-app.C).
To this dataset we added a sequence from GenBank for a fifth species, Eupolybothrus fasciatus
(AY214420) (
Lysis of the tissues was carried out in 50 µl volume of lysis
buffer and proteinase K incubated at 56°C overnight. DNA extraction
followed a standard automated protocol on 96-well glass fibre plates (
Cybertaxonomy. The present paper demonstrates several innovative methods of
semantic tagging and semantic enhancements, text and data processing,
publishing and dissemination in taxonomy, described in more detail in a
forum paper published in the same issue (
All 70 images included in this publication have been deposited in MorphBank (Appendix D doi:10.3897/zookeys.50.504-app.D). All the revised species were registered in ZooBank and Life Science Identifiers (LSID) were assigned to them. Accession numbers were obtained from BOLD (see Appendix C doi:10.3897/zookeys.50.504-app.C for complete metadata) and GenBank for all COI gene sequences. Datasets in spreadsheet format for specimen localities have been shared with the Global Biodiversity Information Facility (GBIF, http://www.gbif.org) via Appendix A doi:10.3897/zookeys.50.504-app.A. To illustrate all records of the species in North Africa interactively in Google Earth, KML files were generated and are available for download as Appendix E doi:10.3897/zookeys.50.504-app.E. The interactive key for identification of all currently valid species of Eupolybothrus was made with DELTA software http://delta-intkey.com (Appendix F doi:10.3897/zookeys.50.504-app.F).
Abbreviations: OD – original description; RD – redescription; K – key; FR – faunistic record; CHL – checklist or catalogue; BD – biological data.
T/TT – Tergite/Tergites, C – Coxa, Tr – Trochanter, PFe – Prefemur, Fe – Femur, Ti – Tibia; Letters a, m, p stand for spines in anterior, medial and posterior positions, respectively; those in brackets indicate the variable spines.
LI, II, III, IV stand for larval stadia 1, 2, 3, 4, respectively. PLI, II, III, etc. stand for post-larval stadia 1, 2, 3, etc. subad. = subadult; juv. – juvenile.
Results Taxonomic account of the currently valid African species of EupolybothrusOrder Lithobiomorpha Pocock, 1895
Family Lithobiidae Newport, 1844
Subfamily Ethopolyinae Chamberlin, 1915
urn:lsid:zoobank.org:act:9A79187E-FC75-4CB7-B634-ABBD4B471D2A
Lithobius grossipes C.L. Koch, 1847, by subsequent designation of Chamberlin (1925). Type locality: Triest, Italy.
Medium- to large-sized Lithobiidae (body length 16–48 mm) with numerous irregularly arranged pores on the last four pairs of coxae; antennal articles always more than 20, from 38 to around 80; ocelli numerous, usually from 1+16 to 1+24, absent or reduced in some troglobitic species; porodont situated laterad to the forcipular coxosternal teeth; tergites with or without posterior triangular projections, tarsi of all legs bipartite; forcipular coxosternite with 5+5–14+14 teeth (usually from 7+7 to 10+10); female gonopod with 2 spurs and simple claw, male gonopods single or bipartite.
Eupolybothrus (Eupolybothrus) andreevi Matic, 1964, Eupolybothrus (Schizopolybothrus) caesar (Verhoeff, 1899), Eupolybothrus (Propolybothrus) dolops Zapparoli, 1998, Eupolybothrus (Schizopolybothrus) excellens (Silvestri, 1894), Eupolybothrus (Eupolybothrus) fasciatus (Newport, 1845), Eupolybothrus (Eupolybothrus) gloriastygis (Absolon, 1916), Eupolybothrus (Eupolybothrus) grossipes (C.L. Koch, 1847), Eupolybothrus kahfi Stoev & Akkari sp. n., Eupolybothrus (Parapolybothrus) herzegowinensis (Verhoeff, 1900), Eupolybothrus (Parapolybothrus) imperialis (Meinert, 1872), Eupolybothrus (Schizopolybothrus) leostygis (Verhoeff, 1899), Eupolybothrus (Eupolybothrus) litoralis (L. Koch, 1867), Eupolybothrus (Eupolybothrus) longicornis (Risso, 1826), Eupolybothrus (Allopolybothrus) nudicornis (Gervais, 1837), Eupolybothrus (Parapolybothrus) obrovensis (Verhoeff, 1930), Eupolybothrus (Mesobothrus) transsylvanicus (Latzel, 1882), Eupolybothrus (Schizopolybothrus) tabularum Verhoeff, 1937, Eupolybothrus (Leptopolybothrus) tridentinus (Fanzago, 1874), Eupolybothrus (Propolybothrus) werneri (Attems, 1902), Eupolybothrus (Mesobothrus) zeus (Verhoeff, 1901).
Eupolybothrus (Schizopolybothrus) acherontis (Verhoeff, 1900), Eupolybothrus acherontis wardaranus (Verhoeff, 1937), Eupolybothrus (Mesobothrus) macedonicus (Verhoeff, 1943), Eupolybothrus osellai Matic, Floca & Hurezeanu, 1992, Eupolybothrus ruffoi Matic, Floca & Hurezeanu, 1992, Eupolybothrus sketi Matic, 1979, Eupolybothrus (Schizopolybothrus) spiniger (Latzel, 1888), Eupolybothrus (Schizopolybothrus) stygis (Folkmanova, 1940), Eupolybothrus valkanovi (Kaczmarek, 1973), Eupolybothrus (Propolybothrus) verrucosus (Sseliwanoff, 1876).
Several taxa assigned to Eupolybothrus
remain species inquirendae. Here we briefly review the current
status of these taxa. Eupolybothrus stygis
was described from Iljina pećina (cave) near Trebinje in Bosnia and
Herzegovina (
urn:lsid:zoobank.org:act:3C17C879-5D17-470E-BD13-C07E14F44534
Figs 1–2Type material of Eupolybothrus cloudsley-thompsoni: 7 ♂♂, 2 ♀♀, 1 juv. of Eupolybothrus cloudsley-thompsoni + 1 juv. Lithobius castaneus Newport, 1844, Tunisia, Roman aqueduct 15 miles south of Tunis, 4.IV.1954, Cloudsley-Thompson leg. Turk Collection, Syntypes. Last pair of legs mounted on a slide, Turk collection 1984.10.1.77 (NHM).
Nontype material: SPAIN: 3 ♂♂, 3 ♀♀, 5 subad. ♂♂, 3 subad. ♀♀, 2 juv., labelled “ Lithobius impressus Granada Meinert” and “ Eupolybothrus impressus (Newport) det. E.H. Eason 1980”, Meinert Collection (ZMUC); ALGERIA: 3 specimens, Djebel Maadid, Kalas Beni Hammaad, 1000 m, 23.X.1989, G. Osella (CMZ); TUNISIA (governorates listed according to their location, from North to South): Bizerte Governorate: 8 ♂♂, 7 ♀♀, 2 juv., Ghar el Melh, garden, Ceratonia siliqua, 37°19'N, 09°51'E, alt. 35 m, in litter, 11.I.2003, N. Akkari leg. (FMNH); 1 ♂, 2 subad. ♀♀, La Grotte beach, 37°19'N, 09°50'E, alt. 5 m, slope facing the sea, halophilous vegetation, under stones, 12.II.2004, N. Akkari leg. (FMNH); 4 ♂♂, 2 ♀♀, Ghar el Melh, 37°19'N, 09°51'E, alt. 35 m, slope with sparse shrubs, under stones, 1.III.2004, N. Akkari leg. (FMNH); 1 ♂, 1 ♀, Ichkeul National Park, inside the park, 37°07.861'N, 09°41.338'E, alt. 5 m, rocks, shrubs, grass, close to the road, under stones, 23.III.2008, P. Stoev, N. Akkari leg. (NMNHS); 1 ♂, 1 ♀, Ichkeul National Park, 37°08.25'N, 09°41.31'E, alt. 0–50 m, Olea europaea- Pistacia lentiscus maquis, 12.III.2009, N. Akkari, H. Enghoff leg. (ZMUC); Béja Governorate: 3 ♂♂, 8 ♀♀, 3 subad. ♂♂, Rahayette (2 km from Sidi Salem Lac), 36°42'N, 09°18'E, alt. 180 m, meadow, shrubs, under stones, 26.XII.2003, N. Akkari leg. (FMNH); 1 ♂, 3 juv., entrance of the city, 36°42.7311'N, 09°19.611'E, alt. 362 m, open area with scattered Eucalyptus, under stones, 24.IV.2005, N. Akkari leg. (FMNH); 5 ♂♂, 7 ♀♀, 7 km from Zahret Médine, 36°46.857'N, 09°01.688'E, alt. 500 m, limestone hill, shrubs, under stones, 20.III.2008, P. Stoev, N. Akkari leg. (NMNHS); 1 ♀, entrance of Béja City, 36°42.311'N, 09°19.611'E, alt. 362 m, open area with scattered Eucalyptus, under stones, 15.IV.2007, N. Akkari leg. (FMNH); Tunis Governorate: 4 ♂♂, 2 juv., Jebel Bou Kornine, close to the asphalt road (highway: Tunis-Hammamète) 17.II.2004, N. Akkari leg. (FMNH); 1 juv., Tunis, 12.II.1903, Lajos Biró leg (HNHM); 1 ♂, 1 subad. ♂, Tunis, 23.II.1903, Lajos Biró leg. (HNHM); 4 ♂♂, 2 ♀♀, Jebel Bou Kornine, 8.IV.2004, N. Akkari leg. (FMNH); 4 ♂♂, 4 ♀♀, Jebel Bou Kornine, 8.IV.2007, N. Akkari leg. (FMNH); 1 ♀, Bou Kornine National Park, 36°42.530'N, 10°20.680'E, alt. 105–150 m, Thuja, Eucalyptus, dry river bed, under stones and logs, 4.III.2008, P. Stoev, N. Akkari leg (NMNHS); Ariana Governorate: 1 ♂, 2 ♀♀, Nahli Park, 36°53'N, 10°09'E, alt. 68 m, suburban habitat with Eucalyptus, Pinus halepensis, under stones, 31.III.2006, N. Akkari leg. (FMNH); 1 ♂, 1 ♀, Sidi Thabet, Jebel Ammar, 10.II.2007, N. Akkari leg. (FMNH); Jendouba Governorate: 1 ♂, Béni Mtir, surroundings of the dam, 36°44'N, 08°44'E, alt. 445 m, shrubs, under stones, 18.II.2007, N. Akkari leg. (FMNH); 3 ♂♂, 3 ♀♀, Béni Mtir, 36°44'N, 08°44'E, alt. 408 m, slope close to the asphalt road, Quercus suber, under stones, 19.II.2007, N. Akkari leg. (FMNH); 3 ♀♀, same locality, under logs, 36°44.583'N, 08°44.832'E, alt. 493 m, 19.II.2007, N. Akkari leg. (FMNH); 5 ♀♀, same locality, 36°44.006'N, 08°44.001'E, alt. 500 m, 19.II.2007, N. Akkari leg. (FMNH); 4 ♂♂, 4 ♀♀, same locality, alt. 503 m, Quercus suber, Erica arborea and Myrtus communis, under stones, 19.II.2007, N. Akkari leg. (FMNH); 3 subad. ♂♂, Béni Mtir and surroundings, 36°43.888'N, 08°44.105'E, alt. 404 m, Quercus suber, close to the road, under stones, 21.III.2008, P. Stoev, N. Akkari leg. (BIO); 4 ♂♂, 3 ♀♀, 2 subad. ♂♂, ruins of the ancient Roman town Bulla Regia, 36°33.506'N, 08°45.356'E, alt. 185 m, under stones, 21.III.2008, P. Stoev, N. Akkari leg. (NMNHS); 2 subad. ♂♂, 1 subad. ♀, Tabarka, the Genoese fort and surroundings, 36°57.838'N, 08°44.680'E, alt. 20–30 m, slope facing the sea, grass, rocks scattered trees, under stones and logs, 22.III.2008, P. Stoev, N. Akkari leg. (NMNHS); 2 ♂♂, 2 ♀♀, Hammam Bourguiba (west of Aïn Draham), 36°45.926'N, 08°35.084'E, alt. 158 m, meadow with scattered trees, under stones, 22.III.2008, P. Stoev, N. Akkari leg. (NMNHS); 1 ♀, 3 km from Hammam Bourguiba (west of Aïn Draham), 36°46.476'N, 08°36.575'E, alt. 322 m, meadow with scattered trees, under stones, 22.III.2008, N. Akkari, P. Stoev leg. (NMNHS); 1 ♂, 2 subad. ♂♂, 3 subad. ♀♀, 9 km from Hammam Bourguiba (west of Aïn Draham), 36°48.046'N, 08°39.544'E, alt. 379 m, pine forest, humid, close to river, under stones, logs and leaf litter, 22.III.2008, P. Stoev, N. Akkari leg. (NMNHS); 2 ♀♀, surroundings of Aïn Draham, collected from under bark of decaying trunk and stones, 31.III.1977, L. Gozmány, S. Mahunka leg. (HNHM); 3 ♂♂, 3 ♀♀, Tabarka, 36°58.105'N, 08°45.356'E, alt. < 40 m, coastal slope below Genoese fort, under stones, 9.III.2009, N. Akkari, H. Enghoff leg. (ZMUC); Nabeul Governorate: 1 ♂, 1 ♀, 1 juv., Cap Bon Peninsula, Hammamète, Olea europaea orchard, under stones, 17.II.2004, N. Akkari leg. (FMNH); 2 ♂♂, 2 ♀♀, Cap Bon Peninsula, Dar Chichou, coniferous forest, under stones with Pinus halepensis, 14.III.2004, N. Akkari leg. (FMNH); 1 ♂, 4 ♀♀, 1 juv., Cap Bon Peninsula, El Haouaria, N37°3, E10°59, alt. 50 m, 28.IV.2004, N. Akkari leg. (FMNH); 1 ♂, 3 ♀♀, 3 juv., Cap Bon Peninsula, Oued el Abid, close to the sea shore, under stones, 9.III.2005, N. Akkari leg. (FMNH); 3 ♂♂, 3 juv., Cap Bon Peninsula, Oued el Abid village, 36°51.804'N, 10°44.701'E, alt. 79 m, 9.III.2005, N. Akkari leg. (FMNH); 1 ♂, Cap Bon Peninsula, Sidi Erraiès, close to a polluted beach, 9.III.2005, N. Akkari leg. (FMNH); 1 ♂, 2 ♀♀, Cap Bon Peninsula, Kélibia, Mansoura beach, 36°50.994'N, 11°07.361'E, alt. 1 m, under stones, 23.III.2005, N. Akkari leg. (FMNH); 3 ♂♂, 1 ♀, 1 subad. ♂, 2 subad. ♀♀, Cap Bon Peninsula, near Oued El Abid Dam, 36°49.901'N, 10°42.378'E, alt. 42 m, grass, stones, under stones, 24.III.2008, N. Akkari, P. Stoev leg. (NMNHS); 12 ♂♂, 10 ♀♀, 1 subad. ♂, 2 juv., Cap Bon Peninsula, near Oued El Abid village, 36°51.804'N, 10°44.711'E, alt. 79 m, Eucalyptus and Pinus forest, under stones, 24.III.2008, P. Stoev, N. Akkari leg. (NMNHS); 1 ♂, Cap Bon Peninsula, 20 km from El Haouaria, 36°56.660'N, 10°53.321'E, alt. 30 m, broad leaf forest, sandy soil, under stones, 24.III.2008, N. Akkari, P. Stoev leg. (BIO); 8 ♂♂, 11 ♀♀, 1 subad. ♀, El Haouaria, the ancient Roman quarry and surroundings, 37°03.448'N, 10°59.869'E, alt. 51 m, slope facing the sea, under stones, 24.III.2008, P. Stoev, N. Akkari leg. (NMNHS); 1 ♂, 1 ♀, Cap Bon Peninsula, El Haouaria, coast, 37°03.448'N, 10°59.869'E, alt. 2 m, rocks, sand, 10–50 m from the water line, under stones, 25.III.2008, N. Akkari, P. Stoev leg. (NMNHS); 2 ♀♀, Cap Bon Peninsula, Kélibia, plage El Mansoura, 36°51.046'N, 11°07.343'E, alt. 5–10 m, approx. 100 m from the water line, Oleander, Mimosa, under stones and in leaf litter, 25.III.2008, P. Stoev, N. Akkari leg. (BIO); 1 ♀, Cap Bon Peninsula, Kélibia, the fort and surroundings, 36°50.337'N, 11°06.841'E, alt. 10–40 m, slope, Eucalyptus, Mimosa, shrubs, under stones, 25.III.2008, N. Akkari, P. Stoev leg. (NMNHS); 2 ♂♂, 2 ♀♀, Cap Bon Peninsula, 7 km from Menzel Bou Zelfa, 36°40.268'N, 10°40.677'E, alt. 236 m, Pinus, Quercus, shrubs, under stones, 25.III.2008, P. Stoev, N. Akkari leg. (BIO, NMNHS); 8 ♂♂, 2 ♀♀, Cap Bon Peninsula, El Haouaria, Roman cave, 1.IV.2007, K. Tajovský leg. (FMNH); 1 juv., Cap Bon Peninsula, Jebel Abderrahman, Olea europaea orchard, under stones, 12.XI.2006, N. Akkari leg. (FMNH); Zaghouan Governorate: 3 ♂♂, 2 ♀♀, Jebel el Fahs, 36°22.39'N, 09°53.41'E, alt. 172 m, under stones, 20.III.2006, N. Akkari leg. (FMNH); 1 ♀, Jebel el Fahs, 36°24.298'N, 10°09.057'E, alt. 166 m, suburbs, Olea europaea orchard, under stones, 25.II.2007, N. Akkari leg. (FMNH); 1 ♀, Jebel Zaghouan, under stones, 17.III.2007, N. Akkari leg. (FMNH); 1 ♂, 1 ♀, 2 subad. ♂, 1 subad. ♀, Jebel Zaghouan, surroundings of the marabout Sidi Bou Gabrine, 36°22.423'N, 10°06.328'E, alt. 642 m, meadows, scattered trees, under stones and in leaf litter, 17.III.2008, N. Akkari leg. (1♂, 1♀ – BIO; 2 subad. ♂♂, 1 subad. ♀ – NMNHS); 1 ♀, Jebel Zaghouan, surroundings of the marabout Sidi Bou Gabrine, 36°22.423'N, 10°06.328'E, alt. 642 m, meadows, scattered trees, under stones, 29.III.2008, P. Stoev, N. Akkari leg. (NMNHS); 1 ♀, Jebel Zaghouan, surroundings of the Gouffre du courant d’air (small limestone cave), 36°21.980'N, 10°05.513'E, alt. 561 m, Quercus ilex, Pistacia lentiscus, Jasminum fructicans, under stones and in leaf litter, 17.III.2008, N. Akkari, P. Stoev leg. (NMNHS); 5 ♂♂, 4 ♀♀, Jebel Zaghouan, collecting along the track between Gouffre Anti Prehistorique (36°21.595'N, 10°05.208'E) and Sidi Bou Gabrine (36°22.423'N, 10°06.328'E), 500–700 m, mixed forest, under stones and in leaf litter, 18.III.2008, N. Akkari, P. Stoev leg. (NMNHS); 2 ♂♂, Jebel Zaghouan, collecting along the track Sidi Bou Gabrine (36°22.423'N, 10°06.328'E) – Sidi Abdel kader Cave (36°22.419'N, 10°06.371'E) – Saida Mannoubia (36°22.650'N, 10°06.332'E) – the asphalt road to Zaghouan (36°22.924'N, 10°06.789'E), alt. 650–780 m, mixed forest, under stones and in leaf litter, 19.III.2008, P. Stoev, N. Akkari leg. (NMNHS); Le Kef Governorate: 4 ♂♂, 2 ♀♀, 1 juv., Tajerouine, Bou Yagoum dam, 35°53'N, 08°53'E, alt. 650–700 m, open dry habitat, under stones, 16.III.2005, N. Akkari leg. (FMNH); 1 ♂, 2 ♀♀, Ferme Shitta, Jebel Eddyr, about 6 km NNE from Le Kef, alt. about 1100 m, collected from sward, mosses, from below stones imbedded in grassy soil, in shaded sites between cliff walls, 28.III.1977, L. Gozmány, S. Mahunka leg. (HNHM); 3 ♂♂, 1 subad. ♂, 1 subad. ♀, 2 juv., Ferme Shitta, Jebel Eddyr, about 7 km NNE from Le Kef, alt. about 1100 m, collected from under rocks at feet of cliff walls, 26.III.1977, L. Gozmány, S. Mahunka leg. (HNHM); 3 ♂♂, 1 ♀, 1 subad. ♂, 1 subad. ♀, Nebeur, about 30 km N from Le Kef, collected on ground and under stones on steep banks and in riverbed, 30.III.1977, L. Gozmány, S. Mahunka leg. (HNHM); 2 subad. ♂♂, 2 juv., 1 larva, Dugga Archeological site, under stones, 30.X.2009, N. Akkari leg. (ZMUC); 1 ♂, South of Le Kef, a flat, immense, fungiform limestone hill, collected from crags, 27.III.1977, L. Gozmány, S. Mahunka leg. (HNHM); Siliana Governorate: 17 ♂♂, 7 ♀♀, 4 subad. ♀♀, Jebel Bargou, 5 km from Bargou (road Bargou – Ouslatia), 36°05.775'N, 09°37.347'E, alt. 571 m, Quercus, Olea, shrubs, under stones, 28.III.2008, N. Akkari, P. Stoev leg. (2 ♂♂, 2 ♀♀ – BIO; remaining in NMNHS); 4 ♂♂, 9 ♀♀, 1 subad. ♀, Jebel Bargou, 50 km from Ouslatia (road Bargou – Ouslatia), 36°06.941'N, 09°39.392'E, alt. 512 m, sparse olive trees, rocks, under stones, 28.III.2008, P. Stoev, N. Akkari leg. (NMNHS); 1 ♂, Kesra, 36°51'N, 09°12'E, alt. 850–900 m, 12.V.2005, coniferous forest, under stones, N. Akkari leg. (FMNH); Kairouan Governorate: 1 subad. ♂, Sbikha village, under stones, 10.XII.2006, N. Akkari leg. (FMNH); 1 ♂, 3 ♀♀, same locality, under stones, 2.II.2007, N. Akkari leg. (FMNH); 1 ♂, 2 subad. ♀♀, 1 juv., 2 larvae, 6 km from Ouslatia, 35°51.785'N, 09°30.972'E, alt. 581 m, sparse olive trees, Roman ruins, bush, open area, stone debris, under stones, 6.III.2008, N. Akkari, P. Stoev leg. (NMNHS); 3 ♂♂, 1 ♀, Thuburbo Majus, open area with shrubs, 30.III.2007, K. Tajovský leg. (FMNH); Sousse Governorate: 3 ♂♂, Sidi Khalifa (67 km from Tunis), open area with scattered shrubs, 17.II.2004, N. Akkari leg. (NMNHS); 2 ♂♂, 2 ♀♀, 2 subad. ♀♀, Hergla, 35°59.735'N, 10°26.300'E, close to the asphalt road, under stones, 22.III.2005, N. Akkari leg. (FMNH); 2 ♂♂, Bou Ficha, Ken, 36°15.511'N, 10°26.617'E, alt. 15 m, close to a saline depression, under stones, 22.III.2005, N. Akkari leg. (FMNH); Monastir Governorate: 3 subad. ♂♂, 3 subad. ♀♀, Békalta, 35°37'N, 11°00'E, alt. 16 m, sandy soil close to the asphalt road, under stones, 30.XI.2003, N. Akkari leg. (FMNH); Kasserine Governorate: 1 juv., Sbeitla, 30 km NW from Kasserine, inside the ruins of the ancient Roman town of Sifetoula, under stones, 7.III.2008, P. Stoev, N. Akkari leg. (NMNHS); 2 ♂♂, 1 ♀, Chambi National Park, surroundings of the park’s guest house, 35°10.139'N, 08°40.486'E, alt. 950 m, sparse trees, bush, Pinus halepensis, under stones, 7.III.2008, N. Akkari, P. Stoev leg. (NMNHS); 1 ♀, 1 subad. ♂, Chambi National Park, surroundings of the park’s guest house, 35°10.139'N, 08°40.486'E, alt. 950–1000 m, Pinus halepensis, Stipa tenacissima, Thuja, under stones, logs and leaf litter of Pinus halepensis, 8.III.2008, P. Stoev, N. Akkari leg. (NMNHS); 1 ♂, Chambi National Park, inside the park, 35°11.901'N, 08°39.505'E, alt. 1291 m, Pinus halepensis, Quercus ilex, Stipa tenacissima, slope, under stones and in leaf litter, 9.III.2008, P. Stoev, N. Akkari leg. (NMNHS); 1 ♂, 1 ♀, juv., Chambi National Park, Chambi peak and its surroundings, 35°12.285'N, 08°40.653'E, alt. 1500–1540 m, rocks, sparse Pinus trees, under stones, 8.III.2008, N. Akkari, P. Stoev leg. (NMNHS); 2 ♀♀, 5 subad. ♂♂, 1 subad. ♀, 1 juv., Chambi National Park, inside the park, 35°11.935'N, 08°40.418'E, alt. 1468 m, Pinus halepensis, Quercus ilex, Stipa tenacissima, slope, under stones and in leaf litter, 9.III.2008, N. Akkari, P. Stoev leg. (NMNHS); 3 ♂♂, 1 ♀, subad. ♂, ♀, 6 juv., Chambi National Park, Chambi peak and its surroundings, 35°12.285'N, 08°40.653'E, alt. 1500–1540 m, Pinus halepensis, Quercus ilex, Stipa tenacissima, under stones and in leaf litter, 9.III.2008, P. Stoev, N. Akkari leg. (2 ♂♂, 2 juv. – BIO; 1 ♂, 1 ♀, subad. ♂, ♀, 4 juv. – NMNHS); Mahdia Governorate: 8 ♂♂, 9 ♀♀, 1 subad. ♀, 1 larva, Mahdia, touristic area, 35°32.796'N, 11°01.662'E, alt. 0 m, scattered palm trees and shrubs close to the road, polluted area not far from agricultural land, under stones, 16.III.2008, N. Akkari, P. Stoev leg. (NMNHS); 2 ♂♂, 2 ♀♀, 1 juv., surroundings of Ksour Essef (17 km from Mahdia), 35°24.824'N, 10°58.026'E, alt. 59 m, olive trees (Olea europaea), grass, stones and shrubs, under stones, 16.III.2008, N. Akkari, P. Stoev leg. (NMNHS); Sfax Governorate: 2 ♂♂, 1 ♀, Sfax, house’s garden, 23.III.2004, N. Akkari leg. (FMNH); Gafsa Governorate: 1 juv., 1 larva, Jebel Bou Ramli, 34°30.877'N, 08°39.731'E, alt. 512 m, deserted rocky plain at the foot of the mountain, scattered trees, Opuntia and palm trees, under stones, 10.III.2008, P. Stoev, N. Akkari leg. (NMNHS); Tozeur Governorate: 1 juv., Midès, 34°24.419'N, 07°54.896'E, alt. 376 m, dry rocky slope, under stones, 10.III.2008, N. Akkari, P. Stoev leg. (NMNHS); Gabès Governorate: 2 juv., Matmata, 33°32.450'N, 09°59.055'E, alt. 384 m, arid biotope, shrubs and stones, under stones, 13.III.2008, P. Stoev, N. Akkari leg. (NMNHS); Tataouine Governorate: 2 juv., surroundings of Tataouine City, 32°55.506'N, 10°26.913'E, alt. 293 m, arid biotope, slope, stones, scattered trees of Pinus (planted), under stones, 13.III.2008, P. Stoev, N. Akkari leg. (NMNHS); 1 larva? (damaged), Ksar Ouled Soltane, 32°47.281'N, 10°30.784'E, alt. 453 m, arid biotope, rocks, stones, close to the village, under stones, 14.III.2008, N. Akkari, P. Stoev leg. (NMNHS).
Length (from anterior margin of cephalic plate to posterior margin of telson) approx. 48 mm in largest specimens; cephalic plate slightly broader than long (Fig. 1a); head up to 2.7 mm long and up to 2.9 mm wide; leg 15 approx. 11.0 mm long, or approx. 27% length of body. Colour (after one and a half years in alcohol) uniformly light chestnut brown; legs and sternites yellow; only forcipular coxosternal teeth and posterior half of forcipular tarsungulum brown.
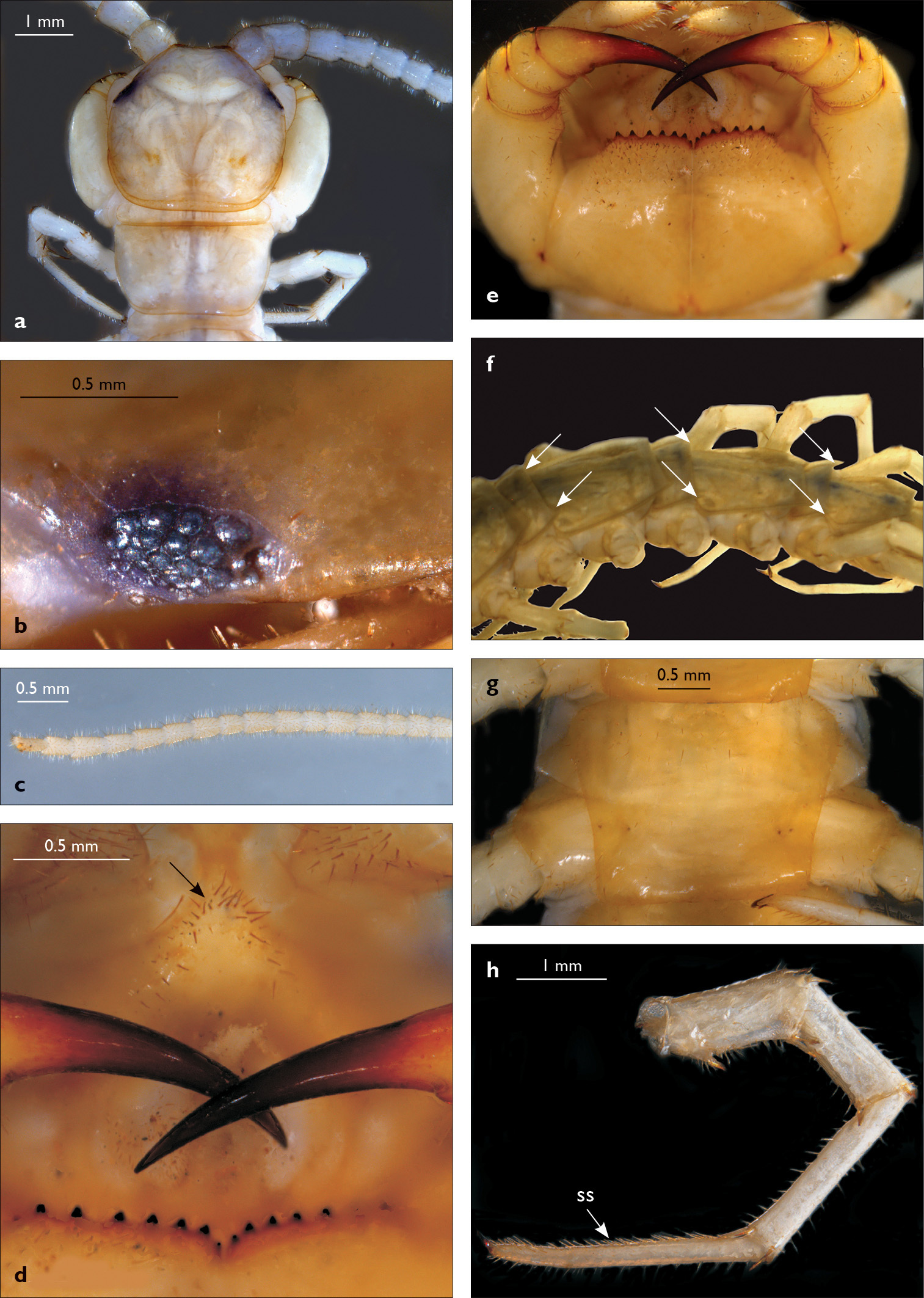
Eupolybothrus nudicornis: a – cephalic plate; b – ocelli and Tömösváry’s organ; c – apical part of antenna; d – clypeus; e – leg 10; f – forcipule; g – TT 6-13; h – sternite 7; i – tarsus 1 and tarsus 2 of leg 15, female from Chambi N.P. Clypeal setae indicated by an arrow (Fig. 1d). Fig. 1f without scale. ss – serial setae; ts – tarsal spine.
Cephalic plate finely punctate, with scattered minute setae, emerging from larger pits that form the punctae; slightly narrower than T1 (Fig. 1a); a median notch contributing to biconvex anterior margin; marginal ridge with a distinct median thickening occupying approx. 10–15% breadth of plate; posterior margin straight; transverse suture situated at about 1/3 of anterior edge; posterior limbs of transverse suture visible, connecting basal antennal article with anterior part of ocellararea. Ocelli: 1+3, 4, 4, 1–1+3, 4, 4, 2 pale, oval to elliptical, in 4 rows. Posterior (major) ocellus elliptical, much larger than seriate ocelli and situated well apart from them. Tömösváry’s organ very small (approx. 1/3 the size of adjacent seriate ocellus), circular, situated on faint sclerotisation lying close to anteriormost ocellus of third ocellar row (Fig. 1b).
Antennae moderately long, approx. 15 mm, reaching midline of T6 when folded backwards; approx. 37% length of body, composed of 43–44 articles; first three articles enlarged, with second being the largest (Fig. 1a); antenna gradually tapering towards the end; articles 5–20 broader than long, ultimate article of antenna about same length as penultimate or slightly longer (Fig. 1c). Basal two articles less setose than the others, which are densely covered with trichoid setae.
Clypeus (Fig. 1d) with a cluster of six medium-sized setae situated asymmetrically at the left half of its apex; central clypeal area smooth, without setae, basal part of clypeus with a single row of setae, lateral clypeal margins with very few dispersed setae.
Forcipule (Fig. 1f): coxosternite subhexagonal, lateral margins feebly convex; anterior margin set off as a rim by furrow that is impressed behind all teeth; coxosternal teeth 5+5, almost equal-sized; median diastema shallow, U-shaped; intradental distance varying, generally increasing towards lateral teeth; porodont arising from a small node at lateral coxosternal margin, situated below the dental rim, and well laterad from lateralmost tooth; base of porodont as thick as adjacent tooth; coxosternite smooth, with one or two rows of setae in close proximity to dental rim; dorsal side of coxosternite with sparse minute setae, the apices of which are not visible from the ventral side. Distal part of tarsungulum about 3–3.5 times longer than proximal part, devoid of setae. Forcipular trochanteroprefemur, femur and tibia fringed with few minute setae (Fig. 1f).
Tergites wrinkled (Fig. 1g); TT 11, 13 with well-developed posterior triangular projections, T9 without triangular projections, posterior angles right-angled; posterior margination poorly developed on all tergites, almost indistinguishable on some; T1 subtrapeziform, slightly wider than cephalic plate, almost as wide as T3. Posterior margin of T1 gently concave, those of TT 3, 5, 8, 10, 12, 14 moderately concave; on intermediate tergite deeply concave; posterior angles of TT 1–6, 8, 10 rounded; those of TT 7, 12, 14 right-angled; tergal setae tiny, almost indistinguishable, in general concentrated on the edges of tergites.
Sternites 1–14 elongated, subtrapeziform, finely punctate, with very few sparse setae, posterior margin convex (Fig. 1h); sternite 15 subrectangular, smooth and more densely setose, especially at posterior margin.
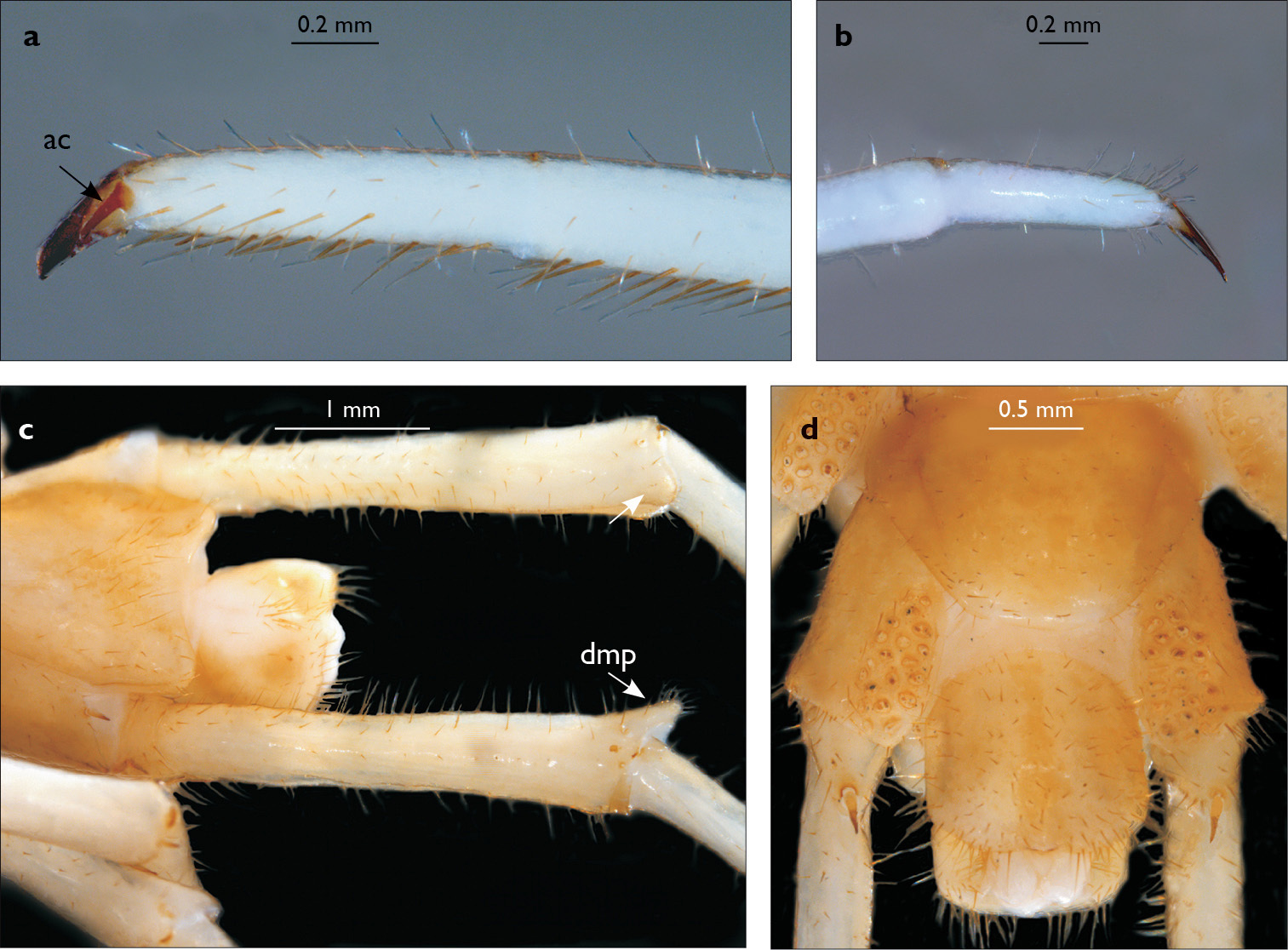
Legs: all legs moderately long (Fig. 1e); leg-pairs 13–15 longer than 1–12; leg 15 longest of all; maximal length of podomeres: coxa 1.2 mm, prefemur 2 mm, femur 1.8 mm, tibia 2.2 mm, tarsus 1 2.2 mm, tarsus 2 1.2 mm, pretarsus 0.3 mm. Tarsus 1 and tarsus 2 of legs 1–13 with two rows of ventral and two rows of lateral (on each side) serial setae (Fig. 1e); serial setae concentrated only on tarsus 2 of leg 14, absent on leg 15 (Fig. 1i). Pretarsus of legs 1–14 with a large principal claw and two smaller and thinner accessory ones emerging dorsally and basally from the principal claw; both accessory claws approx. 1/4–1/5 the length of principal claw, basal one generally smaller and thinner. Pretarsus of leg 15 with a large principal claw only (Fig. 1i). Leg 15 in males with secondary sexual modifications: prefemur moderately enlarged with two paramedian sulci not extending to distal end; distal part of prefemur swollen dorso-medially; inner side of prefemur more densely setose than the outer one (Fig. 2a). Male leg 14 with similar modifications but less pronounced. Spinulation: as in Table 1.
Coxal pores: small, circular, more concentrated on the outer part of pore-field, forming 3–4 irregular rows; only 2–3 pores from the internal row on each coxa larger; around 25–30 on legs 12–14 and about 17 pores on leg 15; pores of inner rows often separated by more than twice their own diameter, those of outermost row usually separated by less than their own diameter (Fig. 2b).
Male first genital sternite with emarginated posterior margin (Fig. 2b), posterior angles broadly rounded, sternal surface densely covered with numerous long brownish setae; gonopod hidden behind the edge of first genital sternite; small, basal part larger covered with six setae, apical part with 2 setae.
Female gonopods with 2+2 moderately long, apically pointed spurs and a simple, falcate claw (Fig. 2c). First article with approx. 14 setae concentrated on small protuberance at its posterior part; posterior half of second article with approx. 20 dorsal and dorso-lateral setae of various sizes; gonopodial claw with 5 moderately long lateral setae.
Eupolybothrus nudicornis, Mahdia (Tunisia), adult male: spinulation of legs.
| Leg | Ventral | Dorsal | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | Tr | PFe | Fe | Ti | C | Tr | PFe | Fe | Ti | |
| 1 | - | - | mp | amp | amp | - | - | amp | a-p | a |
| 2 | - | - | mp | amp | amp | - | - | amp | a-p | a-p\ |
| 3 | - | - | mp | amp | amp | - | - | amp | a-p | a-p\ |
| 4 | - | - | mp | amp | amp | - | - | amp | a-p | a-p\ |
| 5 | - | - | mp | amp | amp | - | - | amp | a-p | a-p\ |
| 6 | - | - | mp | amp | amp | - | - | amp | a-p | a-p\ |
| 7 | - | - | mp | amp | amp | - | - | amp | a-p | a-p\ |
| 8 | - | - | mp | amp | amp | - | - | amp | a-p | a-p\ |
| 9 | - | - | mp | amp | amp | - | - | amp | a-p | a-p\ |
| 10 | - | - | amp | amp | amp | - | - | amp | a-p | a-p\ |
| 11 | - | - | mp | amp | amp | - | - | amp | a-p | a-p\ |
| 12 | - | m | amp | amp | amp | a | - | amp | (a)-p | a-p |
| 13 | - | m | amp | amp | amp | a | - | p | (a)-p | |
| 14 | a | m | amp | amp | a-p | a | - | p | p | |
| 15 | a | m | amp | am | - | a | - | (a)m | p | - |
Eupolybothrus nudicornis: a – prefemora of leg-pair 15, dorsal view b – coxae and male first genital sternite c – female gonopods. Paramedian sulci indicated by arrows (Fig. 2a). mi – medial incision.
- “Pullus” with 10 pairs of legs + 2 incompletely developed pairs (= LIII)
- “Pullus” with 12 pairs of legs + 3 incompletely developed pairs (= LIV)
- “Juvenis” (smaller PL)
- “Junior” (larger PL)
His information is summarized in Table 2. No information on the number of specimens in each group is available. Meinert’s data agree well with our own observations, except for the higher number of forcipular coxosternal teeth in the larval stadia.
Character states in larval and postlarval stadia in Eupolybothrus nudicornis
according to
| Stadium | Body length (mm) | Number of antennal articles | Number of ocelli | Number of forcipular coxosternal teeth |
|---|---|---|---|---|
| Pullus (LIII) | 5-9 | 21 | 3 | 5+5 (also 4+5-4+4) |
| Pullus (LIV) | 24-26 | 4 | ||
| Juvenis | 9.5-11.8 | 25-29 | 4-6 | 4+5, 5+5 |
| Junior | 13.5-20 | 35-42 | 6-8 | 5+5, 6+6 |
Character states in larval and postlarval stadia in Eupolybothrus nudicornis
according to
| Stadium | Number of leg-pairs | Body length (mm) | Number of antennal articles |
|---|---|---|---|
| L0 | 7 | 5-6 | 9 |
| LI | 8 | 6.5 | 11-13 |
| LII | 9 | 7 | 15 |
| LIII | 11 | 7.5 | 17 |
| LIV | 13-14 | 8 | 21 |
| PLI | 15 | 9-11 | 34-36 |
| PLII | 15 | 13-15 | 38 |
| PLIII | 15 | 16-18 | 38-39 |
| PLIV | 15 | 19 | 39-40 |
| PLV | 15 | 21-23 | 40 |
| PLVI | 15 | 27-30 | 41-42 |
| >PLVI | 15 | 33-45 | 42-43 |
Further data on the post-embryonic development based on Tunisian
specimens of Eupolybothrus nudicornis
are provided by Silvestri (
In Table 4 we provide the
character states for the different larval and postlarval stadia obtained
from part of the studied material. The definition of postlarval stadia
follows
Character states in larval and postlarval stadia in Eupolybothrus nudicornis
in Tunisia according to
| Stadium | Number of leg pairs | Body length (♂) | Number of antennal articles | Number of ocelli | Number of forcipular coxosternal teeth | Sex | Locality |
|---|---|---|---|---|---|---|---|
| LIV | 12 | 7 | 26 | 3 | 4+4 | Gafsa, Jebel Bou Ramli | |
| 12 | 8 | 25 | 4 | 4+4 | Archeological site of Dugga | ||
| 12 | 9 | 25 | 5 | 4+4 | 6 km from Ouslatia | ||
| PLI | 15 | 8 | 30 | 4 | 4+4 | Mides | |
| PLII | 15 | 10 | 30 | 5 | 5+5 | 6 km from Ouslatia | |
| PLIII | 15 | 14 | 41 | 7-8 | 5+5 | ♀ | Chambi National Park |
| 15 | 15 | 38 | 6-7 | 5+5 | ♂ | Jebel Bou Kornine | |
| 15 | 16 | 37-38 | 7-8 | 5+5 | ♂ | Archeological site of Dugga | |
| 15 | 17 | - | 7 | 5+5 | ♂ | Jebel Bou Kornine | |
| 15 | 18 | 44-46 | 7-8 | 6+6 | ♂ | Chambi National Park | |
| PLIV | 15 | 18 | 44-46 | 10-411 | 6+6 | ♀ | Chambi National Park |
| 23 | 44 | 10 | 5+5 | ♂ | Tabarka* | ||
| 23 | 40 | 8 | 5+5 | ♀ | Tabrka* | ||
| 23 | 46 | 9 | 6+6 | ♀ | Tabarka* | ||
| 15 | 24 | 40-46 | 11 | 5+6 | ♀ | Beni Mtir | |
| 25 | 44 | 10 | 6+6 | ♀ | Tunis* | ||
| 15 | 26 | 45 | 11 | 5+5 | ♂ | Ghar El Melh | |
| 27 | 42 | 11 | 6+5 | ♂ | Tunis* | ||
| 28 | 41 | 13 | 6+6 | ♀ | Souk el Araba=Jendouba | ||
| 28 | 44 | 10 | 5+5 | ♂ | Tunis* | ||
| PLV | 15 | 28 | 41-44 | 12 | 5+5 | ♂ | Dar Chichou |
| 15 | 25 | 41-42 | 14 | 6+6 | ♂ | Chambi National Park | |
| 29 | 44 | 12 | 5+5 | ♂ | Souk el Arba=Jendouba* | ||
| 30 | 43 | 13 | 5+5 | ♂ | Babouch* | ||
| PLVI | 15 | 30 | 45-47 | 13-14 | 6+5 | ♂ | Ghar El Melh |
| 35 | 44 | 10 | 6+5 | ♂ | Souk el Arba=Jendouba* | ||
| 15 | 35 | 40 | 12-14 | 6+6 | ♂ | Beni Mtir | |
| 15 | 38 | 42-43 | 13-14 | 5+5 | ♀ | Beja | |
| 40 | 41 | 10 | 6+6 | ♀ | Tunis* | ||
| 15 | 40 | - | 14-15 | 5+5 | ♂ | Ghar El Melh | |
| 15 | 42 | - | 11-13 | 5+5 | ♂ | Jebel Bou Kornine | |
| 15 | 43 | 44 | 13 | 6+6 | ♂ | Sidi Khalifa | |
| 15 | 48 | 40-43 | 11-13 | 7+7 | ♂ | Sidi Khalifa |
Eupolybothrus nudicornis
is widespread in the Humid, Subhumid, Semiarid and Arid bioclimatic
zones, according to the bioclimatic division of Tunisia of
Distribution of Eupolybothrus nudicornis in Tunisia.
Known from sea level up to approx. 1500 m. In Italy Eupolybothrus nudicornis has been recorded up to 2500 m altitude (Zapparoli 2006).
W-Mediterranean, according to the chorotype classification of
the W-Palearctic fauna proposed by Vigna Taglianti et al. (
urn:lsid:zoobank.org:act:B9222D42-A69E-47EF-8ACE-7226398E489B
Figs 3–4Holotype: adult ♂, North Tunisia, Zaghouan Governorate, Jebel Zaghouan, Gouffre (chasm) Sidi Bou Gabrine, 36°22.423'N, 10°06.328'E, alt. 642 m, under clay lump, 17.III.2008, P. Stoev leg. (NMNHS). Other material: 1 juv., same locality, date and collector, collected creeping on the wall at the endmost hall (NMNHS).
A species of Eupolybothrus with long antennae, approx. 90% length of body, composed of 68 articles; eyes composed of 18 ocelli; colour uniformly yellow-whitish; anterior margin of forcipular coxosternite with 7+7 teeth; TT 9, 11, 13 with posterior triangular projections; leg 15 56–57% length of body, with a single claw on pretarsus; prefemur of leg 15 with a long, conical dorso-median protuberance emerging from its posterior part and pointing posterior-dorsad; coxal pores generally large, round to ovoid; around 15–20 on legs 12 and 13 and about 20–24 on legs 14 and 15; posterior margin of male first genital sternite straight.
Holotype: Length (from anterior margin of cephalic plate to posterior margin of telson) approx. 30 mm; cephalic plate slightly broader than long (Fig. 3a); length of head: 2.7 mm, breadth: 3 mm; leg 15 aprox. 17 mm long, or 56–57% length of body. Colour generally uniformly yellow-whitish; only forcipular coxosternal teeth, posterior half of forcipular tarsungulum brown; anterior 1/3 of cephalic plate slightly darker yellowish; interrupted black line stretches along the midline of body and can be traced on all but last tergite.
Cephalic plate smooth, wider than T1 (Fig. 3a); a median notch contributing to biconvex anterior margin; marginal ridge with a distinct median thickening occupying almost 50% breadth of plate; posterior margin straight or slightly convex; central part of cephalic plate concave; transverse suture situated at about 1/3 of anterior edge; posterior limbs of transverse suture visible, connecting basal antennal article with anterior part of ocellar area; setae on cephalic plate very few, dispersed, without regular arrangement. Ocelli: 1+3, 4, 5, 5; seriate ocelli greyish, oval to elliptical, in 4 rows: first seriate ocellus of the exteriormost row largest, ocelli of the middle two rows medium-sized, those of inferior row smallest; posterior ocellus as large as the first seriate ocellus. Tömösváry’s organ small, circular, situated on subtriangular sclerotisation immediately below the inferiormost row of seriate ocelli (Fig. 3b).
Left antenna long, approx. 27 mm, reaching or slightly surpassing posterior margin of T12 when folded backwards; 90% length of body, composed of 68 articles; right antenna damaged, composed of at least 34 articles; basal two articles enlarged (Fig. 3a), most articles longer than broad; last 12 articles more elongated than others; ultimate article about same length as penultimate (Fig. 3c). Basal two articles less setose than others, which are densely covered with trichoid setae.
Clypeus with a cluster of about 30–33 long to medium-sized setae situated at apex and near the lateral margin (Fig. 3d).
Forcipule (Fig. 3e): coxosternite subhexagonal, lateral margins feebly convex; anterior margin set off as a rim by furrow that is impressed behind all teeth; coxosternal teeth 7+7, inner tooth smaller than others, its apex well posterior to outer tooth; median diastema small, strongly narrowed by the inner teeth; intradental distance varying, generally increasing towards lateral teeth; porodont arising from a small node below the dental rim, situated posteriad to teeth and well laterad to lateralmost tooth; base of porodont as thick as adjacent tooth or slightly thinner; coxosternite densely setose anteriorly; setae generally long, in approximately 7–8 irregular rows; another row of long setae visible behind anterior margin. Forcipular trochanteroprefemur medially concave with a small subtriangular outgrowth emerging at its posterior part; distal part of tarsungulum about six times longer than proximal part, devoid of setae; forcipular prefemur, femur and tibia fringed with a row of setae (sparse and irregular on the posterior half of prefemoral part).
Tergites (Fig. 3f) generally wrinkled (less so on smaller tergites); TT 9, 11, 13 with well-developed posterior triangular projections, less so on T9; posterior margination lacking on all tergites, poorly visible on last two tergites and on the posterior angles of T1; T1 subtrapeziform, wider than T3, posterior margin transverse. Posterior margin of TT 8, 10, 12, 14 gently concave; posterior angles of TT 1, 2, 3, 4, 5 rounded; those of TT 6, 7, 8 right-angled; pointed on TT 10, 12, and less so also on T14; all tergites covered with sparse, thin but generally long setae, which increase in number towards posterior segments; posterior half of intermediate tergite covered with denser field of such setae.
Sternites smooth, subtrapeziform, with few sparse setae, mainly at lateral margins. Posterior margins straight, slightly convex only on sternites 1 and 15 (Fig. 3g).
Eupolybothrus kahfi sp. n., male, holotype: a – cephalic plate; b – ocelli and Tömösváry’s organ; c – apical part of antenna; d – clypeus; e – forcipule; f – TT 8-14; g – sternite 7; h – leg 10. Figs 3e and f without scales. ss – serial setae. Posterior triangular projections on TT 9, 11 and 13 indicated by arrows (Fig. 3f), clypeal setae indicated by an arrow (Fig. 3d).
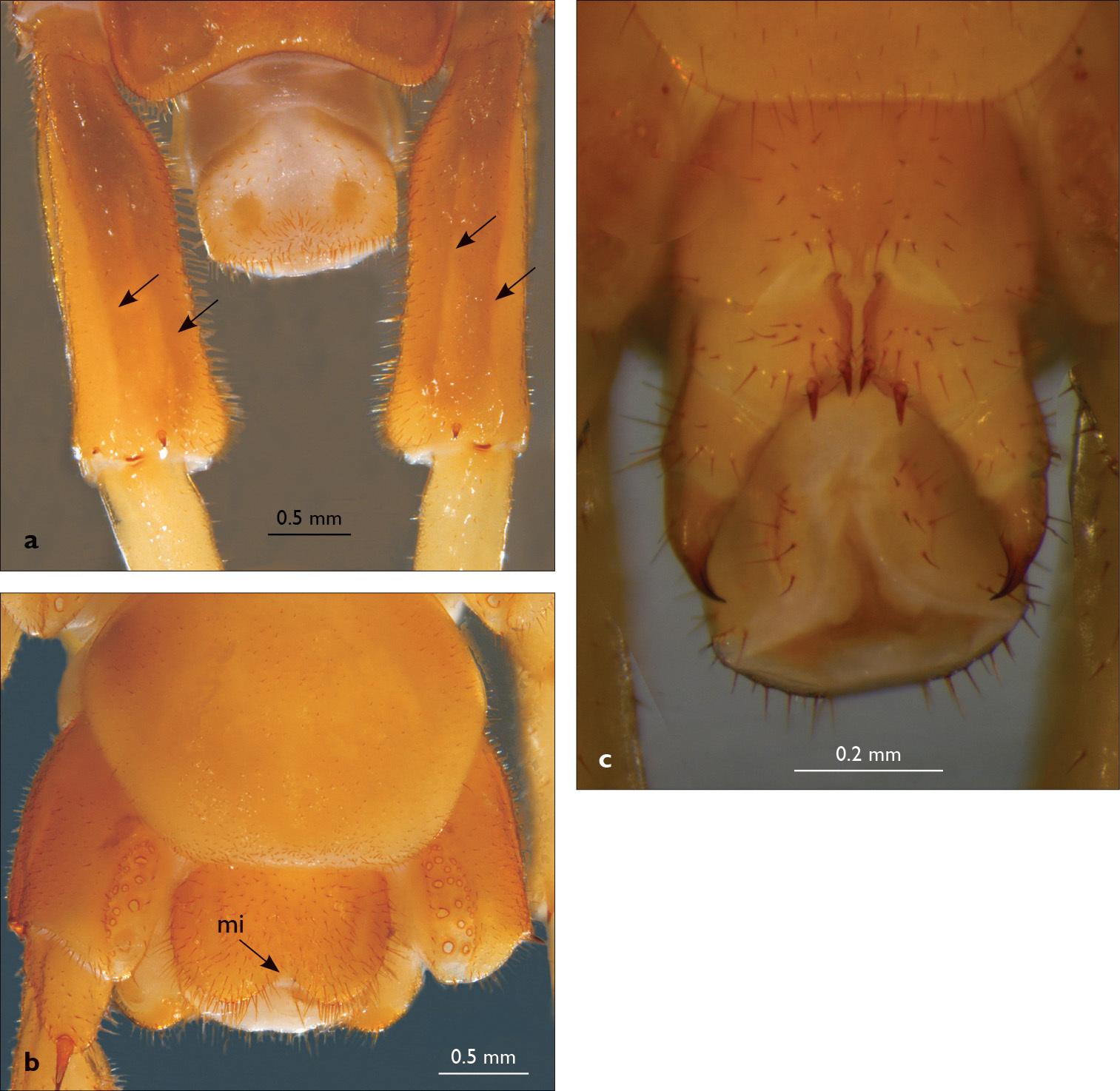
Eupolybothrus kahfi sp. n., male, holotype: a – tarsus 1, tarsus 2 and pretarsus of a midbody leg; b – tarsus 1, tarsus 2 and pretarsus of leg 15; c – prefemora of legs 15, dorso-lateral view; d – coxae and male first genital sternite. ac - accessory claw; dmp - dorso-median protuberance.
Legs: all legs generally elongated (Fig. 3h); legs 14 and 15 much longer than 1–12; leg 13 only slightly longer; leg 15 longest of all; maximal length of podomeres: coxa 1.3 mm, prefemur 3.2 mm, femur 3.2 mm, tibia 3.8 mm, tarsus 1 4.2 mm, tarsus 2 2.3 mm, pretarsus 0.4 mm. Tarsus 1 and tarsus 2 of legs 1–14 with two rows of ventral setae (Fig. 4a). Pretarsus of legs 1–14 with a large principal claw and smaller and thinner accessory claw emerging dorso-laterally; accessory claw half length of the principal claw. Pretarsus of leg 15 with a single claw (Fig. 4b). Leg 15 with secondary sexual modifications: prefemur with a long conical dorso-median protuberance emerging from its posterior part and pointing posterio-dorsad (Fig. 4c), its tip surmounted with a tuft of setae. Leg 14 without particular modifications.
Spinulation: as in Table 5.
Coxal pores: generally large, round to ovoid; 15–20 on legs 12–13 and about 20–24 on legs 14 and 15; pores separated by less than their own diameter, forming 3–4 irregular rows (Fig. 4d).
Male first genital sternite subquadrate (Fig. 4d), fringed with numerous long setae sparsely covering its whole surface, posterior margin not emarginated; gonopod small, hidden behind the edge of first genital sternite, with 8–10 long setae.
Juvenile: pale yellow-whitish, with 15 leg-pairs, most detached; antennae long, approx. 90% of body length, composed of 36–37 articles; ultimate article almost 2.5 times length of penultimate; 5 ocelli; forcipular coxosternite with 5+5 teeth, median diastema shallow, V-shaped; TT 9, 11, 13 with posterior triangular projections; coxal pores: 2, 1, 1, 1.
Eupolybothrus kahfi Stoev & Akkari, sp. n., male, holotype: spinulation of legs
| Leg | Ventral | Dorsal | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | Tr | PFe | Fe | Ti | C | Tr | PFe | Fe | Ti | |
| 1 | - | - | mp | amp | amp | - | - | amp | A | a |
| 2 | - | - | mp | amp | amp | - | - | amp | a-p | a |
| 3 | - | - | mp | amp | amp | - | - | amp | a-p | a-p |
| 4 | - | - | mp | amp | amp | - | - | amp | a-p | a-p |
| 5 | - | - | mp | amp | amp | - | - | amp | a-p | a-p |
| 6 | - | - | (a)mp | amp | amp | - | - | amp | a-p | a-p |
| 7 | - | - | (a)mp | amp | amp | - | - | amp | a-p | a-p |
| 8 | - | - | amp | amp | amp | - | - | amp | a-p | a-p |
| 9 | - | - | amp | amp | amp | - | - | amp | a-p | a-p |
| 10 | - | (m) | amp | amp | amp | - | - | amp | a-p | a-p |
| 11 | - | m | amp | amp | amp | - | - | amp | a-p | a-p |
| 12 | - | m | amp | amp | amp | - | - | amp | a-p | a-p |
| 13 | - | m | amp | amp | amp | - | - | amp | a-p | a-p |
| 14 | - | m | amp | amp | a | a | - | am | a-p | a-p |
| 15 | a | m | amp | am | a | a | - | am | P | - |
derives from the Arabic word kahf (ﮐﮫﻒ) meaning ‘cave’, and kahfi denotes ‘living in cave’.
Eupolybothrus kahfi occurs in a chasm of approximately 30 m depth which after descending continues as a narrow horizontal gallery ending in a small hall. The total length of the cave is approximately 50 m. There are just a few humid spots on the floor, with almost no organic substance. The juvenile specimen was collected creeping on the wall at the end hall, while the adult was found under a lump of clay, approximately one meter below the place of descent. In the cave Eupolybothrus kahfi co-occurs with troglomorphic isopods, spiders of the genus Meta C.L. Koch, 1836, pseudoscorpions of the genus Roncus L. Koch, 1873, harvestmen, troglobitic diplurans, trichopterans and gastropods.
With very few exceptions, Tunisian specimens of Eupolybothrus nudicornis fit the morphological diagnosis of Eupolybothrus nudicornis elongatus well. Eason (1972a) reported on specimens with intermediate characters from Constantine, Algeria, and wrote “…it seems that the characters separating elongatus and impressus are unstable”, and “..…in spite of the intermediate examples from Constantine, it seems advisable to retain, for the time being, the distinction between elongatus and impressus …but they should be regarded as only subspecifically distinct.” Most of the specimens we studied lack triangular projections on T9, though sometimes they were angulated or only slightly projecting behind the rear margin (specimens from Ichkeul National Park, ZMUC). All specimens except for one adult female from Jebel Chambi and one adult female from near Oued El Abid village lacked spines on tarsi of legs 15. Only two specimens out of hundreds possessed tarsal spines. Likewise, the shape of tergite 9 seems to be also infrapopulationally variable. The specimens from Spain (Granada) in Meinert’s collection (ZMUC), which were studied by E.H. Eason in 1980, all lack triangular projections and tarsal spines and should therefore be attributed to Eupolybothrus nudicornis elongatus even if geographically this area is situated within the range of the nominate form. The general colour of the body also varies considerably among the populations, from uniformly dark brown in e.g., the Ichkeul specimens, to uniformly castaneous and dark yellowish-brownish in most of the other examined specimens. Some specimens (e.g., those from near Zahret Médine and Bulla Regia, NMNHS) possess a dark middorsal band.
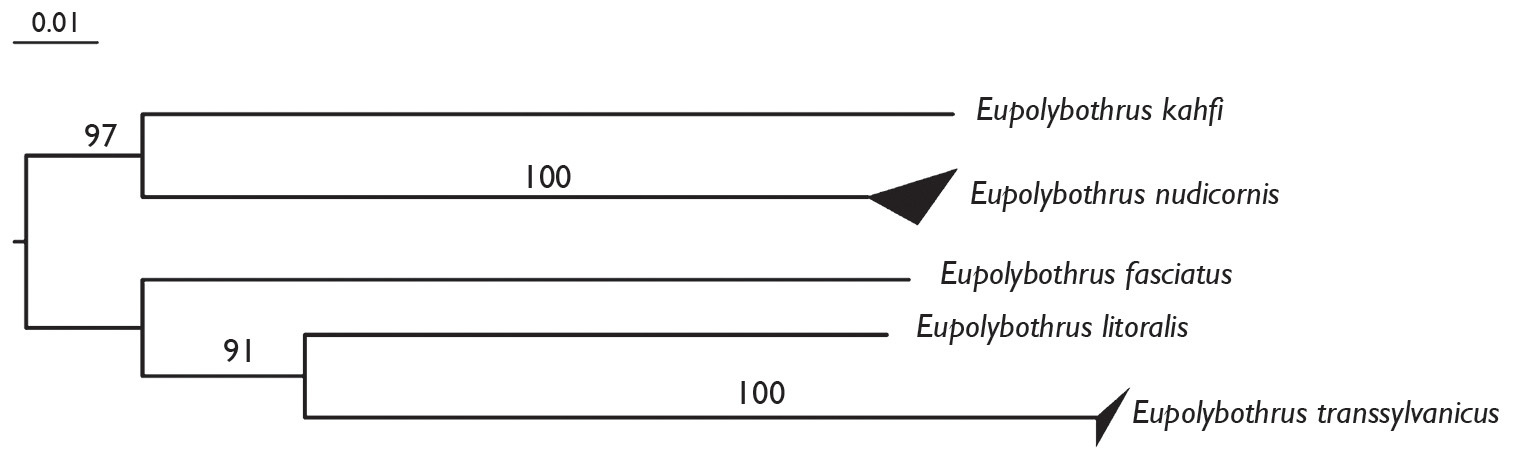
Molecular dataIn order to confirm the delineation of the new species Eupolybothrus kahfi, we used DNA barcoding to bring genetic support to the morphological observations. The COI barcodes examined from 11 specimens (Table 6) among 5 species of Eupolybothrus exhibited a 20.8% mean value for interspecific divergences (Table 7). The lowest value was 16.61% between Eupolybothrus transsylvanicus and Eupolybothrus litoralis, and the highest was 23.98% between Eupolybothrus transsylvanicus and Eupolybothrus nudicornis. By contrast, for the two species for which we were able to measure it, we observed a low infraspecific value, 1.14% for Eupolybothrus nudicornis (sampled from three different populations in Tunisia; see Table 6) and 0.3% for Eupolybothrus transsylvanicus The neighbor joining tree built from this dataset shows the clear separations between the different barcode clusters corresponding to the different species (Fig. 5).
Specimens sequenced for COI and their BOLD and GenBank accession numbers.
| Species | Locality | GenBank accession number | BOLD accession number |
|---|---|---|---|
| Eupolybothrus litoralis | Turkey, Afyon Prov., near village of Akoren | HM065035 | NMNHS-PES-00062 |
| Eupolybothrus nudicornis | Tunisia, Cap Bon Peninsula, 20 km from El Haouaria | HM065036 | NMNHS-PES-00077 |
| Eupolybothrus nudicornis | Tunisia, Cap Bon Peninsula, 7 km from Menzel Bou Zelfa | HM065037 | NMNHS-PES-00079 |
| Eupolybothrus nudicornis | Tunisia, Nabeul, plage El Mansoura | HM065038 | NMNHS-PES-00053 |
| Eupolybothrus nudicornis | Tunisia, Nabeul, plage El Mansoura | HM065039 | NMNHS-PES-00052 |
| Eupolybothrus nudicornis | Tunisia, Jebel Zaghouan, surroundings of the marabout Sidi Bou Gabrine | HM065040 | NMNHS-PES-00045 |
| Eupolybothrus nudicornis | Tunisia, Jebel Zaghouan, surroundings of the marabout Sidi Bou Gabrine | HM065041 | NMNHS-PES-00044 |
| Eupolybothrus kahfi | Tunisia, Jebel Zaghouan, Gouffre Sidi Bou Gabrine | HM065042 | NMNHS-PES-00046 |
| Eupolybothrus transsylvanicus | Bulgaria, Shumen City | HM065043 | NMNHS-PES-00066 |
| Eupolybothrus transsylvanicus | Bulgaria, Shumen City | HM065044 | NMNHS-PES-00065 |
| Eupolybothrus fasciatus | Italy, Fogliano Mt, near Viterbo, Lazio | AY214420 | -------------------------- |
Genetic distances between species within Eupolybothrus (K2P-pairwise).
| 1 | 2 | 3 | 4 | ||
|---|---|---|---|---|---|
| 1 | Eupolybothrus nudicornis | ||||
| 2 | Eupolybothrus litoralis | 21.4 | |||
| 3 | Eupolybothrus transsylvanicus | 23.98 | 16.61 | ||
| 4 | Eupolybothrus kahfi | 19.19 | 22.28 | 23.46 | |
| 5 | Eupolybothrus fasciatus | 21.47 | 18.15 | 20.7 | 21.56 |
Neighbor joining tree (K2P) of 5 species of Eupolybothrus based on the COI 5’ ‘barcoding fragment’. Bootstrap support values are shown on the branches. The upper and lower sides of the triangle represent respectively the maximum and minimum of genetic distances within the species.
Taxonomy. Eupolybothrus kahfi’s
nearest neighbor is Eupolybothrus nudicornis
with 19.19% divergence. This interspecific value is consistent with the
distances observed among the other examined species of the genus. Thus
it contributes genetic support to the delineation of this new species
which appears as a well individualized mitochondrial lineage.
Unfortunately we do not have more specimens of Eupolybothrus kahfi’s
but we can reasonably expect low values for intraspecific variation.
The results for Eupolybothrus nudicornis
and Eupolybothrus transsylvanicus
show low intraspecific divergences for COI by comparison to the
interspecific divergences, confirming the ‘barcoding gap’ described by
No taxonomically significant differences were found between the
syntype specimens of Eupolybothrus cloudsley-thompsoni and Eupolybothrus nudicornis,
which confirms Zapparoli’s (
Post-embryonic development. Information on the post-embryonic development of species of Eupolybothrus
is generally poor, as more comprehensive studies have been published
only for Eupolybothrus nudicornis
(
Distribution. Eupolybothrus nudicornis
is distributed throughout the whole of Maghreb, although from Morocco
and Libya it is so far known only from single localities – near Bab
Berred (Tetouan) (
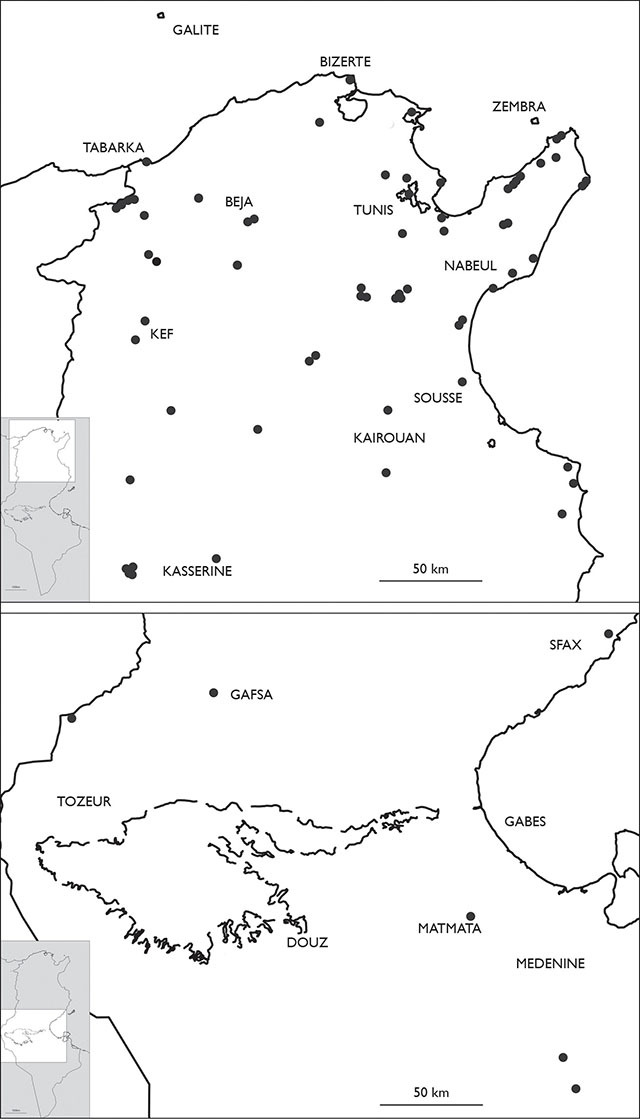
Eupolybothrus kahfi
is known only from its type locality, the cave Sidi Bou Gabrine (Fig. 6a). The cave is situated
in the limestone massif Jebel Zaghouan (Fig. 6b) at a distance of
approximately 500 m from the marabout Sidi Bou Gabrine (Map 4). The southwestern part
of the mountain is composed of Jurassic limestone strata of mostly
Sinemurian to Tithonian age (
a – A view of the entrance of cave Sidi Bou Gabrine, Jebel Zaghouan. b – A view of Jebel Zaghouan, Zaghouan Governorate, NE Tunisia.
Localities of Eupolybothrus nudicornis in Libya and Morocco.
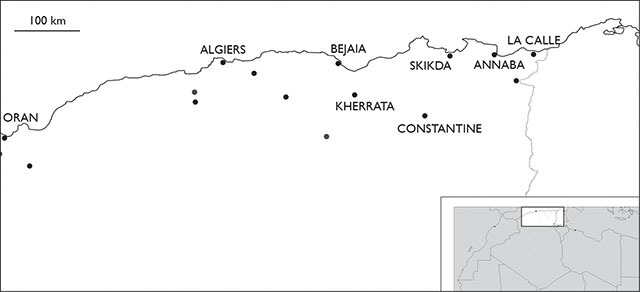
Distribution of Eupolybothrus nudicornis in Algeria.
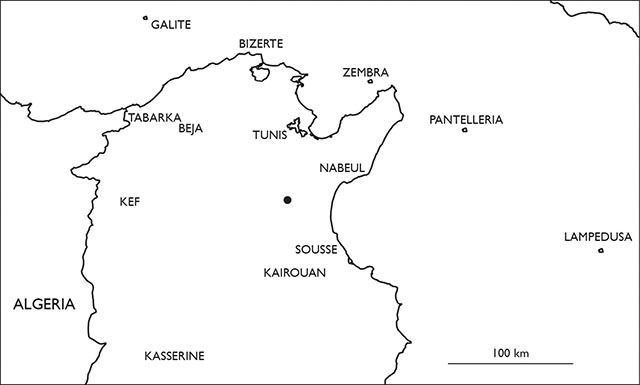
Locality of Eupolybothrus kahfi in Tunisia.
Habitats. In Tunisia Eupolybothrus nudicornis is recorded from coniferous and broad-leaf woods of different composition and dominant structure: 1) oak forests dominated by Quercus suber and Erica arborea; 2) coniferous forests dominated by Pinus halepensis; 3) mixed forests with Pinus halepensis, Quercus ilex and Stipa tenacissima; mixed forests with Olea europaea and Pistacia lentiscus; mixed woods with Eucalyptus and Thuja; 4) Olea europaea orchards. Eupolybothrus nudicornis has been found also in open habitats such as meadows with scattered vegetation, coastal slopes with planted vegetation, rocky terrains overgrown with shrubs not far from the sea (approx. 10–50 m from the water line), coastal sandy habitats with very scattered halophilous vegetation, maquis, arid rocky slopes with shrubs and stones, deserted rocky plains with Opuntia and sparse palm trees, suburban and urban habitats, and ruins.
Unlike Eupolybothrus nudicornis,
Eupolybothrus kahfi
is known only from a cave showing traits of adaptation for life
underground (e.g., long legs and antennae, pale coloration). It is
worth mentioning that still very little is known about the cavernicolous
lithobiomorphs in North Africa. Cave-dwelling lithobiomorphs are
hitherto unknown from Libya and Egypt. Only three species have hitherto
been recorded from caves in Algeria and Morocco, these all being
members of Lithobius
Leach, 1814 (cf.
| 1 (10) | Ocelli (Figs k-1-2) or posterior triangular projections on tergites absent (Fig. k-3) | 2 |
| 2 (5) | Ocelli absent (Fig. k-1), posterior triangular projections at least on TT 9, 11, 13 (Fig. k-4) | 3 |
| 3 (4) | Forcipular coxosternite with 9+9-12+12 teeth (Fig. k-5), forcipular trochanteroprefemur unmodified, 15VCa and 15 DCa spines present, pretarsus of leg 15 with a single claw (Fig. k-6) | Eupolybothrus obrovensis (caves in Italy, Slovenia, Croatia) |
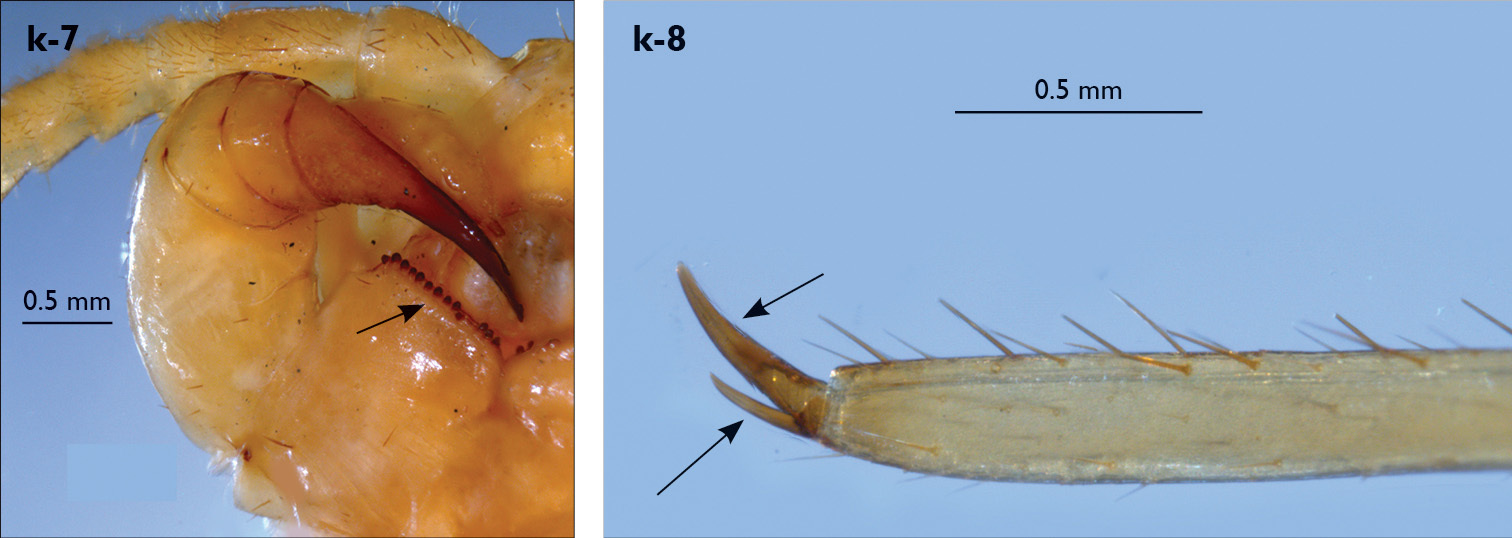
| 4 (3) | Forcipular coxosternite with 13-14 teeth (Fig. k-7), forcipular trochanteroprefemur strongly swollen, 15VCa and 15 DCa spines absent, pretarsus of leg 15 with a principal claw and posterior accessory claw (Fig. k-8) | Eupolybothrus andreevi (cave in Bulgaria) |
| 5 (2) | Ocelli present (Fig. k-9), all tergites without posterior triangular projections (Fig. k-10) | 6 |
| 6 (7) | Pretarsus of leg 15 with accessory claw | Eupolybothrus verrucosus (Moldavia) |
| 7 (6) | Pretarsus of leg 15 without accessory claw | 8 |
| 8 (9) | T1 much broader than head (Fig. k-11), deeply concave posteriorly; forcipular trochanteroprefemur with a dorso-lateral knob (Fig. k-12) | Eupolybothrus dolops (Greece) |
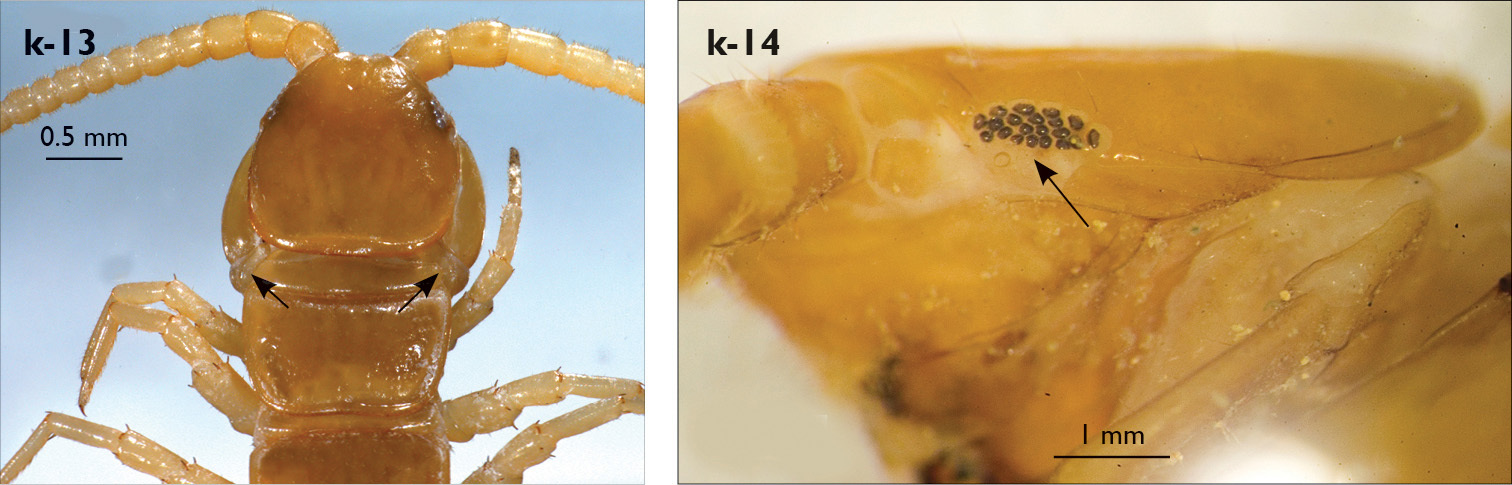
| 9 (8) | T1 as broad or slightly broader than head (Fig. k-13), transverse posteriorly; forcipular trochanteroprefemur without a knob | Eupolybothrus werneri (Greece, Albania) |
| 10 (1) | Ocelli present (Fig. k-14); posterior triangular projections present at least on TT 11 and 13 (Fig. k-15) | 11 |
| 11 (20) | VCm spine present on leg 15 (Fig. k-16) | 12 |
| 12 (13) | Six ill-defined, feebly pigmented ocelli in adults | Eupolybothrus leostygis (caves in Bosnia and Herzegovina, Croatia) |
| 13 (12) | 10-25 pigmented ocelli in adults (Fig. k-17) | 14 |
| 14 (19) | Male leg 15 with a large rounded knob proximate of the middle of the caudal side of the prefemur (Fig. k-18) | 15 |
| 15 (18) | Antennae composed of 74-83 antennal articles | 16 |
| 16 (17) | Prefemoral knob simple (Fig. k-19) | Eupolybothrus acherontis (here possibly also belong the poorly known acherontis wardaranus from FYR Macedonia and Eupolybothrus stygis from Ilijna cave in Bosnia and Herzegovina) (FYR of Macedonia, Bosnia and Herzegovina) |
| 17 (16) | Prefemoral knob apically incised forming two rounded processes densely covered with trichoid setae (Fig. k-20) | Eupolybothrus excellens (Italy) |
| 18 (15) | Antennae composed of 50-60 antennal articles | Eupolybothrus caesar (here possibly also the poorly known Eupolybothrus spiniger from Bosnia and Herzegovina) (Albania, Greece, FYR of Macedonia, Bosnia and Herzegovina) |
| 19 (14) | Male leg 15 without prefemoral knob (Fig. k-21) | Eupolybothrus tabularum (Italy) |
| 20 (11) | VCm spine absent (Fig. k-22) | 21 |
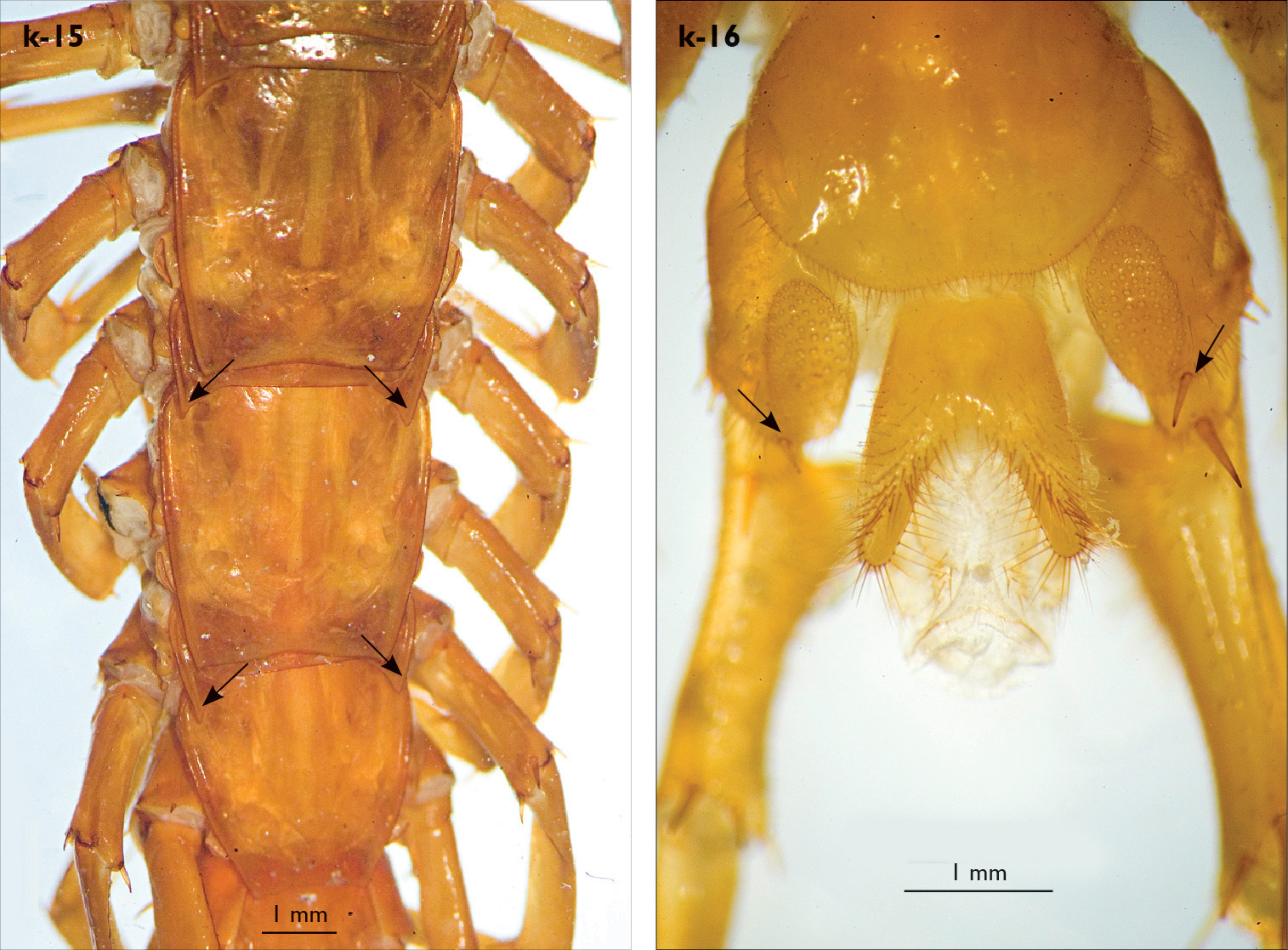
| 21 (28) | Posterior triangular projections present on TT 9, 11, 13 or 11, 13 (Fig. k-23) | 22 |
| 22 (25) | Male gonopods long (Fig. k-24) | 23 |
| 23 (24) | 17 ocelli, last 15 antennal articles shorter, only the ultimate article being 3 times longer than broad, others 2 times at most | Eupolybothrus zeus (here also probably Eupolybothrus sketi from FYR Macedonia) (Greece) |
| 24 (23) | Six-seven ocelli; last 15 articles of antennae elongated, 3 times longer than broad | Eupolybothrus macedonicus (Greece) |
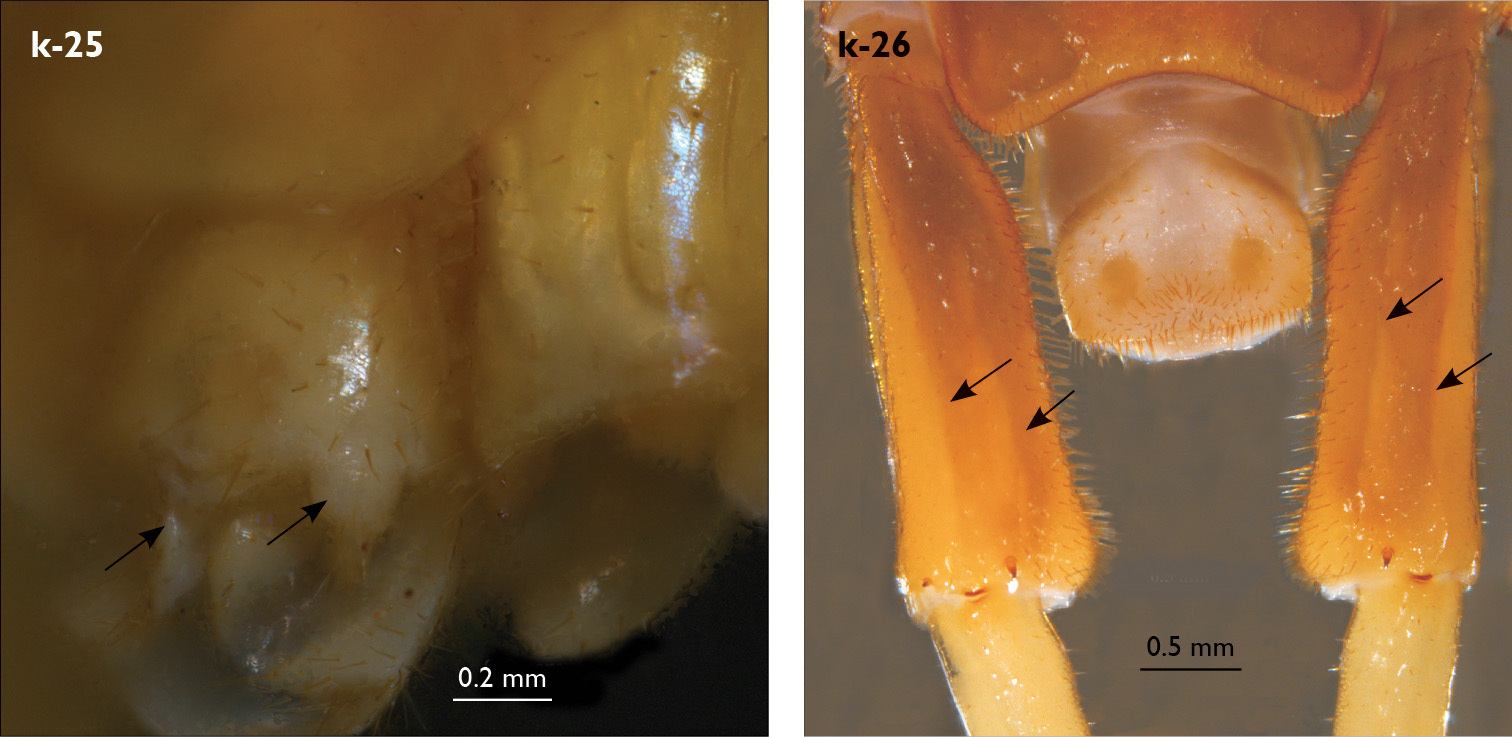
| 25 (22) | Male gonopods short (Fig. k-25) | 26 |
| 26 (27) | Leg 15 approx. 30% length of body; prefemur of leg 15 in male moderately enlarged with two paramedian sulci not extending to posterior margin; posterior part of prefemur swollen dorso-medially (Fig. k-26); posterior margin of male first genital sternite emarginated (Fig. k-22) | Eupolybothrus nudicornis (North Africa from Morocco to Libya, Spain, France, Italy, Malta) |
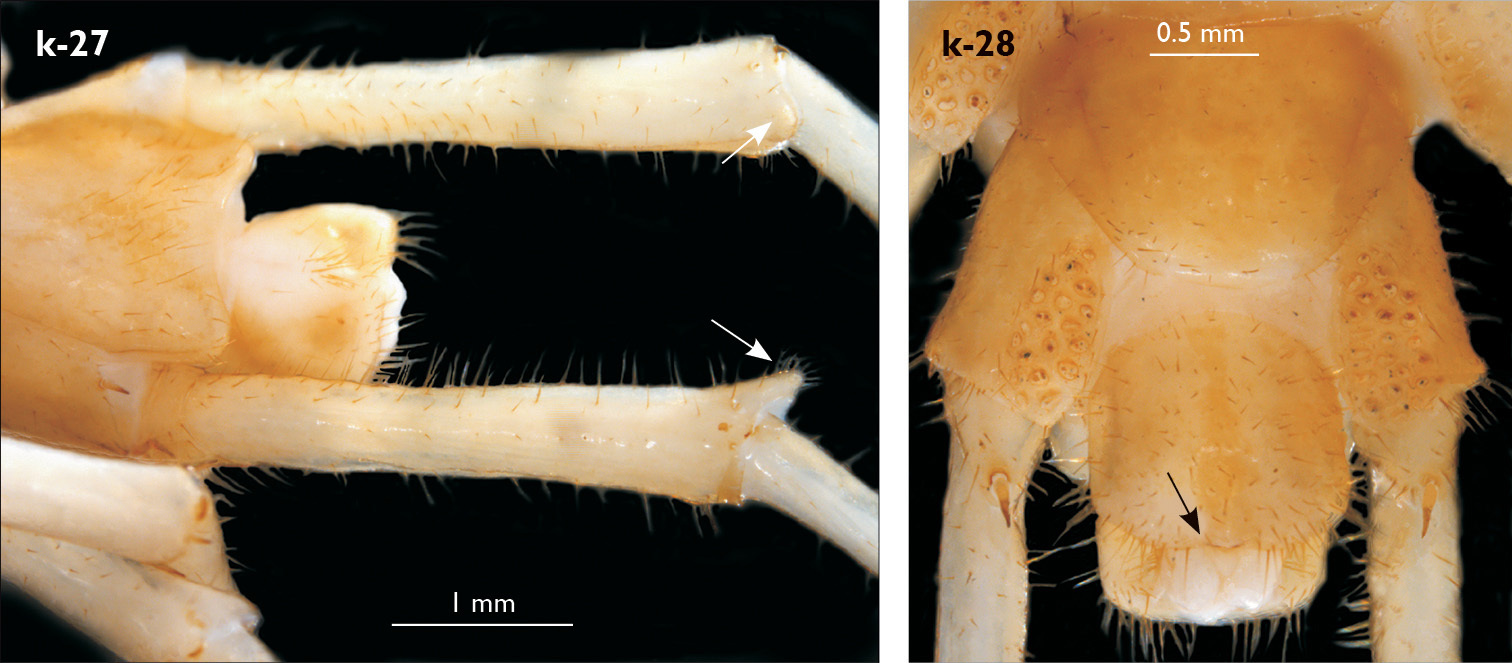
| 27 (26) | Leg 15 approx. 60% length of body; male prefemur 15 with a long, conical dorso-median protuberance (Fig. k-27); posterior margin of male first genital sternite not emarginated (Fig. k-28) | Eupolybothrus kahfi (cave in Tunisia) |
| 28 (21) | Posterior triangular projections present on TT 6, 7, 9, 11, 13 or 7, 9, 11, 13 (Fig. k-29) | 29 |
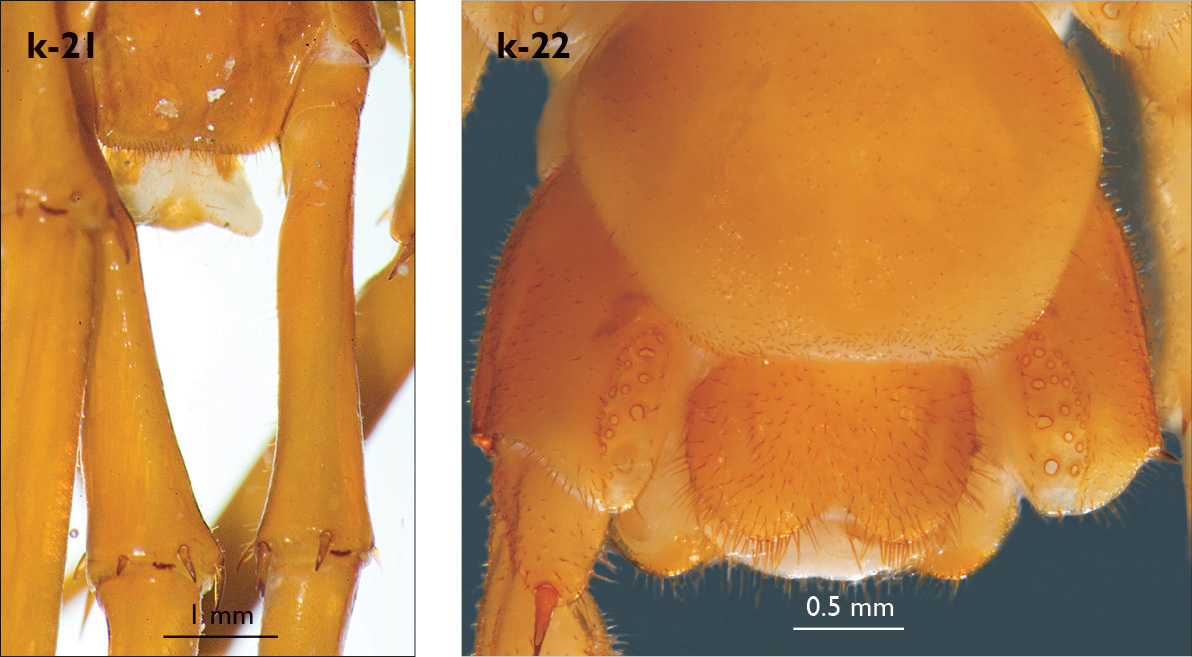
| 29 (32) | Male gonopods short, two-jointed (Fig. k-25), VCa spine on leg 15 present (Fig. k-30), pretarsus of leg 15 without accessory claw | 30 |
| 30 (31) | Prefemur of male leg 15 inflated and strongly expanded medially just proximad of the middle, the protuberance densely covered with setae (Fig. k-31); a densely setose circular dorsomedial area covering almost ? of prefemoral breadth in the position of DPFep spine, which is absent (Fig. k-31) | Eupolybothrus imperialis (Italy) |
| 31 (30) | Prefemur of male leg 15 without such protuberance, evenly expanded along its whole length (Fig. k-32); circular area smaller, covering 1/3 of prefemoral breadth at most (Fig. k-32) | Eupolybothrus herzegowinensis (Albania, Bosnia and Herzegovina, Montenegro) |
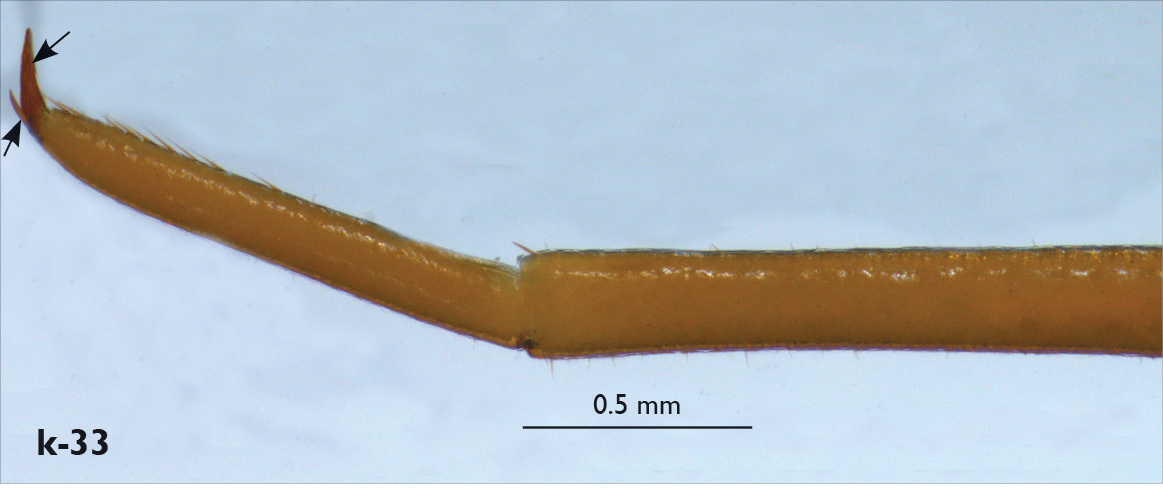
| 32 (29) | Male gonopods long (unknown in Eupolybothrus valkanovi) (Fig. k-24), VCa spine on leg 15 usually absent (rarely only in Eupolybothrus litoralis), pretarsus of leg 15 with accessory claw (Fig. k-33) | 33 |
| 33 (34) | Antennae long, composed of 58-90 articles (Fig. k-34); leg 15 almost as long as body, densely covered with long setae 4-4.5 times diameter of article (Fig. k-35) |
Eupolybothrus gloriastygis
(caves in Bosnia and Herzegovina, Croatia, Montenegro; the record of |
| 34 (33) | Antennae and legs shorter (Fig. k-36), setae on leg 15 shorter (Fig. k-37) | 35 |
| 35 (42) | Pretarsus of leg 15 with accessory apical claw (Fig. k-33) | 36 |
| 36 (39) | Seriate setae on tarsus 2 of leg 15 absent (Fig. k-38) | 37 |
| 37 (38) | Antennae and leg-pair 15 elongated, about 3/4 body length (Fig. k-36); femur of male leg 15 without basal pit (Fig. k-39) | Eupolybothrus longicornis (France, Italy) |
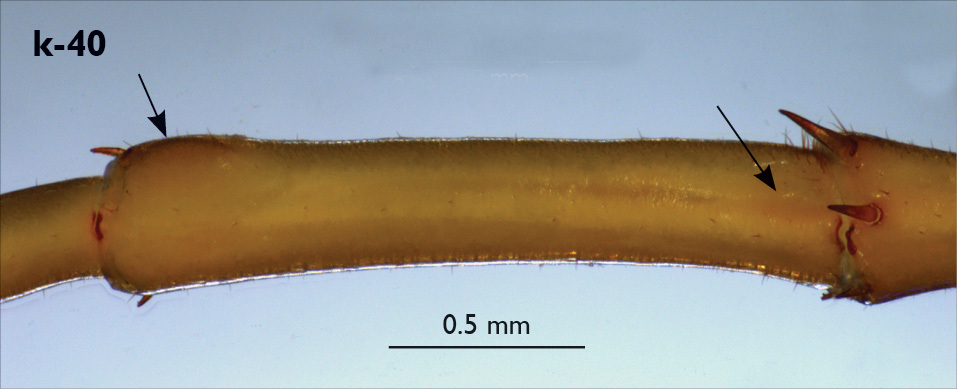
| 38 (37) | Antennae and leg-pair 15 about half body length; femur of male leg 15 with an extensive and deep basal pit (Fig. k-40) | Eupolybothrus litoralis (southern Balkans, Near East) |
| 39 (36) | Seriate setae on tarsus 2 of leg 15 present (Fig. k-33) | 40 |
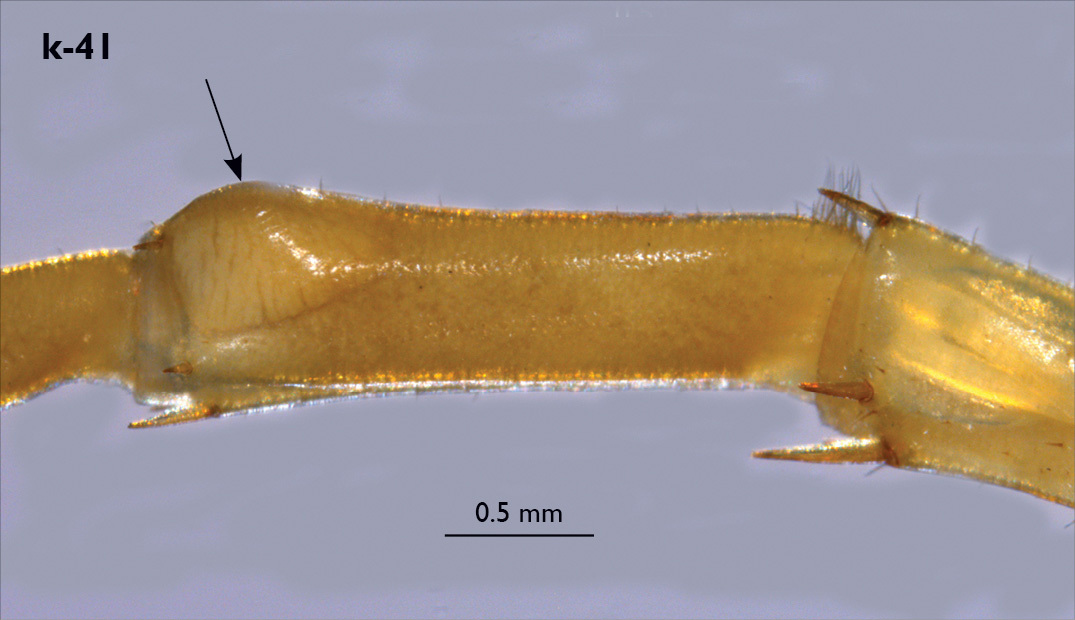
| 40 (41) | Basal pit of femur in male leg 15 extensive and deep; internal dorsal sulcus of femur in male leg 15 not extending to margin of pore-free area which bears a prominent globular swelling (Fig. k-41) | Eupolybothrus fasciatus (Italy, France, uncertain records from the Balkan Peninsula) |
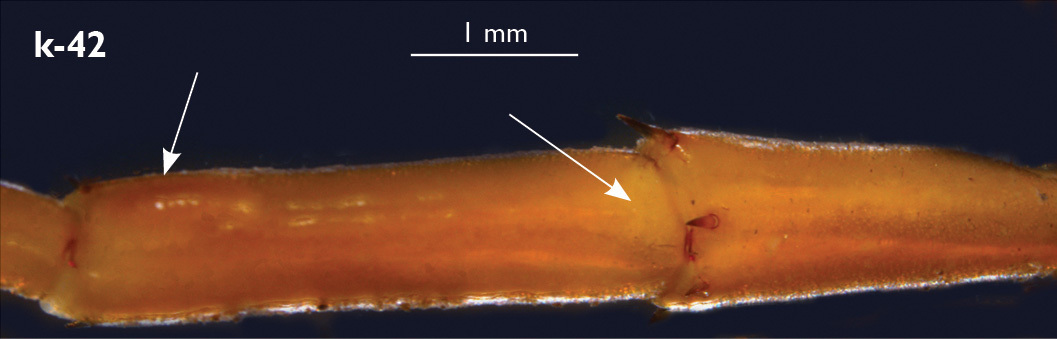
| 41 (40) | Basal pit of femur in male leg 15 small and shallow; internal dorsal sulcus of femur in male leg 15 extending to margin of pore-free area which is not swollen (Fig. k-42) | Eupolybothrus grossipes (France, Italy, Switzerland, Austria, Slovenia, Germany, Serbia?, Romania?) |
| 42 (35) | Pretarsus of leg 15 without accessory apical claw (Fig. k-43) | 43 |
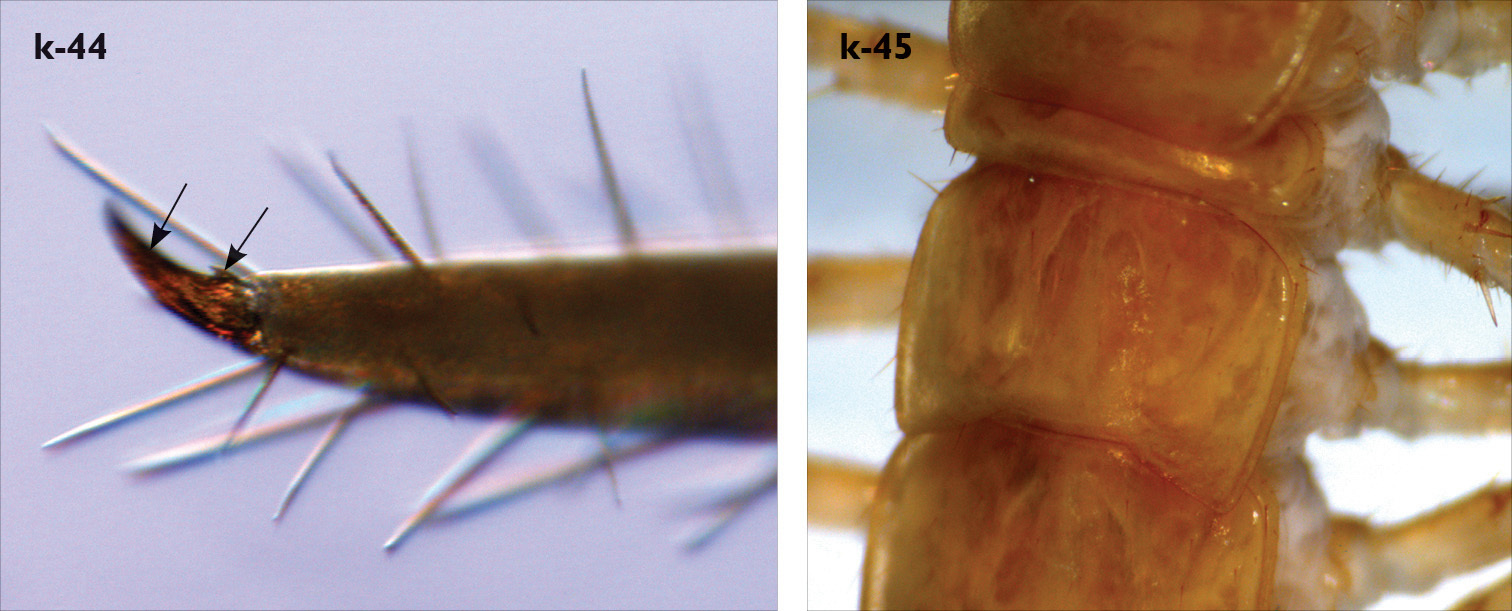
| 43 (44) | Pretarsus of leg 15 with a small accessory claw emerging basally to the principal claw (Fig. k-44), adults: 16-25 mm; T6 without posterior triangular projections (Fig. k-45) | Eupolybothrus tridentinus (Albania, Austria, Bosnia and Herzegovina, Bulgaria, Croatia, Czech Republic, Germany, Hungary, Italy, Romania, Serbia, Montenegro, Slovenia, Switzerland) |
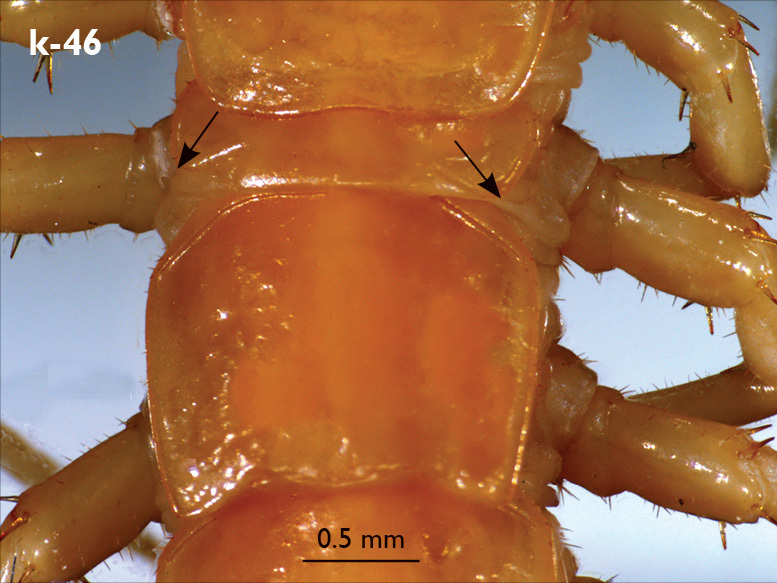
| 44 (43) | Pretarsus of leg 15 without accessory basal claw (Fig. k-43), body length of adults more than 30 mm; T6 with broad posterior triangular projections (Fig. k-46) | Eupolybothrus transsylvanicus (here also probably Eupolybothrus valkanovi known from a single female) (Bosnia and Herzegovina, Bulgaria, Croatia, Greece, Hungary, Romania, Serbia, Montenegro) |
The collecting trip of N. Akkari and P. Stoev in Tunisia in March 2008 was financially supported by the Field Museum Collection Fund, with the logistic help of Petra Sierwald. Atef Belkahla (Tunis, Tunisia) kindly helped with the preparation of maps, while Dicky Sick Ki Yu (Lexington, USA) with the exporting of the interactive key from Delta to Intkey format. Verena Stagl (NHMW) helped to obtain photographs of the type specimens of Eupolybothrus spiniger and nontype specimens of Eupolybothrus obrovensis kept in the NHMW. Zoltán Korsós, Eszter Lazányi, László Dányi (HNHM) and Janet Beccaloni (NHM) provided access to the collections under their care and kindly assisted the visits of P. Stoev to the NHM and HNHM in February and April 2009, respectively. The trips to these museums were financially supported by the European Commission’s (FP 6) Integrated Infrastructure Program SYNTHESYS (GB-TAF, HU-TAF). P. Stoev received financial support from the Bulgarian Minsitry of Education and Research (European Social Fund project No 42) for a research visit to the ZMUC in November 2009. The DNA barcoding part of this work was supported by grants to PDNH from NSERC and from Genome Canada through the Ontario Genomics Institute. We are especially thankful to Robert Mesibov (Launceston, Australia) for his valuable comments on the final draft.
Locality dataset for all published and new records of Eupolybothrus nudicornis and Eupolybothrus kahfi in North Africa. File format: Microsoft Excel (1997-2003). doi:10.3897/zookeys.50.504-app.A
Character dataset for larval and post-larval stadia of Eupolybothrus nudicornis based on published (Meinert 1872, Silvestri 1896, Daas et al. 1996) and new data. Post larval stadia of the new material defined after Daas et al. 1996. File format: Microsoft Excel (1997-2003). doi:10.3897/zookeys.50.504-app.B
BOLD dataset comprising the COI registration numbers of sequenced Eupolybothrus specimens, COI-5P sequence length and locality data. File format: Microsoft Excel (1997-2003). doi:10.3897/zookeys.50.504-app.C
MorphBank dataset of all published figures comprising figure number, species name, locality data, body part and additional metadata. File format: Microsoft Excel (1997-2003). doi:10.3897/zookeys.50.504-app.D
GoogleEarth (http://earth.google.com) interactive map displaying the distribution of Eupolybothrus nudicornis and Eupolybothrus kahfi in North Africa. File format: KML (Keyhole Markup Language) version 2.1 for GoogleEarth. doi:10.3897/zookeys.50.504-app.E
Interactive key for identification of all currently valid species of Eupolybothrus made with Delta software. doi:10.3897/zookeys.50.504-app.F
Copyright notice: All datasets are published under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided that the original author and source are credited.