(C) 2011 Zhi-xiang Pan. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Morphology of the first instar larvae of Collembola has considerably taxonomical and phylogenetic significance. We describe the first instar larvae for the first time in Homidia. External morphology of first instar larvae and adults of Homidia jordanai sp. n. is described based on observations under light and scanning electron microscopes. Most organs of adults bear considerably more setae than the first instar larvae; in addition, first instar larval Homidia lack labial seta R, seta on tenaculum, mucronal spine, and dental spines. The new species is characterized by weakly pigmented body, long antennae subequal to body in length, 1+1 inner macrochaetae on Abd. III, few inner macrochaetae on posterior Abd. IV, and spiny and short seta pi on dental base. Differences between new species and other two similar ones, taxonomical significance of the first instar larvae and the position of Homidia are also discussed.
new species, first instar, scanning electron microscope, chaetotaxy, China
In epimetabolic Collembola, the number of setae and pigmentation often change after moult (
In the present paper, the primary chaetotaxy of Homidia was studied for the first time based on Homidia jordanai sp. n., from East China. Many other morphological details are showed under scanning electron microscope.
Material and methodsAlcohol preserved young and adult specimens, were cleared in lactic acid, mounted on microscope slides in Marc André II solution, and studied using Leica DM2500 and Nikon 80i microscopes. Few specimens, coated with platimum under vacuum conditions, were observed under scanning electron microscope. Photographs were taken with Leica AL2 and Nikon SMZ1000 microscopes using a mounted Nikon DS-Fi1 camera and Hitachi S4800 scanning electron microscope, numbers and letters added with photoshop CS2 (Adobe Inc.), all length data measured with Nikon NIS-Elements Documentation 3.1. First instar larvae were determined by the stability of primary chaetotaxy (e.g. 19/18 common setae on meso-/metathorax,
Abbreviations. Th. –thoracic segment, Abd. –abdominal segment, Ant. –antennal segment, ms –microsensillum/a, s –common sensillum/a, mac –macrochaeta(e), mic –microchaeta(e), SEM –scanning electron microscope, LM –light microscope, Tita I–III –tibiotarsus of fore, mid, and hind legs.
Resultsurn:lsid:zoobank.org:act:7A40BC6A-99BB-4A02-AE92-0899F6556BD8
http://species-id.net/wiki/Homidia_jordanai
Figs 1–80, Tables 1–3♂ on slide, Shaoxin City, Zhuji Country, Dongbaihu, Zhejiang Province, CHINA, 29°34.48'N, 120°24.32'E, 3.X.2009, collection number S4014, collected by Zhi-Xiang Pan and Chen-Chong Si, deposited in Taizhou University.
2 ♂, 11 ♀ and 5 larvae on slide, numerous in alcohol, same data as holotype. 5 paratypes (1 ♂, 1 ♀ on slide, 1 larva and 2 adults in alcohol) deposited in School of Life Sciences, Nanjing University and others in Taizhou University, China.
Named after the famous Spanish entomologist Jordana Rafael (University of Navarra).
Adult. Size. Maximum body length up to 2.3 mm.
Habitus. Ground colour pale yellow in alcohol. Body dorsally without pigment. Coxa and trochanter of all legs with weak blue pigment. Eye patches dark. Antennae gradually darker from Ant. III to Ant. IV (Figs 1, 2).
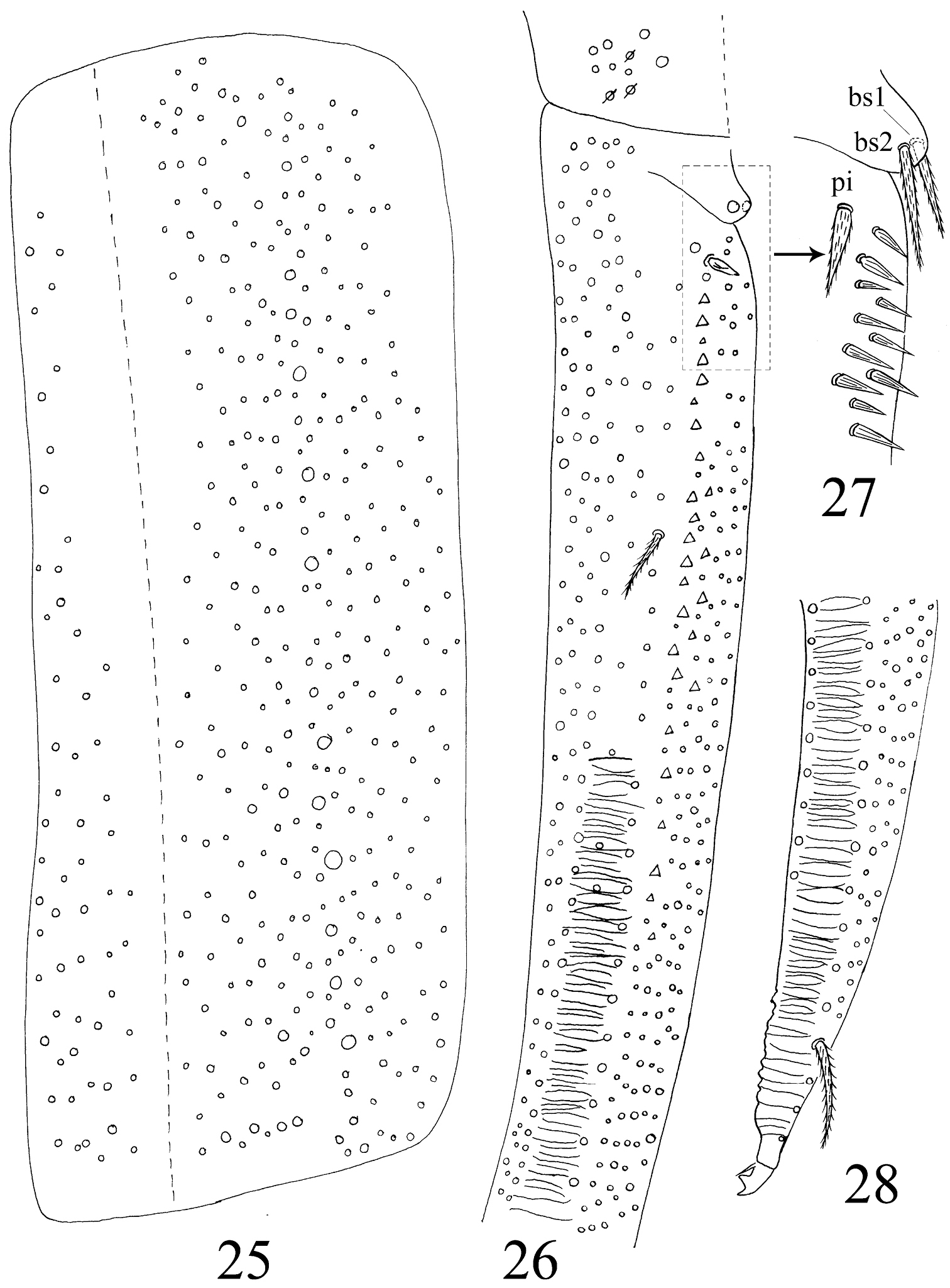
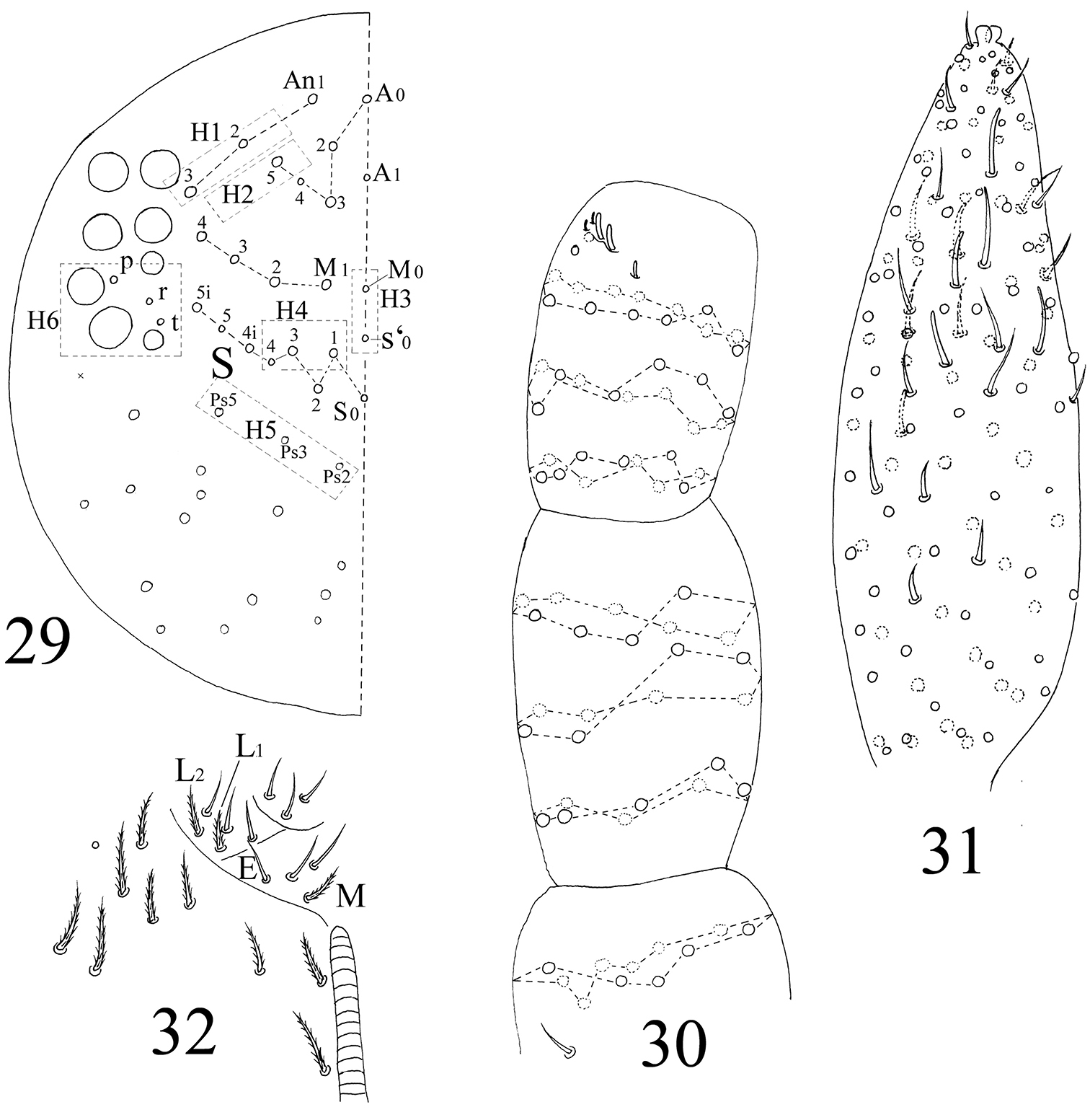
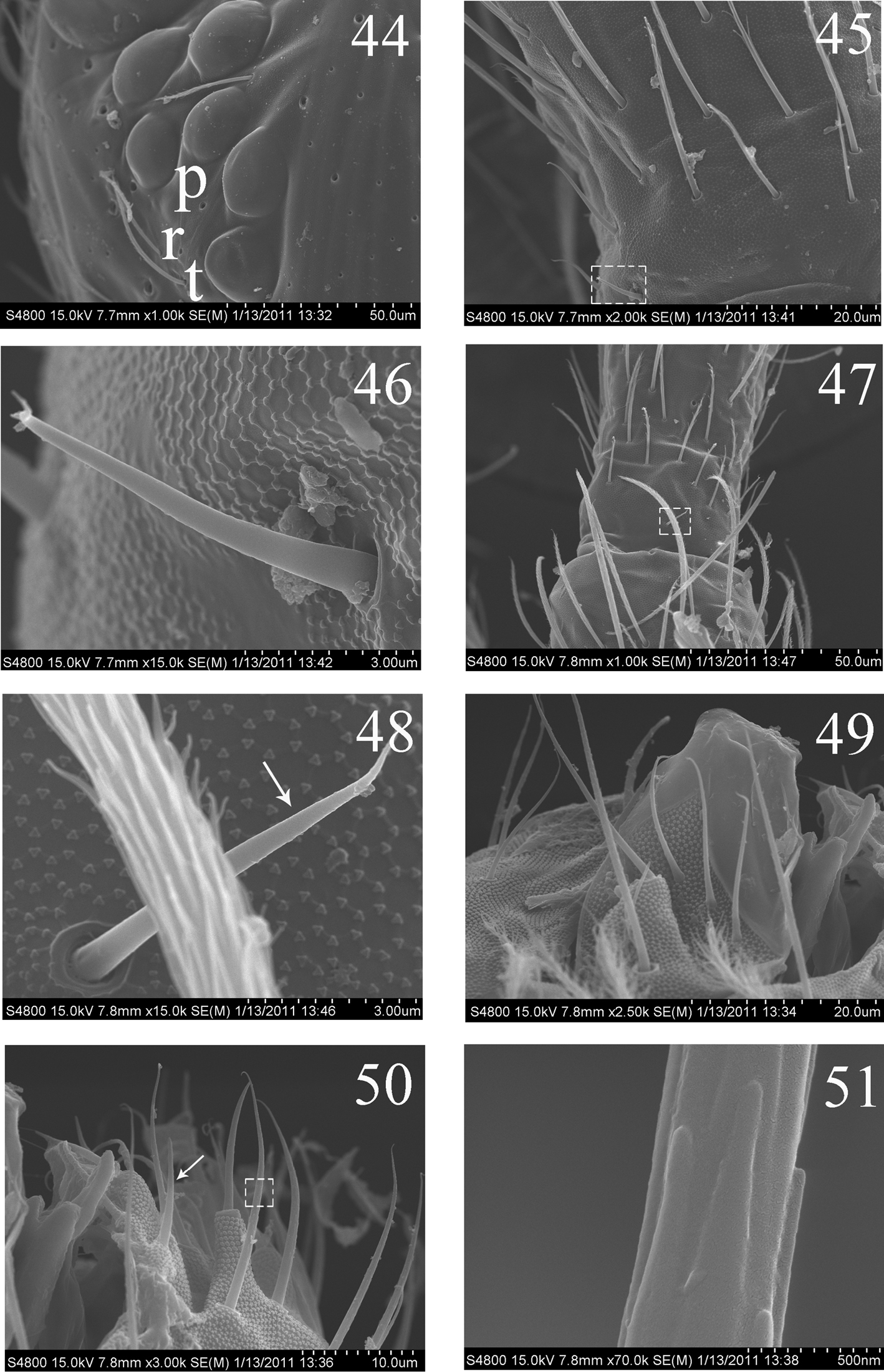
Head. Ommatidia 8+8, G and H smaller than others, and sometimes invisible under LM, interocular setae as p, r, t (Figs 5, 44). Antenna 3.9–4.6 times as long as cephalic diagonal, subequal to body in length, antennal segment ratio as I : II : III : IV = 1 : 1.3–1.5 : 1.3–1.4 : 2.4–3.1. Basal Ant. I with 2 dorsal and 4 ventral spiny setae (Figs 5, 45, 46). Basal Ant. II with 5 smooth setae (Figs 47, 48); distal Ant. II with 4 s (2–3 longer, 1–2 shorter) (Figs 52, 53). Ant. III organ with 2 rod-like s and 3 small s (Figs 54–56); those s also with obvious ridges on surface under SEM. Distal Ant. IV with several types of s (Figs 57–62); apical bulb bilobed (Fig. 6). Dorsal cephalic chaetotaxy with 4An and 7S mac. Clypeus with many ciliate setae (Fig. 5). Labral papillae absent. Prelabral and labral setae as 4/5, 5, 4, all smooth, labium intrusion U-shaped (Fig. 7). Maxillary outer lobe with 1 apical seta, 1 subapical seta and 3 sublobal hairs on sublobal plate; subapical seta slightly longer than apical one (Fig. 49). Labial palp with five papillae A–E, with 0, 5, 0, 4, 4 guard setae, respectively, and 5 smooth proximal smooth setae; lateral process differentiated with blunt tip reaching apex of papilla E (Figs 8, 50, 51). Hyaline plate with 1 main (H) and 2 accessorial (h1, h2) setae. Setal formula of labial base as MREL1L2, seta E smooth, others ciliate (Fig. 9).
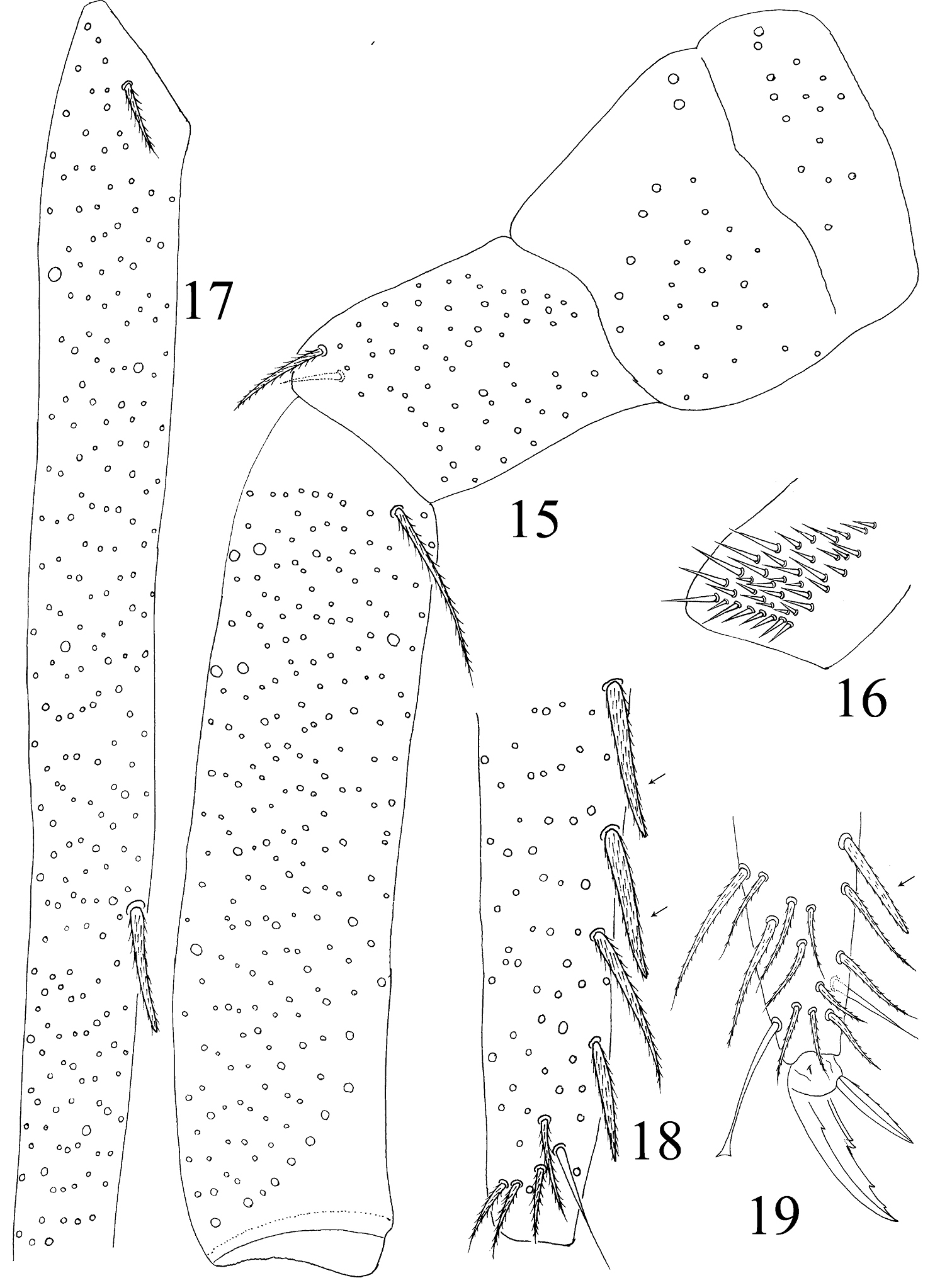
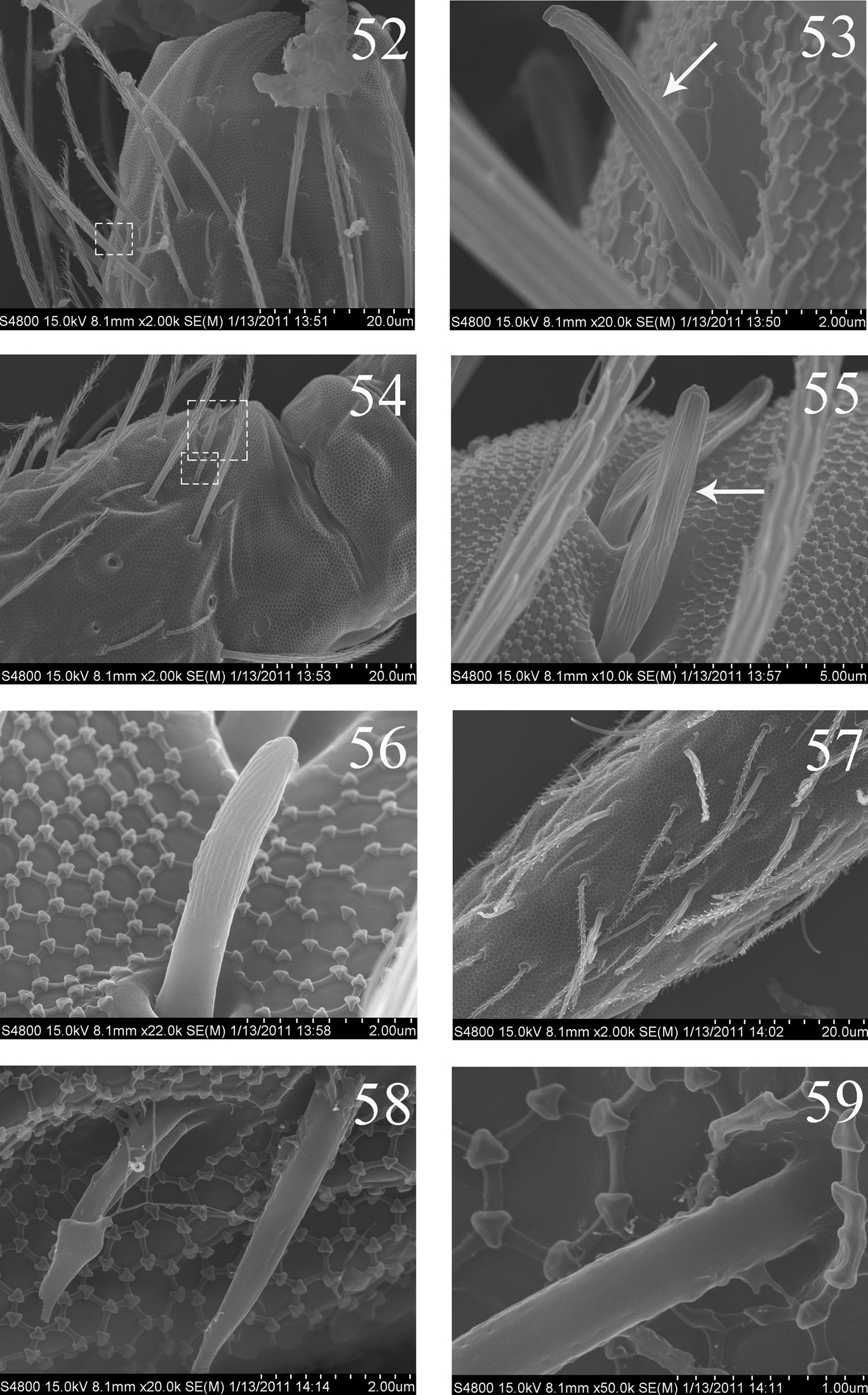
Thorax. Complete s-chaetae of dorsal body as 32/223(>47)3 (examined specimens mostly with 47 s-chaetae on Abd. IV, but some lost during preparation), ms as 10/10100. Th. II with 3 medio-medial (m1, m2, m2i), 3 medio-sublateral (m4, m4i, m4p), 17–18 posterior mac and 3 s-chaetae (ms antero-internal to s); seta p1i2 rarely present, seta p6 as mic. Th. III with 29–32 mac and 2 s-chaetae; setae p1i2 and p4 absent (Fig. 10). Numerous setae on hind leg (Figs 15–19); pseudopores on coxa shown in Fig. 63, but their number unclearly seen. Coxal macrochaetal formula as 3/4+1, 2+1/4+2 (Fig. 11). Trochanteral organ with 36–64 smooth spiny setae (Fig. 16). Inner differentiated tibiotarsal setae slightly ciliate, most distal smooth seta present on hind leg (Figs 18, 19, 65). Tenent hairclavate and subequal to inner edge of unguis in length (Figs 19, 66). Unguis with 4 inner, 2lateral and 1 outer teeth, all tiny. Unguiculus lanceolate with outer edge slightly serrate (Fig. 65). Pretarsus with 1 pair of small spines (Figs 19, 67).
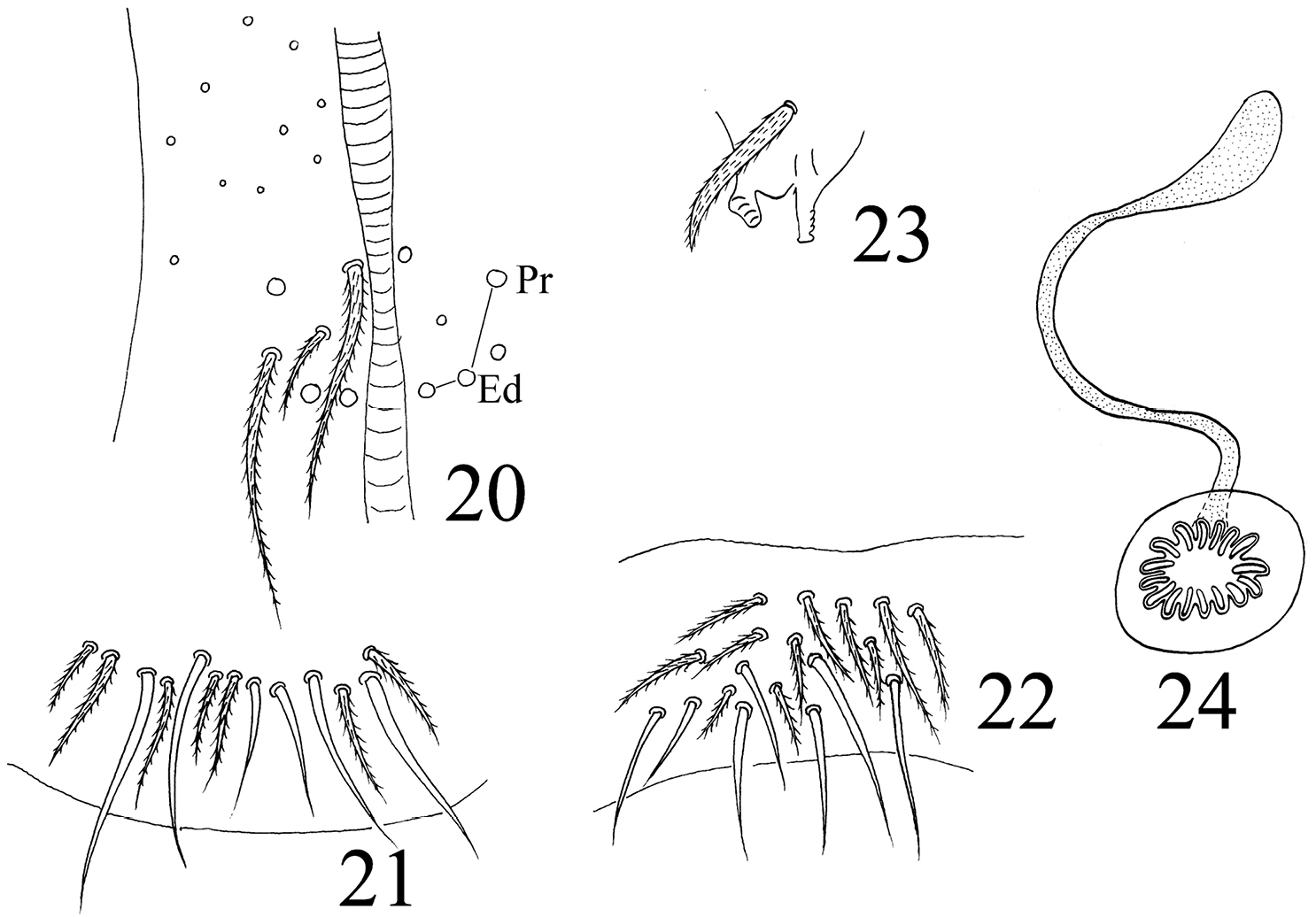
Abdomen. Abd. I with 9 (a2, a3, m2–4, m2i, m4i, m4p, a5) mac and 2 s-chaetae (ms anterio-external to s) (Figs 68, 69). Abd. II with 6 (a2, a3, m3, m3e, m3ea, m3ep) inner, 1 (m5) lateral mac, and 2 s-chaetae. Abd. III with 1 (m3) inner and 4 (am6, pm6, m7a, p6) lateral mac, and 3 s-chaetae (Fig. 12). Abd. IV with more than 47 s-chaetae (2 of normal length and others elongated), 6–9 mac on anterior part and irregularly arranged in a transverse row; posterior part with 2 (3) (B5 and A6, A6 rarely as mic, B4 sometimes present) mac and 1 (B6) mic (Fig. 13). Abd. V with 3 s-chaetae; m3a absent, a5i sometimes absent (Fig. 14). Anterior face of ventral tube with many ciliate setae, including 4+4 mac, line connecting proximal (Pr) and external-distal (Ed) (Chnd and Li 1997) mac oblique to median furrow(Fig. 20); posterior face with 5 or 6 (median with 1 or 2 small) smooth and numerous ciliate setae (Fig. 21); lateral flap with 6–7 smooth and 10–22 ciliate setae (Figs 22, 74, 75). Furcula shown in Figs 25–28. Manubrial plaque with 3 pseudopores, 2 inner and 5–6 outer ciliate setae (Fig. 26). Dens with 20–40 spines (Figs 26, 27, 77); basal sete (
Size. Body length up to 0.7 mm.
Habitus. Ground colour pale white in alcohol. Eye patches dark. Distal antennae slightly pigmented (Figs 3, 4).
Head. Antenna 1.3–1.8 times as long as cephalic diagonal, antennal segment ratio as I : II : III : IV = 1 : 1.4–2.2 : 1.5–2.5 : 3.3–4.3. Ant. I with 11 ciliate and 1 basal spiny setae. Ant. II with 25 ciliate setae. Ant. III with 38 ciliate setae and 5 s-chaetae of Ant. III organ (Figs 30, 78). Ant. IV with numerous ciliate setae and some s-chaetae (Figs 31, 79). Dorsal cephalic chaetotaxy as 3An and 5S mac (Fig. 29). Labium with 3 smooth proximal setae. Setal formula of labial base as MEL1L2, seta E smooth, all others ciliate (Fig. 32). Ommatidia, Ant. IV apical bulb, interocular setae, labral papillae, labrum, maxillary outer lobe, labial palp, hyaline plate same as adults.
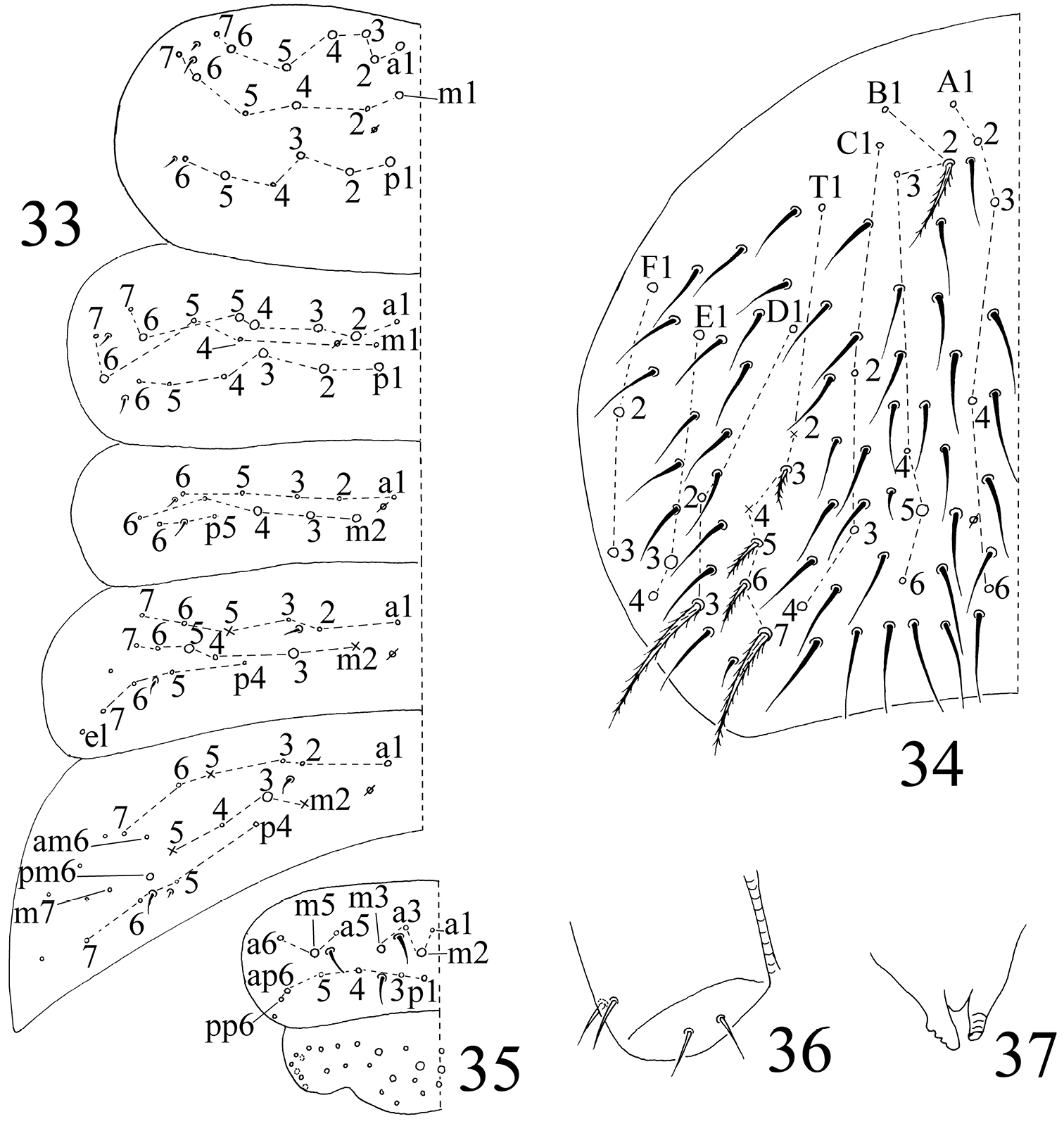
Chaetotaxy. Complete s-chaetae of body as 32/223(50–53)3, ms as 10/10100. Th. II with 13 (a1–6, m1, m4, m6, p1–3, p5) mac, 6 (a7, m2, m5, m7, p4, p6) mic and 3 s-chaetae (ms anterior to s), m1 rarely as mic. Th. III with 9 (a2–6, m6, p1–3) mac, 9 (a1, a7, m1, m4, m5, m7, p4, p6) mic and 2 s-chaetae. Abd. I with 3 (m2–4) mac, 9 (a1–3, a5, a6, m5, m6, p5, p6) mic and 2 s-chaetae (ms antero-external to s). Abd. II with 2 (m3, m5) mac, 13 (a1–3, a6, a7, m4, m6, m7, p4, p7, el) mic, 1 additional mic on lateral and 2 s-chaetae. Abd. III with 1 (m3) mac central, 13 (a1–3, a6, a7, m4, am6, pm6, m7, p4) mic, 3 s-chaetae and 5 lateral additional mic (Fig. 33). Abd. IV with 2 (B5, E3) mac, 27 (A1–4, A6, B1–4, B6, C1–4, T1, T3, T5, D1–3, E1, E4, F1–3) mic and 50–53 s-chaetae (48–51 elongated and 2 of normal length); setae A5 and E2 absent (Fig. 34). Abd. V with total 14 setae and 3 s-chaetae. Abd. VI with 21+21 setae; 3 on middle line (Fig. 35).
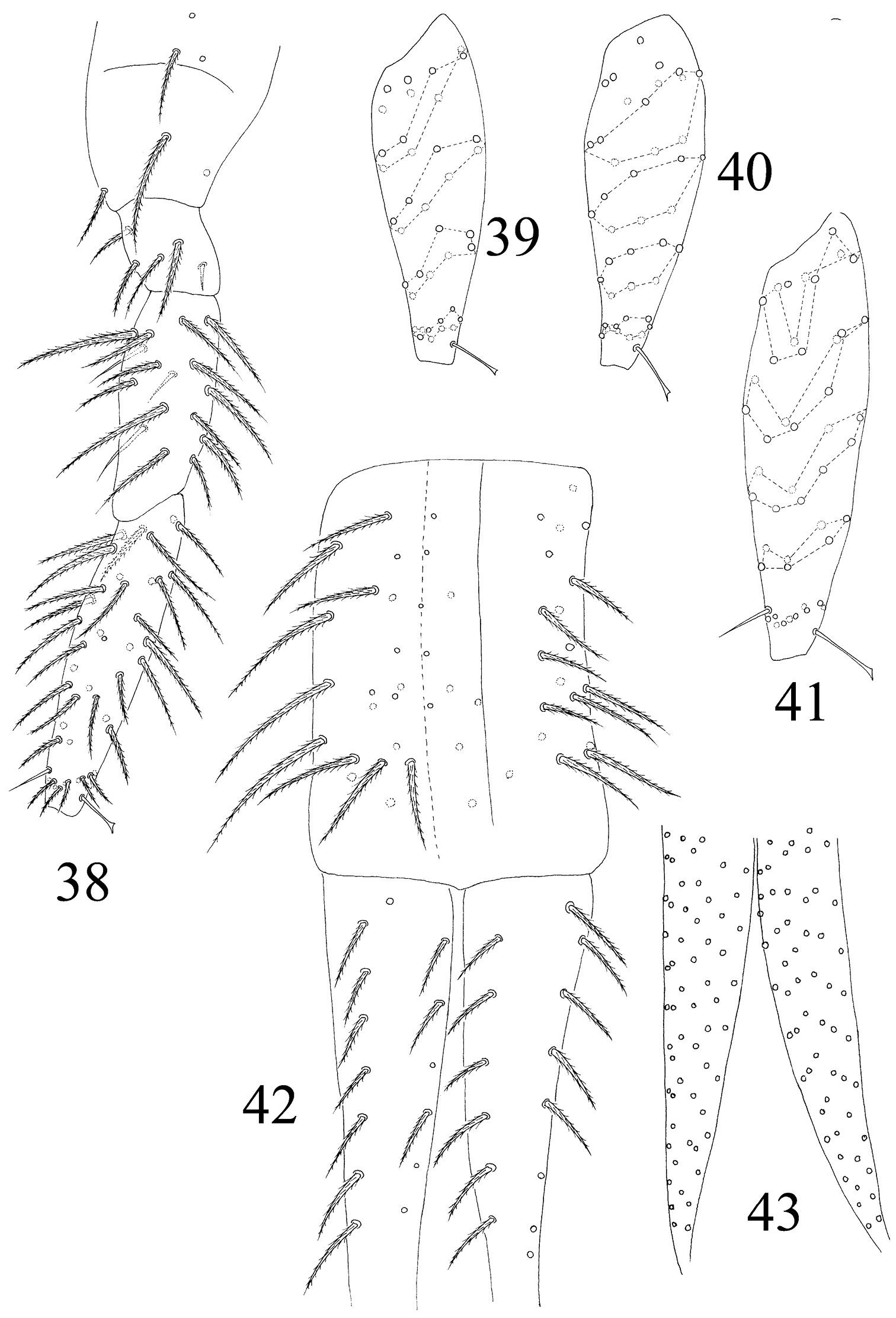
Leg. Coxa I–III with 2, 3, 5 ciliate setae. Trochanter I–III with 4, 5, 4 ciliate and 2, 1, 0 smooth setae, 1 spine on trochanter III. Femur I–III with 10, 16, 14 ciliate and 3, 1, 3 smooth setae. Tita I–III with 38, 41, 46 ciliate setae and 1 tenent hair respectively, 1 supraempodial seta on Tita III (Figs 38–41). Unguis with 4 minute inner and 2lateral teeth. Unguiculus lanceolate with outer edge slightly serrate (Fig. 80).
Ventral tube. Anterior face without seta; posterior face with 2 apical smooth setae; lateral flap with 2 smooth setae (Fig. 36).
Furcula. Manubrum with 24+24 ciliate setae. Manubrial plaque not seen. Dorsal dens with 14–15 (8 in outer 6–7 in inner row) setae, ventral side with about 55 ciliate setae, inner of dens without dental spine, basal setae (bs1 and bs2) absent (Figs 42, 43). Mucro bidentate with subapical tooth obviously larger than apical one; basal spine absent. Tenaculum with 4+4 teeth but without setae on corpus (Fig. 37).
In the leaf litter of Cunninghamia lanceolata (Lambert) and Dicranopteris dichotoma (Thunberg).
The new species is characterized by weak pigment on dorsal body, long antennae subequal to body in length, labial basal seta L1 ciliate, absence of setae m2i2 on Th. II, a1 on Abd. I, a2 on Abd. III, A4–5 on Abd. IV, and dental basal seta pi spiny and shorter than bs. It is similar to Homidia unichaeta Pan, Shi & Zhang, 2010 and Homidia tibetensis Chen & Zhong, 1998 in colour pattern, cephalic chaetotaxy, labrum, coxal formula, chaetotaxy of Abd. II and claw. However, it can be distinguished from them by the length of antennae, labial setal formula, chaetotaxy on Th. II, Abd. I, Abd. III–IV and seta pi on basal dens. Additional differences are listed in Table 1.
Some characters are principally same in the first instar larvae and adults: ommatidia, interocular setae, Ant. III organ, apical bulb on Ant. IV, labrum and labral papillae, labial palp, maxillary outer lobe, claw, bothriotricha and s-chaetoxic pattern on terga.
Characters that develop after the first instar: s on distal part of Ant. II, smooth setae on base of Ant. II, labial seta R, smooth spiny setae on trochanteral organ, mac on anterior face of ventral tube, seta on corpus of tenaculum, pseudopores on manubral plaque, basal spine on mucro and genital plate.
Chaetotaxy become more complicated during postembryonal development, detailed differences between the first instar larvae and adults (apart from chaetotaxy of body tergites) are listed in Table 2. Tergal chaetotaxy of adults becomes much more complicated than that of primary chaetotaxy. In addition to numerous secondary common mic and mac on terga, some primary mic are transformed into mac: m2, m5 and p4 on Th. II, a1, m5, p5 and p6 on Th. III, a2, a3 and a5 on Abd. I, a2 and a3 on Abd. II, am6 and p6 on Abd. III, A6 and B4 on Abd. IV (homology of lateral setae difficult to determine).
Habitus of Homidia jordanai sp. n. 1–2 adults, lateral view 3–4 the first instar larvae 3 dorsal view 4 lateral view.
Homidia jordanai sp. n. 5 dorsal cephalic chaetotaxy 6 apical bulb of Ant. IV 7 labrum 8 labial palp 9 labial base.
. Homidia jordanai sp. n. 10 dorsal chaetotaxy of Th. II–III 11 coxal chaetotaxy formula (A fore leg B mid leg C hind leg) 12–14 dorsalchaetotax 12 Abd. I–III 13 Abd. IV 14 Abd. V.
Hind leg of Homidia jordanai sp. n. 15 coxa, trochanter and femur 16 trochanteral organ 17 basal Tita 18 apical Tita 19 apical Tita and claw.
Homidia jordanai sp. n. 20–22 ventral tube 20 anterior face 21 posterior face 22 lateral flap 23 tenaculum 24 genital plate and spermary.
Furcula of Homidia jordanai sp. n. 25 manubrium 26 manubrial plaque and base of dens 27 inner side of dens (triangles representing spines) 28 apical dens and mucro.
The first instar larval Homidia jordanai sp. n. 29 dorsal cephalic chaetotaxy 30 Ant. I–III 31 Ant. IV 32 labal base
The first instar larval Homidia jordanai sp. n. 33–35 dorsal chaetotaxy 33 Th. II–Abd. III 34 Abd. IV 35 Abd. V–VI 36 ventral tube 37 tenaculum.
The first instar larval Homidia jordanai sp. n. 38 hind leg 39 Tita I 40 Tita II 41 Tita III 42 manubrium and dorsal dens 43 ventral dens.
SEM photos of adult Homidia jordanai sp. n. 44 eye patches 45 base of Ant. I, dorsal side 46 spiny setae on base of Ant. I, dorsal side 47 joint of Ant. I and Ant. II 48 spiny seta on base of Ant. II 49 maxillary outer lobe 50 labial palp 51 micro-architecture of proximal setae.
SEM photos of adult Homidia jordanai sp. n. 52 distal part of Ant. II 53 s on distal part of Ant. II 54 Ant. III organ 55 internal two s of Ant. III organ 56 external s of Ant. III organ 57 Ant. IV 58–59 s on Ant. IV.
SEM photos of adult Homidia jordanai sp. n. 60–62 s of Ant. IV 63 pseudopores of coxa 64 Tita and claw of hind leg 65 supraempodial seta and outer edge of unguiculus 66 distal part of tenent hair 67 spiny seta on pretarsus.
SEM photos of adult Homidia jordanai sp. n. 68 lateral s-chaetae on Abd. I 69 lateral ms on Abd. I 70 three types of setae on Abd. IV 71 bothriotrichum on Abd. IV 72 elongate s-chaetae on Abd. IV 73 ciliate seta on Abd. IV 74 distal part of ventral tube 75 two types of setae on lateral flap of ventral tube.
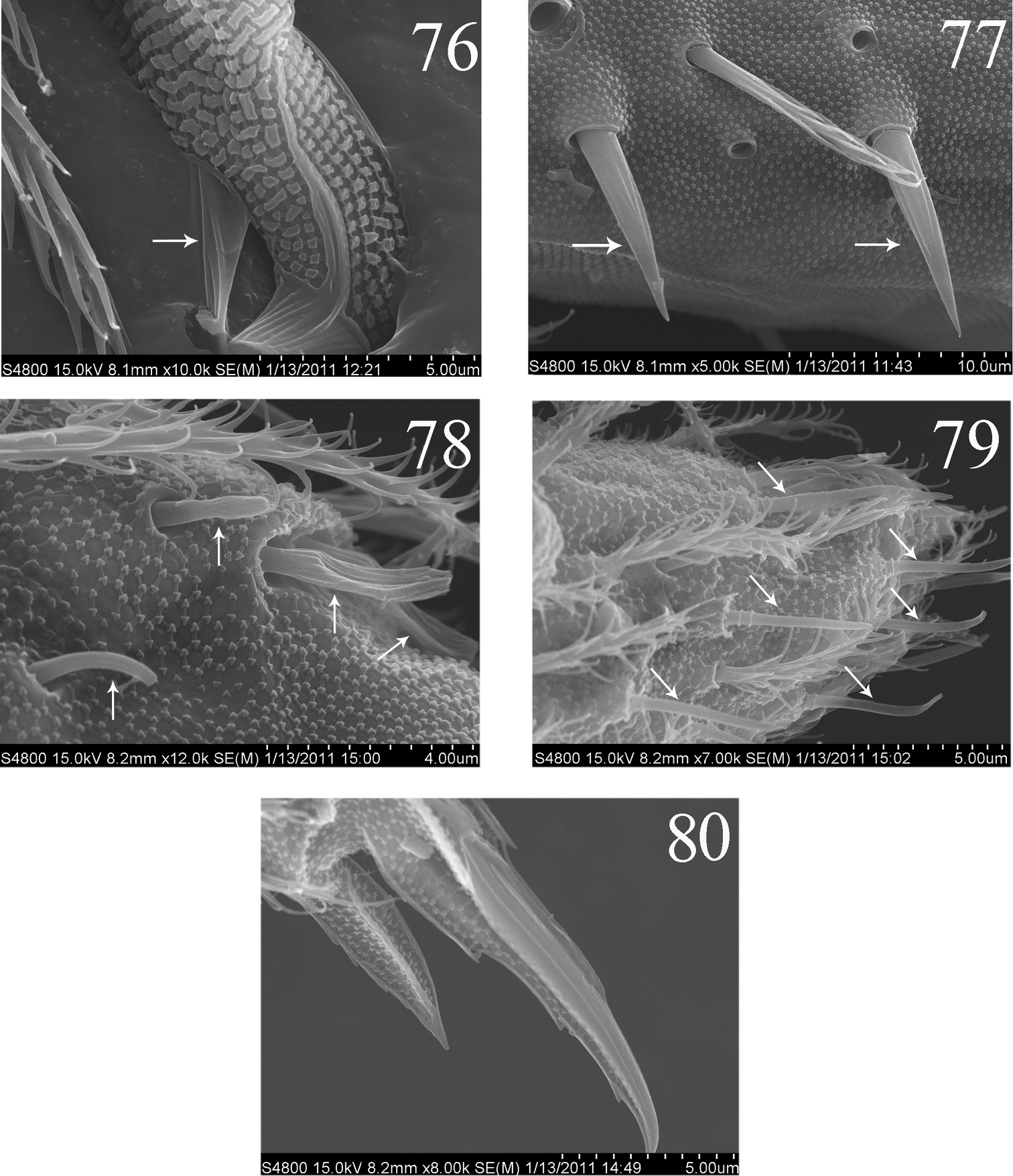
SEM photos of Homidia jordanai sp. n. 76–77 adult 76 partial mucro 77 dental spines 78–80 the first instar larva 78 Ant. III organ 79 distal part of Ant. IV 80 distal part of hind leg.
Main differences between Homidia jordanai sp. n. and two similar species.
| Characters | H. j | H. u | H. t |
| Pigment on dorsal tergite | - | - | slight |
| Antenna length as long as cephalic diagonal | 3.9–4.6 | 1.5–2.5 | about 3.5 times |
| Seta L1 on labial base | ciliate | smooth | smooth |
| Lateral prosess of labial papilla E | reach apex | not reach | ? |
| Seta m2i2 on Th. II | - | + | - |
| Seta a1 on Abd. I | - | + | + |
| Seta a2 on Abd. III | - | + | + |
| Mac on posterior Abd. IV | 2 (rarely 3) | 1 | 2 |
| “Eyebrow” setae on anterior Abd. IV | 6–9 | 3–8 (usually 5–7) | 10–12 |
| Dental spines | 20–40 | 19–23 | 39–54 |
| Comparison of dental basal seta in length | bs > pi | pi > bs | bs > pi |
| Type locality (China) | Zhejiang | Zhejiang | Tibet |
H. j: Homidia jordanai sp. n.; H. u: Homidia unichaeta; H. t: Homidia tibetensis; -: absent; +: present;?: the character unclear.
Differences between the first instar larvae and adults (apart from chaetotaxy of body tergites).
| Characters | First instar larvae | Adults |
| Ground colour | pale white | pale yellow |
| Cephalic chaetotaxy | 3An, 5S | 4An, 7S |
| Spiny setae on basal Ant. I | 1 | 2 dorsal, 4 ventral |
| S on distal Ant. II | - | 4 |
| Proximal setae of labium | 3 | 5 |
| Seta R on labial base | - | + |
| Coxal macrochaetal formula | 2/3/5 | 3/4+1, 3/4+2 |
| Setae on trochanteral organ of hind leg | 1 | 36–64 |
| Inner tibiotarsal setae | strongly ciliate | thicker and slightly serrate |
| Setae on anterior face of ventral tube | - | 4+4 mac and numerous mic |
| Setae on lateral flap of ventral tube | 2 smooth | 6–7 smooth and 10–24 ciliate |
| Setae on posterior face of ventral tube | 2 smooth | 5 or 6 smooth and numerous ciliate |
| Seta on tenaculum | - | 1 |
| “Eyebrow” setae on Abd. IV | - | 6–9 |
| Setae on manubrium | 24+24 | more than 24+24 |
| Pseudopores on manubrial plaque | - | 3 |
| Ciliate setae on manubrial plaque | - | 7–8 |
| Basal setae (bs1 and bs2) on dens | - | + |
| The shape of proximal-inner seta (pi) | normal | spiny |
| Dental spines | - | 20–40 |
| Basal spine of mucro | - | + |
| Genital plate | - | papillate |
-: absent; +: present.
Morphological differences of the first instar larvae among six Entomobyridae species.
| tergite | seta | H. j | O. f | H. n | E. m | P. a | S. d |
| Th. II | m1 | mac | mac | mic | mac | mac | mac |
| m2 | mic | mac | mic | mic | scale | mac | |
| p5 | mac | mac | mic | mic | mac | mac | |
| Th. III | a2 | mac | mic | mic | mic | mic | mac |
| a3 | mac | mic | mic | mic | mic | mic | |
| a4 | mac | mac | mic | mic | mac | mac | |
| m1 | mic | mic | mic | mic | - | mic | |
| m2 | - | - | - | - | mac | - | |
| Abd. I | m2 | mac | mac | mac | mic | mac | mac |
| m4 | mac | mac | mic | mac | mac | mac | |
| m6 | mic | mic | mic | mic | mic | mic | |
| Abd. II | a2 | mic | mac | mic | mic | mac | mac |
| m5 | mac | mac | mic | mac | mac | mac | |
| Abd. III | pm6 | mac | mac | mac | mac | mac | mac |
| p6 | mic | mic | mic | mac | mic | mac | |
| Abd. IV | A4 | mic | - | - | mic | - | - |
| A5 | - | - | - | - | mic | mic | |
| B4 | mic | - | - | mic | mic | mac | |
| B5 | mac | mic | mic | mac | mac | mac | |
| B6 | mic | mic | - | mic | mic | mic | |
| E2 | - | mac | mic | - | mic | mic | |
| E3 | mac | - | - | mac | mic | mac |
H. j: Homidia jordanai sp. n.; O. f: Orchesella flavescens (Bourlet, 1839); H. n: Heteromurus nitidus (Templeton, R in Templeton, R & Westwood, J. O, 1836); E. m: Entomobryoides myrmecophila (Reuter, 1884); P. a: Pseudosinella alba (Packard, 1873); S. d: Seira dowlingi (Wray, 1953); -: absent.
The adult chaetotaxy of Entomobryidae exhibits marked differences among genera or species. Szeptycki (1972) found that the primary chaetotaxy of Entomobryomorpha was almost identical in number and position. Later, he (1979) studied the ontogeny of tergal chaetotaxy of the representative Entomobryidae genera and its preliminary phylogenetic significance at higher level of hierarchy with four subfamilies included in Entomobryidae. Subsequent authors (
We compare the primary dorsal chaetotaxy of the body among Homidia jordanai sp. n. and another five species of family Entomobryidae. The morphology of some primary homologous setae under Szeptycki’s nomenclature exhibits stable differentiation (mac, mic or absent) at the first instar (Table 3), though number and position of them are apparently similar. Among six species, the primary tergal chaetotaxy of Homidia jordanai sp. n. is closest to Entomobryoides myrmecophila, almost identical in number and arrangement except s-chaetae on Abd. IV. Integrating its adult features, such as elongated Abd. IV, four antennal segments and absence of scales, the genus Homidia apparently belongs Entomobryini sensu (
It is still a long way to achieve the correct homology of setae in Entomobryidae. Further exploration at species level could be studied by thorough survey of more species and more characters of first instar, although first instar larvae are usually more difficult to collect than adults and subadults.
Morphology of the “smooth” setae under SEMSpiny setae on antennae are smooth under LM and SEM. S and ms on dorsal body smooth under LM, but not smooth under SEM (Figs 53–62, 68, 69, 72). Guard setae on labial palp, proximal setae, “smooth” setae on ventral tube, tenent hair and supraempodial seta on Tita III are also smooth under LM, but weakly ciliate under SEM (Figs 50, 51, 64–66, 74, 75). We have to carefully describe “smooth” setae in future (
We thank to Dr Frans Janssens (Belgium) and Prof. Christiansen K (USA) who provided some necessary literature for us. The present study was supported by The Zhejiang Provincial Natural Science Foundation of China (Y3100278 and Y3100149) and The Zhejiang Provincial Department of Educational Foundation of China (Y200700872).