Checklist |
|
Corresponding author: Tomáš Scholz ( tscholz@paru.cas.cz ) Academic editor: Boyko Georgiev
© 2017 Philippe V. Alves, Alain de Chambrier, Tomáš Scholz, José L. Luque.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Alves PV, de Chambrier A, Scholz T, Luque JL (2017) Annotated checklist of fish cestodes from South America. ZooKeys 650: 1-205. https://doi.org/10.3897/zookeys.650.10982
|
Abstract
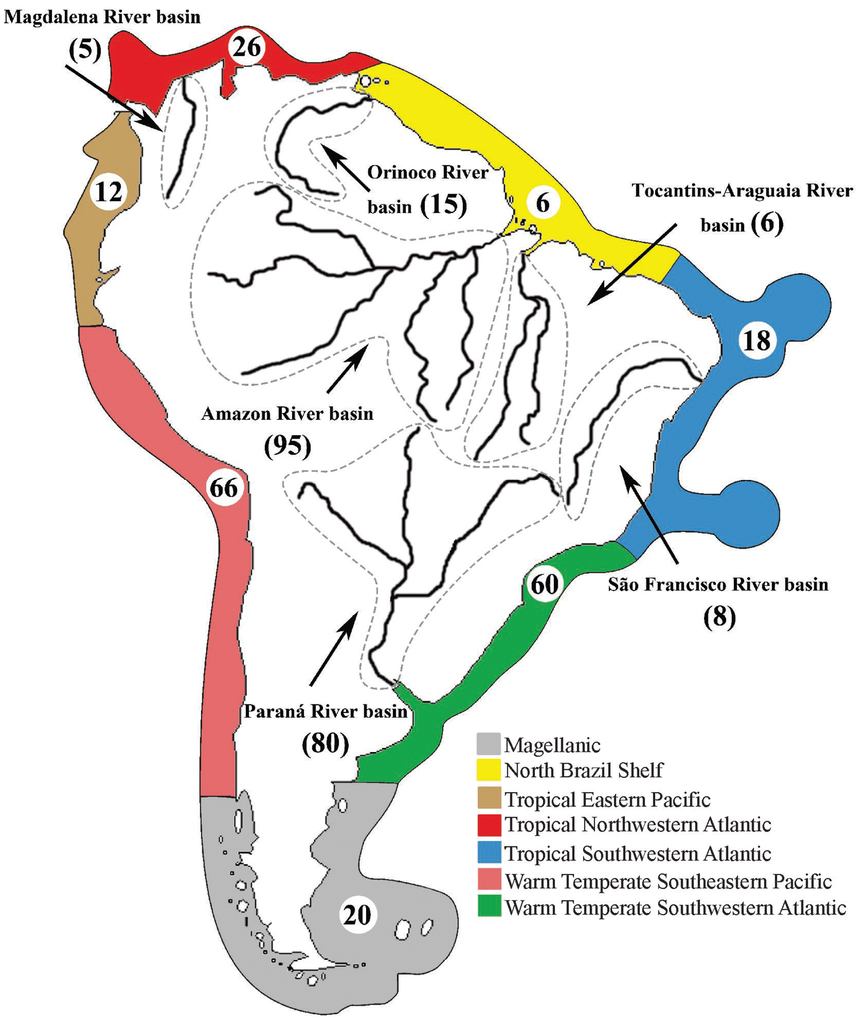
An exhaustive literature search supplemented by a critical examination of records made it possible to present an annotated checklist of tapeworms (Cestoda) that, as adults or larvae (metacestodes), parasitize freshwater, brackish water and marine fishes, i.e. cartilaginous and bony fishes, in South America. The current knowledge of their species diversity, host associations and geographical distribution is reviewed. Taxonomic problems are discussed based on a critical evaluation of the literature and information on DNA sequences of individual taxa is provided to facilitate future taxonomic and phylogenetic studies. As expected, the current knowledge is quite uneven regarding the number of taxa and host-associations reported from the principal river basins and marine ecoregions. These differences may not only reflect the actual cestode richness but may also be due to the research effort that has been devoted to unravelling the diversity of these endoparasitic helminths in individual countries. A total of 297 valid species, 61 taxa identified to the generic level, in addition to unidentified cestodes, were recorded from 401 species of fish hosts. Among the recognized cestode orders, 13 have been recorded in South America, with the Onchoproteocephalidea displaying the highest species richness, representing c. 50% of all species diversity. The majority of records include teleost fish hosts (79%) that harbour larval and adult stages of cestodes, whereas stingrays (Myliobatiformes) exhibit the highest proportion of records (39%) among the elasmobranch hosts. Fish cestodes are ubiquitous in South America, being mostly recorded from the Warm Temperate Southeastern Pacific (WTSP; 31%) for marine hosts and the Amazon River basin (45%) for freshwater ones. The following problems were detected during the compilation of literary data: (i) unreliability of many records; (ii) poor taxonomic resolution, i.e. identification made only to the genus or even family level; (iii) doubtful host identification; and (iv) the absence of voucher specimens that would enable us to verify identification. It is thus strongly recommended to always deposit representative specimens in any type of studies, including faunal surveys and ecological studies. An analysis of the proportion of three basic types of studies, i.e. surveys, taxonomic and ecological papers, has shown a considerable increase of ecological studies over the last decade.
Keywords
Biodiversity, marine ecoregions, river basins, species richness, tapeworms
Introduction
Tapeworms (Cestoda) are a monophyletic assemblage of flatworms (Phylum Platyhelminthes) and they are obligate internal parasites of vertebrates. Their complex life-cycles include one or more intermediate hosts in a wide array of animal phyla (mostly arthropods) and they are exclusively transmitted perorally, i.e. via the food chain (
Cestodes parasitizing elasmobranchs and teleost fishes in at least one stage of development comprise one of the most diverse lineages of tapeworms (
South America is a megadiverse continent, including at least five of the world’s biodiversity ‘hotspots’, more than 30.000 km of coastline and two of the 10 largest freshwater drainage systems of the world, i.e. the Amazon and Paraná River basins, which is reflected in its species-rich ichthyofauna (
Studies on fish cestodes from South America date back to the early 19th Century, when C. A. Rudolphi described Anthocephalus macrourus Rudolphi, 1819 from an unidentified sparid fish and Anthocephalus interruptum Rudolphi, 1819 (both cestodes of the order Trypanorhyncha) from Trichiurus lepturus Linnaeus off the Brazilian coast, even though these species are no longer valid (
Detailed taxonomic studies combining morphological and molecular approaches have recently expanded our knowledge at lower and higher taxonomic levels, mostly under the framework of the National Science Foundation (Planetary Biodiversity Inventory program) funded project called “A survey of the tapeworms (Cestoda: Platyhelminthes) from vertebrate bowels of the earth” (see http://tapewormdb.uconn.edu). This project funded, amongst others, intensive research on fish cestodes in South America, which were mainly undertaken by A. de Chambrier, T. Scholz and A. A. Gil de Pertierra for teleost hosts, and V. A. Ivanov, F. P. L. Marques and F. Reyda for elasmobranch hosts. The present paper aims at addressing the following objectives: (1) to provide for the first time an annotated checklist that summarizes records of cestodes in marine and freshwater fishes from South America, including detailed information on their hosts, site of infection, geographical distribution, stage of development and molecular data; (2) to critically assess some doubtful reports; and (3) to depict the problems that impede a better understanding of the diversity and host associations of cestodes in South America.
Materials and methods
Parasite-host and host-parasite checklists for fish cestodes from South America were compiled on the basis of an exhaustive search of literature published until August 2016; abstracts of meetings, theses and reports without primary data were not considered. The bibliographic search was complemented by the information gathered from Helminthological Abstracts, Host-Parasite Database of the Natural History Museum, London (
The species are arranged according to taxonomic categories and are presented in alphabetical order followed by data on their hosts (species name, class and family), habitat, site of infection, stage of development, marine ecoregion according to
Host species are arranged in taxonomic and then alphabetical order. The scientific names of hosts have been updated based on
The following abbreviations are used for collections:
BMNH
The British Museum (Natural History) Collection at the
MNHB Museum der Naturkunde für Humboldt Universität zu Berlin, Germany
The following abbreviations are used for marine ecoregions according to
JFD Juan Fernández and Desventuradas
NBS North Brazil Shelf
TEP Tropical Eastern Pacific
TNA Tropical Northwestern Atlantic
TSA Tropical Southwestern Atlantic
WTSA Warm Temperate Southwestern Atlantic
WTSP Warm Temperate Southeastern Pacific
The following abbreviations are used for molecular markers:
18S small subunit of the nuclear ribosomal RNA gene
ITS1 first nuclear ribosomal internal transcribed spacer
5.8S 5.8S ribosomal RNA gene
ITS2 second nuclear ribosomal internal transcribed spacer
28S large subunit of the nuclear ribosomal RNA gene
16S large subunit of the mitochondrial ribosomal RNA gene
cox1
cytochrome
The following abbreviation is used for records of metacestodes in the host-parasite list:
L larvae
* Asterisks in the parasite-host list indicate the type species of the genus.
Parasite-Host List
Class Cestoda Rudolphi, 1808
Order Amphilinidea Poche, 1922
Family Amphilinidae Claus, 1879
Nesolecithus janickii Poche, 1922*
[Syns. Monostoma liguloideum Diesing, 1850 (pro parte); Amphilina liguloidea Monticelli, 1892 sensu
Arapaima gigas (Actinopterygii: Arapaimidae); freshwater; body cavity; adult; Amazon River basin; Brazil (Janicki 1908;
Notes: type host.
Schizochoerus liguloideus (Diesing, 1850) Poche, 1922*
[Syns. Monostoma liguloideum Diesing, 1850 (pro parte); Amphilina liguloidea Monticelli, 1892]
Arapaima gigas (Actinopterygii: Arapaimidae); freshwater; body cavity; adult; Amazon River basin; Brazil, Peru (
Notes: type host.
Order Bothriocephalidea Kuchta, Scholz, Brabec & Bray, 2008
Family Bothriocephalidae Blanchard, 1849
Bothriocephalus timii Gil de Pertierra, Arredondo, Kuchta & Incorvaia, 2015
Cottoperca gobio (Actinopterygii: Bovichtidae); marine; intestine, pyloric caeca; adult; Magellanic; Argentina (
Notes: type host. Sequences of 18S (KR780929), 28S (KR780885), 16S (KR780839) and cox1 (KR780787) (
Bothriocephalus sp.
Eleginops maclovinus (Actinopterygii: Eleginopsidae); marine; intestine; adult; WTSP; Chile (
Engraulis anchoita (Actinopterygii: Engraulidae); marine; pyloric caeca; adult; Magellanic, WTSA; Argentina (
Engraulis ringens (Actinopterygii: Engraulidae); marine; intestine; adult; WTSP; Chile (
Helicolenus lengerichi (Actinopterygii: Sebastidae); marine; intestine; adult; WTSP; Chile (
Clestobothrium crassiceps (Rudolphi, 1819) Lühe, 1899*
[Syn. Bothriocephalus crassiceps Rudolphi, 1819]
Aphos porosus (Actinopterygii: Batrachoididae); marine; intestine; adult (immature); WTSP; Chile (
Dissostichus eleginoides (Actinopterygii: Nototheniidae); marine; intestine, pyloric caeca; stage of development not given; Magellanic; Falkland Islands (
Note: R. Kuchta (pers. comm.) suggested that this report might be wrong.
Macruronus magellanicus (Actinopterygii: Merlucciidae); marine; intestine;
adult; Magellanic; Argentina, Chile (
Merluccius gayi gayi (Actinopterygii: Merlucciidae); marine; intestine; adult; WTSP; Chile (
Note:
Merluccius gayi peruanus (Actinopterygii: Merlucciidae); marine; intestine; adult; WTSP; Peru (
Merluccius sp. (Actinopterygii: Merlucciidae); marine; intestine; adult; Magellanic, WTSP; Argentina, Chile (
Note:
Micromesistius australis australis (Actinopterygii: Gadidae); marine; intestine; adult; Magellanic; Chile (
Clestobothrium cristinae Gil de Pertierra, Incorvaia & Arrendondo, 2011
Merluccius hubbsi (Actinopterygii: Merlucciidae); marine; intestine; adult; Magellanic; Argentina (
Notes: type host. R. Kuchta (pers. comm.) suggested that all reports from M. hubbsi before the original description of C. cristinae were erroneously reported as C. crassiceps. Sequences of 18S (KR780948), 28S (KR780901), 16S (KR780862) and cox1 (KR7808301) (
Clestobothrium splendidum Gil de Pertierra, Incorvaia & Arredondo, 2011
Merluccius australis (Actinopterygii: Merlucciidae); marine; intestine; adult; Magellanic; Argentina, Chile (
Notes: type host. R. Kuchta (pers. comm.) suggested that all reports from M. australis before the original description of C. splendidum as well as that of
Schyzocotyle acheilognathi (Yamaguti, 1934) Brabec, Waeschenbach, Scholz, Littlewood & Kuchta, 2015*
[For synonyms, see
Cyprinus carpio (Actinopterygii: Cyprinidae); freshwater; intestine; adult; Paraná State (fishpond), Negro River basin; Argentina, Brazil (
Notes: these reports from South America are probably result of the import of common carp from Europe to Brazil (
Pethia conchonius (Actinopterygii: Cyprinidae); freshwater; intestine; adult; Santa Catarina State; Brazil (
Notes: host reported as Puntius conchonius.
Poecilia reticulata (Actinopterygii: Poeciliidae); freshwater; intestine; adult; Paraná River Basin; Brazil (
Note: tapeworms reported as ‘Pseudophyllidea’, but considered as S. acheilognathi by R. Kuchta (pers. comm.).
Xiphophorus hellerii (Actinopterygii: Poeciliidae); freshwater; intestine; adult; Santa Catarina State; Brazil (
Xiphophorus maculatus (Actinopterygii: Poeciliidae); freshwater; intestine; adult; Santa Catarina State; Brazil (
Notes: P. conchonius, X. hellerii and X. maculatus are ornamental fish imported to South America (
Senga sp.
Astyanax altiparanae (Actinopterygii: Characidae); freshwater; pyloric caeca; adult; Rio das Pedras Farm (lakes); Brazil (
Astyanax scabripinnis (Actinopterygii: Characidae); freshwater; intestine; adult; São Paulo State; Brazil (
Unidentified bothriocephalid cestode
Girella laevifrons (Actinopterygii: Kyphosidae); marine; site of infection not given; adult; WTSP; Chile (
Family Echinophallidae Schumacher, 1914
Neobothriocephalus aspinosus Mateo & Bullock, 1966*
Seriolella violacea (Actinopterygii: Centrolophidae); marine; intestine, stomach; adult; WTSP; Chile, Peru (
Notes: type host; it was originally reported as Neptomenus crassus. Sequences of 18S (KR780944), 28S (KR780897), 16S (KR780857) and cox1 (KR780805) (
Neobothriocephalus sp.
Hippoglossina macrops (Actinopterygii: Paralichthyidae); marine; intestine; adult; WTSP; Chile (
Note: all but one authors reported the cestode as N. aspinosus.
Paralichthys adspersus (Actinopterygii: Paralichthyidae); marine; intestine; adult; WTSP; Chile (
Paralichthys microps (Actinopterygii: Paralichthyidae); marine; intestine; adult; WTSP; Chile (
Parabothriocephalus sp.
Macrourus holotrachys (Actinopterygii: Macrouridae); marine; intestine, pyloric caeca; adult; WTSP: Chile (
Family Triaenophoridae Lönnberg, 1899
Ailinella mirabilis Gil de Pertierra & Semenas, 2006*
Aplochiton zebra (Actinopterygii: Galaxiidae); amphidromous; intestine; adult; Patagonian lakes; Argentina (
Note:
Galaxias maculatus (Actinopterygii: Galaxiidae); amphidromous; intestine; adult; Moreno and Nahuel Huapi Lake systems (Andean-Patagonian region); Argentina (
Notes: type host.
Anchistrocephalus microcephalus (Rudolphi, 1819) Monticelli, 1890*
[For synonyms, see
Mola mola (Actinopterygii: Molidae); marine; intestine; adult; WTSA; Brazil (
Notes: type host. The tapeworms were reported as Amphigonophorus carvalhoi Mendes, 1944.
Mola ramsayi (Actinopterygii: Molidae); marine; intestine; adult; WTSP; Chile (
Anonchocephalus argentinensis Szidat, 1961
Xystreurys rasile (Actinopterygii: Paralichthyidae); marine; intestine; adult; WTSA; Argentina (
Anonchocephalus chilensis (Riggenbach, 1896) Lühe, 1902*
[Syn. Bothriotaenia chilensis Riggenbach, 1896]
Genypterus blacodes (Actinopterygii: Ophidiidae); marine; intestine; adult; WTSA, WTSP; Argentina, Chile (
Notes:
Genypterus brasiliensis (Actinopterygii: Ophidiidae); marine; intestine; adult; WTSA; Argentina, Brazil (
Note:
Genypterus chilensis (Actinopterygii: Ophidiidae); marine; intestine, pyloric caeca; adult; WTSP; Chile (
Note: type host.
Genypterus maculatus (Actinopterygii: Ophidiidae); marine; intestine; adult; WTSP; Chile (
Anonchocephalus patagonicus Suriano & Labriola, 1998
Paralichthys patagonicus (Actinopterygii: Paralichthyidae); marine; intestine; adult; Magellanic; Argentina (Suriano and Labriola, 1998).
Note: type host.
Anonchocephalus sp.
Pinguipes brasilianus (Actinopterygii: Pinguipedidae); marine; intestine; adult; WTSA; Argentina (
Galaxitaenia toloi Gil de Pertierra & Semenas, 2005*
Galaxias platei (Actinopterygii: Galaxiidae); amphidromous; intestine; adult; Moreno Lake system (Patagonian region); Argentina (
Notes: type host.
Unidentified bothriocephalideans
Aplochiton taeniatus (Actinopterygii: Galaxiidae); amphidromous; intestine; adult; Patagonian lakes; Argentina (
Note: reported as Nippotaenia sp. but the nippotaeniids are not found in the Americas (
Cichla monoculus (Actinopterygii: Cichlidae); freshwater; intestine; adult; Rio das Pedras Farm (lakes); Brazil (
Note: reported as B. cuspidatus and considered as misidentification by R. Kuchta (pers. comm.).
Odontesthes smitti (Actinopterygii: Atherinopsidae); marine; intestine; metacestode; Magellanic; Argentina (
Oncorhynchus mykiss (Actinopterygii: Salmonidae); anadromous; intestine; adult; Moreno and Nahuel Huapi lakes (Patagonian region); Argentina (
Note: reported as Nippotaenia sp.
Paralabrax humeralis (Actinopterygii: Serranidae); marine; intestine; adult; WTSP; Chile (
Percichthys trucha (Actinopterygii: Percichthyidae); freshwater; intestine; adult; Moreno and Nahuel Huapi lakes (Patagonian region); Argentina (
Note: reported as Nippotaenia sp.
Percophis brasiliensis (Actinopterygii: Percophidae); marine; mesentery; metacestode; WTSA; Argentina (
Plagioscion squamosissimus (Actinopterygii: Sciaenidae); freshwater; intestine; adult; Amazon River basin; Brazil (
Note: host reported as P. squamosissima and its tapeworms as an unidentified ptychobothriid (
Salvelinus fontinalis (Actinopterygii: Salmonidae); anadromous; intestine; adult; Moreno and Nahuel Huapi lakes (Patagonian region); Argentina (
Note: reported as Nippotaenia sp.
Unidentified bothriocephalideans (identified as ‘Pseudophyllidea’)
Eleginops maclovinus (Actinopterygii: Eleginopsidae); marine; intestine; adult; Magellanic; Falkland Islands (
Paralichthys adspersus (Actinopterygii: Paralichthyidae); marine; intestine; adult; WTSP; Chile (
Prolatilus jugularis (Actinopterygii: Pinguipedidae); marine; intestine; adult; Magellanic; Chile (
Order Caryophyllidea van Beneden in Carus, 1863
[Caryophyllidean tapeworms do not occur in the Neotropical region, where their common hosts, i.e. cyprinid and catostomid fishes, are absent; therefore, these reports need verification]
Unidentified caryophyllideans
Cyprinus carpio (Actinopterygii: Cyprinidae); freshwater; intestine; adult; Paraná State; Brazil (
Note: introduced fish host (
Geophagus brasiliensis (Actinopterygii: Cichlidae); freshwater; intestine; adult; Paraná State (dams); Brazil (
Order Cathetocephalidea Schmidt & Beveridge, 1990
Family Cathetocephalidae Dailey & Overstreet 1973
Cathetocephalus australis Schmidt & Beveridge, 1990
Carcharhinus brachyurus (Elasmobranchii: Carcharhinidae); marine; spiral valve; adult; WTSA; Argentina (
Note: type host.
Cathetocephalus thatcheri Dailey & Overstreet, 1973*
Carcharhinus leucas (Elasmobranchii: Carcharhinidae); marine; spiral valve; adult; WTSP; Peru (
Note: type host.
Family Disculicepitidae Joyeux & Baer, 1935
Disculiceps galapagoensis Nock & Caira, 1988
Carcharhinus longimanus (Elasmobranchii: Carcharhinidae); marine; spiral valve; adult; Galapagos; Ecuador (
Note: type host.
Disculiceps pileatus (Linton, 1890) Joyeux & Baer, 1936*
[Syn. Discocephalum pileatum Linton, 1890]
Carcharhinus porosus (Elasmobranchii: Carcharhinidae); marine; spiral valve; adult; WTSP; Peru (
Note: tapeworms reported as Discocephalum pileatum.
Disculiceps sp.
Aetobatus narinari (Elasmobranchii: Myliobatidae); marine; spiral valve; adult; TNA; Venezuela (
Note: these specimens probably belong to Tylocephalum (Lecanicephalidea), according to
Order Cyclophyllidea van Beneden in Braun, 1900
Family Gryporhynchidae Spassky & Spasskaya, 1973
Glossocercus auritus (Rudolphi, 1819) Bona, 1994
[For synonyms, see
Poecilia reticulata (Actinopterygii: Poeciliidae); freshwater; mesentery; metacestode; Pampulha Dam, Minas Gerais State; Brazil (
Parvitaenia macropeos (Wedl, 1855) Baer & Bona, 1960
[For synonyms, see
Australoheros facetus (Actinopterygii: Cichlidae); freshwater; intestine; metacestode; Pampulha Dam, Minas Gerais State; Brazil (
Valipora campylancristrota (Wedl, 1855) Baer & Bona, 1960
[For synonyms, see
Geophagus brasiliensis (Actinopterygii: Cichlidae); freshwater; gallbladder; metacestode; Paraná State (dams); Brazil (
Hoplosternum littorale (Actinopterygii: Callichthyidae); freshwater; gallbladder; metacestode; Paraná River basin; Brazil (
Prochilodus lineatus (Actinopterygii: Prochilodontidae); freshwater; gallbladder; metacestode; Paraná River basin; Brazil (
Valipora sp.
Crenicichla britskii (Actinopterygii: Cichlidae); freshwater; gallbladder; metacestode; Paraná River basin; Brazil (
Pimelodus maculatus (Actinopterygii: Pimelodidae); freshwater; gallbladder; metacestode; Paraná River basin; Brazil (
Prochilodus argenteus (Actinopterygii: Prochilodontidae); freshwater; gallbladder; metacestode; São Francisco River basin; Brazil (
Unidentified cyclophyllideans
Dormitator maculatus (Actinopterygii: Eleotridae); amphidromous; liver, intestine, gonads; metacestode; TNA; Venezuela (
Percichthys trucha (Actinopterygii: Percichthyidae); freshwater; body cavity; metacestode; Negro River basin (Patagonian region); Argentina (
Satanoperca pappaterra (Actinopterygii: Cichlidae); freshwater; site of infection and stage of development not given; Paraná River basin; Brazil (
Order Diphyllidea van Beneden in Carus, 1863
Family Echinobothriidae Perrier, 1897
Ahamulina catarina Marques, Jensen & Caira, 2012*
Scyliorhinus besnardi (Elasmobranchii: Scyliorhinidae); marine; spiral valve; adult; WTSA; Brazil (
Notes: type host. Sequences of partial 18S (KC860176–KC860180), 28S (KC860128–KC860132) and cox1 (KC860220–KC860224) (
Coronocestus notoguidoi (Ivanov, 1997) Caira, Marques, Jensen, Kuchta & Ivanov, 2013
[Syn. Echinobothrium notoguidoi Ivanov, 1997]
Mustelus schmitti (Elasmobranchii: Triakidae); marine; spiral valve; adult; WSTA; Argentina (
Notes: type host.
Halysioncum euzeti (Campbell & Carvajal, 1980) Caira, Marques, Jensen, Kuchta & Ivanov, 2013
[Syn. Echinobothrium euzeti Campbell & Carvajal, 1980]
Sympterygia lima (Elasmobranchii: Arhynchobatidae) marine; spiral valve; adult; WTSP; Chile (
Notes: type host; it was originally reported as Psammobatis lima.
Halysioncum megacanthum (Ivanov & Campbell, 1998) Caira, Marques, Jensen, Kuchta & Ivanov, 2013
[Syn. Echinobothrium megacanthum Ivanov & Campbell, 1998]
Myliobatis goodei (Elasmobranchii: Myliobatidae); marine; spiral valve; adult; Magellanic; Argentina (
Notes: type host.
Halysioncum pigmentatum (Ostrowski de Núñez, 1971) Caira, Marques, Jensen, Kuchta & Ivanov, 2013
[Syn. Echinobothrium pigmentatum Ostrowski de Núñez, 1971]
Zapteryx brevirostris (Elasmobranchii: Rhinobatidae); marine; spiral valve; adult; WTSA; Argentina (
Note: type host.
Unidentified diphyllideans
Notothenia cf. angustata (Actinopterygii: Nototheniidae); marine; intestine; metacestode; WTSP; Chile (
Sebastes capensis (Actinopterygii: Sebastidae); marine; unspecified site of infection; metacestode; WTSP; Chile (
Note: two morphotypes were distinguished by
Order Diphyllobothriidea Kuchta, Scholz, Brabec & Bray, 2008
Family Diphyllobothriidae Lühe, 1910
Adenocephalus pacificus Nybelin, 1931
[For synonyms, see
Anisotremus scapularis (Actinopterygii: Haemulidae); marine; body cavity, viscera; metacestode; WTSP; Peru (
Ariopsis seemanni (Actinopterygii: Ariidae); brackish, marine; peritoneum; metacestode; WTSP; Peru (
Note:
Cilus gilberti (Actinopterygii: Sciaenidae); marine; viscera; metacestode; WTSP; Peru (
Cynoscion analis (Actinopterygii: Sciaenidae); marine; viscera; metacestode; WTSP; Peru (
Galeichthys peruvianus (Actinopterygii: Ariidae); marine; viscera, peritoneum; metacestode; WTSP; Peru (
Note:
Genypterus maculatus (Actinopterygii: Ophidiidae); marine; viscera; metacestode; WTSP; Peru (
Menticirrhus ophicephalus (Actinopterygii: Sciaenidae); marine; body cavity, viscera; metacestode; WTSP; Peru (
Merluccius gayi peruanus (Actinopterygii: Merlucciidae); marine; peritoneum, viscera, stomach surface; metacestode; WTSP; Peru (
Note:
Paralabrax humeralis (Actinopterygii: Serranidae); marine; site of infection not given; metacestode; WTSP; Peru (
Paralichthys adspersus (Actinopterygii: Paralichthyidae); marine; stomach surface; metacestode; WTSP; Peru (
Paralonchurus peruanus (Actinopterygii: Sciaenidae); marine; intestinal surface; metacestode; WTSP; Peru (
Note: the author reported the host as Polyclemus peruanus.
Sarda chiliensis (Actinopterygii: Scombridae); marine; body cavity; metacestode; WTSP; Peru (
Notes: the genus Adenocephalus Nybelin, 1931 was resurrected by
Sciaena callaensis (Actinopterygii: Sciaenidae); marine; peritoneum, stomach surface; metacestode; WTSP; Peru (
Sciaena deliciosa (Actinopterygii: Sciaenidae); marine; peritoneum, stomach surface, viscera; metacestode; WTSP; Peru (
Note:
Scomberomorus sierra (Actinopterygii: Scombridae); marine; body cavity; metacestode; WTSP; Peru (
Note: host reported as S. maculatus.
Seriolella violacea (Actinopterygii: Centrolophidae); marine; peritoneum; metacestode; WTSP; Peru (
Trachinotus paitensis (Actinopterygii: Carangidae); marine; peritoneum; metacestode; WTSP; Peru (
Trachurus murphyi (Actinopterygii: Carangidae); marine; body cavity; metacestode; WTSP; Peru (
Diphyllobothrium dendriticum (Nitzsch, 1824) Lühe, 1910
[For synonyms, see
Basilichthys australis (Actinopterygii: Atherinopsidae); freshwater; mesentery, liver, muscles; metacestode; Riñihue and Panguipulli Lakes; Chile (
Galaxias maculatus (Actinopterygii: Galaxiidae); amphidromous; liver, body cavity, intestinal surface; metacestode; Nahuel Huapi (Patagonian region), Riñihue Lakes; Argentina, Chile (Ortubay 1994;
Odontesthes mauleanum (Actinopterygii: Atherinopsidae); freshwater; mesentery; metacestode; Panguipulli Lake; Chile (
Oncorhynchus kisutch (Actinopterygii: Salmonidae); anadromous; intestinal surface, mesentery, spleen; metacestode; Llanquihue Lake; Chile (
Oncorhynchus mykiss (Actinopterygii: Salmonidae); anadromous; body cavity, mesentery, internal organs, muscles; metacestode; lakes of Valdivia River basin, Huechulaufquen, Rosario, Moreno and Nahuel Huapi Lakes (Patagonian region), lakes of Chiloé Island; Argentina, Chile (
Notes: tapeworms described as D. microcordiceps by
Percichthys trucha (Actinopterygii: Percichthyidae); freshwater; body cavity, mesentery, internal organs, muscles; metacestode; Riñihue Lake; Chile (
Percilia gillissi (Actinopterygii: Perciliidae); freshwater; body cavity, mesentery, internal organs, muscles; metacestode; Riñihue Lake; Chile (
Salmo salar (Actinopterygii: Salmonidae); anadromous; muscles; metacestode; Nahuel Huapi Lake (Patagonian region); Argentina (
Salmo trutta (Actinopterygii: Salmonidae); anadromous; body cavity; metacestode; Huechulaufquen and Rosario Lakes (Patagonian region), Valdivia River basin; Argentina, Chile (
Salvelinus fontinalis (Actinopterygii: Salmonidae); anadromous; body cavity, muscles; metacestode; Huechulaufquen, Rosario, Moreno, Nahuel Huapi Lakes (Patagonian region); Argentina (
Diphyllobothrium latum (Linnaeus, 1758) Lühe, 1910
[For synonyms see
Basilichthys australis (Actinopterygii: Atherinopsidae); freshwater; muscles; metacestode; Panguipulli Lake; Chile (
Diplomystes camposensis (Actinopterygii: Diplomystidae); freshwater; liver; metacestode; Riñihue Lake; Chile (
Note: the authors reported the tapeworms as Diphyllobothrium sp., but
Galaxias maculatus (Actinopterygii: Galaxiidae); amphidromous; body cavity, muscles; metacestode; Panguipulli and Riñihue Lakes; Chile (
Galaxias platei (Actinopterygii: Galaxiidae); amphidromous; body cavity; metacestode; Valdivia River Basin; Chile (
Odontesthes mauleanum (Actinopterygii: Atherinopsidae); freshwater; liver, gonads, mesentery, muscles; metacestode; Panguipulli Lake; Chile (
Oncorhynchus mykiss (Actinopterygii: Salmonidae); anadromous; body cavity, internal organs, muscles; metacestode; lakes of Valdivia River basin, Huechulaufquen, Rosario, Moreno and Nahuel Huapi Lakes (Patagonian region); Argentina, Chile (
Note: host reported as Salmo gairdneri by some authors.
Percichthys trucha (Actinopterygii: Percichthyidae); freshwater; body cavity, muscles; metacestode; lakes of Valdivia River basin; Chile (
Percichthys sp. (Actinopterygii: Percichthyidae); freshwater; body cavity, pyloric caeca, liver, stomach, gonads, muscles; metacestode; Moreno and Nahuel Huapi Lakes (Patagonian region); Argentina (
Salmo trutta (Actinopterygii: Salmonidae); anadromous; body cavity; metacestode; lakes of Valdivia River basin, Huechulaufquen and Rosario Lakes (Patagonian region); Argentina, Chile (
Note: host reported as Salmo trutta trutta and Salmo trutta fario by some authors.
Salvelinus fontinalis (Actinopterygii: Salmonidae); anadromous; liver; metacestode; Huechulaufquen, Moreno, Nahuel Huapi and Rosario Lakes (Patagonian region); Argentina (
Diphyllobothrium sp.
[Reports from freshwater fishes most likely correspond to D. dendriticum or D. latum (R. Kuchta, pers. comm.). All reports of unidentified diphyllobothrideans are included in this section]
Basilichthys australis (Actinopterygii: Atherinopsidae); freshwater; liver; metacestode; Riñihue Lake; Chile (
Cilus gilberti (Actinopterygii: Sciaenidae); marine; site of infection not given; metacestode; WTSP; Chile (
Cynoscion analis (Actinopterygii: Sciaenidae); marine; body cavity, peritoneum, stomach surface; metacestode; WTSP; Peru (
Engraulis ringens (Actinopterygii: Engraulidae); marine; site of infection not given; metacestode; WTSP; Chile (
Galaxias maculatus (Actinopterygii: Galaxiidae); amphidromous; body cavity, liver; metacestode; Moreno Lake (Patagonian region); Argentina (Ortubay 1994;
Galaxias platei (Actinopterygii: Galaxiidae); amphidromous; liver; metacestode; Riñihue Lake; Chile (
Genypterus brasiliensis (Actinopterygii: Ophidiidae); marine; body cavity, intestinal serosa, intestine, muscles; metacestode; WTSA; Brazil (
Genypterus maculatus (Actinopterygii: Ophidiidae); marine; body cavity, peritoneum, stomach surface; metacestode; WTSP; Chile, Peru (
Lophius gastrophysus (Actinopterygii: Lophiidae); marine; body cavity, intestinal serosa; metacestode; WTSA; Brazil (
Merluccius australis (Actinopterygii: Merlucciidae); marine; stomach wall; metacestode; Magellanic, WTSP; Chile, Falkland Islands (
Merluccius gayi peruanus (Actinopterygii: Merlucciidae); marine; body cavity, mesentery, peritoneum, stomach surface; metacestode: WTSP; Peru (
Merluccius hubbsi (Actinopterygii: Merlucciidae); marine; stomach wall; metacestode; Magellanic; Argentina, Falkland Islands (
Micromesistius australis australis (Actinopterygii: Gadidae); marine; site of infection not given; metacestode; WTSP; Chile (
Odontesthes regia (Actinopterygii: Atherinopsidae); freshwater; liver, gonads; metacestode; Riñihue Lake; Chile (
Oncorhynchus kisutch (Actinopterygii: Salmonidae); anadromous; stomach, spleen, liver, mesentery, gonads; metacestode; Aisén River basin; Chile (
Oncorhynchus mykiss (Actinopterygii: Salmonidae); anadromous; body cavity, internal organs, mesentery, muscles; metacestode; lakes of Valdivia River basin, Moreno and Nahuel Huapi Lakes (Patagonian region), Tarahuin Lake (Chiloe Island); Argentina, Chile (Wolffhügel 1949;
Notes: host reported as S. gairdneri or S. gairdneri irideus by some authors. After experimental infections of small rodents with metacestodes,
Paralichthys isosceles (Actinopterygii: Paralichthyidae); marine; body cavity, mesentery, liver, ovary, stomach; metacestode; WTSA; Brazil (
Note:
Paralonchurus peruanus (Actinopterygii: Sciaenidae); marine; body cavity; metacestode; WTSP; Peru (
Note: host reported as Polyclemus peruanus.
Salmo trutta (Actinopterygii: Salmonidae); anadromous; body cavity, peritoneum, liver, mesentery, muscles; metacestode; Rupanco and Calafquén Lakes, Moreno and Nahuel Huapi Lakes (Patagonian region); Argentina, Chile (
Note: host reported as S. trutta fario or S. trutta trutta by some authors.
Salvelinus fontinalis (Actinopterygii: Salmonidae); anadromous; body cavity, muscles; metacestode; Moreno and Nahuel Huapi Lakes (Patagonian region); Argentina (
Sciaena callaensis (Actinopterygii: Sciaenidae); marine; peritoneum, stomach surface, body cavity; metacestode; WTSP; Peru (
Sciaena deliciosa (Actinopterygii: Sciaenidae); marine; body cavity, intestinal surface; metacestode; WTSP; Peru (
Scomber japonicus (Actinopterygii: Scombridae); marine; site of infection not given; metacestode; WTSP; Peru (
Sebastes capensis (Actinopterygii: Sebastidae); marine; site of infection not given; metacestode; Magellanic; Chile (
Trachurus murphyi (Actinopterygii: Carangidae); marine; liver; metacestode; WTSP; Chile, Peru (
Unidentified ‘Pseudophyllidea’ (larval stages)
[Larval stages found in the body cavity and mesentery are most likely species of Diphyllobothrium (R. Kuchta, pers. comm.)]
Aphos porosus (Actinopterygii: Batrachoididae); marine; body cavity; metacestode; WTSP; Chile (
Balistes capriscus (Actinopterygii: Balistidae); marine; site of infection not given; metacestode; WTSA; Brazil (
Dissostichus eleginoides (Actinopterygii: Nototheniidae); marine; anterior
intestine; metacestode; Magellanic; Chile, Falkland Islands (
Engraulis anchoita (Actinopterygii: Engraulidae); marine; mesentery; metacestode; WTSA; Argentina (
Gobiesox marmoratus (Actinopterygii: Gobiesocidae); marine; site of infection not given; metacestode; WTSP; Chile (
Helcogrammoides chilensis (Actinopterygii: Tripterygiidae); marine; site of infection not given; metacestode; WTSP; Chile (
Hypsoblennius sordidus (Actinopterygii: Blenniidae); marine; intestine; metacestode; Magellanic; Chile (
Macruronus magellanicus (Actinopterygii: Merlucciidae); marine; body cavity; metacestode; Magellanic; Argentina, Chile (
Merluccius gayi gayi (Actinopterygii: Merlucciidae); marine; stomach wall; metacestode; WTSP; Chile (
Merluccius hubbsi (Actinopterygii: Merlucciidae); marine; mesentery; metacestode; Magellanic; Argentina (
Note:
Micromesistius australis australis (Actinopterygii: Gadidae); marine; unspecified site of infection; metacestode; Magellanic; Chile (
Odontesthes regia (Actinopterygii: Atherinopsidae); marine; intestine; metacestode; Magellanic; Chile (
Percophis brasiliensis (Actinopterygii: Percophidae); marine; mesentery; metacestode; WTSA; Argentina, Uruguay (
Prolatilus jugularis (Actinopterygii: Pinguipedidae); marine; intestine; metacestode; Magellanic; Chile (
Scartichthys viridis (Actinopterygii: Bleniidae); marine; site of infection not given; metacestode; WTSA; Chile (
Sicyases sanguineus (Actinopterygii: Gobiesocidae); marine; site of infection not given; metacestode; WTSA; Chile (
Trachurus lathami (Actinopterygii: Carangidae); marine; mesentery; metacestode; WTSA; Argentina, Brazil (
Trachurus murphyi (Actinopterygii: Carangidae); marine; site of infection not given; metacestode; WTSP; Chile (
Order Gyrocotylidea Poche, 1926
Family Gyrocotylidae Benham, 1901
Gyrocotyle maxima MacDonagh, 1927
[Syns. Gyrocotyle meandrica Mendívil-Herrera, 1946; G. urna sensu Manter, 1951; Amphiptyches urna Spencer, 1889]
Callorhinchus callorynchus (Holocephali: Callorhinchidae); marine; spiral valve; adult; WTSA, WTSP; Brazil, Chile, Peru, Uruguay (
Note: tapeworms reported as G. meandrica by
Mustelus schmitti (Elasmobranchii: Triakidae); marine; spiral valve; adult; WTSA; Argentina (
Notes: type host; it was reported as Mustelus asterias, but most likely
Gyrocotyle rugosa Diesing, 1850*
[Syn. Gyrocotyle plana Linton, 1924]
Callorhinchus callorynchus (Holocephali: Callorhinchidae); marine; spiral valve; adult; WTSA, WTSP; Argentina, Chile (
Note: type host.
Unidentified gyrocotylidean
Callorhinchus callorynchus (Holocephali: Callorhinchidae); marine; spiral valve; adult; WTSA; Uruguay (
Order Lecanicephalidea Wardle & McLeod, 1952
Family Aberrapecidae Jensen, Caira, Cielocha, Littlewood & Waeschenbach, 2016
Aberrapex arrhynchum (Brooks, Mayes & Thorson, 1981) Jensen, 2001
[Syn. Discobothrium arrhynchum Brooks, Mayes & Thorson, 1981]
Myliobatis goodei (Elasmobranchii: Myliobatidae); marine; spiral valve; adult; WTSA (estuary of the La Plata River); Uruguay (
Notes: type host.
Family Cephalobothriidae Pintner, 1928
Tylocephalum brooksi Ivanov & Campbell, 2000
Rhinoptera bonasus (Elasmobranchii: Myliobatidae); marine; spiral valve; adult; TNA; Venezuela (
Note: type host.
Tylocephalum sp.
Rhinoptera bonasus (Elasmobranchii: Myliobatidae); marine; spiral valve; adult; TNA; Venezuela (
Family Lecanicephalidae Braun, 1900
Lecanicephalum peltatum Linton, 1890*
Dasyatis americana (Elasmobranchii: Dasyatidae); marine; spiral valve; adult; TEP; Colombia (
Family Paraberrapecidae Jensen, Caira, Cielocha, Littlewood & Waeschenbach, 2016
Paraberrapex atlanticus Mutti & Ivanov, 2016
Squatina guggenheim (Elasmobranchii: Squatinidae); marine; spiral valve; adult; Magellanic, WTSA; Argentina (
Note: type host.
Family Polypocephalidae Meggitt, 1924
Polypocephalus medusia (Linton, 1890) Southwell, 1925
[Syn. Parataenia medusia Linton, 1890]
Dasyatis americana (Elasmobranchii: Dasyatidae) marine; spiral valve; adult; TEP; Colombia (
Order Onchoproteocephalidea Caira, Jensen, Waeschenbach, Olson & Littlewood, 2014
(Syns. Proteocephalidea Mola, 1928; Tetraphyllidea Carus, 1863 pro parte)
Family Onchobothriidae Braun, 1900
Acanthobothrium amazonense Mayes, Brooks & Thorson, 1978
Potamotrygon constellata (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin; Brazil (
Notes: type host; it was reported as P. circularis.
Acanthobothrium americanum Campbell, 1969
Dasyatis americana (Elasmobranchii: Dasyatidae); marine; spiral valve; adult; TNA; Venezuela (
Note: type host.
Acanthobothrium annapinkiense Carvajal & Goldstein, 1971
Zearaja chilensis (Elasmobranchii: Rajidae); marine; spiral valve; adult; Magellanic; Chile (
Note: type host; it was reported as Raja chilensis.
Acanthobothrium atahualpai Marques, Brooks & Barriga, 1997
Gymnura afuerae (Elasmobranchii: Gymnuridae); marine; spiral valve; adult; TEP; Ecuador (
Note: type host.
Acanthobothrium batailloni Euzet, 1955
Myliobatis chilensis (Elasmobranchii: Myliobatidae); marine; spiral valve; adult; WTSP; Chile, Peru (
Acanthobothrium brevissime Linton, 1908
Myliobatis peruvianus (Elasmobranchii: Myliobatidae); marine; spiral valve; adult; WTSP; Peru (
Acanthobothrium campbelli Marques, Brooks & Monks, 1995
Dasyatis longa (Elasmobranchii: Dasyatidae); marine; spiral valve; adult; TEP; Ecuador (
Acanthobothrium cartagenense Brooks & Mayes, 1980
Urobatis jamaicensis (Elasmobranchii: Urotrygonidae); marine; spiral valve; adult; TNA; Colombia (
Note: type host; it was reported as Urolophus jamaicensis.
Acanthobothrium chilense Rego, Vicente & Herrera, 1968
Sarda chiliensis (Actinopterygii: Scombridae); marine; intestine; adult; WTSP; Peru (
Notes: type host. Elasmobranchs are the typical definitive host for Acanthobothrium species (
Acanthobothrium colombianum Brooks & Mayes, 1980
Aetobatus narinari (Elasmobranchii: Myliobatidae); marine; spiral valve; adult; TNA; Colombia (
Note: type host.
Acanthobothrium coquimbense Carvajal & Jeges, 1980
Myliobatis chilensis (Elasmobranchii: Myliobatidae); marine; spiral valve; adult; WTSP; Chile (
Note: type host.
Acanthobothrium costarricense Marques, Brooks & Monks, 1995
Dasyatis longa (Elasmobranchii: Dasyatidae); marine; spiral valve; adult; TEP; Ecuador (
Note: type host.
Acanthobothrium dasybati Yamaguti, 1934
Unidentified ray host (Elasmobranchii); marine; spiral valve; adult (only one immature specimen; WTSA; Brazil (
Note: this species was described from Dasyatis akajei in the Western Pacific (Japanese Sea) and its report from the Brazilian coast needs verification.
Acanthobothrium electricolum Brooks & Mayes, 1978
Narcine brasiliensis (Elasmobranchii: Narcinidae); marine; spiral valve; adult; TNA; Colombia, Venezuela (
Note: type host.
Acanthobothrium fogeli Goldstein, 1964
Gymnura micrura (Elasmobranchii: Gymnuridae); marine; spiral valve; adult; TNA; Venezuela (
Note: type host.
Acanthobothrium gonzalesmugaburoi Severino & Sarmiento, 1979
Myliobatis peruvianus (Elasmobranchii: Myliobatidae); marine; spiral valve; adult; WTSP; Peru (
Note: type host.
Acanthobothrium himanturi Brooks, 1977
Himantura schmardae (Elasmobranchii: Dasyatidae); marine; spiral valve; adult; TNA; Colombia (
Note: type host.
Acanthobothrium holorhini Alexander, 1953
Myliobatis chilensis (Elasmobranchii: Myliobatidae); marine; spiral valve; adult; WTSP; Peru (
Acanthobothrium lintoni Goldstein, Henson & Schlicht, 1968
Narcine brasiliensis (Elasmobranchii: Narcinidae); marine; spiral valve; adult; TNA; Colombia (
Note: type host.
Acanthobothrium lusarmientoi Severino & Verano, 1980
Sympterygia brevicaudata (Elasmobranchii: Arhynchobatidae); marine; spiral valve; adult; WTSP; Peru (
Note: type host; it was reported as Psammobatis caudispina.
Acanthobothrium marplatense Ivanov & Campbell, 1998
Atlantoraja castelnaui (Elasmobranchii: Arhynchobatidae); marine; spiral valve; adult; WTSA; Argentina (
Note: type host; it was reported as Rioraja castelnaui.
Acanthobothrium minusculum Marques, Brooks & Barriga, 1997
Urobatis tumbesensis (Elasmobranchii: Urotrygonidae); marine; spiral valve; adult; TEP; Ecuador (
Note: type host; it was reported as Urolophus tumbesensis.
Acanthobothrium monksi Marques, Brooks & Barriga, 1997
Aetobatus narinari (Elasmobranchii: Myliobatidae); marine; spiral valve; adult; TEP; Ecuador (
Note: type host.
Acanthobothrium obuncum Marques, Brooks & Barriga, 1997
Dasyatis longa (Elasmobranchii: Dasyatidae); marine; spiral valve; adult; TEP; Ecuador (
Note: type host.
Acanthobothrium olseni Dailey & Mudry, 1968
Rhinobatos planiceps (Elasmobranchii: Rhinobatidae); marine; spiral valve; adult; WTSP; Chile, Peru (
Acanthobothrium peruviense Reyda, 2008
Potamotrygon cf. falkneri (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin; Peru (
Note: host reported as Potamotrygon cf. castexi and the tapeworms as Acanthobothrium cf. peruviense.
Potamotrygon motoro (Elasmobranchii: Potamotrygonidae); freshwater; spiral
valve; adult; Amazon River basin; Peru (
Note: type host.
Acanthobothrium psammobati Carvajal & Goldstein, 1969
Psammobatis scobina (Elasmobranchii: Arhynchobatidae); marine; spiral valve; adult; WTSP; Chile, Peru (
Note: type host.
Sympterygia brevicaudata (Elasmobranchii: Arhynchobatidae); marine; spiral
valve; adult; WTSP; Chile (
Acanthobothrium quinonese Mayes, Brooks & Thorson, 1978
Potamotrygon magdalenae (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Magdalena River basin; Colombia (
Notes: type host.
Potamotrygon yepezi (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Maracaibo basin; Venezuela (
Acanthobothrium ramiroi Ivanov, 2005
Potamotrygon motoro (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon and Paraná River basins; Argentina, Peru (
Notes: type host.
Acanthobothrium regoi Brooks, Mayes & Thorson, 1981
Potamotrygon falkneri (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Paraná River basin; Brazil (
Potamotrygon motoro (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Paraná River basin; Brazil (
Potamotrygon orbignyi (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Orinoco River basin; Venezuela (
Note: type host; it was reported as P. hystrix.
Acanthobothrium robustum Alexander, 1953
Rhinobatos planiceps (Elasmobranchii: Rhinobatidae); marine; spiral valve; adult; WTSP; Peru (
Acanthobothrium tasajerasi Brooks, 1977
Dasyatis guttata (Elasmobranchii: Dasyatidae); marine; spiral valve; adult; Maracaibo basin; Venezuela (
Himantura schmardae (Elasmobranchii: Dasyatidae); marine; spiral valve; adult; TNA; Colombia (
Note: type host.
Acanthobothrium terezae Rego & Dias, 1976
Paratrygon aiereba (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin; Brazil (
Notes:
Potamotrygon motoro (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Paraná River basin; Brazil (
Note: type host; it was reported as Paratrygon motoro and Elipesurus sp.
Acanthobothrium tortum (Linton, 1916) Baer & Euzet, 1962
Aetobatus narinari (Elasmobranchii: Myliobatidae); marine; spiral valve; adult; TNA; Venezuela (
Note: type host.
Acanthobothrium urotrygoni Brooks & Mayes, 1980
Dasyatis guttata (Elasmobranchii: Dasyatidae); marine; spiral valve; adult; TNA; Venezuela (
Urotrygon venezuelae (Elasmobranchii: Urotrygonidae); marine; spiral valve; adult; TNA; Colombia (
Note: type host.
Acanthobothrium zapterycum Ostrowski de Núñez, 1971
Zapteryx brevirostris (Elasmobranchii: Rhinobatidae); marine; spiral valve; adult; WTSA; Argentina (
Note: type host.
Acanthobothrium sp.
Myliobatis chilensis (Elasmobranchii: Myliobatidae); marine; spiral valve; adult; WTSP; Peru (
Myliobatis goodei (Elasmobranchii: Myliobatidae); marine; spiral valve; adult; WTSA (La Plata River estuary); Uruguay (
Note: the authors distinguished two morphotypes.
Sympterygia brevicaudata (Elasmobranchii: Arhynchobatidae); marine; spiral valve; adult; WTSP; Chile (
Zapteryx brevirostris (Elasmobranchii: Rhinobatidae); marine; spiral valve; adult; WTSA; Argentina (
Acanthobothroides thorsoni Brooks, 1977*
Dasyatis dipterura (Elasmobranchii: Dasyatidae); marine; spiral valve; adult; WTSP; Peru (
Note: host reported as D. brevis.
Dasyatis guttata (Elasmobranchii: Dasyatidae); marine; spiral valve; adult; TNA; Venezuela (
Himantura schmardae (Elasmobranchii: Dasyatidae); marine; spiral valve; adult; TNA; Colombia (
Note: type host.
Platybothrium auriculatum Yamaguti, 1952
[Syns. Platybothrium baeri Euzet, 1952; Cylindrophorus posteroporus Riser, 1955]
Prionace glauca (Elasmobranchii: Carcharhinidae); marine; spiral valve; adult; TSA, WTSP; Brazil, Chile, Peru (
Note:
Platybothrium sp.
Sphyrna zygaena (Elasmobranchii: Sphyrnidae); marine; spiral valve; adult; WTSP; Peru (
Potamotrygonocestus amazonensis Mayes, Brooks & Thorson, 1981
Potamotrygon constellata (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin; Brazil (
Notes: type host; it was reported as P. circularis.
Potamotrygon falkneri (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Paraná River basin; Brazil (
Potamotrygon motoro (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon and Paraná River basins; Brazil (
Note:
Potamotrygon orbignyi (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon and Orinoco River basins; Brazil, Venezuela (
Note: host reported as P. reticulatus by
Potamotrygon scobina (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin; Brazil (
Potamotrygon yepezi (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Maracaibo basin; Venezuela (
Potamotrygon sp. (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin; Brazil (
Potamotrygonocestus chaoi Marques, Brooks & Araujo, 2003
Plesiotrygon iwamae (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin; Brazil (
Notes: type host.
Potamotrygonocestus fitzgeraldae Marques, Brooks & Araujo, 2003
Paratrygon aiereba (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin; Brazil, Peru (
Notes: type host.
Potamotrygon cf. falkneri (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin; Peru (
Notes: host reported as P. castexi and the tapeworms as P. cf. fitzgeraldae. Sequences of partial 18S (KF685832) and 28S (KF685773) (
Potamotrygon leopoldi (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin; Brazil (
Potamotrygon motoro (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Paraná River basin; Argentina (
Potamotrygon orbignyi (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Paraná River basin; Brazil (
Potamotrygonocestus magdalenensis Brooks & Thorson, 1976*
Potamotrygon magdalenae (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Magdalena River basin; Colombia (
Note: type host.
Potamotrygonocestus marajoara Luchetti, Marques & Charvet-Almeida, 2008
Plesiotrygon iwamae (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin (estuary); Brazil (
Note: type host.
Potamotrygonocestus maurae Marques, Brooks & Araujo, 2003
Potamotrygon orbignyi (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin; Brazil (
Note: type host.
Potamotrygonocestus travassosi Rego, 1979
[Syn. Potamotrygonocestus orinocoensis Brooks, Mayes & Thorson, 1981]
Paratrygon aiereba (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin; Brazil (
Potamotrygon constellata (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin; Brazil (
Potamotrygon falkneri (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Paraná River basin; Brazil (
Potamotrygon motoro (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Paraná River basin; Brazil (
Note: tapeworms reported as Potamotrygonocestus orinocoensis.
Potamotrygon orbignyi (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon and Orinoco River basins; Brazil, Venezuela (
Notes: type host; it was reported as P. reticulatus and P. hystrix. The taxon was considered a species inquirenda by
Potamotrygonocestus sp.
Paratrygon aiereba (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin; Peru (
Note:
Potamotrygon cf. falkneri (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin; Peru (
Note: host reported as Potamotrygon cf. castexi.
Potamotrygon henlei (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Tocantins-Araguaia River basin; Brazil (
Note: these cestodes may represent an undescribed species of the genus (
Potamotrygon motoro (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin; Peru (
Potamotrygon schroederi (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin; Brazil (
Note: these cestodes may represent an undescribed species of the genus (
Family Prosobothriidae Baer & Euzet, 1955
Prosobothrium armigerum Cohn, 1902*
Prionace glauca (Elasmobranchii: Carcharhinidae); marine; spiral valve; adult; WTSP; Peru (
Family Proteocephalidae La Rue, 1911
[Even though recent molecular data suggest that most of the traditionally recognized subfamilies are artificial, i.e. non-monophyletic, we are following Woodland’s subfamilial classification for practical reasons]
Subfamily Corallobothriinae Freze, 1965
Corallotaenia sp.
Ageneiosus pardalis (Actinopterygii: Auchenipteridae); freshwater; intestine; adult (immature specimens); Magdalena River basin; Colombia (
Notes: host reported as A. caucanus. This is the first record of the genus in South America (
Megathylacus jandia Woodland, 1934*
[Syn. Megathylacus brooksi Rego & Pavanelli, 1985]
Zungaro jahu (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Brazil (
Notes: host reported as Paulicea luetkeni or Z. zungaro (for details on the host taxonomic status, see
Zungaro zungaro (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil, Peru (
Notes: type host; it was originally reported as Rhamdia sp., but it is most likely Z. zungaro as discussed by
Megathylacus sp.
Pseudoplatystoma corruscans (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Brazil (
Pseudoplatystoma fasciatum (Actinopterygii: Pimelodidae); freshwater; intestine; adult (also immature specimens); Amazon River basin; Peru (
Note: host reported as P. punctifer by
Megathylacus travassosi Pavanelli & Rego, 1992
Pseudoplatystoma corruscans (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Brazil (
Notes: type host.
Pseudoplatystoma fasciatum (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Brazil (
Sciadocephalus megalodiscus Diesing, 1850*
Cichla kelberi (Actinopterygii: Cichlidae); freshwater; intestine; adult; Paraná
River basin; Brazil (
Note: this host should be synonymized with C. ocellaris based on molecular data (
Cichla monoculus (Actinopterygii: Cichlidae); freshwater; intestine, stomach; adult; Amazon and Paraná River basins; Brazil, Peru (
Notes: type host. C. monoculus should be synonymized with C. ocellaris based on molecular data (
Cichla piquiti (Actinopterygii: Cichlidae); freshwater; intestine; adult; Paraná and
Tocantins-Araguaia River basins; Brazil (
Subfamily Endorchiinae Woodland, 1934
Endorchis auchenipteri de Chambrier & Vaucher, 1999
Auchenipterus osteomystax (Actinopterygii: Auchenipteridae); freshwater; intestine; adult; Paraná River basin; Paraguay (
Note: type host.
Endorchis piraeeba Woodland, 1934*
Brachyplatystoma filamentosum (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil; (
Notes: type host. In type material, Nominoscolex piraeeba and E. piraeeba are mixed on the same slide (
Brachyplatystoma cf. filamentosum (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Peru (
Endorchis sp.
Pimelodus altissimus (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Peru (
Pimelodus cf. maculatus (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Paraguay (
Trachelyopterus striatulus (Actinopterygii: Auchenipteridae); freshwater; intestine; adult; Paraná River basin; Paraguay (
Subfamily Ephedrocephalinae Mola, 1929
Ephedrocephalus microcephalus Diesing, 1850*
[Syn. Rudolphiella microcephalus (Diesing, 1850) Brooks, 1995]
Phractocephalus hemioliopterus (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil (
Notes: type host. Sequences of complete and partial 18S (KC786007, AY551108), respectively; complete ITS2 (AY551143), partial 28S (KC786017, AJ388605), partial 16S (KC785994, AJ389509) and partial cox1 (KC785982) (
Subfamily Monticelliinae Mola, 1929
Ageneiella brevifilis de Chambrier & Vaucher, 1999*
Ageneiosus inermis (Actinopterygii: Auchenipteridae); freshwater; intestine; adult; Paraná River basin; Argentina, Paraguay (
Notes: type host; it was reported as A. brevifilis. Sequences of partial 18S (AY551102), complete ITS2 (AY551138), partial 28S (AJ388600) and 16S (AJ389495) (
Ageneiosus militaris (Actinopterygii: Auchenipteridae); freshwater; intestine; adult; Paraná River basin; Argentina (
Ageneiella sp.
Ageneiosus inermis (Actinopterygii: Auchenipteridae); freshwater; intestine; adult; Amazon River basin; Peru (
Chambriella agostinhoi (Pavanelli & Machado, 1992) Rego, Chubb & Pavanelli, 1999*
[Syn. Goezeella agostinhoi Pavanelli & Machado, 1992]
Pimelodus maculatus (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Brazil (
Notes: tapeworms reported as Goezeella agostinhoi. This report needs verification, but apparently, there are no vouchers deposited in any museum collection.
Zungaro jahu (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná
River basin; Brazil (
Notes: type host; it was reported as Z. zungaro or P. luetkeni and the tapeworms as Goezeella agostinhoi by some authors. Before formal description of the species,
Zungaro zungaro (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Peru (
Chambriella paranaensis (Pavanelli & Rego, 1989) Rego, Chubb & Pavaneli, 1999
[Syns. Goezeella paranaensis Pavanelli & Rego, 1989; Spatulifer paranensis (sic!) (Pavanelli & Rego, 1989) Brooks, 1995]
Hemisorubim platyrhynchos (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon and Paraná River basins; Brazil, Paraguay, Peru (
Chambriella sp.
Brachyplatystoma vaillantii (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Peru (
Note: Chambriella sp 1. sensu
Phractocephalus hemioliopterus (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil, Peru (
Note: Chambriella sp. 2 sensu
Pseudoplatystoma fasciatum (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Peru (
Note: Chambriella sp. 3 sensu
Sorubimichthys planiceps (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil, Peru (
Note: Chambriella sp. 4 sensu
Choanoscolex abscisus (Riggenbach, 1895) La Rue, 1911*
[Syns. Ichthyotaenia abscisa Riggenbach, 1895; Corallobothrium abscissus (sic!) (Riggenbach, 1895) Meggitt, 1927; Proteocephalus abscissus (sic!) (Riggenbach, 1895) Fuhrmann, 1933; Spatulifer abscissus (sic!) (Riggenbach, 1895) Brooks, 1995]
Pseudoplatystoma corruscans (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná and São Francisco River basins; Brazil, Paraguay (
Notes: type host.
Pseudoplatystoma fasciatum (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon, Orinoco, Paraná and São Francisco River basins; Brazil, Peru, Venezuela (
Notes: tapeworms reported as Choanoscolex cf. abscisus by
Rhaphiodon vulpinus (Actinopterygii: Cynodontidae); freshwater; intestine; adult; Paraná River basin; Brazil (
Zungaro jahu (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná
River basin; Brazil (
Notes: host reported as P. luetkeni or Z. zungaro; it was considered an accidental host by
Choanoscolex sp.
Pseudoplatystoma fasciatum (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon and Paraná River basins; Paraguay, Peru (
Pseudoplatystoma tigrinum (Actinopterygii: Pimelodidae); freshwater; intestine; adult (immature specimens); Amazon River basin; Peru (
Sorubimichthys planiceps (Actinopterygii: Pimelodidae); freshwater; intestine; adult (mostly immature specimens); Amazon River basin; Brazil, Peru (
Goezeella danbrooksi de Chambrier, Rego & Mariaux, 2004
[Syn. Goezeella siluri sensu Brooks & Deardorff, 1980]
Ageneiosus pardalis (Actinopterygii: Auchenipteridae); freshwater; intestine; adult; Magdalena River Basin; Colombia (
Note: type host; it was originally reported as A. caucanus, whereas the tapeworms have been reported as Goezeella siluri following
Goezeella siluri Fuhrmann, 1916*
[Syns. Goezeella piramutab Woodland, 1933; Monticellia piramutab (Woodland, 1933) Woodland, 1935; M. siluri (Fuhrmann, 1916) Woodland, 1935; Corallobothrium siluri (Fuhrmann, 1916) Harwood, 1933; Spatulifer piramutab (Woodland, 1933) Brooks & Deardorff, 1980; S. siluri (Fuhrmann, 1916) Brooks, 1995]
Brachyplatystoma vaillantii (Actinopterygii: Pimelodidae); freshwater; intestine; adult (immature and mature specimens); Amazon and Orinoco River basins; Brazil, Venezuela (
Note: the specimens studied by
Cetopsis coecutiens (Actinopterygii: Cetopsidae); freshwater; intestine; adult; Amazon River basin; Brazil (
Note: type host.
Cetopsis othonops (Actinopterygii: Cetopsidae); freshwater; intestine; adult; Orinoco River basin; Venezuela (
Note: host reported as Pseudocetopsis othonops.
Pinirampus pirinampu (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil (
Note: sequences of partial 18S (AY551110), complete ITS2 (AY551146), partial 28S (AJ388612) and 16S (AJ389518) (
Lenhataenia megacephala (Woodland, 1934) de Chambrier & Scholz, 2008*
[Syn. Monticellia megacephala Woodland, 1934]
Rhamdia quelen (Actinopterygii: Heptapteridae); freshwater; intestine, stomach; adult; Chascomus lagoon (Salado River basin); Argentina (
Note: host reported as Rhamdia sapo.
Sorubimichthys planiceps (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil, Peru (
Notes: type host; it was originally reported as Platystomatichthys sturio.
Manaosia bracodemoca Woodland, 1935*
[Syns. Paramonticellia itaipuensis Pavanelli & Rego, 1991; Goezeella nupeliensis Pavanelli & Rego, 1991; Spatulifer nupeliensis (Pavanelli & Rego, 1991) Brooks, 1995]
Hemisorubim platyrhynchos (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Brazil (
Sorubim lima (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon and Paraná River basins; Brazil, Paraguay, Peru (
Notes: type host.
Monticellia amazonica de Chambrier & Vaucher, 1997
[Syns. Nomimoscolex piracatinga Woodland, 1935; Monticellia rugata Rego, 1975 (pro parte); Spatulifer rugata (Rego, 1975) Brooks & Deardorff, 1980; Paramonticellia piracatinga (Woodland, 1935) Brooks, 1995]
Calophysus macropterus (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil, Peru (
Note: type host; it was originally reported as Pimelodus pati (syn. of Luciopimelodus pati according to
Monticellia belavistensis Pavanelli, Machado, Takemoto & dos Santos, 1994
Pterodoras granulosus (Actinopterygii: Doradidae); freshwater; intestine; adult; Amazon and Paraná River basins; Argentina, Brazil, Paraguay, Peru (
Note: type host.
Monticellia coryphicephala (Monticelli, 1891) La Rue, 1911*
[Syns. Taenia coryphicephala Monticelli, 1891; Tetracotylus coryphicephala Monticelli, 1891; Ichthyotaenia coryphicephala (Monticelli, 1891) Lönnberg, 1894; Proteocephalus (Proteocephalus) coryphicephala (Monticelli, 1891) Harwood, 1933]
Salminus brasiliensis (Actinopterygii: Bryconidae); freshwater; intestine; adult; Paraná and São Francisco River basins; Brazil, Paraguay (
Notes: type host; it was originally reported as Silurus sp., but this genus only occurs in the Palaearctic region. Sequences of complete ITS2 (AJ238839), partial 28S (AJ238832) and 16S (AJ238831) (
Salminus franciscanus (Actinopterygii: Bryconidae); freshwater; intestine; adult; São Francisco River basin; Brazil (
Note: host reported as S. brevidens.
Monticellia dlouhyi de Chambrier & Vaucher, 1999
Acestrorhynchus altus (Actinopterygii: Acestrorhynchidae); freshwater; intestine; adult; Paraná River basin; Paraguay (
Note: type host.
Monticellia magna (Rego, Santos & Silva, 1974) de Chambrier & Vaucher, 1997
[Syns. Nomimoscolex magna Rego Santos & Silva, 1974 (pro parte); Monticellia loyolai Pavanelli & Machado, 1992]
Pimelodus albicans (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Argentina (
Pimelodus argenteus (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Argentina (
Pimelodus cf. blochii (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Paraguay (
Pimelodus maculatus (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná and São Francisco River basins; Brazil, Argentina (
Notes: type host; it was reported as Pimelodus clarias. There is a mixture of two species in the type material, originally described as N. magna, which can be differentiated by the position of internal organs (see p. 255 in
Pimelodus cf. maculatus (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Paraguay (
Note: they reported the tapeworms as Monticellia cf. magna.
Monticellia santafesina Arredondo & Gil de Pertierra, 2010
Megalonema platanum (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Argentina (
Note: type host.
Megalonema platycephalum (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Peru (
Monticellia ventrei de Chambrier & Vaucher, 1999
[Syn. Myzophorus admonticellia Woodland 1934 (pro parte)]
Luciopimelodus pati (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Argentina (
Pinirampus pirinampu (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon and Paraná River basins; Brazil, Paraguay, Peru (
Notes: this host is assumed to be the type, because all possible fish hosts cited by
Monticellia sp.
Brycon orbignyanus (Actinopterygii: Bryconidae); freshwater; intestine; adult (immature specimens); Paraná River basin; Paraguay (
Pinirampus pirinampu (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Paraguay (
Pseudoplatystoma corruscans (Actinopterygii: Pimelodidae); freshwater; intestine; adult; São Francisco River basin; Brazil (
Pseudoplatystoma fasciatum (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Paraguay (
Regoella brevis Arredondo, Gil de Pertierra & de Chambrier, 2013*
Pseudoplatystoma fasciatum (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Argentina (
Notes: type host. Sequence of partial 28S (KP729389) (
Spasskyellina lenha (Woodland, 1933) Freze, 1965*
[Syn. Monticellia lenha Woodland, 1933]
Sorubimichthys planiceps (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil, Peru (
Notes: type host. Sequence of 28S (KP729413) under the name Lenhataenia megacephala in the GenBank database – see
Spasskyellina mandi Pavanelli & Takemoto, 1996
[Syn. Monticellia mandi (Pavanelli & Takemoto, 1996) de Chambrier & Vaucher, 1999]
Pimelodus ornatus (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Brazil (
Note: type host.
Spasskyellina spinulifera (Woodland, 1935) Freze, 1965
[Syns. Monticellia spinulifera (Woodland, 1935); M. spinulifer (sic!) of Brooks (1995)]
Pseudoplatystoma corruscans (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Argentina, Brazil, Paraguay (
Note: sequence of 28S (KP729417) (
Pseudoplatystoma fasciatum (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon and Paraná River basins; Brazil, Peru (
Note: type host.
Pseudoplatystoma tigrinum (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Peru (
Sorubim lima (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Brazil (
Spasskyellina sp.
Pimelodus ornatus (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Brazil (
Pseudoplatystoma fasciatum (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Brazil (
Spatulifer maringaensis Pavanelli & Rego, 1989
Hemisorubim platyrhynchos (Actinopterygii: Pimelodidae); freshwater; intestine, stomach; adult; Amazon and Paraná River basins; Brazil, Paraguay, Peru (
Note: type host.
Sorubim lima (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon
and Paraná River basins; Argentina, Brazil, Paraguay, Peru (
Notes:
Spatulifer rugosa (Woodland, 1935) Brooks & Deardorff, 1980
[Syn. Monticellia rugosa Woodland, 1935]
Pseudoplatystoma fasciatum (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon and Paraná River basins; Argentina, Brazil, Peru (
Notes: type host; it was reported as P. punctifer by
Spatulifer surubim Woodland, 1934*
[Syns. Peltidocotyle rugosa sensu Woodland, 1933b nec Diesing, 1850; Spatulifer surubim Woodland, 1934; Monticellia surubim (Woodland, 1934) Woodland, 1935]
Pseudoplatystoma tigrinum (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil (
Note: type host.
Spatulifer sp.
Pseudoplatystoma tigrinum (Actinopterygii: Pimelodidae); freshwater; intestine; adult (immature specimens); Amazon River basin; Peru (
Note: probably Spatulifer surubim according to
Monticelliinae gen. sp.
Phractocephalus hemioliopterus (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Peru (
Subfamily Nupeliinae Pavanelli & Rego, 1991
Nupelia portoriquensis Pavanelli & Rego, 1991*
Sorubim lima (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná
River basin; Brazil, Paraguay (
Notes: type host. Sequence of partial 28S (KP729401) (
Nupelia tomasi de Chambrier & Vaucher, 1999
Trachelyopterus galeatus (Actinopterygii: Auchenipteridae); freshwater; intestine; adult; Paraná River basin; Paraguay (
Note: type host.
Trachelyopterus cf. striatulus (Actinopterygii: Auchenipteridae); freshwater; intestine; adult; Paraná River basin; Paraguay (
Nupelia sp.
Goeldiella eques (Actinopterygii: Heptapteridae); freshwater; intestine; adult; Amazon River basin; Peru (
Subfamily Peltidocotylinae Woodland, 1934
Amazotaenia yvettae de Chambrier, 2001*
Brachyplatystoma capapretum (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil (
Notes: type host; it was reported as B. filamentosum and re-identified by J. Lundberg (pers. comm.).
Brachyplatystoma vaillantii (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil (
Jauella glandicephalus Rego & Pavanelli, 1985*
[Syn. Spatulifer glandicephala (Rego & Pavanelli, 1985) Brooks, 1995]
Zungaro jahu (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná
River basin; Argentina, Brazil, Paraguay (
Notes: type host; it was reported as P. luetkeni or Z. zungaro.
Zungaro zungaro (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Peru (
Luciaella ivanovae Gil de Pertierra, 2009*
Ageneiosus inermis (Actinopterygii: Auchenipteridae); freshwater; intestine; adult; Paraná River basin; Argentina (
Note: type host.
Mariauxiella pimelodi de Chambrier & Rego, 1995*
Pimelodus ornatus (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Brazil, Paraguay (
Pimelodus sp. (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Brazil (
Note: type host.
Mariauxiella piscatorum de Chambrier & Vaucher, 1999
Hemisorubim platyrhynchos (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon and Paraná River basins; Brazil, Paraguay, Peru (
Note: type host.
Peltidocotyle lenha (Woodland, 1933) Woodland, 1934
[Syns. Othinoscolex lenha Woodland, 1933; Othinoscolex myzofer Woodland, 1933; Woodlandiella myzofera (Woodland, 1933) Freze, 1965; Peltidocotyle rugosa of Schmidt, 1986; Rudolphiella lenha (Woodland, 1933) Brooks, 1995]
Sorubimichthys planiceps (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil, Peru (
Notes: type host; it was originally reported as Platystomatichthys sturio. Sequences of partial 18S (AY551122), complete IT2 (AJ238842), partial 28S (AJ238836) and 16S (AJ238827) (
Zungaro jahu (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná
River basin; Argentina, Brazil (
Notes: host reported as Zungaro zungaro or Paulicea luetkeni.
Zungaro zungaro (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil, Peru (
Notes: sequences of complete IT2 (AJ238840, AJ238843), partial 28S (AJ238834, AJ238837) and 16S (AJ238826, AJ238829) (
Peltidocotyle rugosa Diesing, 1850*
Pseudopimelodus mangurus (Actinopterygii: Pseudopimelodidae); freshwater; intestine; adult; locality not given; Argentina (
Note: host reported as Zungaro mangurus.
Pseudoplatystoma corruscans (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Argentina, Brazil (
Note: type host; it was originally reported as Platystoma tigrinum (syn. of Pseudoplatystoma tigrinum), but it does not occur in the Paraná River basin, thus the fish host is assumed to be P. corruscans (see
Pseudoplatystoma fasciatum (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon and Paraná River basins; Brazil, Paraguay, Peru (
Note: sequence of complete 18S (AF286989) and ITS2 (AJ238841), partial 28S (AJ238835, AF286937) and 16S (AJ238828) (
Zungaro jahu (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná
River basin; Brazil (
Notes: host reported as P. luetkeni or Z. zungaro.
Peltidocotyle sp.
Zungaro jahu (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná
River basin; Paraguay (
Note: host reported as Paulicea luetkeni.
Subfamily Proteocephalinae La Rue, 1911
Brayela karuatayi (Woodland, 1934) Rego, 1984*
[Syn. Anthobothrium karuatayi Woodland, 1934]
Platynematichthys notatus (Actinopterygii: Pimelodidae); freshwater; intestine; adult (also immature specimens); Amazon River basin; Brazil, Peru (
Notes: type host; it was originally reported as Glanidium sp.;
Cangatiella arandasi Pavanelli & Machado, 1991*
Trachelyopterus galeatus (Actinopterygii: Auchenipteridae); freshwater; intestine; adult; Paraná River basin; Brazil (
Notes: host also reported as Parauchenipterus galeatus. Sequence of 28S (KP729411) (
Note: type host.
Cangatiella macdonaghi (Szidat & Nani, 1951) Gil de Pertierra & Viozzi, 1999 [Syns. Ichthyotaenia macdonaghi Szidat & Nani, 1951; Proteocephalus macdonaghi (Szidat & Nani, 1951) Yamaguti, 1959]
Odontesthes bonariensis (Actinopterygii: Atherinopsidae); freshwater; intestine; adult; lakes in Buenos Aires and Córdoba Provinces; Argentina (
Notes:
Odontesthes hatcheri (Actinopterygii: Atherinopsidae); freshwater; intestine; adult; Peligrini lake; Argentina (
Notes: type host; it was originally reported as Basilichthys microlepidotus.
Euzetiella tetraphylliformis de Chambrier, Rego & Vaucher, 1999*
Pseudoplatystoma fasciatum (Actinopterygii: Pimelodidae); freshwater; intestine; adult (immature); Amazon River basin; Peru (
Zungaro jahu (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Brazil, Paraguay (
Zungaro zungaro (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil, Peru (
Notes: host originally reported as Paulicea luetkeni (syn. of Z. jahu and Z. zungaro); since the holotype was described from a fish collected in the Amazon River, Z. zungaro should be considered the type host.
Frezella vaucheri Alves, de Chambrier, Scholz & Luque, 2015*
Tocantinsia piresi (Actinopterygii: Auchenipteridae); freshwater; intestine; adult; Amazon River basin; Brazil (
Notes: type host. Sequence of partial 28S (KM387399) (
Margaritaella gracilis Arredondo & Gil de Pertierra, 2012*
Callichthys callichthys (Actinopterygii: Callichthyidae); freshwater; intestine; adult; Paraná River basin; Argentina (
Note: type host.
Proteocephalus bagri Holcman-Spector & Mañé-Garzón, 1988
Rhamdia quelen (Actinopterygii: Heptapteridae); freshwater; intestine; Chis-Chis, Chascomús, Sauce, Diario and Dos Patos lagoons; Argentina, Brazil, Uruguay (
Note: type host; it was originally reported as R. sapo.
Proteocephalus fossatus (Riggenbach, 1895) La Rue, 1911
[Syn. Ichthyotaenia fossata Riggenbach, 1895]
Luciopimelodus pati (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Paraguay (
Note: type host; it was originally reported as Pimelodus pati.
Proteocephalus gibsoni Rego & Pavanelli, 1991
[Syn. Proteocephalus ocellatus sensu Rego & Pavanelli, 1990 nec Proteocephalus ocellatus (Rudolphi, 1802)]
Astronotus ocellatus (Actinopterygii: Cichlidae); freshwater; intestine; adult; Amazon River basin; Peru (
Notes: type host.
Astronotus sp. (Actinopterygii: Cichlidae); freshwater; intestine; adult; Amazon River basin; Brazil (
Geophagus brasiliensis (Actinopterygii: Cichlidae); freshwater; intestine; adult; Paraná and Doce River basins; Brazil (
Proteocephalus hemioliopteri de Chambrier & Vaucher, 1997
[Syns. Myzophorus woodlandi Rego, 1984; Nomimoscolex woodlandi (Rego, 1984) Rego & Pavanelli, 1992]
Phractocephalus hemioliopterus (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil, Peru (
Notes: type host. Sequences of partial 18S (AY551129), complete ITS2 (AY551165) and partial 28S (AJ388622) (
Proteocephalus hobergi de Chambrier & Vaucher, 1999
Oxydoras kneri (Actinopterygii: Doradidae); freshwater; intestine; adult; Amazon
and Paraná River basins; Paraguay, Peru (
Notes: type host. Sequence of partial 28S (AJ275062) (
Oxydoras niger (Actinopterygii: Doradidae); freshwater; intestine; adult; Amazon
River basin; Peru (de Chambrier et al. 2015).
Proteocephalus kuyukuyu Woodland, 1935
Megalodoras uranoscopus (Actinopterygii: Doradidae); freshwater; intestine; adult (immature specimens); Amazon and Orinoco River basins; Peru, Venezuela (
Note:
Oxydoras niger (Actinopterygii: Doradidae); freshwater; intestine; adult (immature specimens); Amazon River basin; Brazil (
Notes: type host; it was reported as Pseudodoras niger, but
Pterodoras granulosus (Actinopterygii: Doradidae); freshwater; intestine; adult (immature specimens); Amazon River basin; Peru (
Note: sequence of partial 28S (KP729388) (
Pterodoras sp. (Actinopterygii: Doradidae); freshwater; intestine; adult (immature specimens); Amazon River basin; Peru (
Proteocephalus macrophallus (Diesing, 1850) La Rue, 1914
[Syns. Taenia macrophalla Diesing, 1850; Ichthyotaenia macrophalla (Diesing, 1850) Riggenbach, 1896]
Cichla kelberi (Actinopterygii: Cichlidae); freshwater; intestine; adult; Paraná and São Francisco River basin; Brazil (
Cichla monoculus (Actinopterygii: Cichlidae); freshwater; intestine; adult; Amazon and Paraná River basins; Brazil, Peru (
Notes: type host; some authors assumed C. ocellaris as the type host, but the taxonomic status of these cichlids is unclear (see notes on p. 33). Sequence of partial 28S (KP729394) (
Cichla ocellaris (Actinopterygii: Cichlidae); freshwater; intestine; adult; Amazon, Orinoco, Paraíba do Sul and Paraná River basins; Brazil, Venezuela (
Cichla piquiti (Actinopterygii: Cichlidae); freshwater; intestine, stomach; adult; Paraná and Tocantins-Araguaia River basins; Brazil (
Cichla sp. (Actinopterygii: Cichlidae); freshwater; intestine; adult; Paraná River basin; Brazil (
Proteocephalus mahnerti de Chambrier & Vaucher, 1999
Hoplerythrinus unitaeniatus (Actinopterygii: Erythrinidae); freshwater; intestine; adult; Paraná River basin; Paraguay (
Note: type host.
Proteocephalus microscopicus Woodland, 1935
Cichla kelberi (Actinopterygii: Cichlidae); freshwater; intestine; adult; Paraná and São Francisco River basins; Brazil (
Cichla monoculus (Actinopterygii: Cichlidae); freshwater; intestine; adult; Amazon and Paraná River basins; Brazil, Peru (
Cichla ocellaris (Actinopterygii: Cichlidae); freshwater; intestine; adult; Amazon River basin; Brazil (
Note: type host.
Cichla piquiti (Actinopterygii: Cichlidae); freshwater; intestine; adult; Paraná and Tocantins-Araguaia River basins; Brazil (
Cichla sp. (Actinopterygii: Cichlidae); freshwater; intestine; adult; Paraná River basin; Brazil (
Proteocephalus pilarensis de Chambrier & Vaucher, 1999
Paraloricaria sp. (Actinopterygii: Loricariidae); freshwater; intestine; adult; Paraná River basin; Paraguay (
Note: type host.
Proteocephalus pimelodi (Gil de Pertierra, 1995) de Chambrier & Vaucher, 1997
[Syn. Nomimoscolex pimelodi Gil de Pertierra, 1995]
Pimelodus maculatus (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Argentina (
Note: type host.
Proteocephalus platystomi Lynsdale, 1959
Pseudoplatystoma sp. (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil (
Note: host originally reported as Platystoma sp.; the specimens collected by Woodland in 1937 were deposited without any identification in BMNH (
Note: type host.
Proteocephalus regoi de Chambrier, Scholz and Vaucher, 1996
Hoplias malabaricus (Actinopterygii: Erythrinidae); freshwater; intestine; adult; Paraná River basin; Paraguay (
Note: type host.
Proteocephalus renaudi de Chambrier & Vaucher, 1994
Franciscodoras marmoratus (Actinopterygii: Doradidae); freshwater; intestine; adult; São Francisco River basin; Brazil (
Platydoras costatus (Actinopterygii: Doradidae); freshwater; intestine; adult; Paraná River basin; Paraguay (
Notes: type host; it does not occur in the Paraná River basin (
Proteocephalus rhamdiae Holcman-Spector & Mañé-Garzón, 1988
Rhamdia quelen (Actinopterygii: Heptapteridae); freshwater; intestine; adult; Chis-Chis, Chascomús, Sauce, Diario and Dos Patos lagoons and Paraná River basin; Argentina, Brazil, Paraguay, Uruguay (
Note: type host; it was originally reported as R. sapo.
Proteocephalus serrasalmus Rego & Pavanelli, 1990
Pygocentrus nattereri (Actinopterygii: Serrasalmidae); freshwater; intestine; adult; Paraná River basin; Brazil (
Note: host originally reported as Serrasalmus nattereri.
Serrasalmus maculatus (Actinopterygii: Serrasalmidae); freshwater; intestine; adult; Paraná River basin; Brazil, Paraguay (
Note: type host; it was originally reported as S. spilopleura.
Proteocephalus soniae de Chambrier & Vaucher, 1994
Platydoras costatus (Actinopterygii: Doradidae); freshwater; intestine; adult; Paraná River basin; Paraguay (
Note: type host; it was most likely mistaken (see notes for P. renaudi on p. 50 for more details).
Proteocephalus sophiae de Chambrier & Rego, 1994
Zungaro zungaro (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil, Peru (
Note: type host; it was originally reported as P. luetkeni.
Proteocephalus vazzolerae Pavanelli & Takemoto, 1995
Leporinus friderici (Actinopterygii: Anostomidae); freshwater; caeca, intestine; adult; Paraná River basin; Brazil (
Leporinus lacustris (Actinopterygii: Anostomidae); freshwater; caeca, intestine; adult; Paraná River basin; Brazil (
Piaractus mesopotamicus (Actinopterygii: Serrasalmidae); freshwater; intestine; adult; Paraná River basin; Brazil (
Note: type host.
Proteocephalus vladimirae de Chambrier & Vaucher, 1999
Pinirampus pirinampu (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Paraguay (
Note: type host.
Proteocephalus sp.
Franciscodoras marmoratus (Actinopterygii: Doradidae); freshwater; intestine; adult; São Francisco River basin; Brazil (
Gymnotus carapo (Actinopterygii: Gymnotidae); freshwater; intestine; adult; Paraiba do Sul River basin; Brasil (
Phractocephalus hemioliopterus (Actinopterygii: Pimelodidae); freshwater; intestine; adult (immature specimens); Amazon River basin; Peru (
Note: they named this morphotype as Proteocephalus sp. 1 and it is probably a new species (
Pimelodus blochii (Actinopterygii: Pimelodidae); freshwater; intestine; adult (immature specimens); Amazon River basin; Peru (
Pimelodus maculatus (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Brazil (
Note:
Platydoras costatus (Actinopterygii: Doradidae); freshwater; intestine; adult; Paraná River basin; Paraguay (
Note: the host species was most likely misidentified (see notes on p. 50 for more details).
Pterodoras granulosus (Actinopterygii: Doradidae); freshwater; intestine; adult (immature specimens); Amazon River basin; Peru (
Note: they named this morphotype as Proteocephalus sp. 2 and it is probably a new species (
Pseudocrepidobothrium chanaorum Arredondo, Gil de Pertierra & de Chambrier, 2014
Pseudoplatystoma fasciatum (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Argentina (
Note: type host; it was reported as P. reticulatum (see the note on p. 36).
Pseudocrepidobothrium eirasi (Rego & de Chambrier, 1995) Rego & Ivanov, 2001*
[Syn. Crepidobothrium eirasi Rego & de Chambrier, 1995]
Phractocephalus hemioliopterus (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil (
Notes: type host. Sequences of partial 18S (AY551106), complete ITS2 (AY551179), partial 28S (AJ388623) and 16S (AJ389494) (
Pseudocrepidobothrium ludovici Ruedi & de Chambrier, 2012
Phractocephalus hemioliopterus (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil (
Note: type host.
Pseudocrepidobothrium sp.
Phractocephalus hemioliopterus (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil (
Notes: tapeworms reported as Crepidobothrium sp. Sequence of complete ITS2 (AJ238838), partial 28S (AJ238833, AJ275063) and 16S (AJ238830) (
Scholzia emarginata (Diesing, 1850) de Chambrier, Rego & Gil de Pertierra, 2005*
[Syns. Tetrabothrium emarginatum Diesing, 1850; Tetrabothrium (Eutetrabothrium) emarginatum Diesing, 1856; Nomimoscolex emarginatum (Diesing, 1850) Rego, Chubb & Pavaneli, 1999; Myzophorus pirarara Woodland, 1935; Nomimoscolex pirarara (Woodland, 1935) Rego & Pavanelli, 1992; Proteocephalus pirarara (Woodland, 1935) de Chambrier & Vaucher, 1997].
Phractocephalus hemioliopterus (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil, Peru (
Notes: type host. Sequences of partial 18S (AY551131, AY551112, KC786006), complete ITS2 (AY551170, AY551148), partial 28S (AJ388616, KC786016), partial 16S (AJ389513, KC785993) and partial cox1 (KC785981) (
Subfamily Rudolphiellinae Woodland, 1935
Rudolphiella lobosa (Riggenbach, 1895) Fuhrmann, 1916*
[Syns. Corallobothrium lobosum Riggenbach, 1895; Ephedrocephalus lobosum (Riggenbach, 1895) Mola, 1906]
Luciopimelodus pati (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Paraguay (
Notes: type host; it was originally reported as Pimelodus pati, but
Megalonema platanum (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Paraguay (
Note:
Rudolphiella myoides (Woodland, 1934) Woodland, 1935
[Syn. Amphilaphorchis myoides Woodland, 1934]
Pinirampus pirinampu (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil (
Note: type host.
Rudolphiella piracatinga (Woodland, 1935) Gil de Pertierra & de Chambrier, 2000
[Syns. Monticellia piracatinga Woodland, 1935; M. rugata Rego, 1975 (pro parte); Rudolphiella rugata (
Calophysus macropterus (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil, Peru (
Notes: host originally described as Pimelodus pati (for details, see
Note: type host.
Rudolphiella piranabu (Woodland, 1934) Woodland, 1935
[Syn. Amphilaphorchis piranabu Woodland, 1934]
Pinirampus pirinampu (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon and Paraná River basins; Brazil (
Note: type host.
Rudolphiella szidati Gil de Pertierra & de Chambrier, 2000
Luciopimelodus pati (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Argentina (
Notes: type host. Sequences of partial and complete 18S (AY551135, AF286990), respectively, complete ITS2 (AY551174), partial 28S (AJ388617, AF286938) and 16S (AJ389517) (
Rudolphiella sp.
Luciopimelodus pati (Actinopterygii: Pimelodidae); freshwater; intestine; adult (including immature specimens); Paraná River basin; Paraguay (
Pinirampus pirinampu (Actinopterygii: Pimelodidae); freshwater; intestine; adult (immature specimens); Amazon and Paraná River basins; Paraguay (
Subfamily Zygobothriinae Woodland, 1933
Amphoteromorphus ninoi Carfora, de Chambrier & Vaucher, 2003
Brachyplatystoma filamentosum (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil (
Note: sequence of 28S (AJ388624) (Chambrier et al. 2004b).
Brachyplatystoma vaillantii (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil (
Notes: type host. Tapeworms reported as Amphoteromorphus piraeeba by
Amphoteromorphus ovalis Carfora, de Chambrier & Vaucher, 2003
Brachyplatystoma cf. filamentosum (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Peru (
Brachyplatystoma sp. (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil (
Note: type host.
Amphoteromorphus parkamoo Woodland, 1935
Zungaro zungaro (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil, Peru (
Notes: type host; it was originally reported as Pseudopimelodus zungaro, but also as Paulicea luetkeni in additional studies. Sequences of partial 18S (AY551103), complete ITS2 (AY551139), partial 28S (AJ388595) and 16S (AJ389523) (
Amphoteromorphus peniculus Diesing, 1850*
Brachyplatystoma rousseauxii (Actinopterygii: Pimelodidae); freshwater; intestine: adult; Amazon River basin; Brazil, Peru (
Notes: type host; it was originally reported as Bagrus goliath, but also as Brachyplatystoma flavicans in additional studies. Sequence of partial 28S (KP729410) (
Amphoteromorphus piraeeba Woodland, 1934
Brachyplatystoma filamentosum (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil (
Notes: type host; tapeworm reported as Amphoteromorphus peniculus by
Amphoteromorphus piriformis Carfora, de Chambrier & Vaucher, 2003
Brachyplatystoma rousseauxii (Actinopterygii: Pimelodidae); freshwater; intestine; adult (also immature specimens); Amazon River basin; Brazil, Peru (
Notes: type host; it was originally reported as Brachyplatystoma flavicans;
Brooksiella praeputialis (Rego, Santos & Silva, 1974) Rego, Chubb & Pavanelli, 1999*
[Syn. Amphoteromorphus praeputialis Rego, dos Santos & Silva, 1974]
Cetopsis coecutiens (Actinopterygii: Cetopsidae); freshwater; intestine; adult; Amazon River basin; Brazil (
Notes: type host;
Cetopsis othonops (Actinopterygii: Cetopsidae); freshwater; intestine; adult; Orinoco River basin; Venezuela (
Note: host reported as Pseudocetopsis othonops.
Gibsoniela mandube (Woodland, 1935) Rego, 1984*
[Syns. Anthobothrium mandube Woodland, 1935; Endorchis (Pseudendorchis) mandube (Woodland, 1935) Yamaguti, 1959; Nomimoscolex mandube (Woodland, 1935) Brooks, 1995]
Ageneiosus inermis (Actinopterygii: Auchenipteridae); freshwater; intestine; adult; Amazon River basin; Brazil, Peru (
Notes: type host; it was reported as A. brevifilis or Pseudoageneiosus brevifilis.
Ageneiosus sp. (Actinopterygii: Auchenipteridae); freshwater; intestine; adult; Amazon River basin; Peru (
Note: sequence of partial 28S (KP729412) (
Gibsoniela meursaulti de Chambrier & Vaucher, 1999
[Syn. Endorchis mandube Woodland, 1935]
Ageneiosus inermis (Actinopterygii: Auchenipteridae); freshwater; intestine; adult; Amazon and Paraná River basins; Argentina, Brazil, Paraguay (
Notes: type host; it was reported as Ageneiosus brevifilis and Pseudoageneiosus brevifilis.
Ageneiosus militaris (Actinopterygii: Auchenipteridae); freshwater; intestine; adult; Paraná River basin; Argentina (
Harriscolex kaparari (Woodland, 1935) Rego, 1987*
[Syns. Nomimoscolex karapari Woodland, 1935; Houssayela karapari (Woodland, 1935) Brooks, 1995]
Brachyplatystoma vaillantii (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Orinoco River basin; Venezuela (
Pseudoplatystoma corruscans (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná and São Francisco River basins; Brazil (
Note: records of Harriscolex kaparari from the Paraná River basin need verification, since H. nathaliae was described from the same river basin and fish host.
Pseudoplatystoma fasciatum (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon and Paraná River basins; Brazil, Peru (
Pseudoplatystoma tigrinum (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil (
Notes: type host. Sequence of partial 28S (AJ275227) and 16S (AJ275223) (
Harriscolex nathaliae Gil de Pertierra & de Chambrier, 2013
Pseudoplatystoma corruscans (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Argentina, Paraguay (
Note: type host;
Harriscolex piramutab (Woodland, 1933) de Chambrier, Kuchta & Scholz, 2015 [Syns. Anthobothrium piramutab Woodland, 1933; Proteocephalus piramutab (Woodland, 1933) Rego, 1984]
Brachyplatystoma vaillantii (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil, Peru (
Note: type host.
Houssayela sudobim (Woodland, 1935) Rego, 1987*
[Syns. Myzophorus sudobim Woodland, 1935; Nomimoscolex woodlandi Freze, 1965 necN. woodlandi Rego & Pavanelli, 1992]
Pseudoplatystoma fasciatum (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil, Peru (
Notes: type host.
Nomimoscolex admonticellia (Woodland, 1934) Rego & Pavanelli, 1992
[Syns. Myzophorus admonticellia Woodland, 1934 (pro parte); Paramonticellia admonticellia (Woodland, 1934) Brooks, 1995; Myzophorus schaefferi Pavanelli and Machado, 1991 (nomen nudum)]
Pinirampus pirinampu (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon and Paraná River basins; Brazil, Peru (
Notes: see p. 40 in the section of M. ventrei for notes on the type host.
Nomimoscolex alovarius Brooks & Deardorff, 1980
Pimelodus blochii (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Magdalena River basin; Colombia (
Note: type host; it was reported as Pimelodus clarias.
Nomimoscolex chubbi (Pavanelli & Takemoto, 1995) de Chambrier & Vaucher, 1997
[Syn. Proteocephalus chubbi Pavanelli & Takemoto, 1995]
Gymnotus carapo (Actinopterygii: Gymnotidae); freshwater; intestine; adult; Paraná River basin; Argentina, Brazil, Paraguay (
Notes: type host. Sequences of partial 28S (AJ388625) and 16S (AJ389524) (
Gymnotus sp. (Actinopterygii: Gymnotidae); freshwater; intestine; adult; Paraná River basin; Brazil (
Nomimoscolex dechambrieri Gil de Pertierra, 2003
Gymnotus carapo (Actinopterygii: Gymnotidae); freshwater; intestine; adult; Paraná River basin; Argentina (
Note: type host.
Nomimoscolex dorad (Woodland, 1935) Freze, 1965
[Syn. Myzophorus dorad Woodland, 1935]
Brachyplatystoma rousseauxii (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil (
Notes: type host; it was reported as Brachyplatystoma flavicans in some studies. Sequences of partial 18S (AY551114), complete ITS2 (AY551150), partial 28S (AJ388613) and 16S (AJ389498) (
Nomimoscolex guillermoi Gil de Pertierra, 2003
Gymnotus carapo (Actinopterygii: Gymnotidae); freshwater; intestine; adult; Paraná River basin; Argentina (
Note: type host.
Nomimoscolex lenha (Woodland, 1933) Woodland, 1935
[Syns. Proteocephalus lenha Woodland, 1933; Paramonticellia lenha (Woodland, 1933) Brooks, 1995]
Sorubimichthys planiceps (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil, Peru (
Notes: type host; it was originally reported as Platystomatichthys sturio. Sequences of partial 18S (AY551115), complete ITS2 (AY551151), partial 28S (AJ388611) and 16S (AJ389499) (
Nomimoscolex lopesi Rego, 1989
[Syn. Paramonticellia lopesi (Rego, 1989) Brooks, 1995]
Pseudoplatystoma fasciatum (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon and Paraná River basins; Argentina, Brazil, Paraguay, Peru (
Note: type host. Sequences of partial 18S (AY551116), complete ITS2 (AY551152), partial 28S (AJ388618) and 16S (AJ389521) (
Nomimoscolex matogrossensis Rego & Pavanelli, 1990
Hoplerythrinus unitaeniatus (Actinopterygii: Erythrinidae); freshwater; body
cavity, intestine, stomach; metacestode; Amazon River basin; Brazil (
Note: the authors did not provide any molecular data on these larvae; therefore, this report needs verification.
Hoplias malabaricus (Actinopterygii: Erythrinidae); freshwater; intestine; adult
and metacestode; Amazon and Paraná River basin; Brazil, Paraguay (
Notes: type host. Sequences of partial 18S (Z98387, Z98386, Z98388), 28S (AJ388614) and 16S (AJ389500) (
Nomimoscolex microacetabula Gil de Pertierra, 1995
Pimelodus albicans (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Argentina (
Pimelodus maculatus (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Argentina (
Note: type host.
Pimelodus ornatus (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Paraguay (
Note: they reported this species as Nomimoscolex cf. microacetabula.
Nomimoscolex pertierrae de Chambrier, Takemoto & Pavanelli, 2006
Pseudoplatystoma corruscans (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná river basin; Brazil (
Note: type host.
Nomimoscolex piraeeba Woodland, 1934*
Brachyplatystoma capapretum (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil (
Notes: type host; it was identified as B. filamentosum. Nomimoscolex piraeeba, N. dorad and N. suspectus were defined as Nomimoscolex (sensu stricto) by
Brachyplatystoma rousseauxii (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil (
Note: host reported as Brachyplatystoma flavicans.
Nomimoscolex semenasae Gil de Pertierra, 2002
Olivaichthys viedmensis (Actinopterygii: Diplomystidae); freshwater; intestine; adult; Moreno and Nahuel Huapi Lakes; Argentina (
Note: type host; it was originally reported as Diplomystes viedmensis.
Nomimoscolex sudobim Woodland, 1935
[Syn. Paramonticellia sudobim (Woodland, 1935) Brooks, 1995]
Pseudoplatystoma corruscans (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Brazil (
Note: records of this species from the Paraná River basin need verification, because N. pertierrae was described from the same river basin and host.
Pseudoplatystoma fasciatum (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon and Paraná River basins; Brazil, Peru (
Notes: type host. Host reported as P. reticulatum by
Pseudoplatystoma tigrinum (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Peru (
Note: they reported this species as Nomimoscolex cf. sudobim.
Nomimoscolex suspectus Zehnder, de Chambrier, Vaucher & Mariaux, 2000
Brachyplatystoma filamentosum (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil (
Notes: type host. Sequences of partial 28S (AJ275067) (
Brachyplatystoma cf. filamentosum (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Peru (
Brachyplatystoma rousseauxii (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil (
Notes: host reported as Brachyplatystoma flavicans. Sequence of partial 28S (AJ275068) (
Brachyplatystoma vaillantii (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil (
Notes: tapeworms reported as Nomimoscolex sp. by
Nomimoscolex sp.
Brachyplatystoma rousseauxii (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin (estuary, Marajó Island); Brazil (
Luciopimelodus pati (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Paraguay (
Pimelodus maculatus (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraíba do Sul, Paraná and São Francisco River basins; Brazil (
Pimelodus ornatus (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Peru (
Pseudoplatystoma corruscans (Actinopterygii: Pimelodidae); freshwater; intestine; adult; São Francisco River basin; Brazil (
Pseudoplatystoma fasciatum (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River Basin; Brazil (
Note: host reported as P. punctifer (see the note on p. 32).
Travassiella jandia (Woodland, 1934) de Chambrier, Scholz & Kuchta, 2014*
[Syns. Proteocephalus jandia Woodland, 1934; Travassiella avitellina Rego & Pavanellli, 1987]
Rhamdia quelen (Actinopterygii: Heptapteridae); freshwater; intestine; adult; Chis-Chis lagoon (Buenos Aires); Argentina (
Note: host reported as Rhamdia sapo.
Zungaro jahu (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Argentina, Brazil, Paraguay (
Notes: host also reported as Zunguro zungaro or Paulicea luetkeni.
Zungaro zungaro (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil, Peru (
Note: type host; it was originally reported as Rhamdia sp.
Unidentified fish (Actinopterygii); freshwater; intestine; adult; Amazon River basin; Brazil (
Zygobothrium megacephalum Diesing, 1850*
Phractocephalus hemioliopterus (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil, Peru (
Notes: type host. Sequences of complete 18S (AF286991) and ITS2 (AY551177), partial 28S (AF286939, AJ388621) and 16S (AJ389508) (
Unidentified proteocephalids
Aequidens tetramerus (Actinopterygii: Cichlidae); freshwater; body cavity; metacestode; Amazon River basin; Brazil (
Aphyocharax anisitsi (Actinopterygii: Characidae); freshwater; site of infection not given; metacestode; Paraná River basin; Brazil (
Astronotus ocellatus (Actinopterygii: Cichlidae); freshwater; body cavity; metacestode; fish farms in Northeast (Ceará, Pernambuco, Piauí and Rio Grande do Norte States); Brazil (
Astyanax altiparanae (Actinopterygii: Characidae); freshwater; body cavity; metacestode; Rio das Pedras Farm (lakes); Brazil (
Auchenipterus nigripinnis (Actinopterygii: Auchenipteridae); freshwater; intestine; adult; Paraná River basin; Brazil (
Brachyplatystoma filamentosum (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil (
Brycon cephalus (Actinopterygii: Bryconidae); freshwater; surface of intestine and pyloric caeca; metacestode; Amazon River basin; Brazil (
Cichla ocellaris (Actinopterygii: Cichlidae); freshwater; body cavity; metacestode; fish farms in Northeastern Brazil (Ceará, Pernambuco, Piauí and Rio Grande do Norte States); Brazil (
Cichlasoma amazonarum (Actinopterygii: Cichlidae); freshwater; intestine; adult; Amazon River basin; Peru (
Note: these tapeworms belong to a new species and genus that will be described in a forthcoming paper.
Colossoma macropomum (Actinopterygii: Serrasalmidae); freshwater; body cavity; metacestode; fish farms in Northeastern Brazil (Ceará, Pernambuco, Piauí and Rio Grande do Norte States); Brazil (
Corydoras atropersonatus (Actinopterygii: Callichthyidae); freshwater; mesentery; metacestode; Amazon River basin; Peru (
Corydoras reticulatus (Actinopterygii: Callichthyidae); freshwater; mesentery; metacestode; Amazon River basin; Peru (
Corydoras sychri (Actinopterygii: Callichthyidae); freshwater; mesentery; metacestode; Amazon River basin; Peru (
Crenicichla lepidota (Actinopterygii: Cichlidae); freshwater; intestine; adult (immature specimens); Paraná River Basin; Paraguay (
Cynoscion striatus (Actinopterygii: Sciaenidae); marine; intestine; metacestode; WTSA; Brazil (
Note: certainly not a larva of the Proteocephalidae.
Cyprinus carpio (Actinopterygii: Cyprinidae); freshwater; body cavity; metacestode; fish farms in Northeastern Brazil (Ceará, Pernambuco, Piauí and Rio Grande do Norte states); Brazil (
Note: introduced fish host (
Galeocharax knerii (Actinopterygii: Characidae); freshwater; intestine; metacestode; Paraná River basin; Brazil (
Geophagus brasiliensis (Actinopterygii: Cichlidae); freshwater; body cavity, gallbladder; metacestode; Paraná River basin; Brazil (
Geophagus proximus (Actinopterygii: Cichlidae); freshwater; body cavity; metacestode; Paraná River basin; Brazil (
Hemisorubim platyrhynchos (Actinopterygii: Pimelodidae); freshwater; mesentery; metacestode; Amazon River basin; Peru (
Hypophthalmichthys nobilis (Actinopterygii: Cyprinidae); freshwater; body cavity; metacestode; fish farms in Northeastern Brazil (Ceará, Pernambuco, Piauí and Rio Grande do Norte States); Brazil (
Note: introduced fish host (
Hypostomus cf. ternetzi (Actinopterygii: Loricariidae); freshwater; intestine; adult (immature specimens); Paraná River Basin; Paraguay (
Iheringichthys labrosus (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Brazil (
Laetacara curviceps (Actinopterygii: Cichlidae); freshwater; body cavity; metacestode; Amazon River basin; Brazil (
Leporellus vittatus (Actinopterygii: Anostomidae); freshwater; site of infection not given; metacestode; Paraná River basin; Brazil (
Leporinus aff. friderici (Actinopterygii: Anostomidae); freshwater; intestine; adult; Paraná River Basin; Paraguay (
Loricariichthys platymetopon (Actinopterygii: Loricariidae); freshwater; body cavity, internal organs; metacestode; Paraná River basin; Brazil (Schäeffer et al. 1992).
Megalonema platanum (Actinopterygii: Pimelodidae); freshwater; intestine; adult (immature specimens); Paraná River Basin; Paraguay (
Oxydoras kneri (Actinopterygii: Doradidae); freshwater; intestine; adult; Paraná River basin; Brazil (
Oreochromis sp. (Actinopterygii: Cichlidae); freshwater; body cavity; metacestode; fish farms in Northeast (Ceará, Pernambuco, Piauí and Rio Grande do Norte States); Brazil (
Note: introduced fish host (
Phractocephalus hemioliopterus (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Amazon River basin; Brazil (
Note: the author reported the presence of operculate eggs released from a contracted proglottid, but this unique report needs confirmation.
Piaractus brachypomus (Actinopterygii: Serrasalmidae); freshwater; body cavity; metacestode; fish farms in northeastern Brazil (Ceará, Pernambuco, Piauí and Rio Grande do Norte States); Brazil (
Note: host reported as Colossoma brachipomum.
Pimelodella gracilis (Actinopterygii: Heptapteridae); freshwater; mesentery; metacestode; Amazon River basin; Peru (
Pimelodus maculatus (Actinopterygii: Pimelodidae); freshwater; intestine; adult (immature specimens), metacestode; Paraná and São Francisco River basins; Brazil, Paraguay (
Pimelodus pohli (Actinopterygii: Pimelodidae); freshwater; intestine; metacestode; São Francisco River basin; Brazil (
Pimelodus sp. (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Brazil (
Pinirampus pirinampu (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Brazil (
Prochilodus brevis (Actinopterygii: Prochilodontidae); freshwater; body cavity; metacestode; fish farms in Northeast (Ceará, Pernambuco, Piauí and Rio Grande do Norte States); Brazil (
Note: host reported as Prochilodus cearensis.
Prochilodus lineatus (Actinopterygii: Prochilodontidae); freshwater; body cavity; metacestode; Paraná River basin; Brazil (
Psellogrammus kennedyi (Actinopterygii: Characidae); freshwater; site of infection not given; metacestode; Paraná River basin; Brazil (
Pseudoplatystoma corruscans (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Brazil (
Pseudoplatystoma fasciatum (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Brazil (
Rhamdia quelen (Actinopterygii: Heptapteridae); freshwater; intestine; adult; Guaíba River estuary (Fortes and Hoffman 1987;
Note: host reported as Rhamdia sapo; in both studies, the authors reported hyperparasitism caused by metacestodes of proteocephalids.
Sardinella sp. (Actinopterygii: Clupeidae); marine; intestine; metacestode; WTSA; Brazil (
Note: certainly not a larva of the Proteocephalidae.
Satanoperca pappaterra (Actinopterygii: Cichlidae); freshwater; intestine; metacestode; Paraná River basin; Brazil (
Trinectes maculatus (Actinopterygii: Achiridae); brackish, freshwater; mesentery; metacestode; Amazon River basin; Peru (
Hybrid fish host (Actinopterygii: Serrasalmidae); freshwater; intestine; metacestode; Amazon River basin; Brazil (
Note: the host is a hybrid of Colossoma macropomum and Piaractus mesopotamicus.
Species inquirendae
Acanthobothroides peruensis López, 1994
Dasyatis dipterura (Elasmobranchii: Dasyatidae); marine; spiral valve; adult; WTSP; Peru (
Notes: type host; it was reported as D. brevis. Only A. thorsoni and A. pacificum Marques, Brooks & Ureña, 1996 are considered valid species in the genus (
Monticellia diesingii (Monticelli, 1891) La Rue, 1911
[Syns. Taenia diesingii Monticelli, 1891; Tetracotylus diesingii Monticelli, 1891; Ichthyotaenia diesingii (Monticelli, 1891) Riggenbach, 1896]
‘Silurus dorgado’ (unknown fish host); freshwater; intestine; adult; unknown
specific locality (
Monticellia macrocotylea (Monticelli, 1891) La Rue, 1911
[Syns. Taenia macrocotylea Monticelli, 1891; Tetracotylus macrocotylea Monticelli, 1891; Ichthyotaenia macrocotylea (Monticelli, 1891) Riggenbach, 1896]
‘Silurus megacephalus’ (unknown fish host); freshwater; intestine; adult; unknown locality (
Nomimoscolex arandasregoi Fortes, 1981
Genidens barbus (Actinopterygii: Ariidae); anadromous; intestine; adult; Guaíba River estuary, WTSA; Brazil (
Note: host reported under four different names, Tachysurus agassizii, T. upsulonophorus, T. barbus and Netuma barba.
Genidens genidens (Actinopterygii: Ariidae); anadromous; intestine; adult; Guaíba River estuary; Brazil (
Note:
Genidens sp. (Actinopterygii: Ariidae); anadromous; intestine; adult; Guaíba River estuary; Brazil (
Note:
Platybothrium parvum Linton, 1901
Sphyrna zygaena (Elasmobranchii: Sphyrnidae); marine; spiral valve; adult; WTSP; Peru (
Note: for details on the taxonomic status of this species, see
Order Phyllobothriidea Caira, Jensen, Waeschenbach, Olson & Littlewood, 2014
Family Phyllobothriidae Braun, 1900
Crossobothrium antonioi Ivanov, 2009
Notorynchus cepedianus (Elasmobranchii: Hexanchidae); marine; spiral valve; adult; WTSA; Argentina (
Note: type host.
Crossobothrium dohrni (Örley, 1885) Ruhnke, 1996
[Syns. Orygmatobothrium dohrni Örley, 1885; Phyllobothrium dohrni (Örley, 1885) Zschokke, 1888]
Hexanchus griseus (Elasmobranchii: Hexanchidae); marine; spiral valve; adult; WTSP; Chile (
Note: tapeworms reported as Phyllobothrium dorhni.
Crossobothrium laciniatum Linton, 1889*
[Syn. Phyllobothrium laciniatum (Linton, 1889) Southwell, 1925]
Hexanchus griseus (Elasmobranchii: Hexanchidae); marine; spiral valve; adult; Magellanic; Chile (
Notes: position of the species within Phyllobothriidae, based on molecular data, is unclear. Sequences of partial 18S (KF685824) and 28S (KF685883) (
Crossobothrium pequeae Ivanov, 2009
Notorynchus cepedianus (Elasmobranchii: Hexanchidae); marine; spiral valve; adult; WTSA; Argentina (
Note: type host.
Nandocestus guariticus (Marques, Brooks & Lasso, 2001) Reyda, 2008*
[Syn. Anindobothrium guariticus Marques, Brooks & Lasso, 2001]
Paratrygon aiereba (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; Amazon and Orinoco River basins; Peru, Venezuela (
Notes: type host.
Potamotrygon cf. falkneri (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult (immature); Amazon River basin; Peru (
Note: host reported as Potamotrygon cf. castexi and it may represent an undescribed species of Potamotrygon (see
Orygmatobothrium juani Ivanov, 2008
Mustelus fasciatus (Elasmobranchii: Carcharhinidae); marine; spiral valve; adult; WTSA; Argentina (
Note: type host.
Orygmatobothrium musteli (van Beneden, 1849) Diesing, 1863*
[Syns. Anthobothrium musteli van Beneden, 1850 (pro parte); Orygmatobothrium versatile (Diesing, 1854) Diesing, 1863; Tetrabothrium versatile Diesing, 1854]
Mustelus mento (Elasmobranchii: Triakidae); marine; spiral valve; adult; WTSP; Chile (
Mustelus whitneyi (Elasmobranchii: Triakidae); marine; spiral valve; adult; WTSP; Peru (
Orygmatobothrium schmittii Suriano & Labriola, 2001
Mustelus schmitti (Elasmobranchii: Triakidae); marine; spiral valve; adult; WTSA; Argentina (
Notes: type host.
Orygmatobothrium sp.
Mustelus mento (Elasmobranchii: Triakidae); marine; spiral valve; adult; WTSP; Peru (
Paraorygmatobothrium angustum (Linton, 1889) Ruhnke, 2011
[Syns. Orygmatobothrium angustum Linton, 1889; Crossobothrium angustum (Linton, 1889) Linton, 1901; Phyllobothrium angustum (Linton, 1889) Euzet, 1952; Scyphophyllidium angustum (Linton, 1889) Riser, 1955]
Alopias vulpinus (Elasmobranchii: Alopiidae); marine; spiral valve; adult; WTSP; Chile (
Note: tapeworms reported as C. angustum.
Prionace glauca (Elasmobranchii: Carcharhinidae); marine; spiral valve; adult; WTSP; Chile, Peru (
Note: tapeworms reported as C. angustum.
Paraorygmatobothrium filiforme (Yamaguti, 1952) Ruhnke, 1996
[Syns. Phyllobothrium filiforme Yamaguti, 1952; Crossobothrium filiforme (Yamaguti, 1952) Williams, 1968]
Carcharhinus longimanus (Elasmobranchii: Carcharhinidae); marine; spiral valve; adult; TSA; Brazil (
Note: tapeworms reported as Phyllobothrium filiforme.
Paraorygmatobothrium prionacis (Yamaguti, 1934) Ruhnke, 1994*
[Syns. Phyllobothrium prionacis Yamaguti, 1934; Crossobothrium prionacis (Yamaguti, 1934) Williams, 1968; Anthobothrium minutum Guiart, 1935]
Prionace glauca (Elasmobranchii: Carcharhinidae); marine; spiral valve; adult; TSA; Brazil (
Notes: type host. Tapeworms reported as P. prionacis.
Paraorygmatobothrium triacis (Yamaguti, 1952) Ruhnke, 1996
[Syns. Phyllobothrium triacis Yamaguti, 1952; Crossobothrium triacis (Yamaguti, 1952) Euzet, 1959]
Mustelus mento (Elasmobranchii: Triakidae); marine; spiral valve; adult; WTSP; Chile (
Note: tapeworms reported as C. triacis.
Mustelus whitneyi (Elasmobranchii: Triakidae); marine; spiral valve; adult; WTSP; Peru (
Note: tapeworms reported as C. triacis.
Phyllobothrium lactuca van Beneden, 1850*
Dipturus trachyderma (Elasmobranchii: Rajidae); marine; spiral valve; adult; WTSP; Chile (
Notes: tapeworms reported as Phyllobothrium cf. lactuca and the host as Raja trachyderma. The identification of this cestode is most likely erroneous, since sharks are the definitive hosts for species of the genus Phyllobothrium van Beneden, 1849 (see
Mustelus mento (Elasmobranchii: Triakidae); marine; spiral valve; adult; WTSP; Chile, Peru (
Notes: tapeworms reported as Phyllobothrium cf. lactuca by
Phyllobothrium sp.
Dipturus flavirostris (Elasmobranchii: Rajidae); marine; spiral valve; adult; WTSP; Chile (
Note: host reported as Raja flavirostris.
Mustelus mento (Elasmobranchii: Triakidae); marine; spiral valve; adult; WTSP; Peru (
Myliobatis goodei (Elasmobranchii: Myliobatidae); marine; spiral valve; adult; WTSA (La Plata River estuary); Uruguay (
Sympterygia bonapartii (Elasmobranchii: Arhynchobatidae); marine; spiral valve; adult; WTSA; Argentina (
Notes: host reported as Psammobatis microps. The author found only specimens without scolex, which she supposed to belong to Phyllobothrium (see
Urophycis brasiliensis (Actinopterygii: Phycidae); marine; mesentery; metacestode: WTSA; Argentina (
Zapteryx brevirostris (Elasmobranchii: Rhinobatidae); marine; spiral valve; metacestode; WTSA; Argentina (
Scyphophyllidium uruguayense Brooks, Marques, Perroni & Sidagis, 1999
Mustelus mento (Elasmobranchii: Triakidae); marine; spiral valve; adult; WTSA; Uruguay (
Note: type host.
Thysanocephalum thysanocephalum (Linton, 1889) Braun, 1900
[Syns. Phyllobothrium thysanocephalum Linton, 1889 nec Thysanocephalum crispum (Linton, 1889) Linton, 1890 (nomen nudum)]
Sphyrna zygaena (Elasmobranchii: Sphyrnidae); marine; spiral valve; adult; WTSP; Peru (
Notes: tapeworms reported as Thysanocephalum crispum. According to
Unidentified phyllobothriideans
Merluccius australis (Actinopterygii: Merlucciidae); marine; intestine; metacestode; Magellanic; Chile, Falkland Islands (
Merluccius hubbsi (Actinopterygii: Merlucciidae); marine; intestine; metacestode; Magellanic; Argentina, Falkland Islands (
Mugil liza (Actinopterygii: Mugilidae); marine; site of infection not given; metacestode; WTSA; Brazil (
Note: host reported as Mugil platanus.
Oncorhynchus mykiss (Actinopterygii: Salmonidae); anadromous; intestine; metacestode; Aisén River basin; Chile (
Prionotus sp. (Actinopterygii: Triglidae); marine; intestine; metacestode; TSA; Brazil (
Urophycis brasiliensis (Actinopterygii: Phycidae); marine; mesentery; metacestode; WTSA; Brazil (
Urophycis mystaceus (Actinopterygii: Phycidae); marine; mesentery; metacestode; WTSA; Brazil (
Urophycis sp. (Actinopterygii: Phycidae); marine; intestine; metacestode; TSA; Brazil (
Taxa incertae sedis
Guidus argentinense Ivanov, 2006*
Bathyraja brachyurops (Elasmobranchii: Arhynchobatidae); marine; spiral valve; adult; WTSA; Argentina (
Notes: type host.
Phyllobothrium sinuosiceps Williams, 1959
Hexanchus griseus (Elasmobranchii: Hexanchidae); marine; spiral valve; adult; WTSP; Chile (
Notes: type host. This species somewhat resembles members of the genus Crossobothrium, but it is treated as incertae sedis by
Order Rhinebothriidea Healy, Caira, Jensen, Webster & Littlewood, 2009
Family Anthocephaliidae Ruhnke, Caira & Cox, 2015
Anthocephalum gracile Linton, 1890*
[Syns. Phyllobothrium centrurum Southwell, 1925; Anthocephalum centrurum (Southwell, 1925) Ruhnke, 1994]
Dasyatis americana (Elasmobranchii: Dasyatidae); marine; spiral valve; adult; TNA; Venezuela (
Note: tapeworms reported as Phyllobothrium centrurum.
Anthocephalum hobergi (Zamparo, Brooks & Barriga, 1999) Marques & Caira, 2016
[Syn. Pararhinebothroides hobergi Zamparo, Brooks & Barriga, 1999]
Urobatis tumbesensis (Elasmobranchii: Urotrygonidae); marine; spiral valve; adult; TEP; Ecuador (
Notes: type host. Sequences of partial 18S (KU295561–KU295564) and 28S (KU295565–KU295568) (
Anthocephalum kingae (Schmidt, 1978) Ruhnke & Seaman, 2009
[Syn. Phyllobothrium kingae Schmidt, 1978]
Dasyatis americana (Elasmobranchii: Dasyatidae); marine; spiral valve; adult; TNA; Colombia (
Note: tapeworms reported as Anthocephalum cf. kingae by Brooks and Mayes (1980).
Urobatis jamaicensis (Elasmobranchii: Urotrygonidae); marine; spiral valve; adult; TNA; Colombia (
Note: type host; it was reported as Urolophus jamaicensis. Tapeworms reported as Anthocephalum cf. kingae by Brooks and Mayes (1980).
Family Echeneibothriidae de Beauchamp, 1905
Echeneibothrium megalosoma Carvajal & Dailey, 1975
Dipturus flavirostris (Elasmobranchii: Rajidae); marine; spiral valve; adult; WTSP; Chile (
Note: host reported as Raja flavirostris.
Zearaja chilensis (Elasmobranchii: Rajidae); marine; spiral valve; adult; WTSP; Chile (
Notes: type host; it was reported as Raja chilensis.
Echeneibothrium multiloculatum Carvajal & Dailey, 1975
Dipturus flavirostris (Elasmobranchii: Rajidae); marine; spiral valve; adult; WTSP; Chile (
Note: host reported as Raja flavirostris.
Zearaja chilensis (Elasmobranchii: Rajidae); marine; spiral valve; adult; WTSP; Chile (
Note: type host; it was reported as Raja chilensis.
Echeneibothrium williamsi Carvajal & Dailey, 1975
Dipturus flavirostris (Elasmobranchii: Rajidae); marine; spiral valve; adult; WTSP; Chile (
Note: host reported as Raja flavirostris.
Zearaja chilensis (Elasmobranchii: Rajidae); marine; spiral valve; adult; WTSP; Chile (
Note: type host; it was reported as Raja chilensis.
Notomegarhynchus navonae Ivanov & Campbell, 2002*
Atlantoraja castelnaui (Elasmobranchii: Arhynchobatidae); marine; spiral valve; adult; WTSA; Argentina (
Note: type host.
Family Rhinebothriidae Euzet, 1953
Rhinebothrium brooksi Reyda & Marques, 2011
Paratrygon aiereba (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin; Brazil (
Notes: type host. Sequences of partial cox1 (JF803719–JF803724) under the name Rhinebothrium sp. 1 (
Potamotrygon orbignyi (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin; Brazil (
Rhinebothrium chilensis Euzet & Carvajal, 1973
Sympterygia bonapartii (Elasmobranchii: Arhynchobatidae); marine; spiral valve; adult; WTSA; Argentina (
Sympterygia lima (Elasmobranchii: Arhynchobatidae); marine; spiral valve; adult; WTSP; Chile (
Note: type host; it was reported as Psammobatis lima.
Rhinebothrium copianullum Reyda, 2008
Paratrygon aiereba (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon and Tocantins-Araguaia River basins; Brazil, Peru (
Notes: type host.
Potamotrygon henlei (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Tocantins-Araguaia River basin; Brazil (
Potamotrygon leopoldi (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin; Brazil (
Note: sequence of cox1 (JF803711) (
Potamotrygon motoro (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult (immature); Amazon River basin; Brazil (
Note: accidental host (
Potamotrygon orbignyi (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin; Brazil (
Potamotrygon schroederi (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult (immature); Amazon River basin; Brazil (
Note: accidental host, according to
Potamotrygon tatianae (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult (immature); Amazon River basin; Peru (
Notes: accidental host. Sequence of cox1 (JF803699) (
Potamotrygon sp. (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon and Tocantins-Araguaia River basins; Brazil (
Notes: the authors distinguished four morphotypes of the host. Sequence of cox1 (JF803702, JF803715–JF803718) (
Rhinebothrium corbatai Menoret & Ivanov, 2011
Potamotrygon motoro (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Paraná River basin; Argentina (
Note: type host.
Rhinebothrium corymbum Campbell, 1975
Dasyatis americana (Elasmobranchii: Dasyatidae); marine; spiral valve; adult; TNA; Venezuela (
Note: type host.
Rhinebothrium fulbrighti Reyda & Marques, 2011
Potamotrygon orbignyi (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin (estuary); Brazil (
Notes: type host. Sequences of cox1 (JF803725, JF803729–JF803734) under the name Rhinebothrium sp. 2 (
Potamotrygon sp. (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin (estuary); Brazil (
Rhinebothrium jaimei Marques & Reyda, 2015
Potamotrygon orbignyi (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin (estuary); Brazil (
Note: type host.
Potamotrygon scobina (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin (estuary); Brazil (
Rhinebothrium leiblei Euzet & Carvajal, 1973
Sympterygia lima (Elasmobranchii: Arhynchobatidae); marine; spiral valve; adult; WTSP; Chile (
Note: type host; it was reported as Psammobatis lima.
Rhinebothrium margaritense Mayes & Brooks, 1981
Dasyatis americana (Elasmobranchii: Dasyatidae); marine; spiral valve; adult; TNA; Venezuela (
Dasyatis guttata (Elasmobranchii: Dasyatidae); marine; spiral valve; adult; TNA; Venezuela (
Note: type host.
Rhinebothrium mistyae Menoret & Ivanov, 2011
Potamotrygon motoro (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Paraná River basin; Argentina (
Note: type host.
Rhinebothrium paratrygoni Rego & Dias, 1976
[Syn. Rhinebothrium paranaensis Menoret & Ivanov, 2009]
Potamotrygon brachyura (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Paraná River basin; Brazil (
Potamotrygon falkneri (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Paraná River basin; Argentina, Brazil, Paraguay (
Note: sequences of cox1 (JF803684, JF803685, JF803687–JF803689, JF803691, JF803692) (
Potamotrygon histrix (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Paraná River basin; Brazil (
Potamotrygon motoro (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon and Paraná River basins; Brazil (
Note: sequences of cox1 (JF803686, JF803690, JF803693) (
Potamotrygon orbignyi (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Orinoco River basin; Venezuela (
Note: host reported as P. hystrix and P. reticulatus (for details, see
Potamotrygon sp. (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon and Paraná River basins; Brazil (
Note: type host; it was reported as Elipesurus sp.;
Rhinebothrium rhinobati Dailey & Carvajal, 1976
Rhinobatos planiceps (Elasmobranchii: Rhinobatidae); marine; spiral valve; adult; WTSP; Chile, Peru (
Note: type host.
Rhinebothrium scobinae Euzet & Carvajal, 1973
Psammobatis scobina (Elasmobranchii: Arhynchobatidae); marine; spiral valve; adult; WTSP; Chile (
Note: type host.
Rhinebothrium tetralobatum Brooks, 1977
Himantura schmardae (Elasmobranchii: Dasyatidae); marine; spiral valve; adult; TNA; Colombia (
Note: type host.
Rhinebothrium sp.
Gobionellus oceanicus (Actinopterygii: Gobiidae); marine; body cavity; metacestode; TSA; Brazil (
Paratrygon aiereba (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin; Peru (
Notes: they reported hyperparasitism caused by metacestodes of proteocephalids. Sequences of partial 28S (AY193880–AY193883) from adult tapeworms and partial 28S (AY193877–AY193879) from the encysted larval forms (
Scomber colias (Actinopterygii: Scombridae); marine; intestine, pyloric caeca; metacestode; WTSA; Brazil (
Note: host reported as S. japonicus, but specimens from the Atlantic were re-assigned as S. colias, according to
Synodus scituliceps (Actinopterygii: Synodontidae); marine; intestine, pyloric caeca; metacestode; WTSP; Peru (
Rhinebothroides campbelli Ivanov, 2004
Potamotrygon motoro (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Paraná River basin; Argentina (
Notes: type host.
Rhinebothroides freitasi (Rego, 1979) Brooks, Mayes & Thorson, 1981
[Syns. Rhinebothrium freitasi Rego, 1979; Rhinebothroides circularisi Mayes, Brooks & Thorson, 1981; Rhinebothroides venezuelensis Brooks, Mayes & Thorson, 1981]
Potamotrygon constellata (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin; Brazil (
Note: host reported as P. circularis, whereas tapeworms were reported as R. circularisi.
Potamotrygon cf. falkneri (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon and Paraná River basins; Brazil, Peru (
Notes:
Potamotrygon henlei (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Tocantins-Araguaia River basin; Brazil (
Potamotrygon leopoldi (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin; Brazil (
Potamotrygon motoro (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon and Paraná River basins; Argentina, Brazil (
Potamotrygon orbignyi (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon and Orinoco River basins; Brazil, Venezuela (
Notes: type host; it was reported as P. hystrix by
Potamotrygon schroederi (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin; Brazil (
Potamotrygon scobina (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin; Brazil (
Potamotrygon yepezi (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Maracaibo basin; Venezuela (
Rhinebothroides glandularis Brooks, Mayes & Thorson, 1981
Potamotrygon henlei (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Tocantins-Araguaia River basin; Brazil (
Potamotrygon motoro (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon and Paraná River basins; Argentina, Brazil (
Note: sequence of partial cox1 (JF803682) (
Potamotrygon orbignyi (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon and Orinoco River basins; Brazil, Venezuela (
Notes: type host; it was originally reported as P. hystrix.
Potamotrygon scobina (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin; Brazil (
Potamotrygon signata (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Parnaíba River basin; Brazil (
Potamotrygon sp. (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon and Orinoco River basins; Venezuela (
Notes:
Rhinebothroides mclennanae Brooks & Amato, 1992
Potamotrygon motoro (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Paraná River basin; Argentina, Brazil (
Notes: type host.
Rhinebothroides moralarai (Brooks & Thorson, 1976) Mayes, Brooks & Thorson, 1981*
[Syn. Rhinebothrium moralarai Brooks & Thorson, 1976]
Potamotrygon magdalenae (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Magdalena River basin; Colombia (
Notes: type host.
Potamotrygon sp. (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin; Brazil (
Note: sequence of partial cox1 (JF803681) (
Rhinebothroides scorzai (Lopez-Neyra & Diaz-Ungria, 1958) Mayes, Brooks & Thorson, 1981
[Syn. Rhinebothrium scorzai Lopez-Neyra & Diaz-Ungria, 1958]
Paratrygon aiereba (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin; Brazil (
Potamotrygon motoro (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon and Paraná River basins; Brazil (
Potamotrygon orbignyi (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Orinoco River basin; Venezuela (Lopez-Neyra and Diaz-Ungria 1958).
Note: type host; it was originally reported as P. hystrix.
Potamotrygon sp. (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin; Brazil (
Note: sequence of partial cox1 (JF803680) (
Rhinebothroides sp.
Paratrygon aiereba (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult (immature); Amazon River basin; Peru (
Potamotrygon cf. falkneri (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon and Paraná River basins; Brazil, Peru (
Notes:
Potamotrygon motoro (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin; Peru (
Note: the authors reported hyperparasitism caused by metacestodes of proteocephalids.
Potamotrygon tatianae (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin; Peru (
Note: sequence of partial cox1 (JF803679) (
Rhodobothrium mesodesmatum (Bahamonde & Lopez, 1962) Campbell & Carvajal, 1979
[Syns. Proboscidosaccus mesodesmatis Bahamonde & Lopez, 1962; Anthobothrium peruanum Rego, Vicente & Herrera, 1968]
Myliobatis chilensis (Elasmobranchii: Myliobatidae); marine; spiral valve; adult; WTSP; Chile, Peru (
Note: type host. Tapeworm originally described by
Myliobatis peruvianus (Elasmobranchii: Myliobatidae); marine; spiral valve; adult; WTSP; Peru (
Sarda chiliensis (Actinopterygii: Scombridae); marine; intestine; adult (?); WTSP; Peru (
Rhodobothrium paucitesticulare Mayes & Brooks, 1981
Rhinoptera bonasus (Elasmobranchii: Myliobatidae); marine; spiral valve; adult; TNA; Venezuela (
Note: type host.
Rhodobothrium pulvinatum Linton, 1889*
[Syns. Anthobothrium pulvinatum Linton, 1890 necA. pulvinatum Linton, 1889 (nomen nudum); Inermiphyllidium pulvinatum (Linton, 1890) Riser, 1955]
Dasyatis americana (Elasmobranchii: Dasyatidae); marine; spiral valve; adult; TNA; Venezuela (
Dasyatis guttata (Elasmobranchii: Dasyatidae); marine; spiral valve; adult; TNA; Venezuela (
Scalithrium magniphallum (Brooks, 1977) Ball, Neifar & Euzet, 2003
[Syn. Rhinebothrium magniphallum Brooks, 1977]
Dasyatis americana (Elasmobranchii: Dasyatidae); marine; spiral valve; adult; TNA; Colombia (
Dasyatis guttata (Elasmobranchii: Dasyatidae); marine; spiral valve; adult; TNA; Venezuela (
Himantura schmardae (Elasmobranchii: Dasyatidae); marine; spiral valve; adult; TNA; Colombia, Venezuela (
Note: type host.
Urobatis jamaicensis (Elasmobranchii: Urotrygonidae); marine; spiral valve; adult; TNA; Colombia (
Notes: host reported as Urolophus jamaicensis.
Urotrygon venezuelae (Elasmobranchii: Urotrygonidae); marine; spiral valve; adult; TNA; Colombia (
Taxa incertae sedis
Anindobothrium anacolum (Brooks, 1977) Marques, Brooks & Lasso, 2001*
[Syn. Caulobothrium anacollum Brooks, 1977]
Himantura schmardae (Elasmobranchii: Dasyatidae); marine; spiral valve; adult; TNA; Colombia (
Notes: type host. The genus Anindobothrium is likely a member of the Rhinebothriidea (see
Anindobothrium lisae Marques, Brooks & Lasso, 2001
Potamotrygon orbignyi (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin; Brazil (
Note: type host.
Phyllobothrium auricula van Beneden, 1858
Myliobatis chilensis (Elasmobranchii: Myliobatidae); marine; spiral valve; adult; WTSP; Peru (
Note: the species is likely a member of the Rhinebothriidea (see
Myliobatis peruvianus (Elasmobranchii: Myliobatidae); marine; spiral valve; adult; WTSP; Peru (
Phyllobothrium discopygi Campbell & Carvajal, 1987
Discopyge tschudi (Elasmobranchii: Torpedinidae); marine; spiral valve; adult; WTSP; Chile (
Notes: type host. This species is likely a member of the Rhinebothriidea (see
Phyllobothrium myliobatidis Brooks, Mayes & Thorson, 1981
Myliobatis goodei (Elasmobranchii: Myliobatidae); marine; spiral valve; adult; WTSA (La Plata River estuary); Uruguay (
Notes: type host. The tapeworm is likely a member of the Rhinebothriidea (see
Order ‘Tetraphyllidea’ Carus, 1863
Anthobothrium laciniatum Linton, 1890
Carcharhinus longimanus (Elasmobranchii: Carcharhinidae); marine; spiral valve; adult; TSA; Brazil (
Anthobothrium galeorhini Suriano, 2002
Galeorhinus galeus (Elasmobranchii: Triakidae); marine; spiral valve; adult; Magellanic; Argentina (
Note: type host.
Calliobothrium australis Ostrowski de Núñez, 1973
Mustelus schmitti (Elasmobranchii: Triakidae); marine; spiral valve; adult; WTSA; Argentina, Uruguay (
Notes: type host. This species was originally described as C. verticillatum australis, but
Calliobothrium verticillatum (Rudolphi, 1819) van Beneden, 1850*
[Syns. Bothriocephalus verticillatus Rudolphi, 1819; Acanthobothrium verticillatum (Rudolphi, 1819) van Beneden, 1849]
Mustelus mento (Elasmobranchii: Triakidae); marine; spiral valve; adult; WTSP; Chile, Peru (
Calliobothrium sp.
Mustelus mento (Elasmobranchii: Triakidae); marine; spiral valve; adult; WTSP; Peru (
Caulobothrium myliobatidis Carvajal, 1977
Myliobatis chilensis (Elasmobranchii: Myliobatidae); marine; spiral valve; adult; WTSP; Chile, Peru (
Note: type host.
Caulobothrium ostrowskiae Brooks, Mayes & Thorson, 1981
Myliobatis goodei (Elasmobranchii: Myliobatidae); marine; spiral valve; adult; WTSA (La Plata River estuary); Uruguay (
Note: type host.
Caulobothrium uruguayense Brooks, Mayes & Thorson, 1981
Myliobatis goodei (Elasmobranchii: Myliobatidae); marine; spiral valve; adult; WTSA (La Plata River estuary); Uruguay (
Note: type host; it was reported as Myliobatis uruguayensis.
Dioecotaenia campbelli Mayes & Brooks, 1981
Rhinoptera bonasus (Elasmobranchii: Myliobatidae); marine; spiral valve; adult; TNA; Venezuela (
Note: type host.
Reesium paciferum (Sproston, 1948) Euzet, 1955*
[Syn. Dinobothrium paciferum Sproston, 1948]
Cetorhinus maximus (Elasmobranchii: Cetorhinidae); marine; spiral valve; adult; WTSP; Peru (
Note: type host.
Serendip deborahae Brooks & Barriga, 1995*
Rhinoptera steindachneri (Elasmobranchii: Myliobatidae); marine; spiral valve; adult; TEP; Ecuador (
Notes: type host. Serendip Brooks & Barriga, 1995 is likely a member of the Rhinebothriidea, according to
Symcallio barbarae (Ivanov & Brooks, 2002) Bernot, Caira & Pickering, 2015
[Syns. Calliobothrium eschrichti of
Mustelus schmitti (Elasmobranchii: Triakidae); marine; spiral valve; adult; WTSA; Argentina, Uruguay (
Note: type host.
Symcallio lintoni (Euzet, 1954) Bernot, Caira & Pickering, 2015
[Syn. Calliobothrium lintoni Euzet, 1954]
Mustelus whitneyi (Elasmobranchii: Triakidae); marine; spiral valve; adult; WTSP; Peru (
Symcallio lunae (Ivanov & Brooks, 2002) Bernot, Caira & Pickering, 2015
[Syns. of Calliobothrium lintoni of
Mustelus schmitti (Elasmobranchii: Triakidae); marine; spiral valve; adult; WTSA; Argentina, Uruguay (
Notes: type host.
Collective group name for larval ‘tetraphyllideans’ and Unidentified taxa
‘ Scolex pleuronectis ’ Müller, 1788; ‘ Scolex polymorphus ’ Rudolphi, 1819; ‘ Scolex sp.’; ‘Tetraphyllidea gen. sp.’
Anchoa tricolor (Actinopterygii: Engraulidae); marine; intestine; metacestode; WTSA; Brazil (
Aphos porosus (Actinopterygii: Batrachoididae); marine; site of infection not given; metacestode; WTSP; Chile (
Aspistor luniscutis (Actinopterygii: Ariidae); marine; site of infection not given; metacestode; WTSA; Brazil (
Note: host reported as Sciadeichthys luniscutis.
Balistes capriscus (Actinopterygii: Balistidae); marine; site of infection not given; metacestode; WTSA; Brazil (
Balistes vetula (Actinopterygii: Balistidae); marine; site of infection not given; metacestode; WTSA; Brazil (
Brachyplatystoma sp. (Actinopterygii: Pimelodidae); freshwater; site of infection not given; metacestode; Amazon River basin (estuary of Amazon River); Brazil (
Caranx latus (Actinopterygii: Carangidae); marine; site of infection not given; metacestode; WTSA; Brazil (
Cilus gilberti (Actinopterygii: Sciaenidae); marine; site of infection not given; metacestode; WTSP; Chile (
Conger orbignianus (Actinopterygii: Congridae); marine; intestine; metacestode; WTSA; Argentina (
Note:
Coryphaena hippurus (Actinopterygii: Coryphaenidae); marine; intestine; metacestode; WTSP; Peru (
Cynoscion guatucupa (Actinopterygii: Sciaenidae); marine; pyloric caeca, intestine; metacestode; WTSA; Argentina (
Dactylopterus volitans (Actinopterygii: Dactylopteridae); marine; mesentery; metacestode; WTSA; Brazil (
Dissostichus eleginoides (Actinopterygii: Nototheniidae); marine; stomach, intestine; metacestode; Magellanic; Falkland Islands (
Eleginops maclovinus (Actinopterygii: Eleginopsidae); marine; intestine; metacestode; Magellanic; Falkland Islands (
Engraulis anchoita (Actinopterygii: Engraulidae); marine; pyloric caeca; metacestode; Magellanic, WTSA; Argentina (
Engraulis ringens (Actinopterygii: Engraulidae); marine; site of infection not
given; metacestode; WTSP; Chile (
Ethmidium maculatum (Actinopterygii: Clupeidae); marine; intestine; metacestode; WTSP; Peru (
Note: host reported as Brevoortia maculata.
Euthynnus alletteratus (Actinopterygii: Scombridae); marine; intestine; metacestode; WTSA; Brazil (
Genidens barbus (Actinopterygii: Ariidae); marine; site of infection not given; metacestode; WTSA; Brazil (
Note:
Genypterus blacodes (Actinopterygii: Ophidiidae); marine; intestine; metacestode; WTSA; Argentina (
Genypterus brasiliensis (Actinopterygii: Ophidiidae); marine; intestine; metacestode; WTSA; Brazil (
Genypterus maculatus (Actinopterygii: Ophidiidae); marine; intestine; metacestode; WTSP; Chile, Peru (
Gobiesox marmoratus (Actinopterygii: Gobiesocidae); marine; site of infection not given; metacestode; WTSP; Chile (
Gymnothorax moringa (Actinopterygii: Muraenidae); marine; site of infection not given; metacestode; WTSA; Brazil (
Haemulon steindachneri (Actinopterygii: Haemulidae); marine; intestine; metacestode; WTSA; Brazil (
Helcogrammoides chilensis (Actinopterygii: Tripterygiidae); marine; site of
infection not given; metacestode; WTSP; Chile (
Hippoglossina macrops (Actinopterygii: Paralichthyidae); marine; intestine; metacestode WTSP; Chile (
Hyporhamphus unifasciatus (Actinopterygii: Hemiramphidae); brackish, marine; body cavity; metacestode; TSA (Itamaracá Island); Brazil (
Katsuwonus pelamis (Actinopterygii: Scombridae); marine; intestine; metacestode; WTSA, WTSP; Brazil, Peru (
Macruronus magellanicus (Actinopterygii: Merlucciidae); marine; intestine; metacestode; Magellanic; Chile (
Menticirrhus americanus (Actinopterygii: Sciaenidae); marine; site of infection not given; metacestode; WTSA; Brazil (
Merluccius australis (Actinopterygii: Merlucciidae); marine; intestine; metacestode; Magellanic; Chile (
Merluccius gayi gayi (Actinopterygii: Merlucciidae); marine; intestine; metacestode; WTSP; Chile (
Merluccius hubbsi (Actinopterygii: Merlucciidae); marine; pyloric caeca, intestine; metacestode; Magellanic, WTSA; Argentina, Uruguay (
Micropogonias furnieri (Actinopterygii: Sciaenidae); marine; intestine; metacestode; WTSA; Brazil (
Mugil cephalus (Actinopterygii: Mugilidae); marine; intestine; metacestode; WTSP; Chile (
Mugil liza (Actinopterygii: Mugilidae); catadromous; intestine; metacestode; WTSA; Brazil (
Notes: host reported as Mugil platanus.
Normanichthys crockeri (Actinopterygii: Normanichthyidae); marine; intestine, ovary; metacestode; Magellanic; Chile (
Notothenia cf. angustata (Actinopterygii: Nototheniidae); marine; intestine; metacestode; WTSP; Chile (
Odontesthes argentinensis (Actinopterygii: Atherinopsidae); brackish, marine; site of infection not given; metacestode; WTSA (Mar Chiquita coastal lagoon); Argentina (
Odontesthes regia (Actinopterygii: Atherinopsidae); brackish, marine; intestine; metacestode; WTSP; Peru (
Note: host reported as O. regia regia.
Odontesthes smitti (Actinopterygii: Atherinopsidae); marine; body cavity, mesentery, stomach; metacestode; Magellanic; Argentina (
Oligoplites palometa (Actinopterygii: Carangidae); marine; intestine; metacestode; WTSA; Brazil (
Oligoplites saliens (Actinopterygii: Carangidae); marine; intestine; metacestode; WTSA; Brazil (
Oligoplites saurus (Actinopterygii: Carangidae); marine; intestine; metacestode; WTSA; Brazil (
Oncorhynchus kisutch (Actinopterygii: Salmonidae); anadromous; intestine; metacestode; lakes in Chiloé Island; Chile (
Orthopristis ruber (Actinopterygii: Haemulidae); marine; intestine; metacestode; WTSA; Brazil (
Pagrus pagrus (Actinopterygii: Sparidae); marine; mesentery; metacestode; WTSA; Brazil (
Paralichthys adspersus (Actinopterygii: Paralichthyidae); marine; intestine; metacestode; WTSP; Chile (
Paralichthys isosceles (Actinopterygii: Paralichthyidae); marine; stomach, intestine; metacestode; WTSA; Argentina, Brazil (
Paralichthys microps (Actinopterygii: Paralichthyidae); marine; intestine; metacestode; WTSP; Chile (
Paralichthys orbignyanus (Actinopterygii: Paralichthyidae); marine; site of
infection not given; metacestode; WTSA (Mar Chiquita coastal lagoon); Argentina (
Paralichthys patagonicus (Actinopterygii: Paralichthyidae); marine; stomach, intestine; metacestode; WTSA; Argentina (
Paralonchurus brasiliensis (Actinopterygii: Sciaenidae); marine; intestine; metacestode; WTSA; Brazil (
Parona signata (Actinopterygii: Carangidae); marine; intestine; metacestode; WTSA; Argentina (
Percophis brasiliensis (Actinopterygii: Percophidae); marine; intestine; metacestode; WTSA; Argentina, Uruguay (
Pinguipes brasilianus (Actinopterygii: Pinguipedidae); marine; intestine; metacestode; WTSA; Argentina, Brazil (
Pomatomus saltatrix (Actinopterygii: Pomatomidae); marine; intestine; metacestode; WTSA; Brazil (
Note: host reported as P. saltator.
Porichthys porosissimus (Actinopterygii: Batrachoididae) marine; intestine; metacestode; WTSA (Bahía Blanca estuary); Argentina (
Notes:
Priacanthus arenatus (Actinopterygii: Priacanthidae); marine; intestine; metacestode; WTSA; Brazil (
Prolatilus jugularis (Actinopterygii: Pinguipedidae); marine; intestine, ovary; metacestode; Magellanic; Chile (
Pseudopercis numida (Actinopterygii: Pinguipedidae); marine; intestine; metacestode; WTSA; Brazil (
Pseudopercis semifasciata (Actinopterygii: Pinguipedidae); marine; intestine; metacestode; Magellanic, WTSA; Argentina, Brazil (
Raneya brasiliensis (Actinopterygii: Ophidiidae); marine; mesentery; metacestode; WTSA; Argentina (
Salmo trutta (Actinopterygii: Salmonidae); anadromous; intestine; metacestode; lakes in Chiloé Island; Chile (
Sarda chiliensis (Actinopterygii: Scombridae); marine; intestine; metacestode; WTSP; Peru (
Note: host reported as S. chiliensis chiliensis.
Sarda sarda (Actinopterygii: Scombridae); marine; intestine; metacestode; WTSA; Brazil (
Sardinella brasiliensis (Actinopterygii: Clupeidae); marine; pyloric caeca (cysts); metacestode; WTSA; Brazil (
Sardinops sagax (Actinopterygii: Clupeidae); marine; intestine; metacestode; WTSP; Peru (
Note: host reported as S. sagax sagax.
Scomber colias (Actinopterygii: Scombridae); marine; body cavity, intestine, stomach; metacestode; WTSA; Argentina, Brazil (
Note: host reported as S. japonicus.
Scomber japonicus (Actinopterygii: Scombridae); marine; intestine; metacestode; WTSP; Peru (
Scomberomorus brasiliensis (Actinopterygii: Scombridae); marine; intestine; metacestode; WTSA; Brazil (
Sebastes capensis (Actinopterygii: Sebastidae); marine; site of infection not
given; metacestode; WTSP; Chile (
Sicyases sanguineus (Actinopterygii: Gobiesocidae); marine; site of infection not given; metacestode; WTSP; Chile (
Stellifer minor (Actinopterygii: Sciaenidae); marine; intestine; metacestode; WTSP; Peru (
Strongylura scapularis (Actinopterygii: Belonidae); marine; intestine; metacestode; WTSP; Peru (
Note: host reported as Belone scapularis.
Synodus scituliceps (Actinopterygii: Synodontidae); marine; intestine; metacestode; WTSP; Peru (
Trachurus lathami (Actinopterygii: Carangidae); marine; intestine; metacestode; WTSA; Argentina, Brazil (
Trachurus murphyi (Actinopterygii: Carangidae); marine; intestine; metacestode; WTSP; Chile, Peru (
Note:
Trichiurus lepturus (Actinopterygii: Trichiuridae); marine; stomach, intestine; metacestode; WTSA; Brazil (
Tylosurus acus acus (Actinopterygii: Belonidae); marine; intestine; metacestode; WTSA; Brazil (
Note: host reported as Tylosurus acus.
Urophycis brasiliensis (Actinopterygii: Phycidae); marine; intestine; metacestode; WTSA; Argentina, Brazil (
Note:
Urophycis mystaceus (Actinopterygii: Phycidae); marine; intestine; metacestode; WTSA; Brazil (
Xystreurys rasile (Actinopterygii: Paralichthyidae); marine; stomach, intestine; metacestode; WTSA; Argentina (
Taxon incertae sedis
Anthobothrium pristis Woodland, 1934
Pristis pristis (Elasmobranchii: Pristidae); brackish, freshwater, marine; spiral valve; adult; Amazon River basin; Brazil (
Note: host reported as P. perotteti.
Order Trypanorhyncha Diesing, 1863
Suborder Trypanoselachoida Olson, Caira, Jensen, Overstreet, Palm & Beveridge, 2010
Superfamily Gymnorhynchoidea Dollfus, 1935
Family Gilquiniidae Dollfus, 1935
Gilquinia squali (Fabricius, 1794) Dollfus, 1930*
[Syns. Taenia squali Fabricius, 1794; Bothriocephalus paleaceus Rudolphi, 1810; Rhynchobothrium tetrabothrium van Beneden, 1849; Tetrarhynchobothrium affine Diesing, 1854; Tetrarhynchus anteroporus Hart, 1936]
Etmopterus granulosus (Elasmobranchii: Etmopteridae); marine; spiral valve; adult (immature); WTSP; Chile (
Note: host reported as Centroscyllium granulosus.
Gilquinia sp.
Micropogonias furnieri (Actinopterygii: Sciaenidae); marine; mesentery; metacestode; WTSA (La Plata River estuary); Argentina (
Note:
Family Gymnorhynchidae Dollfus, 1935
Gymnorhynchus isuri Robinson, 1959
Isurus oxyrinchus (Elasmobranchii: Lamnidae); marine; spiral valve; adult; WTSA; Brazil (
Molicola horridus (Goodsir, 1841) Dollfus, 1935*
[For synonyms, see
Isurus oxyrinchus (Elasmobranchii: Lamnidae); marine; spiral valve; adult; WTSA; Brazil (
Mola ramsayi (Actinopterygii: Molidae); marine; liver; metacestode; WTSP; Chile (
Note: tapeworms reported as Gymnorhynchus (M.) horridus.
Prionace glauca (Elasmobranchii: Carcharhinidae); marine; spiral valve; adult; WTSA; Brazil (
Molicola sp.
Mola mola (Actinopterygii: Molidae); marine; site of infection not given; metacestode; WTSA; Brazil (
Thyrsites atun (Actinopterygii: Gempylidae); marine; muscles; metacestode; WTSP; Chile (
Superfamily Lacistorhynchoidea Guiart, 1927
Family Lacistorhynchidae Guiart, 1927
Callitetrarhynchus gracilis (Rudolphi, 1819) Pintner, 1931*
[For synonyms, see
Balistes capriscus (Actinopterygii: Balistidae); marine; liver, mesentery; metacestode; WTSA; Brazil (
Balistes vetula (Actinopterygii: Balistidae); marine; body cavity, muscle; metacestode; WTSA; Brazil (
Caranx crysos (Actinopterygii: Carangidae); marine; body cavity; metacestode; TSA; Brazil (
Caranx hippos (Actinopterygii: Carangidae); marine; mesentery; metacestode; WTSA; Brazil (
Caranx latus (Actinopterygii: Carangidae); marine; mesentery; metacestode; WTSA; Brazil (
Centropomus undecimalis (Actinopterygii: Centropomidae); amphidromous; peritoneum; metacestode; NBS (Marajó Island); Brazil (
Note: the cestodes were collected by Göldi in 1896.
Chloroscombrus chrysurus (Actinopterygii: Carangidae); marine; body cavity; metacestode; TSA, WTSA; Brazil (
Cynoscion acoupa (Actinopterygii: Sciaenidae); marine; muscles; metacestode; NBS; Brazil (
Cynoscion guatucupa (Actinopterygii: Sciaenidae); marine; body cavity, kidney; metacestode; WTSA; Argentina, Brazil, Uruguay (
Euthynnus alletteratus (Actinopterygii: Scombridae); marine; mesentery; metacestode; WTSA; Brazil (
Genidens barbus (Actinopterygii: Ariidae); marine; body cavity, viscera; metacestode; WTSA; Brazil (
Note: host reported as Netuma barba.
Genypterus brasiliensis (Actinopterygii: Ophidiidae); marine; body cavity, mesentery, muscles; metacestode; WTSA; Brazil (
Haemulon aurolineatum (Actinopterygii: Haemulidae); marine; body cavity, muscles; metacestode; TSA; Brazil (
Harengula clupeola (Actinopterygii: Clupeidae); marine; body cavity; metacestode; TSA; Brazil (
Hemilutjanus macrophthalmos (Actinopterygii: Sciaenidae); marine; surface of
internal organs, serous membrane; metacestode; WTSP; Peru (
Hyporthodus niveatus (Actinopterygii: Serranidae); marine; body cavity; metacestode; WTSA; Brazil (
Note: host reported as Epinephelus niveatus.
Larimus breviceps (Actinopterygii: Sciaenidae); marine; body cavity; metacestode; TSA; Brazil (
Lutjanus synagris (Actinopterygii: Lutjanidae); marine; muscles; metacestode; WTSA; Brazil (
Macrodon ancylodon (Actinopterygii: Sciaenidae); marine; body cavity, kidney, mesentery; metacestode; WTSA; Brazil (
Merluccius gayi peruanus (Actinopterygii: Merlucciidae); marine; mesentery; metacestode; WTSP; Peru (
Micropogonias furnieri (Actinopterygii: Sciaenidae); marine; body cavity, kidney, mesentery; metacestode; TNA, WTSA; Brazil, Venezuela (
Mustelus canis (Elasmobranchii: Triakidae); marine; spiral valve; adult; WTSA; Brazil (
Oligoplites palometa (Actinopterygii: Carangidae); marine; body cavity; metacestode; TSA, WTSA; Brazil (
Oligoplites saurus (Actinopterygii: Carangidae); marine; body cavity; metacestode; WTSA; Brazil (
Opisthonema oglinum (Actinopterygii: Clupeidae); marine; body cavity; metacestode; TSA; Brazil (
Pagrus pagrus (Actinopterygii: Sparidae); marine; body cavity; metacestode; WTSA; Brazil (
Paralichthys isosceles (Actinopterygii: Paralichthyidae); marine; muscles; metacestode; WTSA; Brazil (
Paralichthys patagonicus (Actinopterygii: Paralichthyidae); marine; body cavity, kidney, mesentery, spleen; metacestode; WTSA; Brazil (
Paralonchurus peruanus (Actinopterygii: Sciaenidae); marine; surface of internal organs, serous membrane; metacestode; WTSP; Peru (
Note: host reported as Polyclemus peruanus.
Percophis brasiliensis (Actinopterygii: Percophidae); marine; mesentery; metacestode; WTSA; Argentina, Uruguay (
Pinguipes brasilianus (Actinopterygii: Pinguipedidae); marine; mesentery; metacestode; WTSA; Brazil (
Plagioscion squamosissimus (Actinopterygii: Sciaenidae); freshwater; muscles; metacestode; Amazon River basin; Brazil (
Pomatomus saltatrix (Actinopterygii: Pomatomidae); marine; body cavity, mesentery, peritoneum; metacestode; WTSA; Brazil (
Note: host reported as P. saltator by some authors.
Prionace glauca (Elasmobranchii: Carcharhinidae); marine; spiral valve; metacestode; WTSA; Brazil (
Pseudopercis numida (Actinopterygii: Pinguipedidae); marine; mesentery; metacestode; WTSA; Brazil (
Sardinella brasiliensis (Actinopterygii: Clupeidae); marine; body cavity; metacestode; WTSA; Brazil (
Sciaena deliciosa (Actinopterygii: Sciaenidae); marine; surface of internal organs, serous membrane; metacestode; WTSP; Peru (
Scomberomorus brasiliensis (Actinopterygii: Scombridae); marine; body cavity; metacestode; TSA; Brazil (
Note: host reported as S. maculatus.
Scomberomorus cavalla (Actinopterygii: Scombridae); marine; mesentery; metacestode; WTSA; Brazil (
Selene setapinnis (Actinopterygii: Carangidae); marine; mesentery; metacestode; WTSA; Brazil (
Selene vomer (Actinopterygii: Carangidae); marine; body cavity; metacestode; TSA; Brazil (
Sphyraena guachancho (Actinopterygii: Sphyraenidae); marine; body cavity; metacestode; TSA; Brazil (
Trachurus lathami (Actinopterygii: Carangidae); marine; mesentery; metacestode; WTSA; Argentina, Brazil (
Trichiurus lepturus (Actinopterygii: Trichiuridae); marine; body cavity, mesentery, stomach; WTSA; Brazil (
Umbrina canosai (Actinopterygii: Sciaenidae); marine; site of infection not given; metacestode; WTSA; Brazil (
Urophycis brasiliensis (Actinopterygii: Phycidae); marine; site of infection not given; metacestode; WTSA; Argentina (
Callitetrarhynchus speciosus (Linton, 1897) Carvajal & Rego, 1985
[Syns. Rhynchobothrium speciosum Linton, 1897; Tentacularia pseudodera Schuler, 1938]
Aluterus monoceros (Actinopterygii: Monacanthidae); marine; liver, mesentery; metacestode; WTSA; Brazil (
Balistes capriscus (Actinopterygii: Balistidae); marine; mesentery; metacestode; WTSA; Brazil (
Balistes vetula (Actinopterygii: Balistidae); marine; body cavity, mesentery, muscles; metacestode; WTSA; Brazil (
Cynoscion guatucupa (Actinopterygii: Sciaenidae); marine; mesentery; metacestode; WTSA; Brazil (
Genidens barbus (Actinopterygii: Ariidae); marine; body cavity, viscera; metacestode; WTSA; Brazil (
Note: host reported as Netuma barba.
Micropogonias furnieri (Actinopterygii: Sciaenidae); marine; mesentery; metacestode; WTSA; Brazil (
Pomatomus saltatrix (Actinopterygii: Pomatomidae); marine; body cavity, mesentery; metacestode; WTSA; Brazil (
Note: type host; it was reported as P. saltator by some authors.
Priacanthus arenatus (Actinopterygii: Priacanthidae); marine; mesentery, serosa of intestine and ovary; metacestode; WTSA; Brazil (
Scomberomorus cavalla (Actinopterygii: Scombridae); marine; mesentery; metacestode; WTSA; Brazil (
Sphyrna zygaena (Elasmobranchii: Sphyrnidae); marine; spiral valve; metacestode; WTSA; Brazil (
Stephanolepis hispidus (Actinopterygii: Monacanthidae); marine; mesentery; metacestode; WTSA; Brazil (
Callitetrarhynchus sp.
Balistes capriscus (Actinopterygii: Balistidae); marine; mesentery; metacestode; WTSA; Brazil (
Balistes vetula (Actinopterygii: Balistidae); marine; body cavity, mesentery, muscles; metacestode; WTSA; Brazil (
Conodon nobilis (Actinopterygii: Haemulidae); marine; body cavity; metacestode; WTSA; Brazil (
Dasyrhynchus giganteus (Diesing, 1850) Pintner, 1929
[Syns. Anthocephalus giganteus Diesing, 1850; Rhynchobothrium insigne Linton, 1924; Sbesterium insigne Dollfus, 1929]
Caranx hippos (Actinopterygii: Carangidae); marine; brain; metacestode; NBS, TSA; Brazil (
Oligoplites saliens (Actinopterygii: Carangidae); marine; subcutaneous; metacestode; NBS (estuary of Tapajós River); Brazil (
Notes: type host; it was originally reported as Chorinemus saliens.
Dasyrhynchus pacificus Robinson, 1965
Carcharhinus brachyurus (Elasmobranchii: Carcharhinidae); marine; spiral valve; adult; WTSA; Brazil (
Carcharhinus limbatus (Elasmobranchii: Carcharhinidae); marine; spiral valve; adult; WTSA; Brazil (
Cynoscion guatucupa (Actinopterygii: Sciaenidae); marine; body cavity, haemal
arches, mesentery, kidney; metacestode; WTSA; Argentina, Brazil, Uruguay (
Cynoscion jamaicensis (Actinopterygii: Sciaenidae); marine; mesentery; metacestode; WTSA; Brazil (
Macrodon ancylodon (Actinopterygii: Sciaenidae); marine; body cavity, pericardium, kidney; metacestode; WTSA; Brazil (
Menticirrhus americanus (Actinopterygii: Sciaenidae); marine; body cavity, mesentery, kidney; metacestode; WTSA; Brazil (
Sciaena deliciosa (Actinopterygii: Sciaenidae); marine; peritoneum; metacestode; WTSP; Peru (
Scyliorhinus haeckelii (Elasmobranchii: Scyliorhinidae); marine; spiral valve; adult; WTSA; Brazil (
Sphyrna sp. (Elasmobranchii: Sphyrnidae); marine; spiral valve; adult; WTSA; Brazil (
Floriceps saccatus Cuvier, 1817*
[Syns. Anthocephalus elongatus Rudolphi, 1819; Rhynchobothrium ingens Linton, 1921; R. carangis MacCallum, 1921; Floriceps caballeroi Ceuz-Reyes, 1977]
Aluterus monoceros (Actinopterygii: Monacanthidae); marine; liver, mesentery; metacestode; WTSA; Brazil (
Centropomus nigrescens (Actinopterygii: Centropomidae); amphidromous; peritoneum; metacestode; WTSP; Peru (
Coryphaena hippurus (Actinopterygii: Coryphaenidae); marine; muscles; metacestode; WTSA; Brazil (
Prionace glauca (Elasmobranchii: Carcharhinidae); marine; spiral valve; adult; WTSA; Brazil (
Seriola lalandi (Actinopterygii: Carangidae); marine; muscle; metacestode; WTSP; Chile (
Note: host reported as S. mazatlana.
Grillotia (Christianella) carvajalregorum Menoret & Ivanov, 2009
[Syns. Progrillotia dollfusi Carvajal & Rego 1983; Grillotia (Progrillotia) dollfusi (Carvajal & Rego, 1983) Palm, 2004; G. carvajalregorum Menoret & Ivanov, 2009]
Acanthistius brasilianus (Actinopterygii: Serranidae); marine; mesentery; metacestode; WTSA; Argentina (
Carcharhinus signatus (Elasmobranchii: Carcharhinidae); marine; stomach; adult; WTSA; Brazil (
Conger orbignianus (Actinopterygii: Congridae); marine; intestinal surface, mesentery; metacestode; WTSA; Argentina (
Ctenosciaena gracilicirrhus (Actinopterygii: Sciaenidae); marine; body cavity; metacestode; WTSA; Brazil (
Cynoscion guatucupa (Actinopterygii: Sciaenidae); marine; body cavity, mesentery; metacestode; WTSA; Argentina, Brazil (
Cynoscion jamaicensis (Actinopterygii: Sciaenidae); marine; body cavity; metacestode; WTSA; Brazil (
Cynoscion striatus (Actinopterygii: Sciaenidae); marine; body cavity; metacestode; WTSA; Brazil (
Notes: type host. Carvaval and Rego (1983) re-identified the larvae collected by
Dules auriga (Actinopterygii: Serranidae); marine; mesentery; metacestode; WTSA; Argentina (
Note:
Genypterus brasiliensis (Actinopterygii: Ophidiidae); marine; mesentery; metacestode; WTSA; Brazil (
Note: after morphological re-evalution of specimens deposited by
Heptranchias perlo (Elasmobranchii: Hexanchidae); marine; spiral valve; metacestode; WTSA; Brazil (
Lophius gastrophysus (Actinopterygii: Lophiidae); marine; body cavity; metacestode; WTSA; Brazil (
Macrodon ancylodon (Actinopterygii: Sciaenidae); marine; mesentery, body
cavity; metacestode; WTSA; Brazil (
Menticirrhus americanus (Actinopterygii: Sciaenidae); marine; body cavity; metacestode; WTSA; Brazil (
Menticirrhus littoralis (Actinopterygii: Sciaenidae); marine; body cavity; metacestode; WTSA; Brazil (
Merluccius hubbsi (Actinopterygii: Merlucciidae); marine; mesentery; metacestode; Magellanic; Argentina (
Micropogonias furnieri (Actinopterygii: Sciaenidae); marine; mesentery; metacestode; WTSA; Argentina, Brazil (
Nemadactylus bergi (Actinopterygii: Cheilodactylidae); marine; mesentery; metacestode; WTSA; Argentina (
Paralichthys isosceles (Actinopterygii: Paralichthyidae); marine; body cavity, liver, mesentery, muscles; metacestode; WTSA; Argentina, Brazil (
Paralichthys patagonicus (Actinopterygii: Paralichthyidae); marine; body cavity, mesentery; metacestode; WTSA; Argentina, Brazil (
Paralonchurus brasiliensis (Actinopterygii: Sciaenidae); marine; body cavity; metacestode; WTSA; Brazil (
Parona signata (Actinopterygii: Carangidae); marine; mesentery; metacestode; WTSA; Argentina (
Percophis brasiliensis (Actinopterygii: Percophidae); marine; mesentery; metacestode; WTSA; Argentina (
Pomatomus saltatrix (Actinopterygii: Pomatomidae); marine; mesentery; metacestode; WTSA; Argentina (
Porichthys porosissimus (Actinopterygii: Batrachoididae); marine; mesentery; metacestode; WTSA; Argentina (
Prionotus nudigula (Actinopterygii: Triglidae); marine; mesentery; metacestode; Magellanic; Argentina (
Prionotus punctatus (Actinopterygii: Triglidae); marine; intestinal surface, mesentery; metacestode; WTSA; Argentina, Brazil (Bicudo et al. 2005;
Pseudopercis numida (Actinopterygii: Pinguipedidae); marine; mesentery; metacestode; WTSA; Brazil (
Pseudopercis semifasciata (Actinopterygii: Pinguipedidae); marine; mesentery; metacestode; WTSA; Brazil (
Raneya brasiliensis (Actinopterygii: Ophidiidae); marine; mesentery; metacestode; Magellanic, WTSA; Argentina (
Squalus sp. (Elasmobranchii: Squalidae); marine; spiral valve; adult; WTSA; Brazil (
Squatina guggenheim (Elasmobranchii: Squatinidae); marine; spiral valve; adult; WTSA; Argentina (
Note:
Trachurus lathami (Actinopterygii: Carangidae); marine; mesentery; metacestode; WTSA; Argentina, Brazil (
Umbrina canosai (Actinopterygii: Sciaenidae); marine; body cavity, mesentery; metacestode; WTSA; Argentina, Brazil (
Urophycis brasiliensis (Actinopterygii: Phycidae); marine; mesentery; metacestode; WTSA; Argentina (
Xystreurys rasile (Actinopterygii: Paralichthyidae); marine; body cavity, mesentery; metacestode; WTSA; Argentina, Brazil (
Grillotia (Christianella) minuta (van Beneden, 1849) Guiart, 1931*
[Syns. Tetrarhynchus minutus van Beneden, 1849; Grillotia minuta (van Beneden, 1849); T. smarisgora Wagener, 1854; T. smarismaenae Wagener, 1854; T. smaridum Pintner, 1893; G. angeli Dollfus, 1969; G. bothridiopunctata Dollfus, 1969]
Cynoscion guatucupa (Actinopterygii: Sciaenidae); marine; mesentery; metacestode; WTSA; Argentina, Uruguay (
Notes: tapeworms reported as G. bothridiopunctata. G. (C.) minuta is distributed across the Northeastern Atlantic and morphologically similar to G. (C.) carvajalregorum; therefore, its occurrence in the Southwestern Atlantic is doubtful (
Grillotia (Grillotia) borealis Keeney & Campbell, 2001
Macrourus carinatus (Actinopterygii: Macrouridae); marine; mesentery; metacestode; Magellanic; Argentina (
Note: this species was described from fishes in the North Pacific Ocean and it is morphologically similar to G. patagonica Menoret and Ivanov, 2012; thus, its occurrence in the Southwestern Atlantic is doubtful (
Salilota australis (Actinopterygii: Moridae); marine; mesentery; metacestode; Magellanic; Argentina (
Grillotia (Grillotia) dollfusi Carvajal, 1971
Dipturus flavirostris (Elasmobranchii: Rajidae); marine; spiral valve; adult; WTSP; Chile (
Note: host reported as Raja flavirostris.
Macruronus magellanicus (Actinopterygii: Merlucciidae); marine; body cavity; metacestode; WTSP; Chile (
Merluccius gayi gayi (Actinopterygii: Merlucciidae); marine; body cavity, intestinal serosa, gonads, liver, stomach wall; metacestode; WTSP; Chile (
Merluccius gayi peruanus (Actinopterygii: Merlucciidae); marine; mesentery; metacestode; WTSP; Peru (
Zearaja chilensis (Elasmobrachii: Rajidae); marine; spiral valve; adult; WTSP; Chile (
Notes: type host; it was reported as Raja chilensis.
Grillotia (Grillotia) erinaceus (van Beneden, 1858) Guiart, 1927
[Syns. Tetrarhynchus erinaceus van Beneden, 1858; Rhynchobothrium imparispine Linton, 1897; Grillotia pseuderinaceus Dollfus, 1969; G. recurvispinis Dollfus, 1969]
Conger orbignianus (Actinopterygii: Congridae); marine; mesentery; metacestode; WTSA; Argentina (
Dissostichus eleginoides (Actinopterygii: Nototheniidae); marine; mesentery, stomach, intestine wall; metacestode; Magellanic; Falkland Islands (
Eleginops maclovinus (Actinopterygii: Eleginopsidae); marine; mesentery; metacestode; Magellanic; Falkland Islands (
Porichthys porosissimus (Actinopterygii: Batrachoididae); marine; mesentery; metacestode; WTSA; Argentina (
Note:
Sympterygia bonapartii (Elasmobranchii: Arhynchobatidae); marine; spiral valve; adult; WTSA; Argentina (
Note:
Grillotia (Grillotia) patagonica Menoret & Ivanov, 2012
Cottoperca gobio (Actinopterygii: Bovichtidae); marine; body cavity; metacestode; Magellanic; Argentina (
Nemadactylus bergi (Actinopterygii: Cheilodactylidae); marine; mesentery; metacestode; Magellanic; Argentina (
Patagonotothen brevicauda brevicauda (Actinopterygii: Nototheniidae); marine; mesentery; metacestode; Magellanic; Argentina (
Patagonotothen ramsayi (Actinopterygii: Nototheniidae); marine; mesentery; metacestode; Magellanic; Argentina (
Psammobatis rudis (Elasmobranchii: Arhynchobatidae); marine; spiral valve; adult; Magellanic; Argentina (
Note: type host.
Salilota australis (Actinopterygii: Moridae); marine; mesentery; metacestode; Magellanic; Argentina (
Grillotia heptanchi (Vaullegeard, 1899) Dollfus, 1942 (Grillotia sensu lato)
[For synonyms, see Beveridge and Campbell (2013)]
Genypterus chilensis (Actinopterygii: Ophidiidae); marine; muscles; metacestode; Magellanic; Chile (
Hexanchus griseus (Elasmobranchii: Hexanchidae); marine; spiral valve; adult; WTSP; Chile (
Macruronus magellanicus (Actinopterygii: Merlucciidae); marine; body cavity, viscera; metacestode; Magellanic; Chile (
Merluccius australis (Actinopterygii: Merlucciidae); marine; mesentery, muscles; metacestode; Magellanic; Chile (
Note:
Merluccius gayi gayi (Actinopterygii: Merlucciidae); marine; muscles; metacestode; WTSP; Chile (
Grillotia sp.
Aphos porosus (Actinopterygii: Batrachoididae); marine; body cavity; metacestode; WTSP; Chile (
Bathyraja magellanica (Elasmobranchii: Arhynchobatidae); marine; spiral valve; adult; Magellanic; Argentina (
Note: the specimens are most likely G. (G.) patagonica, but the internal morphology could not be assessed in frozen material, which prevented the precise identification (
Coelorinchus chilensis (Actinopterygii: Macrouridae); marine; stomach; metacestode; JFD; Chile (Pardo-Gandarilhas et al. 2008).
Eleginops maclovinus (Actinopterygii: Eleginopsidae); marine; site of infection not given; metacestode; WTSP; Chile (
Lutjanus analis (Actinopterygii: Lutjanidae); marine; body cavity; metacestode; TSA; Brazil (
Note:
Merluccius australis (Actinopterygii: Merlucciidae); marine; body cavity, mesentery; metacestode; Magellanic; Falkland Islands (
Merluccius hubbsi (Actinopterygii: Merlucciidae); marine; body cavity; metacestode; Magellanic, WTSA; Argentina, Falkland Islands, Uruguay (
Micromesistius australis australis (Actinopterygii: Gadidae); marine; site of infection not given; metacestode; Magellanic; Argentina, Chile, Falkland Islands (
Paralabrax humeralis (Actinopterygii: Serranidae); marine; stomach; metacestode; WTSP; Peru (
Paralichthys orbignyanus (Actinopterygii: Paralichthyidae); marine; site of infection not given; metacestode; WTSA (Mar Chiquita coastal lagoon); Argentina (
Percophis brasiliensis (Actinopterygii: Percophidae); marine; mesentery; metacestode; WTSA; Argentina, Brazil, Uruguay (
Pinguipes brasilianus (Actinopterygii: Pinguipedidae); marine; mesentery; metacestode; Magellanic, WTSA; Argentina, Brazil (
Prionotus nudigula (Actinopterygii: Triglidae); marine; mesentery; metacestode; WTSA; Argentina (
Pseudopercis semifasciata (Actinopterygii: Pinguipedidae); marine; mesentery; metacestode; Magellanic, WTSA; Argentina (
Squatina armata (Elasmobranchii: Squatinidae); marine; spiral valve; adult; WTSP; Peru (
Lacistorhynchus dollfusi Beveridge & Sakanari, 1987
[Syn. Lacistorhynchus tenuis (van Beneden, 1858) (pro parte)]
Cheilotrema fasciatum (Actinopterygii: Sciaenidae); marine; body cavity; metacestode; WTSP; Peru (
Note: host reported as Sciaena fasciata.
Paralichthys adspersus (Actinopterygii: Paralichthyidae); marine; body cavity, muscles; metacestode; WTSP; Chile (
Lacistorhynchus tenuis (van Beneden, 1858) Pintner, 1913*
[Syns. Tetrarhynchus tenuis van Beneden, 1858; Rhynchobothrium heterospine Linton, 1897; Rhynchobothrium bulbifer Linton, 1897]
Anisotremus scapularis (Actinopterygii: Haemulidae); marine; mesentery, muscles; metacestode; WTSP; Peru (
Note:
Cheilodactylus variegatus (Actinopterygii: Cheilodactylidae); marine; mesentery, muscles; metacestode; WTSP; Peru (
Cheilotrema fasciatum (Actinopterygii: Sciaenidae); marine; body cavity; metacestode; WTSP; Peru (
Note: host reported as Sciaena fasciata.
Cilus gilberti (Actinopterygii: Sciaenidae); marine; site of infection not given; metacestode; WTSP; Chile (
Labrisomus philippii (Actinopterygii: Labrisomidae); marine; mesentery, muscles; metacestode; WTSP; Peru (
Merluccius gayi peruanus (Actinopterygii: Merlucciidae); marine; mesentery; metacestode; WTSP; Peru (
Mugil cephalus (Actinopterygii: Mugilidae); marine; mesentery, muscles; metacestode; WTSP; Peru (
Odontesthes regia (Actinopterygii: Atherinopsidae); marine; muscles; metacestode; WTSP; Peru (
Paralichthys patagonicus (Actinopterygii: Paralichthyidae); marine; body cavity; metacestode; WTSA; Argentina (
Triakis maculata (Elasmobranchii: Triakidae); marine; spiral valve; adult; WTSP; Chile (
Lacistorhynchus sp.
Merluccius australis (Actinopterygii: Merlucciidae); marine; muscles; metacestode; Magellanic; Chile, Falkland Island (
Scartichthys viridis (Actinopterygii: Bleniidae); marine; site of infection not
given; metacestode; WTSP; Chile (
Urophycis brasiliensis (Actinopterygii: Phycidae); marine; surface of pyloric
caeca; metacestode; WTSA; Argentina (
Urophycis mystaceus (Actinopterygii: Phycidae); marine; mesentery; metacestode; WTSA; Brazil (
Paragrillotia sp.
Dipturus trachyderma (Elasmobranchii: Rajidae); marine; site of infection and
stage of development not given; WTSP; Chile (
Notes: host reported as Raja trachyderma. Neither were the three known species of Paragrillotia Dollfus, 1969 described from rays nor reported from Southeastern Pacific (see
Pseudogrillotia peruviana Escalante & Carvajal, 1984
Scomberomorus sierra (Actinopterygii: Scombridae); marine; mesentery; metacestode; WTSP; Peru (
Note: type host; it was reported as S. maculatus.
Pseudogrillotia sp.
Pomatomus saltatrix (Actinopterygii: Pomatomidae); marine; mesentery; metacestode; WTSA; Brazil (
Pseudolacistorhynchus noodti Palm, 1995*
[Syn. Rhynchobothrium sp. of Linton 1909, 1924]
Pseudupeneus maculatus (Actinopterygii: Mullidae); marine; body cavity; metacestode; TSA; Brazil (
Note: type host.
Scomberomorus brasiliensis (Actinopterygii: Scombridae); marine; body cavity; metacestode; TSA; Brazil (
Note: host reported as S. maculatus.
Family Pterobothriidae Pintner, 1931
Pterobothrium acanthotruncatum Escalante & Carvajal, 1984
[Syns. Synbothrium hemuloni MacCallum, 1921; Gymnorhynchus gigas (Cuvier, 1817) sensu Southwell (1929); Pterobothrium heteracanthum Diesing, 1850 sensu Palm (1995)]
Coryphaena hippurus (Actinopterygii: Coryphaenidae); marine; gallbladder, mesentery; metacestode; WTSP; Peru (
Note: type host.
Micropogonias furnieri (Actinopterygii: Sciaenidae); marine; body cavity; metacestode; WTSA; Brazil (
Paralonchurus peruanus (Actinopterygii: Sciaenidae); marine; gallbladder, gonads, mesentery, peritoneum; metacestode; WTSP; Peru (
Pterobothrium crassicole Diesing, 1850
[Syns. Synbothrium felis of MacCallum – USNMHC 35978; Synbothrium sp. of MacCallum – USNMHC 35744]
Aspistor luniscutis (Actinopterygii: Ariidae); marine; body cavity; metacestode; WTSA; Brazil (
Bagre marinus (Actinopterygii: Ariidae); marine; body cavity; metacestode; NBS (estuary of Amazon River); Brazil (
Note: host reported as Bagrus marinus.
Brachyplatystoma rousseauxii (Actinopterygii: Pimelodidae); freshwater; body cavity; metacestode; Amazon River basin (estuary); Brazil (
Note: host reported as B. flavicans.
Brachyplatystoma vaillantii (Actinopterygii: Pimelodidae); freshwater; body cavity; metacestode; Amazon River basin (estuary); Brazil (
Citharichthys spilopterus (Actinopterygii: Paralichthyidae); marine; muscles; metacestode; TSA; Brazil (
Cynoscion acoupa (Actinopterygii: Sciaenidae); marine; muscles; metacestode; NBS; Brazil (
Cynoscion leiachus (Actinopterygii: Sciaenidae); marine; body cavity; metacestode; WTSA; Brazil (
Note:
Cynoscion sp. (Actinopterygii: Sciaenidae); marine; site of infection not given; metacestode; WTSA; Brazil (
Genidens barbus (Actinopterygii: Ariidae); marine; muscles; metacestode; WTSA; Brazil (
Note: host reported as Netuma barba.
Gobioides broussonnetii (Actinopterygii: Gobiidae); brackish; mesentery; metacestode; Amazon River basin (estuary, Marajó Island); Brazil (
Micropogonias furnieri (Actinopterygii: Sciaenidae); marine; body cavity, liver, mesentery, peritoneum; metacestode; WTSA; Brazil (
Micropogonias undulatus (Actinopterygii: Sciaenidae); marine; mesentery; metacestode; WTSA; Brazil (
Note: The host is assigned to Tachysurus sp. in the
Oligoplites palometa (Actinopterygii: Carangidae); marine; body cavity; metacestode; WTSA; Brazil (
Note:
Paralichthys isosceles (Actinopterygii: Paralichthyidae); marine; stomach serosa; metacestode; WTSA; Brazil (
Paralichthys patagonicus (Actinopterygii: Paralichthyidae); marine; body cavity, kidney, liver, mesentery, muscles, stomach serosa; metacestode; WTSA; Brazil (
Pimelodus sp. (Actinopterygii: Pimelodidae); freshwater; mesentery; metacestode; Amazon River basin; Brazil (
Notes: type host.
Plagioscion squamosissimus (Actinopterygii: Sciaenidae); freshwater; muscles; metacestode; Amazon River Basin (estuary); Brazil (
Pogonias cromis (Actinopterygii: Sciaenidae); marine; body cavity; metacestode; WTSA; Brazil (
Pomatomus saltatrix (Actinopterygii: Pomatomidae); marine; mesentery; metacestode; WTSA; Brazil (
Scomberomorus cavalla (Actinopterygii: Scombridae); marine; mesentery; metacestode; WTSA; Brazil (
Scorpaena sp. (Actinopterygii: Scorpaenidae); marine; mesentery; metacestode; WTSA; Brazil (
Pterobothrium heteracanthum Diesing, 1850
[Syns. Syndesmobothrium filicolle Linton, 1890; Synbothrium fillicolle Linton, 1897; S. hemuloni MacCallum, 1921; Gymnorhynchus cymbiumi Chincholikar & Shinde, 1977; Neogymnorhynchus platycephali Bilqees & Shah, 1982]
Cynoscion acoupa (Actinopterygii: Sciaenidae); marine; muscles; metacestode; NBS; Brazil (
Cynoscion guatucupa (Actinopterygii: Sciaenidae); marine; body cavity; metacestode; WTSA; Brazil (
Micropogonias furnieri (Actinopterygii: Sciaenidae); marine; body cavity, mesentery, peritoneum; metacestode; TSA, WTSA; Argentina, Brazil (
Note:
Micropogonias undulatus (Actinopterygii: Sciaenidae); marine; gallbladder surface, intestine, mesentery; metacestode; NBS (Amazon River estuary), WTSA; Brazil, Uruguay (
Notes: type host; it was reported as M. lineatus.
Paralichthys isosceles (Actinopterygii: Paralichthyidae); marine; muscles; metacestode; WTSA; Brazil (
Plagioscion squamosissimus (Actinopterygii: Sciaenidae); freshwater; muscles; metacestode; Amazon River basin (estuary); Brazil (
Pogonias cromis (Actinopterygii: Sciaenidae); marine; body cavity; metacestode; WTSA; Brazil (
Pomadasys crocro (Actinopterygii: Haemulidae); marine; muscles; metacestode; NBS (Amazon River estuary); Brazil (
Note: host reported as Pristipoma coro.
Umbrina canosai (Actinopterygii: Sciaenidae); marine; body cavity; metacestode; WTSA; Brazil (
Clupeidae gen. sp. (Actinopterygii); marine; mesentery; metacestode; WTSA; Brazil (
Pterobothrium kingstoni Campbell & Beveridge, 1996
[Syn. Synbothrium lintoni MacCallum, 1921 (pro parte)]
Citharichthys spilopterus (Actinopterygii: Paralichthyidae); marine; body cavity, muscles; metacestode; TSA; Brazil (
Haemulon aurolineatum (Actinopterygii: Haemulidae); marine; muscles; metacestode; TSA; Brazil (
Note: the worms were collected by Palm in Brazil’s Northeastern coast.
Pterobothrium sp.
Conodon nobilis (Actinopterygii: Haemulidae); marine; body cavity; metacestode; WTSA; Brazil (
Cynoscion acoupa (Actinopterygii: Sciaenidae); marine; body cavity; metacestode; WTSA; Brazil (
Cynoscion striatus (Actinopterygii: Sciaenidae); marine; muscles; metacestode; WTSA; Brazil (
Note: metacestode described as Tetrarhynchus fragilis (nomen nudum) and identified as Pterobotrium sp. by
Epinephelus sp. (Actinopterygii: Serranidae); marine; body cavity; metacestode; TNA; Venezuela (
Gymnura sp. (Elasmobranchii: Gymnuridae); marine; spiral valve; adult; WTSA; Brazil (
Macrodon ancylodon (Actinopterygii: Sciaenidae); marine; muscles; metacestode; WTSA; Brazil (
Note:
Menticirrhus americanus (Actinopterygii: Sciaenidae); marine; body cavity; metacestode; WTSA; Brazil (
Note:
Micropogonias furnieri (Actinopterygii: Sciaenidae); marine; body cavity, muscles; metacestode; WTSA; Brazil (
Note:
Mycteroperca bonaci (Actinopterygii: Serranidae); marine; body cavity; metacestode; WTSA; Brazil (
Pagrus pagrus (Actinopterygii: Sparidae); marine; body cavity; metacestode; WTSA; Brazil (
Paralichthys isosceles (Actinopterygii: Paralichthyidae); marine; site of infection not given; metacestode; WTSA; Brazil (
Paralichthys sp. (Actinopterygii: Paralichthyidae); marine; muscles; metacestode; WTSA; Brazil (
Notes:
Pomatomus saltatrix (Actinopterygii: Pomatomidae); marine; intestine; metacestode; WTSA; Brazil (
Note: host reported as P. saltator.
Prionotus sp. (Actinopterygii: Triglidae); marine; body cavity; metacestode; WTSA; Brazil (
Umbrina canosai (Actinopterygii: Sciaenidae); marine; muscles; metacestode; WTSA; Brazil (
Notes:
Siluriform fish (Actinopterygii); marine; body cavity; metacestode; WTSA; Brazil (
Unidentified ray (Elasmobranchii); marine; spiral valve; adult; WTSA; Brazil (
Note:
Unidentified pterobothriids
Bagre bagre (Actinopterygii: Ariidae); marine; site of infection not given; metacestode; NBS; Brazil (
Macrodon ancylodon (Actinopterygii: Sciaenidae); marine; site of infection not given; metacestode; NBS; Brazil (
Superfamily Otobothrioidea Dollfus, 1942
Family Otobothriidae Dollfus, 1942
Otobothrium sp.
Balistes vetula (Actinopterygii: Balistidae); marine; body cavity, muscles; metacestode; WTSA; Brazil (
Paralichthys isosceles (Actinopterygii: Paralichthyidae); marine; body cavity, liver, mesentery, intestine, stomach; metacestode; WTSA; Brazil (
Poecilancistrium caryophyllum (Diesing, 1850) Dollfus, 1929*
[For synonyms, see
Carcharhinus leucas (Elasmobranchii: Carcharhinidae); marine; spiral valve; adult; NBS (estuarine waters); Brazil (
Note: host reported as Prionodon leucas.
Cilus gilberti (Actinopterygii: Sciaenidae); marine; peritoneum; metacestode; WTSP; Peru (
Note: host reported as Sciaena gilberti.
Cynoscion acoupa (Actinopterygii: Sciaenidae); marine; muscles; metacestode; NBS; Brazil (
Macrodon ancylodon (Actinopterygii: Sciaenidae); marine; muscles; metacestode; NBS; Brazil (
Micropogonias altipinnis (Actinopterygii: Sciaenidae); marine; muscles; metacestode; TEP; Ecuador (
Micropogonias furnieri (Actinopterygii: Sciaenidae); marine; body cavity, muscles; metacestode; TNA, WTSA; Brazil, Venezuela (
Plagioscion squamosissimus (Actinopterygii: Sciaenidae); freshwater; muscles; metacestode; Amazon River basin (estuary of Amazon River); Brazil (
Rhizoprionodon lalandii (Elasmobranchii: Carcharhinidae); marine; spiral valve; adult; NBS (estuarine waters); Brazil (
Note: type host; it was reported as Scoliodon lalandii.
Family Pseudotobothriidae Palm, 1995
Pseudotobothrium dipsacum (Linton, 1897)
[Syn. Otobothrium dipsacum Linton, 1897]
Haemulon plumierii (Actinopterygii: Haemulidae); marine; body cavity; metacestode; TSA; Brazil (
Hyporthodus niveatus (Actinopterygii: Serranidae); marine; body cavity; metacestode; WTSA; Brazil (
Note: host reported as Epinephelus niveatus.
Pseudupeneus maculatus (Actinopterygii: Mullidae); marine; body cavity; metacestode; TSA; Brazil (
Suborder Trypanobatoida Olson, Caira, Jensen, Overstreet, Palm & Beveridge, 2010
Superfamily Eutetrarhynchoidea Guiart, 1927
Family Eutetrarhynchidae Guiart, 1927
Dollfusiella acuta Menoret & Ivanov, 2015
Atlantoraja castelnaui (Elasmobranchii: Arhynchobatidae); marine; spiral valve; adult; WTSA; Argentina (
Atlantoraja platana (Elasmobranchii: Arhynchobatidae); marine; spiral valve; adult; Magellanic; Argentina (
Sympterygia acuta (Elasmobranchii: Arhynchobatidae); marine; spiral valve; adult; Magellanic, WTSA; Argentina (
Note: type host.
Sympterygia bonapartii (Elasmobranchii: Arhynchobatidae); marine; spiral valve; adult; WTSA; Argentina (
Dollfusiella musteli (Carvajal, 1974) Beveridge, Neifar & Euzet, 2004
[Syns. Prochristianella musteli Carvajal, 1974; Eutetrarhynchus musteli (Carvajal, 1974) Beveridge, 1990]
Mustelus mento (Elasmobranchii: Triakidae); marine; spiral valve; adult; WTSP; Chile (
Note: type host.
Dollfusiella taminii Menoret & Ivanov, 2014
Psammobatis bergi (Elasmobranchii: Arhynchobatidae); marine; spiral valve; adult; WTSA; Argentina (
Note: type host.
Dollfusiella vooremi (São Clemente & Gomes, 1989) Beveridge, Neifar & Euzet, 2004
[Syn. Eutetrarhynchus vooremi São Clemente & Gomes, 1989]
Mustelus canis (Elasmobranchii: Triakidae); marine; spiral valve; adult; WTSA; Brazil (
Note: type host.
Mustelus schmitti (Elasmobranchii: Triakidae); marine; spiral valve; adult; Magellanic, WTSA; Argentina, Brazil (
Note: tapeworms reported as E. vooremi by
Sympterygia bonapartii (Elasmobranchii: Arhynchobatidae); marine; spiral valve; adult; WTSA; Argentina (
Notes:
Dollfusiella sp.
Micropogonias furnieri (Actinopterygii: Sciaenidae); marine; body cavity; metacestode; WTSA, WTSP; Brazil, Chile (
Note:
Mecistobothrium oblongum Menoret & Ivanov, 2015
Myliobatis goodei (Elasmobranchii: Myliobatidae); marine; spiral valve; adult; Magellanic; Argentina (
Note: type host.
Parachristianella damiani Menoret & Ivanov, 2014
Myliobatis goodei (Elasmobranchii: Myliobatidae); marine; spiral valve; adult; WTSA; Argentina (
Note: type host.
Parachristianella monomegacantha Kruse, 1959
Himantura schmardae (Elasmobranchii: Dasyatidae); marine; spiral valve; adult; TNA; Venezuela (
Note: tapeworms reported as P. cf. monomegacantha.
Rhinobatos planiceps (Elasmobranchii: Rhinobatidae); marine; spiral valve; adult; WTSP; Chile (
Paroncomegas araya (Woodland, 1934) Campbell, Marques & Ivanov, 1999*
[Syns. Tentacularia araya Woodland, 1934; Eutetrarhynchus araya (Woodland, 1934) Rego & Dias, 1976]
Potamotrygon falkneri (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Paraná River basin; Paraguay (
Potamotrygon cf. falkneri (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin; Peru (
Note: host reported as Potamotrygon cf. castexi.
Potamotrygon motoro (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon and Paraná River basins; Argentina, Brazil (
Notes:
Potamotrygon orbignyi (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Orinoco River basin; Venezuela (
Note: host reported as P. reticulatus.
Potamotrygon sp. (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Amazon River basin; Brazil (
Note: type host; it was described as Trygon sp.
Paroncomegas sp.
[Syn. Eutetrarhynchus baeri Lopez-Neyra & Diaz-Ungria, 1958]
Potamotrygon orbignyi (Elasmobranchii: Potamotrygonidae); freshwater; spiral valve; adult; Orinoco River basin; Venezuela (Lopez-Neyra and Diaz-Ungria, 1958).
Note: host reported as P. hystrix.
Prochristianella heteracantha Dailey & Carvajal, 1976
Rhinobatos planiceps (Elasmobranchii: Rhinobatidae); marine; spiral valve; adult; WTSP; Chile, Peru (
Note: type host.
Family Rhinoptericolidae Carvajal & Campbell, 1975
Rhinoptericola megacantha Carvajal & Campbell, 1975*
Rhinoptera bonasus (Elasmobranchii: Myliobatidae); marine; spiral valve; adult; TNA; Venezuela (
Note: type host.
Rhinoptera brasiliensis (Elasmobranchii: Myliobatidae); marine; spiral valve; adult; WTSA; Brazil (
Superfamily Tentacularioidea Poche, 1926
Family Sphyriocephalidae Pintner, 1913
Hepatoxylon megacephalum (Rudolphi, 1819) Dollfus, 1942
[Syns. Tetrarhynchus megacephalus Rudolphi, 1819; Tetrarhynchus scyllium canicula Wagener, 1854]
Trichomycterus punctulatus (Actinopterygii: Trichomycteridae); freshwater; site
of infection not given; metacestode; Lima; Peru (
Hepatoxylon trichiuri (Holten, 1802) Bosc, 1811*
[For synonyms, see
Brama australis (Actinopterygii: Bramidae); marine; gonads, mesentery, muscles; metacestode; WTSP; Chile (
Note: host reported as Lepidotus australis by
Brama japonica (Actinopterygii: Bramidae); marine; body cavity; metacestode; WTSP; Peru (
Coelorinchus chilensis (Actinopterygii: Macrouridae); marine; intestine; metacestode; JFD; Chile (Pardo-Gandarilhas et al. 2008).
Coryphaena hippurus (Actinopterygii: Coryphaenidae); marine; liver, intestinal
serosa, stomach wall; metacestode; WTSA; Brazil (
Dissostichus eleginoides (Actinopterygii: Nototheniidae); marine; body cavity, mesentery, stomach wall; metacestode; Magellanic, WTSP; Chile, Falkland Islands (
Genypterus blacodes (Actinopterygii: Ophidiidae); marine; stomach; metacestode; WTSP; Chile (
Genypterus brasiliensis (Actinopterygii: Ophidiidae); marine; mesentery, muscles; metacestode; WTSA; Brazil (
Genypterus chilensis (Actinopterygii: Ophidiidae); marine; mesentery; metacestode; WTSP; Chile (
Genypterus maculatus (Actinopterygii: Ophidiidae); marine; gonads, mesentery, muscles; metacestode; WTSP; Chile (
Isacia conceptionis (Actinopterygii: Haemulidae); marine; intestinal surface; metacestode; WTSP; Chile (
Lampris guttatus (Actinopterygii: Lampridae); marine; gonads, mesentery, muscles; metacestode; WTSP; Chile (
Note: host reported as Lampris regia.
Macruronus magellanicus (Actinopterygii: Merlucciidae); marine; body cavity, gonads, mesentery, muscles; Magellanic; Chile, Falkland Islands (
Merluccius australis (Actinopterygii: Merlucciidae); marine; body cavity, muscles; metacestode; Magellanic, WTSP; Argentina, Chile, Falkland Islands (
Merluccius gayi gayi (Actinopterygii: Merlucciidae); marine; body cavity, mesentery; metacestode; Magellanic, WTSP; Chile (
Note: host reported as Merluccius gayi by some authors.
Merluccius hubbsi (Actinopterygii: Merlucciidae); marine; body cavity, mesentery; metacestode; Magellanic, WTSA; Argentina, Falkland Islands, Uruguay (
Micromesistius australis australis (Actinopterygii: Gadidae); marine; site of
infection not given; metacestode; Magellanic; Chile, Falkland Islands (
Notacanthus sexspinis (Actinopterygii: Notacanthidae); marine; intestine; metacestode; JFD; Chile (Pardo-Gandarilhas et al. 2008).
Oncorhynchus tshawytscha (Actinopterygii: Salmonidae); anadromous; body cavity; metacestode; Magellanic (Curaco de Vélez); Chile (
Prionace glauca (Elasmobranchii: Carcharhinidae); marine; body cavity, stomach serosa, liver; metacestode; JFD, WTSA, WTSP; Brazil, Chile, Peru (
Pseudopercis semifasciata (Actinopterygii: Pinguipedidae); marine; mesentery; metacestode; Magellanic; Argentina (
Salilota australis (Actinopterygii: Moridae); marine; body cavity; metacestode; Magellanic; Argentina (
Scomber japonicus (Actinopterygii: Scombridae); marine; site of infection not given; metacestode; WTSP; Chile (
Sebastes capensis (Actinopterygii: Sebastidae); marine; site of infection not given; metacestode; WTSP; Chile (
Somniosus pacificus (Elasmobranchii: Somniosidae); marine; intestinal surface; metacestode; WTSP; Chile (
Trachurus murphyi (Actinopterygii: Carangidae); marine; site of infection not given; metacestode; WTSP; Chile (
Hepatoxylon sp.
Helicolenus lengerichi (Actinopterygii: Sebastidae); marine; mesentery; metacestode; WTSP; Chile (
Macrourus holotrachys (Actinopterygii: Macrouridae); marine; visceral cavity; metacestode; WTSP: Chile (
Micromesistius australis australis (Actinopterygii: Gadidae); marine; site of infection not given; metacestode; Magellanic; Argentina, Chile (
Trachurus murphyi (Actinopterygii: Carangidae); marine; site of infection not given; metacestode; WTSP; Chile (
Heterosphyriocephalus tergestinus (Pintner, 1913) Dallarés, Carrassón & Schaeffner, 2016
[Syn. Sphyriocephalus tergestinus Pintner, 1913]
Sarda chilensis (Actinopterygii: Scombridae); marine; stomach; metacestode; WTSP; Peru (
Family Tentaculariidae Poche, 1926
Heteronybelinia annakohnae Pereira & Boeger, 2005
Ctenosciaena gracilicirrhus (Actinopterygii: Sciaenidae); marine; body cavity; metacestode; WTSA; Brazil (
Notes: type host.
Cynoscion guatucupa (Actinopterygii: Sciaenidae); marine; body cavity; metacestode; WTSA; Brazil (
Cynoscion jamaicensis (Actinopterygii: Sciaenidae); marine; body cavity; metacestode; WTSA; Brazil (
Menticirrhus americanus (Actinopterygii: Sciaenidae); marine; body cavity; metacestode; WTSA; Brazil (
Heteronybelinia estigmena (Dollfus, 1960) Palm, 1999*
[For synonyms, see
Cynoscion jamaicensis (Actinopterygii: Sciaenidae); marine; body cavity; metacestode; WTSA; Brazil (
Haemulon plumierii (Actinopterygii: Haemulidae); marine; body cavity; metacestode; TSA; Brazil (
Note: tapeworms reported as Nybelinia senegalensis Dollfus, 1960.
Sphyraena guachancho (Actinopterygii: Sphyraenidae); marine; body cavity; metacestode; TSA; Brazil (
Heteronybelinia mattisi Menoret & Ivanov, 2013
Nemadactylus bergi (Actinopterygii: Cheilodactylidae); marine; mesentery; metacestode; WTSA; Argentina (
Raneya brasiliensis (Actinopterygii: Ophidiidae); marine; mesentery; metacestode; WTSA; Argentina (
Sympterygia bonapartii (Elasmobranchii: Arhynchobatidae); marine; spiral valve; adult; WTSA; Argentina (
Notes: type host.
Heteronybelinia nipponica (Yamaguti, 1952) Palm, 1999
[Syns. Nybelinia nipponica Yamaguti, 1952; N. rougetcampanae Dollfus, 1960; H. rougetcampanae (Dollfus, 1960) Palm, 1999]
Carcharhinus signatus (Elasmobranchii: Carcharhinidae); marine; spiral valve; metacestode; WTSA; Brazil (
Genypterus brasiliensis (Actinopterygii: Ophidiidae); marine; body cavity; metacestode; WTSA; Brazil (
Menticirrhus americanus (Actinopterygii: Sciaenidae); marine; body cavity; metacestode; WTSA; Brazil (
Mullus argentinae (Actinopterygii: Mullidae); marine; body cavity; metacestode; WTSA; Brazil (
Paralichthys isosceles (Actinopterygii: Paralichthyidae); marine; body cavity, intestine, kidney, muscles; metacestode; WTSA; Brazil (
Paralichthys patagonicus (Actinopterygii: Paralichthyidae); marine; stomach; metacestode; WTSA; Brazil (
Sphyrna lewini (Elasmobranchii: Sphyrnidae); marine; spiral valve; adult; WTSA; Brazil (
Note: tapeworms reported as N. (Syngenes) rougetcampanae.
Sphyrna zygaena (Elasmobranchii: Sphyrnidae); marine; spiral valve; metacestode; WTSA; Brazil (
Umbrina canosai (Actinopterygii: Sciaenidae); marine; body cavity; metacestode; WTSA; Brazil (
Xystreurys rasile (Actinopterygii: Paralichthyidae); marine; mesentery, stomach; metacestode; WTSA; Brazil (
Heteronybelinia overstreeti Palm, 2004
Pseudupeneus maculatus (Actinopterygii: Mullidae); marine; body cavity; metacestode; TSA; Brazil (
Note:
Heteronybelinia perideraeus (Shipley & Hornell, 1906) Palm, 1999
[Syns. Tetrarhynchus perideraeus Shipley & Hornell, 1906; Stenobothrium perideraeum (Shipley & Hornell, 1906) Pintner, 1913; Nybelinia dakari Dollfus, 1960]
Notorynchus cepedianus (Elasmobranchii: Hexanchidae); marine; spiral valve; adult; WTSA; Brazil (
Note: host reported as N. pectorosus.
Heteronybelinia yamagutii (Dollfus, 1960) Palm, 1999
[Syn. Nybelinia yamagutii Dollfus, 1960]
Carcharhinus signatus (Elasmobranchii: Carcharhinidae); marine; spiral valve; metacestode; WTSA; Brazil (
Heteronybelinia sp.
Urophycis brasiliensis (Actinopterygii: Phycidae); marine; site of infection not
given; metacestode; WTSA; Brazil (
Mixonybelinia beveridgei (Palm, Walter, Schwerdtfeger & Reimer, 1997) Palm, 1999*
[Syn. Nybelinia beveridgei Palm, Walter, Schwerdtfeger & Reimer, 1997]
Dipturus trachyderma (Elasmobranchii: Rajidae); marine; stomach; metacestode; WTSA; Brazil (
Note: host reported as D. trachydermus.
Genypterus brasiliensis (Actinopterygii: Ophidiidae); marine; liver, mesentery, serosa of stomach; metacestode; WTSA; Brazil (
Mixonybelinia edwinlintoni (Dollfus, 1960) Palm & Walter, 2000
[Syn. Nybelinia edwinlintoni Dollfus, 1960]
Pseudupeneus maculatus (Actinopterygii: Mullidae); marine; body cavity; metacestode; TSA; Brazil (
Mixonybelinia sp.
Lophius gastrophysus (Actinopterygii: Lophiidae); marine; muscles; metacestode; WTSA; Brazil (
Nybelinia africana Dollfus, 1960
Pseudupeneus maculatus (Actinopterygii: Mullidae); marine; body cavity; metacestode; TSA; Brazil (
Nybelinia bisulcata (Linton, 1889) Dollfus, 1929
[Syns. Rhynchobothrium bisulcatum Linton, 1889; Tetrarhynchus bisulcatus (Linton, 1889) Linton, 1890]
Umbrina canosai (Actinopterygii: Sciaenidae); marine; body cavity; metacestode; WTSA; Brazil (
Note: the taxonomic status of this species is problematic since the type material is a mixture of different species under the same name (
Nybelinia erythraea Dollfus, 1960
Paralichthys patagonicus (Actinopterygii: Paralichthyidae); marine; stomach; metacestode; WTSA; Brazil (
Xystreurys rasile (Actinopterygii: Paralichthyidae); marine; stomach; metacestode; WTSA; Brazil (
Nybelinia fayapaulazariahi Reimer, 1980
Rhizoprionodon terraenovae (Elasmobranchii: Carcharhinidae); marine; spiral valve; adult; WTSA; Brazil (
Nybelinia indica Chandra, 1986
Pseudupeneus maculatus (Actinopterygii: Mullidae); marine; body cavity; metacestode; TSA; Brazil (
Nybelinia lingualis (Cuvier, 1817) Dollfus, 1929*
[For synonyms, see
Cynoscion sp. (Actinopterygii: Sciaenidae); marine; mesentery; metacestode; WTSA; Brazil (Ortubay 1944).
Haemulon plumierii (Actinopterygii: Haemulidae); marine; body cavity; metacestode; TSA; Brazil (
Note: tapeworms reported as N. cf. lingualis.
Isurus oxyrinchus (Elasmobranchii: Lamnidae); marine; spiral valve; adult; WTSA; Brazil (
Mustelus canis (Elasmobranchii: Carcharhinidae); marine; spiral valve; metacestode; WTSA; Brazil (
Mustelus schmitti (Elasmobranchii: Carcharhinidae); marine; spiral valve; metacestode; WTSA; Brazil (
Note: tapeworms reported as N. (N.) lingualis.
Oncopterus darwinii (Actinopterygii: Pleuronectidae); marine; intestinal surface; metacestode; WTSA; Argentina (
Paralichthys isosceles (Actinopterygii: Paralichthyidae); marine; stomach, intestine, mesentery, spleen serosa, muscles; metacestode; WTSA; Brazil (
Paralichthys patagonicus (Actinopterygii: Paralichthyidae); marine; body cavity, mesentery, stomach; metacestode; WTSA; Argentina, Brazil (
Porichthys porosissimus (Actinopterygii: Batrachoididae); marine; body cavity; metacestode; WTSA; Argentina (
Pseudupeneus maculatus (Actinopterygii: Mullidae); marine; body cavity; metacestode; TSA; Brazil (
Selene vomer (Actinopterygii: Carangidae); marine; body cavity; metacestode; TSA; Brazil (
Note: tapeworms reported as N. cf. lingualis.
Sympterygia bonapartii (Elasmobranchii: Arhynchobatidae); marine; spiral valve; metacestode; WTSA; Argentina (
Trachurus murphyi (Actinopterygii: Carangidae); marine; wall of pharynx; metacestode; WTSP; Chile (
Xystreurys rasile (Actinopterygii: Paralichthyidae); marine; body cavity, mesentery, stomach; metacestode; WTSA; Brazil (
Nybelinia surmenicola Okada in Dollfus, 1929
Hippoglossina macrops (Actinopterygii: Paralichthyidae); marine; intestine; metacestode; WTSP; Chile (
Merluccius gayi gayi (Actinopterygii: Merlucciidae); marine; body cavity, mesentery; metacestode; WTSP; Chile (
Paralichthys adspersus (Actinopterygii: Paralichthyidae); marine; body cavity, gill arches, muscles; metacestode; WTSP; Chile (
Trachurus murphyi (Actinopterygii: Carangidae); marine; site of infection not given; metacestode; WTSP; Chile, Peru (
Nybelinia sp.
Aphos porosus (Actinopterygii: Batrachoididae); marine; body cavity; metacestode; WTSP; Chile (
Balistes capriscus (Actinopterygii: Balistidae); marine; mesentery; metacestode; WTSA; Brazil (
Brama australis (Actinopterygii: Bramidae); marine; site of infection not given; metacestode; WTSP; Chile (
Brama japonica (Actinopterygii: Bramidae); marine; body cavity; metacestode; WTSP; Peru (
Caranx hippos (Actinopterygii: Carangidae); marine; mesentery; metacestode; WTSA; Brazil (
Caranx latus (Actinopterygii: Carangidae); marine; mesentery; metacestode; WTSA; Brazil (
Cilus gilberti (Actinopterygii: Sciaenidae); marine; site of infection not given; metacestode; WTSP; Chile (
Conger orbignianus (Actinopterygii: Congridae); marine; intestinal surface, mesentery; metacestode; WTSA; Argentina (
Coryphaena hippurus (Actinopterygii: Coryphaenidae); marine; body cavity, stomach; metacestode; WTSP; Peru (
Cynoscion analis (Actinopterygii: Sciaenidae); marine; intestine, surface of stomach; metacestode; WTSP; Peru (
Cynoscion guatucupa (Actinopterygii: Sciaenidae); marine; body cavity, mesentery; metacestode; WTSA; Brazil (
Dactylopterus volitans (Actinopterygii: Dactylopteridae); marine; mesentery; metacestode; WTSA; Brazil (
Diapterus rhombeus (Actinopterygii: Gerreidae); marine; mesentery; metacestode; WTSA; Brazil (
Genypterus brasiliensis (Actinopterygii: Ophidiidae); marine; body cavity, mesentery, intestinal serosa; metacestode; WTSA; Brazil (
Genypterus maculatus (Actinopterygii: Ophidiidae); marine; site of infection not given; metacestode; WTSP; Chile (
Hippoglossina macrops (Actinopterygii: Paralichthyidae); marine; gill arches; metacestode; WTSP; Chile (
Isacia conceptionis (Actinopterygii: Haemulidae); marine; mesentery; metacestode; WTSP; Peru (
Lophius gastrophysus (Actinopterygii: Lophiidae); marine; mesentery, body
cavity, muscles; metacestode; WTSA; Brazil (
Macrodon ancylodon (Actinopterygii: Sciaenidae); marine; mesentery; metacestode; WTSA; Brazil (
Merluccius gayi peruanus (Actinopterygii: Merlucciidae); marine; intestine; metacestode; WTSP; Peru (
Merluccius hubbsi (Actinopterygii: Merlucciidae); marine; mesentery; metacestode; Magellanic; Argentina (
Micropogonias furnieri (Actinopterygii: Sciaenidae); marine; mesentery; metacestode; TSA; Brazil (
Mola ramsayi (Actinopterygii: Molidae); marine; intestine; metacestode; WTSP; Chile (
Note: the authors distinguished two different morphotypes.
Mullus argentinae (Actinopterygii: Mullidae); marine; mesentery; metacestode; WTSA; Argentina, Brazil (
Odontesthes regia (Actinopterygii: Atherinopsidae); marine; site of infection not given; metacestode; WTSP; Chile (
Note: host reported as Austromenidia laticlava.
Paralabrax humeralis (Actinopterygii: Serranidae); marine; site of infection not given; metacestode; WTSP; Chile (
Paralichthys adspersus (Actinopterygii: Paralichthyidae); marine; intestine; metacestode; WTSP; Chile (
Paralichthys isosceles (Actinopterygii: Paralichthyidae); marine; intestine, stomach; metacestode; WTSA; Argentina, Brazil (
Paralichthys microps (Actinopterygii: Paralichthyidae); marine; intestine; metacestode; WTSP; Chile (
Paralichthys patagonicus (Actinopterygii: Paralichthyidae); marine; intestine, stomach; metacestode; WTSA; Argentina (
Paralonchurus brasiliensis (Actinopterygii: Sciaenidae); marine; mesentery; metacestode; WTSA; Brazil (
Percophis brasiliensis (Actinopterygii: Percophidae); marine; mesentery; metacestode; WTSA; Argentina, Brazil, Uruguay (
Note:
Pomatomus saltatrix (Actinopterygii: Pomatomidae); marine; site of infection not given; metacestode; WTSA; Brazil (
Notes: host reported as P. saltator.
Prionotus punctatus (Actinopterygii: Triglidae); marine; intestine; metacestode; WTSA; Brazil (
Note:
Pseudopercis numida (Actinopterygii: Pinguipedidae); marine; mesentery; metacestode; WTSA; Brazil (
Pseudopercis semifasciata (Actinopterygii: Pinguipedidae); marine; mesentery; metacestode; Magellanic, WTSA; Argentina (
Raneya brasiliensis (Actinopterygii: Ophidiidae); marine; site of infection not given; metacestode; Magellanic, WTSA; Argentina (
Sarda chilensis (Actinopterygii: Scombridae); marine; site of infection not given; metacestode; WTSP; Peru (
Sardinella brasiliensis (Actinopterygii: Clupeidae); marine; body cavity; metacestode; WTSA; Brazil (
Sciaena deliciosa (Actinopterygii: Scombridae); marine; body cavity, intestinal surface; metacestode; WTSP; Peru (
Scomber japonicus (Actinopterygii: Scombridae); marine; site of infection not given; metacestode; WTSP; Chile (
Scomberomorus brasiliensis (Actinopterygii: Scombridae); marine; mesentery; metacestode; WTSA; Brazil (
Selene setapinnis (Actinopterygii: Carangidae); marine; mesentery; metacestode; WTSA; Brazil (
Seriolella porosa (Actinopterygii: Centrolophidae); marine; body cavity; metacestode; Magellanic; Argentina (
Sympterygia bonapartii (Elasmobranchii: Arhynchobatidae); marine; spiral valve; adult; WTSA; Argentina (
Note: host reported as Psammobatis microps.
Trachurus lathami (Actinopterygii: Carangidae); marine; mesentery; metacestode; WTSA; Brazil (
Trachurus murphyi (Actinopterygii: Carangidae); marine; body cavity, mesentery; metacestode; WTSP; Chile, Peru (
Umbrina canosai (Actinopterygii: Sciaenidae); marine; site of infection not given; metacestode; WTSA; Brazil (
Urophycis brasiliensis (Actinopterygii: Phycidae); marine; mesentery; metacestode; WTSA; Brazil (
Note:
Urophycis mystaceus (Actinopterygii: Phycidae); marine; mesentery; metacestode; WTSA; Brazil (
Xystreurys rasile (Actinopterygii: Paralichthyidae); marine; intestine, stomach; metacestode; WTSA; Argentina (
Tentacularia coryphaenae Bosc, 1797*
[For synonyms, see
Carcharhinus longimanus (Elasmobranchii: Carcharhinidae); marine; spiral valve; adult; TSA, WTSA; Brazil (
Carcharhinus obscurus (Elasmobranchii: Carcharhinidae); marine; spiral valve; adult; WTSA; Brazil (
Carcharodon carcharias (Elasmobranchii: Lamnidae); marine; duodenum; adult; specific locality not given; Brazil (
Centropomus nigrescens (Actinopterygii: Centropomidae); amphidromous; peritoneum; metacestode; WTSP; Peru (
Coryphaena equiselis (Actinopterygii: Coryphaenidae); marine; body cavity; metacestode; specific locality not given; Brazil (
Coryphaena hippurus (Actinopterygii: Coryphaenidae); marine; body cavity, liver, gonads, muscles; metacestode; WTSA, WTSP; Brazil, Peru (
Note: type host.
Genypterus brasiliensis (Actinopterygii: Ophidiidae); marine; mesentery; metacestode; WTSA; Brazil (
Katsuwonus pelamis (Actinopterygii: Scombridae); marine; abdominal flaps, body cavity, muscles; metacestode; WTSA, WTSP; Brazil, Peru (
Lophius gastrophysus (Actinopterygii: Lophiidae); marine; muscles; metacestode; WTSA; Brazil (
Merluccius gayi peruanus (Actinopterygii: Merlucciidae); marine; mesentery; metacestode; WTSP; Peru (
Polyprion oxygeneios (Actinopterygii: Polyprionidae); marine; peritoneal cavity; metacestode; JFD; Chile (
Prionace glauca (Elasmobranchii: Carcharhinidae); marine; stomach, spiral valve; adult; JFD, WTSA, WTSP; Brazil, Chile, Peru (
Scomber japonicus (Actinopterygii: Scombridae); marine; body cavity; metacestode; WTSP; Chile, Peru (
Scomberomorus cavalla (Actinopterygii: Scombridae); marine; mesentery; metacestode; WTSA; Brazil (
Trachurus murphyi (Actinopterygii: Carangidae); marine; mesentery; metacestode; WTSP; Chile, Peru (
Carangidae gen. sp. (Actinopterygii: Carangidae); marine; kidney, mesentery; metacestode; WTSP; Peru (
Note: material is deposited in H. Palm’s personal collection.
Unidentified tentaculariids
Dules auriga (Actinopterygii: Serranidae); marine; mesentery; metacestode; WTSA; Argentina (
Paralichthys isosceles (Actinopterygii: Paralichthyidae); marine; site of infection not given; metacestode; WTSA; Brazil (
Urophycis brasiliensis (Actinopterygii: Phycidae); marine; mesentery; metacestode; WTSA; Argentina, Brazil (
Xystreurys rasile (Actinopterygii: Paralichthyidae); marine; intestine, mesentery; metacestode; WTSA; Argentina (
Unidentified trypanorhynchs
Antimora rostrata (Actinopterygii: Moridae); marine; visceral cavity; metacestode; WTSP: Chile (
Auxis thazard (Actinopterygii: Scombridae); marine; body cavity; metacestode; WTSA; Brazil (
Balistes vetula (Actinopterygii: Balistidae); marine; body cavity; metacestode; WTSA; Brazil (
Brama australis (Actinopterygii: Bramidae); marine; site of infection not given; metacestode; WTSP; Chile (
Note:
Caranx crysos (Actinopterygii: Carangidae); marine; body cavity; metacestode; WTSA; Brazil (
Carcharhinus limbatus (Elasmobranchii: Carcharhinidae); marine; site of infection not given; adult; WTSA; Brazil (
Note: host reported as Carcharias limbatus.
Carcharodon carcharias (Elasmobranchii: Lamnidae); marine; site of infection not given; adult; WTSA; Brazil (
Note: host reported as Carcharias lamia.
Centropomus undecimalis (Actinopterygii: Centropomidae); amphidromous; intestine; metacestode; Paraíba do Sul River basin (estuary of Guandú River); Brazil (
Coryphaenoides ariommus (Actinopterygii: Macrouridae); marine; visceral cavity; metacestode; WTSP: Chile (
Cynoscion acoupa (Actinopterygii: Scianidae); marine; mesentery, peritoneum; metacestode; WTSA; Brazil (
Cynoscion jamaicensis (Actinopterygii: Sciaenidae); marine; body cavity; metacestode; WTSA; Brazil (
Cynoscion leiarchus (Actinopterygii: Scianidae); marine; mesentery; metacestode; WTSA; Brazil (
Cynoscion striatus (Actinopterygii: Scianidae); marine; body cavity; metacestode; WTSA; Brazil (
Note:
Cynoscion sp. (Actinopterygii: Scianidae); marine; muscles; metacestode; TNA; Venezuela (
Note: tapeworms reported as Tetrarhynchus fragilis.
Dissostichus eleginoides (Actinopterygii: Nototheniidae); marine; site of infection not given; metacestode; Magellanic; Falkland Islands (
Epinephelus morio (Actinopterygii: Serranidae); marine; liver, mesentery, muscles, peritoneum; metacestode; WTSA; Brazil (
Note: host reported as Cerna morio.
Epinephelus sp. (Actinopterygii: Serranidae); marine; muscles; metacestode; TNA; Venezuela (
Note: tapeworms reported as Tetrarhynchus fragilis.
Euthynnus alletteratus (Actinopterygii: Scombridae); marine; liver, mesentery, peritoneum; metacestode; WTSA; Brazil (
Note: host reported as Gymnosarda alleterada.
Genidens barbus (Actinopterygii: Ariidae); marine; body cavity; metacestode; WTSA; Brazil (
Note: host reported as Netuma barba.
Genidens sp. (Actinopterygii: Ariidae); marine; mesentery; metacestode; WTSA; Brazil (
Note: host reported as Thachysurus sp. (sic!)
Genypterus blacodes (Actinopterygii: Ophidiidae); marine; mesentery; metacestode; WTSP; Chile (
Genypterus brasiliensis (Actinopterygii: Ophidiidae); marine; mesentery; metacestode; WTSA; Argentina (
Genypterus maculatus (Actinopterygii: Ophidiidae); marine; site of infection not given; metacestode; WTSP; Chile (
Helicolenus lengerichi (Actinopterygii: Sebastidae); marine; intestinal serosa, mesentery, peritoneum, stomach serosa; WTSP; Chile (
Hyporthodus niveatus (Actinopterygii: Serranidae); marine; liver, mesentery, peritoneum; metacestode; WTSA; Brazil (
Note: host reported as Garrupa niveata.
Lophius gastrophysus (Actinopterygii: Lophiidae); marine; body cavity; metacestode; WTSA; Brazil (
Lutjanus analis (Actinopterygii: Lutjanidae); marine; body cavity; metacestode; TSA; Brazil (
Macrodon ancylodon (Actinopterygii: Sciaenidae); marine; body cavity; metacestode; WTSA; Brazil (
Macrourus holotrachys (Actinopterygii: Macrouridae); marine; visceral cavity; metacestode; WTSP; Chile (
Masturus lanceolatus (Actinopterygii: Molidae); marine; liver; metacestode; TSA (estuary of Una River); Brazil (
Micropogonias furnieri (Actinopterygii: Scianidae); marine; body cavity, mesentery, peritoneum; metacestode; WTSA; Brazil, Uruguay (
Notes:
Micropogonias undulatus (Actinopterygii: Scianidae); marine; mesentery, peritoneum; metacestode; WTSA; Brazil (
Note: host reported as Micropogon undulatus.
Mola mola (Actinopterygii: Molidae); marine; oral cavity, heart, intestine, liver, muscles, stomach; metacestode; TSA; Brazil (
Mycteroperca bonaci (Actinopterygii: Serranidae); marine; liver, mesentery, peritoneum; metacestode; WTSA; Brazil (
Note: host reported as Epinephelus bonaci.
Notothenia cf. angustata (Actinopterygii: Nototheniidae); marine; body cavity; metacestode; WTSP; Chile (
Orthopristis ruber (Actinopterygii: Haemulidae); marine; mesentery, peritoneum; metacestode; WTSA; Brazil (
Pagrus pagrus (Actinopterygii: Sparidae); marine; body cavity; metacestode; WTSA; Brazil (
Paralabrax humeralis (Actinopterygii: Serranidae); marine; site of infection not given; metacestode; WTSP; Peru (
Pomatomus saltatrix (Actinopterygii: Pomatomidae); marine; mesentery, intestine; peritoneum; metacestode; WTSA; Brazil (
Note: host reported as Cheilodipterus saltator and P. saltator by
Porichthys porosissimus (Actinopterygii: Batrachoididae); marine; liver, mesentery; metacestode; WTSA; Brazil (
Pseudopercis numida (Actinopterygii: Pinguipedidae); marine; mesentery, peritoneum; metacestode; WTSA; Brazil (
Rhinobatos percellens (Elasmobranchii: Rhinobatidae); marine; site of infection not given; adult; WTSA; Brazil (
Note: host reported as 'cação viola', vernacular name of R. percellens.
Rhizoprionodon terraenovae (Elasmobranchii: Carcharhinidae); marine; site of infection not given; adult; WTSA; Brazil (
Note: host reported as 'cação alecrim' vernacular name of R. terraenovae.
Sardinella brasiliensis (Actinopterygii: Clupeidae); marine; body cavity; metacestode; WTSA; Brazil (
Sardinella sp. (Actinopterygii: Clupeidae); marine; liver; metacestode; WTSA; Brazil (
Scomber colias (Actinopterygii: Scombridae); marine; body cavity, mesentery, peritoneum, stomach; metacestode; WTSA; Brazil (
Selene vomer (Actinopterygii: Carangidae); marine; mesentery, peritoneum; metacestode; WTSA; Brazil (
Trachurus murphyi (Actinopterygii: Carangidae); marine; site of infection not given; metacestode; WTSP; Chile (
Trichiurus lepturus (Actinopterygii: Trichiuridae); marine; muscle; metacestode; WTSA; Brazil (
Urophycis brasiliensis (Actinopterygii: Phycidae); marine; body cavity; metacestode; WTSA; Brazil (
Sciaenidae gen. sp. (Actinopterygii: Sciaenidae); site of infection not given; metacestode; WTSA; Brazil (
Note: host reported as ‘pescadinha’, vernacular name of sciaenid fishes.
Species inquirendae
Otobothrium cysticum (Mayer, 1842) Dollfus, 1942
[Syn. Tetrarhynchus cysticus Mayer, 1842]
Genypterus brasiliensis (Actinopterygii: Ophidiidae); marine; body cavity, mesentery; metacestode; WTSA; Brazil (
Note:
Pagrus pagrus (Actinopterygii: Sparidae); marine; body cavity; metacestode; WTSA; Brazil (
Pomatomus saltatrix (Actinopterygii: Pomatomidae); marine; body cavity; metacestode; WTSA; Brazil (
Note: host reported as P. saltator.
Scomberomorus brasiliensis (Actinopterygii: Scombridae); marine; body cavity; metacestode; TSA; Brazil (
Note:
Sphyraena guachancho (Actinopterygii: Sphyraenidae); marine; body cavity; metacestode; TSA; Brazil (
Pterobothrium fragile (Diesing, 1850) Dollfus, 1942
[Syns. Synbothrium fragile Diesing, 1850; Syndesmobothrium fragile (Diesing, 1850) Diesing, 1863; Pterobothrium (Synbothrium) fragile (Diesing, 1850) sensu
Pristis pristis (Elasmobranchii: Pristidae); marine; intestine; adult; NBS (estuary of Amazon River); Brazil (
Notes: host reported as P. perotteti. Type specimens collected by J. Natterer are poorly preserved and the validity of P. fragile needs confirmation (see
Pterobothrium interruptum (Rudolphi, 1819) Diesing, 1850
[Syn. Anthocephalus interruptum Rudolphi, 1819]
Trichiurus lepturus (Actinopterygii: Trichiuridae); marine; body cavity; metacestode; type locality not given; Brazil (
Note: the type material deposited in MNHB was destroyed during the World War II (
Nomen nudum
Pterobothrium macrourum (Rudolphi, 1819) Diesing, 1850*
[Syn. Anthocephalus macrourus Rudolphi, 1819]
Sparidae gen. sp. (Actinopterygii: Sparidae); marine; liver, mesentery; metacestode; type locality not given; Brazil (
Note: type specimens collected by Olfers are barely recognized as cestodes and basic taxonomic information is missing in the original description; therefore,
Unidentified cestodes
Acestrorhynchus altus (Actinopterygii: Acestrorhynchidae); freshwater; intestine; stage of development not given; Paraná River basin; Brazil (
Ageneiosus inermis (Actinopterygii: Auchenipteridae); freshwater; site of infection and stage of development not given; Amazon and Paraná River basins; Brazil (
Note: hosts reported as A. valenciennesi and Pseudoageneiosus brevifilis by
Astyanax altiparanae (Actinopterygii: Characidae); freshwater; mesentery; metacestode; Paraná River basin; Brazil (
Astyanax bimaculatus (Actinopterygii: Characidae); freshwater; site of infection and stage of development not given; Paraná River basin; Brazil (
Astyanax sp. (Actinopterygii: Characidae); freshwater; site of infection and stage of development not given; Amazon River basin; Brazil (
Australoheros facetus (Actinopterygii: Cichlidae); freshwater; site of infection and stage of development not given; Doce River basin; Brazil (
Brachyplatystoma sp. (Actinopterygii: Pimelodidae); freshwater; site of infection and stage of development not given; Paraná River basin; Brazil (
Brama australis (Actinopterygii: Bramidae); marine; site of infection not given; metacestode; WTSP; Chile (
Brevoortia aurea (Actinopterygii: Clupeidae); marine; site of infection not given; metacestode; WTSA; Argentina (
Notes: host collected in a coastal lagoon.
Calophysus macropterus (Actinopterygii: Pimelodidae); freshwater; site of infection and stage of development not given; Amazon River basin; Brazil (
Caranx hippos (Actinopterygii: Carangidae); brackish, marine; site of infection and stage of development not given; TSA; Brazil (
Centropomus sp. (Actinopterygii: Centropomidae); amphidromous; site of infection and stage of development not given; Doce River basin; Brazil (
Cetopsis coecutiens (Actinopterygii: Cetopsidae); freshwater; site of infection and stage of development not given; Amazon River basin; Brazil (
Cichla ocellaris (Actinopterygii: Cichlidae); freshwater; site of infection not given; adult; Pereira de Miranda fishpond, Ceará State; Brazil (
Cichlasoma bimaculatum (Actinopterygii: Cichlidae); freshwater; site of infection not given; adult; Pereira de Miranda fishpond, Ceará State, Paraná River basin; Brazil (
Colossoma macropomum (Actinopterygii: Serrasalmidae); freshwater; site of infection not given; metacestode; Paraná River basin; Brazil (
Coryphaena hippurus (Actinopterygii: Coryphaenidae); brackish, marine; site of infection and stage of development not given; TSA; Brazil (
Crenicichla haroldoi (Actinopterygii: Cichlidae); freshwater; intestine; adult (immature); Paraná River basin; Brazil (
Cynoscion guatucupa (Actinopterygii: Sciaenidae); marine; site of infection not given; metacestode; WTSA; Argentina (
Dules auriga (Actinopterygii: Serranidae); marine; mesentery; metacestode; WTSA; Argentina (
Galaxias maculatus (Actinopterygii: Galaxiidae); amphidromous; intestine; metacestode; Maullin River basin; Chile (
Galeocharax knerii (Actinopterygii: Characidae); freshwater; intestine; adult (immature); Paraná River basin; Brazil (
Geophagus brasiliensis (Actinopterygii: Characidae); freshwater; site of infection and stage of development not given; Doce River basin; Brazil (
Gymnocharacinus bergii (Actinopterygii: Characidae); freshwater; site of infection not given; metacestode; Negro River Basin; Argentina (
Gymnotus carapo (Actinopterygii: Gymnotidae); freshwater; site of infection and stage of development not given; Doce River basin; Brazil (
Note: host reported as Giton fasciatus.
Gymnotus inaequilabiatus (Actinopterygii: Gymnotidae); freshwater; intestine; stage of development not given; Paraná River basin; Brazil (
Harengula sp. (Actinopterygii: Clupeidae); marine; site of infection and stage of development not given; TSA; Brazil (
Hemisorubim platyrhynchos (Actinopterygii: Pimelodidae); freshwater; mesentery, intestine; metacestode, adult; Amazon and Paraná River basins; Brazil, Peru (
Hoplerythrinus unitaeniatus (Actinopterygii: Erythrinidae); freshwater; site of infection and stage of development not given; Paraná River basin; Brazil (
Hoplias malabaricus (Actinopterygii: Erythrinidae); freshwater; site of infection and stage of development not given; Paraná River basin; Brazil (
Lagocephalus laevigatus (Actinopterygii: Tetraodontidae); brackish, marine; site of infection and stage of development not given; TSA; Brazil (
Leporinus obtusidens (Actinopterygii: Anostomidae); freshwater; intestine; adult; Paraná River basin; Brazil (
Loricariichthys sp. (Actinopterygii: Loricariidae); freshwater; intestine; adult (immature); Paraná River basin; Brazil (
Lutjanus jocu (Actinopterygii: Lutjanidae); brackish, freshwater; marine; site of infection and stage of development not given; TSA; Brazil (
Macrodon ancylodon (Actinopterygii: Sciaenidae); marine; mesentery, muscles; metacestode; WTSA; Brazil (
Merluccius australis (Actinopterygii: Merlucciidae); marine; mesentery, muscles; metacestode; WTSP; Chile (
Micropogonias furnieri (Actinopterygii: Sciaenidae); marine; mesentery, muscles; metacestode; WTSA; Brazil (
Micropogonias sp. (Actinopterygii: Sciaenidae); marine; site of infection and stage of development not given; TSA; Brazil (
Oligoplites saurus (Actinopterygii: Carangidae); brackish, marine; site of infection and stage of development not given; TSA; Brazil (
Oreochromis niloticus (Actinopterygii: Cichlidae); freshwater; site of infection not given; metacestode; Pereira de Miranda fishpond, Ceará and Paraná States; Brazil (
Note: introduced fish host (
Paralichthys isosceles (Actinopterygii: Paralichthyidae); marine; site of infection not given; metacestode; WTSA; Brazil (
Piaractus mesopotamicus (Actinopterygii: Characidae); freshwater; site of infection and stage of development not given; Paraná River basin; Brazil (
Pimelodella gracilis (Actinopterygii: Heptapteridae); freshwater; intestine, mesentery; adult, metacestode; Amazon and Paraná River basins; Brazil (
Pimelodella lateristriga (Actinopterygii: Heptapteridae); freshwater; intestine; adult; Paraná River basin; Brazil (
Pimelodus maculatus (Actinopterygii: Pimelodidae); freshwater; intestine; adult, metacestode; Paraná River basin; Brazil (
Notes: host reported as P. clarias by all authors.
Pimelodus ortmanni (Actinopterygii: Pimelodidae); freshwater; intestine; metacestode; Paraná River basin; Brazil (
Pinirampus pirinampu (Actinopterygii: Pimelodidae); freshwater; site of infection and stage of development not given; Paraná River basin; Brazil (
Plagioscion squamosissimus (Actinopterygii: Sciaenidae); freshwater; mesentery, intestine; metacestode, adult (immature); Amazon, Paraná and Tocantins-Araguaia River basins; Brazil (
Poecilia vivipara (Actinopterygii: Poeciliidae); freshwater; site of infection not given; adult; Pereira de Miranda fishpond, Ceará State; Brazil (
Potamotrygon motoro (Elasmobranchii: Potamotrygonidae); freshwater; intestine; adult; Paraná River basin; Brazil (
Notes:
Potamotrygon orbignyi (Elasmobranchii: Potamotrygonidae); freshwater; site of infection and stage of development not given; Amazon River basin; Brazil (
Note: host reported as Paratrygon hystrix.
Prochilodus argenteus (Actinopterygii: Prochilodontidae); freshwater; intestinal serosa; metacestode; São Francisco River Basin; Brazil (
Pseudopercis semifasciata (Actinopterygii: Pinguipedidae); marine; stomach wall; metacestode; Magellanic, WTSA; Argentina (
Pseudoplatystoma fasciatum (Actinopterygii: Pimelodidae); freshwater; intestine; adult; Paraná River basin; Brazil (
Pseudoplatystoma sp. (Actinopterygii: Pimelodidae); freshwater; site of infection and stage of development not given; Amazon and Paraná River basins; Brazil (
Note:
Raneya brasiliensis (Actinopterygii: Ophidiidae); marine; mesentery; metacestode; Magellanic, WTSA; Argentina (
Rhaphiodon vulpinus (Actinopterygii: Cynodontidae); freshwater; site of infection and stage of development not given; Paraná River basin; Brazil (
Salilota australis (Actinopterygii: Moridae); marine; body cavity; metacestode; Magellanic; Argentina (
Salminus brasiliensis (Actinopterygii: Bryconidae); freshwater; intestine; adult (immature); Paraná River basin; Brazil (
Scomber colias (Actinopterygii: Scombridae); brackish, marine; site of infection
and stage of development not given; TSA; Brazil (
Selene vomer (Actinopterygii: Carangidae); brackish, marine; site of infection and stage of development not given; TSA; Brazil (
Sphoeroides testudineus (Actinopterygii: Tetraodontidae); brackish, marine; site of infection and stage of development not given; TSA; Brazil (
Steindachneridion parahybae (Actinopterygii: Pimelodidae); freshwater; site of infection and stage of development not given; Paraná River basin; Brazil (
Note: this fish is found only in the Paraíba do Sul and Jequitinhonha River basins (
Sternopygus macrurus (Actinopterygii: Sternopygidae); freshwater; intestine; adult; Paraná River basin; Brazil (
Tilapia rendalli (Actinopterygii: Cichlidae); freshwater; intestine; metacestode; Ingá Lake, Paraná State, Brazil (
Trachurus murphyi (Actinopterygii: Carangidae); marine; body cavity; metacestode; WTSP; Chile (
Umbrina coroides (Actinopterygii: Sciaenidae); brackish, marine; site of infection and stage of development not given; TSA; Brazil (
Xystreurys rasile (Actinopterygii: Paralichthyidae); marine; mesentery; metacestode; WTSA; Argentina (
Zungaro jahu (Actinopterygii: Pimelodidae); freshwater; site of infection and stage of development not given; Paraná River basin; Brazil (
Note: host reported as P. luetkeni.
Host-Parasite List
Phylum Chordata
Class Actinopterygii
Order Anguilliformes
Family Congridae
Conger orbignianus Valenciennes: Grillotia (Christianella) carvajalregorum (L), G. (Grillotia) erinaceus (L), Nybelinia sp. (L), ‘Scolex spp.’ (L)
Family Muraenidae
Gymnothorax moringa (Cuvier): ‘Scolex spp.’ (L)
Order Atheriniformes
Family Atherinopsidae
Basilichthys australis Eigenmann: Diphyllobothrium dendriticum (L), D. latum (L), Diphyllobothrium sp. (L).
Odontesthes argentinensis (Valenciennes): ‘Scolex spp.’ (L)
Odontesthes bonariensis (Valenciennes): Cangatiella macdonaghi
Odontesthes hatcheri (Eigenmann): Cangatiella macdonaghi
Odontesthes mauleanum (Steindachner): Diphyllobothrium dendriticum (L), D. latum (L), Diphyllobothrium sp. (L).
Odontesthes regia (Humboldt): Diphyllobothrium sp. (L), Lacistorhynchus tenuis (L), Nybelinia sp. (L), unidentified ‘pseudophyllidean’ (L), ‘Scolex spp.’ (L)
Odontesthes smitti (Lahille): unidentified bothriocephalidean (L), ‘Scolex spp.’ (L)
Order Aulopiformes
Family Synodontidae
Synodus scituliceps Jordan & Gilbert: Rhinebothrium sp. (L), ‘Scolex spp.’ (L)
Order Batrachoidiformes
Family Batrachoididae
Aphos porosus (Valenciennes): Clestobothrium crassiceps, Grillotia sp. (L), Nybelinia sp. (L), unidentified ‘pseudophyllidean’ (L), ‘Scolex spp.’ (L)
Porichthys porosissimus (Cuvier): Grillotia (Christianella) carvajalregorum (L), G. (Grillotia) erinaceus (?) (L), Nybelinia lingualis (L), unidentified trypanorhynch (L), ‘Scolex spp.’ (L)
Order Beloniformes
Family Belonidae
Strongylura scapularis (Jordan & Gilbert): ‘Scolex spp.’ (L)
Tylosurus acus acus (Lacépède): ‘Scolex’ spp. (L)
Family Hemiramphidae
Hyporhamphus unifasciatus (Ranzani): ‘Scolex spp.’ (L)
Order Characiformes
Family Acestrorhynchidae
Acestrorhynchus altus Menezes: Monticellia dlouhyi, unidentified cestode
Family Anostomidae
Leporellus vittatus (Valenciennes): unidentified proteocephalid
Leporinus friderici (Bloch): Proteocephalus vazzolerae, unidentified proteocephalid
Leporinus lacustris Amaral Campos: Proteocephalus vazzolerae
Leporinus obtusidens (Valenciennes): unidentified cestode
Family Bryconidae
Brycon cephalus (Günther): unidentified proteocephalid
Brycon orbignyanus (Valenciennes): Monticellia sp.
Salminus brasiliensis (Cuvier): Monticellia coryphicephala, unidentified cestode
Salminus franciscanus Lima & Britski: Monticellia coryphicephala
Family Characidae
Aphyocharax anisitsi Eigenmann & Kennedy: unidentified proteocephalid
Astyanax altiparanae Garutti & Britski: Senga sp., unidentified proteocephalid, unidentified cestode
Astyanax bimaculatus (Linnaeus): unidentified cestode
Astyanax scabripinnis (Jenyns): Senga sp.
Astyanax sp.: unidentified cestode
Galeocharax knerii (Steindachner): unidentified cestode
Gymnocharacinus bergii Steindachner: unidentified cestode
Psellogrammus kennedyi (Eigenmann): unidentified proteocephalid
Family Cynodontidae
Rhaphiodon vulpinus Spix & Agassiz: Choanoscolex abscisus, unidentified cestode
Family Erythrinidae
Hoplerythrinus unitaeniatus (Spix & Agassiz): Nomimoscolex matogrossensis (?), Proteocephalus mahnerti, unidentified cestode
Hoplias malabaricus (Bloch): Nomimoscolex matogrossensis, Proteocephalus regoi, unidentified cestode
Family Prochilodontidae
Prochilodus argenteus Spix & Agassiz: Valipora sp. (L), unidentified cestode
Prochilodus brevis Steindachner: unidentified proteocephalid
Prochilodus lineatus (Valenciennes): unidentified proteocephalid, Valipora campylancristrota (L)
Family Serrasalmidae
Colossoma macropomum (Cuvier): unidentified proteocephalid, unidentified cestode
Colossoma macropomum × Piaractus mesopotamicus: unidentified proteocephalid
Piaractus brachypomus (Cuvier): unidentified proteocephalid
Piaractus mesopotamicus (Holmberg): Proteocephalus vazzolerae, unidentified cestode
Pygocentrus nattereri Kner: Proteocephalus serrasalmus
Serrasalmus maculatus Kner: Proteocephalus serrasalmus
Order Clupeiformes
Family Clupeidae
Brevoortia aurea (Spix & Agassiz): unidentified cestode
Ethmidium maculatum (Valenciennes): ‘Scolex spp.’ (L)
Harengula clupeola (Cuvier): Callitetrarhynchus gracilis (L)
Harengula sp.: unidentified cestode
Opisthonema oglinum (Lesueur): Callitetrarhynchus gracilis (L)
Sardinella brasiliensis (Steindachner): Callitetrarhynchus gracilis (L), Nybelinia sp. (L), unidentified trypanorhynch, ‘Scolex spp.’ (L)
Sardinella sp.: unidentified proteocephalid, unidentified trypanorhynch
Sardinops sagax (Jenyns): ‘Scolex spp.’ (L)
Clupeidae gen. sp.: Pterobothrium heteracanthum (L)
Family Engraulidae
Anchoa tricolor (Spix & Agassiz): ‘Scolex spp.’ (L)
Engraulis anchoita Hubbs & Marini: Bothriocephalus sp., unidentified ‘pseudophyllidean’, ‘Scolex spp.’ (L)
Engraulis ringens Jenyns: Bothriocephalus sp., Diphyllobothrium sp. (L), ‘Scolex spp.’ (L)
Order Cypriniformes
Family Cyprinidae
Cyprinus carpio Linnaeus: Schyzocotyle acheilognathi, unidentified caryophyllidean, unidentified proteocephalid
Hypophthalmichthys nobilis (Richardson): unidentified proteocephalid
Pethia conchonius (Hamilton): Schyzocotyle acheilognathi
Order Cyprinodontiformes
Family Poeciliidae
Poecilia reticulata Peters: Glossocercus auritus (L), Schyzocotyle acheilognathi
Poecilia vivipara Bloch & Schneider: unidentified cestode
Xiphophorus hellerii Heckel: Schyzocotyle acheilognathi
Xiphophorus maculatus (Günther): Schyzocotyle acheilognathi
Order Gadiformes
Family Gadidae
Micromesistius australis australis Norman: Clestobothrium crassiceps, Diphyllobothrium sp. (L), Grillotia sp. (L), Hepatoxylon trichiuri (L), Hepatoxylon sp. (L), unidentified ‘pseudophyllidean’
Family Macrouridae
Coelorinchus chilensis Gilbert & Thompson: Grillotia sp. (L), Hepatoxylon trichiuri (L)
Coryphaenoides ariommus Gilbert & Thompson: unidentified trypanorhynch
Macrourus carinatus (Günther): Grillotia (Grillotia) borealis (?) (L)
Macrourus holotrachys Günther: Hepatoxylon sp. (L), Parabothriocephalus sp. (L),
unidentified trypanorhynch
Family Merlucciidae
Macruronus magellanicus Lönnberg: Clestobothrium crassiceps, Grillotia (Grillotia) dollfusi (L), G. heptanchi (L), Hepatoxylon trichiuri (L), unidentified ‘pseudophyllidean’, ‘Scolex spp.’ (L)
Merluccius australis (Hutton): Clestobothrium splendidum, Diphyllobothrium sp. (L), Grillotia heptanchi (L), Grillotia sp. (L), Hepatoxylon trichiuri (L), Lacistorhynchus sp. (L), unidentified phyllobothriidean, ‘Scolex spp.’ (L), unidentified cestode
Merluccius gayi gayi (Guichenot): Clestobothrium crassiceps, Grillotia (Grillotia) dollfusi (L), G. heptanchi (L), Hepatoxylon trichiuri (L), Nybelinia surmenicola (L), unidentified ‘pseudophyllidean’, ‘Scolex spp.’ (L)
Merluccius gayi peruanus (Ginsburg): Adenocephalus pacificus (L), Callitetrarhynchus gracilis (L), Clestobothrium crassiceps, Diphyllobothrium sp. (L), Grillotia (Grillotia) dollfusi (L), Lacistorhynchus tenuis (L), Nybelinia sp. (L), Tentacularia coryphaenae (L)
Merluccius hubbsi Marini: Clestobothrium cristinae, Diphyllobothrium sp. (L), Grillotia (Christianella) carvajalregorum (L), Grillotia sp. (L), Hepatoxylon trichiuri (L), Nybelinia sp. (L), unidentified phyllobothriidean, unidentified ‘pseudophyllidean’, ‘Scolex spp.’ (L)
Merluccius sp.: Clestobothrium crassiceps
Family Moridae
Antimora rostrata (Günther): unidentified trypanorhynch
Salilota australis (Günther): Grillotia (Grillotia) borealis (?) (L), G. (G.) patagonica (L), Hepatoxylon trichiuri (L), unidentified cestode
Family Phycidae
Urophycis brasiliensis (Kaup): Callitetrarhynchus gracilis (L), Grillotia (Christianella) carvajalregorum (L), Heteronybelinia sp. (L), Lacistorhynchus sp. (L), Nybelinia sp. (L), Phyllobothrium sp. (L), unidentified phyllobothriidean, unidentified trypanorhynch, ‘Scolex spp.’ (L)
Urophycis mystaceus Ribeiro: Lacistorhynchus sp. (L), Nybelinia sp. (L), unidentified phyllobothriidean, ‘Scolex spp.’ (L)
Urophycis sp.: unidentified phyllobothriidean
Order Gobiesociformes
Family Gobiesocidae
Gobiesox marmoratus Jenyns: unidentified ‘pseudophyllidean’, ‘Scolex spp.’ (L)
Sicyases sanguineus Müller & Troschel: unidentified ‘pseudophyllidean’, ‘Scolex spp.’ (L)
Order Gymnotiformes
Family Gymnotidae
Gymnotus carapo Linnaeus: Nomimoscolex chubbi, N. dechambrieri, N. guillermoi, Proteocephalus sp., unidentified cestode
Gymnotus inaequilabiatus (Valenciennes): unidentified cestode
Gymnotus sp.: Nomimoscolex chubbi
Family Sternopygidae
Sternopygus macrurus (Bloch & Schneider): unidentified cestode
Order Lampriformes
Family Lampridae
Lampris guttatus (Brünnich): Hepatoxylon trichiuri (L)
Order Lophiiformes
Family Lophiidae
Lophius gastrophysus Miranda Ribeiro: Diphyllobothrium sp. (L), Grillotia (Christianella) carvajalregorum (L), Mixonybelinia sp. (L), Nybelinia sp. (L), Tentacularia coryphaenae (L), unidentified trypanorhynch
Ordem Mugiliformes
Family Mugilidae
Mugil cephalus Linnaeus: Lacistorhynchus tenuis (L), ‘Scolex spp.’ (L)
Mugil liza Valenciennes: unidentified phyllobothriidean, ‘Scolex spp.’ (L)
Order Notacanthiformes
Family Notacanthidae
Notacanthus sexspinis Richardson: Hepatoxylon trichiuri (L)
Order Ophidiiformes
Family Ophidiidae
Genypterus blacodes (Forster): Anonchocephalus chilensis, Hepatoxylon trichiuri (L), unidentified trypanorhynch, ‘Scolex spp.’ (L)
Genypterus brasiliensis Regan: Anonchocephalus chilensis, Callitetrarhynchus gracilis (L), Diphyllobothrium sp. (L), Grillotia (Christianella) carvajalregorum (L), Hepatoxylon trichiuri (L), Heteronybelinia nipponica (L), Mixonybelinia beveridgei (L), Nybelinia sp. (L), Otobothrium cysticum (L), Tentacularia coryphaenae (L), unidentified trypanorhynch, ‘Scolex spp.’ (L)
Genypterus chilensis (Guichenot): Anonchocephalus chilensis, Grillotia heptanchi (L), Hepatoxylon trichiuri (L)
Genypterus maculatus (Tschudi): Adenocephalus pacificus (L), Anonchocephalus chilensis, Diphyllobothrium sp. (L), Hepatoxylon trichiuri (L), Nybelinia sp. (L), unidentified trypanorhynch, ‘Scolex spp.’
Raneya brasiliensis (Kaup): Grillotia (Christianella) carvajalregorum (L), Heteronybelinia mattisi (L), Nybelinia sp. (L), ‘Scolex spp.’ (L), unidentified cestode
Order Osmeriformes
Family Galaxiidae
Aplochiton taeniatus Jenyns: unidentified bothriocephalidean
Aplochiton zebra Jenyns: Ailinella mirabilis
Galaxias maculatus (Jenyns): Ailinella mirabilis, Diphyllobothrium dendriticum (L), D. latum (L), Diphyllobothrium sp. (L), unidentified cestode
Galaxias platei Steindachner: Diphyllobothrium latum (L), Diphyllobothrium sp. (L), Galaxitaenia toloi
Order Osteoglossiformes
Family Arapaimidae
Arapaima gigas (Schinz): Nesolecithus janickii, Schizochoerus liguloideus
Order Perciformes
Family Blenniidae
Hypsoblennius sordidus (Bennett): unidentified ‘pseudophyllidean’
Scartichthys viridis (Valenciennes): Lacistorhynchus sp. (L), unidentified ‘pseudophyllidean’
Family Bovichtidae
Cottoperca gobio (Günther): Bothriocephalus timii, Grillotia (Grillotia) patagonica (L)
Family Bramidae
Brama australis Valenciennes: Hepatoxylon trichiuri (L), Nybelinia sp. (L), unidentified trypanorhynch, unidentified cestode
Brama japonica Hilgendorf: Hepatoxylon trichiuri (L), Nybelinia sp. (L)
Family Carangidae
Caranx crysos (Mitchill): Callitetrarhynchus gracilis (L), unidentified trypanorhynch
Caranx hippos (Linnaeus): Callitetrarhynchus gracilis (L), Dasyrhynchus giganteus (L), Nybelinia sp. (L), unidentified cestode
Caranx latus Agassiz: Callitetrarhynchus gracilis (L), Nybelinia sp. (L), ‘Scolex spp.’ (L)
Chloroscombrus chrysurus (Linnaeus): Callitetrarhynchus gracilis (L)
Oligoplites palometa (Cuvier): Callitetrarhynchus gracilis (L), Pterobothrium crassicole (L), ‘Scolex spp.’ (L)
Oligoplites saliens (Bloch): Dasyrhynchus giganteus (L), ‘Scolex spp.’ (L)
Oligoplites saurus (Bloch & Schneider): Callitetrarhynchus gracilis (L), ‘Scolex spp.’ (L), unidentified cestode
Parona signata (Jenyns): Grillotia (Christianella) carvajalregorum (L), ‘Scolex spp.’ (L)
Selene setapinnis (Mitchill): Callitetrarhynchus gracilis (L), Nybelinia sp. (L)
Selene vomer (Linnaeus): Callitetrarhynchus gracilis (L), Nybelinia lingualis (L), unidentified trypanorhynch, unidentified cestode
Seriola lalandi Valenciennes: Floriceps saccatus (L)
Trachinotus paitensis Cuvier: Adenocephalus pacificus (L)
Trachurus lathami Nichols: Callitetrarhynchus gracilis (L), Grillotia (Christianella) carvajalregorum (L), Nybelinia sp. (L), unidentified ‘pseudophyllidean’, ‘Scolex spp.’ (L)
Trachurus murphyi Nichols: Adenocephalus pacificus (L), Diphyllobothrium sp. (L), Hepatoxylon trichiuri (L), Hepatoxylon sp. (L), Nybelinia lingualis (L), N. surmenicola (L), Nybelinia sp. (L), Tentacularia coryphaenae (L), unidentified ‘pseudophyllidean’, unidentified trypanorhynch, ‘Scolex spp.’ (L), unidentified cestode
Carangidae gen. sp.: Tentacularia coryphaenae (L)
Family Centrolophidae
Seriolella porosa Guichenot: Nybelinia sp. (L).
Seriolella violacea Guichenot: Adenocephalus pacificus (L), Neobothriocephalus aspinosus
Family Centropomidae
Centropomus nigrescens Günther: Floriceps saccatus (L), Tentacularia coryphaenae (L)
Centropomus undecimalis (Bloch): Callitetrarhynchus gracilis (L), unidentified trypanorhynch
Centropomus sp.: unidentified cestode
Family Cheilodactylidae
Cheilodactylus variegatus Valenciennes: Lacistorhynchus tenuis (L)
Nemadactylus bergi (Norman): Grillotia (Christianella) carvajalregorum (L), G. (Grillotia) patagonica (L), Heteronybelinia mattisi (L)
Family Cichlidae
Aequidens tetramerus (Heckel): unidentified proteocephalid
Astronotus ocellatus (Agassiz): Proteocephalus gibsoni, unidentified proteocephalid
Astronotus sp.: Proteocephalus gibsoni
Australoheros facetus (Jenyns): Parvitaenia macropeos (L), unidentified cestode
Cichla kelberi Kullander & Ferreira: Proteocephalus macrophallus, P. microscopicus, Sciadocephalus megalodiscus
Cichla monoculus Spix: Proteocephalus macrophallus, P. microscopicus, Sciadocephalus megalodiscus, unidentified bothriocephalidean
Cichla ocellaris Schneider: Proteocephalus macrophallus, P. microscopicus, unidentified proteocephalid, unidentified cestode
Cichla piquiti Kullander & Ferreira: Proteocephalus macrophallus, P. microscopicus, Sciadocephalus megalodiscus
Cichla sp.: Proteocephalus macrophallus, P. microscopicus
Cichlasoma amazonarum Kullander: unidentified proteocephalid (new species and genus, see the parasite-host list)
Cichlasoma bimaculatum (Linnaeus): unidentified cestode
Crenicichla britskii Kullander: Valipora sp. (L)
Crenicichla haroldoi Luengo & Britski: unidentified cestode
Crenicichla lepidota Heckel: unidentified proteocephalid
Geophagus brasiliensis (Quoy & Gaimard): unidentified caryophyllidean, Proteocephalus gibsoni, Valipora campylancristrota (L), unidentified proteocephalid, unidentified cestode
Geophagus proximus (Castelnau): unidentified proteocephalid
Laetacara curviceps (Ahl): unidentified proteocephalid
Oreochromis niloticus (Linnaeus): unidentified cestode
Oreochromis sp.: unidentified proteocephalid
Satanoperca pappaterra (Heckel): unidentified cyclophyllidean, unidentified proteocephalid
Tilapia rendalli (Boulenger): unidentified cestode
Family Coryphaenidae
Coryphaena equiselis Linnaeus: Tentacularia coryphaenae (L)
Coryphaena hippurus Linnaeus: Floriceps saccatus (L), Hepatoxylon trichiuri (L),
Pterobothrium acanthotruncatum, Nybelinia sp., Tentacularia coryphaenae, ‘Scolex spp.’, unidentified cestode
Family Eleginopsidae
Eleginops maclovinus (Cuvier): Bothriocephalus sp., Grillotia (Grillotia) erinaceus (L), Grillotia sp. (L), unidentified bothriocephalidean, ‘Scolex spp.’ (L)
Family Eleotridae
Dormitator maculatus (Bloch): unidentified cyclophyllidean (L)
Family Gempylidae
Thyrsites atun (Euphrasen): Molicola sp. (L)
Family Gerreidae
Diapterus rhombeus (Cuvier): Nybelinia sp. (L)
Family Gobiidae
Gobioides broussonnetii Lacépède: Pterobothrium crassicole (L)
Gobionellus oceanicus (Pallas): Rhinebothrium sp. (L)
Family Haemulidae
Conodon nobilis (Linnaeus): Callitetrarhynchus sp. (L), Pterobothrium sp. (L)
Haemulon aurolineatum Cuvier: Callitetrarhynchus gracilis (L), Pterobothrium kingstoni (L)
Haemulon plumierii (Lacépède): Heteronybelinia estigmena (L), Nybelinia lingualis (L), Pseudotobothrium dipsacum (L)
Haemulon steindachneri (Jordan & Gilbert): ‘Scolex spp.’ (L)
Isacia conceptionis (Cuvier): Hepatoxylon trichiuri (L), Nybelinia sp. (L)
Orthopristis ruber (Cuvier): unidentified trypanorhynch, ‘Scolex spp.’ (L)
Pomadasys crocro (Cuvier): Pterobothrium heteracanthum (L)
Family Kyphosidae
Girella laevifrons (Tschudi): unidentified bothriocephalidean
Family Labrisomidae
Labrisomus philippii (Steindachner): Lacistorhynchus tenuis (L)
Family Lutjanidae
Lutjanus analis (Cuvier): Grillotia sp. (L), unidentified trypanorhynch
Lutjanus jocu (Bloch & Schneider): unidentified cestode
Lutjanus synagris (Linnaeus): Callitetrarhynchus gracilis (L)
Family Mullidae
Mullus argentinae Hubbs & Marini: Heteronybelinia nipponica (L), Nybelinia sp. (L)
Pseudupeneus maculatus (Bloch): Heteronybelinia overstreeti (L), Mixonybelinia edwinlintoni (L), Nybelinia africana (L), N. indica (L), N. lingualis (L), Pseudolacistorhynchus noodti (L), Pseudotobothrium dipsacum (L)
Family Nototheniidae
Dissostichus eleginoides Smitt: Clestobothrium crassiceps (?), Grillotia (Grillotia) erinaceus (L), Hepatoxylon trichiuri (L), unidentified ‘pseudophyllidean’, unidentified trypanorhynch, ‘Scolex spp.’ (L)
Notothenia cf. angustata Hutton: unidentified diphyllidean, unidentified trypanorhynch, ‘Scolex spp.’ (L)
Patagonotothen brevicauda brevicauda (Lönnberg): Grillotia (Grillotia) patagonica (L)
Patagonotothen ramsayi (Regan): Grillotia (Grillotia) patagonica (L)
Family Percichthyidae
Percichthys trucha (Valenciennes): Diphyllobothrium dendriticum (L), D. latum (L), unidentified bothriocephalidean, unidentified cyclophyllidean (L)
Percichthys sp.: Diphyllobothrium latum (L)
Family Perciliidae
Percilia gillissi Girard: Diphyllobothrium dendriticum (L)
Family Percophidae
Percophis brasiliensis Quoy & Gaimard: Callitetrarhynchus gracilis (L), Grillotia (Christianella) carvajalregorum (L), Grillotia sp. (L), Nybelinia sp. (L), unidentified bothriocephalidean, unidentified ‘pseudophyllidean’, ‘Scolex spp.’ (L)
Family Pinguipedidae
Pinguipes brasilianus Cuvier: Anonchocephalus sp., Callitetrarhynchus gracilis (L), Grillotia sp. (L), ‘Scolex spp.’ (L)
Prolatilus jugularis (Valenciennes): ‘Scolex spp. (L)’, unidentified bothriocephalidean unidentified ‘pseudophyllidean’
Pseudopercis numida Miranda Ribeiro: Callitetrarhynchus gracilis (L), Grillotia (Christianella) carvajalregorum (L), Nybelinia sp. (L), unidentified trypanorhynch, ‘Scolex spp.’ (L)
Pseudopercis semifasciata (Cuvier): Grillotia (Christianella) carvajalregorum (L), Grillotia sp. (L), Hepatoxylon trichiuri (L), Nybelinia sp. (L), ‘Scolex spp.’ (L), unidentified cestode
Family Polyprionidae
Polyprion oxygeneios (Schneider & Forster): Tentacularia coryphaenae (L)
Family Pomatomidae
Pomatomus saltatrix (Linnaeus): Callitetrarhynchus gracilis (L), C. speciosus (L), Grillotia (Christianella) carvajalregorum (L), Nybelinia sp. (L), Otobothrium cysticum (L), Pseudogrillotia sp. (L), Pterobothrium crassicole (L), Pterobothrium sp. (L), unidentified trypanorhynch, ‘Scolex spp.’ (L)
Family Priacanthidae
Priacanthus arenatus Cuvier: Callitetrarhynchus speciosus (L), ‘Scolex spp.’ (L)
Family Sciaenidae
Cheilotrema fasciatum Tschudi: Lacistorhynchus dollfusi (L), L. tenuis (L)
Cilus gilberti (Abbott): Adenocephalus pacificus (L), Diphyllobothrium sp. (L), Lacistorhynchus tenuis (L), Poecilancistrium caryophyllum (L), Nybelinia sp. (L), ‘Scolex spp.’ (L)
Ctenosciaena gracilicirrhus (Metzelaar): Grillotia (Christianella) carvajalregorum (L), Heteronybelinia annakohnae (L)
Cynoscion acoupa (Lacépède): Callitetrarhynchus gracilis (L), Poecilancistrium caryophyllum (L), Pterobothrium crassicole (L), P. heteracanthum (L), Pterobothrium sp. (L), unidentified trypanorhynch
Cynoscion analis (Jenyns): Adenocephalus pacificus (L), Diphyllobothrium sp. (L), Nybelinia sp. (L)
Cynoscion guatucupa (Cuvier): Callitetrarhynchus gracilis (L), C. speciosus (L), Dasyrhynchus pacificus (L), Grillotia (Christianella) carvajalregorum (L), G. (C.) minuta (?) (L), Heteronybelinia annakohnae (L), Nybelinia sp. (L), Pterobothrium heteracanthum (L),‘Scolex spp.’ (L), unidentified cestode
Cynoscion jamaicensis (Vaillant & Bocourt): Dasyrhynchus pacificus (L), Grillotia (Christianella) carvajalregorum (L), Heteronybelinia annakohnae (L), H. estigmena (L), unidentified trypanorhynch
Cynoscion leiarchus (Cuvier): Pterobothrium crassicole (L), unidentified trypanorhynch
Cynoscion striatus (Cuvier): Grillotia (Christianella) carvajalregorum (L), Pterobothrium sp. (L), unidentified proteocephalid, unidentified trypanorhynch
Cynoscion sp.: Nybelinia lingualis (L), Pterobothrium crassicole (L), unidentified trypanorhynch
Larimus breviceps Cuvier: Callitetrarhynchus gracilis (L)
Macrodon ancylodon (Bloch & Schneider): Callitetrarhynchus gracilis (L), Dasyrhynchus pacificus (L), Grillotia (Christianella) carvajalregorum (L), Nybelinia sp. (L), Poecilancistrium caryophyllum (L), Pterobothrium sp. (L), unidentified trypanorhynch, unidentified cestode
Menticirrhus americanus (Linnaeus): Dasyrhynchus pacificus (L), Grillotia (Christianella) carvajalregorum (L), Heteronybelinia annakohnae (L), H. nipponica (L), Pterobothrium sp. (L), ‘Scolex spp.’ (L)
Menticirrhus littoralis (Holbrook): Grillotia (Christianella) carvajalregorum (L)
Menticirrhus ophicephalus (Jenyns): Adenocephalus pacificus (L)
Micropogonias altipinnis (Günther): Poecilancistrium caryophyllum (L)
Micropogonias furnieri (Desmarest): Callitetrarhynchus gracilis (L), C. speciosus (L), Dollfusiella sp. (L), Gilquinia sp. (L), Grillotia (Christianella) carvajalregorum (L), Nybelinia sp. (L), Poecilancistrium caryophyllum (L), Pterobothrium acanthotruncatum (L), P. crassicole (L), P. heteracanthum (L), Pterobothrium sp. (L), unidentified trypanorhynch, ‘Scolex spp.’ (L), unidentified cestode
Micropogonias undulatus (Linnaeus): Pterobothrium crassicole (L), P. heteracanthum (L), unidentified trypanorhynch
Micropogonias sp.: unidentified cestode
Paralabrax humeralis (Valenciennes): Adenocephalus pacificus (L), Grillotia sp. (L), Nybelinia sp. (L), unidentified bothriocephalidean, unidentified trypanorhynch
Paralonchurus brasiliensis (Steindachner): Grillotia (Christianella) carvajalregorum (L), Nybelinia sp. (L), ‘Scolex spp.’ (L)
Paralonchurus peruanus (Steindachner): Adenocephalus pacificus (L), Callitetrarhynchus gracilis (L), Diphyllobothrium sp. (L), Pterobothrium acanthotruncatum (L)
Plagioscion squamosissimus (Heckel): Callitetrarhynchus gracilis (L), Pterobothrium crassicole (L), P. heteracanthum (L), Poecilancistrium caryophyllum (L), unidentified bothriocephalidean, unidentified cestode
Pogonias cromis (Linnaeus): Pterobothrium crassicole (L), P. heteracanthum (L)
Sciaena callaensis Hildebrand: Adenocephalus pacificus (L), Diphyllobothrium sp. (L).
Sciaena deliciosa (Tschudi): Adenocephalus pacificus (L), Callitetrarhynchus gracilis (L), Dasyrhynchus pacificus (L), Diphyllobothrium sp. (L), Nybelinia sp. (L)
Umbrina canosai Berg: Callitetrarhynchus gracilis (L), Grillotia (Christianella) carvajalregorum (L), Heteronybelinia nipponica (L), Nybelinia bisulcata (?) (L), Nybelinia sp. (L), Pterobothrium heteracanthum (L), Pterobothrium sp. (L)
Umbrina coroides Cuvier: unidentified cestode
Sciaenidae gen. sp.: unidentified trypanorhynch
Family Scombridae
Auxis thazard (Lacépède): unidentified trypanorhynch
Euthynnus alletteratus (Rafinesque): Callitetrarhynchus gracilis (L), unidentified trypanorhynch, ‘Scolex spp.’ (L)
Katsuwonus pelamis (Linnaeus): Tentacularia coryphaenae (L), ‘Scolex spp.’ (L)
Sarda chiliensis (Cuvier): Adenocephalus pacificus (L), Acanthobothrium chilense, Nybelinia sp. (L), Rhodobothrium mesodesmatum (L), Sphyriocephalus tergestinus (L), ‘Scolex spp.’ (L)
Scomber colias Gmelin: Rhinebothrium sp. (L), unidentified trypanorhynch, unidentified cestode, ‘Scolex spp.’ (L)
Scomber japonicus Houttuyn: Diphyllobothrium sp. (L), Hepatoxylon trichiuri (L), Nybelinia sp. (L), Tentacularia coryphaenae (L), unidentified trypanorhynch, ‘Scolex spp.’ (L)
Scomberomorus brasiliensis Collette, Russo & Zavala-Camin: Callitetrarhynchus gracilis (L), Nybelinia sp. (L), Otobothrium cysticum (L), Pseudolacistorhynchus noodti (L), ‘Scolex spp.’ (L)
Scomberomorus cavalla (Cuvier): Callitetrarhynchus gracilis (L), C. speciosus (L), Pterobothrium crassicole (L), Tentacularia coryphaenae (L)
Scomberomorus sierra Jordan & Starks: Adenocephalus pacificus (L), Pseudogrillotia peruviana (L)
Stellifer minor (Tschudi): ‘Scolex spp.’ (L)
Family Serranidae
Acanthistius brasilianus (Cuvier): Grillotia (Christianella) carvajalregorum (L)
Dules auriga Cuvier: Grillotia (Christianella) carvajalregorum (L), unidentified trypanorhynch, unidentified cestode
Epinephelus morio (Valenciennes): unidentified trypanorhynch
Epinephelus sp.: Pterobothrium sp. (L), unidentified trypanorhynch
Hemilutjanus macrophthalmos (Tschudi): Callitetrarhynchus gracilis (L)
Hyporthodus niveatus (Valenciennes): Callitetrarhynchus gracilis (L), Pseudotobothrium dipsacum (L), unidentified trypanorhynch
Mycteroperca bonaci (Poey): Pterobothrium sp. (L), unidentified trypanorhynch
Family Sparidae
Pagrus pagrus (Linnaeus): Callitetrarhynchus gracilis (L), Pterobothrium sp. (L), Otobothrium cysticum (L), unidentified trypanorhynch, ‘Scolex spp.’ (L)
Sparidae gen. sp.: Pterobothrium macrourum (L)
Family Sphyraenidae
Sphyraena guachancho Cuvier: Callitetrarhynchus gracilis (L), Heteronybelinia estigmena (L), Otobothrium cysticum (L)
Family Trichiuridae
Trichiurus lepturus Linnaeus: Callitetrarhynchus gracilis (L), Pterobothrium interruptum (L), unidentified trypanorhynch, ‘Scolex spp.’ (L)
Family Tripterygiidae
Helcogrammoides chilensis (Cancino): unidentified ‘pseudophyllidean’, ‘Scolex spp.’ (L)
Order Pleuronectiformes
Family Achiridae
Trinectes maculatus (Bloch & Schneider): unidentified cestode
Family Paralichthyidae
Citharichthys spilopterus Günther: Pterobothrium crassicole (L), P. kingstoni (L)
Hippoglossina macrops Steindachner: Neobothriocephalus sp., Nybelinia surmenicola (L), Nybelinia sp. (L), ‘Scolex spp.’ (L)
Paralichthys adspersus (Steindachner): Adenocephalus pacificus (L), Lacistorhynchus dollfusi (L), Neobothriocephalus sp., Nybelinia surmenicola (L), Nybelinia sp. (L), unidentified bothriocephalidean, ‘Scolex spp.’ (L)
Paralichthys isosceles Jordan: Callitetrarhynchus gracilis (L), Grillotia (Christianella) carvajalregorum (L), Diphyllobothrium sp. (L), Heteronybelinia nipponica (L), Nybelinia lingualis (L), Nybelinia sp. (L), Otobothrium sp. (L), Pterobothrium crassicole (L), P. heteracanthum (L), Pterobothrium sp. (L), unidentified trypanorhynch, ‘Scolex spp.’ (L), unidentified cestode
Paralichthys microps (Günther): Neobothriocephalus sp., Nybelinia sp. (L), ‘Scolex spp.’ (L)
Paralichthys orbignyanus (Valenciennes): Grillotia sp. (L), ‘Scolex spp.’ (L)
Paralichthys patagonicus Jordan: Anonchocephalus patagonicus, Callitetrarhynchus gracilis (L), Grillotia (Christianella) carvajalregorum (L), Heteronybelinia nipponica (L), Lacistorhynchus tenuis (L), Nybelinia erythraea (L), N. lingualis (L), Nybelinia sp. (L), Pterobothrium crassicole (L), ‘Scolex spp.’ (L)
Paralichthys sp.: Pterobothrium sp. (L)
Xystreurys rasile (Jordan): Anonchocephalus argentinensis, Grillotia (Christianella) carvajalregorum (L), Heteronybelinia nipponica (L), Nybelinia erythraea (L), N. lingualis (L), Nybelinia sp. (L), unidentified trypanorhynch, ‘Scolex spp.’ (L), unidentified cestode
Family Pleuronectidae
Oncopterus darwinii Steindachner: Nybelinia lingualis (L)
Order Salmoniformes
Family Salmonidae
Oncorhynchus kisutch (Walbaum): Diphyllobothrium dendriticum (L), Diphyllobothrium sp. (L), ‘Scolex spp.’ (L)
Oncorhynchus mykiss (Walbaum): Diphyllobothrium dendriticum (L), D. latum (L), Diphyllobothrium sp. (L), unidentified bothriocephalidean, unidentified phyllobothriidean
Oncorhynchus tshawytscha (Walbaum): Hepatoxylon trichiuri (L)
Salmo salar Linnaeus: Diphyllobothrium dendriticum (L)
Salmo trutta Linnaeus: Diphyllobothrium dendriticum (L), D. latum (L), Diphyllobothrium sp. (L), ‘Scolex spp.’ (L)
Salvelinus fontinalis (Mitchill): Diphyllobothrium dendriticum (L), D. latum (L), Diphyllobothrium sp. (L), unidentified bothriocephalidean
Order Scorpaeniformes
Family Dactylopteridae
Dactylopterus volitans (Linnaeus): Nybelinia sp. (L), ‘Scolex spp.’ (L)
Family Normanichthyidae
Normanichthys crockeri Clark: ‘Scolex spp.’ (L)
Family Scorpaenidae
Scorpaena sp.: Pterobothrium crassicole (L)
Family Sebastidae
Helicolenus lengerichi Norman: Bothriocephalus sp., Hepatoxylon sp. (L), unidentified trypanorhynch
Sebastes capensis (Gmelin): Diphyllobothrium sp. (L), Hepatoxylon trichiuri (L), unidentified diphyllidean, ‘Scolex spp.’ (L)
Family Triglidae
Prionotus nudigula Ginsburg: Grillotia (Christianella) carvajalregorum (L), Grillotia sp. (L)
Prionotus punctatus (Bloch): Grillotia (Christianella) carvajalregorum (L), Nybelinia sp. (L)
Prionotus sp.: Pterobothrium sp. (L), unidentified phyllobothriidean
Order Siluriformes
Family Ariidae
Ariopsis seemanni (Günther): Adenocephalus pacificus (L)
Aspistor luniscutis (Valenciennes): Pterobothrium crassicole (L), ‘Scolex spp.’ (L)
Bagre bagre (Linnaeus): unidentified trypanorhynch
Bagre marinus (Mitchill): Pterobothrium crassicole (L)
Galeichthys peruvianus Lütken: Adenocephalus pacificus (L)
Genidens barbus (Lacépède): Callitetrarhynchus gracilis (L), C. speciosus (L), Nomimoscolex arandasregoi, Pterobothrium crassicole (L), unidentified trypanorhynch, ‘Scolex spp.’ (L)
Genidens genidens (Cuvier): Nomimoscolex arandasregoi
Genidens sp.: Nomimoscolex arandasregoi, unidentified trypanorhynch
Family Auchenipteridae
Ageneiosus inermis (Linnaeus): Ageneiella brevifilis, Ageneiella sp., Gibsoniela mandube, G. meursaulti, Luciaella ivanovae, unidentified cestode
Ageneiosus militaris Valenciennes: Ageneiella brevifilis, Gibsoniela meursaulti
Ageneiosus pardalis Lütken: Corallotaenia sp. (?), Goezeella danbrooksi
Ageneiosus sp.: Gibsoniela mandube
Auchenipterus nigripinnis (Boulenger): unidentified proteocephalid
Auchenipterus osteomystax (Miranda Ribeiro): Endorchis auchenipteri
Tocantinsia piresi (Miranda Ribeiro): Frezella vaucheri
Trachelyopterus galeatus (Linnaeus): Cangatiella arandasi, Nupelia tomasi
Trachelyopterus striatulus (Steindachner): Endorchis sp.
Trachelyopterus cf. striatulus: Nupelia tomasi
Family Callichthyidae
Callichthys callichthys (Linnaeus): Margaritaella gracilis
Corydoras atropersonatus Weitzman & Nijssen: unidentified proteocephalid
Corydoras reticulatus Fraser-Brunner: unidentified proteocephalid
Corydoras sychri Weitzman: unidentified proteocephalid
Hoplosternum littorale (Hancock): Valipora campylancristrota (L)
Family Cetopsidae
Cetopsis coecutiens (Lichtenstein): Brooksiella praeputialis, Goezeella siluri, unidentified cestode
Cetopsis othonops (Eigenmann): Brooksiella praeputialis, Goezeella siluri
Family Diplomystimidae
Diplomystes camposensis Arratia: Diphyllobothrium latum (L)
Olivaichthys viedmensis (MacDonagh): Nomimoscolex semenasae
Family Doradidae
Franciscodoras marmoratus (Lütken): Proteocephalus renauldi, Proteocephalus sp.
Megalodoras uranoscopus (Eigenmann & Eigenmann): Proteocephalus kuyukuyu
Oxydoras kneri Bleeker: Proteocephalus hobergi, unidentified proteocephalid
Oxydoras niger (Valenciennes): Proteocephalus hobergi, P. kuyukuyu
Platydoras costatus (Linnaeus): Proteocephalus renaudi (?), P. soniae (?), Proteocephalus sp. (?)
Pterodoras granulosus (Valenciennes): Monticellia belavistensis, Proteocephalus kuyukuyu, Proteocephalus sp.
Pterodoras sp.: Proteocephalus kuyukuyu
Family Heptapteridae
Goeldiella eques (Müller & Troschel): Nupelia sp.
Pimelodella gracilis (Valenciennes): unidentified proteocephalid, unidentified cestode
Pimelodella lateristriga (Lichtenstein): unidentified cestode
Rhamdia quelen (Quoy & Gaimard): Lenhataenia megacephala, Proteocephalus bagri, P. rhamdiae, Travassiella jandia, unidentified proteocephalid
Family Loricariidae
Hypostomus cf. ternetzi (Boulenger): unidentified proteocephalid
Loricariichthys platymetopon Isbrücker & Nijssen: unidentified proteocephalid
Loricariichthys sp.: unidentified cestode
Paraloricaria sp.: Proteocephalus pilarensis
Family Pimelodidae
Brachyplatystoma capapretum Lundberg & Akama: Amazotaenia yvettae, Endorchis piraeeba
Brachyplatystoma filamentosum (Lichtenstein): Amphoteromorphus ninoi, A. piraeeba, Nomimoscolex piraeeba, N. suspectus, unidentified proteocephalid
Brachyplatystoma cf. filamentosum: Amphoteromorphus ovalis, Endorchis piraeeba, Nomimoscolex suspectus
Brachyplatystoma rousseauxii (Castelnau): Amphoteromorphus peniculus, A. piriformis, Nomimoscolex dorad, N. piraeeba, N. suspectus, Nomimoscolex sp., Pterobothrium crassicole (L)
Brachyplatystoma vaillantii (Valenciennes): Amazotaenia yvettae, Amphoteromorphus
ninoi, Chambriella sp., Goezeella siluri, Harriscolex piramutab, Nomimoscolex suspectus, Pterobothrium crassicole (L)
Brachyplatystoma sp.: Amphoteromorphus ovalis, ‘Scolex spp.’ (L), unidentified cestode
Calophysus macropterus (Lichtenstein): Monticellia amazonica, Rudolphiella piracatinga, unidentified cestode
Hemisorubim platyrhynchos (Valenciennes): Chambriella paranaensis, Manaosia bracodemoca, Mariauxiella piscatorum, Spatulifer maringaensis, unidentified cestode
Iheringichthys labrosus (Lütken): unidentified proteocephalid
Luciopimelodus pati (Valenciennes): Monticellia ventrei, Nomimoscolex sp., Proteocephalus fossatus, Rudolphiella lobosa (?), R. szidati, Rudolphiella sp.
Megalonema platanum (Günther): Monticellia santafesina, Rudolphiella lobosa, unidentified proteocephalid
Megalonema platycephalum Eigenmann: Monticellia santafesina
Phractocephalus hemioliopterus (Schneider): Chambriella sp., Ephedrocephalus microcephalus, Proteocephalus hemioliopteri, Proteocephalus sp., Pseudocrepidobothrium eirasi, P. ludovici, Pseudocrepidobothrium sp., Scholzia emarginata, Zygobothrium megacephalum, Monticelliinae gen. sp., unidentified proteocephalid
Pimelodus albicans (Valenciennes): Monticellia magna, Nomimoscolex microacetabula
Pimelodus altissimus Eigenmann & Pearson: Endorchis sp.
Pimelodus argenteus Perugia: Monticellia magna
Pimelodus blochii Valenciennes: Nomimoscolex alovarius, Proteocephalus sp.
Pimelodus cf. blochii: Monticellia magna (?)
Pimelodus maculatus Lacépède: Chambriella agostinhoi, Monticellia magna, Nomimoscolex microacetabula, Nomimoscolex sp., Proteocephalus pimelodi, Proteocephalus sp., Valipora sp. (L), unidentified proteocephalid, unidentified cestode
Pimelodus cf. maculatus: Endorchis sp., Monticellia magna
Pimelodus ornatus Kner: Mariauxiella pimelodi, Nomimoscolex microacetabula, Nomimoscolex sp., Spasskyellina mandi, Spasskyellina sp.
Pimelodus ortmanni Haseman: unidentified cestode
Pimelodus pohli Ribeiro & Lucena: unidentified proteocephalid
Pimelodus sp.: Mariauxiella pimelodi, Pterobothrium crassicole (L), unidentified proteocephalid
Pinirampus pirinampu (Spix & Agassiz): Goezeella siluri, Monticellia ventrei, Monticellia sp., Nomimoscolex admonticellia, Proteocephalus vladimirae, Rudolphiella myoides, R. piranabu, Rudolphiella sp., unidentified proteocephalid, unidentified cestode
Platynematichthys notatus (Jardine): Brayela karuatayi
Pseudoplatystoma corruscans (Agassiz): Choanoscolex abscisus, Harriscolex kaparari (?), H. nathaliae, Megathylacus travassosi, Megathylacus sp., Monticellia sp., Nomimoscolex pertierrae, Nomimoscolex sudobim (?), Nomimoscolex sp., Peltidocotyle rugosa, Spasskyellina spinulifera, unidentified proteocephalid
Pseudoplatystoma fasciatum (Linnaeus) (sensu lato): Chambriella sp., Choanoscolex abscisus, Choanoscolex sp., Euzetiella tetraphylliformis, Harriscolex kaparari, Houssayela sudobim, Megathylacus travassosi, Megathylacus sp., Monticellia sp., Nomimoscolex lopesi, N. sudobim, Nomimoscolex sp., Peltidocotyle rugosa, Pseudocrepidobothrium chanaorum, Regoella brevis, Spasskyellina spinulifera, Spasskyellina sp., Spatulifer rugosa, unidentified proteocephalid, unidentified cestode
Pseudoplatystoma tigrinum (Valenciennes): Choanoscolex sp., Harriscolex kaparari, Nomimoscolex sudobim, Spasskyellina spinulifera, Spatulifer surubim, Spatulifer sp.
Pseudoplatystoma sp.: Proteocephalus platystomi, unidentified cestode
Sorubim lima (Bloch & Schneider): Manaosia bracodemoca, Nupelia portoriquensis, Spasskyellina spinulifera, Spatulifer maringaensis
Sorubimichthys planiceps (Spix & Agassiz): Chambriella sp., Choanoscolex sp., Lenhataenia megacephala, Nomimoscolex lenha, Peltidocotyle lenha, Spasskyellina lenha
Steindachneridion parahybae (Steindachner): unidentified cestode
Zungaro jahu (Ihering): Chambriella agostinhoi, Choanoscolex abscisus, Euzetiella tetraphylliformis, Jauella glandicephalus, Megathylacus jandia, Peltidocotyle lenha, P. rugosa, Peltidocotyle sp., Travassiella jandia, unidentified cestode
Zungaro zungaro (Humboldt): Amphoteromorphus parkamoo, Chambriella agostinhoi, Euzetiella tetraphylliformis, Jauella glandicephalus, Megathylacus jandia, Peltidocotyle lenha, Proteocephalus sophiae, Travassiella jandia
Family Pseudopimelodidae
Pseudopimelodus mangurus (Valenciennes): Peltidocotyle rugosa
Family Trichomycteridae
Trichomycterus punctulatus Valenciennes: Hepatoxylon megacephalum (L)
Unidentified siluriform fish
‘Silurus dorgado’: Monticellia diesingii
‘Silurus megacephalus’: Monticellia macrocotylea
Siluriform fish: Pterobothrium sp. (L)
Order Tetraodontiformes
Family Balistidae
Balistes capriscus Gmelin: Callitetrarhynchus gracilis (L), C. speciosus (L), Callitetrarhynchus sp. (L), Nybelinia sp. (L), unidentified ‘pseudophyllidean’, ‘Scolex spp.’ (L)
Balistes vetula Linnaeus: Callitetrarhynchus gracilis (L), C. speciosus (L), Callitetrarhynchus sp. (L), Otobothrium sp. (L), unidentified trypanorhynch, ‘Scolex spp.’ (L)
Family Molidae
Masturus lanceolatus Liénard: unidentified trypanorhynch
Mola mola (Linnaeus): Anchistrocephalus microcephalus, Molicola sp. (L), unidentified trypanorhynch
Mola ramsayi (Giglioli): Anchistrocephalus microcephalus, Molicola horridus (L), Nybelinia sp. (L)
Family Monacanthidae
Aluterus monoceros (Linnaeus): Callitetrarhynchus speciosus (L), Floriceps saccatus (L)
Stephanolepis hispidus (Linnaeus): Callitetrarhynchus speciosus (L)
Family Tetraodontidae
Lagocephalus laevigatus (Linnaeus): unidentified cestode
Sphoeroides testudineus (Linnaeus): unidentified cestode
Class Chondrichthyes
Subclass Elasmobranchii
Order Carcharhiniformes
Family Carcharhinidae
Carcharhinus brachyurus (Günther): Cathetocephalus australis, Dasyrhynchus pacificus
Carcharhinus leucas (Müller & Henle): Cathetocephalus thatcheri, Poecilancistrium caryophyllum
Carcharhinus limbatus (Müller & Henle): Dasyrhynchus pacificus, unidentified trypanorhynch
Carcharhinus longimanus (Poey): Anthobothrium laciniatum, Disculiceps galapagoensis, Paraorygmatobothrium filiforme, Tentacularia coryphaenae
Carcharhinus obscurus (Lesueur): Tentacularia coryphaenae
Carcharhinus porosus (Ranzani): Disculiceps pileatus
Carcharhinus signatus (Poey): Grillotia (Christianella) carvajalregorum, Heteronybelinia nipponica (L), H. yamagutii (L)
Prionace glauca (Linnaeus): Callitetrarhynchus gracilis (L), Floriceps saccatus, Hepatoxylon trichiuri (L), Molicola horridus, Paraorygmatobothrium angustum, P. prionacis, Platybothrium auriculatum, Prosobothrium armigerum, Tentacularia coryphaenae
Rhizoprionodon lalandii (Müller & Henle): Poecilancistrium caryophyllum
Rhizoprionodon terraenovae (Richardson): Nybelinia fayapaulazariahi, unidentified trypanorhynch
Family Scyliorhinidae
Scyliorhinus besnardi Springer & Sadowsky: Ahamulina catarina
Scyliorhinus haeckelii (Miranda Ribeiro): Dasyrhynchus pacificus
Family Sphyrnidae
Sphyrna lewini (Griffith & Smith): Heteronybelinia nipponica
Sphyrna zygaena (Linnaeus): Callitetrarhynchus speciosus (L), Heteronybelinia nipponica (L), Platybothrium parvum, Platybothrium sp., Thysanocephalum thysanocephalum
Sphyrna sp.: Dasyrhynchus pacificus
Family Triakidae
Galeorhinus galeus (Linnaeus): Anthobothrium galeorhini
Mustelus canis (Mitchill): Callitetrarhynchus gracilis, Dollfusiella vooremi, Nybelinia lingualis (L)
Mustelus fasciatus (Garman): Orygmatobothrium juani
Mustelus mento Cope: Calliobothrium verticillatum, Calliobothrium sp., Dollfusiella musteli, Orygmatobothrium musteli, Orygmatobothrium sp., Paraorygmatobothrium triacis, Phyllobothrium lactuca, Phyllobothrium sp., Scyphophyllidium uruguayense
Mustelus schmitti Springer: Calliobothrium australis, Coronocestus notoguidoi, Dollfusiella vooremi, Gyrocotyle maxima (?), Nybelinia lingualis, Orygmatobothrium schmittii, Symcallio barbarae, S. lunae
Mustelus whitneyi Chirichigno: Orygmatobothrium musteli, Paraorygmatobothrium triacis, Symcallio lintoni
Triakis maculata Kner & Steindachner: Lacistorhynchus tenuis
Order Hexanchiformes
Family Hexanchidae
Heptranchias perlo (Bonnaterre): Grillotia (Christianella) carvajalregorum (L)
Hexanchus griseus (Bonnaterre): Crossobothrium dohrni, C. laciniatum, Grillotia heptanchi, Phyllobothrium sinuosiceps
Notorynchus cepedianus (Péron): Crossobothrium antonioi, C. pequeae, Heteronybelinia perideraeus
Order Lamniformes
Family Alopiidae
Alopias vulpinus (Bonnaterre): Paraorygmatobothrium angustum
Family Cetorhinidae
Cetorhinus maximus (Gunnerus): Reesium paciferum
Family Lamnidae
Carcharodon carcharias (Linnaeus): Tentacularia coryphaenae, unidentified trypanorhynch
Isurus oxyrinchus Rafinesque: Gymnorhynchus isuri, Molicola horridus, Nybelinia lingualis
Order Myliobatiformes
Family Dasyatidae
Dasyatis americana Hildebrand & Schroeder: Acanthobothrium americanum, Anthocephalum gracile, A. kingae, Lecanicephalum peltatum, Polypocephalus medusia, Rhinebothrium corymbum, R. margaritense, Rhodobothrium pulvinatum, Scalithrium magniphallum
Dasyatis dipterura (Jordan & Gilbert): Acanthobothroides peruensis, A. thorsoni
Dasyatis guttata (Bloch & Schneider): Acanthobothrium tasajerasi, A. urotrygoni, Acanthobothroides thorsoni, Rhinebothrium margaritense, Rhodobothrium pulvinatum, Scalithrium magniphallum
Dasyatis longa (Garman): Acanthobothrium campbelli, A. costarricense, A. obuncum
Himantura schmardae (Werner): Acanthobothrium himanturi, A. tasajerasi, Acanthobothroides thorsoni, Anindobothrium anacolum, Parachristianella monomegacantha, Rhinebothrium tetralobatum, Scalithrium magniphallum
Family Gymnuridae
Gymnura afuerae (Hildebrand): Acanthobothrium atahualpai
Gymnura micrura (Bloch & Schneider): Acanthobothrium fogeli
Gymnura sp.: Pterobothrium sp.
Family Myliobatidae
Aetobatus narinari (Euphrasen): Acanthobothrium colombianum, A. monksi, A. tortumDisculiceps sp. (?)
Myliobatis chilensis Philippi: Acanthobothrium batailloni, A. coquimbense, A. holorhini, Acanthobothrium sp., Caulobothrium myliobatidis, Phyllobothrium auricula, Rhodobothrium mesodesmatum
Myliobatis goodei Garman: Aberrapex arrhynchum, Acanthobothrium sp., Caulobothrium ostrowskiae, C. uruguayense, Halysioncum megacanthum, Mecistobothrium oblongum, Parachristianella damiani, Phyllobothrium myliobatidis, Phyllobothrium sp.
Myliobatis peruvianus Garman: Acanthobothrium brevissime, A. gonzalesmugaburoi, Phyllobothrium auricula, Rhodobothrium mesodesmatum
Rhinoptera bonasus (Mitchill): Dioecotaenia campbelli, Rhinoptericola megacantha, Rhodobothrium paucitesticulare, Tylocephalum brooksi, Tylocephalum sp.
Rhinoptera brasiliensis Müller: Rhinoptericola megacantha
Rhinoptera steindachneri Evermann & Jenkins: Serendip deborahae
Family Potamotrygonidae
Paratrygon aiereba (Müller & Henle): Acanthobothrium terezae, Nandocestus guariticus, Potamotrygonocestus fitzgeraldae, P. travassosi, Potamotrygonocestus sp., Rhinebothrium brooksi, R. copianullum, Rhinebothrium sp., Rhinebothroides scorzai, Rhinebothroides sp.
Plesiotrygon iwamae Rosa, Castello & Thorson: Potamotrygonocestus chaoi, P. marajoara
Potamotrygon brachyura (Günther): Rhinebothrium paratrygoni
Potamotrygon constellata (Vaillant): Acanthobothrium amazonense, Potamotrygonocestus amazonensis, P. travassosi, Rhinebothroides freitasi
Potamotrygon falkneri Castex & Maciel: Acanthobothrium regoi, Paroncomegas araya, Potamotrygonocestus amazonensis, P. travassosi, Rhinebothrium paratrygoni
Potamotrygon cf. falkneri: Acanthobothrium peruviense, Nandocestus guariticus, Paroncomegas araya, Potamotrygonocestus fitzgeraldae, Potamotrygonocestus sp., Rhinebothroides freitasi, Rhinebothroides sp.
Potamotrygon henlei (Castelnau): Potamotrygonocestus sp., Rhinebothrium copianullum, Rhinebothroides freitasi, R. glandularis
Potamotrygon histrix (Müller & Henle): Rhinebothrium paratrygoni
Potamotrygon leopoldi Castex & Castello: Potamotrygonocestus fitzgeraldae, Rhinebothrium copianullum, Rhinebothroides freitasi
Potamotrygon magdalenae (Duméril): Acanthobothrium quinonese, Potamotrygonocestus magdalenensis, Rhinebothroides moralarai
Potamotrygon motoro (Müller & Henle): Acanthobothrium peruviense, A. ramiroi, A. regoi, A. terezae, Paroncomegas araya, Potamotrygonocestus amazonensis, P. fitzgeraldae, P. travassosi, Potamotrygonocestus sp., Rhinebothrium copianullum, R. corbatai, R. mistyae, R. paratrygoni, Rhinebothroides campbelli, R. freitasi, R. glandularis, R. mclennanae, R. scorzai, unidentified cestode
Potamotrygon orbignyi (Castelnau): Acanthobothrium regoi, Anindobothrium lisae, Paroncomegas araya, Paroncomegas sp., Potamotrygonocestus amazonensis, P. fitzgeraldae, P. maurae, P. travassosi, Rhinebothrium brooksi, R. copianullum, R. fulbrighti, R. jaimei, R. paratrygoni, Rhinebothroides freitasi, R. glandularis, R. scorzai, unidentified cestode
Potamotrygon schroederi Fernández-Yépez: Potamotrygonocestus sp., Rhinebothrium copianullum, Rhinebothroides freitasi
Potamotrygon scobina Garman: Potamotrygonocestus amazonensis, Rhinebothrium jaimei, Rhinebothroides freitasi, R. glandularis
Potamotrygon signata Garman: Rhinebothroides glandularis
Potamotrygon tatianae Silva & Carvalho: Rhinebothrium copianullum, Rhinebothroides sp.
Potamotrygon yepezi Castex & Castello: Acanthobothrium quinonese, Potamotrygonocestus amazonensis, Rhinebothroides freitasi
Potamotrygon sp.: Paroncomegas araya, Potamotrygonocestus amazonensis, Rhinebothrium copianullum, R. fulbrighti, R. paratrygoni, Rhinebothroides glandularis, R. moralarai, R. scorzai
Family Urotrygonidae
Urobatis jamaicensis (Cuvier): Acanthobothrium cartaginense, Anthocephalum kingae, Scalithrium magniphallum
Urobatis tumbesensis (Chirichigno & McEachran): Acanthobothrium minusculum, Anthocephalum hobergi
Urotrygon venezuelae Schultz: Acanthobothrium urotrygoni, Scalithrium magniphallum
Order Pristiformes
Family Pristidae
Pristis pristis (Linnaeus): Anthobothrium pristis, Pterobothrium fragile
Order Rajiformes
Family Arhynchobatidae
Atlantoraja castelnaui (Miranda Ribeiro): Acanthobothrium marplatense, Dollfusiella acuta, Notomegarhynchus navonae
Atlantoraja platana (Günther): Dollfusiella acuta
Bathyraja brachyurops (Fowler): Guidus argentinense
Bathyraja magellanica (Philippi): Grillotia sp.
Psammobatis bergi Marini: Dollfusiella taminii
Psammobatis rudis Günther: Grillotia (Grillotia) patagonica
Psammobatis scobina (Philippi): Acanthobothrium psammobati, Rhinebothrium scobinae
Sympterygia acuta Garman: Dollfusiella acuta
Sympterygia bonapartii Müller & Henle: Dollfusiella acuta, D. vooremi (?), Grillotia (Grillotia) erinaceus (?), Heteronybelinia mattisi, Nybelinia lingualis, Nybelinia sp., Phyllobothrium sp., Rhinebothrium chilensis
Sympterygia brevicaudata (Cope): Acanthobothrium lusarmientoi, A. psammobati, Acanthobothrium sp.
Sympterygia lima (Poeppig): Halysioncum euzeti, Rhinebothrium chilensis, R. leiblei
Family Rajidae
Dipturus flavirostris (Philippi): Echeneibothrium megalosoma, E. multiloculatum, E. williamsi, Grillotia (Grillotia) dollfusi, Phyllobothrium sp.
Dipturus trachyderma (Krefft & Stehmann): Mixonybelinia beveridgei (L), Paragrillotia sp. (?), Phyllobothrium lactuca (?)
Zearaja chilensis (Guichenot): Acanthobothrium annapinkiense, Echeneibothrium megalosoma, E. multiloculatum, E. williamsi, Grillotia (Grillotia) dollfusi
Family Rhinobatidae
Rhinobatos percellens (Walbaum): unidentified trypanorhynch
Rhinobatos planiceps Garman: Acanthobothrium olseni, A. robustum, Parachristianella monomegacantha, Prochristianella heteracantha, Rhinebothrium rhinobati
Zapteryx brevirostris (Müller & Henle): Acanthobothrium zapterycum, Acanthobothrium sp., Halysioncum pigmentatum, Phyllobothrium sp. (L)
Order Squaliformes
Family Etmopteridae
Etmopterus granulosus (Günther): Gilquinia squali
Family Somniosidae
Somniosus pacificus Bigelow & Schroeder: Hepatoxylon trichiuri (L)
Family Squalidae
Squalus sp.: Grillotia (Christianella) carvajalregorum
Order Squatiniformes
Family Squatinidae
Squatina armata (Philippi): Grillotia sp.
Squatina guggenheim Marini: Grillotia (Christianella) carvajalregorum, Paraberrapex atlanticus
Order Torpediniformes
Family Narcinidae
Discopyge tschudii Heckel: Phyllobothrium discopygi
Narcine brasiliensis (Olfers): Acanthobothrium electricolum, A. lintoni
Unidentified ray order
Unidentified ray: Acanthobothrium dasybati (?), Pterobothrium sp.
Subclass Holocephali
Order Chimaeriformes
Family Callorhinchidae
Callorhinchus callorynchus (Linnaeus): Gyrocotyle maxima, G. rugosa
Results and discussion
The database compiled from the available literature on fish cestodes in South America comprises records of 297 species recognized as valid as well as unidentified ones included in 120 genera and 32 families, associated with 401 cartilaginous and bony fish hosts (Tables
Survey of fish cestodes from South America according to their high taxonomic level classification.
| Order | Family | No. of genera | No. of species | Identified to generic level | No. of sequences* |
|---|---|---|---|---|---|
| Amphilinidea | Amphilinidae | 2 | 2 | 0 | 4 |
| Bothriocephalidea | Bothriocephalidae | 4 | 5 | 2 | 12 |
| Echinophallidae | 2 | 1 | 2 | 4 | |
| Triaenophoridae | 4 | 6 | 1 | 1 | |
| Cathetocephalidea | Cathetocephalidae | 1 | 2 | 0 | 0 |
| Disculicepitidae | 1 | 2 | 1 | 0 | |
| Cyclophyllidea | Gryporhynchidae | 3 | 2 | 1 | 0 |
| Diphyllidea | Echinobothriidae | 3 | 5 | 0 | 12 |
| Diphyllobothriidea | Diphyllobothriidae | 2 | 3 | 1 | 26 |
| Gyrocotylidea | Gyrocotylidae | 1 | 2 | 0 | 0 |
| Lecanicephalidea | Aberrapecidae | 1 | 1 | 0 | 0 |
| Cephalobothriidae | 1 | 1 | 1 | 0 | |
| Lecanicephalidae | 1 | 1 | 0 | 0 | |
| Paraberrapecidae | 1 | 1 | 0 | 0 | |
| Polypocephalidae | 1 | 1 | 0 | 0 | |
| Onchoproteocephalidea | Onchobothriidae | 4 | 45 | 3 | 3 |
| Prosobothriidae | 1 | 1 | 0 | 0 | |
| Proteocephalidae | 38 | 102 | 15 | 164 | |
| Phyllobothriidea | Phyllobothriidae | 7 | 15 | 2 | 7 |
| Rhinebothriidea | Anthocephaliidae | 1 | 3 | 0 | 6 |
| Echeneibothriidae | 2 | 4 | 0 | 0 | |
| Rhinebothriidae | 4 | 24 | 2 | 57 | |
| ‘Tetraphyllidea’ | incertae sedis | 7 | 13 | 1 | 3 |
| Trypanorhyncha | Eutetrarhynchidae | 5 | 9 | 2 | 2 |
| Gilquiniidae | 1 | 1 | 1 | 0 | |
| Gymnorhynchidae | 2 | 2 | 1 | 0 | |
| Lacistorhynchidae | 9 | 16 | 5 | 0 | |
| Otobothriidae | 2 | 1 | 1 | 0 | |
| Pseudotobothriidae | 1 | 1 | 0 | 0 | |
| Pterobothriidae | 1 | 4 | 1 | 0 | |
| Rhinoptericolidae | 1 | 1 | 0 | 0 | |
| Sphyriocephalidae | 2 | 3 | 1 | 0 | |
| Tentaculariidae | 4 | 17 | 3 | 0 | |
| Total | 120 | 297 | 61 | 301 |
| Class | Subclass | Order | No. of genera | No. of species | No. of cestodes reported* |
|---|---|---|---|---|---|
| ACTINOPTERYGII | Neopterygii | Anguilliformes | 2 | 2 | 2 |
| Atheriniformes | 2 | 7 | 4 | ||
| Aulopiformes | 1 | 1 | 0 | ||
| Batrachoidiformes | 2 | 2 | 4 | ||
| Beloniformes | 3 | 3 | 0 | ||
| Characiformes | 18 | 27 | 8 | ||
| Clupeiformes | 8 | 9 | 2 | ||
| Cypriniformes | 3 | 3 | 1 | ||
| Cyprinodontiformes | 2 | 4 | 2 | ||
| Gadiformes | 9 | 14 | 14 | ||
| Gobiesociformes | 2 | 2 | 0 | ||
| Gymnotiformes | 2 | 3 | 3 | ||
| Lampriformes | 1 | 1 | 1 | ||
| Lophiiformes | 1 | 1 | 2 | ||
| Mugiliformes | 1 | 2 | 1 | ||
| Notacanthiformes | 1 | 1 | 1 | ||
| Ophidiiformes | 2 | 5 | 11 | ||
| Osmeriformes | 2 | 4 | 4 | ||
| Osteoglossiformes | 1 | 1 | 2 | ||
| Perciformes | 87 | 125 | 46 | ||
| Pleuronectiformes | 6 | 9 | 14 | ||
| Salmoniformes | 3 | 6 | 3 | ||
| Scorpaeniformes | 6 | 6 | 3 | ||
| Siluriformes | 45 | 68 | 95 | ||
| Tetraodontiformes | 7 | 9 | 5 | ||
| CHONDRICHTHYES | Elasmobranchii | Carcharhiniformes** | 8 | 21 | 44 |
| Hexanchiformes** | 3 | 3 | 8 | ||
| Lamniformes** | 4 | 4 | 6 | ||
| Myliobatiformes*** | 11 | 33 | 86 | ||
| Pristiformes*** | 1 | 1 | 2 | ||
| Rajiformes*** | 8 | 17 | 30 | ||
| Squaliformes** | 3 | 2 | 3 | ||
| Squatiniformes** | 1 | 2 | 2 | ||
| Torpediniformes*** | 2 | 2 | 3 | ||
| Holocephali | Chimaeriformes | 1 | 1 | 2 | |
| TOTAL | 259 | 401 | 414 |
The tapeworm with the widest spectrum of definitive hosts is Rhinebothroides freitasi (Rhinebothriidea) that parasitizes nine species of stingrays of the genus Potamotrygon, even though it exhibits only a mesostenoxenous specificity, i.e. occurrence limited to a single host genus. Conversely, members of five orders, namely Amphilinidea, Cathetocephalidea, Diphyllidea, Lecanicephalidea, ‘Tetraphyllidea’ and most likely Gyrocotylidea (see the checklist records for details), showed only a single fish host (oioxenous specificity). It is also worth noting the usually broad spectrum of intermediate teleost hosts for metacestodes, mainly diphyllobothriideans, ‘tetraphyllideans’ and trypanorhynchs, which is reflected in the higher number of actinopterygian (315) than chondrichthyean (86) hosts. However, the stingray Potamotrygon motoro harbours the highest number of cestodes (17) belonging to the species-rich genera Acanthobothrium Blanchard, 1848, Potamotrygonocestus Brooks & Thorson, 1976, Rhinebothrium Linton, 1890 and Rhinebothroides Mayes, Brooks & Thorson, 1981, in addition to Paroncomegas araya.
A total of 208 species of tapeworms are found across seven major ecoregions of South American coast (one additional species is found in Galapagos), being the highest species richness reported from WTSP (66) and WTSA (60), whereas 209 species are found throughout six major river basins of South America (Fig.
The number of taxonomic studies has been steadily growing since 1940, but only 16 papers were based on an integrated taxonomy approach, using molecular data as an important tool. The number of general parasitological surveys has also increased since the beginning of the last century, whereas ecological studies have launched the first publications only in the mid-sixties, with a peak in the last sixteen years, noticeably higher than the previous period (Fig.
Taxonomic resolution
Among the genera of fish cestodes reported from South America, one half was either identified only at generic level or they were specifically identified in some reports and at generic level in others. The numerous papers published in the last 30 years, mostly those ecological ones (see Fig.
The accurate identification of larval stages of cestodes is usually challenging, because they lack key morphological traits that are present in their adult forms, and studies dealing with their genetic characterization are rare in South America (
One of the main obstacles that hampers our understanding of the diversity of fish cestodes in South America is the deficient knowledge of their life cycles and failure to match the morphologically amorphous or divergent larval forms to their adult stages; to date, no life cycle studies have been undertaken in this continent.
Unlike the poor taxonomic resolution of marine larvae from teleosts, adult forms, typically those infecting freshwater catfishes (Siluriformes) and potamotrygonid stingrays (Potamotrygonidae), have been fairly well-documented (
Elasmobranch and teleost fish hosts
Contrasting the generally poorly-known diversity of fish cestodes in South America, some groups of hosts have been extensively studied compared to others. Among the elasmobranch hosts, the stingrays (Myliobatiformes) have been steadily examined for tapeworms, exhibiting the highest proportion of records (39%), which were mainly reported from marine and freshwater systems (e.g.
According to
Accurate identification of fish hosts
During the development of this checklist, we have faced several examples of problematic identification and controversial taxonomy of hosts, which may compromise the reliability of any parasitological survey and limit our understanding of host specificity, the relationship between parasite and host phylogenies, as well as the establishment of trophic links elucidated by life-cycle studies (
Therefore, we recommend that parasitologists keep a piece of host tissue in a molecular-grade ethanol for sequencing and to work in synergy with fish taxonomists to be as accurate as possible in fish identification, as already advocated by
Conclusions
Acknowledgements
The authors are indebted to Roman Kuchta (České Budějovice) for providing helpful comments on the manuscript and Radmila Řepová (České Budějovice) for her help with bibliographic references. Special thanks are extended to Arlene Jones (St. Albans) for revising the English version of the manuscript. This study was supported by the 'Ciência sem fronteiras' Brazilian program visitant researcher modality (No. 135/2012) (stay of TS in Brazil at the Universidade Federal Rural do Rio de Janeiro) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) grants to JLL (Nos. 474077/2011-0, 304254/2011-8, 402665/2012-0); also supported by the National Science Foundation PBI awards Nos. 0818696 and 0818823, Institute of Parasitology (institutional support RVO 60077344) and Czech Science Foundation (P505/12/G112). PVA was supported by a postgraduate fellowship from CNPq.
References
- Abdallah VD, Luque JL, Alves DR, Paraguassú AR (2002) Aspectos quantitativos das infrapopulações de metazoários parasitos da cavalinha, Scomber japonicus (Osteichthyes: Scombridae) do litoral do Estado do Rio de Janeiro, Brasil. Revista Universidade Rural, Série Ciências da Vida 22: 103–107.
- Ahid SMM, Filgueira KD, Fonseca ZAAS, Soto-Blanco B, Oliveira MF (2009) Ocorrência de parasitismo em Mola mola (Linnaeus, 1758) por metazoários no litoral do Rio Grande do Norte, Brasil. Acta Veterinaria Brasilica 3: 43–47. https://doi.org/10.21708/avb.2009.3.1.1118
- Alarcos AJ, Etchegoin JA (2010) Parasite assemblages of estuarine-dependent marine fishes from Mar Chiquita coastal lagoon (Buenos Aires Province, Argentina). Parasitology Research 107: 1083–1091. https://doi.org/10.1007/s00436-010-1974-z
- Alarcos AJ, Ivanov VA, Sardella NH (2006) Distribution patterns and interactions of cestodes in the spiral intestine of the narrownose smooth-hound shark, Mustelus schmitti Springer, 1939 (Chondrichthyes, Carcharhiniformes). Acta Parasitologica 51: 100–106. https://doi.org/10.2478/s11686-006-0015-7
- Alarcos AJ, Pereira AN, Taborda NL, Luque JL, Timi JT (2016) Parasitological evidence of stocks of Paralichthys isosceles (Pleuronectiformes: Paralichthyidae) at small and large geographical scales in South American Atlantic coasts. Fisheries Research 173: 221–228. https://doi.org/10.1016/j.fishres.2015.07.018
- Alarcos AJ, Timi JT (2012) Parasite communities in three sympatric flounder species (Pleuronectiformes: Paralichthyidae). Parasitology Research 110: 2155–2166. https://doi.org/10.1007/s00436-011-2741-5
- Alarcos AJ, Timi JT (2013) Stocks and seasonal migrations of the flounder Xystreurys rasile as indicated by its parasites. Journal of Fish Biology 83: 531–541. https://doi.org/10.1111/jfb.12190
- Albuquerque MC, Santos MD, Monteiro CM, Martins AN, Ederli NB, Brasil-Sato MC (2008) Helmintos endoparasitos de Pimelodus maculatus Lacépède, 1803 (Siluriformes, Pimelodidae) de duas localidades (Lagoa e Calha) do Rio Guandu, Estado do Rio de Janeiro, Brasil. Revista Brasileira de Parasitologia Veterinária 17: 113–119.
- Alcântara NM, Tavares-Dias M (2015) Structure of the parasites communities in two Erythrinidae fish from Amazon river system (Brazil). Revista Brasileira de Parasitologia Veterinária 24: 183–190. https://doi.org/10.1590/S1984-29612015039
- Alves DR, Luque JL (1999) Aspectos quantitativos das infrapopulações de metazoários parasitos de indivíduos jovens da corvina, Micropogonias furnieri (Osteichthyes: Sciaenidae) do litoral do Estado do Rio de Janeiro, Brasil. Contribuições Avulsas sobre a História Natural do Brasil, Série Zoologia 10: 1–4.
- Alves DR, Luque JL (2000) Metazoários parasitos de Micropogonias furnieri (Osteichthyes: Sciaenidae) do litoral do Estado do Rio de Janeiro, Brasil. Parasitología al Día 24: 40–45. https://doi.org/10.4067/S0716-07202000000100006
- Alves DR, Luque JL (2001a) Community ecology of the metazoan parasites of white croaker, Micropogonias furnieri (Osteichthyes: Sciaenidae), from the coastal zone of the State of Rio de Janeiro, Brazil. Memórias do Instituto Oswaldo Cruz 96: 145–153. https://doi.org/10.1590/S0074-02762001000200002
- Alves DR, Luque JL (2001b) Aspectos quantitativos das infrapopulações de metazoários parasitos de Micropogonias furnieri (Osteichthyes: Sciaenidae) do litoral do Estado do Rio de Janeiro, Brasil. Parasitología al Día 25: 30–35. https://doi.org/10.4067/S0716-07202001000100006
- Alves DR, Luque JL (2006) Ecologia das comunidades de metazoários parasitos de cinco espécies de escombrídeos (Perciformes: Scombridae) do litoral do estado do Rio de Janeiro, Brasil. Revista Brasileira de Parasitologia Veterinária 15: 167–181.
- Alves DR, Luque JL, Abdallah VD (2003) Metazoan parasites of chub mackerel, Scomber japonicus Houttuyn (Osteichthyes: Scombridae), from the coastal zone of the State of Rio de Janeiro, Brazil. Revista Brasileira de Parasitologia Veterinária 12: 164–170.
- Alves DR, Luque JL, Paraguassú AR (2002a) Metazoários parasitos do "congro-rosa" Genypterus brasiliensis Regan, 1903 (Osteichthyes, Ophidiidae) do litoral do Estado do Rio de Janeiro, Brasil. Revista Brasileira de Zoociências 4: 133–142.
- Alves DR, Luque JL, Paraguassú AR (2002b) Community ecology of the metazoan parasites of pink cusk-eel, Genypterus brasiliensis (Osteichthyes: Ophidiidae), from the coastal zone of the State of Rio de Janeiro, Brazil. Memórias do Instituto Oswaldo Cruz 97: 683–689. https://doi.org/10.1590/S0074-02762002000500018
- Alves DR, Luque JL, Paraguassú AR, Jorge DS, Viñas RA (2002c) Ecologia da comunidade de metazoários parasitos da abrótea, Urophycis mystaceus Ribeiro, 1903 (Osteichthyes, Phycidae), do litoral do Estado do Rio de Janeiro. Revista Brasileira de Zoociências 4: 19–30.
- Alves DR, Paraguassú AR, Luque JL (2004) Metazoários parasitos da abrótea, Urophycis brasiliensis (Kaup, 1858),(Osteichthyes: Phycidae) do litoral do Estado do Rio de Janeiro, Brasil. Revista Brasileira de Parasitologia Veterinária 13: 49–55.
- Alves DR, Paraguassú AR, Luque JL (2005) Community ecology of the metazoan parasites of the grey triggerfish, Balistes capriscus Gmelin, 1789 and queen triggerfish B. vetula Linnaeus, 1758 (Osteichthyes: Balistidae) from the State of Rio de Janeiro, Brazil. Revista Brasileira de Parasitologia Veterinária 14: 71–77.
- Alves PV, de Chambrier A, Scholz T, Luque JL (2015) A new genus and species of proteocephalidean tapeworm (Cestoda), first parasite found in the driftwood catfish Tocantinsia piresi (Siluriformes: Auchenipteridae) from Brazil. Folia Parasitologica 62: 006. https://doi.org/10.14411/fp.2015.006
- Amato JFR, São Clemente SC, Oliveira GA (1990) Tentacularia coryphaenae Bosc, 1801 (Eucestoda: Trypanorhyncha) in the inspection and technology of the skipjack tuna, Katsuwonus pelamis (L.) (Pisces: Scombridae). Atlantica 12: 73–77.
- Andrade SMS, Malta JCO, Ferraz E (2001) Parasitological fauna of matrinchã fries Brycon cephalus (Günther, 1869) collected in Negro and Solimões Rivers, in Central Amazônia. Acta Amazonica 31: 263–263. https://doi.org/10.1590/1809.4392200-1312273
- Araújo CSO, Tavares-Dias M, Gomes ALS, Andrade SMS, Lemos JRG, Oliveira AT, Cruz WR, Affonso EG (2009) Infecções parasitárias e parâmetros sanguíneos em Arapaima gigas Schinz, 1822 (Arapaimidae) cultivados no estado do Amazonas, Brasil. In: Tavares-Dias M (Ed.) Manejo e sanidade de peixes em cultivo. Embrapa Amapá, Macapá, 389–424.
- Araújo ME, Silva-Falcão EC, Falcão PD, Marques VM, Joca IR (2010) Stranding of Masturus lanceolatus (Actinopterygii: Molidae) in the estuary of the Una River, Pernambuco, Brazil: natural and anthropogenic causes. Marine Biodiversity Records 3: e69. https://doi.org/10.1017/S1755267210000588
- Armas G (1983) Estudio de los endoparásitos (cestodos y nematodos) de Paralabrax humeralis Cuvier & Valenciennes, 1828, pez serránido de consumo humano em el Perú. Revista Ciencias Veterinárias Colegio Médico Eduardo Aragua (Venezuela) 12: 56–66.
- Arredondo NJ, de Chambrier A, Gil de Pertierra AA (2013) A new genus and species of the Monticelliinae (Eucestoda: Proteocephalidea), a parasite of Pseudoplatystoma fasciatum (Pisces: Siluriformes) from the Paraná River basin (Argentina), with comments on microtriches of proteocephalideans. Folia Parasitologica 60: 248–256. https://doi.org/10.14411/fp.2013.028
- Arredondo NJ, Gil de Pertierra AA (2008) The taxonomic status of Spatulifer cf. maringaensis Pavanelli & Rego, 1989 (Eucestoda: Proteocephalidea) from Sorubim lima (Bloch & Schneider) (Pisces: Siluriformes), and the use of the microthrix pattern in the discrimination of Spatulifer spp. Systematic Parasitology 70: 223–236. https://doi.org/10.1007/s11230-008-9142-x
- Arredondo NJ, Gil de Pertierra AA (2010) Monticellia santafesina n. sp. (Cestoda: Proteocephalidea), a parasite of Megalonema platanum (Günther) (Siluriformes: Pimelodidae) in the Paraná River basin, Argentina. Systematic Parasitology 76: 103–110. https://doi.org/10.1007/s11230-010-9238-y
- Arredondo NJ, Gil de Pertierra AA (2012) Margaritaella gracilis gen. n. et sp. n. (Eucestoda: Proteocephalidea), a parasite of Callichthys callichthys (Pisces: Siluriformes) from the Paraná River basin, Argentina. Folia Parasitologica 59: 99–106. https://doi.org/10.14411/fp.2012.015
- Arredondo NJ, Gil de Pertierra AA, de Chambrier A (2014) A new species of Pseudocrepidobothrium (Cestoda: Proteocephalidea) from Pseudoplatystoma reticulatum (Pisces: Siluriformes) in the Paraná River basin (Argentina). Folia Parasitologica 61: 462–472. https://doi.org/10.14411/fp.2014.051
- Azevedo GB, Madi RR, Ueta MT (2007) Metazoários parasitas de Astyanax altiparanae (Pisces: Characidae) na Fazenda Rio das Pedras, Campinas, SP, Brasil. Bioikos 21: 89–96.
- Azevedo RK, Abdallah VD, Luque JL (2010) Acanthocephala, Annelida, Arthropoda, Myxozoa, Nematoda and Platyhelminthes parasites of fishes from the Guandu river, Rio de Janeiro, Brazil. Check List 6: 659–667.
- Azevedo RK, Abdallah VD, Luque JL (2011) Biodiversity of fish parasites from Guandu River, southeastern Brazil: an ecological approach. Neotropical Helminthology 5: 185–199.
- Bachmann F, Greinert JA, Bertelli PW, Silva Filho HH, Lara NOT, Ghiraldelli L, Martins ML (2007) Parasitofauna de Pimelodus maculatus (Osteichthyes: Pimelodidae) do rio Itajaí-Açu em Blumenau, Estado de Santa Catarina, Brasil. Acta Scientiarum: Biological Sciences 29: 109–114. https://doi.org/10.4025/actascibiolsci.v29i1.159
- Baer JG (1969) Diphyllobothrium pacificum, a tapeworm from sea lions endemic in man along the coastal area of Peru. Journal of the Fisheries Board of Canada 26: 717–723. https://doi.org/10.1139/f69-071
- Bahamonde N, Lopez MT (1962) Proboscidosaccus mesodesmatis n. sp. parásito de Mesodesma donacium Lamarck. Investigaciones Zoológicas Chilenas 8: 43–56.
- Balboa L, George-Nascimento M (1998) Variaciones ontogenéticas y entre años en las infracomunidades de parásitos metazoos de dos especies de peces marinos de Chile. Revista Chilena de Historia Natural 71: 27–37.
- Bandoni SM, Brooks DR (1987) Revision and phylogenetic analysis of the Amphilinidea Poche, 1922 (Platyhelminthes: Cercomeria: Cercomeromorpha). Canadian Journal of Zoology 65: 1110–1128. https://doi.org/10.1139/z87-175
- Békési L, Feitosa VA, Cabral FAB (1992) Metacestodosis caused by plerocercoids of Proteocephalidea (Cestoda) in fish fry cultured in large scale in the Brazilian Northeast. Parasitologia Hungarica 25: 9–13.
- Bellay S, Ueda BH, Takemoto RM, Lizama MAP, Pavanelli GC (2012) Fauna parasitária de Geophagus brasiliensis (Perciformes: Cichlidae) em reservatórios do estado do Paraná, Brasil. Revista Brasileira de Biociências 10: 74–78.
- Bernot JP, Caira JN, Pickering M (2015) The dismantling of Calliobothrium (Cestoda: Tetraphyllidea) with erection of Symcallio n. gen. and description of two new species. Journal of Parasitology 101: 167–181. https://doi.org/10.1645/14-571.1
- Bertullo V (1965) Infestación masiva de músculos de corvina (Micropogom opercularis L.) por Tretrarhynchus fragilis (Diesing). Revista del Instituto de Investigaciones Pesqueras 1: 344–348.
- Bethular A, Mancini M, Salinas V, Echaniz S, Vignatti A, Larriestra A (2014) Alimentación, condición corporal y principales parásitos del pejerrey (Odontotesthes bonaeriensis) del embalse San Roque (Argentina). Biología Acuática 30: 59–68.
- Beveridge I, Campbell RA (1993) A revision of Dasyrhynchus Pintner (Cestoda: Trypanorhyncha), parasitic in elasmobranch and teleost fishes. Systematic Parasitology 24: 129–157. https://doi.org/10.1007/BF00009597
- Beveridge I, Campbell RA (2007) Revision of the Grillotia erinaceus (van Beneden, 1858) species complex (Cestoda: Trypanorhyncha), with the description of G. brayi n. sp. Systematic Parasitology 68: 1–31. https://doi.org/10.1007/s11230-006-9082-2
- Beveridge I, Justine J (2007a) Paragrillotia apecteta n. sp. and redescription of P. spratti (Campbell & Beveridge, 1993) n. comb. (Cestoda, Trypanorhyncha) from hexanchid and carcharhinid sharks off New Caledonia. Zoosystema 29: 381–391.
- Beveridge I, Justine J (2007b) Redescriptions of four species of Otobothrium Linton, 1890 (Cestoda: Trypanorhyncha), including new records from Australia, New Caledonia and Malaysia, with the description of O. parvum n. sp. Zootaxa 1587: 1–25.
- Beveridge I, Sakanari JA (1987) Lacistorhynchus dollfusi sp. nov. (Cestoda: Trypanorhyncha) in elasmobranch fishes from Australian and North American coastal waters. Transactions of the Royal Society of South Australia 111: 147–154.
- Bicudo AJ, Tavares LE, Luque JL (2004) Metazoários parasitos da cabrinha Prionotus punctatus (Bloch, 1793) (Osteichthyes: Triglidae) do litoral do Estado do Rio de Janeiro, Brasil. Revista Brasileira de Parasitologia Veterinária 14: 27–33.
- Bittencourt LS, Pinheiro DA, Cárdenas MQ, Fernandes BM, Tavares-Dias M (2014) Parasites of native Cichlidae populations and invasive Oreochromis niloticus (Linnaeus, 1758) in tributary of Amazonas River (Brazil). Revista Brasileira de Parasitologia Veterinária 23: 44–54. https://doi.org/10.1590/S1984-29612014006
- Boni TA, Padial AA, Prioli SMAP, Lucio LC, Maniglia TC, Bignotto TS, Panarari-Antunes RS, Prioli RA, Prioli AJ (2011) Molecular differentiation of species of the genus Zungaro (Siluriformes, Pimelodidae) from the Amazon and Paraná-Paraguay River basins in Brazil. Genetics and Molecular Research 10: 2795– 2805. https://doi.org/10.4238/2011.November.10.2
- Brabec J, Waeschenbach A, Scholz T, Littlewood DTJ, Kuchta R (2015) Molecular phylogeny of the Bothriocephalidea (Cestoda): molecular data challenge morphological classification. International Journal for Parasitology 45: 761–771. https:// doi 10.1016/j.ijpara.2015.05.006
- Braicovich PE, Luque JL, Timi JT (2012) Geographical patterns of parasite infracommunities in the rough scad, Trachurus lathami Nichols, in the southwestern Atlantic Ocean. Journal of Parasitology 98: 768–777. https://doi.org/10.1645/GE-2950.1
- Braicovich PE, Timi JT (2008) Parasites as biological tags for stock discrimination of the Brazilian flathead Percophis brasiliensis in the south‐west Atlantic. Journal of Fish Biology 73: 557–571. https://doi.org/10.1111/j.1095-8649.2008.01948.x
- Braicovich PE, Timi JT (2010) Seasonal stability in parasite assemblages of the Brazilian flathead, Percophis brasiliensis (Perciformes: Percophidae): predictable tools for stock identification. Folia Parasitologica 57: 206–212. https://doi.org/10.14411/fp.2010.027
- Braicovich PE, Timi JT (2015) Homogeneity of parasite assemblages of Dules auriga (Serranidae) in hydrographically heterogeneous sites. Journal of Fish Biology 86: 1363–1376. https://doi.org/10.1111/jfb.12648
- Brasil-Sato MC (2003) Parasitos de peixes da bacia do São Francisco. In: Godinho HP, Godinho AL (Eds) Águas, peixes e pescadores do São Francisco das Minas Gerais. PUC Minas, Belo Horizonte, 149–165.
- Bravo S, Almonacid C, Oyarzo C, Silva MT (2007) The parasite fauna of Galaxias maculatus in the estuary of Maullin River, Chile. Bulletin of the European Association of Fish Pathologists 27: 10–17.
- Bray RA (1994) Order Nippotaeniidea. In: Khalil LF, Jones A, Bray RA (Eds) Keys to the Cestode Parasites of Vertebrates. CAB International, Wallingford, 253–255.
- Bray RA, Jones A, Andersen KI (1994) Order Pseudophyllidea Carus, 1863. In: Khalil LF, Jones A, Bray RA (Eds) Keys to the cestode parasites of vertebrates. CAB International, Wallingford, 205–247.
- Brickle P, MacKenzie K (2007) Parasites as biological tags for Eleginops maclovinus (Teleostei: Eleginopidae) around the Falkland Islands. Journal of Helminthology 81: 147–153. https://doi.org/10.1017/S0022149X07750514
- Brickle P, MacKenzie K, Pike A (2006) Variations in the parasite fauna of the Patagonian toothfish (Dissostichus eleginoides Smitt, 1898), with length, season, and depth of habitat around the Falkland Islands. Journal of Parasitology 92: 282–291. https://doi.org/10.1645/GE-539R.1
- Brooks DR (1977) Six new species of tetraphyllidean cestodes, including a new genus, from a marine stingray Himantura schmardae (Werner, 1904) from Colombia. Proceedings of the Helminthological Society of Washington 44: 51–59.
- Brooks DR, Amato JFR (1992) Cestode parasites in Potamotrygon motoro (Natterer) (Chondrichthyes: Potamotrygonidae) from southwestern Brazil, including Rhinebothroides mclennanae n. sp. (Tetraphyllidea: Phyllobothriidae), and a revised host-parasite checklist for helminths inhabiting Neotropical freshwater stingrays. Journal of Parasitology 78: 393–398. https://doi.org/10.2307/3283633
- Brooks DR, Barriga R (1995) Serendip deborahae n. gen. and n. sp. (Eucestoda: Tetraphyllidea: Serendipidae n. fam.) in Rhinoptera steindachneri Evermann and Jenkins, 1891 (Chondrichthyes: Myliobatiformes: Myliobatidae) from Southeastern Ecuador. Journal of Parasitology 81: 80–84.
- Brooks DR, Deardorff TL (1980) Three proteocephalid cestodes from Colombian siluriform fishes, including Nomimoscolex alovarius sp. n. (Monticelliidae: Zygobothriinae). Proceedings of the Biological Society of Washington 47: 15–21.
- Brooks DR, Marques F, Perroni C, Sidagis C (1999) Scyphophyllidium uruguayense n. sp. (Eucestoda: Tetraphyllidea) in Mustelus mento (Cope, 1877) (Chondrichthyes: Carcharhiniformes: Triakidae) from La Paloma, Uruguay. Journal of Parasitology 85: 490–494. https://doi.org/10.2307/3285784
- Brooks DR, Mayes MA (1978) Acanthobothrium electricolum sp. n. and A. lintoni Goldstein, Henson, and Schlicht 1969 (Cestoda: Tetraphyllidea) from Narcine brasiliensis (Olfers) (Chondrichthyes: Torpedinidae) in Colombia. Journal of Parasitology: 617–619. https://doi.org/10.2307/3279945
- Brooks DR, Mayers MA (1980) Cestodes in four species of euryhaline stingrays from Colombia. Proceedings of the Helminthological Society of Washington 47: 22–29.
- Brooks DR, Mayes MA, Thorson TB (1981a) Cestode parasites in Myliobatis goodei Garman (Myliobatiformes: Myliobatidae) from Rio de la Plata, Uruguay, with a summary of cestodes collected from South American elasmobranchs during 1975-1979. Proceedings of the Biological Society of Washington 93: 1239–1252.
- Brooks DR, Mayes MA, Thorson TB (1981b) Systematic review of cestodes infecting freshwater stingrays (Chondrichthyes: Potamotrygonidae) including four new species from Venezuela. Proceedings of the Biological Society of Washington 48: 43–64.
- Brooks DR, Rasmussen G (1984) Proteocephalidean cestodes from Venezuelan siluriform fishes, with a revised classification of the Monticelliidae. Proceedings of the Biological Society of Washington 97: 748–760.
- Brooks DR, Thorson TB (1976) Two tetraphyllidean cestodes from the freshwater stingray Potamotrygon magdalenae Dumeril 1852 (Chondrichthyes: Potamotrygonidae) from Colombia. Journal of Parasitology 62: 943–947. https://doi.org/10.230-7/3279188
- Brown J, Brickle P, Scott BE (2013) The parasite fauna of the Patagonian toothfish Dissostichus eleginoides off the Falkland Islands. Journal of Helminthology 87: 501–509. https://doi.org/10.1017/S0022149X12000636
- Bueno GBF, Aguiar JCC, Santos SMC (2014) Community structure of metazoan parasites of Trichiurus lepturus (Perciformes, Trichiuridae) from Ubatuba, Southwestern Atlantic Ocean, Brazil. Acta Scientiarum: Biological Sciences 36: 357–364. https://doi.org/10.4025/actascibiolsci.v36i3.21908
- Caira JN, Jensen K (2014) A digest of elasmobranch tapeworms. Journal of Parasitology 100: 373–391. https://doi.org/10.1645/14-516.1
- Caira JN, Jensen K (2015) Insights on the identities of sharks of the Rhizoprionodon acutus (Elasmobranchii: Carcharhiniformes) species complex based on three new species of Phoreiobothrium (Cestoda: Onchoproteocephalidea). Zootaxa 4059: 335–350. https://doi.org/10.11646/zootaxa.4059.2.5
- Caira JN, Jensen K, Barbeau E (Eds) (2012) Global Cestode Database. World Wide Web electronic publication. http://www.tapewormdb.uconn.edu/ [accessed 1.V.2016]
- Caira JN, Jensen K, Waeschenbach A, Olson PD, Littlewood DTJ (2014) Orders out of chaos – molecular phylogenetics reveals the complexity of shark and stingray tapeworm relationships. International Journal for Parasitology 44: 55–73. https://doi.org/10.1016/j.ijpara.2013.10.004
- Caira JN, Littlewood DTJ (2013) Worms, Platyhelminthes. In: Levin SA (Ed.) Encyclopedia of Biodiversity. Academic Press, San Diego, 437–469. https://doi.org/10.1016/B978-0-12-384719-5.00166-0
- Caira JN, Marques FPL, Jensen K, Kuchta R, Ivanov VA (2013) Phylogenetic analysis and reconfiguration of genera in the cestode order Diphyllidea. International Journal for Parasitology 43: 621–639. https://doi.org/10.1016/j.ijpara.2013.03.001
- Caira JN, Orringer DJ (1995) Additional information on the morphology of Potamotrygonocestus magdalenensis (Tetraphyllidea: Onchobothriidae) from the freshwater stingray Potamotrygon magdalenae in Colombia. Journal of the Helminthological Society of Washington 62: 22–26.
- Campbell RA, Beveridge I (1996) Revision of the family Pterobothriidae Pintner, 1931 (Cestoda: Trypanorhyncha). Invertebrate Systematics 10: 617–662. https://doi.org/10.1071/IT9960617
- Campbell RA, Beveridge I (2002) The genus Acanthobothrium (Cestoda: Tetraphyllidea: Onchobothriidae) parasitic in Australian elasmobranch fishes. Invertebrate Systematics 16: 237–344. https://doi.org/10.1071/IT01004
- Campbell RA, Carvajal JG (1979) Synonymy of the phyllobothriid genera Rhodobothrium Linton, 1889, Inermiphyllidium Riser, 1955, and Sphaerobothrium Euzet, 1959 (Cestoda: Tetraphyllidea). Proceedings of the Helminthological Society of Washington 46: 88–97.
- Campbell RA, Carvajal JG (1980) Echinobothrium euzeti, a new cestode from the spiral valve of a Chilean elasmobranch. Proceedings of the Helminthological Society of Washington 47: 165–167.
- Campbell RA, Carvajal JG (1987) Phyllobothrium discopygi n. sp. (Cestoda: Tetraphyllidea) from Chile, with a critical comparison of the affinities of P. auricula van Beneden, 1858 and P. foliatum Linton, 1890. Systematic Parasitology 10: 159–164. https://doi.org/10.1007/BF00009621
- Campbell RA, Marques FPL, Ivanov VA (1999) Paroncomegas araya (Woodland, 1934) n. gen. et comb. (Cestoda: Trypanorhyncha: Eutetrarhynchidae) from the freshwater stingray Potamotrygon motoro in South America. Journal of Parasitology 85: 313–320. https://doi.org/10.2307/3285640
- Campos CM, Fonseca VE, Takemoto RM, Moraes FR (2008) Fauna parasitária de cachara Pseudoplatystoma fasciatum (Siluriforme: Pimelodidae) do rio Aquidauana, Pantanal Sul Mato-grossense, Brasil. Acta Scientiarum: Biological Sciences: 91–96. https://doi.org/10.4025/actascibiolsci.v30i1.1469
- Campos CM, Fonseca VE, Takemoto RM, Moraes FR (2009a) Ecology of the parasitic endohelminth community of Pseudoplatystoma fasciatum (Linnaeus, 1776) (Siluriformes: Pimelodidae) from the Aquidauana River, Pantanal, State of Mato Grosso do Sul, Brazil. Brazilian Journal of Biology 69: 93–99. https://doi.org/10.1590/S1519-69842009000100011
- Campos CM, Moraes JRE, Rondini J, Moraes FR (2009b) Histopatologia do intestino de Pseudoplatystoma fasciatum (Osteichthyes, Pimelodidae) parasitados com cestodas proteocefalídeos e nematodas. Boletim do Instituto de Pesca: 153–158.
- Carballo MC, Cremonte F, Navone GT, Timi JT (2012) Similarity in parasite community structure may be used to trace latitudinal migrations of Odontesthes smitti along Argentinean coasts. Journal of Fish Biology 80: 15–28. https://doi.org/10.1111/j.1095-8649.2011.03125.x
- Carballo MC, Navone GT, Cremonte F (2011) Parasites of the silversides Odontesthes smitti and Odontesthes nigricans (Pisces: Atherinopsidae) from Argentinean Patagonia. Comparative Parasitology 78: 95–103. https://doi.org/10.1654/4445.1
- Cardoso TP, Salgado RL, Andrade PF, São Clemente SC, Lima FC (2006) Nematóides da família Anisakidae e cestóides da ordem Trypanorhyncha em peixes teleósteos comercializados no estado do Rio de Janeiro. Revista Brasileira de Ciência Veterinária 13: 98–101.
- Carfora M, de Chambrier A, Vaucher C (2003) Le genre Amphoteromorphus (Cestoda: Proteocephalidea), parasite de poissons-chats d’Amérique tropicale: étude morphologique et approche biosystématique par électrophorèse des protéines. Revue Suisse de Zoologie 110: 381–409. https://doi.org/10.5962/bhl.part.80190
- Carvajal JG (1971) Grillotia dollfusi sp. n. (Cestoda: Trypanorhyncha) from the skate, Raja chilensis, from Chile, and a note on G. heptanchi. Journal of Parasitology 57: 1269–1271. https://doi.org/10.2307/3277978
- Carvajal JG (1974) Records of cestodes from Chilean sharks. Journal of Parasitology 60: 29–34. https://doi.org/10.2307/3278674
- Carvajal JG (1977) Description of the adult and larva of Caulobothrium myliobatidis sp. n. (Cestoda: Tetraphyllidea) from Chile. Journal of Parasitology 63: 99–103. https://doi.org/10.2307/3280111
- Carvajal JG, Barros C, Whittaker FH (1985) Scanning electron microscopy of scolices of some tetraphyllidean cestodes in Chilean skates. Microscopía Electrónica y Biología Celular 9: 23–33.
- Carvajal JG, Barros C, Whittaker FH (1987) Scanning electron microscopy of the scolex of the plerocercus Callitetrarhynchus gracilis (Rudolphi, 1819) (Cestoda: Trypanorhyncha). Journal of Parasitology 73: 1265–1267. https://doi.org/10.2307/3282323
- Carvajal JG, Campbell RA (1979) Identificación de las larvas de cestodos tetrarínquidos presentes en las merluzas y congrios de Puerto Montt, Chile. Boletín Chileno de Parasitología 34: 65–67.
- Carvajal JG, Cattan PE (1978) Occurrence of the plerocercus of Grillotia dollfusi Carvajal 1971 (Cestoda: Trypanorhyncha) in the Chilean hake Merluccius gayi (Guichenot 1848). Journal of Parasitology 64: 695–695. https://doi.org/10.2307/3279962
- Carvajal JG, Cattan PE, Castillo C, Schatte P (1979) Larval anisakids and other helminths in the hake, Merluccius gayi (Guichenot) from Chile. Journal of Fish Biology 15: 671–677. https://doi.org/10.1111/j.1095-8649.1979.tb03676.x
- Carvajal JG, Dailey MD (1975) Three new species of Echeneibothrium (Cestoda: Tetraphyllidea) from the skate, Raja chilensis Guichenot, 1848, with comments on mode of attachment and host specificity. Journal of Parasitology 61: 89–94. https://doi.org/10.2307/3279115
- Carvajal JG, Goldstein RJ (1969) Acanthobothrium psammobati sp. n. (Cestoda: Tetraphyllidea: Onchobothriidae) from the skate, Psammobatis scobina (Chondrichthyes: Rajidae) from Chile. Zoologischer Anzeiger 182: 432–435.
- Carvajal JG, Goldstein RJ (1971) Acanthobothrium annapinkiensis n. sp. (Cestoda: Tetraphyllidea: Onchobothriidae) from the skate, Raja chilensis (Chondrichthyes: Rajidae) from Chile. Zoologischer Anzeiger 186: 158–162.
- Carvajal JG, Jeges JG (1980) Cestode parasites of Myliobatis chilensis with a description of a new species of Acanthobothrium. Anales del Centro de Ciencias del Mar y Limnologia 7: 51–56.
- Carvajal JG, Rego AA (1983) Progrillotia dollfusi sp. n. (Cestoda: Trypanorhyncha) parasito de pescada do litoral brasileiro. Memórias do Instituto Oswaldo Cruz 78: 231–234. https://doi.org/10.1590/S0074-02761983000200012
- Carvajal JG, Rego AA (1985) Critical studies on the genus Callitetrarhynchus (Cestoda: Trypanorhyncha) with recognition of Rhynchobothrium speciosum Linton, 1897 as a valid species of the genus Callitetrarhynchus. Systematic Parasitology 7: 161–167. https://doi.org/10.1007/BF00011449
- Carvajal JG, Ruíz G (1987) Fijación de dos especies de Acanthobothrium (Cestoda: Tetraphyllidea) a la válvula espiral de rayas del género Sympterygia: estudio de la interfase parásito-huésped. Parasitología al Día 11: 49–55.
- Carvalho AR, Luque JL (2011) Seasonal variation in metazoan parasites of Trichiurus lepturus (Perciformes: Trichiuridae) of Rio de Janeiro, Brazil. Brazilian Journal of Biology 71: 771–782. https://doi.org/10.1590/S1519-69842011000400024
- Carvalho-Costa LF, Piorski NM, Willis SC, Galetti PM, Ortí G (2011) Molecular systematics of the neotropical shovelnose catfish genus Pseudoplatystoma Bleeker 1862 based on nuclear and mtDNA markers. Molecular Phylogenetics and Evolution 59: 177–194. https://doi.org/10.1016/j.ympev.2011.02.005
- Cattan PE (1977) El congrio dorado Genypterus blacodes, Schneider, nuevo registro en Chile, para Hepatoxylon trichiuri Holten, 1802 (Cestoda, Trypanorhyncha). Boletín Chileno de Parasitología 32: 92–93.
- Cattan PE, Carvajal JG, Torres D, Yáñez JL (1979) Primera comunicación sobre cestodos Trypanorhyncha en peces del archipiélago de Juan Fernández. Boletín Chileno de Parasitología 34: 44–46.
- Ceccarelli PS, Adriano EA, Santos SMC, Rego RF, Silva LOL (2006) Levantamento quali-quantitativo da fauna parasitológica de peixes do Pantanal Mato-Grossense. In: Centro Nacional de Pesquisa e Gestão de Recursos Pesqueiros Continentais-Cepta (Ed.) Pesquisas patológicas e genéticas em recursos pesqueiros da Bacia do Alto Paraguai. IBAMA, Pirassununga, 16–116.
- Chambers CB, Cribb TH, Jones MK (2000) Tetraphyllidean metacestodes of teleosts of the Great Barrier Reef, and the use of in vitro cultivation to identify them. Folia Parasitologica 47: 285–292. https://doi.org/10.14411/fp.2000.050
- Chávez RA, González MT, Oliva ME, Valdivia IM (2012) Endoparasite fauna of five Gadiformes fish species from the coast of Chile: host ecology versus phylogeny. Journal of Helminthology 86: 10–15. https://doi.org/10.1017/S0022149X10000921
- Chávez RA, Valdivia IM, Oliva ME (2007) Local variability in metazoan parasites of the pelagic fish species, Engraulis ringens: implications for fish stock assessment using parasites as biological tags. Journal of Helminthology 81: 113–116. https://doi.org/10.1017/S0022149X07726573
- Chemes SB, Takemoto RM (2011) Diversity of parasites from middle Paraná system freshwater fishes, Argentina. International Journal of Biodiversity and Conservation 3: 249–266.
- Chero J, Cruces C, Iannacone J, Sáez G, Alvariño L, Rodríguez C, Rodríguez H, Tuesta E, Pacheco A, Huamani N (2014a) Índices parasitológicos de la merluza peruana Merluccius gayi peruanus (Ginsburg, 1954) (Perciformes: Merlucciidae) adquiridos del terminal pesquero de Ventanilla, Callao, Perú. Neotropical Helminthology 8: 141–162.
- Chero J, Cruces C, Iannacone J, Sáez G, Sanchez L, Minaya D, Alvariño L, Mendoza-Vidaurre C, Luque JL (2015) First record of Unitubulotestis pelamydis (Trematoda: Didymozoidae) and Sphyriocephalus tergestinus (Cestoda: Sphyriocephalidae) in eastern Pacific bonito, Sarda chiliensis (Perciformes: Scombridae) in Peru. Neotropical Helminthology 9: 313–323.
- Chero J, Iannacone J, Cruces C, Sáez G, Alvariño L (2014b) Comunidad de metazoos parásitos de la corvina Cilus gilberti (Abbott, 1899) (Perciformes: Sciaenidae) en la zona costera de Chorrillos, Lima, Perú. Neotropical Helminthology 8: 163–182.
- Chero J, Sáez G, Iannacone J, Aquino W (2014c) Aspectos ecológicos de los helmintos parásitos de lorna Sciaena deliciosa (tschudi, 1846) (Perciformes: Sciaenidae) adquiridos del terminal pesquero de Ventanilla, Callao, Perú. Neotropical Helminthology 8: 59–76.
- Choudhury A, Aguirre-Macedo ML, Curran SS, Ostrowski de Núñez M, Overstreet RM, Pérez-Ponce de León G, Santos CP (2016) Trematode diversity in freshwater fishes of the Globe II: 'New World'. Systematic Parasitology 93: 271–282. https://doi.org/10.1007/s11230-016-9632-1
- Cordeiro AS, Luque JL (2004) Community ecology of the metazoan parasites of Atlantic moonfish, Selene setapinnis (Osteichthyes: Carangidae) from the coastal zone of the State of Rio de Janeiro, Brazil. Brazilian Journal of Biology 64: 399–406. https://doi.org/10.1590/S1519-69842004000300004
- Cordeiro AS, Luque JL (2005) Metazoários parasitos do coió Dactylopterus volitans (Linnaeus, 1758) (Osteichthyes: Dactylopteridae) do litoral do Estado do Rio de Janeiro, Brasil. Acta Scientiarum: Biological Sciences 27: 119–123. https://doi.org/10.4025/actascibiolsci.v27i2.1320
- Cortés Y, Muñoz G (2008) Infracomunidades de parásitos eumetazoos del bagre de mar Aphos porosus (Valenciennes, 1837) (Actinopterygii: Batrachoidiformes) en Chile central. Revista de Biología Marina y Oceanografía 43: 255–263. https://doi.org/10.4067/S0718-19572008000200004
- Cortés Y, Muñoz G (2009) Metazoan parasite infracommunities of the toadfish Aphos porosus (Pisces: Batrachoidiformes) in central Chile: how variable are they over time? Journal of Parasitology 95: 753–756. https://doi.org/10.1645/GE-1651.1
- Cousin JCB, Pereira JJr, Gonzales JF (2006) Histopatologia no fígado de Prionace glauca (Chondrichthyes, Squaliformes, Carcharhinidae) causada por Hepatoxylon trichiuri (Eucestoda, Trypanorhyncha). Biociências 11: 167–172.
- Cremonte F, Sardella NH (1997) The parasita Fauna of Scomber japonicus Houttuyn, 1782 (Pisces: Scombridae) in two zones of the Argentine Sea. Fisheries Research 31: 1–9. https://doi.org/10.1016/S0165-7836(97)00024-6
- Cruces C, Chero J, Iannacone J, Diestro A, Sáez G, Alvariño L (2014) Metazoos parásitos de "caballa" Scomber japonicus Houttuyn, 1782 (Perciformes: Scombridae), del puerto de Chicama, La Libertad, Perú. Neotropical Helminthology 8: 357–381.
- Cruces C, Chero J, Iannacone J, Sáez G, Alvariño L (2015) Comunidad de endohelmintos parásitos del trambollo de boca amarilla Labrisomus philippii (Steindachner, 1866) (Perciformes: Labrisomidae) de la costa central del Perú. The Biologist 13: 91–109.
- Dailey MD, Carvajal JG (1976) Helminth parasites of Rhinobatos planiceps Garman 1880, including two new species of cestodes, with comments on host specificity of the genus Rhinebothrium Linton 1890. Journal of Parasitology 62: 939–942. https://doi.org/10.2307/3279187
- de Chambrier A (1990) Redescription de Proteocephalus paraguayensis (Rudin, 1917) (Cestoda: Proteocephalidae) parasite de Hydrodynastes gigas (Dum., Bibr. & Dum., 1854) du Paraguay. Systematic Parasitology 16: 85–87. https://doi.org/10.1007/BF00009608
- de Chambrier A (2001) A new tapeworm from the Amazon, Amazotaenia yvettae gen. n., sp. n., (Eucestoda: Proteocephalidea) from the siluriform fishes Brachyplatystoma filamentosum and B. vaillanti (Pimelodidae). Revue Suisse de Zoologie 108: 303–316. https://doi.org/10.5962/bhl.part.79632
- de Chambrier A (2003) Systematic status of Manaosia bracodemoca Woodland, 1935 and Paramonticellia itaipuensis Pavanclli et Rego, 1991 (Eucestoda: Proteocephalidea), parasites of Sorubim lima (Siluriformes: Pimelodidae) from South America. Folia Parasitologica 50: 121–127. https://doi.org/10.14411/fp.2003.021
- de Chambrier A, Gil de Pertierra AA (2002) Redescription of Travassiella avitellina Rego & Pavanelli, 1987 (Proteocephalidea: Monticelliidae, Zygobothriinae), a parasite of Paulicea luetkeni (Siluriformes) from South America. Memórias do Instituto Oswaldo Cruz 97: 657–661. https://doi.org/10.1590/S0074-02762002000500013
- de Chambrier A, Kuchta R, Scholz T (2015a) Tapeworms (Cestoda: Proteocephalidea) of teleost fishes from the Amazon River in Peru: additional records as an evidence of unexplored species diversity. Revue Suisse de Zoologie 122: 149–163. https://doi.org/10.5281/zenodo.14580
- de Chambrier A, Rego AA (1994) Proteocephalus sophiae n. sp. (Cestoda: Proteocephalidae), a parasite of the siluroid fish Paulicea luetkeni (Pisces: Pimelodidae) from the Brazilian Amazon. Revue Suisse de Zoologie 101: 361–368. https://doi.org/10.5962/bhl.part.79911
- de Chambrier A, Rego AA (1995) Mariauxiella pimelodi n. g., n. sp. (Cestoda: Monticelliidae): A parasite of pimelodid siluroid fishes from South America. Systematic Parasitology 30: 57–65. https://doi.org/10.1007/BF00009245
- de Chambrier A, Rego AA, Gil de Pertierra AA (2005) Redescription of two cestodes (Eucestoda: Proteocephalidea) parasitic in Phractocephalus hemioliopterus (Siluriformes) from the Amazon and erection of Scholzia gen. n. Revue Suisse de Zoologie 112: 735–752. https://doi.org/10.5962/bhl.part.80323
- de Chambrier A, Rego AA, Mariaux J (2004a) Redescription of Brooksiella praeputialis and Goezeella siluri (Eucestoda: proteocephalidea), parasites of Cetopsis coecutiens (Siluriformes) from the Amazon and proposition of Goezeella dankbrooksi sp. n. Revue Suisse de Zoologie 111: 111–120. https://doi.org/10.5962/bhl.part.80230
- de Chambrier A, Rego AA, Vaucher C (1999) Euzetiella tetraphylliformis n. gen., n. sp., (Eucestoda: Proteocephalidae), parasite du poisson d’eau douce néotropical Paulicea luetkeni (Siluriforme, Pimelodidae). Parasite 6: 43–47. https://doi.org/10.1051/parasite/1999061043
- de Chambrier A, Scholz T (2005) Redescription of Houssayela sudobim (Woodland, 1935) (Cestoda: Proteocephalidea), a parasite of Pseudoplatystoma fasciatum (Pisces: Siluriformes) from the River Amazon. Systematic Parasitology 62: 161–169. https://doi.org/10.1007/s11230-005-5487-6
- de Chambrier A, Scholz T (2008) Tapeworms (Cestoda: Proteocephalidea) of firewood catfish Sorubimichthys planiceps (Siluriformes: Pimelodidae) from the Amazon River. Folia Parasitologica 55: 17–28. https://doi.org/10.14411/fp.2008.004
- de Chambrier A, Scholz T, Kuchta R (2014) Taxonomic status of Woodland’s enigmatic tapeworms (Cestoda: Proteocephalidea) from Amazonian catfishes: back to museum collections. Systematic Parasitology 87: 1–19. https://doi.org/10.1007/s11230-013-9457-0
- de Chambrier A, Scholz T, Kuchta R, Posel P, Mortenthaler M, Guardia CC (2006a) Tapeworms (Cestoda: Proteocephalidea) of fishes from the Amazon River in Peru. Comparative Parasitology 73: 111–120. https://doi.org/10.1654/4182.1
- de Chambrier A, Scholz T, Vaucher C (1996) Tapeworms (Cestoda: Proteocephalidea) of Hoplias malabaricus (Pisces: Characiformes, Erythrinidae) in Paraguay: description of Proteocephalus regoi sp. n., and redescription of Nomimoscolex matogrossensis. Folia Parasitologica 43: 133–140.
- de Chambrier A, Takemoto RM, Pavanelli GC (2006b) Nomimoscolex pertierrae n. sp. (Eucestoda: Proteocephalidea), a parasite of Pseudoplatystoma corruscans (Siluriformes: Pimelodidae) in Brazil and redescription of N. sudobim Woodland, 1935, a parasite of P. fasciatum. Systematic Parasitology 64: 191–202. https://doi.org/10.1007/s11230-006-9031-0
- de Chambrier A, Vaucher C (1994) Etude morpho-anatomique et génétique de deux nouveaux Proteocephalus Weinland, 1858 (Cestoda: Proteocephalidae) parasites de Platydoras costatus (L.), poisson siluriforme du Paraguay. Systematic Parasitology 27: 173–185. https://doi.org/10.1007/BF00008479
- de Chambrier A, Vaucher C (1997) Révision des cestodes (Monticelliidae) décrits par Woodland (1934) chez Brachyplatystoma filamentosum avec redéfinition des genres Endorchis Woodland, 1934 et Nomimoscolex Woodland, 1934. Systematic Parasitology 37: 219–233. https://doi.org/10.1023/A:1005863808627
- de Chambrier A, Vaucher C (1999) Proteocephalidae et Monticelliidae (Eucestoda: Proteocephalidea) parasites de poissons d’eau douce au Paraguay, avec descriptions d’un genre nouveau et de dix espèces nouvelles. Revue Suisse de Zoologie 106: 165–240. https://doi.org/10.5962/bhl.part.80074
- de Chambrier A, Waeschenbach A, Fisseha M, Scholz T, Mariaux J (2015b) A large 28S rDNA-based phylogeny confirms the limitations of established morphological characters for classification of proteocephalidean tapeworms (Platyhelminthes, Cestoda). ZooKeys 500: 25–59. https://doi.org/10.3897/zookeys.500.9360
- de Chambrier A, Zehnder M, Vaucher C, Mariaux J (2004b) The evolution of the Proteocephalidea (Platyhelminthes, Eucestoda) based on an enlarged molecular phylogeny, with comments on their uterine development. Systematic Parasitology 57: 159–171. https://doi.org/10.1023/B:SYPA.0000019083.26876.34
- Dias FJE, São Clemente SC, Knoff M (2009) Cestóides Trypanorhyncha parasitos de peroá, Balistes capriscus Gmelin, 1789 comercializados no estado do Rio de Janeiro, Brasil. Revista Brasileira de Ciência Veterinária 16: 19–21. https://doi.org/10.4322/rbcv.2014.163
- Dias FJE, São Clemente SC, Knoff M (2010) Nematoides anisaquídeos e cestoides Trypanorhyncha de importância em saúde pública em Aluterus monoceros (Linnaeus, 1758) no Estado do Rio de Janeiro, Brasil. Revista Brasileira de Parasitologia Veterinária 19: 94–97. https://doi.org/10.1590/S1984-29612010000200005
- Dias FJE, São Clemente SC, Pinto RM, Knoff M (2011a) Anisakidae nematodes and Trypanorhyncha cestodes of hygienic importance infecting the king mackerel Scomberomorus cavalla (Osteichthyes: Scombridae) in Brazil. Veterinary Parasitology 175: 351–355. https://doi.org/10.1016/j.vetpar.2010.10.014
- Dias LNS, Paiva RS, São Clemente SC, Rodrigues AE, Peralta ASL, Matos ER (2011b) Cestóides Trypanorhyncha parasitos de scianideos de importância comercial, capturados no litoral amazônico, Brasil. Revista Brasileira de Ciência Veterinária 18: 3–5. https://doi.org/10.4322/rbcv.2014.111
- Diesing KM (1850) Systema Helminthum, vol I. Braumüller, Vienna, 679 pp.
- Diesing KM (1855) Sechzehn Gattungen von Binnenwürmern und ihre Arten. Denkschriften der Kaiserlichen Akademie der Wissenschaften, Mathematisch-Naturwissenschaftliche Classe 9: 171–185.
- Diesing KM (1856) Zwanzig Arten von Cephalocotyllen. Denkschriften der Kaiserlichen Akademie der Wissenschaften, Mathematisch-Naturwissenschaftliche Classe 12: 23–38.
- Dobson A, Lafferty KD, Kuris AM, Hechinger RF, Jetz W (2008) Homage to Linnaeus: how many parasites? How many hosts? Proceedings of the National Academy of Sciences 105: 11482–11489. https://doi.org/10.1073/pnas.0803232105
- Dollfus RP (1930) Sur les Tétrarhynches. I. Définition des genres (suite). Mémoires de la Société Zoologique de France 29: 139–216.
- Dollfus RP (1942) Études critiques sur les Tétrarhynques du Muséum de Paris. Archives du Muséum National d’Histoire Naturelle, Paris, 466 pp.
- Drago FB (2012) Community structure of metazoan parasites of silverside, Odontesthes bonariensis (Pisces, Atherinopsidae) from Argentina. Iheringia: Série Zoologia 102: 26–32. https://doi.org/10.1590/S0073-47212012000100004
- Durán LE, Oliva ME (1980) Estudio parasitológico en Merluccius gayi peruanus Gingsburg, 1954. Boletín Chileno de Parasitología 35: 18–21.
- Eiras JC, Rego AA, Pavanelll GC (1986) Histopathology in Paulicea lutkeni (Pisces: Pimelodidae) resulting from infections with Megathylacus brooksi and Jauela glandicephalus (Cestoda: Proteocephalidae). Journal of Fish Biology 28: 359–365. https://doi.org/10.1111/j.1095-8649.1986.tb05172.x
- Escalante H (1983) Larvas plerocercoides de Diphyllobothridae Lühe, 1910: hallazgo en peces marinos de consumo humano en la costa peruana. Boletín Chileno de Parasitología 38: 50–52.
- Escalante H (1986) Cestodes de elasmobranquios de la costa peruana. Revista de Ciencias (Lima) 74: 70–74.
- Escalante H, Arigaza P, Moreno B (1987) Metacéstodos de tetrafilídeos em teleosteos em la zona norte del mar peruano. Rebiol 6: 15–23.
- Escalante H, Carvajal JG (1981) Cestodos tetrafilididos de peces del genero Mustelus en la costa peruana. Boletín Chileno de Parasitología 36: 76–78.
- Escalante H, Carvajal JG (1984) Larval trypanorhynch cestodes from Peruvian teleost fishes, with descriptions of two new species. Studies on Neotropical Fauna and Environment 19: 185–194. https://doi.org/10.1080/01650528409360658
- Escalante H, Miranda H (1986) Diphyllobothrium pacificum: hallazgo de larvas plerocercoides en peces marinos del Perú y desarrollo de formas adultas del parásito en Canis familiaris. Boletín Chileno de Parasitología 41: 7–13.
- Euzet L, Carvajal JG (1973) Rhinebothrium (Cestoda, Tetraphyllidea) parasites de raies du genre Psammobatis au Chili. Bulletin du Museum National d’Histoire Naturelle, Paris 137: 779–787.
- Faria A, Silva D (1934) Garoupa vermelha de Abrolhos e São Tomé "Garoupa Bichada" Tetrarhynchus. Primeiro Congresso Nacional de Pesca, Rio de Janeiro 1: 237–250.
- Feijó LMF, Rodrigues HO, Rodrigues SS (1979) Contribuição ao estudo da fauna helmintológica de sardinhas (Sardinella sp.) do litoral do Estado do rio de Janeiro. Atas da Sociedade de Biologia do Rio de Janeiro 20: 23–27.
- Felizardo NN, Torres EJL, Fonsesca MCG, Pinto RM, Gomes DC, Knoff M (2010) Cestodes of the flounder Paralichthys isosceles Jordan, 1890 (Osteichthyes – Paralichthyidae) from the state of Rio de Janeiro, Brazil. Neotropical Helminthology 4: 113–126.
- Fernández J (1985) Estudio parasitológico de Merluccius australis (Hutton, 1872) (Pisces: Merluccidae): aspectos sistemáticos, estadísticos y zoogeográficos. Boletín de la Sociedad de Biología de Concepción 56: 31–41.
- Fernández J (1987) Los parásitos de la lisa Mugil cephalus L., en Chile: sistemática y aspectos poblacionales (Perciformes: Mugilidae). Gayana Zoología 51: 3–58.
- Fernández J, Villalba C, Albiña A (1986) Parásitos del pejegallo, Callorhynchus callorhynchus (L.), en Chile: aspectos biológicos y sistemáticos. Biología Pesquera 15: 63–73.
- Fernández MV, Brugni NL, Viozzi GP, Semenas L (2010) The relationship between fish assemblages and the helminth communities of a prey fish, in a group of small shallow lakes. Journal of Parasitology 96: 1066–1071. https://doi.org/10.1645/GE-2380.1
- Fernández MV, Semenas L, Viozzi GP (2012) Parasites of the “Peladilla,” Aplochiton zebra (Osmeriformes: Galaxiidae), from Patagonia (Argentina and Chile). Comparative Parasitology 79: 231–237. https://doi.org/10.1654/4561.1
- Ferraris CJJr (2007) Checklist of catfishes, recent and fossil (Osteichthyes: Siluriformes), and catalogue of siluriform primary types. Zootaxa 1418: 1–628.
- Ferreira MF, São Clemente SC, Tortelly R, Lima FC, Nascimento ER, Oliveira GA, Lima AR (2006) Parasitas da ordem Trypanorhyncha: sua importância na inspeção sanitária do pescado. Revista Brasileira de Ciência Veterinária 13: 190–193. https://doi.org/10.4322/rbcv.2014.297
- Flores K, George-Nascimento M (2009) Las infracomunidades de parásitos de dos especies de Scartichthys (Pisces: Blenniidae) en localidades cercanas del norte de Chile. Revista Chilena de Historia Natural 82: 63–71. https://doi.org/10.4067/S0716-078X2009000100004
- Fonseca MCG, São Clemente SC, Felizardo NN, Gomes DC, Knoff M (2012) Trypanorhyncha cestodes of hygienic-sanitary importance infecting flounders Paralichthys patagonicus Jordan, 1889 and Xystreurys rasile (Jordan, 1891) of the Neotropical region, Brazil. Parasitology Research 111: 865–874. https://doi.org/10.1007/s00436-012-2912-z
- Fortes E (1981) Descrição de quatro espécies novas de helmintos de bagres (Pisces, Bagridae) do estuário do Guaíba, Porto Alegre, RS, Brasil. Arquivos da Faculdade de Veterinária UFRGS 9: 69–78.
- Fortes E, Hoffmann RP (1987) Hiperparasitismo de cestódeos de Rhamdia sapo Valenciennes, 1840 do estuário do Guaíba, Rio Grande do Sul, Brasil. Revista Brasileira de Medicina Veterinária 9: 114.
- Fortes E, Hoffmann RP (1995) Levantamento da fauna parasitária de peixes do lago Guaíba, Porto Alegre, Rio Grande do Sul, Brasil. Revista Brasileira de Medicina Veterinária 17: 107–111.
- Franceschini L, Zago AC, Zocoller-Seno MC, Veríssimo-Silveira R, Ninhaus-Silveira A, Silva RJ (2013) Endohelminths in Cichla piquiti (Perciformes, Cichlidae) from the Paraná River, São Paulo State, Brazil. Revista Brasileira de Parasitologia Veterinária 22: 475–484. https://doi.org/10.1590/S1984-29612013000400006
- Freze VI (1965) [Proteocephalata. Tapeworms of Fish, Amphibians and Reptiles]. In: Skryabin KI (Ed.) [Principles of Cestodology. Vol. 5]. Izdatel'stvo «Nauka«, Moscow, 538 pp. [In Russian] [English translation, Israel Program of Scientific Translation, 1969, Cat. No. 1853. v + 597 pp.]
- Froese R, Pauly D (Eds) (2016) FishBase. World Wide Web electronic publication. http://www.fishbase.org, version (06/2016) [accessed 1.V.2016]
- Fuhrmann O (1916) Eigentümliche Fischcestoden. Zoologischer Anzeiger 46: 385–398.
- Fuhrmann O (1934) Vier Diesing’sche Typen (Cestoda). Revue Suisse de Zoologie 41: 545–564.
- Fuster de Plaza ML, Boschi EE (1957) Desnutrición y deformaciones vertebrales en pejerreyes de los embalses de Córdoba. Ministerio de Agricultura y Ganaderıa. Departamento de Investigaciones Pesqueras, Buenos Aires, 26 pp.
- Garcías F, Mendoza R, George-Nascimento M (2001) Variación entre años de las infracomunidades de parásitos metazoos de la corvina Cilus gilberti (Pisces: Sciaenidae) en Chile. Revista Chilena de Historia Natural 74: 833–840. https://doi.org/10.4067/S0716-078X2001000400010
- George‐Nascimento M (1996) Populations and assemblages of parasites in hake, Merluccius gayi, from the southeastern Pacific Ocean: stock implications. Journal of Fish Biology 48: 557–568. https://doi.org/10.1111/j.1095-8649.1996.tb01452.x
- George-Nascimento M (2000) Geographical variations in the jack mackerel Trachurus symmetricus murphyi populations in the southeastern Pacific Ocean as evidenced from the associated parasite communities. Journal of Parasitology 86: 929–932. https://doi.org/10.1645/0022-3395(2000)086[0929:GVITJM]2.0.CO;2
- George-Nascimento M, Arancibia H (1992) Stocks ecológicos del jurel (Trachurus symmetricus murphyi Nichols) en tres zonas de pesca frente a Chile, detectados mediante comparación de su fauna parasitaria y morfometría. Revista Chilena de Historia Natural 65: 453–470.
- George-Nascimento M, Arancibia H (1994) La fauna parasitaria y la morfometría de la merluza austral Merluccius australis (Hutton) como indicadoras de unidades de stock. Biología Pesquera 23: 31–47.
- George-Nascimento M, Garcías F, Muñoz G (2002) Parasite body volume and infracommunity patterns in the southern pomfret Brama australis (Pisces: Bramidae). Revista Chilena de Historia Natural 75: 835–839. https://doi.org/10.4067/S0716-078X2002000400016
- George-Nascimento M, Huet B (1984) Una aproximación ecológica al estudio del parasitismo en el congrio negro Genypterus maculatus (Tschudi) (Pisces: Ophidiidae). Biología Pesquera 13: 23–30.
- George-Nascimento M, Iriarte JL (1989) Las infracomunidades de parásitos metazoos del chancharro Helicolenus lengerichi Norman, 1937 (Pisces, Scorpaenidae): un ensamble no interactivo de especies. Revista Chilena de Historia Natural 62: 217–227.
- George-Nascimento M, Mellado A, Saavedra S, Carvajal JG (2009) Variabilidad de las comunidades de parásitos metazoos del róbalo Eleginops maclovinus (Cuvier & Valenciennes, 1830) (Pisces: Eleginopidae) en Chile. Revista Chilena de Historia Natural 82: 199–207. https://doi.org/10.4067/S0716-078X2009000200003
- George-Nascimento M, Moscoso D (2013) Variación local y geográfica de las infracomunidades de parásitos de la anchoveta Engraulis ringens en Chile. Revista de Biología Marina y Oceanografía 48: 207–212. https://doi.org/10.4067/S0718-19572013000100020
- George-Nascimento M, Moscoso D, Niklitschek E, González K (2011) Variación geográfica de las comunidades de parásitos de la merluza de tres aletas Micromesistius australis al sur de Sudamérica. Revista de Biología Marina y Oceanografía 46: 53–58. https://doi.org/10.4067/S0718-19572011000100007
- George-Nascimento M, Oliva M (2015) Fish population studies using parasites from the Southeastern Pacific Ocean: considering host population changes and species body size as sources of variability of parasite communities. Parasitology 142: 25–35. https://doi.org/10.1017/S0031182014001127
- George-Nascimento M, Ortiz E (1982) Nuevos registros de huésped para el plerocercoide de Hepatoxylon trichiuri (Holten, 1802) (Cestoda: Trypanorhyncha) en peces marinos chilenos. Parasitología al Día 6: 39.
- Gibson DI (1994) Order Amphilinidea Poche, 1922. In: Khalil LF, Jones A, Bray RA (Eds) Keys to the Cestode Parasites of Vertebrates. CAB International, Wallingford, 3–10.
- Gibson DI, Bray RA, Harris EA (Compilers) (2005) Host-Parasite Database of the Natural History Museum, London. World Wide Web electronic publication. http://www.nhm.ac.uk/research-curation/scientific-resources/taxonomy-systematics/host-parasites/ [accessed 1.IV.2016]
- Gil de Pertierra AA (1995) Nomimoscolex microacetabula sp. n. y N. pimelodi sp. n. (Cestoda: Proteocephalidea) parasitos de Siluriformes del Rio de la Plata. Neotrópica 41: 19–26.
- Gil de Pertierra AA (2002a) Nomimoscolex semenasae n. sp. (Proteocephalidea: Monticelliidae), a cestode parasite of Diplomystes viedmensis (Pisces: Siluriformes) from the Patagonian region of Argentina. Systematic Parasitology 53: 183–190. https://doi.org/10.1023/A:1021151225726
- Gil de Pertierra AA (2002b) Redescription of Proteocephalus bagri and P. rhamdiae (Cestoda: Proteocephalidac), parasites of Rhamdia quelen (Siluriformes: Pimelodidae) from South America, with comments on morphological variation. Folia Parasitologica 49: 55–66. https://doi.org/10.14411/fp.2002.012
- Gil de Pertierra AA (2003) Two new species of Nomimoscolex (cestoda: Proteocephalidea, Monticelliidae) from Gymnotus carapo (Pisces: gymnotiformes) in Argentina. Memórias do Instituto Oswaldo Cruz 98: 345–352. https://doi.org/10.1590/S0074-02762003000300009
- Gil de Pertierra AA (2004) Redescription of Monticellia magna (Rego, dos Santos & Silva, 1974) (Eucestoda: Monticelliidae) parasite of Pimelodus spp. (Pisces: Siluriformes) from Argentina, and morphological study of microtriches. Revue Suisse de Zoologie 111: 11–20. https://doi.org/10.5962/bhl.part.80222
- Gil de Pertierra AA (2005) Comparative study of the microtriches of adult cestodes (Proteocephalidea: Monticelliidae), and some comments on their systematic value. Zoologischer Anzeiger 243: 295–304. https://doi.org/10.1016/j.jcz.2005.01.002
- Gil de Pertierra AA (2009) Luciaella ivanovae n. g., n. sp. (Eucestoda: Proteocephalidea: Peltidocotylinae), a parasite of Ageneiosus inermis (L.) (Siluriformes: Auchenipteridae) in Argentina. Systematic Parasitology 73: 71–80. https://doi.org/10.1007/s11230-009-9174-x
- Gil de Pertierra AA, Arredondo NJ, Kuchta R, Incorvaia IS (2015) A new species of Bothriocephalus Rudolphi, 1808 (Eucestoda: Bothriocephalidea) from the channel bull blenny Cottoperca gobio (Günther) (Perciformes: Bovichtidae) on the Patagonian shelf off Argentina. Systematic Parasitology 90: 247–256. https://doi.org/10.1007/s11230-015-9549-0
- Gil de Pertierra AA, de Chambrier A (2000) Rudolphiella szidati sp. n. (Proteocephalidea: Monticelliidae, Rudolphiellinae) parasite of Luciopimelodus pati (Valenciennes, 1840) (Pisces: Pimelodidae) from Argentina with new observations on Rudolphiella lobosa (Riggenbach, 1895). Revue Suisse de Zoologie 107: 81–95. https://doi.org/10.5962/bhl.part.80119
- Gil de Pertierra AA, de Chambrier A (2013) Harriscolex nathaliae n. sp. (Cestoda: Proteocephalidea) from Pseudoplatystoma corruscans (Siluriformes: Pimelodidae) in the Paraná River Basin, Argentina. Journal of Parasitology 99: 480–486. https://doi.org/10.1645/12-11.1
- Gil de Pertierra AA, Incorvaia IS, Arredondo NJ (2011) Two new species of Clestobothrium (Cestoda: Bothriocephalidea), parasites of Merluccius australis and M. hubbsi (Gadiformes: Merlucciidae) from the Patagonian shelf of Argentina, with comments on Clestobothrium crassiceps (Rudolphi, 1819). Folia Parasitologica 58: 121–134. https://doi.org/10.14411/fp.2011.012
- Gil de Pertierra AA, Ostrowski de Núñez M (1990) Seasonal dynamics and maturation of the cestode Proteocephalus jandia (Woodland, 1933) in the catfish (Rhamdia sapo). Acta Parasitologica Polonica 35: 305–313.
- Gil de Pertierra AA, Semenas LG (2005) Galaxitaenia toloi n. gen., n. sp. (Eucestoda: Pseudophyllidea) from Galaxias platei (Pisces: Osmeriformes, Galaxiidae), in the Patagonian region of Argentina. Journal of Parasitology 91: 900–908. https://doi.org/10.1645/GE-437R.1
- Gil de Pertierra AA, Semenas LG (2006) Ailinella mirabilis gen. n., sp. n. (Eucestoda: Pseudophyllidea) from Galaxias maculatus (Pisces: Galaxiidae) in the Andean-Patagonian region of Argentina. Folia Parasitologica 53: 276–286. https://doi.org/10.14411/fp.2006.034
- Gil de Pertierra AA, Viozzi GP (1999) Redescription of Cangatiella macdonaghi (Szidat y Nani, 1951) comb. nov. (Cestoda: Proteocephalidae) a parasite of the atheriniform fish Odontesthes hatcheri (Eigenmann, 1909) from the Patagonian region of Argentina. Neotrópica 45: 13–20.
- Gomes DC, Fabio SP, Rolas FJT (1972) Contribution to the knowledge of the parasites of fishes in Guanabara State. Memórias do Instituto Oswaldo Cruz 70: 541–553. https://doi.org/10.1590/S0074-02761972000400005
- Gomes DC, Knoff M, São Clemente SC, Lanfredi RM, Pinto RM (2005) Taxonomic reports of Homeacanthoidea (Eucestoda: Trypanorhyncha) in lamnid and sphyrnid elasmobranchs collected off the coast of Santa Catarina, Brazil. Parasite 12: 15–22. https://doi.org/10.1051/parasite/2005121015
- Gonçalves RA, Oliveira MSB, Neves LR, Tavares-Dias M (2016) Seasonal pattern in parasite infracommunities of Hoplerythrinus unitaeniatus and Hoplias malabaricus (Actinopterygii: Erythrinidae) from the Brazilian Amazon. Acta Parasitologica 61: 119–129. https://doi.org/10.1515/ap-2016-0016
- González H, Garrido V, Landeta M, Martens P (1980) New data for the identification of Diphyllobothrium sp. in Lake Rupanco, Chile. Boletín Chileno de Parasitología 35: 10–14.
- González H, Garrido V, Martens P, Aguirrebeña R (1978) Identificación de Diphyllobothrium sp. en especies salmonideas del lago Rupanco, Chile. Boletín Chileno de Parasitología 33: 25–34.
- González L, Carvajal JG (1994) Estudio parasitológico de Merluccius australis (Hutton, 1972) del mar interior de Aysén. Investigación Pesquera 38: 75–85.
- González MT, Acuña E, Oliva ME (2001) Metazoan parasite fauna of the bigeye flounder, Hippoglossina macrops, from northern Chile. Influence of host age and sex. Memórias do Instituto Oswaldo Cruz 96: 1049–1054. https://doi.org/10.1590/S0074-02762001000800004
- González MT, Barrientos C, Moreno C (2006) Biogeographical patterns in endoparasite communities of a marine fish (Sebastes capensis Gmelin) with extended range in the Southern Hemisphere. Journal of Biogeography 33: 1086–1095. https://doi.org/10.1111/j.1365-2699.2006.01488.x
- González MT, Poulin R (2005a) Nested patterns in parasite component communities of a marine fish along its latitudinal range on the Pacific coast of South America. Parasitology 131: 569–577. https://doi.org/10.1017/S0031182005007900
- González MT, Poulin R (2005b) Spatial and temporal predictability of the parasite community structure of a benthic marine fish along its distributional range. International Journal for Parasitology 35: 1369–1377. https://doi.org/10.1016/j.ijpara.2005.07.016
- González MT, Vásquez R, Acuna E (2008) Biogeographic patterns of metazoan parasites of the bigeye flounder, Hippoglossina macrops, in the Southeastern Pacific coast. Journal of Parasitology 94: 429–435. https://doi.org/10.1645/GE-1265.1
- Graça RJ, Machado MH (2007) Ocorrência e aspectos ecológicos de metazoários parasitos de peixes do Lago do Parque do Ingá, Maringá, Estado do Paraná. Acta Scientiarum: Biological Sciences 29: 321–326. https://doi.org/10.4025/actascibiolsci. v29i3. 507
- Guagliardo S, Schwerdt C, Galeano N, González R, Tanzola RD (2014) Helminthic assemblages of Seriolella porosa Guichenot 1848 (Pisces: Centrolophidae) from San Matías Gulf (Argentina). Neotropical Helminthology 8: 84–96.
- Guagliardo S, Tanzola D, Schwerdt C, Galeano N (2009) Host-parasite relationships between Porichthys porosissimus (Pisces) and cestode larvae of the Scolex group. Helminthologia 46: 162–167. https://doi.org/10.2478/s11687-009-0031-x
- Guidelli G, Tavechio WLG, Takemoto RM, Pavanelli GC (2006) Fauna parasitária de Leporinus lacustris e Leporinus friderici (Characiformes, Anostomidae) da planície de inundação do alto rio Paraná, Brasil. Acta Scientiarum: Biological Sciences 28: 281–290. https://doi.org/10.4025/actascibiolsci.v28i3.228
- Guidelli G, Tavechio WLG, Takemoto RM, Pavanelli GC (2011) Relative condition factor and parasitism in anostomid fishes from the floodplain of the Upper Paraná River, Brazil. Veterinary Parasitology 177: 145–151. https://doi.org/10.1016/j.vetpar.2010.11.035
- Guidelli GM, Isaac A, Takemoto RM, Pavanelli GC (2003) Endoparasite infracommunities of Hemisorubim platyrhynchos (Valenciennes, 1840) (Pisces: Pimelodidae) of the Baía River, upper Paraná River floodplain, Brazil: specific composition and ecological aspects. Brazilian Journal of Biology 63: 261–268. https://doi.org/10.1590/S1519-69842003000200011
- Healy CJ (2003) A revision of Platybothrium Linton, 1890 (Tetraphyllidea: Onchobothriidae), with a phylogenetic analysis and comments on host-parasite associations. Systematic Parasitology 56: 85–139. https://doi.org/10.1023/A:1026135528505
- Healy CJ, Caira JN, Jensen K, Webster BL, Littlewood DTJ (2009) Proposal for a new tapeworm order, Rhinebothriidea. International Journal for Parasitology 39: 497–511. https://doi.org/10.1016/j.ijpara.2008.09.002
- Henríquez VP, González MT (2014) Patterns of variation in parasite component communities and infracommunities of a littoral fish species from the northern coast of Chile. Journal of Helminthology 88: 89–96. https://doi.org/10.1017/S0022149X12000788
- Henríquez VP, González MT, Licandeo R, Carvajal JG (2011) Metazoan parasite communities of rock cod Eleginops maclovinus along southern Chilean coast and their use as biological tags at a local spatial scale. Journal of Fish Biology 79: 1851–1865. https://doi.org/10.1111/j.1095-8649.2011.03126.x
- Hermida M, Carvalho BFL, Cruz C, Saraiva A (2014) Parasites of the mutton snapper Lutjanus analis (Perciformes: Lutjanidae) in Alagoas, Brazil. Revista Brasileira de Parasitologia Veterinária 23: 241–243. https://doi.org/10.1590/S1984-29612014023
- Hernández-Orts JS, Scholz T, Brabec J, Kuzmina T, Kuchta R (2015) High morphological plasticity and global geographical distribution of the Pacific broad tapeworm Adenocephalus pacificus (syn. Diphyllobothrium pacificum): molecular and morphological survey. Acta Tropica 149: 168–178. https://doi.org/10.1016/j.actatropica.2015.05.017
- Holcman-Spector B, Mañé-Garzón F (1988) Two new species of the genus Proteocephalus Weinland, 1858 (Proteocephalidea, Eucestoda) of freshwater fishes Rhamdia sapo (Valenciennes, 1840), from Uruguay. Parasitología al Día 12: 148–154.
- Hypša V, Škeříková A, Scholz T (2005) Phylogeny, evolution and host–parasite relationships of the order Proteocephalidea (Eucestoda) as revealed by combined analysis and secondary structure characters. Parasitology 130: 359–371. https://doi.org/10.1017/S0031182004006638
- Iannacone J (2003) Three metazoan parasites of palm ruff Seriolella violacea Guichenot (Pisces, Centrolophidae), Callao, Peru. Revista Brasileira de Zoologia 20: 257–260. https://doi.org/10.1590/S0101-81752003000200014
- Iannacone J, Alvariño L (2009) Dinámica poblacional de la diversidad parasitaria de la “Cabrilla” Paralabrax humeralis (Teleostei: Serranidae) en Chorrillos, Lima, Perú. Neotropical Helminthology 3: 73–88.
- Iannacone J, Alvariño L (2013) Parasitological indices of Pacific pomfret Brama japonica Hilgendorf, 1878 (Osteichthyes, Bramidae) acquired at the fishing terminal of Chorrillos Lima, Peru. Neotropical Helminthology 7: 117–132.
- Iannacone J, Alvariño L, Chero J, Sáez G (2015) Comunidad parasitaria de cabinza Isacia conceptionis (Cuvier & Valenciennes, 1830) (Perciformes: Haemulidae) en la Zona de Chorrillos, Lima, Perú. Revista de Investigaciones Veterinarias del Perú 26: 96–110. https://doi.org/10.15381/rivep.v26i1.10943
- Iannacone J, Avila-Peltroche J, Rojas-Perea S, Salas-Sierralta M, Neira-Cruzado K, Palomares-Torres R, Valdivia-Alarcón S, Pacheco-Silva A, Benvenutto-Vargas V, Ferrario-Bazalar V (2011) Dinámica poblacional de los parásitos metazoos del pez guitarra del pacífico Rhinobatos planiceps (Batoidea: Rajiformes) de la zona costera marina de Lima, Perú. Neotropical Helminthology 5: 265–278.
- Isaac A, Guidelli GM, França JG, Pavanelli GC (2004) Composição e estrutura das infracomunidades endoparasitárias de Gymnotus spp. (Pisces: Gymnotidae) do rio Baía, Mato Grosso do Sul, Brasil. Acta Scientiarum: Biological Sciences 26: 453–462. https://doi.org/10.4025/actascibiolsci.v26i4.1527
- Ivanov VA (1997) Echinobothrium notoguidoi n. sp. (Cestoda: Diphyllidea) from Mustelus schmitti (Chondrichthyes: Carcharhiniformes) in the Argentine Sea. Journal of Parasitology 83: 913–916. https://doi.org/10.2307/3284288
- Ivanov VA (2004) A new species of Rhinebothroides Mayes, Brooks & Thorson, 1981 (Cestoda: Tetraphyllidea) from the ocellate river stingray in Argentina, with amended descriptions of two other species of the genus. Systematic Parasitology 58: 159–174. https://doi.org/10.1023/B:SYPA.0000032933.71645.1e
- Ivanov VA (2005) A new species of Acanthobothrium (Cestoda: Tetraphyllidea: Onchobothriidae) from the ocellate river stingray, Potamotrygon motoro (Chondrichthyes: Potamotrygonidae), in Argentina. Journal of Parasitology 91: 390–396. https://doi.org/10.1645/GE-354R1
- Ivanov VA (2006) Guidus n. gen. (Cestoda: Tetraphyllidea), with description of a new species and emendation of the generic diagnosis of Marsupiobothrium. Journal of Parasitology 92: 832–840. https://doi.org/10.1645/GE-767R.1
- Ivanov VA (2008) Orygmatobothrium spp. (Cestoda: Tetraphyllidea) from triakid sharks in Argentina: redescription of Orygmatobothrium schmittii and description of a new species. Journal of Parasitology 94: 1087–1097. https://doi.org/10.1645/GE-1482.1
- Ivanov VA (2009) New species of Crossobothrium (Cestoda: Tetraphyllidea) from the broadnose sevengill shark, Notorynchus cepedianus, in Argentina. Journal of Parasitology 95: 1479–1488. https://doi.org/10.1645/GE-2096.1
- Ivanov VA, Brooks DR (2002) Calliobothrium spp. (Eucestoda: Tetraphyllidea: Onchobothriidae) in Mustelus schmitti (Chondrichthyes: Carcharhiniformes) from Argentina and Uruguay. Journal of Parasitology 88: 1200–1213. https://doi.org/10.2307/3285494
- Ivanov VA, Campbell RA (1998a) A new species of Acanthobothrium van Beneden, 1849 (Cestoda: Tetraphyllidea) from Rioraja castelnaui (Chondrichthyes: Rajoidei) in coastal waters of Argentina. Systematic Parasitology 40: 203–212. https://doi.org/10.1023/A:1006049404646
- Ivanov VA, Campbell RA (1998b) Echinobothrium megacanthum sp. n. (Cestoda: Diphyllidea) from the eagle ray Myliobatis goodei (Chondrichthyes: Rajoidei) from the Patagonian shelf of Argentina. Folia Parasitologica 45: 225–229.
- Ivanov VA, Campbell RA (2000) Emendation of the generic diagnosis of Tylocephalum (Cestoda: Lecanicephalidea: Tetragonocephalidae), and description of Tylocephalum brooksi n. sp. Journal of Parasitology 86: 1085–1092. https://doi.org/10.2307/3284827
- Ivanov VA, Campbell RA (2002) Notomegarhynchus navonae n. gen. and n. sp. (Eucestoda: Tetraphyllidea), from skates (Rajidae: Arhynchobatinae) in the Southern Hemisphere. Journal of Parasitology 88: 340–349. https://doi.org/10.1645/0022-3395(2002)088[0340:NNNGAN]2.0.CO;2
- Janicki C (1808) Über den Bau von Amphilina liguloidea Diesing. Zeitschrift für Wissenschaftliche Zoologie 89: 568–597. http://www.biodiversitylibrary.org/part/26850
- Jara CA (1998) Prevalencia e intensidad de parasitismo por helmintos en cuatro especies de peces de la zona norte del mar peruano. Revista Peruana de Parasitología 13: 76–83.
- Jensen K (2001) Four new genera and five new species of lecanicephalideans (Cestoda: Lecanicephalidea) from elasmobranchs in the Gulf of California, Mexico. Journal of Parasitology 87: 845–861. https://doi.org/10.1645/0022-3395(2001)087[0845:FNGAFN]2.0.CO;2
- Jensen K, Bullard SA (2010) Characterization of a diversity of tetraphyllidean and rhinebothriidean cestode larval types, with comments on host associations and life-cycles. International Journal for Parasitology 40: 889–910. https://doi.org/10.1016/j.ijpara.2009.11.015
- Jensen K, Caira JN, Cielocha JJ, Littlewood DTJ, Waeschenbach A (2016) When proglottids and scoleces conflict: phylogenetic relationships and a family-level classification of the Lecanicephalidea (Platyhelminthes: Cestoda). International Journal for Parasitology 46: 291–310. https://doi.org/10.1016/j.ijpara.2016.02.002
- Jerônimo GT, Ventura AS, Pádua SBD, Satake F, Ishikawa MM, Martins ML (2013) Parasitofauna de cachara cultivado em tanque-rede no Rio Paraguai. Pesquisa Agropecuária Brasileira: 1163–1166. https://doi.org/10.1590/S0100-204X2013000800054
- Karling LC, Isaac A, Affonso IP, Takemoto RM, Pavanelli GC (2013a) The impact of a dam on the helminth fauna and health of a neotropical fish species Salminus brasiliensis (Cuvier 1816) from the upper Paraná River, Brazil. Journal of Helminthology 87: 245–251. https://doi.org/10.1017/S0022149X1200034X
- Karling LC, Lacerda ACF, Takemoto RM, Pavanelli GC (2013b) Ecological relationships between endoparasites and the fish Salminus brasiliensis (Characidae) in a Neotropical floodplain. Neotropical Helminthology 7: 219–230.
- Kennedy CR, Andersen KI (1982) Redescription of Anchistrocephalus microcephalus (Rudolphi) (Cestoda, Pseudophyllidea) from the sunfish Mola mola. Zoologica Scripta 11: 101–105. https://doi.org/10.1111/j.1463-6409.1982.tb00522.x
- Khalil LF, Jones A, Bray RA (1994) Keys to the Cestode Parasites of Vertebrates. CAB international, Wallingford, 768 pp.
- Knoff M, Luque JL, Amato JF (1997) Community ecology of the metazoan parasites of grey mullets, Mugil platanus (Osteichthyes: Mugilidae) from the littoral of the state of Rio de Janeiro, Brazil. Revista Brasileira de Biologia 57: 441–454.
- Knoff M, São Clemente SC, Andrada CDG, Lima FC, Padovani RES, Fonseca MCG, Neves RCF, Gomes DC (2008) Cestóides Pseudophyllidea parasitos de congro-rosa, Genypterus brasiliensis Regan, 1903 comercializados no estado do Rio de Janeiro, Brasil. Revista Brasileira de Ciência Veterinária 15: 28–32. https://doi.org/10.4322/rbcv.2014.192
- Knoff M, São Clemente SC, Fonseca MCG, Felizardo NN, Pinto RM, Gomes DC (2011) Cestodes Diphyllobothriidea parasitizing blackfin goosefish, Lophius gastrophysus Miranda-Ribeiro, 1915. Arquivo Brasileiro de Medicina Veterinária e Zootecnia 63: 1033–1038. https://doi.org/10.1590/S0102-09352011000400035
- Knoff M, São Clemente SC, Pinto RM, Gomes DC (2002) Prevalência e intensidade de infecção de cestóides Trypanorhyncha de elasmobrânquios nos estados do Paraná e Santa Catarina, Brasil. Parasitología Latinoamericana 57: 149–157. https://doi.org/10.4067/S071777122002000300012
- Knoff M, São Clemente SC, Pinto RM, Gomes DC (2004a) Registros taxonômicos de cestóides Trypanorhyncha/Homeacanthoidea em elasmobrânquios coletados na costa do Estado do Paraná, Brasil. Parasitología Latinoamericana 59: 31–36. https://doi.org/10.4067/S0717-77122004000100006
- Knoff M, São Clemente SC, Pinto RM, Lanfredi RM, Gomes DC (2004b) New records and expanded descriptions of Tentacularia coryphaenae and Hepatoxylon trichiuri homeacanth trypanorhynchs (Eucestoda) from carcharhinid sharks from the State of Santa Catarina off-shore, Brazil. Revista Brasileira de Parasitologia Veterinária 13: 73–80.
- Knoff M, São Clemente SC, Pinto RM, Lanfredi RM, Gomes DC (2004c) Taxonomic reports of Otobothrioidea (Eucestoda, Trypanorhyncha) from elasmobranch fishes of the southern coast off Brazil. Memórias do Instituto Oswaldo Cruz 99: 31–36. https://doi.org/10.1590/S0074-02762004000100006
- Knoff M, São Clemente SC, Pinto RM, Lanfredi RM, Gomes DC (2007) Redescription of Gymnorhynchus isuri (Cestoda: Trypanorhyncha) from Isurus oxyrinchus (Elasmobranchii: Lamnidae). Folia Parasitologica 54: 208–214. https://doi.org/10.14411/fp.2007.028
- Koch KR, Jensen K, Caira JN (2012) Three new genera and six new species of lecanicephalideans (Cestoda) from eagle rays of the genus Aetomylaeus (Myliobatiformes: Myliobatidae) from northern Australia and Borneo. Journal of Parasitology 98: 175–198. https://doi.org/10.1645/GE-2798.1
- Kohn A, Fernandes BMM (1987) Estudo comparativo dos helmintos parasitos de peixes do Rio Mogi Guassu, coletados nas excursões realizadas entre 1927 e 1985. Memórias do Instituto Oswaldo Cruz 82: 483–500. https://doi.org/10.1590/S0074-02761987000400006
- Kohn A, Fernandes BMM, Baptista-Farias DMF, Cohen SC, Santos AL, Pamplona-Basilio MC, Vieira MJAF, Feitosa VA (2004) Prevalência de helmintos parasitos dos peixes do açude Pereira de Miranda e dos viveiros do DNOCS (Pentecoste, Ceará, Brasil). Revista Brasileira de Ciência Veterinária 11: 55–57. https://doi.org/10.4322/rbcv.2014.344
- Kohn A, Fernandes BMM, Macedo B, Abramson B (1985) Helminths parasites of freshwater fishes from Pirassununga, SP, Brazil. Memórias do Instituto Oswaldo Cruz 80: 327–336. https://doi.org/10.1590/S0074-02761985000300009
- Kohn A, Fernandes BMM, Pipolo HV, Godoy MP (1988) Helmintos parasitos de peixes das usinas hidreléticas da Eletrosul (Brasil). II: Reservatórios de Salto Osório e de Salto Santiago, Bacia do Rio Iguaçu. Memórias do Instituto Oswaldo Cruz 83: 299–303. https://doi.org/10.1590/S0074-02761988000300006
- Kohn A, Moravec F, Cohen SC, Canzi C, Takemoto RM, Fernandes BMM (2011) Helminths of freshwater fishes in the reservoir of the Hydroelectric Power Station of Itaipu, Paraná, Brazil. Check List 7: 681–690. https://doi.org/10.15560/7.5.681
- Kuchta R, Scholz T (2007) Diversity and distribution of fish tapeworms of the “Bothriocephalidea” (Eucestoda). Parassitologia 49: 129–146.
- Kuchta R, Scholz T, Bray RA (2008) Revision of the order Bothriocephalidea Kuchta, Scholz, Brabec & Bray, 2008 (Eucestoda) with amended generic diagnoses and keys to families and genera. Systematic Parasitology 71: 81–136. https://doi.org/10.1007/s11230-008-9153-7
- Kuchta R, Serrano-Martínez ME, Scholz T (2015) Pacific broad tapeworm Adenocephalus pacificus as a causative agent of globally reemerging diphyllobothriosis. Emerging Infectous Diseases 21: 1697–1703. https://doi.org/10.3201/eid2110.150516
- Kullander SO, Ferreira EJG (2006) A review of the South American cichlid genus Cichla, with descriptions of nine new species (Teleostei: Cichlidae). Ichthyological Exploration of Freshwaters 17: 289–398.
- Kuraiem BP, Knoff M, Felizardo NN, Gomes DC, São Clemente SC (2016) Callitetrarhynchus speciosus (Linton, 1897) Carvajal & Rego, 1985 Trypanorhyncha (Cestoda) parasitizing Priacanthus arenatus (Cuvier, 1829) (Osteichthyes, Priacanthidae) from Rio de Janeiro coast, Brazil. Neotropical Helminthology 10: 33–40.
- La Rue GR (1911) A revision of the cestode family Proteocephalidae. Zoologischer Anzeiger 38: 473–482. http://www.biodiversitylibrary.org/item/95304#page/485/mode/1up
- La Rue GR (1914) A revision of the cestode family Proteocephalidae. Illinois Biological Monographs 1: 1–349. https://doi.org/10.5962/bhl.title.16792
- Lacerda ACF, Takemoto RM, Pavanelli GC (2008) Digenea, Nematoda, Cestoda, and Acanthocephala, parasites in Potamotrygonidae (Chondrichthyes) from the upper Paraná River floodplain, states of Paraná and Mato Grosso do Sul, Brazil. Check List 4: 115–122. https://doi.org/10.15560/4.2.115
- Lacerda ACF, Takemoto RM, Pavanelli GC (2009) Ecology of endoparasites of the fluvial stingray Potamotrygon falkneri (Chondrichthyes: Potamotrygonidae) from the upper Paraná River floodplain, Brazil. Brazilian Journal of Biology 69: 297–303. https://doi.org/10.1590/S1519-69842009000200009
- Lacerda ACF, Takemoto RM, Poulin R, Pavanelli GC (2013) Parasites of the fish Cichla piquiti (Cichlidae) in native and invaded Brazilians basins: release not from the enemy, but from its effects. Parasitology Research 112: 279–288. https://doi.org/10.1007/s00436-012-3135-z
- Lacerda ACF, Takemoto RM, Tavares-Dias M, Poulin R, Pavanelli GC (2012) Comparative parasitism of the fish Plagioscion squamosissimus in native and invaded river basins. Journal of Parasitology 98: 713–717. https://doi.org/10.1645/GE-2882.1
- Lanfranchi AL, Rossin MA, Timi JT (2009) Parasite infracommunities of a specialized marine fish species in a compound community dominated by generalist parasites. Journal of Helminthology 83: 373–378. https://doi.org/10.1017/S0022149X09390069
- Leible MD, Carvajal JG, Fuentealba MC (1990) Polimorfismo en Raja (Dipturus) flavirostris Philippi, 1892: análisis morfológico y parasitario. Boletín de la Sociedad de Biología de Concepción 61: 93–102.
- Lima FC, São Clemente SC, Mesquita EF (1997) Pseudocistos cecais associados a parasitose por Scolex pleuronectis em sardinha-verdadeira (Sardinella brasiliensis). Parasitología al Día 21: 58–60.
- Littlewood DTJ, Bray RA, Waeschenbach A (2015) Phylogenetic patterns of diversity in the cestodes and trematodes. In: Morand S, Krasnov BR, Littlewood DTJ (Eds) Parasite Diversity and Diversification: Evolutionary Ecology Meets Phylogenetics. Cambridge University Press, Cambridge, 304–319. https://doi.org/10.1017/CBO9781139794749.020
- Lizama MAP, Takemoto RM, Pavanelli GC (2005) Influence of host sex and age on infracommunities of metazoan parasites of Prochilodus lineatus (Valenciennes, 1836) (Prochilodontidae) of the upper Paraná River floodplain, Brazil. Parasite 12: 299–304. https://doi.org/10.1051/parasite/2005124299
- Lizama MAP, Takemoto RM, Pavanelli GC (2006) Parasitism influence on the hepato, splenosomatic and weight/length relation and relative condition factor of Prochilodus lineatus (Valenciennes, 1836) (Prochilodontidae) of the Upper Paraná River floodplain, Brazil. Revista Brasileira de Parasitologia Veterinária 15: 116–122.
- Lizama MAP, Takemoto RM, Pavanelli GC (2008) Ecological aspects of metazoan parasites of Astyanax altiparanae Garutti & Britski, 2000 (Characidae) of the upper Paraná River floodplain, Brazil. Boletim do Instituto de Pesca 34: 527–533.
- Llerena CZ, Chávez AV, Casas EA (2001) Frecuencia de larvas Diphyllobothriidae y larvas Anisakidae en peces marinos comerciales del terminal pesquero de Ventanilla-Callao. Revista de Investigaciones Veterinarias del Perú 12: 58–62.
- Lopes LPC, Varella AMB, Malta JCO (2009) Metazoan parasites of Pseudoplatystoma punctifer (Linnaeus, 1766) and Pseudoplatystoma tigrinum (Spix & Agassiz, 1829) Siluriformes: Pimelodidae) of the Central Amazon Basin, Brazil. Biologia Geral e Experimental 9: 3–15.
- López E (1994) Acanthobothroides peruensis n. sp. (Tetraphyllidea: Onchobothriidae) parásito de Dasyatis brevis G. (Rajiformes: Dasyatidae) de la costa del Perú. Biotempo 1: 19–20.
- López de McDonald E, Tantaleán MV (1985) Contribución al conocimiento de la helmintofauna de peces marinos de la costa peruana. Parasitología al Día 9: 40–43.
- López-Neyra CR, Diaz-Ungría C (1958) Cestodes de Venezuela. V. Cestodes de vertebrados Venezolanos. Novedades Cientificas: Contribuciones Ocasionales del Museo de Historia Natural La Salle 23: 1–42.
- Luchetti NM, Marques FPL, Charvet-Almeida P (2008) A new species of Potamotrygonocestus Brooks & Thorson, 1976 (Eucestoda: Tetraphyllidea) from Plesiotrygon iwamae Rosa, Castello & Thorson (Mylliobatoidea: Potamotrygonidae) and a redescription of Potamotrygonocestus chaoi Marques, Brooks & Araujo, 2003. Systematic Parasitology 70: 131–145. https://doi.org/10.1007/s11230-008-9135-9
- Luque JL (1991) Formas larvarias de helmintos parásitos en especies marinas del Perú. Parasitología al Día 15: 43–48.
- Luque JL, Alves DR (2001) Ecologia das comunidades de metazoários parasitos do xaréu, Caranx hippos (Linnaeus) e do xerelete, Caranx latus Agassiz (Osteichthyes, Carangidae) do litoral do Estado do Rio de Janeiro, Brasil. Revista Brasileira de Zoologia 18: 399–410. https://doi.org/10.1590/S0101-81752001000200011
- Luque JL, Alves DR, Ribeiro RS (2003) Community ecology of the metazoan parasites of banded croaker, Paralonchurus brasiliensis (Osteichthyes: Sciaenidae), from the coastal zone of the State of Rio de Janeiro, Brazil. Acta Scientiarum: Biological Sciences 25: 273–278. https://doi.org/10.4025/actascibiolsci.v25i2.2009
- Luque JL, Amato JFR, Takemoto RM (1995) Helminth larval stages in Orthopristis ruber and Haemulon steindachneri (Osteichthyes: Haemulidae) from the coast of the state of Rio de Janeiro, Brazil. Revista Brasileira de Biologia 55: 33–38.
- Luque JL, Amato JFR, Takemoto RM (1996a) Comparative analysis of the communities of metazoan parasites of Orthopristis ruber and Haemulon steindachneri (Osteichthyes: Haemulidae) from the southeastern Brazilian litoral: I. structure and influence of the size and sex of hosts. Revista Brasileira de Biologia 56: 279–292.
- Luque JL, Amato JFR, Takemoto RM (1996b) Comparative analysis of the communities of metazoan parasites of Orthopristis ruber and Haemulon steindachneri (Osteichthyes: Haemulidae) from the southeastern Brazilian litoral: II diversity, interspecifc associations, and distribution of the gastrointestinal parasites. Revista Brasileira de Biologia 56: 292–302.
- Luque JL, Chaves ND (1999) Ecologia da comunidade de metazoários parasitos da anchova Pomatomus saltator (Linnaeus) (Osteichthyes, Pomatomidae) do litoral do estado do Rio de Janeiro, Brasil. Revista Brasileira de Zoologia 16: 711–723. https://doi.org/10.1590/S0101-81751999000300010
- Luque JL, Cordeiro AS, Oliva ME (2010) Metazoan parasites as biological tags for stock discrimination of whitemouth croaker Micropogonias furnieri. Journal of Fish Biology 76: 591–600. https://doi.org/10.1111/j.1095-8649.2009.02515.x
- Luque JL, Felizardo NN, Tavares LER (2008) Community ecology of the metazoan parasites of namorado sandperches, Pseudopercis numida Miranda-Ribeiro, 1903 and P. semifasciata Cuvier, 1829 (Perciformes: Pinguipedidae), from the coastal zone of the State of Rio de Janeiro, Brazil. Brazilian Journal of Biology 68: 269–278. https://doi.org/10.1590/S1519-69842008000200007
- Luque JL, Porrozzi F, Alves DR (2002) Community ecology of the metazoan parasites of Argentine goatfish, Mullus argentinae (Osteichthyes: Mullidae), from the coastal zone of the state of Rio de Janeiro, Brazil. Revista Brasileira de Parasitologia Veterinária 11: 33–38.
- Luque JL, Poulin R (2004) Use of fish as intermediate hosts by helminth parasites: a comparative analysis. Acta Parasitologica 49: 353–361.
- Luque JL, Poulin R (2007) Metazoan parasite species richness in Neotropical fishes: hotspots and the geography of biodiversity. Parasitology 134: 865–878. https://doi.org/10.1017/S0031182007002272
- Lynsdale JA (1959) On a new species of Proteocephalus from Brazil. Journal of Helminthology 33: 145–150. https://doi.org/10.1017/S0022149X00033381
- MacDonagh E (1927) Parásitos de peces comestibles – III. Dos cestodarios: Gyrocotyle rugosa del "Pez gallo" y Gyrocotyle maxima n. sp. del "Gatuso". La Semana Médica 34: 1232–1235.
- MacDonagh E (1932) Sobre una Ichthyotaenia y oncosfera del pejerrey. La Semana Médica 25: 1‒16.
- Machado MH, Pavanelli GC, Takemoto RM (1994) Influence of host’s sex and size on endoparasitic infrapopulations of Pseudoplatystoma corruscans and Schizodon borelli (Osteichthyes) of the high Paraná River, Brazil. Revista Brasileira de Parasitologia Veterinária 3: 143–148.
- Machado MH, Pavanelli GC, Takemoto RM (1995) Influence of the type of environment and of the hydrological level variation in endoparasitic infrapopulations of Pseudoplatystoma corruscans (Agassiz) and Schizodon borelli (Boulenger) (Osteichthyes) of the high Paraná River, Brazil. Revista Brasileira de Zoologia 12: 961–976. https://doi.org/10.1590/S0101-81751995000400023
- Machado MH, Pavanelli GC, Takemoto RM (1996) Structure and diversity of endoparasitic infracommunities and the trophic level of Pseudoplatystoma corruscans and Schizodon borelli (Osteichthyes) of the high Paraná River. Memórias do Instituto Oswaldo Cruz 91: 441–448. https://doi.org/10.1590/S0074-02761996000400010
- Machado PM, Almeida SC, Pavanelli GC, Takemoto RM (2000) Ecological aspects of endohelminths parasitizing Cichla monoculus Spix, 1831 (Perciformes: Cichlidae) in the Paraná River near Porto Rico, State of Paraná, Brazil. Comparative Parasitology 67: 210–217.
- MacKenzie K, Brickle P, Hemmingsen W, George-Nascimento M (2013) Parasites of hoki, Macruronus magellanicus, in the Southwest Atlantic and Southeast Pacific Oceans, with an assessment of their potential value as biological tags. Fisheries Research 145: 1–5. https://doi.org/10.1016/j.fishres.2013.03.008
- MacKenzie K, Longshaw M (1995) Parasites of the hakes Merluccius australis and M. hubbsi in the waters around the Falkland Islands, southern Chile, and Argentina, with an assessment of their potential value as biological tags. Canadian Journal of Fisheries and Aquatic Sciences 52: 213–224. https://doi.org/10.1139/f95-529
- Mancini M, Bucco C, Salinas V, Larriestra A, Tanzola R, Guagliardo S (2008) Seasonal variation of parasitism in pejerrey Odontesthes bonariensis (Atheriniformes, Atherinopsidae) from La Viña reservoir (Córdoba, Argentina). Revista Brasileira de Parasitologia Veterinária 17: 28–32. https://doi.org/10.1590/S1984-29612008000100006
- Mancini M, Grosman F (1998) Aspectos poblacionales del pejerrey Odontesthes bonariensis en el embalse Río Tercero, Córdoba. Natura Neotropicalis 2: 137–143.
- Mariaux J (1998) A molecular phylogeny of the Eucestoda. Journal of Parasitology 84: 114–124. https://doi.org/10.2307/3284540
- Marques FPL, Brooks DR (2003) Taxonomic revision of Rhinebothroides (Eucestoda: Tetraphyllidea: Phyllobothriidae), parasites of Neotropical freshwater stingrays (Rajiformes: Myliobatoidei: Potamotrygonidae). Journal of Parasitology 89: 994–1017. https://doi.org/10.1645/GE-3059
- Marques F, Brooks DR, Ureña HM (1996) Two new species of tetraphyllidean cestodes in Himantura pacifica (Chondrichthyes: Myliobatiformes: Dasyatididae) from the northwest coast of Costa Rica. Journal of Parasitology 82: 302–306. https://doi.org/10.2307/3284165
- Marques FPL, Brooks DR, Araújo MLG (2003) Systematics and phylogeny of Potamotrygonocestus (Platyhelminthes, Tetraphyllidea, Onchobothriidae) with descriptions of three new species from freshwater potamotrygonids (Myliobatoidei, Potamotrygonidae). Zoologica Scripta 32: 367–396. https://doi.org/10.1046/j.1463-6409.2003.00111.x
- Marques FPL, Brooks DR, Barriga R (1997) Six species of Acanthobothrium (Eucestoda: Tetraphyllidea) in stingrays (Chondrichthyes: Rajiformes: Myliobatoidei) from Ecuador. Journal of Parasitology 83: 475–484. https://doi.org/10.2307/3284414
- Marques FPL, Brooks DR, Lasso CA (2001) Anindobothrium n. gen. (Eucestoda: Tetraphyllidea) inhabiting marine and freshwater potamotrygonid stingrays. Journal of Parasitology 87: 666–672. https://doi.org/10.2307/3285110
- Marques FPL, Caira JN (2016) Pararhinebothroides – neither the sister-taxon of Rhinebothroides nor a valid genus. Journal of Parasitology 102: 249–259. https://doi.org/10.1645/15-894
- Marques FPL, Jensen K, Caira JN (2012) Ahamulina n. gen. (Cestoda: Diphyllidea) from the polkadot catshark, Scyliorhinus besnardi (Carcharhiniformes: Scyliorhinidae), off Brazil. Zootaxa: 51–59.
- Marques FPL, Reyda FB (2015) Rhinebothrium jaimei sp. n. (Eucestoda: Rhinebothriidea: Rhinebothriidae): a new species from Neotropical freshwater stingrays (Potamotrygonidae). Folia Parasitologica 62: 057. https://doi.org/10.14411/fp.2015.057
- Martins ML, Pereira JJr, de Chambrier A, Mouriño JLP (2011) Infection levels of proteocephalidean cestodes in Cichla piquiti (Osteichthyes: Cichlidae) of the Volta Grande Reservoir, Minas Gerais, Brazil, relative to host body weight and gender. Journal of Helminthology 85: 462–467. https://doi.org/10.1017/S0022149X10000830
- Martins ML, Pereira JJr, de Chambrier A, Yamashita MM (2009) Proteocephalid cestode infection in alien fish, Cichla piquiti Kullander and Ferreira, 2006 (Osteichthyes: Cichlidae), from Volta Grande reservoir, Minas Gerais, Brazil. Brazilian Journal of Biology 69: 189–195. https://doi.org/10.1590/S1519-69842009000100025
- Mateo E, Bullock WL (1966) Neobothriocephalus aspinosus gen. et sp. n. (Pseudophyllidea: Parabothriocephalidae), from the Peruvian marine fish, Neptomenus crassus. Journal of Parasitology 52: 1070–1073. https://doi.org/10.2307/3276350
- Mattos DPBG, Verícimo MA, Lopes LMS, São Clemente SC (2015) Immunogenic activity of the fish tapeworm Pterobothrium heteracanthum (Trypanorhyncha: Pterobothriidae) in BALB/c mice. Journal of Helminthology 89: 203–207. https://doi.org/10.1017/S0022149X13000795
- Mayes MA, Brooks DR (1981) Cestode parasites of some Venezuelan stingrays. Proceedings of the Biological Society of Washington 93: 1230–1238.
- Mayes MA, Brooks DR, Thorson TB (1978) Two new species of Acanthobothrium van Beneden 1849 (Cestoidea: Tetraphyllidea) from freshwater stingrays in South America. Journal of Parasitology 64: 838–841. https://doi.org/10.2307/3279513
- Mayes MA, Brooks DR, Thorson TB (1981) Two new tetraphyllidean cestodes from Potamotrygon circularis Garman (Chondrichthyes: Potamotrygonidae) in the Itacuaí River, Brazil. Proceedings of the Biological Society of Washington 48: 38–42.
- Mendes MV (1944) Sobre Cestoda de teleósteos marinhos. Boletim da Faculdade de Filosofia, Ciências e Letras da Universidade de São Paulo, Série Zoologia 43: 173–187.
- Mendívil-Herrera J (1946) Gyrocotyle meandrica n. sp., del intestino espiral de pez gallo, Callorhynchus callorhynchus (L.). Comunicaciones Zoologicas del Museo de Historia Natural de Montevideo 36: 1–12.
- Menoret A, Ivanov VA (2009a) A new species of tetraphyllidean (Cestoda) from the largespot river stingray, Potamotrygon falkneri (Potamotrygonidae: Chondrichthyes), from the Paraná Basin. Journal of Parasitology 95: 994–999. https://doi.org/10.1645/GE-1850.1
- Menoret A, Ivanov VA (2009b) New name for Progrillotia dollfusi Carvajal et Rego, 1983 (Cestoda: Trypanorhyncha): description of adults from Squatina guggenheim (Chondrichthyes: Squatiniformes) off the coast of Argentina. Folia Parasitologica 56: 284–294. https://doi.org/10.14411/fp.2009.033
- Menoret A, Ivanov VA (2011) Descriptions of two new freshwater Neotropical species of Rhinebothrium (Cestoda: Rhinebothriidea) from Potamotrygon motoro (Chondrichthyes: Potamotrygonidae). Folia Parasitologica 58: 178–186. https://doi.org/10.14411/fp.2011.018
- Menoret A, Ivanov VA (2012) Description of plerocerci and adults of a new species of Grillotia (Cestoda: Trypanorhyncha) in teleosts and elasmobranchs from the Patagonian shelf off Argentina. Journal of Parasitology 98: 1185–1199. https://doi.org/10.1645/GE-3107.1
- Menoret A, Ivanov VA (2013) A new species of Heteronybelinia (Cestoda: Trypanorhyncha) from Sympterygia bonapartii (Rajidae), Nemadactylus bergi (Cheilodactylidae) and Raneya brasiliensis (Ophidiidae) in the south-western Atlantic, with comments on host specificity of the genus. Journal of Helminthology 87: 467–482. https://doi.org/10.1017/S0022149X12000545
- Menoret A, Ivanov VA (2014) Eutetrarhynchid trypanorhynchs (Cestoda) from elasmobranchs off Argentina, including the description of Dollfusiella taminii sp. n. and Parachristianella damiani sp. n., and amended description of Dollfusiella vooremi (Sao Clemente et Gomes, 1989). Folia Parasitologica 61: 411–431. https://doi.org/10.14411/fp.2014.056
- Menoret A, Ivanov VA (2015) Trypanorhynch cestodes (Eutetrarhynchidae) from batoids along the coast of Argentina, including the description of new species in Dollfusiella Campbell et Beveridge, 1994 and Mecistobothrium Heinz et Dailey, 1974. Folia Parasitologica 62: 058. https://doi.org/10.14411/fp.2015.058
- Mesquita RLB, Santos SMC, Ceccarelli PS, Luque JL (2012) Metazoários endoparasitos de Salminus brasiliensis (Cuvier, 1816) (Characiformes: Characidae) do rio Mogi Guaçu, SP, Brasil. Revista Brasileira de Zoociências 14: 95–102.
- Miloslavich P, Klein E, Díaz JM, Hernández CE, Bigatti G, Campos L, Artigas F, Castillo J, Penchaszadeh PE, Neill PE, Carranza A, Retana MV, Díaz de Astarloa JM, Lewis M, Yorio P, Piriz ML, Rodríguez D, Yoneshigue-Valentin Y, Gamboa L, Martín A (2011) Marine biodiversity in the Atlantic and Pacific coasts of South America: knowledge and gaps. PLoS ONE 6: e14631. https://doi.org/10.1371/journal.pone.0014631
- Mola P (1906) Di alcune specie poco studiate o mal note di Cestodi. Annuario del Museo Zoologico della R Università di Napoli 2: 1–12.
- Monteiro CM, Santos MD, Zuchi NA, Brasil-Sato MC (2009) Ecological parameters of the endohelminths in relation to size and sex of Prochilodus argenteus (Actinopterygii: Prochilodontidae) from the Upper São Francisco River, Minas Gerais, Brazil. Zoologia (Curitiba) 26: 753–757. https://doi.org/10.1590/S1984-46702009000400021
- Monticelli FS (1891) Noticie su di alcune specie di Taenia. Bollettino della Società dei Naturalisti in Napoli 5: 151–174.
- Monticelli FS (1892) Appunti sui Cestodaria. Atti della Reale Accademia delle Scienze Fisiche e Matematiche, Napoli 5: 67–78.
- Moreira J, Paschoal F, Cezar AD, Luque JL (2015) Community ecology of the metazoan parasites of Brazilian sardinella, Sardinella brasiliensis (Steindachner, 1879) (Actinopterygii: Clupeidae) from the coastal zone of the State of Rio de Janeiro, Brazil. Brazilian Journal of Biology 75: 736–741. https://doi.org/10.1590/1519-6984.00114
- Moreira LHA, Takemoto RM, Pagotto JPA, Pavanelli GC (2014) Endoparasite community structure of three fish species in tributary streams of the river Pirapó, Paraná state, Brazil. Neotropical Helminthology 8: 97–109.
- Moreira ST, Ito KF, Takemoto RM, Pavanelli GC (2005) Ecological aspects of the parasites of Iheringichthys labrosus (Lütken, 1874) (Siluriformes: Pimelodidae) in reservoirs of Paraná basin and upper Paraná floodplain, Brazil. Acta Scientiarum: Biological Sciences 27: 317–322. https://doi.org/10.4025/actascibiolsci.v27i4.1265
- Moreno AZ, Fuentes JL, Mago Y, Chinchilla O (2008) Descripción, taxonomía e índices ecológicos de parásitos en peces de la laguna de los Mártires, Isla de Margarita, Venezuela. SABER, Revista Multidisciplinaria del Consejo de Investigación de la Universidad de Oriente 20: 3–11.
- Müller MI, Madi RR, Ueta MT (2008) Primeiro registro de ocorrência de cestódeos da família Bothriocephalidae Blanchard, 1849 (Pseudophyllidea), parasitando Cichla monoculus (Cichlidae) nas lagoas da Fazenda Rio das Pedras, Campinas (SP). Bioikos 22: 45–49.
- Muñoz G (2014) Parasites communities in the clingfish Gobiesox marmoratus from central Chile. Acta Parasitologica 59: 108–114. https://doi.org/10.2478/s11686-014-0215-5
- Muñoz G, Delorme N (2011) Variaciones temporales de las comunidades de parásitos en peces intermareales de Chile central: hospedadores residentes vs temporales. Revista de Biología Marina y Oceanografía 46: 313–327. https://doi.org/10.4067/S0718-19572011000300003
- Muñoz G, Garcías F, Valdebenito V, George-Nascimento M (2001) Parasitofauna y alimentación de Notothenia cf. angustata Hutton, 1875 (Pisces: Nototheniidae) en el intermareal de dos localidades del Golfo de Arauco, Chile. Boletín Chileno de Parasitología 56: 29–33. https://doi.org/10.4067/S0365-94022001000100008
- Muñoz G, Olmos V (2008) Revisión bibliográfica de especies endoparásitas y hospedadoras de sistemas acuáticos de Chile. Revista de Biología Marina y Oceanografía 43: 173–245. https://doi.org/10.4067/S0718-19572008000200002
- Muñoz G, Randhawa HS (2011) Monthly variation in the parasite communities of the intertidal fish Scartichthys viridis (Blenniidae) from central Chile: are there seasonal patterns? Parasitology Research 109: 53–62. https://doi.org/10.1007/s00436-010-2220-4
- Muñoz G, Zamora L (2011) Ontogenetic variation in parasite infracommunities of the clingfish Sicyases sanguineus (Pisces: Gobiesocidae). Journal of Parasitology 97: 14–19. https://doi.org/10.1645/GE-2445.1
- Muñoz SA, George-Nascimento M (2008) The effect of Anonchocephalus chilensis Riggenbach (Eucestoda: Bothriocephalidea) on infracommunity patterns in Genypterus maculatus Tschudi (Osteichthyes: Ophidiidae). Journal of Helminthology 82: 221–226. https://doi.org/10.1017/S0022149X08960788
- Mutti LD, Ivanov VA (2016) A new species of Paraberrapex Jensen, 2001 (Cestoda: Lecanicephalidea) from Squatina guggenheim Marini (Squatiniformes: Squatinidae) off Argentina. Folia Parasitologica 63: 007. https://doi.org/10.14411/fp.2016.007
- Myers N, Mittermeier RA, Mittermeier CG, Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403: 853–858. https://doi.org/10.1038/35002501
- Ñacari LA, Oliva ME (2016) Metazoan parasites of deep-sea fishes from the South Eastern Pacific: exploring the role of ecology and host phylogeny. Deep Sea Research Part I: Oceanographic Research Papers 115: 123–130. https://doi.org/10.1016/j.dsr.2016.06.002
- Napoleão SR, Antonucci AM, Amorim AF, Takemoto RM (2015) Occurrence of Rhinoptericola megacantha (Cestoda, Trypanorhyncha) in new host and new location. Arquivo Brasileiro de Medicina Veterinária e Zootecnia 67: 1175–1177. https://doi.org/10.1590/1678-4162-7015
- Naylor GJP, Caira JN, Jensen K, Rosana KAM, White WT, Last PR (2012) A DNA sequence-based approach to the identification of shark and ray species and its implications for global elasmobranch diversity and parasitology. Bulletin of the American Museum of Natural History 367: 1–262. https://doi.org/10.1206/754.1
- Neghme M, Bertín V (1951) Diphyllobothrium latum en Chile. IV Estado actual de las investigaciones epidemiológicas. Revista Chilena de Higiene y Medicina Preventiva 13: 8–11.
- Neghme M, Bertín V, Tagle I, Silva R, Artigas J (1950) Diphyllobothrium latum en Chile. II Primera encuesta en el lago Colico. Boletín de Infecciones Parasitarias Chilenas 5: 16–17.
- Niklitschek EJ, Secor DH, Toledo P, Lafon A, George-Nascimento M (2010) Segregation of SE Pacific and SW Atlantic southern blue whiting stocks: integrating evidence from complementary otolith microchemistry and parasite assemblage approaches. Environmental Biology of Fishes 89: 399–413. https://doi.org/10.1007/s10641-010-9695-9
- Nock AM, Caira JN (1988) Disculiceps galapagoensis n. sp. (Lecanicephalidea: Disculicepitidae) from the shark, Carcharhinus longimanus, with comments on D. pileatus. Journal of Parasitology 74: 153–158. https://doi.org/10.2307/3282492
- Oliva ME (1982) Parásitos en peces marinos de la zona de Antofagasta. Ciencia y Tecnología del Mar 6: 45–51.
- Oliva ME (1994) Parasites of the Chilean jack mackerel Trachurus symmetricus murphyi (Pisces: Carangidae). Memórias do Instituto Oswaldo Cruz 89: 363–364. https://doi.org/10.1590/S0074-02761994000300011
- Oliva ME (1999) Metazoan parasites of the jack mackerel Trachurus murphyi (Teleostei, Carangidae) in a latitudinal gradient from South America (Chile and Peru). Parasite 6: 223–230. https://doi.org/10.1051/parasite/1999063223
- Oliva ME (2001) Metazoan parasites of Macruronus magellanicus from southern Chile as biological tags. Journal of Fish Biology 58: 1617–1622. https://doi.org/10.1006/jfbi.2001.1569
- Oliva ME, Ballón I (2002) Metazoan parasites of the Chilean hake Merluccius gayi gayi as a tool for stock discrimination. Fisheries Research 56: 313–320. https://doi.org/10.1016/S0165-7836(01)00329-0
- Oliva ME, Castro RE, Burgos R (1996) Parasites of the flatfish Paralichthys adspersus (Steindachner, 1867) (Pleuronectiformes) from northern Chile. Memórias do Instituto Oswaldo Cruz 91: 301–306. https://doi.org/10.1590/S0074-02761996000300009
- Oliva ME, Fernández I, Oyarzun C, Murillo C (2008a) Metazoan parasites of the stomach of Dissostichus eleginoides Smitt 1898 (Pisces: Notothenidae) from southern Chile: a tool for stock discrimination? Fisheries Research 91: 119–122. https://doi.org/10.1016/j.fishres.2007.11.012
- Oliva ME, González MT, Acuña E (2004) Metazoan parasite fauna as a biological tag for the habitat of the flounder Hippoglossina macrops from northern Chile, in a depth gradient. Journal of Parasitology 90: 1374–1377. https://doi.org/10.1645/GE-317R
- Oliva ME, Luque JL (1998) Metazoan parasite infracommunities in five sciaenids from the central Peruvian coast. Memórias do Instituto Oswaldo Cruz 93: 175–180. https://doi.org/10.1590/S0074-02761998000200007
- Oliva ME, Luque JL (2002) Endohelminth parasites of the trambollo Labrisomus philippii (Steindachner) (Osteichthyes: Labrisomidae) from the central Peruvian coast. Comparative Parasitology 69: 100–104. https://doi.org/10.1654/1525-2647(2002)069[0100:EPOTTL]2.0.CO;2
- Oliva ME, Valdivia IM, Costa G, Freitas N, Pinheiro de Carvalho MA, Sánchez L, Luque JL (2008b) What can metazoan parasites reveal about the taxonomy of Scomber japonicus Houttuyn in the coast of South America and Madeira Islands? Journal of Fish Biology 72: 545–554. https://doi.org/10.1111/j.1095-8649.2007.01725.x
- Oliveira MT (1985) Infestação da pescada-foguete, Macrodon ancylodon (Bloch, 1801) Jordan, Evermann e Clark, 1830, e da corvina Micropogon furnieri (Desmarest, 1822) Jordan, 1884 família Sciaenidae do litoral Sul do Brasil, por larvas de Cestoda. Higiene Alimentar 4: 191–201.
- Oliveira SAL, São Clemente SC, Benigno RNM, Knoff M (2009) Poecilancistrium caryophyllum (Diesing, 1850) (Cestoda, Trypanorhyncha), parasite of Macrodon ancylodon (Bloch & Schneider, 1801) from the Northern littoral of Brazil. Revista Brasileira de Parasitologia Veterinária 18: 71–73. https://doi.org/10.4322/rbpv.01804014
- Olson PD, Caira JN (1999) Evolution of the major lineages of tapeworms (Platyhelminthes: Cestoidea) inferred from 18S ribosomal DNA and elongation factor-1α. Journal of Parasitology: 1134–1159. https://doi.org/10.2307/3285679
- Olson PD, Caira JN, Jensen K, Overstreet RM, Palm HW, Beveridge I (2010) Evolution of the trypanorhynch tapeworms: parasite phylogeny supports independent lineages of sharks and rays. International Journal for Parasitology 40: 223–242. https://doi.org/10.1016/j.ijpara.2009.07.012
- Olson PD, Littlewood DTJ, Bray RA, Mariaux J (2001) Interrelationships and evolution of the tapeworms (Platyhelminthes: Cestoda). Molecular Phylogenetics and Evolution 19: 443–467. https://doi.org/10.1006/mpev.2001.0930
- Ortubay SG, Semenas LG, Ubeda CA, Quaggiotto AE, Viozzi GP (1994) Catálogo de peces dulceacuícolas de la Patagonia Argentina y sus parásitos metazoos. Direccion de Pesca, Subsecretaria de Recursos Naturales, San Carlos de Bariloche, 110 pp.
- Ostrowski de Núñez M (1971) Estudios preliminares sobre la fauna parasitaria de algunos elasmobranquios del litoral bonaerense, Mar del Plata, Argentina. I. Cestodes y trematodes de Psammobatis microps (Günther) y Zapteryx brevirostris (Müller y Henle). Physis 30: 425–446.
- Ostrowski de Núñez M (1973) Estudios preliminares sobre la fauna parasitaria de algunos elasmobranquios del litoral bonaerense, Mar del Plata, Argentina. II. Cestodes de Mustelus schmitti Springer 1939. Physis 32: 1–14.
- Palm HW (1997) Trypanorhynch cestodes of commercial fishes from northeast Brazilian coastal waters. Memórias do Instituto Oswaldo Cruz 92: 69–79. https://doi.org/10.1590/S0074-02761997000100014
- Palm HW (2004) The Trypanorhyncha Diesing, 1863. PKSPL-IPB Press, Bogor, 710 pp.
- Palm HW, Walter T (2000) Tentaculariid cestodes (Trypanorhyncha) from the Muséum d’Histoire Naturelle, Paris. Zoosystema 22: 641–666.
- Paraguassú AR, Luque JL, Alves DR (2002) Community ecology of the metazoan parasites of red porgy, Pagrus pagrus (L., 1758) (Osteichthyes, Sparidae), from the coastal zone, state of Rio de Janeiro, Brazil. Acta Scientiarum: Biological Sciences 24: 461–467.
- Pardo-Gandarillas MC, González K, Ibáñez CM, George-Nascimento M (2008) Parasites of two deep-sea fish Coelorynchus chilensis (Pisces: Macrouridae) and Notacanthus sexspinis (Pisces: Notacanthidae) from Juan Fernández Archipelago, Chile. Marine Biodiversity Records 1: e76. https://doi.org/10.1017/S1755267207007944
- Paschoal F, Cezar AD, Luque JL (2015) Checklist of metazoan associated with grunts (Perciformes, Haemulidae) from the Nearctic and Neotropical regions. Check List 11: 1501. https://doi.org/10.15560/11.1.1501
- Pavanelli GC, Machado MH (1990) Cangatiella arandasi gen. n. sp. n. (Cestoda-Proteocephalidae), parasito de Parauchenipterus galeatus (Siluriformes-Auchenipteridae) do rio Paraná, PR. Revista Brasileira de Zoologia 7: 531–534. https://doi.org/10.1590/S0101-81751990000400012
- Pavanelli GC, Machado MH (1991) Proteocefalídeos parasitos de peixes, em especial pimelodídeos do rio Paraná, Paraná. Revista Unimar 13: 163–175.
- Pavanelli GC, Machado MH (1992) Goezeella agostinhoi sp. n. e Monticellia loyolai sp. n., cestóides proteocefalídeos parasitas de peixes pimelodídeos do rio Paraná, Paraná, Brasil. Revista Brasileira de Parasitologia Veterinária 1: 45–50.
- Pavanelli GC, Machado MH, Takemoto RM, Santos LC (1994) A new species of proteocephalidean cestode, Monticellia belavistensis sp. n., parasite of Pterodoras granulosus (Valenciennes) (Pisces, Doradidae) from Itaipu reservoir and Paraná River, Paraná, Brazil. Revista Brasileira de Zoologia 11: 587–595. https://doi.org/10.1590/S0101-81751994000400001
- Pavanelli GC, Rego AA (1989) Novas espécies de proteocefalídeos (Cestoda) de Hemisorubim platyrhynchos (Pisces: Pimelodidae) do Estado do Paraná. Revista Brasileira de Biologia 49: 381–386.
- Pavanelli GC, Rego AA (1991) Cestóides proteocefalídeos de Sorubim lima (Schneider, 1801) (Pisces: Pimelodidae) do rio Paraná e reservatório de Itaipu. Revista Brasileira de Biologia 51: 7–12.
- Pavanelli GC, Rego AA (1992) Megathylacus travassosi sp. n. and Nomimoscolex sudobim Woodland, 1935 (Cestoda-Proteocephalidea) parasites of Pseudoplatystoma corruscans (Agassiz, 1829) (Siluriformes-Pimelodidae) from the Itaipu reservoir and Paraná River, Paraná state, Brazil. Memórias do Instituto Oswaldo Cruz 87: 191–195. https://doi.org/10.1590/S0074-02761992000500036
- Pavanelli GC, Takemoto RM (1995) New species of Proteocephalus (Cestoda-Proteocephalidae) parasitic in fishes from the Paraná River, Paraná, Brazil. Memórias do Instituto Oswaldo Cruz 90: 593–596. https://doi.org/10.1590/S0074-02761995000500009
- Pavanelli GC, Takemoto RM (1996) Spasskyellina mandi n. sp. (Proteocephalidea: Monticelliidae), parasite of Pimelodus ornatus Kner, 1857 (Pisces: Pimelodidae) of the Paraná River, Paraná, Brazil. Memórias do Instituto Oswaldo Cruz 91: 723–726. https://doi.org/10.1590/S0074-02761996000600013
- Pavanelli GC, Takemoto RM (2000) Aspects of the ecology of proteocephalid cestodes, parasites of Sorubim lima (Pimelodidae), of the upper Paraná River, Brazil: II. Interspecific associations and distribution of gastrintestinal parasites. Revista Brasileira de Biologia 60: 585–590. https://doi.org/10.1590/S0034-71082000000400007
- Pereira AN, Pantoja C, Luque JL, Timi JT (2014) Parasites of Urophycis brasiliensis (Gadiformes: Phycidae) as indicators of marine ecoregions in coastal areas of the South American Atlantic. Parasitology Research 113: 4281–4292. https://doi.org/10.1007/s00436-014-4106-3
- Pereira JJr (1993) O complexo de espécies de Trypanorhyncha (Cestoda), em corvinas Micropogonias furnieri do Rio Grande do Sul. Arquivos da Faculdade de Veterinária UFRGS 21: 58–70.
- Pereira JJr (1998) Trypanorhyncha (Cercomeromorpha, Eucestoda) nos Sciaenidae (Neopterygii, Perciformes) do Litoral do Rio Grande do Sul: sistemática, estrutura das comunidades componentes e sua utilização como indicadores da estrutura trófica da assembléia hospedeira. PhD thesis, Curitiba, Brazil: Universidade Federal do Paraná.
- Pereira JJr (2000) New morphologic data on Anonchocephalus chilensis (Riggenbach, 1896) (Triaenophoridae: Pseudophyllidea: Cestoda) and emendation of genus diagnosis. Comunicaçoes do Museu de Ciencias e Tecnologia da PUCRS, Serie Zoologia 13: 99–104.
- Pereira JJr, Boeger WA (2005) Larval tapeworms (Platyhelminthes, Cestoda) from sciaenid fishes of the southern coast of Brazil. Zoosystema 27: 5–25.
- Pérez I, Chávez A, Casas E (1999) Presencia de formas parasitarias en peces comerciales del mar peruano. Revista de Investigaciones Veterinarias del Perú 10: 34–38.
- Piazza RS, Martins ML, Guiraldelli L, Yamashita MM (2006) Parasitic diseases of freshwater ornamental fishes commercialized in Florianópolis, Santa Catarina, Brazil. Boletim do Instituto de Pesca 32: 51–57.
- Pinto HA, Melo AL (2011a) Metacestodes of Parvitaenia macropeos (Cyclophyllidea, Gryporhynchidae) in Australoheros facetus (Pisces, Cichlidae) in Brazil. Neotropical Helminthology 5: 279–283.
- Pinto HA, Melo AL (2011b) Metacestodes of Glossocercus auritus (Cyclophyllidea, Gryporhynchidae) in Poecilia reticulata (Pisces, Poeciliidae) from Brazil. Revista Brasileira de Parasitologia Veterinária 20: 161–164. https://doi.org/10.1590/S1984-29612011000200012
- Pinto RM, Knoff M, São Clemente SC, Lanfredi RM, Gomes DC (2006) The taxonomy of some Poecilacanthoidea (Eucestoda: Trypanorhyncha) from elasmobranchs off the southern coast of Brazil. Journal of Helminthology 80: 291–298. https://doi.org/10.1079/JOH2006339
- Piorski NM, Garavello JC, Arce HM, Sabaj-Pérez MH (2008) Platydoras brachylecis, a new species of thorny catfish (Siluriformes: Doradidae) from northeastern Brazil. Neotropical Ichthyology 6: 481–494. https://doi.org/10.1590/S1679-62252008000300021
- Poche F (1922) Zur Kenntnis der Amphilinidea. Zoologischer Anzeiger 54: 276–287. http://www.biodiversitylibrary.org/part/68459
- Porto CJS, São Clemente SC, Freitas MQ, São Clemente RRB, Knoff M, Matos E (2009) Pterobothrium crassicolle (Eucestoda: Trypanorhyncha) em corvinas, Micropogonias furnieri, comercializadas no município de Niterói, Rio de Janeiro, Brasil. Revista Brasileira de Ciência Veterinária 16: 133–135.
- Poulin R, Besson AA, Morin MB, Randhawa HS (2016) Missing links: testing the completeness of host-parasite checklists. Parasitology 143: 114–122. https://doi.org/10.1017/S0031182015001559
- Poulin R, Leung TLF (2010) Taxonomic resolution in parasite community studies: are things getting worse? Parasitology 137: 1967–1973. https://doi.org/10.1017/S0031182010000910
- Rabey JS (1973) Un cestode parasito del tubo digestivo del bagresapo (Rhamdia sapo) de la laguna de Chascomús, Provincia de Buenos Aires. Physis 32: 115–119.
- Rauque CA, Viozzi GP, Semenas LG (2003) Component population study of Acanthocephalus tumescens (Acanthocephala) in fishes from Lake Moreno, Argentina. Folia Parasitologica 50: 72–78. https://doi.org/10.14411/fp.2003.013
- Rego AA (1975) Estudos de cestóides de peixes do Brasil. 2ª Nota: revisão do gênero Monticellia La Rue, 1911 (Cestoda, Proteocephalidae). Revista Brasileira de Biologia 35: 567–586.
- Rego AA (1977) Cestóides parasitas de Carcharinus longimanus (Poey, 1861). Revista Brasileira de Biologia 37: 847–852.
- Rego AA (1979) Contribuição ao conhecimento dos helmintos de raias fluviais Paratrygonidae. Revista Brasileira de Biologia 39: 879–890.
- Rego AA (1984a) Proteocephalidea from Amazonian freshwater fishes: new systematic arrangement for the species described by Woodland as Anthobothrium (Tetraphyllidea). Acta Amazonica 14: 86–94. https://doi.org/10.1590/1809-43921984142094
- Rego AA (1984b) Proteocefalídeos (Cestoda) de Phractocephalus hemioliopterus, peixe da Amazônia. Memórias do Instituto Oswaldo Cruz 79: 257–261. https://doi.org/10.1590/S0074-02761984000200013
- Rego AA (1987a) Redescription of Pterobothrium crassicole Diesing, 1850 (Cestoda: Trypanorhyncha) and revalidation of the species. Memórias do Instituto Oswaldo Cruz 82: 51–53. https://doi.org/10.1590/S0074-02761987000100008
- Rego AA (1987b) Cestóides proteocefalídeos do Brasil. Reorganização taxonômica. Revista Brasileira de Biologia 47: 203–212
- Rego AA (1989) Cestóides proteocefalídeos de “cachara”, Pseudoplatystoma fasciatus (L.) (Pisces, Pimelodidae) de Mato Grosso. Memórias do Instituto Oswaldo Cruz 84: 455–461. https://doi.org/10.1590/S0074-02761989000800080
- Rego AA (1990) Cestóides proteocefalídeos parasitas de pintado, Pseudoplatystoma corruscans (Agassiz) (Pisces, Pimelodidae). Ciência e Cultura 42: 997–1002.
- Rego AA (1991) Redescription of Nomimoscolex piraeeba Woodland, 1934 (Cestoda, Proteocephalidea), from the Amazon catfishes, Brachyplatystoma spp. with proposal of synonyms and invalidation of Endorchiinae and Endorchis. Memórias do Instituto Oswaldo Cruz 86: 229–232. https://doi.org/10.1590/S0074-02761991000200013
- Rego AA (1992) Redescription of Gibsoniela mandube (Woodland, 1935) (Cestoda: Proteocephalidea), a parasite of Ageneiosus brevifilis (Pisces: Siluriformes), and reappraisal of the classification of the Proteocephalideans. Memórias do Instituto Oswaldo Cruz 87: 417–422. https://doi.org/10.1590/S0074-02761992000300012
- Rego AA (1997) Senga sp., occurrence of a pseudophyllid cestode in a Brazilian freshwater fish. Memórias do Instituto Oswaldo Cruz 92: 607. https://doi.org/10.1590/s0074-02761997000500008
- Rego AA (1999) Scolex morphology of proteocephalid cestodes parasites of Neotropical freshwater fishes. Memórias do Instituto Oswaldo Cruz 94: 37–52. https://doi.org/10.1590/S0074-02761999000100011
- Rego AA (2002) Cestóides proteocefalídeos parasitas de Pseudoplatystoma (Pisces, Pimelodidae) da América do Sul. Revista Brasileira de Zoociências 4: 269–282.
- Rego AA (2004) Current state of knowledge of cestodes from Neotropical freshwater fishes and rays. Revista Brasileira de Zoociências 6: 61–79.
- Rego AA, Chubb JC, Pavanelli GC (1999a) Cestodes in South American freshwater teleost fishes: keys to genera and brief description of species. Revista Brasileira de Zoologia 16: 299–367. https://doi.org/10.1590/S0101-81751999000200003
- Rego AA, de Chambrier A (1995) Crepidobothrium eirasi n. sp. (Cestoda, Proteocephalidae), a parasites of the siluroid fish Phractocephalus hemioliopterus (Schneider, 1801) (Pisces, Pimelodidae) from the Brazilian Amazon. Revue Suisse de Zoologie 102: 3–11. https://doi.org/10.5962/bhl.part.80457
- Rego AA, Dias PL (1976) Estudos de cestóides de peixes do Brasil. 3ª Nota: cestóides de raias fluviais Paratrygonidae. Revista Brasileira de Biologia 36: 941–956.
- Rego AA, Gibson DI (1989) Hyperparasitism by helminths: new records of cestodes and nematodes in proteocephalid cestodes from South American siluriform fishes. Memórias do Instituto Oswaldo Cruz 84: 371–376. https://doi.org/10.1590/S0074-02761989000300012
- Rego AA, Ivanov VA (2001) Pseudocrepidobothrium eirasi (Rego and de Chambrier, 1995) gen. n. and comb. nov. (Cestoda, Proteocephalidea), parasite of a South American freshwater fish, and comparative cladistic analysis with Crepidobothrium spp. Acta Scientiarum: Biological Sciences 23: 363–367.
- Rego AA, Machado PM, Pavanelli G (1999b) Sciadocephalus megalodiscus Diesing, 1850 (Cestoda: Corallobothriinae), a parasite of Cichla monoculus Spix, 1831 (Cichlidae), in the Paraná river, State of Paraná, Brazil. Journal of the Helminthlogical Society of Washington 66: 133–137.
- Rego AA, Mayer MT (1976) Ocorrência de duas espécies de tetrafilídeos em tubarão da costa brasileira e considerações sobre os gêneros Cylindrophorus, Platybothrium e Phoreiobothrium (Cestoda, Tetraphyllidea). Revista Brasileira de Biologia 36: 321–328.
- Rego AA, Pavanelli G (1985) Jauella glandicephalus gen. n., sp. n. e Megathylacus brooksi sp. n., cestóides patogênicos para o jaú, Paulicea luetkeni, peixe pimelodídeo. Revista Brasileira de Biologia 45: 643–652.
- Rego AA, Pavanelli G (1987) Cestóides proteocefalídeos do jaú, Paulicea luetkeni, peixe pimelodídeo do Brasil. Revista Brasileira de Biologia 47: 357–361.
- Rego AA, Pavanelli G (1990) Novas espécies de cestóides proteocefálideos: parasitas de peixes näo Siluriformes. Revista Brasileira de Biologia 50: 91–101.
- Rego AA, Pavanelli G (1991) Proteocephalus gibsoni nom. nov. for Proteocephalus ocellatus Rego and Pavanelli, 1990 preoccupied by Proteocephalus ocellatus (Rudolphi, 1802). Revista Brasileira de Biologia 51: 701.
- Rego AA, Pavanelli GC (1992) Redescription of Nomimoscolex admonticellia (Woodland), comb. n (Cestoda: Proteocephalidea) parasite of Pinirampus pirinampu (Spix), a freshwater siluriform fish. Revista Brasileira de Zoologia 9: 283–289. https://doi.org/10.1590/S0101-81751992000200015
- Rego AA, Santos CP (1983) Helmintofauna de cavalas, Scomber japonicus Houtt, do Rio de Janeiro. Memórias do Instituto Oswaldo Cruz 78: 443–448. https://doi.org/10.1590/S0074-02761983000400008
- Rego AA, dos Santos JC, Silva PP (1974) Estudos de cestóides de peixes do Brasil. Memórias do Instituto Oswaldo Cruz 72: 187–204. https://doi.org/10.1590/S0074-02761974000200004
- Rego AA, Schaeffer GV (1999) Histopatologia das lesões de Brachyplatystoma spp. (Pisces: Siluriformes) por Nomimoscolex piraeeba Woodland, 1934 (Cestoda: Proteocephalidea). Revista Brasileira de Ciência Veterinária 6: 98–100. https://doi.org/10.4322/rbcv.2015.144
- Rego AA, Vicente JJ (1988) Excursão científica à zona do Pantanal, Estado de Mato Grosso, para coleta de helmintos. Ciência e Cultura 40: 65–68.
- Rego AA, Vicente JJ, Herrera NI (1968) Sôbre dois novos parasitos de peixe da costa do Peru (Cestoda, Tetraphyllidea). Memórias do Instituto Oswaldo Cruz 66: 145–149. https://doi.org/10.1590/S0074-02761968000200002
- Reis RE (2013) Conserving the freshwater fishes of South America. International Zoo Yearbook 47: 65–70. https://doi.org/10.1111/izy.12000
- Revenga JE (1993) Diphyllobothrium dendriticum and Diphyllobothrium latum in fishes from southern Argentina: association, abundance, distribution, pathological effects, and risk of human infection. Journal of Parasitology 79: 379–383. https://doi.org/10.2307/3283573
- Revenga JE, Perfumo CJ, Ubeda CA, Semenas LG (1995) Difilobotriasis en salmónidos introducidos en el Parque y Reserva Nacional Nahuel Huapi, Argentina: patología de las lesiones producidas por Diphyllobothrium spp. Archivos de Medicina Veterinaria 23: 115–122.
- Revenga JE, Semenas LG (1991) Difilobotriasis en salmónidos introducidos en el Parque y Reserva Nacional Nahuel Huapi, Argentina: morfología de plerocercoides. Archivos de Medicina Veterinaria 23: 157–164.
- Revenga JE, Torres PF, Baiz M (2005) Impact of a caged-trout farm on parasites of Galaxias maculatus in Lake Moreno, southern Argentina. Journal of Parasitology 91: 707–709. https://doi.org/10.1645/ge-441r
- Reyda FB (2008) Intestinal helminths of freshwater stingrays in southeastern Peru, and a new genus and two new species of cestode. Journal of Parasitology 94: 684–699. https://doi.org/10.1645/GE-1230.1
- Reyda FB, Marques FPL (2011) Diversification and species boundaries of Rhinebothrium (Cestoda; Rhinebothriidea) in South American freshwater stingrays (Batoidea; Potamotrygonidae). PLoS ONE 6: e22604. https://doi.org/10.1371/journal.pone.0022604
- Reyda FB, Olson PD (2003) Cestodes of cestodes of Peruvian freshwater stingrays. Journal of Parasitology 89: 1018–1024. https://doi.org/10.1645/ge-3143
- Reyes-Piraino X (1982) Presencia de Hepatoxylon trichiuri (holten, 1802) (Cestoda: Trypanorhyncha) en Oncorhynchus tshawytscha y Somniosus pacificus capturados en Chile. Investigaciones Marinas (Valparaíso) 10: 41–43.
- Ribeiro RS, Luque JL, Alves DR (2002) Aspectos quantitativos dos parasitos da Maria-luiza, Paralonchurus brasiliensis (Osteichthyes: Sciaenidae), do litoral do Estado do Rio de Janeiro. Revista Universidade Rural, Série Ciências da Vida 22: 151–154.
- Ribeiro TS, Lizama MAP, Takemoto RM (2014) Metazoan endoparasites diversity of Pseudoplatystoma corruscans (Siluriformes: Pimelodidae) as an indicator of environmental alterations on a tropical aquatic system. Acta Parasitologica 59: 398–404. https://doi.org/10.2478/s11686-014-0260-0
- Ribeiro TS, Takemoto RM (2014) Resposta inflamatória do pintado à infecção por Nomimoscolex pertierrae (Eucestoda: Proteocephalidea). Boletim do Instituto de Pesca 40: 111–120.
- Riffo R (1991) La fauna de parásitos metazoos del lenguado de ojos grandes Hippoglossina macrops Steindachner, 1876 (Pisces: Bothidae): una aproximación ecológica. Medio Ambiente 11: 54–60.
- Riffo R (1994) Composición taxonómica y características cuantitativas de la fauna de parásitos metazoos del congrio dorado Genypterus blacodes Schneider 1801. Medio Ambiente 12: 27–31.
- Riffo R (1995) Análisis comparativo de la fauna de parásitos metazoos de dos especies de lenguados congenéricos y sintópicos: Paralichthys microps Gunther 1881 y Paralichthys adspersus Steindachner 1867 (Pleuronectiformes: Bothidae) en la Bahía Concepción, Chile. Medio ambiente 12: 51–59.
- Riggenbach E (1895) Beiträge zur Kenntnis der Taenien der Süsswasserfische. Centralblatt für Bakteriologie und Parasitenkunde 18: 609–613.
- Riggenbach E (1896a) Bemerkungen über das Genus Bothriotaenia Railliet. Centralblatt für Bakteriologie, Parasitenkunde und Infektionskrankheiten 20: 222–231.
- Riggenbach E (1896b) Das Genus Ichthyotaenia. Revue Suisse de Zoologie 4: 165–277. https://doi.org/10.5962/bhl.part.70783
- Ringuelet R (1943) Piscicultura del pejerrey o Atherinicultura. Suelo Argentino, Buenos Aires, 162 pp.
- Rivera G, Sarmiento L (1990) Cestodes en peces elasmobranquios en Pucusana. Boletin de Lima 12: 87–88.
- Rocha CA, Rocha CAMJr, da Silva IHF, Alcântara ME, Drago Bisneto MQ, Baker PKB (2016) Ecological aspects of helminth fauna of Brachyplatystoma rousseauxii (Siluriformes: Pimelodidae) from Bay of Marajó, Para State, Brazil. Veterinária e Zootecnia 23: 243–250.
- Rodrigues HO, Rodrigues SS, Faria Z (1990) Contribution to the knowledge of the helminthological fauna of vertebrates of Maricá, Rio de Janeiro State, Brazil. Memórias do Instituto Oswaldo Cruz 85: 115-116. https://doi.org/10.1590/S0074-02761990000100020
- Rodríguez L, Balboa L, George-Nascimento M (2000) Parasitismo en la caballa Scomber japonicus Houttuyn, 1782 y el jurel Trachurus symmetricus murphyi Nichols, 1920 frente a Chile central. Biología Pesquera 28: 15–21.
- Rodríguez L, George-Nascimento M (1996) La fauna de parásitos metazoos del bacalao de profundidad Dissostichus eleginoides Smitt, 1898 (Pisces: Nototheniidae) en Chile central: aspectos taxonómicos, ecológicos y zoogeográficos. Revista Chilena de Historia Natural 69: 21–33.
- Rodríguez T, Tantaleán MV (1980) A study of the helminths of elasmobranch fishes of the Peruvian coast. 1. New reports of tetraphyllideans. Boletin Peruano de Parasitologia 2: 71–75.
- Rossin MA, Timi JT (2010) Parasite assemblages of Nemadactylus bergi (Pisces: Latridae): the role of larval stages in the short-scale predictability. Parasitology Research 107: 1373–1379. https://doi.org/10.1007/s00436-010-2011-y
- Rozas M, Bohle H, Sandoval A, Ildefonso R, Navarrete A, Bustos P (2012) First molecular identification of Diphyllobothrium dendriticum plerocercoids from feral rainbow trout (Oncorhynchus mykiss) in Chile. Journal of Parasitology 98: 1220–1226. https://doi.org/10.1645/jp-ge-3097.1
- Rudolphi CA (1819) Entozoorum synopsis cui accedunt mantesia duplex et indices locupletissimi. Rücker. S. A, Berolini, 811 pp. https://doi.org/10.5962/bhl.title.9157
- Ruedi V, de Chambrier A (2012) Pseudocrepidobothrium ludovici sp n. (Eucestoda: Proteocephalidea), a parasite of Phractocephalus hemioliopterus (Pisces: Pimelodidae) from Brazilian Amazon. Revue Suisse de Zoologie 119: 137–147. https://doi.org/10.5962/bhl.part.150326
- Ruhnke TR (2011) Tapeworms of Elasmobranchs (part III). A monograph on the Phyllobothriidae. Bulletin of the University of Nebraska State Museum 25: 1–208.
- Ruhnke TR, Caira JN (2009) Two new species of Anthobothrium van Beneden, 1850 (Tetraphyllidea: Phyllobothriidae) from carcharhinid sharks, with a redescription of Anthobothrium laciniatum Linton, 1890. Systematic Parasitology 72: 217–227. https://doi.org/10.1007/s11230-008-9168-0
- Ruhnke TR, Caira JN, Cox A (2015) The cestode order Rhinebothriidea no longer family-less: a molecular phylogenetic investigation with erection of two new families and description of eight new species of Anthocephalum. Zootaxa 3904: 51–81. https://doi.org/10.11646/zootaxa.3904.1.3
- Ruhnke TR, Workman RE (2013) Two new species and a new phyllobothriid cestode genus from sharks of the genus Negaprion Whitley (Carcharhiniformes). Systematic Parasitology 85: 37–48. https://doi.org/10.1007/s11230-013-9411-1
- Sabas CSS, Brasil-Sato MC (2014) Helminth fauna parasitizing Pimelodus pohli (Actinopterygii: Pimelodidae) from the upper São Francisco River, Brazil. Revista Brasileira de Parasitologia Veterinária 23: 375–382. https://doi.org/10.1590/S1984-29612014067
- Sabas CSS, Luque JL (2003) Metazoan parasites of weakfish, Cynoscion guatucupa and Macrodon ancylodon (Osteichthyes: Sciaenidae), from the coastal zone of the State of Rio de Janeiro, Brazil. Revista Brasileira de Parasitologia Veterinária 12: 171–178.
- Santos CAML, Zogbi EPV (1971) Infestation of fish in Brazil with Tetrarhynchus fragilis larvae. In: Kreuzer R (Ed.) Fish Inspection and Quality Control. Fishing News (Books) Ltd. London, 262–264.
- Santos-Clapp MD, Brasil-Sato MC (2014) Parasite community of Cichla kelberi (Perciformes, Cichlidae) in the Tres Marias Reservoir, Minas Gerais, Brazil. Revista Brasileira de Parasitologia Veterinária 23: 367–374. https://doi.org/10.1590/s1984-29612014059
- Santos MD, Brasil-Sato MC (2004) Parasitos metazoários de Franciscodoras marmoratus (Reinhardt, 1874), "serrudo" (Siluriformes: Doradidae) do rio São Francisco, Brasil. Revista Brasileira de Parasitologia Veterinária 13: 18–22.
- Santos MD, Brasil-Sato MC (2006) Parasitic community of Fransciscodoras marmoratus (Reinhardt, 1874) (Pisces: Siluriformes, Doradidae) from the upper Sao Francisco River, Brazil. Brazilian Journal of Biology 66: 931–938. https://doi.org/10.1590/S1519-69842006000500019
- Santos MD, Lemos-Pita SRLC, Brasil-Sato MC (2007) Metazoan parasite fauna of Pimelodus maculatus La Cépède, 1803 (Siluriformes, Pimelodidae) from the Guandu river, Rio de Janeiro State, Brazil. Acta Scientiarum: Biological Sciences 29: 101–107. https://doi.org/10.4025/actascibiolsci. v29i1. 130
- Santos RBS, Tavares-Dias M (2010) Células sanguíneas e resposta hematológica de Oxydoras niger (Pisces, Doradidae) oriundos da bacia do médio rio Solimões, estado do Amazonas (Brasil), naturalmente parasitados. Boletim do Instituto de Pesca 36: 283–292.
- Santos RS, Roumbedakis K, Marengoni NG, Takahashi HK, Pimenta FDA, Melo CMR, Martins ML (2011) Proteocephalid cestode infection in tucunaré Cichla sp. (Osteichthyes: Cichlidae) from Paraná River, São Paulo. Arquivo Brasileiro de Medicina Veterinária e Zootecnia 63: 584–590. https://doi.org/10.1590/S0102-09352011000300008
- Santos SMC, Ceccarelli PS, Rêgo RF (2003) Helmintos em peixes do Pantanal sul-mato-grossense: primeira expedição do Programa Pantanal. Boletim Técnico do CEPTA 16: 15–26.
- São Clemente SC (1986a) Plerocercos da ordem Trypanorhyncha, parasitos de corvina Micropogonias furnieri (Desmarest) no litoral do Estado do Rio de Janeiro. Atas da Sociedade de Biologia do Rio de Janeiro 26: 29–36.
- São Clemente SC (1986b) Prevalência e intensidade média de infecção de plerocercos de Trypanorhyncha, parasitando corvina Micropogonias furnieri (Desmarest) no litoral do Rio de Janeiro. Atas da Sociedade de Biologia do Rio de Janeiro 26: 37–44.
- São Clemente SC (1987) Plerocercos de cestóides da ordem Trypanorhyncha em corvina Micropogonias furnieri (Desmarest) e sua importância na inspeção sanitária do pescado. Arquivos Fluminense de Medicina Veterinária 2: 82–83.
- São Clemente SC, Coelho MRT, Serra-Freire NM (1991a) Cestóides parasitos de bagreNetuma barba (Lacépède, 1803) pescados no litoral do Rio de Janeiro e comercializados para consumo humano. Arquivos da Universidade Federal Rural do Rio de Janeiro 14: 27–34.
- São Clemente SC, Gomes DC (1989a) Dasyrhynchus pacificus Robinson, 1965 (Trypanorhyncha: Dasyrhynchidae) description of the adult form. Memórias do Instituto Oswaldo Cruz 84: 113–116. https://doi.org/10.1590/S0074-02761989000100020
- São Clemente SC, Gomes DC (1989b) Trypanorhyncha from sharks of southern Brazilian coast: Eutetrarhynchus vooremi sp. n. and two other species parasites of Mustelus (Pisces, Triakidae). Memórias do Instituto Oswaldo Cruz 84: 475–481. https://doi.org/10.1590/S0074-02761989000800083
- São Clemente SC, Gomes DC (1992) Description of the adult form of Nybelinia (Syngenes) rougetcampanae Dollfus, 1960 and some new data on N. (N.) bisulcata (Linton, 1889) (Trypanorhyncha: Tentaculariidae). Memórias do Instituto Oswaldo Cruz 87: 251–255. https://doi.org/10.1590/S0074-02761992000500047
- São Clemente SC, Gomes DC, Serra-Freire NM (1991b) Prevalência e intensidade de infecção de helmintos da ordem Trypanorhyncha em elasmobrânquios no litoral sul do Brasil. Parasitología al Día 15: 9–14.
- São Clemente SC, Knoff M, Lima FC, Andrada CDG, Felizardo NN, Padovani RES, Gomes DC (2007) Cestóides Trypanorhyncha parasitos de peixe sapo-pescador, Lophius gastrophysus Miranda-Ribeiro, 1915 comercializados no estado do Rio de Janeiro, Brasil. Revista Brasileira de Parasitologia Veterinária 16: 37–42.
- São Clemente SC, Knoff M, Padovani RES, Lima FC, Gomes DC (2004) Cestóides Trypanorhyncha parasitos de Congro-rosa, Genypterus brasiliensis Regan, 1903 comercializados nos municípios de Niterói e Rio de Janeiro, Brasil. Revista Brasileira de Parasitologia Veterinária 13: 97–102.
- São Clemente SC, Lima FC, Uchoa CMA (1995) Parasitos de Balistes vetula (L.) e sua importância na inspeção do pescado. Revista Brasileira de Ciência Veterinária 2: 39–41. https://doi.org/10.4322/rbcv.2015.020
- São Clemente SC, Matos E, Uchoa CMA, Matos P (1993) Trypanorhynch plerocerci in fish of commercial importance in Brazil. Parasitología al Día 17: 52–53.
- São Clemente SC, Pereira JJr, Knoff M, Silva CM, Fernandez JG, Cousin JC (2001) Hepatoxylon trichiuri (Holten, 1802) Dollfus, 1942, Hepatoxylidae Dollfus, 1940 (Eucestoda: Trypanorhyncha) em Prionace glauca (Linnaeus, 1758), do litoral do estado do Rio Grande do Sul e em Coryphaena hippurus Linnaeus, 1758, do litoral do estado do Rio de Janeiro, Brasil. Parasitología al Día 25: 135–137. https://doi.org/10.4067/S0716-07202001000300013
- São Clemente SC, Silva CM, Gottschalk S (1997) Prevalência e intensidade de infecção de cestóides Trypanorhyncha em anchovas, Pomatomus saltatrix (L.) do litoral do Rio de Janeiro, Brasil. Parasitología al Día 21: 54–57.
- Sardella NH, Avendaño MF, Timi JT (1998) Parasite communities of Genypterus blacodes and G. brasiliensis (Pisces: Ophidiidae) from Argentina. Helminthologia 35: 209–218.
- Sardella NH, Timi JT (1996) Parasite communities of Merluccius hubbsi from the Argentinian-Uruguayan common fishing zone. Fisheries Research 27: 81–88. https://doi.org/10.1016/0165-7836(95)00460-2
- Sardella NH, Timi JT (2004) Parasites of Argentine hake in the Argentine Sea: population and infracommunity structure as evidence for host stock discrimination. Journal of Fish Biology 65: 1472–1488. https://doi.org/10.1111/j.0022-1112.2004.00572.x
- Schäffer GV, Rego AA, Pavanelli GC (1992) Peritoneal and visceral cestode larvae in Brazilian freshwater fishes. Memórias do Instituto Oswaldo Cruz 87: 257–258. https://doi.org/10.1590/S0074-02761992000500048
- Scholz T, Bray RA, Kuchta R, Řepová R (2004) Larvae of gryporhynchid cestodes (Cyclophyllidea) from fish: a review. Folia Parasitologica 51: 131–152. https://doi.org/10.14411/fp.2004.018
- Scholz T, de Chambrier A, Kuchta R (2008) Redescription of the tapeworm Monticellia amazonica de Chambrier et Vaucher, 1997 (Cestoda, Proteocephalidea), a parasite of Calophysus macropterus (Siluriformes, Pimelodidae) from the Amazon River. Acta Parasitologica 53: 30–35. https://doi.org/10.2478/s11686-008-0004-0
- Scholz T, de Chambrier A, Kuchta R, Littlewood DT, Waeschenbach A (2013) Macrobothriotaenia ficta (Cestoda: Proteocephalidea), a parasite of sunbeam snake (Xenopeltis unicolor): example of convergent evolution. Zootaxa 3640: 485–499. https://doi.org/10.11646/zootaxa.3640.3.12
- Scholz T, de Chambrier A, Prouza A, Royero R (1996) Redescription of Proteocephalus macrophallus, a parasite of Cichla ocellaris (Pisces: Cichlidae) from South America. Folia Parasitologica 43: 287–291.
- Scholz T, Garcia HH, Kuchta R, Wicht B (2009) Update on the human broad tapeworm (genus Diphyllobothrium), including clinical relevance. Clinical Microbiology Reviews 22: 146–160. https://doi.org/10.1128/CMR.00033-08
- Scholz T, Kuchta R, Williams C (2011) Bothriocephalus acheilognathi. In: P. T. K., Woo, Buchmann K (Eds) Fish Parasites: Pathobiology and Protection. CAB International, Oxfordshire, UK, 282–297.
- Scholz T, Priemer J (1989) Notes to the synonymy of cestodes of the genus Proteocephalus Weinland, 1858 from perches P. percae (Müller, 1780) or P. ocellatus (Rudolphi, 1802)? Folia Parasitologica 36: 69–70.
- Semenas L, Kreiter A (1995) Epidemiología de la difilobotriasis en la región Andino Patagónica. Revista de la Asociación Bioquímica Argentina 59: 203–206.
- Sepúlveda F, Marín SL, Carvajal JG (2004) Metazoan parasites in wild fish and farmed salmon from aquaculture sites in southern Chile. Aquaculture 235: 89–100. https://doi.org/10.1016/j.aquaculture.2003.09.015
- Serrano-Martínez E, Tantaleán MV, Leguía GP, Quispe MH, Casas GCV (2015) Parásitos en Arapaima gigas de la Amazonía Peruana según grupo etario. Revista de Investigaciones Veterinarias del Perú 26: 303–309. https://doi.org/10.15381/rivep.v26i2.11014
- Severino RL, Sarmiento LB (1979) Nueva especie del género Acanthobothrium van Beneden 1849; Cestode: Tetraphyllidea de Myliobatis peruvianus Garman 1913. Revista de Ciencias (Lima) 71: 38–43.
- Severino RL, Verano RM (1980) Acanthobothrium lusarmientoi n. sp. (Cestoda: Tetraphyllidea: Onchobothriidae). Psammobatis caudispina Hildebrand, 1941 (Chondrichtyes: Rajiidae) de Perú. Revista de Ciencias (Lima) 72: 21–27.
- Silva A, Tavares-Dias M, Jerônimo G, Martins M (2011) Parasite diversity in Oxydoras niger (Osteichthyes: Doradidae) from the basin of Solimões River, Amazonas state, Brazil, and the relationship between monogenoidean and condition factor. Brazilian Journal of Biology 71: 791–796. https://doi.org/10.1590/S1519-69842011000400026
- Silva ACSJr (2010) Parasitismo por cestoides da ordem Trypanorhyncha na musculatura de Plagioscion squamosissimus pescada branca (Perciforme: Sciaenidae), comercializados em Macapá, AP. Ciência Animal Brasileira 11: 737–742. https://doi.org/10.5216/cab.v11i3.8495
- Silva CM, São Clemente SC (2001) Nematóides da família Anisakidae e cestóides da ordem Trypanoryncha em filés de dourado (Coryphaena hippurus) e ariocó (Lutjanus synagris) e sua importância na inspeçäo de pescado. Higiene Alimentar 15: 75–79.
- Silva LO, Luque JL, Alves DR (2000a) Metazoários parasitos do peixe espada, Trichiurus lepturus (Osteichthyes: Trichiuridae) do litoral do Estado do Rio de Janeiro, Brasil. Parasitología al Día 24: 97–101. https://doi.org/10.4067/S0716-07202000000300005
- Silva LO, Luque JL, Alves DR, Paraguassú AR (2000b) Ecologia da comunidade de metazoários parasitos do peixe-espada Trichiurus lepturus Linnaeus (Osteichthyes, Trichiuridae) do litoral do Estado do Rio de Janeiro, Brasil. Revista Brasileira de Zoociências 2: 115–133.
- Silva RM, Tavares-Dias M, Dias MWR, Dias MKR, Marinho RGB (2013) Parasitic fauna in hybrid tambacu from fish farms. Pesquisa Agropecuária Brasileira 48: 1049–1057. https://doi.org/10.1590/S0100-204X2013000800034
- Soares IA, Vieira FM, Luque JL (2014) Parasite community of Pagrus pagrus (Sparidae) from Rio de Janeiro, Brazil: evidence of temporal stability. Revista Brasileira de Parasitologia Veterinária 23: 216–223. https://doi.org/10.1590/S1984-29612014047
- Soto J, Carvajal JG (1979) Parásitos cestodos de algunos peces comerciales de Antofagasta, Chile. Boletín Chileno de Parasitología 34: 67–71.
- Spalding MD, Fox HE, Allen GR, Davidson N, Ferdaña ZA, Finlayson M, Halpern BS, Jorge MA, Lombana AL, Lourie SA, Martin KD, McManus E, Molnar J, Recchia CA, Robertson J (2007) Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. BioScience 57: 573–583. https://doi.org/10.1641/B570707
- Suriano DM (1966) Estudio de la fauna parasitaria de “Micropogon opercularis” en relación con problemas zoogeográficos del Atlantico Sur. Comunicaciones del Museo Argentino de Ciencias Naturales “Bernardo Rivadavia” 1: 31–47.
- Suriano DM (2002) Anthobothrium galeorhini n. sp. (Eucestoda: Tetraphyllidea) a parasite of Galeorhinus galeus (Triakidae) from the Argentine coast. Parasite 9: 121–125. https://doi.org/10.1051/parasite/2002092121
- Suriano DM, Labriola JB (1998) Redescription of Anonchocephalus chilensis (Riggenbach, 1896) (Pseudophyllidea: Triaenophoridae) and description of A. patagonicus n. sp. Boletín Chileno de Parasitología 53: 73–77.
- Suriano DM, Labriola JB (2001a) Redescription of Cathetocephalus australis Schmidt et Beveridge, 1990 (Cestoda, Cathetocephalidae) parasite of Carcharinus brachyurus (Gunther) (Pisces, Carcharhiniformes) from the southwestern Atlantic Ocean. Acta Parasitologica 46: 276–279.
- Suriano DM, Labriola JB (2001b) A new Orygmatobothrium Diesing, 1863 (Eucestoda, Tetraphyllidea) parasite of Mustelus schmitti Springer, 1939 (Carcharhiniformes, Triakidae) from the southwestern Atlantic Ocean. Zoosystema 23: 669–674.
- Szidat L (1955) La fauna parásitos de Merluccius hubbsi como carácter auxiliar para la solución de los problemas sistemáticos y zoogeográficos del género Merluccius. Comunicaciones del Instituto Nacional de Investigaciones de Ciencias Naturales, Buenos Aires 3: 1–54
- Szidat L (1960) La parasitología como ciencia auxiliar para develar problemas hidrobiológicos, zoogeográficos y geofísicos del Atlántico Sudamericano. Libro Homenaje al Dr. Eduardo Caballero. Instituto de Biología, UNAM, 577–594.
- Szidat L (1961) Versuch einer Zoogeographie des Süd-Atlantik mit Hilfe von Leitparasiten der Meeresfische. Parasitologische Schriftenreihe 13: 1–98.
- Szidat L (1964) Vergleichende Helminthologische Untersuchungen an den argentinischen grossmöwen Larus marinus dominicanus Lichtenstein und Larus ridibundus maculipennis Lichtenstein nebst neuen Beobachtungen über die Artbildung bei Parasiten. Zeitschrift für Parasitenkunde 24: 351–414. https://doi.org/10.1007/BF00260454
- Szidat L (1969) Los parasitos de la “Palometa” Parona signata (Jenyns, 1842) Berg, 1895 y su aplicacion a problemas zoogeograficos del Atlantico sur. Neotrópica 15: 125–131.
- Szidat L, Nani A (1951) Diplostomiasis cerebralis del pejerrey: una grave epizootia que afecta a la economía nacional producida por larvas de trematodes que destruyen el cerebro de los pejerreyes. Revista del Instituto Nacional de Investigación de las Ciencias Naturales 1: 324–384
- Szidat L, Soria MF (1957) Difilobotriasis en nuestro país. Sobre una nueva especie de Sparganum, parásita de salmónidos y de Diphyllobothrium, parásita de gaviotas del lago Nahuel Huapi. Boletín del Museo Argentino de Ciencias Naturales 9: 1–22.
- Tagle I (1951) Parásitos en la merluza. Boletin Informativo de Parasitologia del Chile 6: 8–9.
- Takemoto RM, Amato JFR, Luque JL (1996a) Comparative analysis of the metazoan parasite communities of leatherjackets, Oligoplites palometa, O. saurus, and O. saliens (Osteichthyes: Carangidae) from Sepetiba Bay, Rio de Janeiro, Brazil. Revista Brasileira de Biologia 56: 639–650.
- Takemoto RM, Amato JFR, Luque JL (1996b) Larvas de Eucestoda parasitas de Oligoplites (Osteichthyes, Carangidae) do litoral do Estado do Rio de Janeiro, Brasil. Revista Unimar 18: 283–291.
- Takemoto RM, Pavanelli GC (1994) Ecological aspects of proteocephalidean cestodes parasites of Paulicea luetkeni (Steindachner) (Osteichthyes: Pimelodidae) from the Paraná River, Paraná, Brazil. Revista Unimar 16: 17–26.
- Takemoto RM, Pavanelli GC (1996) Proteocephalidean cestodes in the freshwater fish Cichla monoculus from the Paraná River, Brazil. Studies on Neotropical Fauna and Environment 31: 123–127. https://doi.org/10.1076/snfe.31.2.123.13326
- Takemoto RM, Pavanelli GC (2000) Aspects of the ecology of proteocephalid cestodes parasites of Sorubim lima (Pimelodidae) of the upper Paraná River, Brazil: I. Structure and influence of host’s size and sex. Revista Brasileira de Biologia 60: 577–584. https://doi.org/10.1590/S0034-71082000000400006
- Takemoto RM, Pavanelli GC, Lizama MAP, Lacerda ACF, Yamada FH, Moreira LHA, Ceschini TL, Bellay S (2009) Diversity of parasites of fish from the Upper Paraná River floodplain, Brazil. Brazilian Journal of Biology 69: 691–705. https://doi.org/10.1590/S1519-69842009000300023
- Tantaleán MV (1975) Hallazgo de larvas plerocercoides de Diphyllobothriidae Lühe (Cestoda) en peces del mar peruano. Boletín Chileno de Parasitología 30: 18–20.
- Tantaleán MV (1991) Nuevos helmintos parasitos en peces elasmobranquio de la costa peruana. Boletin de Lima 13: 25–28.
- Tantaleán MV, Huiza A (1994) Sinopsis de los parásitos de peces marinos de la costa peruana. Biotempo 1: 53–101.
- Tantaleán MV, Rodríguez J (1987) Nuevos registros de helmintos parásitos de peces elasmobranquios de las costas del Perú. Revista de Biologia Tropical 35: 167–168.
- Tanzola R, Guagliardo S (2000) Helminth fauna of the Argentine conger, Conger orbignyanus (Pisces: Anguilliformes). Helminthologia 37: 229–232.
- Tanzola RD, Guagliardo SE, Brizzola SM, Arias MV (1997) Helminth fauna of Porichthys porosissimus (Pisces: Batrachoidiformes) in the estuary of Bahia Blanca Argentina. Helminthologia 34: 221–227.
- Tanzola RD, Guagliardo SE, Brizzola SM, Arias MV, Botte SE (1998) Parasite assemblage of Sympterygia bonapartei (Pisces: Rajidae), an endemic skate of the Southwest Atlantic. Helminthologia 35: 123–129.
- Tavares LER, Bicudo AJA, Luque JL (2004) Metazoan parasites of the needlefish Tylosurus acus (Lacépède, 1803) (Osteichthyes: Belonidae) from the coastal zone of the State of Rio de Janeiro, Brazil Revista Brasileira de Parasitologia Veterinária 13: 36–40.
- Tavares LER, Luque JL (2004) Community ecology of the metazoan parasites of white sea catfish, Netuma barba (Osteichthyes: Ariidae), from the coastal zone of the state of Rio de Janeiro, Brazil. Brazilian Journal of Biology 64: 169–176. https://doi.org/10.1590/S1519-69842004000100019
- Tavares LER, Luque JL (2008) Similarity between metazoan parasite communities of two sympatric brackish fish species from Brazil. Journal of Parasitology 94: 985–989. https://doi.org/10.1645/GE-1460.1
- Tavares LER, Luque JL, Bicudo AJA (2005) Community ecology of metazoan parasites of the anchovy Anchoa tricolor (Osteichthyes: Engraulidae) from the coastal zone of the State of Rio de Janeiro, Brazil. Brazilian Journal of Biology 65: 533–540. https://doi.org/10.1590/S1519-69842005000300019
- Tavares LER, Luque JL, Neto SLB (2001) Ecologia da comunidade de metazoários parasitos do olho-de-cão Priacanthus arenatus (Cuvier, 1829) (Osteichthyes, Priacanthidae) do litoral do estado do Rio de Janeiro, Brasil. Revista Brasileira de Zoociências 3: 45–59.
- Thatcher VE (2006) Amazon Fish Parasites. Pensoft Publishers, Sofia-Moscow, 508 pp.
- Timi JT (2003) Parasites of Argentine anchovy in the south‐west Atlantic: latitudinal patterns and their use for discrimination of host populations. Journal of Fish Biology 63: 90–107. https://doi.org/10.1046/j.1095-8649.2003.00131.x
- Timi JT, Lanfranchi AL (2009a) The metazoan parasite communities of the Argentinean sandperch Pseudopercis semifasciata (Pisces: Perciformes) and their use to elucidate the stock structure of the host. Parasitology 136: 1209–1219. https://doi.org/10.1017/S0031182009990503
- Timi JT, Lanfranchi AL (2009b) The importance of the compound community on the parasite infracommunity structure in a small benthic fish. Parasitology Research 104: 295–302. https://doi.org/10.1007/s00436-008-1191-1
- Timi JT, Lanfranchi AL (2013) Ontogenetic changes in heterogeneity of parasite communities of fish: disentangling the relative role of compositional versus abundance variability. Parasitology 140: 309–317. https://doi.org/10.1017/S0031182012001606
- Timi JT, Lanfranchi AL, Etchegoin JA (2009) Seasonal stability and spatial variability of parasites in Brazilian sandperch Pinguipes brasilianus from the Northern Argentine Sea: evidence for stock discrimination. Journal of Fish Biology 74: 1206–1225. https://doi.org/10.1111/j.1095-8649.2009.02190.x
- Timi JT, Lanfranchi AL, Etchegoin JA, Cremonte F (2008) Parasites of the Brazilian sandperch Pinguipes brasilianus Cuvier: a tool for stock discrimination in the Argentine Sea. Journal of Fish Biology 72: 1332–1342. https://doi.org/10.1111/j.1095-8649.2008.01800.x
- Timi JT, Lanfranchi AL, Luque JL (2010a) Similarity in parasite communities of the teleost fish Pinguipes brasilianus in the southwestern Atlantic: infracommunities as a tool to detect geographical patterns. International Journal for Parasitology 40: 243–254. https://doi.org/10.1016/j.ijpara.2009.07.006
- Timi JT, Luque JL, Poulin R (2010b) Host ontogeny and the temporal decay of similarity in parasite communities of marine fish. International Journal for Parasitology 40: 963–968. https://doi.org/10.1016/j.ijpara.2010.02.005
- Timi JT, Luque JL, Sardella NH (2005) Parasites of Cynoscion guatucupa along South American Atlantic coasts: evidence for stock discrimination. Journal of Fish Biology 67: 1603–1618. https://doi.org/10.1111/j.1095-8649.2005.00867.x
- Timi JT, Poulin R (2003) Parasite community structure within and across host populations of a marine pelagic fish: how repeatable is it? International Journal for Parasitology 33: 1353–1362. https://doi.org/10.1016/S0020-7519(03)00203-0
- Torres P (1990) Primeros registros de endohelmintos parásitos en el salmón coho Oncorhynchus kisutch (Walbaum), introducido en Chile. Archivos de Medicina Veterinaria 22: 105–107.
- Torres P, Aedo E, Figueroa L, Siegmund I, Silva R, Navarrete N, Puga S, Marín F, Aedo E (2000) Infección por helmintos parásitos en salmón coho, Oncorhynchus kisutch, durante su retorno al río Simpson, Chile. Boletín Chileno de Parasitología 55: 31–35. https://doi.org/10.4067/S0365-94022000000100009
- Torres P, Contreras A, Figueroa L, Franjola R, González H, Martín R (1977) Investigaciones sobre Pseudophyllidea (Carus, 1813) en el sur de Chile. I. Estudio preliminar sobre infección por plerocercoides de Diphyllobothrium sp. em Salmo gairdneri Richardson, 1836 del lago Calafquén (39°32"S, 72°09"O), Chile. Boletín Chileno de Parasitología 32: 73–80.
- Torres P, Contreras A, Revenga J, Fritz N (1993) Helminth parasites in fishes from Valdivia and Tornagaleones river estuaries in the south of Chile. Memórias do Instituto Oswaldo Cruz 88: 491–492. https://doi.org/10.1590/S0074-02761993000300021
- Torres P, Cubillos V, Aedo E, Silva R, Garrido O, Aedo JE (1995) Prevalencia y aspectos patológicos de la difilobotriasis en salmones de retorno, Oncorhynchus kisutch de Coyhaique, XI Región de Chile. Archivos de Medicina Veterinaria 27: 107–114.
- Torres P, Cubillos V, Gesche W, Rebolledo C, Montefusco A, Miranda JC, Arenas J, Mira A, Nilo M, Abello C (1991) Difilobotriasis en salmónidos introducidos en lagos del sur de Chile. Aspectos patológicos, relación con infección humana, animales domésticos y aves piscívoras. Archivos de Medicina Veterinaria 23: 165–183.
- Torres P, Cuevas C, Tang M, Barra M, Franjola R, Navarrete N, Montefusco A, Otth L, Wilson G, Puga S, Figueroa L, Cerda O (2004) Introduced and native fishes as infection foci of Diphyllobothrium spp. in humans and dogs from two localities at Lake Panguipulli in Southern Chile. Comparative Parasitology 71: 111–117. https://doi.org/10.1654/4119
- Torres P, Figueroa L, Franjola R (1983) Pseudophyllidea in the South of Chile. IX. Types of plerocercoids in trouts from five lakes and new cases of Diphyllobothrium latum in man and D. pacificum in a dog. International Journal of Zoonoses 10: 15–21.
- Torres P, Franjola R, Contreras LF (1982) Pseudophyllidea (Carus, 1813) en el sur de Chile: VII. Distribución estacional de la infección por plerocercoides en Salmo gairdneri (Richardson) del lago Calafquén. Zentralblatt für Veterinärmedizin Reihe B 29: 67–75. https://doi.org/10.1111/j.1439-0450.1982.tb01190.x
- Torres P, Franjola R, Figueroa L, Schlatter R, González H, Contreras B, Martin R (1981) Researches on Pseudophyllidea (Carus, 1813) in the south of Chile. IV Occurrence of Diphyllobothrium dendriticum (Nitzch). Journal of Helminthology 55: 173–188. https://doi.org/10.1017/S0022149X00026833
- Torres P, Franjola R, Pérez J, Auad S, Uherek F, Miranda JC, Flores L, Riquelme J, Salazar S, Hermosilla C, Rojo R (1989a) Epidemiología de la difilobotriasis en la cuenca del río Valdivia, Chile. Revista de Saúde Pública 23: 45–57. https://doi.org/10.1590/S0034-89101989000100007
- Torres P, Gesche W, Montefusco A, Miranda J, Dietz P, Huijse R (1998) Diphyllobothriosis humana y en peces del lago Riñihue, Chile: efecto de la actividad educativa, distribución estacional y relación con sexo, talla y dieta de los peces. Archivos de Medicina Veterinaria 30: 31–45. https://doi.org/10.4067/S0301-732X1998000100004
- Torres P, Leyán V, Puga S (2012) Prevalence, intensity, and abundance of infection and pathogenesis caused by diphyllobothriosis in vulnerable, native fish and introduced trout in Lake Panguipulli, Chile. Journal of Wildlife Diseases 48: 937–950. https://doi.org/10.7589/2011-08-235
- Torres P, Lopez JC, Cubillos V, Lobos C, Silva R (2002) Visceral diphyllobothriosis in a cultured rainbow trout, Oncorhynchus mykiss (Walbaum), in Chile. Journal of Fish Diseases 25: 375–379. https://doi.org/10.1046/j.1365-2761.2002.00381.x
- Torres P, Puga S (2011) Comparative efficacy of candling and glass plate compression for detection of diphyllobothriosis in rainbow trout (Oncorhynchus mykiss) musculature. Revue Scientifique et Technique (International Office of Epizootics) 30: 831–837.
- Torres P, Puga S, Castillo L, Lamilla J, Miranda J (2014) Helmintos, myxozoos y microsporidios en músculos de peces comercializados frescos y su importancia como riesgo potencial para la salud humana en la ciudad de Valdivia, Chile. Archivos de Medicina Veterinaria 46: 83–92. https://doi.org/10.4067/S0301-732X2014000100012
- Torres P, Quintanilla J, Rozas M, Miranda P, Ibarra R, San Martín M, Raddatz B, Wolter M, Villegas A, Canobra C, Hausdorf M, Silva R (2010) Endohelminth parasites from salmonids in intensive culture from southern Chile. Journal of Parasitology 96: 669–670. https://doi.org/10.1645/GE-2211.1
- Torres P, Roman C, Figueroa L, Franjola R (1980) Plerocercoids of Diphyllobothrium (Cobbold) in fishes and identification of copepods in plankton from Calafquen Lake, Chile. Indian Journal of Parasitology 4: 207–208.
- Torres P, Ruíz E, Rebolledo C, Mira A, Cubillos V, Navarrete N, Gesche W, Montefusco A, Valdés L, Alberdi A (1990) Parasitism in fishes and human riverside communities of the Huillinco and Natri lakes (Great Island of Chiloe), Chile. Boletín Chileno de Parasitología 45: 47–55.
- Torres P, Torres J, Garrido O, Thibaut I (1989b) Investigaciones sobre Pseudophyllidea (Carus, 1813) en el sur de Chile. X. Observaciones experimentales sobre la coexistencia de plerocercoides de Diphyllobothrium latum (L.) y D. dendriticum (Nitzch) en salmonidos de la cuenca del rio Valdívia. Archivos de Medicina Veterinaria 21: 51–57.
- Travassos L (1940) Relatório da terceira excursão a zona da Estrada de Ferro Noroeste do Brasil realizada em Fevereiro e Março de 1940: I-Introdução. Memórias do Instituto Oswaldo Cruz 35: 607–696. https://doi.org/10.1590/S0074-02761940000300013
- Travassos L (1944) Relatório da excursão do Instituto Oswaldo Cruz ao Município de Santa Teresa, no Estado do Espírito Santo, em agôsto e setembro de 1943. Memórias do Instituto Oswaldo Cruz 40: 121–128. https://doi.org/10.1590/S0074-02761944000200002
- Travassos L (1945) Relatório da excursão do Instituto Oswaldo Cruz ao Rio Paraná (Porto Cabral), em março e abril de 1944. Memórias do Instituto Oswaldo Cruz 42: 151–165. https://doi.org/10.1590/S0074-02761945000100010
- Travassos L (1947) Relatório da excursão do Instituto Oswaldo Cruz realizada no Estado de S. Paulo em novembro e dezembro de 1946. Memórias do Instituto Oswaldo Cruz 45: 619–627. https://doi.org/10.1590/S0074-02761947000300009
- Travassos L, Pinto C, Muniz J (1927) Excursão scientifica ao Estado de Matto Grosso na zona do Pantanal (Margens dos Rios S. Lourenço e Cuyabá) realisada em 1922. Memórias do Instituto Oswaldo Cruz 20: 249–269. https://doi.org/10.1590/S0074-02761927000200004
- Travassos L, Teixeira de Freitas JF (1940) Relatório da excursão científica realisada na zona da Estrada de Ferro Noroeste do Brasil em Julho de 1939. Memórias do Instituto Oswaldo Cruz 35: 525–556. https://doi.org/10.1590/S0074-02761940000300004
- Travassos L, Teixeira de Freitas JF (1941) Relatório da quinta excursão do Instituto Oswaldo Cruz, realizada à zona da Estrada de Ferro Noroeste do Brasil, em janeiro de 1941: II Pesquisas Parasitológicas. Memórias do Instituto Oswaldo Cruz 36: 272–300. https://doi.org/10.1590/S0074-02761941000300003
- Travassos L, Teixeira de Freitas JF (1942) Relatório da sexta excursão do Instituto Oswaldo Cruz, realizada à zona da Estrada de Ferro Noroeste do Brasil, em novembro de 1941. Memórias do Instituto Oswaldo Cruz 37: 259–286. https://doi.org/10.1590/S0074-02761942000300004
- Travassos L, Teixeira de Freitas JF (1943) Relatório da sétima excursão científica do Instituto Oswaldo Cruz, realizada a zona da Estrada de Ferro Noroeste do Brasil, em maio de 1942. Memórias do Instituto Oswaldo Cruz 38: 385–412. https://doi.org/10.1590/S0074-02761943000300007
- Travassos L, Teixeira de Freitas JF (1948) Relatório da excursão do Instituto Oswaldo Cruz ao norte do Estado do Espírito Santo, junto ao Parque de Reserva e Refúgio Soóretama, em fevereiro e março de 1948. Memórias do Instituto Oswaldo Cruz 46: 605–631. https://doi.org/10.1590/S0074-02761948000300006
- Travassos L, Teixeira de Freitas JF (1964) Pesquisas helmintológicas, realizadas em Maicuru, Estado do Pará. Museu Paraense Emílio Goeldi, Belém, 16 pp.
- Travassos L, Teixeira de Freitas JF, Bührnheim PF (1967) Relatório da excursão do Instituto Oswaldo Cruz ao estado do Espírito Santo em novembro de 1964. Boletím do Museu de Biologia Prof Mello Leitâo (Zoologia) 31: 1‒54
- Tresierra A, Escalante H, Benitez J (1986) Hábitos alimenticios y fauna helmintológica de tres especies de elasmobranquios del mar peruano. Rebiol 6: 35–46.
- Tyler GA (2006) Tapeworms of elasmobranchs (Part II). A monograph on the Diphyllidea (Platyhelminthes: Cestoda). Bulletin of the University of Nebraska State Museum 20: 1–142.
- Valdivia IM, Chávez RA, Oliva ME (2007) Metazoan parasites of Engraulis ringens as tools for stock discrimination along the Chilean coast. Journal of Fish Biology 70: 1504–1511. https://doi.org/10.1111/j.1095-8649.2007.01429.x
- Vales DG, García NA, Crespo EA, Timi JT (2011) Parasites of a marine benthic fish in the Southwestern Atlantic: searching for geographical recurrent patterns of community structure. Parasitology Research 108: 261–272. https://doi.org/10.1007/s00436-010-2052-2
- Vásquez-Ruiz CE, Jara-Campos CA (2012) Prevalencia e intensidad parasitaria en Coryphaena hippurus y Mugil cephalus (Teleostei) desembarcados en los puertos Salaverry y Paita (Perú). Sciéndo 15: 22–32.
- Vergara LA, George-Nascimento M (1982) Contribucion al estudio del parasitismo en el congrio colorado Genypterus chilensis (Guichenot, 1848). Boletín Chileno de Parasitología 37: 9–14.
- Vicente JJ, Fernandes GL (1978) Contribuição ao conhecimento dos helmintos de Bagre bagre (linnaeus, 1766) Fowler, 1841 e de Macrodon ancylodon (Bloch, 1801) Jordan, Evermann e Clark, 1930, no litoral da ilha de São Luís, Estado do Maranhão, Brasil. Boletim do Laboratório de Hidrobiologia 2: 91–95.
- Vicente JJ, Pinto RM, Aguilera O (1989) On Dichelyne (Cucullanellus) elongatus (Tornquist, 1931) Petter, 1974: South American correlated species (Nematoda, Cucullanidae) and some other helminths of Micropogonias furnieri (Desmarest, 1823) (Pisces, Sciaenidae). Memórias do Instituto Oswaldo Cruz 84: 357–361. https://doi.org/10.1590/S0074-02761989000300010
- Vicente JJ, Santos E (1974) Alguns helmintos de peixes do litoral norte fluminense-II. Memórias do Instituto Oswaldo Cruz 72: 173–180. https://doi.org/10.1590/S0074-02761974000200002
- Videira M, Velasco M, Dias L, Matos P, Almeida HDF, Costa ML, São Clemente SC, Matos E (2013) Gobioides broussonnetii (Gobiidae): a new host for Pterobothrium crassicolle (Trypanorhyncha) on Marajó Island, northern Brazil. Revista Brasileira de Parasitologia Veterinária 22: 398–401. https://doi.org/10.1590/S1984-29612013000300013
- Villalba CS, Fernández JB (1985) Parásitos de Mola ramsayi (Giglioli, 1883) (Pisces: Molidae) en Chile. Boletín de la Sociedad de Biología de Concepción 56: 71–78.
- Viozzi G, Semenas L, Brugni N, Flores VR (2009) Metazoan parasites of Galaxias maculatus (Osmeriformes: Galaxiidae) from Argentinean Patagonia. Comparative Parasitology 76: 229–239. https://doi.org/10.1654/4328.1
- Vogelsang EG, Mayaudon TH (1959) Contribucion al estudio de la parasitología animal en Venezuela (XXIII). Revista de Medicina Veterinaria y Parasitología 18: 5–9.
- Waeschenbach A, Webster BL, Littlewood DT (2012) Adding resolution to ordinal level relationships of tapeworms (Platyhelminthes: Cestoda) with large fragments of mtDNA. Molecular Phylogenetics and Evolution 63: 834–847. https://doi.org/10.1016/j.ympev.2012.02.020
- Waicheim A, Blasetti G, Cordero P, Rauque C, Viozzi G (2014) Macroparasites of the invasive fish, Cyprinus carpio, in Patagonia, Argentina. Comparative Parasitology 81: 270–275. https://doi.org/10.1654/1525-2647-81.2.270
- Whittaker FH, Carvajal JG (1980) Scanning electron microscopy of scolices of some cestodes from elasmobranchs. Proceedings of the Helminthological Society of Washington 47: 256–259.
- Whittaker FH, Carvajal JG, Apkarian R (1982) Scanning electron microscopy of the scolex of Grillotia dollfusi Carvajal 1971 (Cestoda: Trypanorhyncha). Journal of Parasitology 68: 1173–1175. https://doi.org/10.2307/3281120
- Wicht B, Yanagida T, Scholz T, Ito A, Jiménez JA, Brabec J (2010) Multiplex PCR for differential identification of broad tapeworms (Cestoda: Diphyllobothrium) infecting humans. Journal of Clinical Microbiology 48: 3111–3116. https://doi.org/10.1128/JCM.00445-10
- Willis SC, Macrander J, Farias IP, Ortí G (2012) Simultaneous delimitation of species and quantification of interspecific hybridization in Amazonian peacock cichlids (genus Cichla) using multi-locus data. BMC Evolutionary Biology 12: 96. https://doi.org/10.1186/1471-2148-12-96
- Wolffügel K (1949) ¿Es autóctono el Diphyllobothrium en Chile? Boletín de la Sociedad de Biología de Concepción 24: 85–89.
- Woodland WNF (1933a) On a new subfamily of proteocephalid cestodes–the Othinoscolecinae–from the Amazon siluroid fish Platystomatichthys sturio (Kner). Parasitology 25: 491–500. https://doi.org/10.1017/S0031182000019739
- Woodland WNF (1933b) On the anatomy of some fish cestodes described by Diesing from the Amazon. Quarterly Journal of Microscopical Science 76: 175–208.
- Woodland WNF (1933c) On two new cestodes from the Amazon siluroid fish Brachyplatystoma vaillanti Cuv. and Val. Parasitology 25: 485–490. https://doi.org/10.1017/S0031182000019727
- Woodland WNF (1934a) On the Amphilaphorchidinae, a new subfamily of proteocephalid cestodes, and Myzophorus admonticellia, gen. et sp. n., parasitic in Pirinampus spp. from the Amazon. Parasitology 26: 141–149. https://doi.org/10.1017/S0031182000023441
- Woodland WNF (1934b) On some remarkable new cestodes from the Amazon siluroid fish, Brachyplatystoma filamentosum (Lichtenstein). Parasitology 26: 268–277. https://doi.org/10.1017/S0031182000023556
- Woodland WNF (1934c) On six new cestodes from Amazon fishes. Proceedings of the Zoological Society of London 104: 33–44. https://doi.org/10.1111/j.1469-7998.1934.tb06218.x
- Woodland WNF (1935a) Additional cestodes from the Amazon siluroids pirarará, dorad, and sudobim. Proceedings of the Zoological Society of London 104: 851–862. https://doi.org/10.1111/j.1096-3642.1934.tb01669.x
- Woodland WNF (1935b) Some more remarkable cestodes from Amazon siluroid fish. Parasitology 27: 207–225. https://doi.org/10.1017/S0031182000023556
- Woodland WNF (1935c) Some new proteocephalids and a ptychobothriid (Cestoda) from the Amazon. Proceedings of the Zoological Society of London 105: 619–623. https://doi.org/10.1111/j.1096-3642.1935.tb01685.x
- Yamada FH, Takemoto RM (2013) Metazoan parasite fauna of two peacock-bass cichlid fish in Brazil. Check List 9: 1371–1377. https://doi.org/10.15560/9.6.1371
- Yamada FH, Takemoto RM, Pavanelli GC (2007) Ecological aspects of ectoparasites from the gills of Satanoperca pappaterra (Heckel, 1840) (Cichlidae) from the upper Paraná river floodplain, Brazil. Acta Scientiarum: Biological Sciences 29: 331–336. https://doi.org/10.4025/actascibiolsci.v29i3.555
- Yáñez P (1950) Observación de un Dibothriorhynchus parásito del azulejo. Revista de Biología Marina 2: 165–166.
- Zago AC, Franceschini L, Zocoller-Seno MC, Veríssimo-Silveira R, Maia AAD, Ikefuti CV, Silva RJ (2013) The helminth community of Geophagus proximus (Perciformes: Cichlidae) from a tributary of the Paraná River, Ilha Solteira Reservoir, São Paulo State, Brazil. Journal of Helminthology 87: 203–211. https://doi.org/10.1017/S0022149X12000223
- Zamparo D, Brooks DR, Barriga R (1999) Pararhinebothroides hobergi n. gen. n. sp. (Eucestoda: Tetraphyllidea) in Urobatis tumbesensis (Chondrichthyes: Myliobatiformes) from coastal Ecuador. Journal of Parasitology 85: 534–539. https://doi.org/10.2307/3285791
- Zehnder MP, de Chambrier A (2000) Morphological and molecular analyses of the genera Peltidocotyle Diesing 1850 and Othinoscolex Woodland 1933, and a morphological study of Woodlandiella Freze, 1965 (Eucestoda, Proteocephalidea), parasites of South American siluriform fishes (Pimelodidae). Systematic Parasitology 46: 33–43. https://doi.org/10.1023/A:1006252601201
- Zehnder MP, de Chambrier A, Vaucher C, Mariaux J (2000) Nomimoscolex suspectus n. sp. (Eucestoda: Proteocephalidea: Zygobothriinae) with morphological and molecular phylogenetic analyses of the genus. Systematic Parasitology 47: 157–172. https://doi.org/10.1023/A:1006465026316
- Zehnder MP, Mariaux J (1999) Molecular systematic analysis of the order Proteocephalidea (Eucestoda) based on mitochondrial and nuclear rDNA sequences. International Journal for Parasitology 29: 1841–1852. https://doi.org/10.1016/S0020-7519(99)00122-8